Abstract
Many multinucleated giant cells are well-known to form from macrophage origin. Those formed from other cell types are less described, but may be as prevalent in pathological tissue. Giant multinucleated cells derived from secondary and primary fibroblast sources in various cultures with similar characteristics to foreign body giant cells are reported. Secondary-transformed NIH 3T3 fibroblasts rapidly fuse within 24 hours in contact co-cultures with RAW 264.7 immortalized macrophages, while 3T3 mono-cultures, non-contact (transwell) co-cultures, and macrophage-conditioned media-treated 3T3 mono-cultures all do not fuse. Primary-derived murine fibroblasts also form multinucleated cells, both in the presence or absence of co-cultured macrophages that increase during long-term culture (5–30 days). In contrast to 3T3 fusion, this primary cell phenomenon is not due to fibroblast fusion, but rather to nuclear division without cytokinesis. That these multinucleated fibroblasts can originate via different mechanisms may influence and distinguish their behaviors in conditions under which they may arise, including various in vitro culture assays, and in certain fibroblastic pathologies such as the foreign body response, fibrosis, cancer and aged tissue.
Keywords: fibroblast, macrophage, co-culture, foreign body response, animal model, cell culture
1. Introduction
Fibroblasts are the most common cell type in the body and can become altered in various pathologies such as fibrosis, cancer, aging, and the foreign body response (FBR) [1–9]. One phenotypic alteration that is not widely acknowledged, but possible is fibroblast multinucleation [6–8]. Additionally, several other multinucleated giant cells including foreign body giant cells (FBGCs), Langhans’ cells, and osteoclasts, all commonly derived from a macrophage cell origin are known [10].
Significantly, FBGCs can form from the fusion of multiple monocytes/macrophages [11] during the FBR, mounted by the host against implanted biomedical materials [12]. The FBR can cause device failure due to degradation by enzymes secreted by macrophages and abnormal collagen production by fibroblasts, resulting in an impeding collagen capsule [9, 12, 13]. Macrophages and fibroblasts are both primary FBR effector cells [9, 12], though unlike macrophages, fibroblasts are not commonly considered to form multinucleated giant cells. However, the mileu surrounding implants is abnormal, can be toxic to cells [14], has been speculated to stimulate tumorigenesis [15, 16], and can elicit altered cell phenotypes such as FBGCs [17]. This unusual environment may also prompt fibroblasts to alter their phenotype and form multinucleated giant cells.
Previous studies have identified multinucleated cells in vivo ostensibly of fibroblast origin [6, 7], one describing cells appearing as “bizarre, atypical fibroblasts with hyperchromatic and large, pleomorphic nuclei and multinucleated floret-like giant cells”[8]. Several studies describe the presence of multinucleated fibroblasts in vitro [18–21] and in vivo in pathologies such as fibrosis and cancer and in aged tissue [1–8]. However, whether these cells multinucleate in vitro and in vivo via fusion similar to FBGCs [11] or through nuclear division without cytokinesis [18] is unclear.
This study definitively identifies fibroblasts that become multinucleated through both mechanisms—fusion and mitosis without cytokinesis—depending on fibroblast phenotype and culture conditions. Immortalized secondary fibroblasts formed multinucleate cells via fusion with other fibroblasts during contact co-culture with secondary-derived macrophages after 24 hours. Primary fibroblasts formed multinucleate cells in mono-culture after becoming senescent and undergoing nuclear division without cytokinesis.
2. Methods and materials
2.1. Cell Culture
2.1.1. Secondary cell culture
Macrophage-like cell line RAW 264.7 and fibroblast-like cell line NIH 3T3 were purchased from the American Type Culture Collection (TIB-71 for RAW and CRL-1658 for 3T3 ATCC, Manassas, VA) and cultured in 96-well tissue culture-treated polystyrene plates (BD Falcon, San Jose, CA) unless otherwise specified, during contact co-culture experiments at 37°C with 5% supplemental CO2 for 24–72 hours. Fibroblasts were used at passages 6–30 and macrophages were used below passage 10. RAW cells were passaged by scraping with a rubber policeman. 3T3 cells were passaged using TripLE (Invitrogen, Carlsbad, CA). For optimal cell fusion, 2×104 fibroblasts and 1×104 macrophages were plated into co-culture wells. This same number of fibroblasts and macrophages were also plated into control wells for comparison. Cells were always cultured in complete media (Dulbecco’s modified eagle medium (DMEM) with 10% fetal bovine serum, and 1% antibiotic/antimycotic (Invitrogen, Carlsbad, CA). Secondary NIH 3T3 fibroblasts were cultured for 24–72 hours under four different conditions: 1) in direct co-culture contact with secondary RAW 264.7 macrophages, 2) co-culture but separated from RAW cells by a Transwell® microporous insert (RAWs in insert and 3T3s in well beneath), 3) in RAW-conditioned complete media, and 4) alone, devoid of macrophage signaling [22]. Mono-cultured RAW cells in complete media served as a control. Non-contact co-cultures utilized 3 µm porosity plasma-treated polycarbonate Transwell® Permeable Supports (Corning, Corning, NY) in 12-well tissue-culture treated polystyrene plates (BD Falcon, San Jose, CA). Cell numbers were scaled linearly with respect to surface area from the 96-well culture dishes to accommodate larger sized wells. In the same 12-well plates all 4 culture conditions—i.e., mono-culture, conditioned media, contact co-culture, and Transwell® co-culture—were also employed. Macrophage-conditioned media was collected after 24 hours of exposure to RAW cells (density=1×105 cells/well) and placed over mono-cultured fibroblasts in 12-well plates. New conditioned media was collected from the original RAW culture well every day for 3 days to be placed over the conditioned media-treated fibroblasts.
2.1.2. Murine primary cell sourcing
Specific-pathogen-free, 2–3 month-old male C57BL/6 mice were purchased from Jackson Laboratory (Bar Harbor, ME). Animals were kept in the University of Utah animal facility, and provided water, mouse chow, bedding, and modes of enrichment ad libitum throughout this study. Animals were euthanized via CO2.
2.1.3. Primary macrophage cell culture
Bone marrow cells were collected from the femurs and tibias of 2–3 month-old male C57BL/6 mice and differentiated into bone marrow macrophages (BMMΦs) using a previously described method [23, 24]. On day 7, the cells were removed from surfaces by incubation in Ca+2/Mg+2-free phosphate-buffered saline solution and scraping with a rubber policeman. Harvested cells were spun at 500 rcf for 5 minutes to form a pellet and then resuspended in DMEM with 10% heat-inactivated fetal bovine serum, 10% L929-conditioned media, 1% antibiotic/antimycotic, 1% MEM nonessential amino acids, 1% HEPES, and 1% sodium pyruvate. Also in 96-well plates, 1×104 primary macrophages per well were seeded alone or in contact co-culture with primary fibroblasts. Alternatively, primary monocyte-like cells were obtained by using the bone marrow cells on day 1, 2, or 4, before complete macrophage differentiation was expected [25–27]. Pure monocytes were obtained using the EasySepR Magnetic Mouse Monocyte Enrichment Kit (Stemcell Technologies Inc., Vancouver, BC) from bone marrow cells according to the manufacturer’s instructions, typically producing monocyte purity of 80%–93%.
2.1.4. Primary fibroblast cell culture
Primary fibroblasts were obtained post-mortem from ear dermal tissue of 2–3 month-old freshly sacrificed male C57BL/6 mice. Ear tissue was clipped at the base of the ear and soaked in 70% ethanol for 5 minutes and then rinsed in sterile phosphate-buffered saline (BD Falcon, San Jose, CA). Tissues were placed in a Petri dish and diced using a sterile razor, placed into 2 ml of 5 mg/ml collagenase solution in DMEM and incubated for 2 hours in a 37°C water bath with agitation [25–27] then filtered though a 70 µm cell filter (BD, San Jose, CA). An equal portion of complete media was added to the filtrate and spun at 500 rcf for 5 minutes to create a cell pellet. Supernatant was aspirated off and the cell pellet was resuspended in primary cell media (DMEM with 10% fetal bovine serum, 1% antibiotic/antimycotic, and 1% MEM nonessential amino acids (Invitrogen, Carlsbad, CA), placed in T75 cell culture flasks (BD Falcon, San Jose, CA), and incubated at 37°C with 5% supplemental CO2. Cells required 3–7 days to become confluent and were subsequently passaged and used in further experiments. Cells were passaged by incubation with TripleE (Invitrogen, Carlsbad, CA). Primary fibroblasts (1×104 cells per well in 96-well plates, characterized by anti-CD14 and anti-vimentin labeling, as well as Oil Red O post-differentiation, vide infra) were cultured alone or in physical contact with primary macrophages for up to 30 days. On different occasions, primary ear-derived fibroblasts were also co-cultured with either secondary RAW 264.7 cells, primary monocyte-like cells or pure monocytes, to determine if more monocytic cells could prompt cell fusion.
