Abstract
Background. The current US national stockpile of influenza H5 vaccine was produced using the antigen from the strain A/Vietnam/1203/2004 (a clade 1 H5 virus). Recent H5 disease has been caused by antigenically divergent H5 viruses, including A/Indonesia/05/2005 (a clade 2 H5 virus).
Methods. The influence of schedule on the antibody response to 2 doses of H5 vaccines (one a clade 1 hemagglutinin protein [HA] vaccine and one a clade 2 HA vaccine) containing 90 μg of antigen was evaluated in healthy adults 18–49 years of age.
Results. Two doses of vaccine were required to induce antibody titers ≥1:10 in most subjects. Accelerated schedules were immunogenic, and antibody developed after vaccinations on days 0 and 7, 0 and 14, and 0 and 28, with the day 0 and 7 schedule inducing lower titers than those induced with the other schedules. With mixed vaccine schedules of clade 1 followed by clade 2 vaccine administration, the first vaccination primed for a heterologous boost. The heterologous response was improved when the second vaccination was given 6 months after the first, compared with the response when the second vaccination was given after an interval of 1 month.
Conclusions. An accelerated vaccine schedule of injections administered at days 0 and 14 was as immunogenic as a vaccine schedule of injections at days 0 and 28, but both schedules were inferior to a vaccine schedule of injections administered at 0 and 6 months for priming for heterologous vaccine boosting.
Clinical Trial Registry Number: NCT00703053
BACKGROUND
Severe disease in humans due to avian influenza viruses of the H5N1 subtype has increased concern regarding the potential emergence of these viruses in pandemic form [1, 2]. Planning for control of such pandemics is of vital importance, and a cornerstone of this planning is the development of effective vaccines against H5N1infection. However, the relatively low frequencies of immune response seen in previous studies of inactivated H5N1 vaccines in humans [3–5] and the likely need for a 2-dose schedule of vaccination pose significant obstacles to vaccine approaches for pandemic response.
In one previous study, serum antibody responses to a 2-dose schedule of an H5 vaccine generated from the avian influenza A/Duck/Singapore/97 virus were modest, and the use of the adjuvant MF59 was required [3]. However, when these same subjects were revaccinated 16 months later with a single dose of the same vaccine and the adjuvant MF59, brisk responses occurred, and the additional dose resulted in titers that were much higher than those seen after the initial dose series [6]. In addition, subjects who were revaccinated with the A/Duck/Singapore/97 vaccine developed significant levels of antibody against the antigenic variants A/Vietnam/04 and A/Indonesia/05 after vaccination [7].
Results from another study [8] have suggested that a single 90-μg dose of A/Vietnam/04 vaccine in heterologously primed subjects resulted in higher titers of hemagglutination-inhibiting antibody (HAI) and neutralizing antibody than were seen after 2 doses (2 doses of 90 μg) in previously unprimed subjects. The US national stockpile contains 20 million doses of A/Vietnam/04 vaccine, and we sought to evaluate the value of priming with this older H5 strain on the subsequent boost with more-contemporary H5 antigen (A/Indonesia/05). We also examined accelerated vaccine schedules to determine the minimum time needed between priming and boosting.
METHODS
Vaccines
The rgA/Vietnam/1203/2004 Batch #04-067 clade 1 vaccine (“rg” indicates a reverse-genetics modified virus; referred to hereafter as A/Vietnam/04 vaccine) is a monovalent submit influenza vaccine that has been approved by the US Food and Drug Administration (FDA) for human use. It consists of a 90-μg/mL dose for intramuscular administration (see the package insert for details) [11].
The investigational inactivated monovalent subvirion clade 2 influenza vaccine was produced using influenza virus A/Indonesia/05/2005 PR8-IBCDC-RG2 (hereafter referred to as A/Indonesia/05 vaccine) by Sanofi Pasteur, under contract to the US Department of Health and Human Services. This investigational vaccine was produced using full-scale processes and pilot plant processes nearly identical to those used to manufacture A/Vietnam/04 vaccine. The vaccine contained the preservative thimerosal at a concentration of ∼0.01% and gelatin as a stabilizer at a concentration of ∼0.05%.
