Abstract
Objective
Evaluate glucose control and clinical outcomes in diabetic burn ICU patients.
Methods
We reviewed 462 civilian patients admitted to our burn ICU over four years. Exclusion criteria were age<18, admission because of skin infection, incomplete records, and military patients. Subjects were labeled as diabetic if they had a diagnosis of diabetes documented in their medical records. Otherwise they were labeled as non-diabetic. Diabetics (n=57) were compared to non-diabetics (n=405). Admission glucose levels were obtained from chemistries. Point-of-care devices provided the remaining glucose values. While in the burn ICU hyperglycemia for all patients was treated using intensive insulin therapy with a target blood glucose goal of 80–110mg/dL. Mann-Whitney U, Chi-square, and multivariate regressions were used for statistical analysis (p≤0.05).
Results
Diabetics were older (60±15 vs 44±17years) with higher admission glucose (196±81 vs 133±52mg/dL), mean glucose (147±37 vs 122±24mg/dL), glucose variability (30±11 vs 22±11%), and fewer ICU-free days (18±12 vs 20±11). After multivariate regression analyses age, ISS, TBSA, admission glucose, and mean glucose significantly affected the number of ventilator-free days, ICU-free days, and hospital-free days. Glucose variability was associated with hospital-free days only. Age, ISS, and TBSA significantly influenced mortality whereas a pre-existing diagnosis of diabetes was not associated with any clinical outcomes.
Conclusions
Admission blood glucose is higher and blood glucose is more difficult to control in diabetic burn ICU patients. A pre-existing diagnosis of diabetes does not influence clinical outcomes in critically ill burn patients.
Keywords: hyperglycemia, diabetes, burns, critically ill, ICU, outcomes, mortality
Introduction
Since 2001, multiple investigators have demonstrated that hyperglycemia is associated with poorer clinical outcomes and increased mortality in a variety of critically ill medical and surgical patient populations1–12. More recently, poorer clinical outcomes and increased mortality have not only been associated with hyperglycemia but also with high blood glucose variability and loss of blood glucose profile complexity13–16. Intensive insulin therapy has been adopted as the best way to minimize hyperglycemia and its associated clinical outcomes in many medical and surgical critical care settings. As of 2008 approximately 73% of American Burn Association (ABA) verified burn centers in the United States had implemented intensive insulin therapy protocols17.
Critically ill burn patients, compared to other trauma patients, exhibit extreme physiologic and metabolic derangements18–19. The presence of hyperglycemia after burns has been associated with a state of great physiologic and metabolic stress since the 1930s and is referred to as stress-induced hyperglycemia, pseudodiabetes of burns, or burns-stress diabetes18–21. However, only patient age and total body surface area (TBSA) burned have been directly associated with mortality in most critically ill burn patient populations22–24.
Like hyperglycemia, the role of diabetes in critical illness has been documented. Yet, the association between a pre-existing diagnosis of diabetes and clinical outcomes in critically ill adults is still not clear. Several authors have asserted that critically ill medical and surgical patients with a pre-existing diagnosis of diabetes are more likely to have infectious complications, worse clinical outcomes, and increased mortality25–31. Others have suggested that only hyperglycemia, not a pre-existing diagnosis of diabetes, is associated with infectious complications, worse clinical outcomes, and increased mortality in critically ill patients31–40. While hyperglycemia has been associated with increased mortality after burn injury10,12,19–21 a pre-existing diagnosis of diabetes has only been associated with infectious complications and poorer clinical outcomes in critically ill burn patients39–40. Recognizing the relative lack of data on diabetic burn intensive care unit (ICU) patients, we investigated the association between a pre-existing diagnosis of diabetes, blood glucose control, clinical outcomes, and mortality in critically ill burn patients.
Methods
After approval was obtained from the Brooke Army Medical Center Institutional Review Board, we conducted a retrospective review of all civilian patients admitted to the United States Army Institute of Surgical Research (USAISR) burn ICU over a four year period. The medical records of the USAISR burn ICU were queried for all patients admitted to the burn ICU from January 1, 2006 to December 31, 2009. Patients were excluded from the study if they were younger than 18 years old, were military patients, had bacterial skin infections as the reason for admission to the burn ICU (e.g. toxic epidermal necrolysis syndrome, Stevens-Johnson syndrome, etc), or had incomplete clinical data (missing lab values or incomplete medical histories; figure 1). Hyperglycemia for all patients in the burn ICU was treated using intensive insulin therapy with a target blood glucose level of 80–110mg/dL.
Figure 1.