2.2. Cell labeling
2.2.1. H&E
A hematoxylin and eosin (H&E) stain (Fisher Scientific, Kalamazoo, MI) was employed according to manufacturer’s instructions to stain for nuclei and cytoplasm respectively.
2.2.2. Fluorescent cell labeling
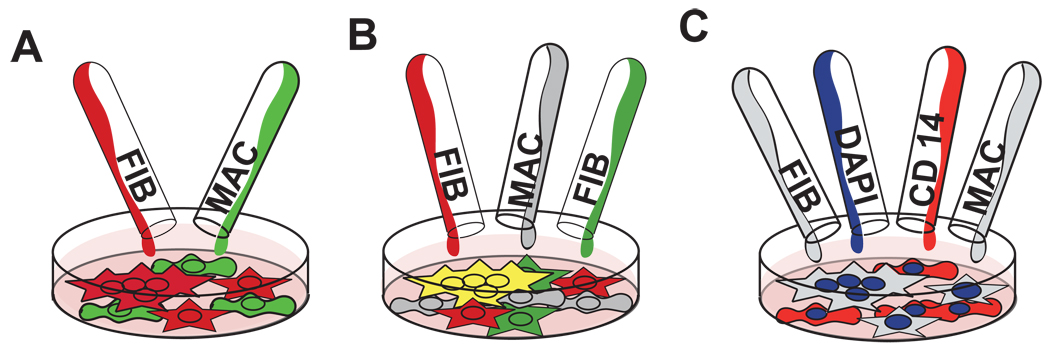
For fixed fluorescence labeling, cells were fixed in 4% paraformaldehyde (Sigma-Aldrich, St. Louis, MO) for 10 minutes at room temperature and labeled with rhodamine-phalloidin and counterstained with 4,6-diamidino-2-phenylindole (DAPI) (Molecular Probes, Eugene, OR) according to manufacturer’s instructions. Live cell in situ fluorescent detection used either a green Vybrant® CFDA SE Cell Tracer Kit or a red Cell Trace Far Red DDAO-SE long-lived intracellular dye (Invitrogen, Carlsbad, CA) according to manufacturer’s instructions. These dyes label 100% of cells (data not shown) and have been shown not to transfer to neighboring cells [28]. Macrophages were labeled with the green live dye, while fibroblasts were labeled with the red live dye prior to seeding in both mono- and co-cultures in order to distinguish fibroblast and macrophage populations during co-culture and determine cellular origin of the resulting multinucleated cells (Scheme 1A). These same dyes and experimental procedure were used to determine FBGC fusion [11]. Analogously two fibroblast populations were labeled with either red or green live cell dye prior to seeding in mono- and co-cultures to determine fibroblast fusion by dye co-localization (Scheme 1B). These same procedures were employed for both secondary- and primary-derived fibroblast cultures.
Scheme 1.
Multinucleate cell origin was followed by fluorescent cell labeling. A) Macrophages were labeled green and fibroblasts were labeled red prior to co-culture. B) Cell fusion was visualized as a co-localization of two different labeled fibroblast populations (red and green) with macrophages receiving no dye (grey). Cell co-localization of red and green dyes appears yellow. C) After 24 hours, macrophage and fibroblast cultures were labeled with DAPI (blue) and macrophage-specific anti-CD14(red) to identify possible macrophage contamination within the plated fibroblast population.
2.2.3. Antibody labeling
A phycoerythrin-conjugated macrophage marker, anti-CD14 (clone Sa2–8, IgG2a, diluted 1:100, eBioscience, San Diego, CA) [29] was added to control RAW cells, 3T3 cells, and co-cultures of RAW and 3T3 cells to determine possible inadvertent macrophage contamination in fibroblast cultures and to confirm multinucleate cell origins (Scheme 1C). This marker was also added to primary-derived macrophages and fibroblasts to determine multinucleate cell origin. Cyanine3-tagged fibroblast marker anti-vimentin [30] (clone V9, IgG1, diluted 1:100, Sigma, St. Louis, MO) was added to primary fibroblasts to assert phenotype and multinucleate cell origin.
2.2.4. Senescence
Both primary and secondary macrophages and fibroblasts and primary adipose-derived stem cells (ASCs, isolation and characterization described in Supplementary Information) were stained with a senescence-labeling kit staining for beta-galactosidase [31] (Cell Signaling Technology, Danvers, MA) according to manufacturer’s instructions.
2.2.5. Cell Apoptosis
Primary fibroblasts were cultured for 3 days prior to apoptosis testing. Positive control fibroblasts were incubated with 1 mg/ml bupivicaine (Hospira, Lake Forest, IL) for 2 hours at 37°C. Primary fibroblasts were labeled with Poly Caspases FLICA™ in vitro Apoptosis Detection Kit (Immunochemistry Technologies, Bloomington, MN) according to manufacturer’s instructions.
2.2.6. Mycoplasma Assay
Mycoplasma testing was performed using DAPI labeling, according to standard protocols [32–34].
2.2.7. TRAP Assay
Secondary fibroblast-derived multinucleated cells were stained using a tartrate resistant acid phosphatase (TRAP) assay (Sigma-Aldrich, St. Louis, MO), specific for osteoclasts [35], according to manufacturer’s instructions.
2.3. Imaging
Fluorescent, brightfield, and colored microscopy images of cells in culture were acquired using a Nikon Eclipse TE2000-U microscope equipped with fluorescent optics, CCD camera, and Metamorph and Q Capture Pro software. Confocal images were captured using a FV1000 IX81 Olympus confocal microscope. Fluorescence and confocal images were used to identify dye co-localization within cells. At least 9 replicates from 3 separate cell experiments were imaged to determine representative image samples of all experiments in this study. Experiments producing multinucleated fibroblasts were repeated at least 6 times.
2.4. Video
Confocal time-lapse video was acquired using a Nikon A1 Confocal microscope over 24–48 hours. In videos taken from 0–24 hours, cultured cells did not readily adhere to the surface of the plate, most likely due to microscope micromotion (results not shown). Four 20X fields were digitally stitched together into a mosaic for the video included in this study.
2.5. Statistics
Numbers of giant cells per frame, percent nuclei fused, and percent multinucleated cells between short-term co-cultures and long-term mono-cultures compared to short-term mono-cultured fibroblasts were evaluated using a student’s t-test with significance defined as p < 0.05. A Single-Factor ANOVA was utilized to determine significance between groups of samples. A post-hoc student’s t-test was used to determine statistically significant differences between samples (p < 0.05). Cell counts were taken from 15X objective images. Three frames per replicate were counted and the mean was used for analysis. At least 3 independent replicates were counted with independent replicates defined as different experiments using different mice for primary cells, and different passage numbers for secondary cells.
3. Results
3.1. Mycoplasma detection
All cell cultures, both those derived from primary and secondary sources, stained negative for mycoplasma contamination using DAPI fluorescence (data not shown).
3.2. Secondary-derived multinucleate cells
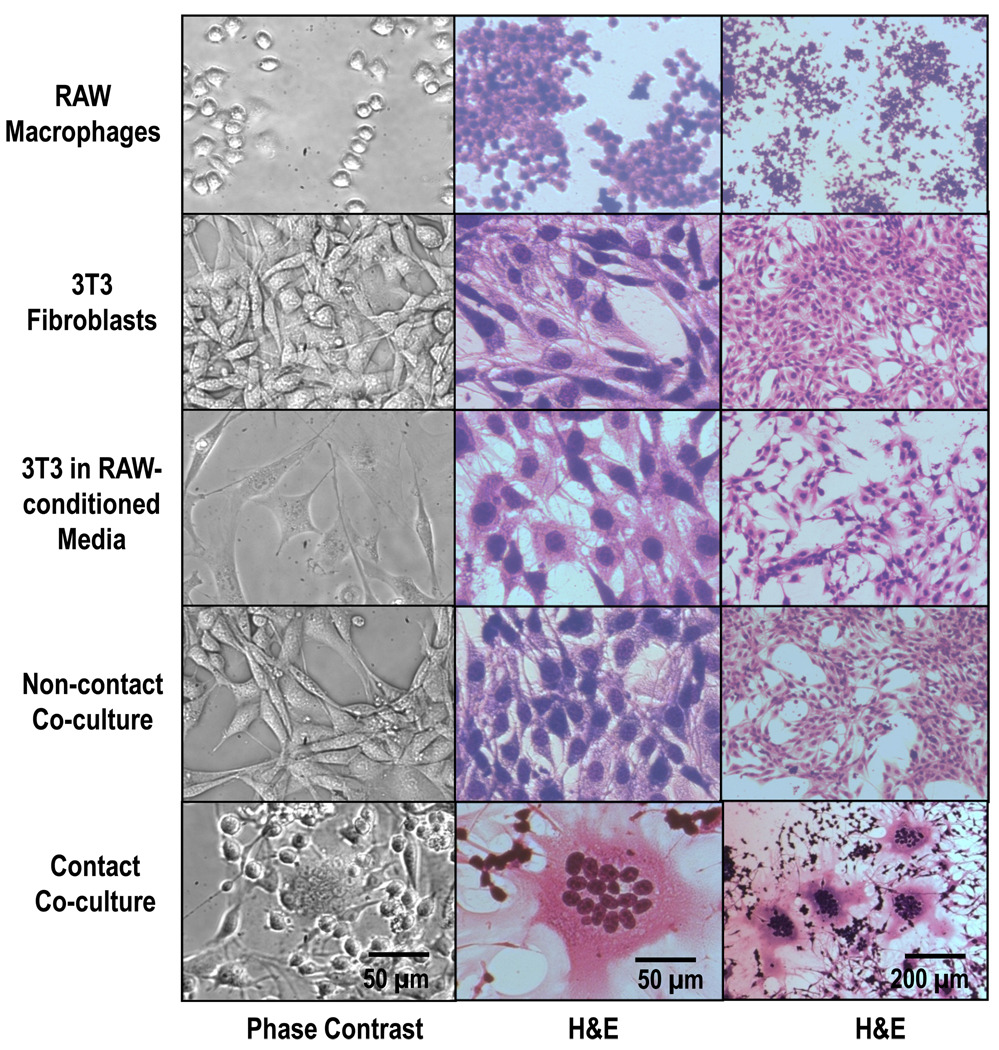
Figure 1 shows multinucleated cells appearing during contact co-culture with secondary RAW macrophages (Row 5). RAW and 3T3 cells cultured alone (Rows 1&2, respectively) and 3T3s in the presence of RAW-conditioned media (Row 3) do not form multinucleated cells. In order to test for signaling effects of short-lived excreted cytokines, 3T3s were co-cultured with RAWs separated by Transwell® inserts (Row 4), where the permeable polyester membrane prevents physical contact of each cell type, but permits mass transport of soluble culture components. No formation of multinucleated fibroblasts occurred in this co-culture system.