The investigational vaccine was essentially identical to the A/Vietnam/04 clade 1 H5N1 vaccine, except for the following: it contains the A/Indonesia/05/2005 H5N1 strain, and the formulation of study vaccines proceeded with no further addition of the preservative thimerosal or gelatin stabilizer. Therefore, the thimerosal and gelatin content of each formulation was reduced in proportion to the dilution factor (based on the hemagglutinin protein [HA] target quality) of the individual formulation, and the vaccine was considered as not containing preservative. The A/Indonesia/05 vaccine was supplied in 0.5 mL unit-dose vials as a sterile solution containing 90 μg of H5 HA per 0.5-mL dose. For one group, a combination of the 2 H5 vaccines was administered as 45 μg of each vaccine in 2 separate injections (0.5 mL of A/Vietnam/04 vaccine or 0.25 mL of A/Indonesia/05 vaccine) in the same extremity.
Study Design
The study evaluated the dose and schedules of unadjuvanted inactivated subvirion H5N1 vaccines belonging to the same or different clades in H5-naive, healthy adults 18–49 years of age and without exposure to H5 vaccine or a history of H5 virus infection. The study also determined whether boosting of subjects given the inactivated influenza rg A/Vietnam/1203/04 vaccine with a heterologous antigen inactivated influenza rg A/Indonesia/05/05 resulted in broader or higher immune responses, compared with boosting by the homologous antigen. The influence of vaccine schedule was examined by evaluating the immune response to 2 doses of H5 vaccine given at intervals <1 month apart. Safety data were collected.
The study was conducted as a randomized, prospective-controlled, multi-center trial. H5-naive, healthy adult subjects were randomized to receive varying schedules (two 90-μg doses separated by 7, 14, 28, or 180 days) and clades (clade 1 followed by clade 1, clade 1 followed by clade 2, clade 2 followed by clade 2, or a combination of clades 1 and 2 [45 μg of each vaccine] followed by a combination of clades 1 and 2) of unadjuvanted inactivated subvirion H5N1 vaccines (Table 1). Subjects with occupational or recreational exposure to poultry, including raising chickens or hunting and handling wild fowl, were excluded. Written informed consent was obtained from all subjects.
Table 1.
Vaccine Antigen and Dose for Each of 9 Groups of Subjects
| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7a | 8 | 9 |
| Day 0 | 90 μg VN | 90 μg VN | 90 μg VN | 90 μg VN | 90 μg I | 90 μg VN | 45 μg VN and 45 μg I | 90 μg I | 90 μg VN |
| Day 7 | … | 90 μg VN | … | … | … | … | … | … | … |
| Day 14 | … | … | 90 μg VN | … | … | … | … | … | … |
| Day 28 | … | … | … | 90 μg VN | 90 μg I | 90 μg I | 45 μg VN and 45 μg I | … | … |
| Day 180 | … | … | … | … | … | … | … | 90 μg I | 90 μg I |
NOTE. Booster vaccinations at one follow-up time were administered for 8 of the groups as indicated. Boosters were not given to group 1. I, inactivated rg A/Indonesia/05/05 H5N1 (clade 2); VN, inactivated rg A/Vietnam/1203/04, H5N1 (clade 1).
Group 7 vaccines were administered as separate injections in the same arm at day 0 and day 28.
Vaccine preparation and administration were performed by an unblinded vaccine administrator who was not involved in subsequent study procedures. A vaccine dose of 90 μg was delivered by deep intramuscular injection with a 1-inch needle. A vaccine dose of 45 μg of each antigen was delivered as 2 separate deep intramuscular injections in the same arm, one above the other, ∼2 cm apart. Because study products were administered in different volumes, the subject was asked to look away when receiving the injection. The unblinded administrator attempted to conceal the syringe as much as possible to limit the ability of the blinded staff and the subject to see the volume of vaccine being administered. All clinical assessments were performed by study personnel blinded to the degree possible, noting that varying schedules, the number of vaccination sites evaluated, and the product volumes administered were inherently unblinding.