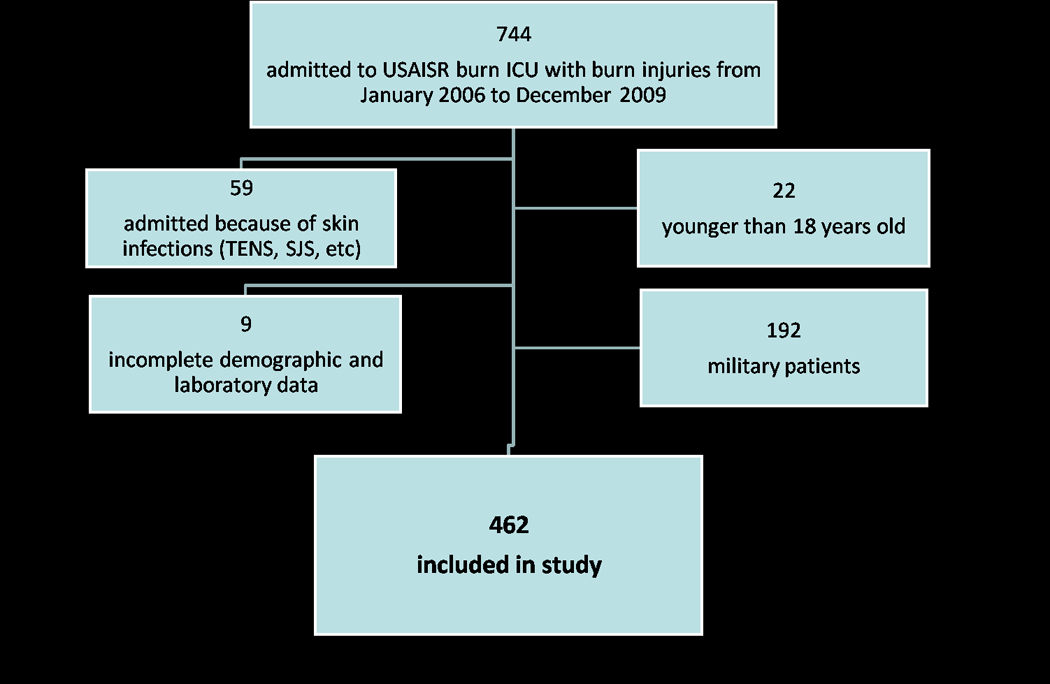
Consort diagram for subject selection.
All demographic, blood glucose, and clinical outcomes data were obtained from each patient’s electronic medical record via a database query performed by the USAISR information technology department. Analyzed demographic data consisted of age, gender, diabetes status, injury severity score (ISS), and total body surface area (TBSA) burned. Subjects were labeled as diabetic if they had a diagnosis of diabetes documented in their medical records. Otherwise they were labeled as non-diabetic. Admission blood glucose values were collected from admission chemistry and electrolyte laboratory panels. All other glucose values were obtained from bedside point-of-care glucometers and arterial blood gas measurement devices. Our institution has previously demonstrated that anemia evidenced by low hematocrit levels is associated with falsely elevated point-of-care glucometer values potentially masking hypoglycemia41. However, while most of the point-of-care glucose values analyzed in our study were corrected for the hematocrit effect42, some were not. As a result, both corrected and uncorrected glucose values were included in the study since both types of values were used by the burn ICU nurses for patient care. After all blood glucose values had been obtained, mean glucose levels for each subject were calculated for his/her entire hospital stay. Blood glucose variability for each subject was derived by calculating a percent coefficient of variation (standard deviation divided by the mean then multiplied by 100). Mean daily blood glucose level for each subject was calculated using daily blood glucose levels averaged over the entire hospital stay. Mean AM blood glucose level for each subject was calculated using blood glucose levels that were drawn from 4AM to 8AM and averaged over the entire hospital stay. The frequency of hypoglycemia was assessed by dividing the number of hypoglycemic measurements, defined as <80mg/dL, by the total number of measurements for each subject. The efficacy of intensive insulin therapy was calculated by dividing the number of out-of-range blood glucose measurements (<80mg/dL or >110mg/dL) by the total number of blood glucose measurements for each subject. Clinical outcomes data was collected from each patient’s electronic medical record. Primary outcome measures that were investigated were ventilator days, ventilator-free days (30 day maximum), ICU days, ICU-free days (30 day maximum), hospital days, hospital-free days (60 day maximum), and mortality. Ventilator-free days, ICU-free days, and hospital-free days were calculated by subtracting ventilator days, ICU days, and hospital days from 30, 30, and 60, respectively. Once all the demographic, blood glucose, and clinical outcomes data had been compiled, we created a study database using Excel and Access (Microsoft, Redmond, WA) which facilitated data integrity verification and data analysis.
Statistical analysis was performed by using the SPSS statistical analysis software package (International Business Machines, Chicago, IL). Diabetics were compared to non-diabetics. Differences between groups with regards to gender, diabetic status, number of subjects with TBSA>20%, number of subjects with full thickness (3rd degree burns), and mortality were calculated using Chi-square analysis (p≤0.05). Due to the non-parametric distribution of the data Mann-Whitney U tests were employed to analyze differences between groups with regards to age, admission blood glucose level, mean blood glucose level, blood glucose variance, , mean daily blood glucose level, mean 6AM blood glucose level, percent of measurements that were hypoglycemic, percent of measurements that were out of range (80–110mg/dL), ISS, TBSA, ventilator days, ventilator-free days, ICU days, ICU-free days, hospital days, and hospital-free days (p≤0.05). Clinical variables that were significantly different between survivors and non-survivors were input into regression models to determine each variable’s contribution to clinical outcomes. Multivariate linear regression models were used to analyze the effects of those variables on ventilator-free days, ICU-free days, and hospital-free days (p≤0.05). Similarly, a multivariate logistic regression model was used to analyze the influence of those same variables on mortality (p≤0.05).
Results
The total number of patients admitted to the burn ICU with burn injuries from January 1, 2006 to December 31, 2009 was 744. A total of 462 subjects satisfied the inclusion criteria and were included in the study. Of these 462 subjects, 57 (12%) had a pre-existing diagnosis of diabetes documented in their medical records upon admission to the burn ICU and 405 subjects (88%) did not have any documented medical history of diabetes. Out of the 109,930 blood glucose measurements that were analyzed for all subjects, 67,193 were corrected for the hematocrit effect whereas 42,737 were not. Sixty-five (14%) of the subjects died and 397 (86%) of the subjects lived.