Figure 1.
Phase contrast and hematoxilin and eosin (H&E)-stained cell images. Cultured 3T3 fibroblasts form multinucleated cells in contact co-culture with RAW macrophages (bottom row), but not during mono-culture (top 2 rows), treatment with conditioned media (3rd row) or non-contact co-culture (4th row). Images shown after 3 days of culture in complete media.
Multinucleated cells have been reported in vivo to have “increased nuclear-cytoplasmic ratio, pale pink scant cytoplasm, and indistinct cell boundaries (with rosette arrangement of hyperchromatic nuclei)”[8] similar to those seen in this study (Figure 1, Row 5). This can be seen both with live cells (Figure 1, column 1) and fixed cells stained with H&E (Figure 1, Columns 2&3). 3T3 cells begin multinucleation immediately upon adherence to tissue culture surfaces in the presence of RAWs (data not shown) and is readily apparent at 24 hours (Figure 2).
Figure 2.
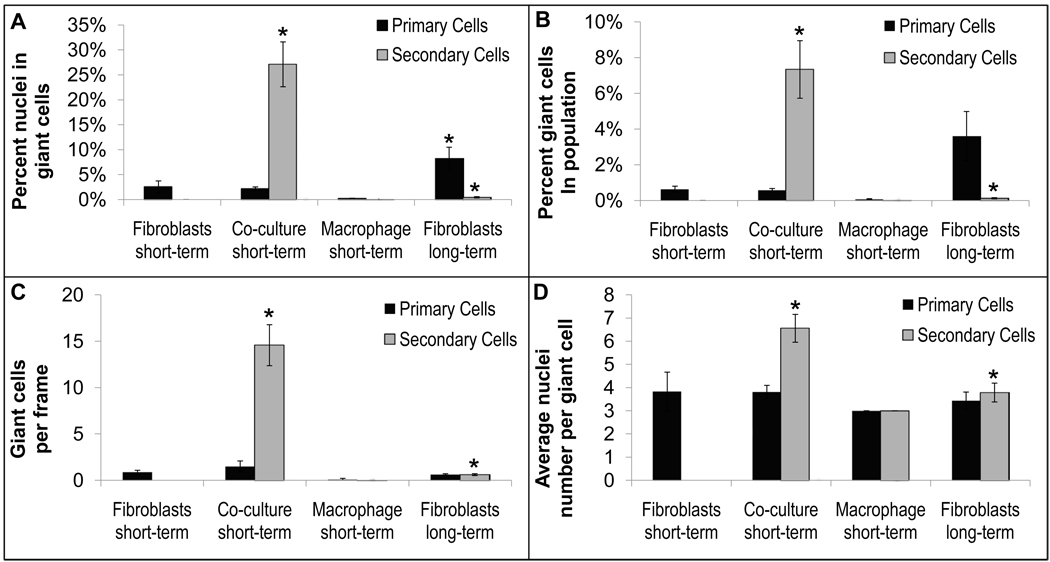
Multinucleate cell characterization. A) Percent nuclei found in multinucleate giant cells, B) percent multinucleate giant cells found in cell population, C) number of multinucleate giant cells per frame, and D) average number of nuclei per giant cell for primary- and secondary-derived fibroblasts and macrophages alone (short-term, 1–3 days, and long-term, 30 days) and in co-culture (short-term, 1–3 days) without the addition of exogenous cytokines. So few giant cells per frame in the long-term fibroblast culture condition is due to the increase in size of those fibroblasts compared to those cultured for short-term, consequently decreasing the overall number of cells per frame (see Figure 7). Data represent the mean ± SEM from 3 independent replicates. Significance (p < 0.05) was analyzed between primary and secondary fibroblast short-term monocultures and short-term co-cultures and between primary and secondary fibroblast short-term monocultures and long-term monocultures. Images were taken with a 15X objective and yielded a frame size of 260, 891 µm2.
3.3. Multinucleate cell origin
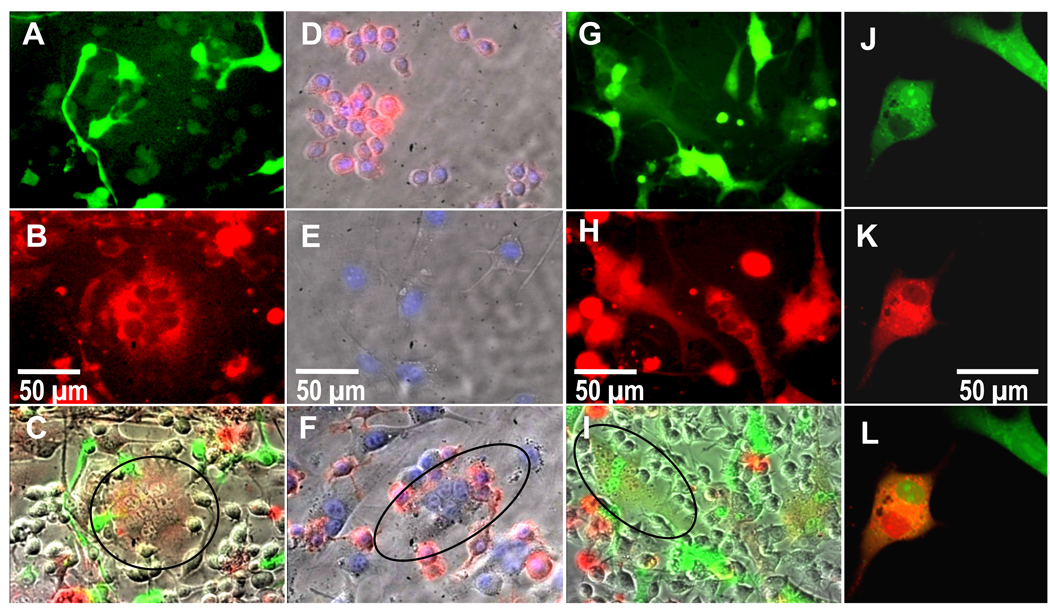
Figure 3 shows RAW cells (Figure 3A) labeled with a cytoplasmic fluorescent green dye prior to co-culture with fibroblasts, and 3T3s (Figure 3B) labeled with a cytoplasmic fluorescent red dye prior to co-culture with macrophages. Resulting multinucleated cells (Figure 3C) exhibited red fluorescence, with no detectable green fluorescence. Additionally, as proof of negligible macrophage contamination in the fibroblast population, anti-CD14 added to the mixed population readily bound all mono-cultured RAW cells (Figure 3D) but not mono-cultured fibroblasts (Figure 3E). In the co-culture system, no multinucleated cells were fluorescently labeled by anti-CD14 (Figure 3F). Other external macrophage markers analyzed, including MHC-II, CD40, CD18, CD11b, and F4/80, were analyzed (data not shown). However, anti-CD14 provided the most reliable and prominent labeling and was thus presented in this study.
Figure 3.