Subjects recorded their daily oral temperature and any systemic and local adverse events or serious adverse events that occurred within the week following vaccination. Subjects received a follow-up telephone call at 1–3 days after vaccination (approximately day 2 after dosing) to obtain information on adverse events and serious adverse events. A Safety Monitoring Committee (SMC) reviewed adverse events and reactogenicity data and made recommendations about the safety of the vaccine for individuals, groups, or the entire protocol.
To answer the question of influence of schedule and boosting antigen, the schedule of vaccine administration and collection of serum for assessment of antibody responses varied in this study, depending on the group and the responses being studied (Tables 1, 2). Serum samples were tested in a fully blinded manner at a central laboratory. Immunogenicity responses were measured by serum hemagglutination inhibiting (HI) antibody and microneutralizing (Neut) antibody titers against the A/Vietnam/1203/04 and A/Indonesia/05/05 H5N1 viruses, as previously described [9, 10]. The geometric mean titer (GMT) of duplicate results for each specified time point was used for all immunogenicity calculations. Serum samples with antibody titers of <1:10 were assigned values of 5 for purposes of mean titer calculations.
Table 2.
Number of Subjects with Serum Samples Drawn for Each of the 9 Groups in the Study, by Visit Day
| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| Visit Day | VNDay 07 | VN/VNDay 0/7 | VN/VNDay 0/14 | VN/VNDay 0/28 | I/IDay 0/28 | VN/IDay 0/28 | VN+I/VN+IDay 0/28 | I/IDay 0 | VN/IDay 0/180 |
| 0 | 94 | 26 | 24 | 102 | 50 | 46 | 51 | 51 | 47 |
| 7 | … | 26 | … | … | … | … | … | … | … |
| 14 | … | 26 | 24 | … | … | … | … | … | … |
| 21 | … | 26 | 24 | 102 | … | … | … | … | … |
| 28 | 94 | 26 | 23 | 101 | 49 | 46 | 50 | 51 | 47 |
| 35 | … | 26 | 24 | 95 | … | … | … | … | … |
| 42 | … | … | 24 | 93 | … | … | … | … | … |
| 49 | … | … | … | 93 | … | … | … | … | … |
| 56 | … | … | … | 95 | 45 | 43 | 48 | … | … |
| 180 | 91 | … | … | 95 | … | … | … | 49 | 46 |
| 208 | … | 25 | 23 | … | 44 | 41 | 47 | 46 | 43 |
| 365 | … | … | … | … | … | … | … | 44 | 42 |
NOTE. Vaccination and vaccine schedule are indicated under the group number. Day refers to the days of vaccination with VN, I, or both. I, inactivated rg A/Indonesia/05/05 H5N1 (clade 2); VN, inactivated rg A/Vietnam/1203/04, H5N1 (clade 1).
Statistics
Exact confidence intervals were determined for all proportional end points. GMTs of antibody and their confidence intervals were computed by transforming results to a logarithmic scale, assuming asymptotic normality conditions were satisfied on this scale, and converting back to the original scale. Analyses are based on a modified intent-to-treat population. Ten subjects are not included in the analyses because they did not provide a post-vaccination blood sample. One subject is also excluded at all timepoints because of steroid receipt prior to a post-vaccination blood draw. Twenty-one subjects who did not receive dose 2 and one subject who was improperly dosed at vaccination 2 were dropped from analyses beginning with dose 2. Five subjects were also removed from analyses subsequent to dose 2 because of receipt of vaccines (n = 4) or steroids (n = 1) prohibited by the protocol. Other protocol violations, such as “out-of-window” vaccinations, did not impact inclusion in or exclusion from the study population. For both serum HI and Neut antibody titers, the GMT of duplicate assays done in parallel in one test were calculated, with 95% confidence intervals, for each dose group and follow-up blood draw. Between-group comparisons were made on the basis of these end points for serum HI or Neut, evaluated at 28 days after dose 2 for all subjects or at 14 days after dose 2 for accelerated schedules.
