Abstract
The effect of long chain 2-alkylaminoethyl-1,1-bisphosphonates against proliferation of the clinically more relevant form of Trypanosoma cruzi, the etiologic agent of American trypanosomiasis (Chagas’ disease), and against tachyzoites of Toxoplasma gondii was investigated. Particularly, compound 26 proved to be an extremely potent inhibitor against the intracellular form of T. cruzi, exhibiting IC50 values at the nanomolar range. This cellular activity was associated with a strong inhibition of the enzymatic activity of T. cruzi farnesyl diphosphate synthase (TcFPPS), which constitutes a valid target for Chagas’ disease chemotherapy. Compound 26 was an effective agent against T. cruzi (amastigotes) exhibiting an IC50 value of 0.67 μM, while this compound showed an IC50 value of 0.81 μM against the target enzyme TcFPPS. This drug was less effective against the enzymatic activity of T. cruzi solanesyl diphosphate synthase TcSPPS showing an IC50 value of 3.2 μM. Interestingly, compound 26 was also very effective against T. gondii (tachyzoites) exhibiting IC50 values of 6.23 μM. This cellular activity was also related to the inhibition of the enzymatic activity towards the target enzyme TgFPPS (IC50 = 0.29 μM) As bisphosphonate-containing compounds are FDA-approved drugs for the treatment of bone resorption disorders, their potential low toxicity makes them good candidates to control different tropical diseases.
The hemoflagellated protozoan parasite Trypanosoma cruzi is the etiological agent of Chagas’ disease or American trypanosomiasis, which is an endemic disease widespread from southern United States to southern Argentina. It has been estimated that close to 18 million people are infected and over 40 million are at risk of infection by T. cruzi.1 The central nervous system is the most frequently affected site in AIDS patients, with meningoencephalitis occurring approximately in 75% of cases. The next normally affected organ is the heart with myocarditis.
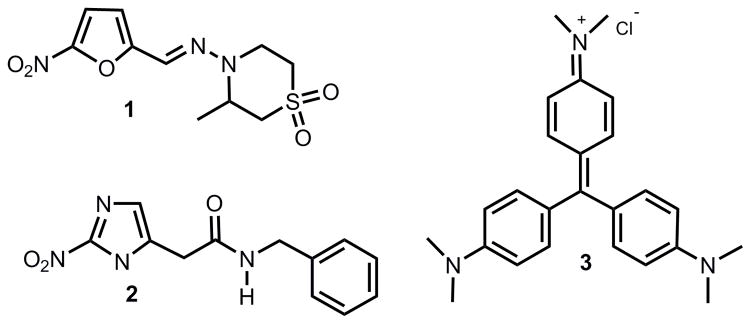
In addition, since people migrate from endemic areas, the possibility for cases in developed nations will also escalate. Chemotherapy for Chagas’ disease still remains unsatisfactory due to limited efficacy and common side effects of the currently available drugs such as nifurtimox (1), now discontinued, and benznidazole (2), which present toxicity associated with their continued use.2–4
The parasite has a complex life cycle involving blood-sucking Reduviid insects and mammals.5 It multiplies in the insect gut as an epimastigote form and is spread as a non-dividing metacyclic trypomastigote from the insect feces by contamination of intact mucosa or wounds produced by the blood-sucking activity of the vector. In the mammalian host, T. cruzi multiplies intracellularly as the amastigote form and is subsequently released into the bloodstream as a non-dividing trypomastigote.5 Transmission of Chagas’ disease could also occur via the placenta or by blood transfusion.6 This latter mechanism is responsible for the occurrence of Chagas’ disease in developed countries where the disease is not endemic.6,7 For this reason, it is very important to have an efficient agent to eradicate the bloodstream trypomastigotes from blood banks as well. Crystal violet (3), the only drug employed for blood sterilization and discovered for that purpose some decades ago,8 suffers from some disadvantages, since it was shown to be carcinogenic in in vivo assays (Figure 1).9
Figure 1.
Current drugs for the treatment of Chagas’ disease and blood sterilization.
Different enzymes involved in the biosynthesis of ergosterol10 and farnesyl diphosphate,11,12 and in protein prenylation,13 have been reported to be excellent targets against pathogenic parasites. Farnesyl diphosphate synthase of T. cruzi (TcFPPS), for example, has been demonstrated to be the target of bisphosphonates that have activity in vitro and in vivo against T. cruzi.14 The gene encoding this enzyme has been cloned and sequenced and the protein expressed and biochemically characterized.14,15 In addition, the crystal structure of TcFPPS at 2 Å resolution have been published.16 Moreover, solanesyl diphosphate synthase, another important prenyltransferase in T. cruzi (TcSPPS), which is involved in the synthesis of ubiquinone, is another potential target for chemotherapy.17
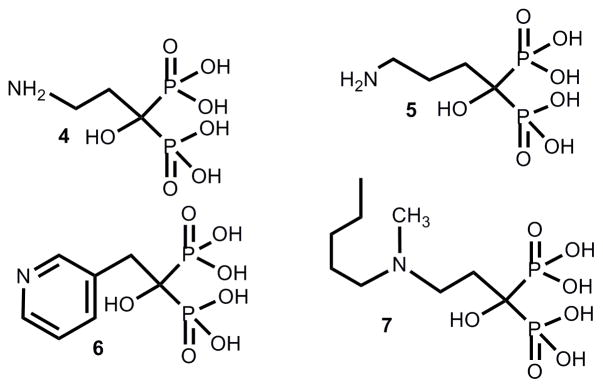
Geminal phosphonates are pyrophosphate analogues in which a methylene group replaces the oxygen atom bridge between the two phosphorus atoms of the pyrophosphate moiety. The substitution of carbon with different side chains has given rise to a large family of compounds. Unlike pyrophosphates, bisphosphonates possess better metabolic stability because they are not recognized by pyrophosphatases and are also stable to hydrolysis under acidic media. Several bisphosphonates are potent inhibitors of bone resorption and are in clinical use for the treatment and prevention of osteoporosis, Paget’s disease, hypercalcemia, tumor bone metastases, and other bone diseases.18,19 Selective action on bone is based on the binding of the bisphosphonate moiety to the bone mineral.18,19 It has been postulated that the acidocalcisomes are equivalent in composition to the bone mineral and that accumulation of bisphosphonates in these organelles, as they do in bone mineral, assists their antiparasitic action.20 Representative bisphosphonates, such as pamidronate (4), alendronate (5), risedronate (6), and ibandronate (7), act by a mechanism that lead to osteoclast apoptosis.21 The site of action of aminobisphosphonates has been narrowed down to the isoprenoid pathway and more specifically, to an inhibition of protein prenylation.22
Rationale
Aminobisphosphonates were initially found to be potent inhibitors of T. cruzi proliferation in vitro and in vivo without toxicity to the host cells.23 Lately, different bisphosphonates were found to be effective growth inhibitors of pathogenic trypanosomatids other than T. cruzi, such as T. brucei rhodesiense, Leishmania donovani, and L. mexicana and Apicomplexan parasites such as Toxoplasma gondii and Plasmodium falciparum.20,24–30 In vivo assays of bisphosphonates have shown that risedronate can significantly increase survival of T. cruzi-infected mice.31 In view of the above results, it is possible to assume that bisphosphonates are potential candidates for chemotherapy of neglected diseases. In addition, bisphosphonates have the advantage that their synthesis is straightforward and inexpensive. It is reasonable to assume a low toxicity for bisphosphonate-containing drugs bearing in mind that many bisphosphonate compounds are FDA-approved drugs for the long-term treatment of several bone disorders.