Fluorescence images showing A) RAW macrophages labeled green, B) 3T3 fibroblasts labeled red, and C) an overlay of brightfield and red and green channels showing a red (circled), and therefore fibroblastic, multinucleated cell. Fluorescence images showing D) macrophages and E) fibroblasts incubated with DAPI (blue) and also fluorescently labeled anti-CD14 (red), revealing F) fibroblasts that stain negative for CD14, are multinucleated (circled), and are therefore not macrophages. Fluorescent G–I) and confocal J–K) images revealing co-localization of a red-labeled population of fibroblasts fused with a green-labeled population of fibroblasts in the presence of non-labeled macrophages, where G and J are the green channel, H and K are the red channel, and I and L are overlays of I) brightfield and red and green fluorescent channels and L) red and green channels (co-localization experiments with 3 replicates each were repeated 3 times). Images shown after 24 hours of culture.
3.4. Secondary fibroblast fusion
Separate 3T3 fibroblasts cultures containing either green or red cytoplasmic dyes were added simultaneously to non-labeled RAW macrophage cultures (Figure 3 G–L). Figure 3 I&L shows that resulting multinucleated cells exhibit both red and green nuclei with yellow (i.e. both red and green co-localization) cell bodies. A video of these cells fusing between 24 and 48 hours in culture is available online in Supplementary Data. This video also shows these cells to be highly motile, traveling hundreds of microns over the course of 24 hours. Figure 4 shows still frames from that video and the corresponding approximate cell trajectories of one tracked multinucleated cell.
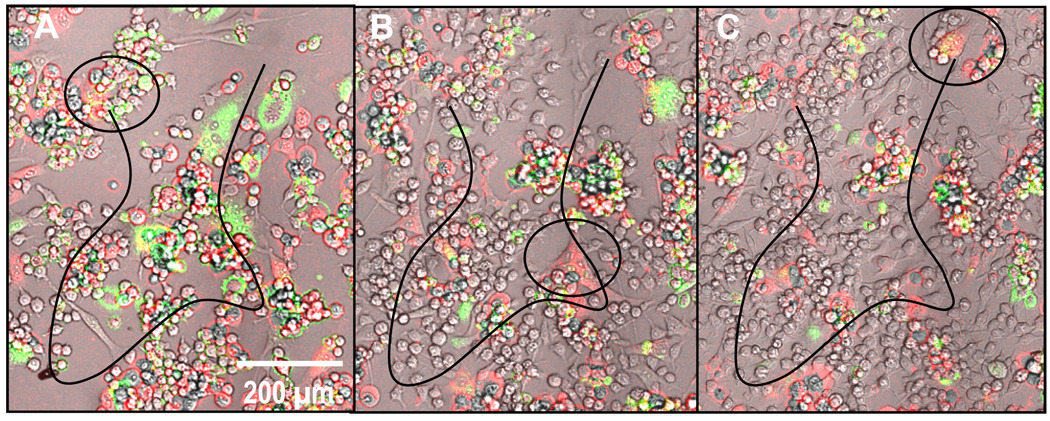
Figure 4.
Frames A–C show the approximate motility trajectory (black line) of a secondary-derived multinucleated fibroblast (circled) traveling hundreds of microns over the course of 24 hours (from 24–28 hours). This time-lapse image series also appears to show the fibroblast beginning as only a few red-labeled cells and ending with several more nuclei including those from green-labeled fibroblasts (full video available online in supplementary data).
Multinucleated cells derived from 3T3 fibroblasts exhibit similar qualities to FBGCs, with enlarged cytoplasms, multiple cellular adhesions, and multiple centric nuclei [36]. However, they do not possess punctate podosomal actin [17, 37] but do possess prominent stress fibers, features consistent with fibroblasts [38] but contrary to FBGCs and osteoclasts (Figure 5A). Furthermore, secondary-derived multinucleated cells in this study did not stain positive for macrophage-marker CD14 (Figure 3 D–F) or osteoclast-marker TRAP (Supplementary Figure 1).
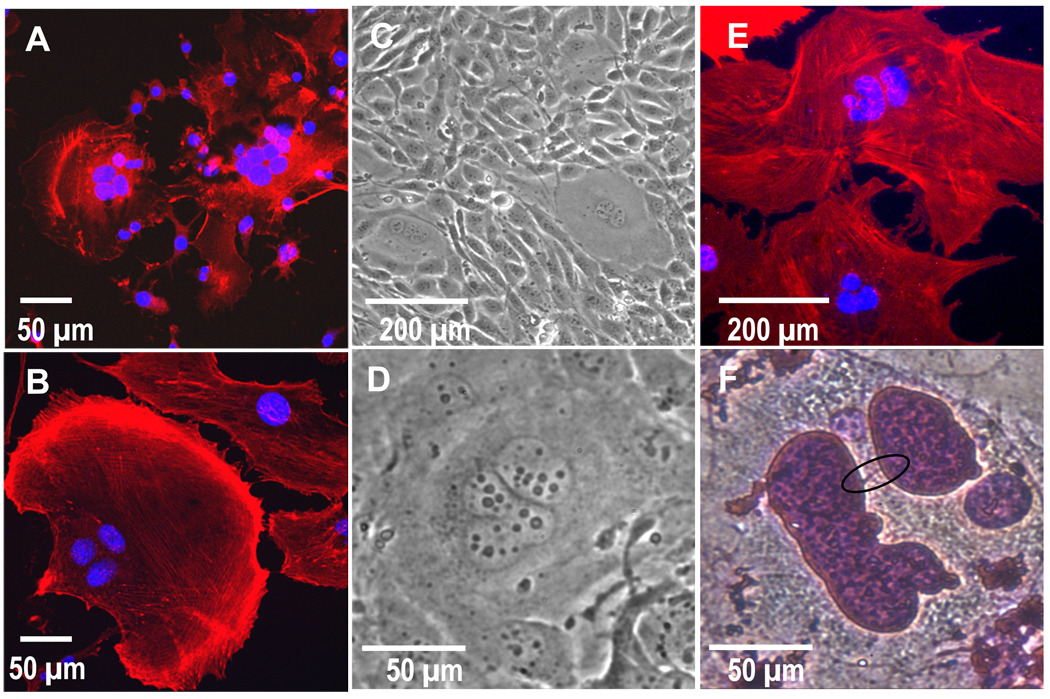
Figure 5.
Actin (red) and nuclei (blue) labeling for A) secondary-derived multinucleate fibroblasts and B) primary-derived multinucleate fibroblasts. These images show prominent stress fibers (more prevalent in primary versus secondary multinucleate cells) and the absence of podosomes surrounding single and multinucleate cells, features contrary to those seen in macrophage-derived FBGCs. Secondary multinucleate cells are shown after 1 day and primary cells are shown after 30 days of culture. C&D) Phase contrast images (C 10X and D 40X) of secondary fibroblasts which spontaneously formed polymorphonuclear multinucleated cells after >20 passages and culture for >5 days. E&F) Primary fibroblasts contain several pleomorphic and budding nuclei seen in E) a confocal image of DAPI (blue) and phalloidin (red) labeled cells and F) an H&E stained cell (purple line, potentially chromatin, circled in black),shown after 30 days in culture.
Interestingly after passages greater than 20, 3T3s cultured on the same surface for extended periods of time (>5 days) began forming multinucleated cells even in the absence of macrophages (Figure 5 C–D). The spontaneously formed multinucleated cells frequently had nuclei that appeared polymorphonuclear.
3.5. Primary-derived multinucleate cells
Primary murine ear dermal fibroblast isolations were confirmed to be dominantly fibroblastic using several cell phenotype assays (see Supplementary Information and Supplementary Figure 4).
Figure 6 shows that primary fibroblasts cultured in the presence of primary bone marrow-derived macrophages (Figure 6 A–C) or secondary-derived RAW cells (Figure 6 D–F) also become multinucleated, while the macrophages alone do not over the same culture time period. No noticeable differences between fibroblast fusion rates in the presence of either primary macrophages or monocytes were seen (data not shown). Primary-derived multinucleated cells are not macrophage-like. Primary macrophages containing cytoplasmic green dye cultured with primary fibroblasts containing cytoplasmic red dye for 24 hours yield multinucleated cells with only red fluorescence (Figure 6 G–I). Additionally, while both primary macrophages and fibroblasts stained positive for CD14, upregulated in macrophages [29, 39], primary BMMΦs stained strongly positive (Figure 6J), while fibroblasts exhibited only very dim fluorescence (Figure 6K). Differentiation between the two cell types is clear. Additionally, fibroblasts stained positive for the fibroblast-specific marker, vimentin, including cells with multiple nuclei (Figure 6 L&O).