RESULTS
A total of 505 subjects were enrolled, 209 (41%) of whom were men and 296 (59%) of whom were women. The majority, 399, of subjects were white (79%), 73 (14%) were black, 18 (4%) were Asian, and the remainder were of other races. The mean age was 34.3 years (range, 18.2–49.8 years). Four hundred and ninety-one subjects were vaccinated and had serum obtained at time 0 for HAI testing (Table 2).
Adverse reactions were largely local and most often present on day 0 (Figure 1). Systemic reactions were mostly mild or moderate (Figure 2) and did not cluster in any single group preferentially (data not shown). Two unrelated serious adverse events occurred; one subject died from drowning, and another subject was hospitalized with diverticulitis.
Figure 1.
Occurrence of any adverse reaction after vaccination dose 1 (A) or vaccination dose 2 (B) among all groups. Severity of reactions is also indicated. Mild, does not interfere with daily activity; moderate, interferes with daily activity; severe, prevents daily activity. Vac, vaccination.
Figure 2.
Occurrence of 6 types of systemic reactions and any systemic reactions, as well as 6 types of local reactions and any local reactions, among all subjects in all groups after vaccination dose 1 (A) or vaccination dose 2 (B). Mild, does not interfere with daily activity; moderate, interferes with daily activity; severe, prevents daily activity. For redness (mm) and swelling (mm), mild, moderate, and severe refer to small (<20-mm), medium (20–50-mm), or large (>50-mm) diameter, respectively, of the indicated sign of adverse event. Elev, elevated; Temp, temperature; Vac, vaccination.
Immunogenicity results are presented for geometric mean HI antibody to A/Vietnam/04 antigen (Figure 3) or A/Indonesia/05 antigen (Figure 4) and in Figures 5 and 6 for geometric mean Neut antibody to A/Vietnam/04 virus or A/Indonesia/05 virus.
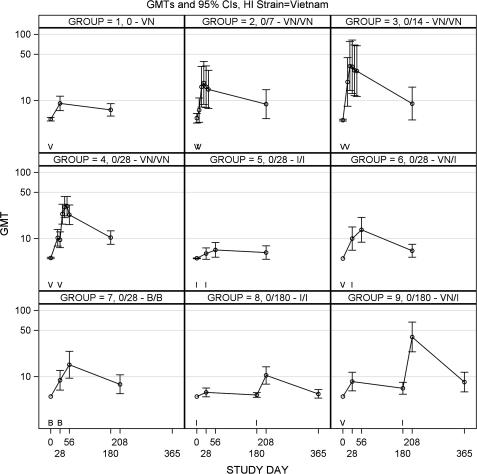
Figure 3.
Geometric mean titer (GMT) plots of serum hemagglutinating inhibiting (HI) antibody to A/Vietnam/04 antigen among the 9 vaccine groups are shown. Symbols on the x-axis of each subpanel indicate the day of vaccination with A/Vietnam/04 vaccine (V), A/Indonesia/05 vaccine (I), or both (B). Statistical comparisons (a) to assess vaccine schedule in group 4 versus group 2 (P = .99) and group 4 versus group 3 (P = .94), (b) to assess monovalent vaccine versus bivalent vaccine in group 4 versus group 7 (P = .61), (c) to assess heterologous boosting in group 4 versus group 6 (P = .37), (d) to assess heterologous boosting after a long rest in group 4 versus group 9 (P = .31) and group 6 versus group 9 (P = .009), and (e) to assess heterologous boost versus bivalent vaccine in group 6 versus group 7 (P >.99).
Figure 4.
Geometric mean titer (GMT) plots of serum hemagglutinating inhibiting (HI) antibody to A/Indonesia/05 antigen among the 9 vaccine groups are shown. Symbols on the x-axis of each subpanel indicate the day of vaccination with A/Vietnam/04 vaccine (V), A/Indonesia/05 vaccine (I), or both (B). Statistical comparisons (a) to assess long-interval vaccine schedule in group 5 versus group 8 (P = .03), (b) to assess monovalent vaccine versus bivalent vaccine in 5 versus group 7 (P >.99),= (c) to assess heterologous boost after a 1-month interval in group 5 versus group 6 (P = .001), (d) to assess heterologous boost after a long rest interval in group 5 versus group 9 (P = .59) and group 8 versus group 9 (P = .72), and (e) to assess interval of heterologous boost in group 6 versus group 9 (P≤.001).