Subjects with a pre-existing diagnosis of diabetes were older and had higher admission blood glucose levels, mean blood glucose levels, blood glucose variability, mean daily blood glucose levels, mean AM blood glucose levels, number of hypoglycemic measurements, and out-of-range blood glucose measurements compared to non-diabetics (table 1). ISS, TBSA, number of subjects with TBSA>20%, and number of subjects with full thickness (3rd degree) burns was not significantly different between subjects with a pre-existing diagnosis of diabetes and non-diabetics (table 1). Ventilator-free days and hospital-free days were not different but ICU-free days were fewer in subjects with a pre-existing diagnosis of diabetes as computed by univariate statistical analysis (table 1). More subjects in the diabetic group died (21%) compared to those in the non-diabetic group (13%). This difference was not statistically significant (table 1).
Table 1.
Demographic data for diabetics and non-diabetics. Median (interquartile range).
| Diabetic Median (IQR) |
Non-diabetic Median (IQR) |
p-value | |
|---|---|---|---|
| n | 57 | 405 | N/A |
| Men (%)‡ | 72 | 83 | 0.07 |
| Age (years)* | 58 (50–71) | 43 (31–54) | <0.05 |
| ISS* | 9 (1–16) | 9 (4–18) | 0.16 |
| TBSA (%)* | 12 (6–27) | 15 (6–26) | 0.53 |
| TBSA>20% (%)‡ | 32 | 34 | 0.77 |
| Full thickness/3rd degree burns (%)‡ | 35 | 46 | 0.12 |
| Admission blood glucose (mg/dL)* | 179 (127–251) | 119 (103–148) | <0.05 |
| Mean blood glucose (mg/dL)* | 143 (117–163) | 119 (108–127) | <0.05 |
| Blood glucose variability (%)* | 28 (22–37) | 22 (14–27) | <0.05 |
| Mean daily blood glucose (mg/dL)* | 143 (118–164) | 119 (109–128) | <0.05 |
| Mean AM blood glucose (mg/dL)* | 124 (112–129) | 112 (103–122) | <0.05 |
| Hypoglycemic measurements (%)* | 5 (2–8) | 1 (0–6) | <0.05 |
| Measurements out-of-range (%)* | 76 (59–90) | 62 (47–75) | <0.05 |
| Ventilator days* | 1 (0–8) | 1 (0–4) | 0.36 |
| Ventilator-free days* | 29 (6–30) | 29 (26–30) | 0.25 |
| ICU days* | 4 (2–10) | 3 (2–9) | 0.16 |
| ICU-free days* | 23 (0–28) | 27 (14–28) | <0.05 |
| Hospital days* | 12 (4–29) | 9 (3–25) | 0.70 |
| Hospital-free days* | 36 (0–56) | 48 (17–56) | 0.13 |
| Mortality (%)‡ | 21 | 13 | 0.11 |
Mann-Whitney U Test, p≤0.05
Chi-square test, p≤0.05
To assess the effects of confounding factors on clinical outcomes, subjects who survived were compared to subjects who died. Non-survivors were older and had higher admission blood glucose levels, mean blood glucose levels, blood glucose variability, ISS, and TBSA compared to survivors (table 2). The non-survivor group had a greater number of subjects with a pre-existing diagnosis of diabetes (18%) compared to the non-survivor group (11%). This difference was not statistically significant, however (table 2). Age, gender, ISS, TBSA, admission blood glucose levels, mean blood glucose levels, blood glucose variability, and diabetes diagnosis status were input into multivariate linear and logistic regression models. The number of ventilator-free days, ICU-free days, and hospital-free days was affected by age, ISS, TBSA, admission blood glucose levels, and mean blood glucose levels (tables 3–5). Blood glucose variability was associated with hospital-free days only (table 5). Age, ISS, and TBSA significantly contributed to mortality (p<0.05 for all variables; table 6). A pre-existing diagnosis of diabetes did not influence ventilator-free days (p=0.98; table 3), ICU-free days (p=0.73; table 4), hospital-free days (p=0.81; table 5), or mortality (p=0.77; table 6).
Table 2.
Demographic data for non-survivors and survivors. Median (interquartile range).