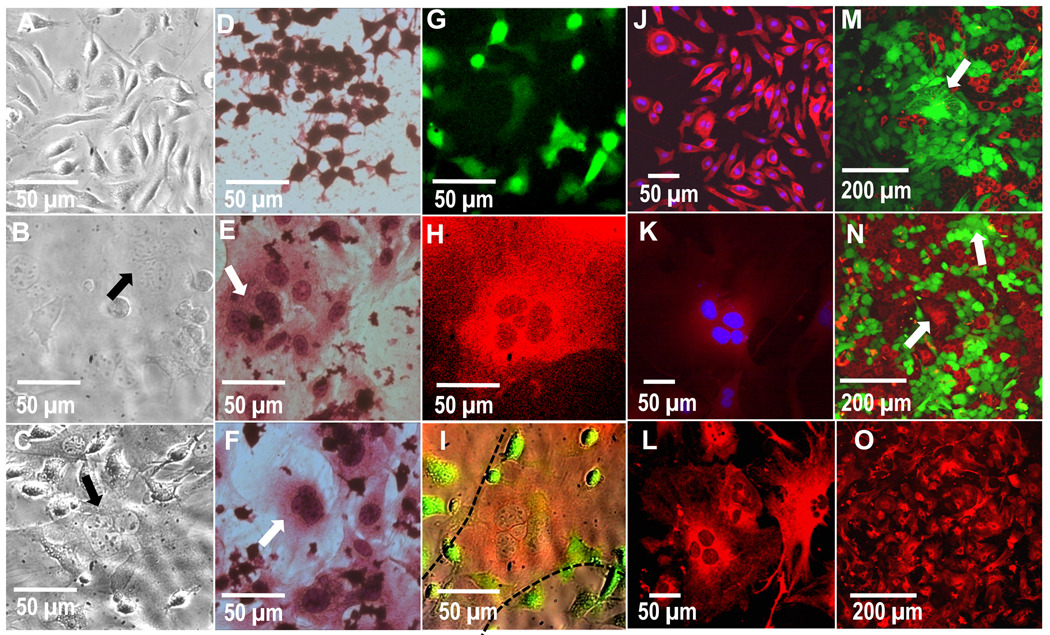
Figure 6.
Phase contrast images of A) primary-derived BMMΦs alone, B) primary fibroblasts alone and C) contact co-culture of BMMΦs and fibroblasts. Note multinucleate cells in both the mono-culture fibroblasts and contact co-cultures (arrows). H&E-stained images showing D) secondary-derived RAWs cultured alone, E) ear fibroblast cultured alone and F) contact co-culture of RAW cells and primary fibroblasts. Multinucleate cells can be seen in fibroblast mono-culture as well as contact co-culture with RAW cells (arrows). G–I) Fluorescent images of contact co-cultured primary macrophages (green channel, G), fibroblasts (Red channel, H) and an overlay of red, green and brightfield channels (I). These images show that the multinucleated cell is red and therefore of fibroblastic origin (H and delineated by dotted lines in I). Confocal images of primary-derived J) BMMΦs and K) fibroblasts labeled with DAPI (blue) and macrophage-marker CD14 (red), revealing a strong CD14 stain in macrophages but not fibroblasts. L and O) show 40X and 20X confocal images, respectively, of primary-derived fibroblasts labeled with fibroblast-marker vimentin (red), showing that the majority of cells in this culture stain positive for fibroblasts, including multinucleate cells. M and N) Confocal images of mono-cultured primary fibroblasts pre-labeled with red or green long-lived intracellular fluorescence prior to culture, where the multinucleated cells (arrows) found were either green or red, but not both (representative images from 3 separate experiments and a total of 9 replicates). This lack of co-localization indicates an absence of fusion to produce cell multinucleation. Images are shown after 3 days of culture.
Figure 6 also shows primary multinucleated cells in mono-cultures of fibroblasts containing no added macrophages. No significant differences are noted between fibroblasts cultured alone or during co-culture both at short time points (<3 days) (Figure 2). Primary multinucleated cells do not appear to form via cell-cell fusion. Figure 6 M&N shows two populations of primary fibroblasts labeled green or red prior to co-culture together. Resulting multinucleated cells were either green or red, but not both, i.e., yellow, demonstrating that primary multinucleated cells do not appear to form via cell-cell fusion.
3.6. Primary multinucleate cell morphology
Multinucleated cells from fibroblasts can be seen as early as 1 day post-culture, though the frequency of multinucleated cells and number of nuclei per cells increases at days 5–30 as seen in Figures 2 and 7. Nonetheless, even after extended culture periods (5–30 days), the frequency of multinucleated cells from primary fibroblasts was far less than those from secondary-derived fibroblasts during co-culture at short time periods (24 hours, Figure 2).
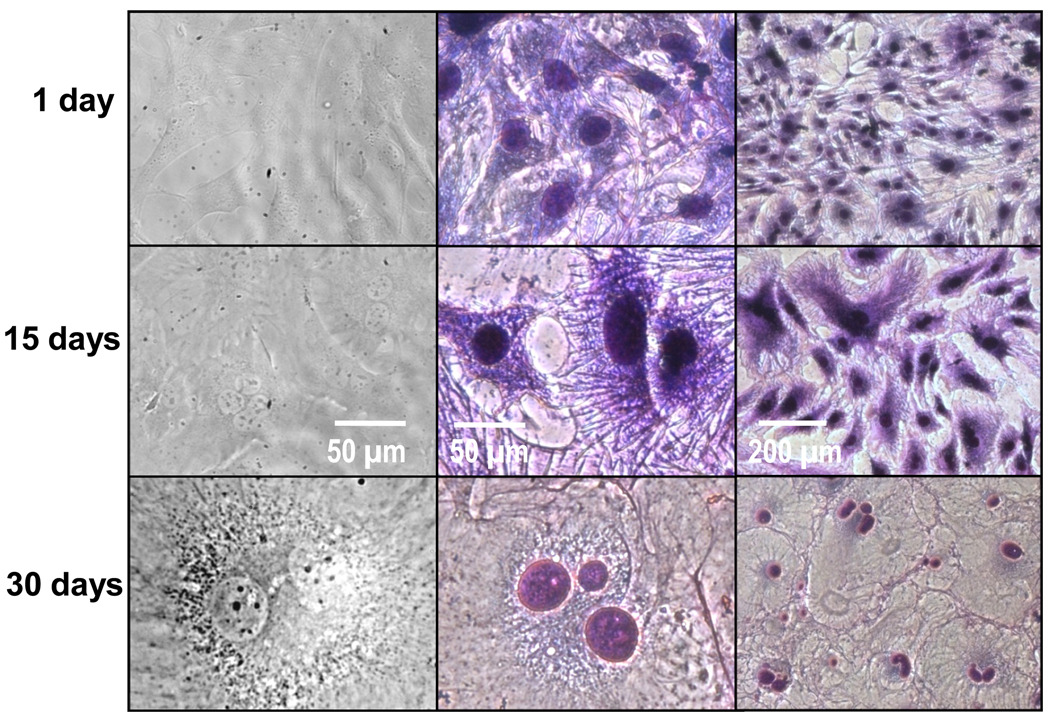
Figure 7.
Phase contrast (column 1) and H&E-stained (columns 2 &3) cell images show changes in primary fibroblast morphology over time. Columns 1 and 2 are at a 40× magnification and column 3 is at a 10× magnification. Day 1 cells possessed spindle and stellate morphologies. By Day 15, cells began to develop larger cell bodies and nuclei. By day 30, the cells possessed very large cell bodies, with some containing multiple and pleomorphic nuclei.
Over time, multinucleated cells from primary-derived fibroblasts change their morphology, developing larger, more-extended cell bodies and even developing what appears to be polymorphonuclei (Figure 5 E&F). Cultured adipose-derived stem cells (ASCs) (Supplementary Figure 2, description of ASC isolation and identification are found in Supplementary Information) and cardiomyoblasts (data not shown) were also observed to form multinucleate cells under identical culture conditions, occasionally possessing polymorphonuclei as well. Interestingly, primary-derived multinucleated cells possessed prominent stress fibers but lacked punctate podosomal actin (Figure 5B), consistent with fibroblasts [38], but not FBGCs or osteoclasts [17, 37].