Figure 5.
Geometric mean titer (GMT) plots of serum microneutralizing (Neut) antibody to A/Vietnam/04 antigen among the 9 vaccine groups are shown. Symbols on the x-axis of each subpanel indicate the day of vaccination with A/Vietnam/04 vaccine (V), A/Indonesia/05 vaccine (I), or both (B). Statistical comparisons (a) to assess vaccine schedule in group 4 versus group 2 (P = .03) and in group 4 versus group 3 (P = .74), (b) to assess monovalent vaccine versus bivalent vaccine in group 4 versus group 7 (P = .006), (c) to assess heterologous boosting in group 4 versus group 6 (P = .01), (d) to assess heterologous boosting after a long rest group 4 versus group 9 (P≤.001; and group 6 versus group 9 (P≤.001), and (e) to assess heterologous boost versus bivalent vaccine in group 6 versus group 7 (P <.99).
Figure 6.
Geometric mean titer (GMT) plots of serum microneutralizing (Neut) antibody to A/Indonesia/05 antigen among the 9 vaccine groups are shown. Symbols on the x-axis of each subpanel indicate the day of vaccination with A/Vietnam/04 vaccine (V), A/Indonesia/05 vaccine (I), or both (B). Statistical comparisons (a) to assess long-interval vaccine schedule in group 5 versus group 8 (P≤.001), (b) to assess monovalent vaccine versus bivalent vaccine in group 5 versus group 7 (P>.99), (c) to assess heterologous boost after a 1-month interval in group 5 versus group 6 (P≤.001), (d) to assess heterologous boost after a long rest interval in group 5 versus group 9 (P = .93) and group 8 versus group 9 (P = .002), and (e) to assess interval of heterologous boost in group 6 versus group 9 (P≤.001).
Accelerated Vaccine Schedule
The schedule of vaccine administration influenced the antibody responses to A/Vietnam/04 vaccine. Subjects given 2 doses of A/Vietnam/04 vaccine separated by only 7 days (Figure 3) had low maximal GMT HI (18.5) at day 21 (14 days after dose 2). Subjects given 2 doses of A/Vietnam/04 vaccine separated by 14 (group 3) or 28 days (group 4) had higher maximal GMT, compared with that for the day 0 and 7 (group 2) schedule (HI of 33.2 on day 21 for the day 0 and 14 schedule, and HI of 30.9 on day 49 for the day 0 and 28 schedule), but this did not achieve statistical significance. The Neut data recapitulated these findings, except that the Neut antibody response to the day 0 and 7 schedule was significantly lower than the response to the day 0 and 28 schedule (Figure 5; group 2 vs 4, P= .029).
Long Rest Interval
The vaccine schedule was examined for A/Indonesia/05 vaccine for the day 0 and 28 schedule (group 5; Figure 4) and day 0 and 180 schedule (group 8; Figure 4). The longer interval between doses resulted in higher HI (GMT for day 208 vs day 56, 58.7 vs 27.6; P = .030) and Neut antibody (185.4 vs 63.8; Figure 6; P < .001).
Cross-Reacting Antibody
Little cross-reacting antibody against A/Indonesia/05 antigen was induced by 2 doses of A/Vietnam/04 vaccine (groups 2, 3, and 4; Figures 4 and 6), and similarly, little or no cross-reacting antibody to A/Vietnam/04 antigen was induced by 2 doses of A/Indonesia/05 vaccine (groups 5 and 8; Figures 3 and 5). This was true regardless of the vaccine schedule, and it was true for both HI and Neut antibody.