Of special interest are 2-alkylaminoethyl-1,1-bisphosphonates derived from fatty acids, which were shown to be potent growth inhibitors against the clinically more relevant form of T. cruzi exhibiting IC50 values at the nanomolar range.26 This class of bisphosphonates has proven to be more efficient than 1-hydroxy- and 1-amino-bisphosphonates as antiparasitic agents.26 Compound 8 (Figure 3) appears as the main member of bisphosphonates derived from fatty acids,26–31 with an IC50 value of 0.84 μM.26 This cellular activity is associated with the inhibition of the enzymatic activity of the target enzyme TcFPPS,26 being a competitive inhibitor28 with an IC50 value of 0.49 μM.26 The scope of this type of bisphosphonates is very broad, because compound 8 also inhibits the enzymatic activity of T. gondii FPPS (IC50 = 0.14 μM),26 and exhibits in vitro inhibitory action against tachyzoites of T. gondii (IC50 = 9.37 μM).26 As it was mentioned before, the target of 2-alkylaminoethyl-1,1-bisphosphonates is FPPS and to a lesser extent, SPPS. Previous studies have indicated that selectivity towards SPPS increases as the chain length increases.26 In fact, compound 8 exhibits IC50 = 1.35 μM towards TcSPPS, while compound 10 presents IC50 values of 1.01 μM and 0.25 μM against FPPS and SDPS, respectively.26
Figure 3.
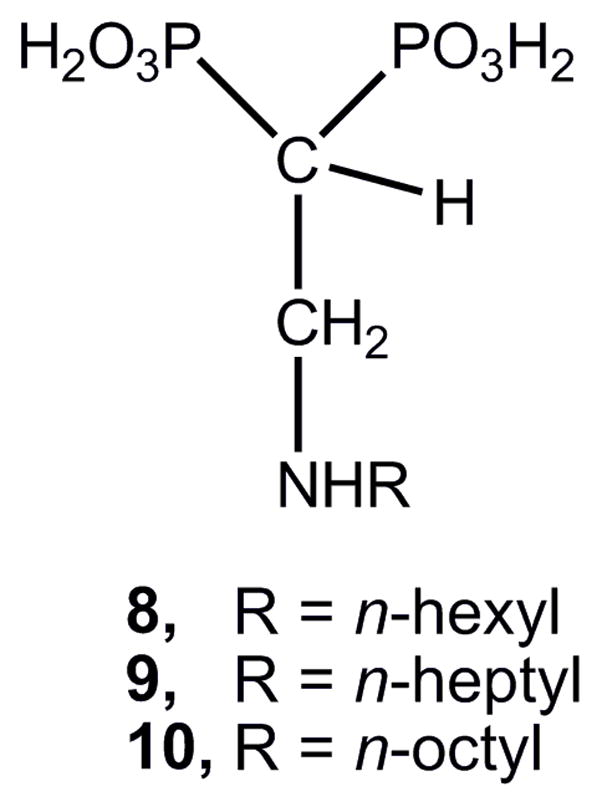
Representative members of 1-[2-(alkylamino)ethyl] 1,1-bisphosphonic acid derivatives.
We have demonstrated that 2-alkylaminoethyl-1,1-bisphosphonates were by far more potent than the parent compounds 1-aminoalkyl-, 1-hydroxyalkyl-, and 1-alkyl-1,1-bisphosphonates.26 The 2-alkylaminoethyl-1,1-bisphosphonate derivatives are isosteric analogues of 1-alkyl-1,1-bisphosphonates, in which an amino group replaces the methylene group at the C-3 position. These aminobisphosphonates were originally designed in order to maintain the ability to coordinate Mg2+ in a tridentate manner as 1-hydroxy-and 1-amino- derivatives do.26 However, preliminary studies on the interaction of inhibitor 9 (IC50 = 58 nM) with TcFPPS based on the X-ray crystallographic structure of 9–TcFPPS have indicated that the nitrogen atom did not coordinate32 the with Mg2+ present at the active site of the target enzyme.33,34 The tridentate coordination structure is circumvented to the hydroxyl groups bonded to the phosphorus atoms either for 2-alkylaminoethyl- or 1-hydroxy-1,1-bisphosphonates.35,36 In addition, the X-ray structure of the complex of risedronate with TcFPPS indicated that the residue Asp250 forms a hydrogen bond with the hydroxyl group present at the C-1 position of the molecule of risedronate, fact not possible with the 2-aminoalkyl derivatives.16,26 Taking into account the above results, it would seem of interest to carry out chain length variations on compound 8 taken as a reference structure (Figure 3). Base on our previous work,26 the resulting 1-[2- (alkylamino)ethyl] bisphosphonates were evaluated against both T. cruzi and T. gondii cells and towards their target enzymes TcFPPS, TcSPPS, and TgFPPS.
Results and Discussion
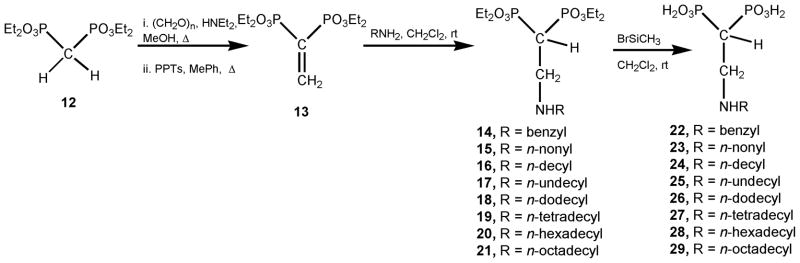
The title compounds 22–29 were straightforwardly prepared employing tetraethyl ethenylidenebisphosphonate (compound 13) as a Michael acceptor,37 which in turn was easily prepared from tetraethyl methylenebisphosphonate (compound 12) in two steps according to a slightly modified Degenhart protocol.38,39 Then, compound 13 was reacted with the corresponding n-alkylamine via a 1,4-conjugated addition reaction to afford the respective Michael adducts (14–21). Once these synthetic precursors were available, they were hydrolyzed by treatment with bromotrimethylsilane in methylene chloride40 to afford the free bisphosphonic acids (22–29). The synthesis of this new 2-alkylaminoethyl gem-bisphosphonates is presented in Scheme 1. Compounds 22–29 were evaluated as growth inhibitors against the amastigote form of T. cruzi, the clinically more relevant form of the parasite. Benznidazole, a well-known sterol biosynthesis inhibitor, was used as a positive control.41 In addition, the correlation of the cellular activity with the action against its target enzyme (TcFPPS) as well as TcSPPS was studied. Besides, based on previous studies on structurally related bisphosphonates against the opportunistic pathogen T. gondii,42,43 this series of new aminobisphosphonic acids was evaluated against T. gondii and its target enzyme TgFPPS.