| Non-survivors Median (IQR) |
Survivors Median (IQR) |
p-value | |
|---|---|---|---|
| n | 65 | 397 | N/A |
| Men (%)‡ | 66 | 84 | <0.05 |
| Age (years)* | 57 (47–78) | 43 (31–54) | <0.05 |
| ISS* | 25 (16–34) | 9 (2–16) | <0.05 |
| TBSA (%)* | 42 (20–62) | 12 (5–22) | <0.05 |
| TBSA>20% (%)‡ | 74 | 27 | <0.05 |
| Full thickness/3rd degree burns (%)‡ | 75 | 40 | <0.05 |
| Admission blood glucose (mg/dL)* | 164 (138–217) | 119 (102–146) | <0.05 |
| Mean blood glucose (mg/dL)* | 121 (113–146) | 119 (108–129) | <0.05 |
| Blood glucose variability (%)* | 26 (23–32) | 21 (14–27) | <0.05 |
| Mean daily blood glucose (mg/dL)* | 122 (114–147) | 120 (108–130) | <0.05 |
| Mean AM blood glucose (mg/dL)* | 114 (108–128) | 114 (102–125) | 0.17 |
| Hypoglycemic measurements (%)* | 5 (2–9) | 1 (0–6) | <0.05 |
| Measurements out-of-range (%)* | 65 (58–79) | 63 (46–78) | <0.05 |
| Ventilator days* | 8 (2–29) | 0 (0–2) | <0.05 |
| Ventilator-free days* | 0 (0–0) | 30 (28–30) | <0.05 |
| ICU days* | 10 (2–31) | 3 (2–7) | <0.05 |
| ICU-free days* | 0 (0–0) | 27 (23–28) | <0.05 |
| Hospital days* | 11 (2–33) | 10 (4–25) | 0.75 |
| Hospital-free days* | 0 (0–0) | 50 (35–56) | <0.05 |
| Diabetic (%)‡ | 18 | 11 | 0.11 |
Mann-Whitney U Test, p≤0.05
Chi-square test, p≤0.05
Table 3.
Multivariate linear regression model for ventilator-free days (p≤0.05).
| Ventilator-free days* | β (SE) | CI | p-value |
|---|---|---|---|
| Age (years) | −0.17 (0.02) | −0.22–−0.13 | <0.05 |
| Gender | 0.16 (0.98) | −1.76–2.10 | 0.87 |
| ISS | −0.25 (0.06) | −0.37–−0.14 | <0.05 |
| TBSA (%) | −0.18 (0.04) | −0.26–−0.10 | <0.05 |
| TBSA>20% (%) | 0.72 (1.31) | −1.86–3.29 | 0.58 |
| Full thickness/3rd degree burns (%) | −0.90 (0.85) | −2.56–0.77 | 0.29 |
| Admission glucose (mg/dL) | −0.05 (0.01) | −0.07–−0.04 | <0.05 |
| Mean glucose (mg/dL) | 0.09 (0.03) | 0.04–0.14 | <0.05 |
| Glucose variability (%) | 0.06 (0.05) | −0.02–0.15 | 0.15 |
| Hypoglycemic measurements (%) | −0.03 (0.06) | −0.14–0.09 | 0.65 |
| Measurements out-of-range (%) | −0.00 (0.02) | −0.05–0.04 | 0.90 |
| Diabetes diagnosis (yes or no) | −0.04 (1.27) | −2.43–2.50 | 0.98 |
β= beta coefficient; SE=standard error; CI=95% confidence interval for β coefficient
Table 5.
Multivariate linear regression model for hospital-free days (p≤0.05).
| Hospital-free days* | β (SE) | CI | p-value |
|---|---|---|---|
| Age (years) | −0.28 (0.04) | −0.36–−0.19 | <0.05 |
| Gender | 2.96 (1.77) | −0.53–6.44 | 0.10 |
| ISS | −0.34 (0.11) | −0.55–−0.13 | <0.05 |
| TBSA (%) | −0.35 (0.07) | −0.50–−0.21 | <0.05 |
| TBSA>20% (%) | −2.75 (2.38) | −7.42–1.93 | 0.25 |
| Full thickness/3rd degree burns (%) | −9.97 (1.54) | −12.98–−6.95 | <0.05 |
| Admission glucose (mg/dL) | −0.07 (0.01) | −0.10–−0.04 | <0.05 |
| Mean glucose (mg/dL) | 0.24 (0.05) | 0.15–0.33 | <0.05 |
| Glucose variability (%) | −0.24 (0.08) | −0.40–−0.08 | <0.05 |
| Hypoglycemic measurements (%) | 0.04 (0.10) | −0.17–0.24 | 0.72 |
| Measurements out-of-range (%) | −0.05 (0.04) | −0.12–0.03 | 0.24 |
| Diabetes diagnosis (yes or no) | −1.13 (2.31) | −5.67–3.42 | 0.63 |
β= beta coefficient; SE=standard error; CI=95% confidence interval for β coefficient
Table 6.
Multivariate logistic regression model for mortality (p≤0.05).
| Mortality* | β (SE) | CI | p-value |
|---|---|---|---|
| Age (years) | −0.09 (0.01) | 0.90–0.94 | <0.05 |
| Gender | −0.86 (0.44) | 0.18–1.00 | 0.05 |
| ISS | −0.11 (0.03) | 0.85–0.95 | <0.05 |
| TBSA (%) | −0.06 (0.02) | 0.91–0.97 | <0.05 |
| TBSA>20% (%) | −1.29 (0.70) | 0.07–1.09 | 0.07 |
| Full thickness/3rd degree burns (%) | 0.53 (0.47) | 0.68–4.24 | 0.25 |
| Admission glucose (mg/dL) | −0.01 (0.00) | 0.99–1.00 | 0.09 |
| Mean glucose (mg/dL) | 0.01 (0.01) | 0.98–1.04 | 0.62 |
| Glucose variability (%) | 0.00 (0.03) | 0.95–1.06 | 0.93 |
| Hypoglycemic measurements (%) | −0.05 (0.03) | 0.89–1.02 | 0.19 |
| Measurements out-of-range (%) | 0.01 (0.02) | 0.97–1.05 | 0.69 |
| Diabetes diagnosis (yes or no) | −0.11 (0.58) | 0.29–2.82 | 0.86 |
β= beta coefficient; SE=standard error; CI=95% confidence interval for β coefficient
Table 4.