One dose of A/Vietnam/04 vaccine was priming for a response to a booster with heterologous vaccine, A/Indonesia/05. Despite the lack of developing cross-reacting antibody after 2 doses of A/Vietnam/04 vaccine, one dose of A/Vietnam/04 vaccine followed by 1 dose of the heterologous vaccine A/Indonesia/05 resulted in antibody to both A/Vietnam/04 antigen and A/Indonesia/05 antigen (group 6, HI GMT 13.6 to A/Vietnam/04 vaccine and 10.1 to A/Indonesia/05 vaccine at day 56; Figures 3 and 4). Although antibodies were present at lower titers than that seen with 2 doses of homologous vaccine, this did not achieve statistical significance for A/Vietnam/04 antigen; however, this was significant for A/Indonesia/05 antigen (A/Vietnam/04 antigen HI GMT on day 56, 22.9 for group 4 vs 13.6 for group 6; P = .372; A/Indonesia/05 vaccine HI GMT on day 56, 27.6 for group 5 vs 10.1 for group 6; P = .001). These data were also reflected in the Neut antibody results (Figures 5 and 6; P < .05 for each comparison).
Combining both antigens in half doses resulted in responses to both viruses. HI antibody to A/Indonesia/05 antigen after A/Indonesia/05 vaccine alone or after combined 45 μg of A/Vietnam/04 vaccine plus 45 μg of A/Indonesia/05 vaccine at days 0 and 28 was not significantly different (group 5 vs group 7, P>.99). A/Vietnam/04 vaccine response was lower but not significantly so, compared with the response to the combined regimen (group 6 vs group 7, P >.99).
Cross-priming by A/Vietnam/04 vaccine for a response to A/Indonesia/05 vaccine was long lived. A/Vietnam/04 vaccine at day 0 followed by A/Indonesia/05 vaccine at day 180 resulted in antibody to A/Indonesia/05 antigen (GMT for group 9, 41.3) compared with 2 doses of A/Indonesia/05 vaccine at days 0 and 180 (GMT for group 8, 58.7) or 2 doses of A/Indonesia/05 vaccine at days 0 and 28 (GMT for group 5, 27.6). The longer interval between doses improved the magnitude of the cross-priming response to A/Indonesia/05 vaccine (HI GMT for group 6 on day 56 vs group 9 on day 208, 10.1 vs 41.3; P < .001).
The longer interval between doses also resulted in higher immune responses to the primary antigen, A/Vietnam/04 after a heterologous boost with A/Indonesia/05 vaccine. A/Vietnam/04 vaccine followed by A/Indonesia/05 vaccine at day 28 gave a GMT of HI to A/Vietnam/04 antigen of 13.6 (group 6), but A/Vietnam/04 vaccine followed by A/Indonesia/05 vaccine at day 180 gave a GMT of HI to A/Vietnam/04 vaccine of 39.7 at day 208 (group 9, P = .009).
Discussion
Although the immune responses are modest in this study, the results suggest several general principles for vaccination against potential avian pandemic influenza. To induce antibody titers that are expected to provide protection against influenza virus with novel HA antigens requires 2 doses of vaccine. Accelerated vaccination schedules as short as doses administered on days 0 and 7 induce some antibody, but doses at administered at days 0 and 14 or days 0 and 28 were better, and no differences were found between levels of antibody induced between day 0 and 14 and day 0 and 28 schedules. It will be important to examine adjuvanted vaccine to see whether these same principles apply but with larger antibody responses.
The longer interval (180 days) between priming and boosting vaccine doses gave the best antibody responses, although in a fast-moving pandemic, this is unlikely to be an option. Significantly higher titers of antibodies were found when 6 months elapsed between priming with one H5 antigen and boosting with another H5, compared with the day 0 and 28 schedule or simultaneous administration of antigens. Preimmunization of at risk persons with one dose of antigenically distantly related H5 should be considered as a public health option to reduce the need to use 2 doses of vaccine in the event of a pandemic.
One dose of heterologous vaccine primed for a different antigen of the same HA subtype. In this regard, the experience with H5 is very much like the clinical experience with 2009 H1 vaccine, in which persons 10 years of age and older responded to a single dose of 2009 H1 HA subunit vaccine. Presumably, previous H1 infection, although antigenically distant for the 2009 H1, had primed them for a response to the new virus [12]. Despite 2009 H1 HA being very distant antigenically from recent seasonal H1 viruses, the responses to a single dose (15 μg) were vigorous in older children and adults [12].