Scheme 1.
Compound 26 resulted to be an extremely potent growth inhibitor against the clinically more relevant form of T. cruzi exhibiting an IC50 value of 0.67 μM, significantly more potent than benznidazole taken as positive control (IC50 = 2.77 μM). This cellular activity was associated with the inhibition of the enzymatic activity towards the target enzyme TcFPPS possessing an IC50 value of 0.81 μM. Compounds 25 and 28 were also potent growth inhibitors of T. cruzi (amastigotes) with IC50 values of 5.13 μM and 2.19 μM, respectively. The inhibition of the enzymatic activity of 25 towards the target enzyme qualitatively correlated with their inhibitory action against growth of amastigotes of T. cruzi as well. Interestingly, compound 26 did not exhibit superior inhibitory action against TcSPPS as might be expected by its chain length having an IC50 value of 3.18 μM. Compound 26 was not only a potent antiparasitic agent against T. cruzi, but also against the opportunistic parasite T. gondii. Certainly, this drug exhibited an extremely potent inhibition of the enzymatic activity of TgFPPS at the low nanomolar range (IC50 = 93 nM), efficacy comparable with risedronate (IC50 = 74 nM). This enzymatic activity correlated well with the cellular activity exerted by this compound against tachyzoites of T. gondii (IC50 = 6.23 μM). Compound 24, in spite of being a potent inhibitor of the enzymatic activity of TgFPPS at the low nanomolar range (IC50 = 68 nM), was practically devoid of antiparasitic activity against T. gondii cells (Table 1).
Table 1.
| Comp | IC50 (μM) | IC50 (μM) | IC50 (μM) | IC50 (μM) | IC50 (μM) |
|---|---|---|---|---|---|
| TcFPPS | TgFPPS | TcSPPS | T. cruzi amastigotes | T. gondii tachyzoites | |
| 22 | >1 | >10 | >10 | >10 | |
| 23 | 0.430 ± 0.088 | 0.440 ± 0.070 | - | >10 | >10 |
| 24 | >1 | 0.068 ± 0.036 | >10 | >10 | >10 |
| 25 | 0.856 ± 0.137 | 0.868 ± 0.545 | 3.081 ± 0.5896 | 5.126 ± 1.915 | >10 |
| 26 | 0.811 ± 0.226 | 0.093 ± 0.024 | 3.182 ± 1.0544 | 0.670 ± 0.086 | 6.23 |
| 27 | >1 | 0.292 ± 0.170 | - | >10 | 11.27 |
| 28 | - | - | 2.189 ± 0.325 | 4.13 | |
| 29 | - | - | - | >10 | 5.29 |
| risedronate | 0.027 ± 0.003 | 0.074 ± 0.017 | - | - | |
| 10 | - | 0.272 ± 0.037 | - | ||
| benznidazole | - | - | 2.768 ± 0.488 | ||
It can be concluded that long-chain analogues of 2-alkylaminoethyl-1,1-bisphosphonates such as compound 26 were of the great effectiveness against both T. cruzi and the target enzyme TcFPPS. The designed compounds maintained the ability of the lower members of this family of compounds as antiparasitic agents. Compound 26 arose as the main member of these new set of drugs. Surprisingly, it was not possible to establish a biological activity/chain length relationship. In fact, compound 8 with a linear 6-carbons in its structure exhibited similar cellular and enzymatic than 26. Most of the synthetic drugs proved to be inhibitors of the enzymatic activity of TcSPPS but to a lesser extent than TcFPPS. Finally, some of these 1-[2-(alkylamino)ethyl] bisphosphonic acids were shown to be effective anti-T. gondii agents indicating the broad scope of this family of compounds. Work aimed at optimizing the chemical structure of 1-(3-azaalkyl)-1,1 bisphosphonic acids such as compound 26 and other closely related analogues is currently being pursued in our laboratory.
Experimental Section
General
The glassware used in air- and/or moisture-sensitive reactions was flame-dried and reactions were carried out under an argon atmosphere. Unless otherwise noted, chemicals were commercially available and used without further purification. Solvents were distilled before use. Dichloromethane was distilled from phosphorus pentoxide. Nuclear magnetic resonance spectra were recorded with a Bruker AM-500 MHz spectrometer. The 1H NMR spectra are referenced with respect to the residual CHCl3 proton of the solvent CDCl3 at δ = 7.26 ppm. Coupling constants are reported in Hz. 13C NMR spectra were fully decoupled and are referenced to the middle peak of the solvent CDCl3 at δ = 77.0 ppm. 31P NMR spectra are referenced with respect to the peak of 85% H3PO4 as external reference. Splitting patterns are designated as s, singlet; d, doublet; t, triplet; q, quadruplet; dd, double doublet, etc. Melting points were determined with a Fisher–Johns apparatus and are uncorrected. IR spectra were recorded with a Nicolet Magna 550 spectrometer. Analytical TLC was performed on commercial 0.2 mm aluminum-coated silica gel plates (F254) and visualized by 254 nm UV or immersion in an aqueous solution of (NH4)6Mo7O24·4H2O (0.04 M), Ce(SO4)2 (0.003 M) in concentrated H2SO4 (10%). Elemental analyses were conducted by UMYMFOR (CONICET-FCEyN). The results were within ±0.4% of the theoretical values.
Synthesis of 1-[2-alkylaminoethyl]-1,1-bisphosphonic acids
General Procedure
A solution of compound 13 (10 mmol) in anhydrous methylene chloride (10 mL) was treated with the corresponding amine (10 mmol) under an argon atmosphere. The reaction mixture was stirred at room temperature overnight. The solvent was evaporated and the residue was purified by column chromatography (silica gel) employing hexane–EtOAc (17:3) as eluent for all compounds. Then, to a solution of the resulting tetraethyl ester (14–21, 1 equivalent) in anhydrous methylene chloride was added dropwise trimethylsilyl bromide (10 equivalents) in an argon atmosphere. The reaction mixture was stirred at room temperature for 48 h. After cooling at 0 °C, anhydrous methanol (10 mL) was added, and the resulting mixture was allowed to reach room temperature. The solution was then concentrated under reduced pressure. The residue was dissolved in dry methanol (10 mL) and subsequently concentrated under reduced pressure twice. The solvent was evaporated and the residue was crystallized from ethanol-water.
Tetraethyl 1-[(Benzylamino)ethyl] 1,1-bisphosphonate (14)
Colorless oil; IR (film, cm−1) 3425, 2982, 2932, 2905, 1639, 1626, 1456, 1391, 1215, 1049, 951, 795, 750, 700; 1H NMR (500.13 MHz, CDCl3) δ 1.32 (t, J = 7.0 Hz, 12H, H-2′), 2.67 (tt, J = 23.6, 5.7 Hz, 1H, H-1), 3.16 (dt, J = 16.9, 5.6 Hz, 2H, H-2), 3.81 (s, 2H, H-4), 4.17 (m, 8H, H-1′), 7.34 (m, 5H, aromatic protons); 13C NMR (125.77 MHz, CDCl3) δ 16.4 (d, J = 3.8 Hz, C-2′), 37.7 (t, J = 132.5 Hz, C-1), 45.0 (t, J = 4.3 Hz, C-2), 53.2 (C-4), 62.7 (dd, J = 31.5, 6.6 Hz, C-1′), 126.9 (Ph), 128.1 (Ph), 128.4 (Ph), 139.9 (Ph); 31P NMR (202.46 MHz, CDCl3) δ 22.66. HRMS (ESI) Calcd. for (C17H32NO6P2) [M+H]+: 408.1705; found 408.1684.