Multivariate linear regression model for ICU-free days (p≤0.05).
| ICU-free days* | β (SE) | CI | p-value |
|---|---|---|---|
| Age (years) | −0.18 (0.02) | −0.23–−0.14 | <0.05 |
| Gender | 0.01 (0.96) | −1.87–1.90 | 0.99 |
| ISS | −0.21 (0.06) | −0.32–−0.10 | <0.05 |
| TBSA (%) | −0.15 (0.04) | −0.23–−0.07 | <0.05 |
| TBSA>20% (%) | −1.72 (1.29) | −4.25–0.80 | 0.18 |
| Full thickness/3rd degree burns (%) | −3.41 (0.83) | −5.04–−1.77 | <0.05 |
| Admission glucose (mg/dL) | −0.04 (0.01) | −0.05–−0.02 | <0.05 |
| Mean glucose (mg/dL) | 0.11 (0.03) | 0.06–0.15 | <0.05 |
| Glucose variability (%) | −0.03 (0.04) | −0.12–0.06 | 0.49 |
| Hypoglycemic measurements (%) | 0.01 (0.06) | −0.10–0.11 | 0.92 |
| Measurements out-of-range (%) | −0.03 (0.02) | −0.07–0.02 | 0.24 |
| Diabetes diagnosis (yes or no) | −0.14 (1.25) | −2.59–2.32 | 0.91 |
β= beta coefficient; SE=standard error; CI=95% confidence interval for β coefficient
Discussion
Hyperglycemia at the time of admission to the ICU, poor ICU blood glucose control, and increased blood glucose variability are associated with clinical outcomes and increased mortality in a variety of critically ill medical and surgical patients1–12,19–21,26–27,29–31,33,35–38. The findings of admission hyperglycemia and ICU hyperglycemia in our study of burn patients are consistent with the stress-induced hyperglycemia and pseudodiabetes of burns that are widely known to occur in all critically ill burn patients18–21. After admission to the burn ICU, patients received intensive insulin therapy with a target blood glucose level of 80–110mg/dL to treat their hyperglycemia. When comparing the diabetics to the non-diabetics in our study population, we found that the burn patients with a pre-existing diagnosis of diabetes were older with higher admission blood glucose levels, higher mean blood glucose levels, and increased blood glucose variability when compared to the critically ill burn patients without a pre-existing diagnosis of diabetes. Our findings are consistent with previous studies that examine hyperglycemia in patients with burn injuries10–21,39–40 and indicate that blood glucose control in our critically ill burn patients is difficult from the moment that they are injured and admitted to the burn ICU. However, our study is limited because we did not have a reliable method to confirm the history of diabetes in our patients. Regardless, using patients’ past medical history to identify diabetic and non-diabetic patients should at least capture those patients who would be most severely affected by diabetes or for whom this information would be available according to the current standard of care. The finding of no difference even in this identified population with presumably more severe disease suggests that a pre-existing diagnosis of diabetes has minimal effects on outcomes in the severely burned. This may be due to the finding that glucose control is almost universally altered after severe injury even in those who were previously normal, thus, all become “diabetic”. The risk of type II error also exists; however, the population is of enough size that a potential factor with a large contribution should have been detected.
Although the effect of hyperglycemia on clinical outcomes and mortality in burn patients is apparent, the effect of diabetes on the same patient population is still unclear and controversial10–21, 39–40. As mentioned above, hyperglycemia in the ICU is associated with poor clinical outcomes and increased mortality1–12, 19–21, 26–27, 29–31, 33, 35–38. However, it is unclear why patients with a pre-existing diagnosis of diabetes who have consistently elevated blood glucose levels do not have worse clinical outcomes compared to non-diabetics in our study. This phenomenon has previously been demonstrated by Egi et al and the NICE-SUGAR investigators who showed that hyperglycemia in critically ill diabetic patients is likely biologically different than in non-diabetics and therefore does not significantly influence clinical outcomes and mortality as expected36, 43. Clinical outcomes measured by ventilator-free days, hospital-free days, and mortality were not different between the diabetic and non-diabetic burn ICU patients in our study. The diabetic group had fewer ICU-free days compared to the non-diabetic group and this was the only clinical outcome measure that was different between the two groups. This result is similar to results from previous investigators who demonstrated a positive relationship between pre-existing diabetes and worse clinical outcomes in a variety of critically ill patients25, 27–31, 33–35, 39–40. This consistency is not surprising since the patients from our study and the patients from the previous positive studies are demographically similar and have similar illness severities. On the other hand, a pre-existing diagnosis of diabetes had a negative relationship with our study’s other clinical outcomes and did not influence ventilator-free days, hospital-free days, and mortality in our critically ill burn patients. These negative findings are consistent with previous studies that have demonstrated no relationship between pre-existing diabetes, clinical outcomes, and mortality in critically ill patients25, 28–29, 31–37, 38–40. The lack of a significant relationship between diabetes and outcomes in our study and in previous studies is most likely the result of other clinical factors, such as age, ISS, and TBSA, overwhelming the effects of pre-existing diabetes. This scenario is highly plausible since the patients in the previous studies are similar to the patients in our study in terms of demographics and illness severity. Finally, our study is different from the studies that report a positive relationship between pre-existing diabetes and increased mortality26–27,30. Unlike our study’s patients, the patients in Langdon’s27 and Smiley’s30 studies were pre-operative, peri-operative, and post-operative surgical patients who were not necessarily critically ill during their hospitalization. Capes26 only demonstrated an association between pre-existing diabetes and mortality in critically ill patients whose diabetes was poorly controlled (≥180mg/dL). We did not know the quality and extent of pre-hospital blood glucose control in any of our patients, which is one of our study’s limitations. Similarly, a recent study by Vincent et al about diabetes and increased ICU mortality does not examine the quality of pre-hospital blood glucose control either34. Pre-hospital blood glucose control can be determined using admission hemoglobin A1c levels which reflect the average blood glucose level for the previous 8–12 weeks. Hemoglobin A1c levels should be drawn at the time patients are admitted to the burn ICU so that they can be stratified into well-controlled, poorly controlled, and undiagnosed diabetics. After stratification each group can be studied to determine if the quality of pre-hospital blood glucose control contributes to clinical outcomes and mortality in diabetic burn ICU patients. Moreover, knowledge of a patient’s admission hemoglobin A1c level would also help physicians and nurses determine the quality of pre-hospital blood glucose control and predict inpatient blood glucose physiology and clinical outcomes44–50.