3.7. Primary multinucleate cell senescence
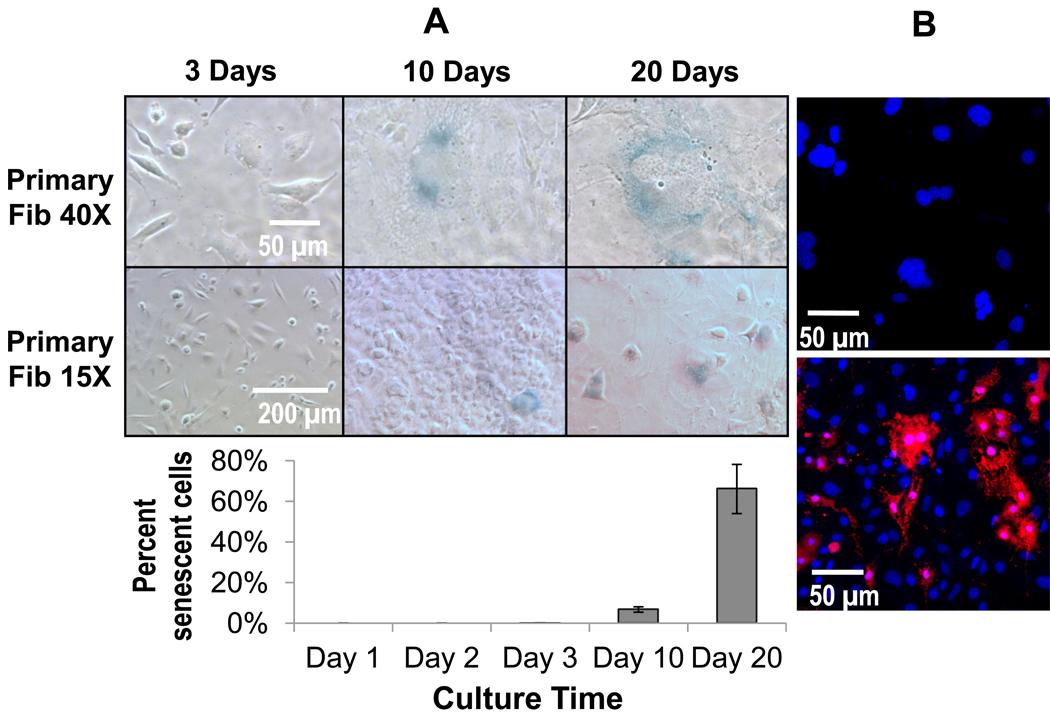
A senescence assay [31] was employed for primary fibroblasts on Days 1, 2, 3, 10, and 20. Fibroblasts stained positive for senescence first on Days 10 and 20 (Figure 8, Panel A). ASCs, also seen to create multinucleate cells, were also tested for senescence during the same time frame and began staining positive for senescence as early as Day 1, though not prevalently until Day 3 onward (Supplementary Figure 3). Secondary-derived multinucleate cells were also tested with this assay and found to exhibit no detectable positive senescence in either 3T3s or RAWs during either co-culture or mono-culture (data not shown). Significantly, all multinucleate cells regardless of the culture time period stained positive for senescence and negative for apoptosis (Figure 8, Panel B).
Figure 8.
Panel A) Senescence staining (blue) in primary fibroblasts after 3, 10, and 20 days expressed qualitatively as representative images and quantitatively in graph below. These data show that at longer time points near day 10, senescence becomes prevalent in primary fibroblasts. Data are represented as the mean ± SEM from 3 independent replicates. Importantly, all multinucleated fibroblasts regardless of time point, stained positive for senescence. Panel B) Confocal images showing DAPI (blue) and apoptosis staining (red) in primary fibroblasts (top image) left untreated and (bottom image) treated with bupivicaine as a positive control. Multinucleate fibroblastic cells even with highly polymorphic nuclei did not stain positive for apoptosis. Images shown after 3 days in culture.
4. Discussion
The majority of multinucleated cells are believed to originate from macrophages [10]. However fibroblasts, the most prevalent cell type in the body, are also capable of forming multinucleate cells both in vitro [18] and in vivo [1–8]. Though some studies claim they form via fusion [4, 7] and others mitosis without cytokinesis [18], this study found that both of these mechanisms can create multinucleated fibroblasts, depending on cell sourcing and culture conditions.
Secondary fibroblast-derived multinucleated cells formed when 3T3 fibroblasts were in direct physical contact with secondary-derived RAW macrophages after 24 hours (Figure 1, Row 5). This was not only seen during co-culture between RAW macrophages and 3T3 fibroblasts but also in identical co-cultures between RAW and L929 fibroblast cells (data not shown). Importantly, cell multinucleation in secondary cultures did not occur 1) during 3T3 fibroblast mono-culture in either complete or RAW macrophage-conditioned complete media, or in non-contact Transwell® macrophage co-cultures with soluble media-phase diffusive exchange between macrophages and fibroblasts. Consistent with a previous study, contact co-cultures of RAW 264.7 macrophages and NIH-3T3 fibroblasts formed multinucleate cells while those with 3T3 fibroblasts and primary bone-derived macrophages did not [22]. Additionally, cell multinucleation in secondary cultures did not occur when either cell type was allowed to adhere prior to adding the other cell type (data not shown). Lack of observed cell fusion in secondary cell cultures of both macrophage-conditioned media and Transwell® co-cultures indicates that cell fusion requires 3T3 fibroblasts in physical contact with RAW macrophages rather than just soluble signal exchange [22]. Necessity for physical cell-cell contact may suggest involvement of external cell membrane receptors such as inter-cellular adhesion molecule (ICAM) 1 on observed secondary-cell multinucleation. ICAM is deemed responsible for many interactions between macrophages and fibroblasts [40], and significantly, fusion between macrophages to form FBGCs [41]. In this study, cultured RAW macrophages were very motile, making contact with many cells over a 24-hour period. Surprisingly, multinucleated cells derived from 3T3 fibroblasts were also found to be highly motile, traveling hundreds of microns during the same 24-hour period (Figure 4 and video in Supplementary Data). Cell mobility in these secondary-derived cultures, enabling facile macrophage-fibroblast and fibroblast-fibroblast interactions, increases the likelihood of physical stimulation of 3T3 fibroblasts by RAW macrophages and subsequent fusion with other 3T3 fibroblasts (Figure 3 I&L).
Multinucleated cells from RAW macrophage contact co-cultures with 3T3 fibroblasts arise from the fibroblast, not macrophage, population. 3T3 fibroblast cultures do not contain contaminating macrophages: CD14 antibodies specifically bound RAW macrophages but not 3T3 fibroblasts and multinucleated cells. Assayed for TRAP, a prominent marker for multinucleate osteoclasts [35], cultured RAW macrophages, 3T3 fibroblasts, and multinucleate cells all stained negative (supplementary data), indicating that these cells were not osteoclastic.
In secondary 3T3 cell contact co-cultures with RAW macrophages, multinucleation is shown to result from fusion of two or more fibroblasts. Two separate populations of secondary-derived fibroblasts labeled with either a red or green cytoplasmic dye prior to plating with non-labeled secondary-derived macrophages, an experiment analogous to that used to determine macrophage fusion to FBGCs [11], produced multinucleated cells with both red and green nuclei and yellow (red plus green) cell bodies. Co-localization of both red and green dyes was seen with both fluorescent and laser scanning confocal microscopy (Figure 3 I&L, respectively). Red and green coloration occupied the same volume, shape, and focal plane (optical cross-section was 0.45 µm, less than a cell thickness), confirming cytoplasm fusion (Figure 3L). All secondary multinucleated cells displayed this color co-localization, with multinucleate cell density being approximately 15 giant cells per 15X frame (Figure 2). A 24-hour time-lapse video (supplementary data) shows a red 3T3 fibroblast traveling approximately 1 mm and apparently fusing with several green and red 3T3 fibroblasts to accumulate approximately 5 nuclei that, conjoined together, move within the same membrane containing both red and green fluorescence.
Primary ear-derived dermal fibroblasts formed multinucleated cells in physical contact with primary bone marrow-derived macrophages (Figure 6 A–C), secondary RAW macrophages (Figure 6 D–F) and primary bone marrow monocyte-like cells and monocytes (data not shown) in 24–72 hours. Primary and secondary macrophages formed extremely rare or no multinucleated cells during the same culture time under these conditions (Figure 2). Primary fibroblasts loaded with a red cytoplasmic dye and primary macrophages containing a green cytoplasmic dye produce red multinucleated cells (Figure 6 G–I) (i.e., from the fibroblast population, not from macrophages). Interestingly, primary fibroblasts also form multinucleated cells in mono-culture (complete absence of macrophages, shown by very dim CD14 labeling (Figure 6K) over the same time frame, increasing in their density over 30 culture days (Figure 2). Though primary fibroblasts are commonly cultured, multinucleated fibroblasts are not commonly reported, likely due to the fact that any rarely occurring multinucleated cells may be dismissed as contaminating cells or phenotypic anomalies. Figure 6 M&N shows mono-cultured primary fibroblasts loaded with either red or green cytoplasmic dye prior to seeding. In these representative images, multinucleated cells display only one color (either red or green) supporting a non-fusion mechanism to create multinucleated cells in primary fibroblast cultures. Similar multinucleated cells are also observed in primary ASCs (Supplementary Figure 2) and cardiomyoblasts (data not shown).
This observed multinucleation event that retains color fidelity in primary fibroblasts is consistent with nuclear division without cytokinesis. This phenomenon has been described previously [18–21], and the dye co-localization studies performed here further substantiate this phenomenon. This asserted nuclear division in the absence of cytokinesis is also supported by Supplementary Figure 5 E&F, showing the presence of globular or pleomorphic nuclei sharing a membrane with one or more nuclei. In vivo presence of multinucleated giant fibroblasts with pleomorphic nuclei has also been reported in a fibroma [8]. Polymorphonuclei exhibiting only 1 nucleus but with multiple lobes are also possible. Figure 5F shows a thread-like feature (circled) that may represent a strand of chromatin often seen connecting lobes of a polymorphonucleus [42]. This has also been seen previously in fibroblasts cultured in vitro, displaying tumor-like phenotypes [43]. After high passage numbers (>20), and extended culture beyond 5 days, secondary 3T3 fibroblasts in monoculture also occasionally express multiple nuclei and develop amorphic nuclei similar to those seen in the mono-cultured primary cells (Figure 5 C&D). That this type of multinucleation is more apparent in primary fibroblasts after 1 week in mono-culture and in secondary fibroblasts at high passage numbers in mono-culture, both in cells that appear to be non-dividing, is likely due to ageing. Previous work found multinucleated fibroblasts with pleomorphic nuclei in 17% of fibroblasts in periodontal ligaments of aged mice (20 months), while no such cells were seen in young mice (5 weeks) [7].