Tetraethyl 1-[(n-Non-1-ylamino)ethyl] 1,1-bisphosphonate (15)
Colorless oil; 1H NMR (500.13 MHz, CDCl3) δ 0.85 (t, J = 7.0 Hz, 3H, H-12), 1.22 (m, 12H, -CH2-), 1.32 (t, J = 7.1 Hz, 12H, H-2′), 1.44 (p, J = 7.2 Hz, 2H, H-5), 2.54 (t, J = 7.2 Hz, 2H, H-4), 2.62 (tt, J = 23.6, 6.0 Hz, 1H, H-1), 3.10 (dt, J = 16.7, 5.9 Hz, 2H, H-2), 4.16 (m, 8H, H-1′); 13C NMR (125.77 MHz, CDCl3) δ 14.0 (C-12), 16.3 (dd, J = 6.4, 2.7 Hz, C-2′), 22.6 (C-11), 26.8 (C-6), 27.3 (C-7), 29.2 (C-8), 29.5 (C-10), 29.9 (C-9), 31.8 (C-5), 37.4 (t, J = 132.2 Hz, C-1), 45.7 (t, J = 4.1 Hz, C-2), 49.2 (C-4), 62.6 (dd, J = 32.3, 6.8 Hz, C-1′); 31P NMR (202.46 MHz, CDCl3) δ 22.78.
Tetraethyl 1-[(n-Dec-1-ylamino)ethyl] 1,1-bisphosphonate (16)
Colorless oil; IR (film, cm−1) 3444, 3332, 2972, 2956, 2925, 2854, 1649, 1569, 1467, 1392, 1369, 1247, 1164, 1026, 970, 798, 532; 1H NMR (500.13 MHz, CDCl3) δ 0.88 (t, J = 7.0 Hz, 3H, H-13), 1.23 (m, 14H, -CH2-), 1.35 (t, J = 7.1 Hz, 12H, H-2′), 1.47 (p, J = 7.1 Hz, 2H, H-5), 2.57 (t, J = 7.2 Hz, 2H, H-4), 2.65 (tt, J = 23.5, 5.9 Hz, 1H, H-1), 3.12 (dt, J = 16.5, 5.9 Hz, 2H, H-2), 4.16 (m, 8H, H-1′); 13C NMR (125.77 MHz, CDCl3) δ 14.1 (C-13), 16.3 (dd, J = 6.4, 2.7 Hz, C-2′), 22.6 (C-12), 26.8 (C-9), 27.3 (C-6), 29.3 (C-10), 29.5 (C-8), 29.5 (C-7), 29.9 (C-11), 31.9 (C-5), 37.4 (t, J = 132.2 Hz, C-1), 45.7 (t, J = 4.0 Hz, C-2), 49.2 (C-4), 62.6 (dd, J = 31.3, 6.8 Hz, C-1′); 31P NMR (202.46 MHz, CDCl3) δ 22.75. HRMS (ESI) Calcd. for (C20H46NO6P2) ) [M+H]+: 458.2800; found: 458.2813.
Tetraethyl 1-[(n-Undec-1-ylamino)ethyl] 1,1-bisphosphonate (17)
Colorless oil; IR (film, cm−1) 3433, 2926, 2854, 1639, 1468, 1391, 1219, 1053, 951, 797; 1H NMR (500.13 MHz, CDCl3) δ 0.88 (t, J = 6.9 Hz, 3H, H-14), 1.26 (m, 16H, -CH2-), 1.35 (t, J = 7.1 Hz, 12H, H-2′), 1.46 (p, J = 7.5 Hz, 2H, H-5), 2.57 (t, J = 7.2 Hz, 2H, H-4), 2.65 (tt, J = 23.1, 5.7 Hz, 1H, H-1), 3.13 (dt, J = 16.6, 5.9 Hz, 2H, H-2), 4.18 (m, 8H, H-1′); 13C NMR (125.77 MHz, CDCl3) δ 14.1 (C-14), 16.4 (dd, J = 6.4, 2.8 Hz, C-2′), 22.7 (C-13), 26.9 (C-10), 27.3 (C-6), 29.3 (C-11), 29.5 (C-8), 29.6 (C-7), 29.9 (C-12), 31.9 (C-5), 33.5, 37.5 (t, J = 132.6 Hz, C-1), 45.8 (t, J = 4.1 Hz, C-2), 49.3 (C-4), 62.6 (dd, J = 31.8, 7.3 Hz, C-1′); 31P NMR (202.46 MHz, D2O-d6) δ 22.79.
Tetraethyl 1-[(n-Dodec-1-ylamino)ethyl] 1,1-bisphosphonate (18)
Colorless oil; IR (film, cm−1) 3450, 2924, 2854, 1468, 1391, 1221, 1053, 953, 797, 559; 1H NMR (500.13 MHz, CDCl3) δ 0.83 (t, J = 7.0 Hz, 3H, H-15), 1.21 (m, 18H, -CH2-), 1.30 (t, J = 7.1 Hz, 12H, H-2′), 1.42 (p, J = 7.1 Hz, 2H, H-5), 2.53 (t, J = 7.2 Hz, 2H, H-4), 2.64 (tt, J = 23.5, 5.9 Hz, 1H, H-1), 3.10 (dt, J = 16.5, 5.8 Hz, 2H, H-2), 4.16 (m, 8H, H-1′); 13C NMR (125.77 MHz, CDCl3) δ 14.0 (C-15), 16.3 (dd, J = 6.4, 2.7 Hz, C-2′), 22.6 (C-14), 26.8 (C-11), 27.3 (C-6), 29.3 (C-12), 29.5 (C-8), 29.6 (C-7), 29.9 (C-13), 31.8 (C-5), 37.4 (t, J = 132.2 Hz, C-1), 45.7 (t, J = 4.1 Hz, C-2), 49.2 (C-4), 62.5 (dd, J = 32.7, 6.3 Hz, C-1′); 31P NMR (202.46 MHz, CDCl3) δ 22.78.