Conclusions
Burn patients with a pre-existing diagnosis of diabetes have higher blood glucose levels at time of admission to the burn ICU. They also have higher mean blood glucose levels and increased blood glucose variability during their ICU stay that indicates managing and controlling their blood glucose levels is difficult even with intensive insulin therapy. A pre-existing diagnosis of diabetes does not appear to influence selected clinical outcomes as demonstrated in this retrospective study.
Acknowledgments
Supported by grants from:
The National Institutes of Health (1 R01 GM063120-04) and The Technologies for Metabolic Monitoring (TMM)/Julia Weaver Fund, A Congressionally Directed Program Jointly Managed by the USA MRMC, NIH, NASA, and the Juvenile Diabetes Research Foundation and Combat Casualty Care Division United States Army Medical Research and Materiel Command
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Alejandra Mora, Email: Alex.Mora1@us.army.mil.
Steven E Wolf, Email: Steven.Wolf@amedd.army.mil.
Charles E Wade, Email: Charles.Wade@amedd.army.mil.
References
- 1.Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. The New England Journal of Medicine. 2001;345(19):1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 2.Bochicchio GV, Salzano L, Joshi M, et al. Admission preoperative glucose is predictive of morbidity and mortality in trauma patients who require immediate operative intervention. The American Surgeon. 2005;71(2):171–174. doi: 10.1177/000313480507100215. [DOI] [PubMed] [Google Scholar]
- 3.Finney SJ, Zekveld C, Elia A, et al. Glucose control and mortality in critically ill patients. The Journal of the American Medical Association. 2003;290(15):2041–2047. doi: 10.1001/jama.290.15.2041. [DOI] [PubMed] [Google Scholar]
- 4.Krinsley JS. Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clinic proceedings. Mayo Clinic. 2003;78(12):1471–1478. doi: 10.4065/78.12.1471. [DOI] [PubMed] [Google Scholar]
- 5.Laird AM, Miller PR, Kilgo PD, et al. Relationship of early hyperglycemia to mortality in trauma patients. The Journal of Trauma. 2004;56(5):1058–1062. doi: 10.1097/01.ta.0000123267.39011.9f. [DOI] [PubMed] [Google Scholar]
- 6.Bochicchio GV, Sung J, Joshi M, et al. Persistent hyperglycemia is predictive of outcome in critically ill trauma patients. The Journal of Trauma. 2005;58(5):921–924. doi: 10.1097/01.ta.0000162141.26392.07. [DOI] [PubMed] [Google Scholar]
- 7.Sung J, Bochicchio GV, Joshi M, et al. Admission hyperglycemia is predictive of outcome in critically ill trauma patients. The Journal of Trauma. 2005;59(1):80–83. doi: 10.1097/01.ta.0000171452.96585.84. [DOI] [PubMed] [Google Scholar]
- 8.Bochicchio GV, Joshi M, Bochicchio KM, et al. Early hyperglycemic control is important in critically injured trauma patients. The Journal of Trauma. 2007;63(6):1353–1358. doi: 10.1097/TA.0b013e31815b83c4. discussion 1358–9. [DOI] [PubMed] [Google Scholar]
- 9.Scalea TM, Bochicchio GV, Bochicchio KM, et al. Tight glycemic control in critically injured trauma patients. Annals of Surgery. 2007;246(4):605–610. doi: 10.1097/SLA.0b013e318155a789. discussion 610–2. [DOI] [PubMed] [Google Scholar]
- 10.Gore DC, Chinkes D, Heggers J, et al. Association of hyperglycemia with increased mortality after severe burn injury. The Journal of Trauma. 2001;51(3):540–544. doi: 10.1097/00005373-200109000-00021. [DOI] [PubMed] [Google Scholar]
- 11.Ipaktchi K, Arbabi S. Advances in burn critical care. Critical Care Medicine. 2006;34(9 Suppl):S239–S244. doi: 10.1097/01.CCM.0000232625.63460.D4. [DOI] [PubMed] [Google Scholar]
- 12.Ballian N, Rabiee A, Andersen DK, et al. Glucose metabolism in burn patients: The role of insulin and other endocrine hormones. Burns. 2010 doi: 10.1016/j.burns.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Pidcoke HF, Salinas J, Wanek SM, et al. Patterns of exogenous insulin requirement reflect insulin sensitivity changes in trauma. American Journal of Surgery. 2007;194(6):798–803. doi: 10.1016/j.amjsurg.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 14.Pidcoke HF, Wanek SM, Rohleder LS, et al. Glucose variability is associated with high mortality after severe burn. The Journal of Trauma. 2009;67(5):990–995. doi: 10.1097/TA.0b013e3181baef4b. [DOI] [PubMed] [Google Scholar]