The results of the present study confirm the usefulness of vaccination with an H5 strain that is antigenically out of date. Despite the significant antigenic drift over time, the A/Vietnam/04 vaccine primed for an antibody response to A/Indonesia/05 vaccine. The large doses of antigen used in the present study (90 μg) might be reduced by using adjuvant. The present study did not evaluate lower doses of antigen. Previously primed individuals might respond to lower doses of H5 HA vaccine, and this needs to be determined in clinical trials. The subjects primed with 1 or 2 doses of Vietnam/04 vaccine in groups 1 and 4 in the present study may be suitable to evaluate additional strategies of subsequent boosts with or without adjuvant in a follow-up study.
Funding
National Institutes of Health contract numbers HHSN272200800003C (to R.B.B), HHSN272200800005C (to M.J.M.), and HHSN272200800004C (to L.A.J.)
References
- 1.Tran TH, Nguyen TL, Nguyen TD, et al. Avian influenza A (H5N1) in 10 patients in Vietnam. N Engl J Med. 2004;350:1179–88. doi: 10.1056/NEJMoa040419. [DOI] [PubMed] [Google Scholar]
- 2.Ungchusak K, Auewarakul P, Dowell SF, et al. Probable person-to-person transmission of avian influenza A (H5N1) N Engl J Med. 2005;352:333–40. doi: 10.1056/NEJMoa044021. [DOI] [PubMed] [Google Scholar]
- 3.Nicholson KG, Colegate AE, Podda A, et al. Safety and antigenicity of non-adjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a randomised trial of two potential vaccines against H5N1 influenza. Lancet. 2001;357:1937–43. doi: 10.1016/S0140-6736(00)05066-2. [DOI] [PubMed] [Google Scholar]
- 4.Treanor JJ, Wilkinson BE, Masseoud F, et al. Safety and immunogenicity of a recombinant hemagglutinin vaccine for H5 influenza in humans. Vaccine. 2001;19:1732–7. doi: 10.1016/s0264-410x(00)00395-9. [DOI] [PubMed] [Google Scholar]
- 5.Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N Engl J Med. 2006;54:1343–51. doi: 10.1056/NEJMoa055778. [DOI] [PubMed] [Google Scholar]
- 6.Stephenson I, Nicholson KG, Colegate AE, et al. Boosting immunity to influenza H5N1 with MF59-adjuvanted H5N3 A/Duck/Singapore/97 vaccine in a primed human population. Vaccine. 2003;21:1687–93. doi: 10.1016/s0264-410x(02)00632-1. [DOI] [PubMed] [Google Scholar]
- 7.Stephenson I, Bugarini R, Nicholson KG, et al. Cross-reactivity to highly pathogenic avian influenza H5N1 viruses after vaccination with nonadjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a potential priming strategy. J Infect Dis. 2005;191:1210–5. doi: 10.1086/428948. [DOI] [PubMed] [Google Scholar]
- 8.Goji NA, Nolan C, Hill H, et al. Immune responses of healthy subjects to a single dose of intramuscular inactivated influenza A/Vietnam/1203/2004 (H5N1) vaccine after priming with an antigenic variant. J Infect Dis. 2008;198:635–41. doi: 10.1086/590916. [DOI] [PubMed] [Google Scholar]
- 9.Noah DL, Hill H, Hines D, White EL, Wolff MC. Qualification of the hemagglutination inhibition assay in support of pandemic influenza vaccine licensure. Clin Vaccine Immunol. 2009;16:558–66. doi: 10.1128/CVI.00368-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rowe T, Abernathy RA, Hu-Primmer J, et al. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J Clin Microbiol. 1999;37:937–43. doi: 10.1128/jcm.37.4.937-943.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanofi Pasteur. Influenza virus vaccine, H5N1. Full prescribing information. http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM112836.pdf. Accessed 23 June 2010. [Google Scholar]
- 12.National Center for Immunization, Respiratory Diseases, CDC. Use of influenza A(H1N1) 2009 monovalent vaccine. Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR. 2009;58:1–8. [PubMed] [Google Scholar]