Tetraethyl 1-[(n-Tetradec-1-ylamino)ethyl] 1,1-bisphosphonate (19)
Colorless oil; 1H NMR (500.13 MHz, CDCl3) δ 0.85 (t, J = 6.9 Hz, 3H, H-17), 1.22 (m, 22H, -CH2-), 1.32 (t, J = 7.1 Hz, 12H, H-2′), 1.44 (p, J = 7.1 Hz, 2H, H-5), 2.55 (t, J = 7.2 Hz, 2H, H-4), 2.62 (tt, J = 23.4, 5.9 Hz, 1H, H-1), 3.10 (dt, J = 16.5, 5.9 Hz, 2H, H-2), 4.16 (m, 8H, H-1′); 13C NMR (125.77 MHz, CDCl3) δ 14.1 (C-17), 16.3 (dd, J = 6.4, 2.7 Hz, C-2′), 22.6 (C-16), 26.8 (C-13), 27.3 (C-6), 29.2 (C-14), 29.5 (C-7), 29.5 (C-11), 29.6 (C-8) 29.6 (C-14) 29.9 (C-9, C-10, C-15), 31.9 (C-5), 37.4 (t, J = 132.6 Hz, C-1), 45.7 (t, J = 4.0 Hz, C-2), 49.2 (C-4), 62.5 (dd, J = 31.8, 6.4 Hz, C-1′); 31P NMR (202.46 MHz, CDCl3) δ 22.78.
Tetraethyl 1-[(n-Hexadec-1-ylamino)ethyl] 1,1-bisphosphonate (20)
Colorless oil; IR (film, cm−1) 3450, 2924, 2854, 1639, 1468, 1391, 1221, 1053, 953, 797; 1H NMR (500.13 MHz, CDCl3) δ 0.88 (t, J = 7.1 Hz, 3H, H-19), 1.25 (m, 26H, -CH2-), 1.34 (t, J = 7.1 Hz, 12H, H-2′), 1.46 (p, J = 6.8 Hz, 2H, H-5), 2.57 (t, J = 7.2 Hz, 2H, H-4), 2.64 (tt, J = 23.3, 5.9 Hz, 1H, H-1), 3.10 (td, J = 8.3, 6.0 Hz, 2H, H-2), 4.18 (m, 8H, H-1′); 13C NMR (125.77 MHz, CDCl3) δ 14.1 (C-19), 16.4 (dd, J = 6.4, 2.7 Hz, C-2′), 22.7 (C-18), 26.9 (C-15), 27.3 (C-6), 29.3 (C-16), 29.5 (C-8), 29.7 (-CH2-), 29.9 (C-17), 31.9 (C-5), 37.5 (t, J = 132.2 Hz, C-1), 45.8 (t, J = 4.1 Hz, C-2), 49.3 (C-4), 62.6 (dd, J = 32.2, 6.8 Hz, C-1′); 31P NMR (202.46 MHz, CDCl3) δ 22.80
Tetraethyl 1-[(n-Octadec-1-ylamino)ethyl] 1,1-bisphosphonate (21)
Colorless oil; IR (film, cm−1) 3435, 2920, 2853, 1639, 1468, 1391, 1221, 1053, 953, 797, 735, 557; 1H NMR (500.13 MHz, CDCl3) δ 0.88 (t, J = 7.0 Hz, 3H, H-21), 1.26 (m, 32H, -CH2-), 1.34 (t, J = 7.1 Hz, 12H, H-2′), 1.47 (p, J = 7.0 Hz, 2H, H-5), 2.57 (t, J = 7.2 Hz, 2H, H-4), 2.65 (tt, J = 23.4, 5.9 Hz, 1H, H-1), 3.13 (dt, J = 16.5, 5.9 Hz, 2H, H-2), 4.18 (m, 8H, H-1′); 13C NMR (125.77 MHz, CDCl3) δ 14.1 (C-21), 16.4 (dd, J = 5.9, 3.2 Hz, C-2′), 22.7 (C-20), 26.8 (C-17), 27.3 (C-6), 29.3 (C-18), 29.5 (C-8), 29.6 (C-7), 29.7 (-CH2-), 29.9 (C-19), 31.9 (C-5), 37.5 (t, J = 132.2 Hz, C-1), 45.7 (t, J = 4.1 Hz, C-2), 49.2 (C-4), 62.6 (dd, J = 31.8, 6.3 Hz, C-1′); 31P NMR (202.46 MHz, CDCl3) δ 22.79.
1-[(Benzylamino)ethyl] 1,1-bisphosphonic Acid (22)
White solid; mp 210–212 °C; (KBr, cm−1) 3089, 2920, 2856, 2353, 2322, 1604, 1460, 1290, 1265, 1209, 1176, 1006, 956, 811, 773, 750, 711; 1H NMR (500.13 MHz, CDCl3) δ 2.41 (m, 1H, H-1), 3.37 (m, 2H, H2), 4.20 (s, 2H, H-4), 7.40 (m, 5H, aromatic protons); 31P NMR (202.46 MHz, CDCl3) δ 15.48. HRMS (ESI) Calcd. for (C9H16O6NP2) [M+H]+: 296.0453; found 296.0448. Anal. Calcd. for (C9H15O6NP2) C, 36.62; H, 5.12; N 4.75. Found C, 36.58; H, 5.07; N, 4.61.
1-[(n-Non-1-ylamino)ethyl] 1,1-bisphosphonic Acid (23)
White solid; mp 193–194 °C; IR (KBr, cm−1) 3082, 2952, 2927, 2856, 1460, 1272, 1184, 999, 972, 954, 709; 1H NMR (500.13 MHz, DMSO) δ 0.85 (t, J = 6.8 Hz, 3H, H-11), 1.25 (m, 12H, -CH2-), 1.54 (m, 2H, H-5), 2.20 (dist t, J = 20.7 Hz, 1H, H-1), 2.91 (m, 2H, H-4), 3.20 (m, 2H, H-2); 13C NMR (125.77 MHz, DMSO) δ 13.9 (C-12), 22.1 (C-11), 25.7 (C-6), 25.9 (C-7), 28.5 (C-8), 28.6 (C-10), 28.8 (C-9), 31.3 (C-5), 44.5 (C-2), 46.5 (C-4); 31P NMR (202.46 MHz, DMSO) δ 15.28; HRMS (ESI) Calcd. for (C11H28O6NP2F) [M+H]+: 332.1392; found 332.1378. Anal. Calcd. for (C11H27O6NP2) C, 39.88; H, 8.21; N, 4.23. Found C, 39.78; H, 8.30; N, 4.33.
1-[(n-Dec-1-ylamino)ethyl] 1,1-bisphosphonic Acid (24)
White solid; mp 127–128 °C; (KBr, cm−1) 2961, 2921, 2854, 1468, 1192, 1161, 1033, 906, 811, 522; 1H NMR (500.13 MHz, DMSO-d6) δ 0.84 (t, J = 6.7 Hz, 3H, H-13), 1.24 (m, 16H, -CH2-), 1.52 (p, J = 7.5 Hz, 2H, H-5), 2.22 (tt, J = 20.7, 7.3 Hz, 1H, H-1), 2.90 (t, J = 7.3 Hz, 2H, H-4), 3.19 (dt, J = 14.0, 7.4 Hz, 2H, H-2); 13C NMR (125.77 MHz, DMSO-d6) δ 14.0 (C-13), 22.1 (C-12), 25.7 (C-6), 25.9 (C-7), 28.6 (C-8), 28.7 (C-10), 28.9 (C-9), 28.9 (C-11), 31.3 (C-5), 44.7 (C-2), 46.5 (C-4); 31P NMR (202.46 MHz, DMSO-d6) δ 11.51. HRMS (ESI) Calcd. for (C11H27O6P2FNa) [M+Na]+: 354.1211; found 354.1192.