- 15.Lundelin K, Vigil L, Bua S, et al. Differences in complexity of glycemic profile in survivors and nonsurvivors in an intensive care unit: a pilot study. Critical Care Medicine. 2010;38(3):849–854. doi: 10.1097/CCM.0b013e3181ce49cf. [DOI] [PubMed] [Google Scholar]
- 16.Hermanides J, Vriesendorp TM, Bosman RJ, et al. Glucose variability is associated with intensive care unit mortality. Critical Care Medicine. 2010;38(3):838–842. doi: 10.1097/CCM.0b013e3181cc4be9. [DOI] [PubMed] [Google Scholar]
- 17.Mann EA, Pidcoke HF, Salinas J, et al. The impact of intensive insulin protocols and restrictive blood transfusion strategies on glucose measurement in American Burn Association (ABA) verified burn centers. Journal of Burn Care & Research. 2008;29(5):718–723. doi: 10.1097/BCR.0b013e3181848c74. [DOI] [PubMed] [Google Scholar]
- 18.Wolfe RR. Herman Award Lecture, 1996: relation of metabolic studies to clinical nutrition--the example of burn injury. The American Journal of Clinical Nutrition. 1996;64(5):800–808. doi: 10.1093/ajcn/64.5.800. [DOI] [PubMed] [Google Scholar]
- 19.Atiyeh BS, Gunn SWA, Dibo SA. Metabolic implications of severe burn injuries and their management: a systematic review of the literature. World Journal of Surgery. 2008;32(8):1857–1869. doi: 10.1007/s00268-008-9587-8. [DOI] [PubMed] [Google Scholar]
- 20.Mecott GA, Al-Mousawi AM, Gauglitz GG, et al. The role of hyperglycemia in burned patients: evidence-based studies. Shock. 2010;33(1):5–13. doi: 10.1097/SHK.0b013e3181af0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piggott H. Hyperglycaemia and diabetes after burns. Lancet. 1965;1(7405) [PubMed] [Google Scholar]
- 22.Wibbenmeyer LA, Amelon MJ, Morgan LJ, et al. Predicting survival in an elderly burn patient population. Burns. 2001;27(6):583–590. doi: 10.1016/s0305-4179(01)00009-2. [DOI] [PubMed] [Google Scholar]
- 23.Pham TN, Kramer CB, Wang J, et al. Epidemiology and outcomes of older adults with burn injury: an analysis of the National Burn Repository. Journal of Burn Care & Research. 2009;30(1):30–36. doi: 10.1097/BCR.0b013e3181921efc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahar P, Wasiak J, Bailey M, et al. Clinical factors affecting mortality in elderly burn patients admitted to a burns service. Burns. 2008;34(5):629–636. doi: 10.1016/j.burns.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Morricone L, Ranucci M, Denti S, et al. Diabetes and complications after cardiac surgery: comparison with a non-diabetic population. Acta Diabetologica. 1999;36(1–2):77–84. doi: 10.1007/s005920050149. [DOI] [PubMed] [Google Scholar]
- 26.Capes SE, Hunt D, Malmberg K, et al. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. 2000;355(9206):773–778. doi: 10.1016/S0140-6736(99)08415-9. [DOI] [PubMed] [Google Scholar]
- 27.Langdon CD, Shriver RL. Clinical issues in the care of critically ill diabetic patients. Critical Care Nursing Quarterly. 2004;27(2):162–171. doi: 10.1097/00002727-200404000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Bochicchio GV, Joshi M, Bochicchio K, et al. Incidence and impact of risk factors in critically ill trauma patients. World Journal of Surgery. 2006;30(1):114–118. doi: 10.1007/s00268-005-0203-x. [DOI] [PubMed] [Google Scholar]
- 29.Rady MY, Johnson DJ, Patel BM, et al. Influence of individual characteristics on outcome of glycemic control in intensive care unit patients with or without diabetes mellitus. Mayo Clinic Proceedings. Mayo Clinic. 2005;80(12):1558–1567. doi: 10.4065/80.12.1558. [DOI] [PubMed] [Google Scholar]
- 30.Smiley DD, Umpierrez GE. Perioperative glucose control in the diabetic or nondiabetic patient. Southern Medical Journal. 2006;99(6):580–589. doi: 10.1097/01.smj.0000209366.91803.99. quiz 590–1. [DOI] [PubMed] [Google Scholar]
- 31.Stoeckle M, Kaech C, Trampuz A, et al. The role of diabetes mellitus in patients with bloodstream infections. Swiss Medical Weekly. 2008;138(35–36):512–519. doi: 10.4414/smw.2008.12228. [DOI] [PubMed] [Google Scholar]
- 32.Vardakas KZ, Siempos II, Falagas ME. Diabetes mellitus as a risk factor for nosocomial pneumonia and associated mortality. Diabetic Medicine. 2007;24(10):1168–1171. doi: 10.1111/j.1464-5491.2007.02234.x. [DOI] [PubMed] [Google Scholar]
- 33.Stegenga ME, Vincent JL, Vail GM, et al. Diabetes does not alter mortality or hemostatic and inflammatory responses in patients with severe sepsis. Critical Care Medicine. 2010;38(2):539–545. doi: 10.1097/CCM.0b013e3181c02726. [DOI] [PubMed] [Google Scholar]