Aging has been shown to manifest as replicative senescence [44], and is suggested to result from damage to the mitotic machinery of dividing cells [45]. To prove if multinucleation events in primary fibroblasts are correlated with cellular age, as seen previously in fibroblasts [18], primary fibroblasts were cultured for 20 days in complete media, and stained for β-galactosidase, a common marker for replicative senescence [31]. At Day 10, (i.e., approximate time when multinucleation in primary cells was observed to increase) senescent cells also became more frequent and all multinucleated cells, regardless of the culture time point, stained positive for senescence (Figures 2 and 8). Both multinucleation and polyploidy (nuclear replication without nuclear division) have been seen in senescent fibroblasts [18, 19]. Multinucleation and polyploidy are also well-known in trophoblasts [46, 47] and cancerous tissue [20, 43]. Though senescence has been proposed as a mechanism to prevent cells from oncogenesis [48], the nuclear material in senescent multinucleate fibroblasts is considered highly unstable, and on occasion cells can escape senescence by a nuclear budding process known as neosis [20, 21, 49]. During neosis multinucleate senescent cells can shed karyoplasts to become highly mitotically active and tumorigenic Raju cells [20, 21, 49]. Interestingly, this nuclear budding process has also been reported in osteoclasts in order to create mononuclear cells from a multinucleate osteoclast [50].
The two types of multinucleated fibroblasts identified in this study in vitro in both secondary and primary fibroblasts correlate with those proposed in vivo due to fusion [4, 7] and nuclear division without cellular fission [43, 45]. Depending on conditions, some multinucleated fibroblasts found in vivo seen in aged tissue, fibrosis, and fibromas [1–8] may be senescent cells either undergoing nuclear division without cytokinesis or fusion-derived fibroblasts, although direct evidence for either mechanism is scant. A clinical cancer study described multinucleated fibroblast histology with large numbers of nuclei (7–20), less prominent actin staining, and a prevalence of 10–30% multinucleated cells per microscopic field [3], characteristics similar to fused secondary fibroblasts in this study. Multinucleate fibroblasts with polymorphic nuclei seen in vivo [7, 8, 43] are better compared to the primary multinucleate fibroblasts formed from nuclear division without cytokinesis as reported in this study. Cells resulting from each mechanism are multinucleate, yet as they derive from different pathways, they may possess distinct traits characteristic of the pathologies in which they arise. Understanding these distinctions should provide better insight into the etiology of pathologies such as fibrosis, cancer, aging, and the FBR, where multinucleate fibroblasts may play a significant role.
Secondary fibroblasts in macrophage co-culture did not form the same type of multinucleated cells as primary cells in co- or mono-cultures. Secondary cells formed multinucleated cells readily after 1 day, while primary cell multinucleation required several days and was never as frequent (Figure 2). Additionally, far more nuclei per cell were observed in multinucleate cells produced from secondary cells than from primary cells (Figure 2), but primary cells possessed more prominent stress fibers (Figure 5). Most interestingly, secondary cells fused to form multinucleated cells that stained negative for senescence (data not shown), while primary cells did not appear to fuse but became multinucleated instead by nuclear division without cytokinesis, correlated with replicative senescence. These differences most likely reside in secondary cells that are transformed, passaged many times, and display oncogenic phenotypes, such as rapid proliferation, lack of contact inhibition and immortalization. Distinct behaviors between primary and secondary fibroblasts in such cell-cell fusions should be considered both in model culture studies employing or clinical histopathological observations invoking this cell type.
5. Conclusions
Multinucleated cells are shown to form in both secondary- and primary-derived fibroblasts cultures in vitro under distinct conditions. Differences are noted in multinucleation mechanism between cultured fibroblasts from primary and secondary sources. Secondary cells produce multinucleation by fusion of multiple fibroblasts only in direct contact culture with macrophages. Primary cells do not multinucleate by fusion, but rather from senescent cells no longer undergoing cytokinesis, and in the absence of co-cultured macrophages. Clinical studies identify multinucleated fibroblasts with pleomorphic nuclei, similar to those derived from primary cells here, as well as the frequent appearance of claimed-fused fibroblasts similar to those from immortalized fibroblast cultures in this study, suggesting that both types of fibroblast-dependent multinucleation may be present in vivo in different pathologies. Understanding the impetus for the formation of each atypical multinucleated fibroblast type is essential in understanding multinucleate fibroblast involvement in pathologies such as the FBR, aging, and fibrotic diseases.
Supplementary Material
Acknowledgements
We acknowledge J.M. Anderson and T.R. Kyriakides for scientific critique and expert insight. This research was supported by National Institute of Health grant R01EB000894.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Powell CM, Cranor ML, Rosen PP. Multinucleated stromal giant cells in mammary fibroepithelial neoplasms. A study of 11 patients. Arch Pathol Lab Med. 1994;118:912–916. [PubMed] [Google Scholar]
- 2.Ryska A, Reynolds C, Keeney GL. Benign tumors of the breast with multinucleated stromal giant cells. Immunohistochemical analysis of six cases and review of the literature. Virchows Arch. 2001;439:768–775. doi: 10.1007/s004280100470. [DOI] [PubMed] [Google Scholar]
- 3.Tse GM, Law BK, Chan KF, Mas TK. Multinucleated stromal giant cells in mammary phyllodes tumours. Pathology. 2001;33:153–156. [PubMed] [Google Scholar]
- 4.El-Labban NG, Lee KW. Myofibroblasts in central giant cell granuloma of the jaws: an ultrastructural study. Histopathology. 1983;7:907–918. doi: 10.1111/j.1365-2559.1983.tb02305.x. [DOI] [PubMed] [Google Scholar]
- 5.Min KW, Gillies E. Multinucleated giant stromal tumor of the omentum: report of a case with immunohistochemical and ultrastructural investigation. Ultrastruct Pathol. 1996;20:89–99. doi: 10.3109/01913129609023243. [DOI] [PubMed] [Google Scholar]
- 6.Regezi JA, Courtney RM, Kerr DA. Fibrous lesions of the skin and mucous membranes which contain stellate and multinucleated cells. Oral Surg Oral Med Oral Pathol. 1975;39:605–614. doi: 10.1016/0030-4220(75)90202-9. [DOI] [PubMed] [Google Scholar]
- 7.Cho MI, Garant PR. Formation of multinucleated fibroblasts in the periodontal ligaments of old mice. Anat Rec. 1984;208:185–196. doi: 10.1002/ar.1092080205. [DOI] [PubMed] [Google Scholar]
- 8.Hassanein A, Telang G, Benedetto E, Spielvogel R. Subungual myxoid pleomorphic fibroma. Am J Dermatopathol. 1998;20:502–505. doi: 10.1097/00000372-199810000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Anderson JM. Chapter 4 Mechanisms of inflammation and infection with implanted devices. Cardiovasc Pathol. 1993;2:33S–41S. [Google Scholar]
- 10.Anderson JM. Multinucleated giant cells. Curr Opin Hematol. 2000;7:40–47. doi: 10.1097/00062752-200001000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Helming L, Gordon S. Macrophage fusion induced by IL-4 alternative activation is a multistage process involving multiple target molecules. Eur J Immunol. 2007;37:33–42. doi: 10.1002/eji.200636788. [DOI] [PubMed] [Google Scholar]
- 12.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wisniewski N, Reichert M. Methods for reducing biosensor membrane biofouling. Colloids Surf B Biointerfaces. 2000;18:197–219. doi: 10.1016/s0927-7765(99)00148-4. [DOI] [PubMed] [Google Scholar]
- 14.Moilanen E, Moilanen T, Knowles R, Charles I, Kadoya Y, al-Saffar N, et al. Nitric oxide synthase is expressed in human macrophages during foreign body inflammation. Am J Pathol. 1997;150:881–887. [PMC free article] [PubMed] [Google Scholar]