1-[(n-Undec-1-ylamino)ethyl] 1,1-bisphosphonic Acid (25)
White solid; mp 170–172 °C; (KBr, cm−1) 3101, 2923, 2854, 2322, 1693, 1467, 1272, 1182, 1002, 950, 705; 1H NMR (200.13 MHz, D2SO4) δ 0.84 (m, 3H, H-14), 1.21 (m, 16H, -CH2-), 1.70 (m, 2H, H-5), 3.21 (m, 2H, H-4), 3.60–3.71 (m, 3H, H-1, H-2); 13C NMR (50.3 MHz, D2SO4) δ 13.9 (C-14), 22.6 (C-13), 26.0 (C-6, C-7), 29.1 (C-8, C-9, C-10, C-11), 29.3 (C-12), 31.8 (C-5), 43.2 (C-2), 51.7 (C-4); 31P NMR (202.46 MHz, D2O) δ 14.37. HRMS (ESI) Calcd. for (C13H32O6NP2) [M+H]+: 360.1705; found 360.1691. Anal. Calcd. for (C13H31O6NP2) C, 43.45; H, 8.70; N, 3.90. Found C, 43.69; H, 8.52; N, 4.23.
1-[(n-Dodec-1-ylamino)ethyl] 1,1-bisphosphonic Acid (26)
White solid; mp 177–178 °C; IR (KBr, cm−1) 3092, 2922, 2853, 1470, 1005, 953, 706; 1H NMR (500.13 MHz, DMSO-d6) δ 0.84 (t, J = 6.8 Hz, 3H, H-15), 1.23 (m, 22H, -CH2-), 1.53 (p, J = 6.9 Hz, 2H, H-5), 2.18 (tt, J = 20.3, 7.4 Hz, 1H, H-1), 2.90 (t, J = 7.2 Hz, 2H, H-4), 3.16 (dt, J = 14.0, 7.1 Hz, 2H, H-2); 13C NMR (50.3 MHz, DMSO-d6) δ 14.1 (C-15), 22.1 (C-14), 25.7 (C-6), 25.9 (C-7), 28.5 (C-8), 28.7 (C-12), 28.9 (C-11), 29.0 (C-10), 29.0 (C-9), 29.1 (C-13), 31.3 (C-5), 44.4 (C-2), 46.4 (C-4); 31P NMR (202.46 MHz, DMSO-d6) δ 15.28. HRMS (ESI) Calcd. for (C14H34O6NP2) [M+H]+: 374.1861; found 374.1844. Anal. Calcd. for (C14H33O6NP2) C, 45.04; H, 8.91; N, 3.75. Found C, 44.80; H, 8.82; N, 3.81.
1-[(n-Tetradec-1-ylamino)ethyl] 1,1-bisphosphonic Acid (27)
White solid; mp 171–173 °C; IR (KBr, cm−1) 3082, 2920, 2852, 2318, 1469. 1272, 1186, 999, 956, 705; 1H NMR (500.13 MHz, DMSO-d6) δ 0.86 (t, J = 6.5 Hz, 3H, H-16), 1.25 (m, 22H, -CH2-), 1.54 (p, J = 6.8 Hz, 2H, H-5), 2.17 (tt, J = 20.2, 7.1 Hz, 1H, H-1), 2.94 (t, J = 7.2 Hz, 2H, H-4), 3.20 (dt, J = 13.9, 7.1 Hz, 2H, H-2); 31P NMR (202.46 MHz, D2O) δ 15.70. HRMS (ESI) Calcd. for (C16H37O6NP2Na) [M+Na]+: 424.1994; found 424.2000. Anal. Calcd. for (C16H37O6NP2) C, 47.87; H, 9.29; N, 3.49. Found C, 47.64; H, 9.34; N, 3.56.
1-[(n-Hexadec-1-ylamino)ethyl] 1,1-bisphosphonic Acid (28)
White solid; 172–174 °C; IR (KBr, cm−1) 3083, 2956, 2918, 2852, 1693, 1469, 1272, 1182, 1002, 952, 914, 806, 705; 1H NMR (200.13 MHz, D2SO4) δ 0.84 (m, 3H, H-19), 1.30 (m, 26H, -CH2-), 1.72 (m, 2H, H-5), 3.21 (m, 2H, H-4), 3.70 (m, 3H, H-1, H-2); 13C NMR (50.3 MHz, D2SO4) δ 14.1 (C-19), 22.8 (C-18), 26.1 (C-6, C-7), 29.6 (C-8, C-9), 29.9 (-CH2-), 32.2 (C-5), 43.4 (C-2), 51.8 (C-4). HRMS (ESI) Calcd. for (C18H42NO6P2) [M+H]+: 430.2487; Found: 430.2468. Anal. Calcd. for (C18H41O6NP2) C, 50.34; H, 9.62; N, 3.26. Found C, 50.88; H, 9.54; N, 3.69.
1-[(n-Octadec-1-ylamino)ethyl] 1,1-bisphosphonic Acid (29)
mp 121–123 °C; IR (KBr, cm−1) 2920, 2850, 1598, 1469, 1272, 1182, 1006, 954, 705; 1H NMR (200.13 MHz, D2SO4) δ 0.84 (m, 3H, H-21), 1.30 (m, 30H, -CH2-), 1.70 (m, 2H, H-5), 3.20 (m, 2H, H-4), 3.70 (m, 3H, H-1, H-2). HRMS (ESI) Calcd. for (C20H27O6P2Na) [M+Na]+: 354.1211; found 354.1192.
Drug Screening
T. cruzi amastigotes assays
Gamma-irradiated (2,000 Rads) Vero cells (3.4 × 104 cells/well) were seeded in 96 well plates (black, clear bottom plates from Greiner Bio-One) in 100 μL RPMI media (Sigma) with 10 % FBS. Plates were incubated overnight at 35 °C and 7 % CO2. After overnight incubation, Vero cells were challenged with 3.4 × 105 trypomastigotes/well (CL strain overexpressing a tdTomato red fluorescent protein) in 50 μL volume and incubated for 5 h at 35 °C and 7 % CO2. After infection, cells were washed once with Hanks solution (150 μL/well) to eliminate any extracellular parasites and compounds were added in serial dilutions in RPMI media in 150 μL volumes. Each dilution was tested in quadruplicate. Each plate also contained controls with host cells and no parasites (for background check), controls with two representative drug dilutions and no parasites (for cytotoxicity assays), and controls with parasites and no drugs (positive control). For each plate, benznidazole was also used as a positive control at 3.5 and 1.5 μM. After drug addition, plates were incubated at 35 °C and 7 % CO2. At day 3 post-infection, plates were assayed for fluorescence.44 IC50 values were determined by nonlinear regression analysis using SigmaPlot.