- 34.Vincent JL, Preiser JC, Sprung CL, et al. Insulin-treated diabetes is not associated with increased mortality in critically ill patients. Critical Care. 2010;14:R12. doi: 10.1186/cc8866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Umpierrez GE, Isaacs SD, Bazargan N, et al. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. The Journal of Clinical Endocrinology and Metabolism. 2002;87(3):978–982. doi: 10.1210/jcem.87.3.8341. [DOI] [PubMed] [Google Scholar]
- 36.Egi M, Bellomo R, Stachowski E, et al. Blood glucose concentration and outcome of critical illness: the impact of diabetes. Critical Care Medicine. 2008;36(8):2249–2255. doi: 10.1097/CCM.0b013e318181039a. [DOI] [PubMed] [Google Scholar]
- 37.Whitcomb BW, Pradhan EK, Pittas AG, et al. Impact of admission hyperglycemia on hospital mortality in various intensive care unit populations. Critical Care Medicine. 2005;33(12):2772–2777. doi: 10.1097/01.ccm.0000189741.44071.25. [DOI] [PubMed] [Google Scholar]
- 38.Van den Berghe G, Wilmer A, Milants I, et al. Intensive insulin therapy in mixed medical/surgical intensive care units: benefit versus harm. Diabetes. 2006;55(11):3151–3159. doi: 10.2337/db06-0855. [DOI] [PubMed] [Google Scholar]
- 39.McCampbell B, Wasif N, Rabbitts A, et al. Diabetes and burns: retrospective cohort study. The Journal of Burn Care & Rehabilitation. 2002;23(3):157–166. doi: 10.1097/00004630-200205000-00004. [DOI] [PubMed] [Google Scholar]
- 40.Maghsoudi H, Aghamohammadzadeh N, Khalili N. Burns in diabetic patients. International Journal of Diabetes in Developing Countries. 2008;28(1):19–25. doi: 10.4103/0973-3930.41982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pidcoke H, Wade C, Mann E, et al. Anemia causes hypoglycemia in intensive care unit patients due to error in single-channel glucometers: methods of reducing patient risk. Critical Care Medicine. 2010;38(2):471–476. doi: 10.1097/CCM.0b013e3181bc826f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mann E, Salinas J, Pidcoke H, et al. Error rates resulting from anemia can be corrected in multiple commonly used point-of-care glucometers. The Journal of Trauma. 2008;64(1):15–20. doi: 10.1097/TA.0b013e318160b9e4. [DOI] [PubMed] [Google Scholar]
- 43.NICE-SUGAR Study Investigators. Finfer S, Chittock D, et al. Intensive versus conventional glucose control in critically ill patients. The New England Journal of Medicine. 2009;360(13):1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 44.Yamamoto-Honda R, Kitazato H, Hashimoto S, et al. Distribution of blood glucose and the correlation between blood glucose and hemoglobin A1c levels in diabetic outpatients. Endocrine Journal. 2008;55(5):913–923. doi: 10.1507/endocrj.k08e-071. [DOI] [PubMed] [Google Scholar]
- 45.Cakmak M, Cakmak N, Cetemen S, et al. The value of admission glycosylated hemoglobin level in patients with acute myocardial infarction. The Canadian Journal of Cardiology. 2008;24(5):375–378. doi: 10.1016/s0828-282x(08)70600-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gornik I, Gornik O, Gasparovic V. HbA1c is outcome predictor in diabetic patients with sepsis. Diabetes Research and Clinical Practice. 2007;77(1):120–125. doi: 10.1016/j.diabres.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 47.Hadjadj S, Coisne D, Mauco G, et al. Prognostic value of admission plasma glucose and HbA in acute myocardial infarction. Diabetic Medicine. 2004;21(4):305–310. doi: 10.1111/j.1464-5491.2004.01112.x. [DOI] [PubMed] [Google Scholar]
- 48.Greci LS, Kailasam M, Malkani S, et al. Utility of HbA(1c) levels for diabetes case finding in hospitalized patients with hyperglycemia. Diabetes Care. 2003;26(4):1064–1068. doi: 10.2337/diacare.26.4.1064. [DOI] [PubMed] [Google Scholar]
- 49.Gustafsson UO, Thorell A, Soop M, et al. Haemoglobin A1c as a predictor of postoperative hyperglycaemia and complications after major colorectal surgery. The British Journal of Surgery. 2009;96(11):1358–1364. doi: 10.1002/bjs.6724. [DOI] [PubMed] [Google Scholar]
- 50.O'Sullivan CJ, Hynes N, Mahendran B, et al. Haemoglobin A1c (HbA1C) in non-diabetic and diabetic vascular patients. Is HbA1C an independent risk factor and predictor of adverse outcome? European Journal of Vascular and Endovascular Surgery. 2006;32(2):188–197. doi: 10.1016/j.ejvs.2006.01.011. [DOI] [PubMed] [Google Scholar]