- 15.Brand KG, Buoen LC, Johnson KH, Brand I. Etiological factors, stages, and the role of the foreign body in foreign body tumorigenesis: a review. Cancer Res. 1975;35:279–286. [PubMed] [Google Scholar]
- 16.Tazawa H, Tatemichi M, Sawa T, Gilibert I, Ma N, Hiraku Y, et al. Oxidative and nitrative stress caused by subcutaneous implantation of a foreign body accelerates sarcoma development in Trp53+/− mice. Carcinogenesis. 2007;28:191–198. doi: 10.1093/carcin/bgl128. [DOI] [PubMed] [Google Scholar]
- 17.DeFife KM, Jenney CR, Colton E, Anderson JM. Cytoskeletal and adhesive structural polarizations accompany IL-13-induced human macrophage fusion. J Histochem Cytochem. 1999;47:65–74. doi: 10.1177/002215549904700107. [DOI] [PubMed] [Google Scholar]
- 18.Walen KH. Human diploid fibroblast cells in senescence; cycling through polyploidy to mitotic cells. In Vitro Cell Dev Biol Anim. 2006;42:216–224. doi: 10.1290/0603019.1. [DOI] [PubMed] [Google Scholar]
- 19.Ohshima S. Abnormal mitosis in hypertetraploid cells causes aberrant nuclear morphology in association with H2O2-induced premature senescence. Cytometry A. 2008;73:808–815. doi: 10.1002/cyto.a.20604. [DOI] [PubMed] [Google Scholar]
- 20.Sundaram M, Guernsey DL, Rajaraman MM, Rajaraman R. Neosis: a novel type of cell division in cancer. Cancer Biol Ther. 2004;3:207–218. doi: 10.4161/cbt.3.2.663. [DOI] [PubMed] [Google Scholar]
- 21.Walen KH. Budded karyoplasts from multinucleated fibroblast cells contain centrosomes and change their morphology to mitotic cells. Cell Biol Int. 2005;29:1057–1065. doi: 10.1016/j.cellbi.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 22.Holt DJ, Chamberlain LM, Grainger DW. Cell-cell signaling in co-cultures of macrophages and fibroblasts. Biomaterials. 2010;31:9382–9394. doi: 10.1016/j.biomaterials.2010.07.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Godek ML, Sampson JA, Duchsherer NL, McElwee Q, Grainger DW. Rho GTPase protein expression and activation in murine monocytes/macrophages is not modulated by model biomaterial surfaces in serum-containing in vitro cultures. J Biomater Sci Polym Ed. 2006;17:1141–1158. doi: 10.1163/156856206778530731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rhoades ER, Orme IM. Similar responses by macrophages from young and old mice infected with Mycobacterium tuberculosis. Mech Ageing Dev. 1998;106:145–153. doi: 10.1016/s0047-6374(98)00113-4. [DOI] [PubMed] [Google Scholar]
- 25.Shao C, Deng L, Henegariu O, Liang L, Raikwar N, Sahota A, et al. Mitotic recombination produces the majority of recessive fibroblast variants in heterozygous mice. Proc Natl Acad Sci U S A. 1999;96:9230–9235. doi: 10.1073/pnas.96.16.9230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szulc J, Wiznerowicz M, Sauvain MO, Trono D, Aebischer P. A versatile tool for conditional gene expression and knockdown. Nat Methods. 2006;3:109–116. doi: 10.1038/nmeth846. [DOI] [PubMed] [Google Scholar]
- 27.Marino G, Salvador-Montoliu N, Fueyo A, Knecht E, Mizushima N, Lopez-Otin C. Tissue-specific autophagy alterations and increased tumorigenesis in mice deficient in Atg4C/autophagin-3. J Biol Chem. 2007;282:18573–18583. doi: 10.1074/jbc.M701194200. [DOI] [PubMed] [Google Scholar]
- 28.Hodgkin PD, Lee JH, Lyons AB. B cell differentiation and isotype switching is related to division cycle number. J Exp Med. 1996;184:277–281. doi: 10.1084/jem.184.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 30.Chang HY, Chi JT, Dudoit S, Bondre C, van de Rijn M, Botstein D, et al. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc Natl Acad Sci U S A. 2002;99:12877–12882. doi: 10.1073/pnas.162488599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Severino J, Allen RG, Balin S, Balin A, Cristofalo VJ. Is beta-galactosidase staining a marker of senescence in vitro and in vivo? Exp Cell Res. 2000;257:162–171. doi: 10.1006/excr.2000.4875. [DOI] [PubMed] [Google Scholar]
- 32.Russell WC, Newman C, Williamson DH. A simple cytochemical technique for demonstration of DNA in cells infected with mycoplasmas and viruses. Nature. 1975;253:461–462. doi: 10.1038/253461a0. [DOI] [PubMed] [Google Scholar]
- 33.Jung H, Wang SY, Yang IW, Hsueh DW, Yang WJ, Wang TH, et al. Detection and treatment of mycoplasma contamination in cultured cells. Chang Gung Med J. 2003;26:250–258. [PubMed] [Google Scholar]
- 34.Uphoff CC, Brauer S, Grunicke D, Gignac SM, MacLeod RA, Quentmeier H, et al. Sensitivity and specificity of five different mycoplasma detection assays. Leukemia. 1992;6:335–341. [PubMed] [Google Scholar]
- 35.Wang Y, Grainger DW. siRNA knock-down of RANK signaling to control osteoclast-mediated bone resorption. Pharm Res. 2010;27:1273–1284. doi: 10.1007/s11095-010-0099-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McNally AK, Anderson JM. Beta1 and beta2 integrins mediate adhesion during macrophage fusion and multinucleated foreign body giant cell formation. Am J Pathol. 2002;160:621–630. doi: 10.1016/s0002-9440(10)64882-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akisaka T, Yoshida H, Inoue S, Shimizu K. Organization of cytoskeletal F-actin, G-actin, and gelsolin in the adhesion structures in cultured osteoclast. J Bone Miner Res. 2001;16:1248–1255. doi: 10.1359/jbmr.2001.16.7.1248. [DOI] [PubMed] [Google Scholar]
- 38.Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 39.Chamberlain LM, Godek ML, Gonzalez-Juarrero M, Grainger DW. Phenotypic non-equivalence of murine (monocyte-) macrophage cells in biomaterial and inflammatory models. J Biomed Mater Res A. 2009;88:858–871. doi: 10.1002/jbm.a.31930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steinhauser ML, Kunkel SL, Hogaboam CM, Evanoff H, Strieter RM, Lukacs NW. Macrophage/fibroblast coculture induces macrophage inflammatory protein-1alpha production mediated by intercellular adhesion molecule-1 and oxygen radicals. J Leukoc Biol. 1998;64:636–641. doi: 10.1002/jlb.64.5.636. [DOI] [PubMed] [Google Scholar]
- 41.Fais S, Burgio VL, Silvestri M, Capobianchi MR, Pacchiarotti A, Pallone F. Multinucleated giant cells generation induced by interferon-gamma. Changes in the expression and distribution of the intercellular adhesion molecule-1 during macrophages fusion and multinucleated giant cell formation. Lab Invest. 1994;71:737–744. [PubMed] [Google Scholar]
- 42.Briggs DK. The individuality of nuclear chromatin with particular reference to polymorphonuclear neutrophil leukocytes. Blood. 1958;13:986–1000. [PubMed] [Google Scholar]
- 43.Gisselsson D, Bjork J, Hoglund M, Mertens F, Dal Cin P, Akerman M, et al. Abnormal nuclear shape in solid tumors reflects mitotic instability. Am J Pathol. 2001;158:199–206. doi: 10.1016/S0002-9440(10)63958-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson FB, Sinclair DA, Guarente L. Molecular biology of aging. Cell. 1999;96:291–302. doi: 10.1016/s0092-8674(00)80567-x. [DOI] [PubMed] [Google Scholar]
- 45.Ly DH, Lockhart DJ, Lerner RA, Schultz PG. Mitotic misregulation and human aging. Science. 2000;287:2486–2492. doi: 10.1126/science.287.5462.2486. [DOI] [PubMed] [Google Scholar]
- 46.Soloveva V, Linzer DI. Differentiation of placental trophoblast giant cells requires downregulation of p53 and Rb. Placenta. 2004;25:29–36. doi: 10.1016/S0143-4004(03)00215-7. [DOI] [PubMed] [Google Scholar]
- 47.Zybina EV, Zybina TG, Bogdanova MS, Stein GI. Whole-genome chromosome distribution during nuclear fragmentation of giant trophoblast cells of Microtus rossiaemeridionalis studied with the use of gonosomal chromatin arrangement. Cell Biol Int. 2005;29:1066–1070. doi: 10.1016/j.cellbi.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 48.O'Brien W, Stenman G, Sager R. Suppression of tumor growth by senescence in virally transformed human fibroblasts. Proc Natl Acad Sci U S A. 1986;83:8659–8663. doi: 10.1073/pnas.83.22.8659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mosieniak G, Sikora E. Poliploidy: the link between senescence and cancer. Curr Pharm Des. 2010;16:734–740. doi: 10.2174/138161210790883714. [DOI] [PubMed] [Google Scholar]
- 50.Solari F, Domenget C, Gire V, Woods C, Lazarides E, Rousset B, et al. Multinucleated cells can continuously generate mononucleated cells in the absence of mitosis: a study of cells of the avian osteoclast lineage. J Cell Sci. 1995;108(Pt 10):3233–3241. doi: 10.1242/jcs.108.10.3233. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.