T. gondii tachyzoites assays
Experiments on T. gondii tachyzoites were carried out as described previously45 using T.gondii tachyzoites expressing red fluorescence protein.46 Cells were routinely maintained in hTerT cells grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum, 2 mM glutamine, 1 mM pyruvate, at 37 °C in a humid 5% CO2 atmosphere. Confluent monolayers grown in 96-well black plates with optical bottoms (Falcon/Becton-Dickinson, Franklin Lakes, NJ) were used and drugs dissolved in the same medium and serially diluted in the plates. Freshly isolated tachyzoites were filtered through a 3 μm filter and passed through a 22 gauge needle, before use. The cultures were inoculated with 104 tachyzoites/well in the same media. The plates were incubated at 37 °C and read daily in a Molecular Devices fluorescence plate reader. To preserve sterility the plates were read with covered lids, and both excitation (510 nm) and emission (540 nm) were read from the bottom.47 For the calculation of the IC50, the percent of growth inhibition was plotted as a function of drug concentration by fitting the values to the function: I = Imax C / (IC50 + C), where I is the percent inhibition, Imax = 100% inhibition, C is the concentration of the inhibitor, and IC50 is the concentration for 50% growth inhibition.
TcFPPS and TgFPPS Assays and Product Analysis
For TcFPPS14,15,48 100 μL of assay buffer (10 mM Hepes, pH 7.4, 5 mM MgCl2, 2 mM dithiothreitol, 100 μM [4-14C]IPP (10 μCi/μmol)), and 100 μM DMAPP were prewarmed to 37 °C. The assay was initiated by the addition of recombinant protein (10–20 ng). The assay was allowed to proceed for 30 min at 37 °C and was quenched by the addition of 6 M HCl (10 μL). The reactions were made alkaline with 6.0 M NaOH (15 μL), diluted in water (0.7 mL), and extracted with hexane (1 mL). The hexane solution was washed with water and transferred to a scintillation vial for counting. One unit of enzyme activity was defined as the activity required to incorporate 1 nmol of [4-14C]IPP into [14-14C]FPP in 1 min. For TgFPPS the assay conditions were as described above except that the buffer contained 1 mM MgCl2.
TcSPPS assay
The activity of the enzyme was determined by a radiometric assay based on that described before.49 Briefly, 100 μL of assay buffer (100 mM Tris-HCl buffer, pH 7.4, 1 mM MgCl2, 1% (v/v) Triton X-100, 100 μM [4-14C]IPP (10 μCi/μmol)), and 50 μM GGPP was prewarmed to 37 °C. The assay was initiated by the addition of 10–20 ng of recombinant protein. The assay was allowed to proceed for 30 min at 37 °C and was quenched by chilling quickly in an ice bath. The reaction products were extracted with 1 ml of 1-butanol saturated with water. The organic layer was washed with water saturated with NaCl, and transferred to a scintillation vial with 4 ml of scintillation solution Ecolume for counting. One unit of enzyme activity was defined as the activity required to incorporate 1 nmol of [4-14C]IPP into [4-14C]FPP in 1 min.
Supplementary Material
Figure 2.
Chemical structure of representative FDA-approved bisphosphonates clinically employed for different bone disorders.
Acknowledgments
This work was supported by grants from the National Research Council of Argentina (PIP 1888), ANPCyT (PICT 2008 #1690), and the Universidad de Buenos Aires (X-191) to J.B.R., and the U.S. National Institutes of Health to R. D. (AI-082542) and S. N. J. M. (AI-068467). V. S. R. was supported in part by a training grant of the Ellison Medical Foundation to the Center for Tropical and Emerging Global Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Referencias
- 1.Urbina JA, Docampo R. Trends Parasitol. 2003;19:495–501. doi: 10.1016/j.pt.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Urbina JA. Acta Tropica. 2010;115:55–68. doi: 10.1016/j.actatropica.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 3.Urbina JA. Drugs Fut. 2010;35:409–420. [Google Scholar]
- 4.García Liñares G, Ravaschino EL, Rodriguez JB. Curr Med Chem. 2006;13:335–360. doi: 10.2174/092986706775476043. [DOI] [PubMed] [Google Scholar]
- 5.Brener Z. Annu Rev Microbiol. 1973;27:347–382. doi: 10.1146/annurev.mi.27.100173.002023. [DOI] [PubMed] [Google Scholar]
- 6.Kirchhoff LV. New Engl J Med. 1993;329:639–644. doi: 10.1056/NEJM199308263290909. [DOI] [PubMed] [Google Scholar]
- 7.Galel SA, Kirchhoff LV. Transfusion. 1996;36:227–231. doi: 10.1046/j.1537-2995.1996.36396182140.x. [DOI] [PubMed] [Google Scholar]
- 8.Nussenzweig V, Sonntag R, Biancalana A, Pedreira de Fleitas JL, Amato Neto V, Kloetzel J. Hospital (Rio de Janeiro) 1953;44:731–744. [PubMed] [Google Scholar]
- 9.Docampo R, Moreno SNJ. Rev Biochem Toxicol. 1985;7:159–204. [Google Scholar]
- 10.Urbina JA. Mem Inst Oswaldo Cruz. 1999;94(Suppl 1):349–355. doi: 10.1590/s0074-02761999000700068. [DOI] [PubMed] [Google Scholar]
- 11.Docampo R, Moreno SN. Curr Drug Targets Infect Disord. 2001;1:51–61. doi: 10.2174/1568005013343191. [DOI] [PubMed] [Google Scholar]
- 12.Docampo R, Moreno SNJ. Curr Pharm Des. 2008;14:882–888. doi: 10.2174/138161208784041079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gelb MH, Van Voorhis WC, Buckner FS, Yokoyama K, Eastman R, Carpenter EP, Panethymitaki C, Brown KA, Smith DF. Mol Biochem Parasitol. 2003;726:155–163. doi: 10.1016/s0166-6851(02)00282-7. [DOI] [PubMed] [Google Scholar]
- 14.Montalvetti A, Bailey BN, Martin MB, Severin GW, Oldfield E, Docampo R. J Biol Chem. 2001;276:33930–33937. doi: 10.1074/jbc.M103950200. [DOI] [PubMed] [Google Scholar]
- 15.Montalvetti A, Fernandez A, Sanders JM, Ghosh S, Van Brussel E, Oldfield E, Docampo R. J Biol Chem. 2003;278:17075–17083. doi: 10.1074/jbc.M210467200. [DOI] [PubMed] [Google Scholar]
- 16.Gabelli SB, McLellan JS, Montalvetti A, Oldfield E, Docampo R, Amzel LM. Proteins. 2006;62:80–88. doi: 10.1002/prot.20754. [DOI] [PubMed] [Google Scholar]
- 17.Ferella M, Montalvetti A, Rohloff P, Miranda K, Fang J, Reina S, Kawamukai M, Búa J, Nilsson D, Pravia C, Katzin A, Cassera MB, Aslund L, Andersson B, Docampo R, Bontempi EJ. J Biol Chem. 2006;281:39339–39348. doi: 10.1074/jbc.M607451200. [DOI] [PubMed] [Google Scholar]
- 18.Reszka AA, Rodan GA. Mini Rev Med Chem. 2004;4:711–719. [PubMed] [Google Scholar]
- 19.Reszka AA, Rodan GA. Curr Osteoporos Rep. 2003;1:45–52. doi: 10.1007/s11914-003-0008-5. [DOI] [PubMed] [Google Scholar]
- 20.Martin MB, Grimley JS, Lewis JC, Heath HT, III, Bailey BN, Kendrick H, Yardley V, Caldera A, Lira R, Urbina JA, Moreno SNJ, Docampo R, Croft SL, Oldfield E. J Med Chem. 2001;44:909–916. doi: 10.1021/jm0002578. [DOI] [PubMed] [Google Scholar]
- 21.Hughes DE, Wright KR, Uy HL, Sasaki A, Yoneda T, Roodman GD, Mundy GR, Boyce BF. J Bone Miner Res. 1995;10:1478–1487. doi: 10.1002/jbmr.5650101008. [DOI] [PubMed] [Google Scholar]
- 22.Rogers MJ, Frith JC, Luckman SP, Coxon FP, Benford HL, Monkkonen J, Auriola S, Chilton KM, Russell RG. Bone. 1999;24:73S–79S. doi: 10.1016/s8756-3282(99)00070-8. [DOI] [PubMed] [Google Scholar]
- 23.Urbina JA, Moreno B, Vierkotter S, Oldfield E, Payares G, Sanoja C, Bailey BN, Yan W, Scott DA, Moreno SNJ, Docampo R. J Biol Chem. 1999;274:33609–33615. doi: 10.1074/jbc.274.47.33609. [DOI] [PubMed] [Google Scholar]
- 24.Martin MB, Sanders JM, Kendrick H, De Luca-Fradley K, Lewis JC, Grimley JS, Van Brussel EM, Olsen JR, Meints GA, Burzynska A, Kafarski P, Croft SL, Oldfield E. J Med Chem. 2002;45:2904–2914. doi: 10.1021/jm0102809. [DOI] [PubMed] [Google Scholar]
- 25.Ghosh S, Chan JM, Lea CR, Meints GA, Lewis JC, Tovian ZS, Flessner RM, Loftus TC, Bruchhaus I, Kendrick H, Croft SL, Kemp RG, Kobayashi S, Nozaki T, Oldfield E. J Med Chem. 2004;47:175–187. doi: 10.1021/jm030084x. [DOI] [PubMed] [Google Scholar]
- 26.Szajnman SH, García Liñares GE, Li Z-H, Galizzi M, Jiang C, Bontempi E, Ferella M, Moreno SNJ, Docampo R, Rodriguez JB. Bioorg Med Chem. 2008;16:3283–3290. doi: 10.1016/j.bmc.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szajnman SH, Bailey BN, Docampo R, Rodriguez JB. Bioorg Med Chem Lett. 2001;11:789–792. doi: 10.1016/s0960-894x(01)00057-9. [DOI] [PubMed] [Google Scholar]
- 28.Szajnman SH, Montalvetti A, Wang Y, Docampo R, Rodriguez JB. Bioorg Med Chem Lett. 2003;13:3231–3235. doi: 10.1016/s0960-894x(03)00663-2. [DOI] [PubMed] [Google Scholar]
- 29.Szajnman SH, Ravaschino EL, Docampo R, Rodriguez JB. Bioorg Med Chem Lett. 2005;15:4685–4690. doi: 10.1016/j.bmcl.2005.07.060. [DOI] [PubMed] [Google Scholar]
- 30.Ling Y, Sahota G, Odeh S, Chan JMW, Araujo FG, Moreno SNJ, Oldfield E. J Med Chem. 2005;48:3130–3140. doi: 10.1021/jm040132t. [DOI] [PubMed] [Google Scholar]
- 31.Bouzahzah B, Jelicks LA, Morris SA, Weiss LM, Tanowitz HB. Parasitol Res. 2005;96:184–187. doi: 10.1007/s00436-005-1331-9. [DOI] [PubMed] [Google Scholar]
- 32.Amzel LM. Personal communication.
- 33.Hosfield DJ, Zhang Y, Dougan DR, Broun A, Tari LW, Swanson RV, Finn J. J Biol Chem. 2003;278:18401–18407. doi: 10.1074/jbc.C300511200. [DOI] [PubMed] [Google Scholar]
- 34.Tarshis LC, Proteau PJ, Kellogg BA, Sacchettini JC, Poulter CD. Proc Natl Acad Sci USA. 1996;93:15018–15023. doi: 10.1073/pnas.93.26.15018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang C-H, Gabelli SB, Oldfield E, Amzel LM. Proteins. 2010;78:888–899. doi: 10.1002/prot.22614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cao R, Chen CK-M, Guo R-T, Wang AH-J, Oldfield E. Proteins. 2008;73:431–439. doi: 10.1002/prot.22066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szajnman SH, García Liñares GE, Moro P, Rodríguez JB. Eur J Org Chem. 2005:3687–3696. [Google Scholar]
- 38.Degenhardt CR, Burdsall DC. J Org Chem. 1986;51:3488–3490. [Google Scholar]
- 39.Bulman Page PC, Jonathan PG, Moore JPG, Mansfield I, McKenzie MJ, Bowler WB, Gallagher JA. Tetrahedron. 2001;57:1837–1847. [Google Scholar]
- 40.Lazzarato L, Rolando B, Lolli ML, Tron GC, Fruttero R, Gasco A, Deleide G, Guenther HL. J Med Chem. 2005;48:1322–1329. doi: 10.1021/jm040830d. [DOI] [PubMed] [Google Scholar]
- 41.Urbina JA, Concepcion JL, Montalvetti A, Rodriguez JB, Docampo R. Antimicrob Agents Chemother. 2003;47:2047–2050. doi: 10.1128/AAC.47.6.2047-2050.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ling Y, Sahota G, Odeh S, Chan JMW, Araujo FG, Moreno SNJ, Oldfield E. J Med Chem. 2005;48:3130–3140. doi: 10.1021/jm040132t. [DOI] [PubMed] [Google Scholar]
- 43.Rodrigues CO, Scott DA, Bailey B, de Souza W, Benchimol M, Moreno B, Urbina JA, Oldfield E, Moreno SNJ. Biochem J. 2000;349:737–745. doi: 10.1042/bj3490737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Canavaci AM, Bustamante JM, Padilla AM, Pereza Brandan CM, Simpson LJ, Xu D, Boehlke CL, Tarleton RL. PLOS Negl Trop Dis. 2010;4:e740. doi: 10.1371/journal.pntd.0000740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gubbels MJ, Li C, Striepen B. Antimicrob Agents Chemother. 2003;43:309–316. doi: 10.1128/AAC.47.1.309-316.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agrawal S, van Dooren GG, Beatty WL, Striepen B. J Biol Chem. 2009;284:33683–33691. doi: 10.1074/jbc.M109.044024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ravaschino EL, Docampo R, Rodriguez JB. J Med Chem. 2006;49:426–435. doi: 10.1021/jm050922i. [DOI] [PubMed] [Google Scholar]
- 48.Ogura K, Nishino T, Shinka T, Seto S. Methods Enzymol. 1985;110:167–171. [Google Scholar]
- 49.Rilling HC. Eukaryotic prenyltransferases. Methods Enzymol. 1985;110:145–152. doi: 10.1016/s0076-6879(85)10069-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.