Abstract
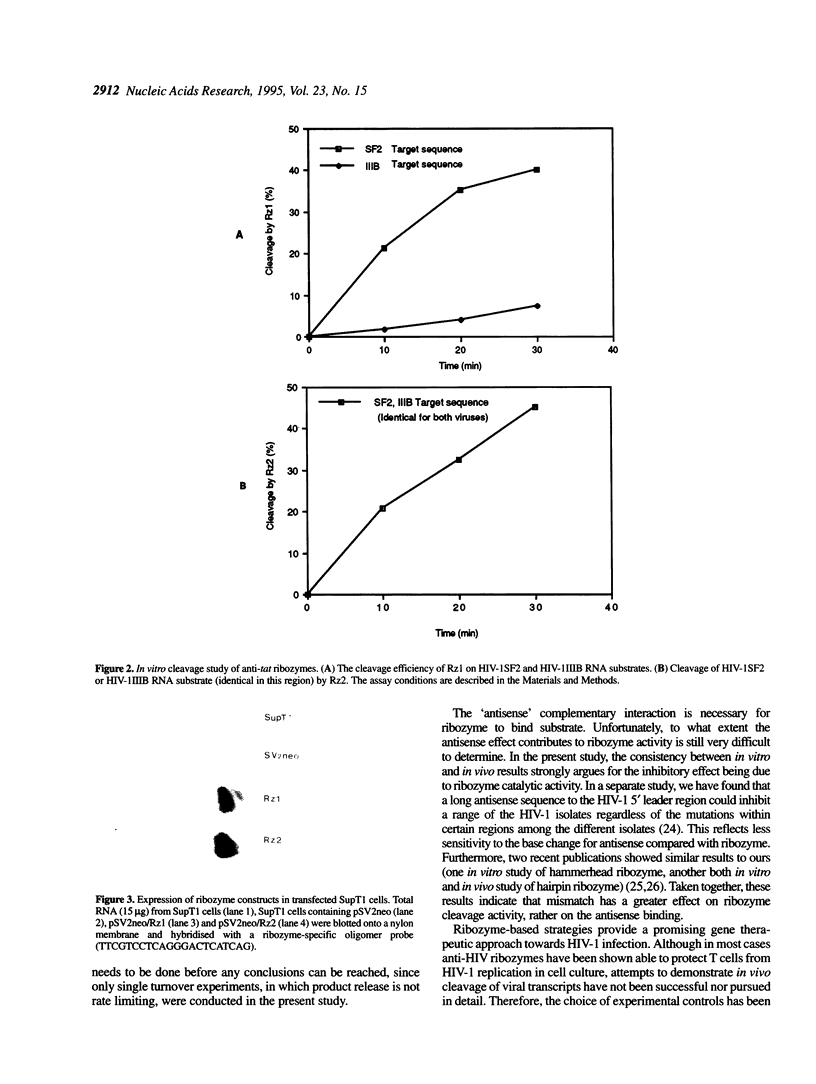
The structural motif formed between a hammerhead ribozyme and its substrate consists of three RNA double helices in which the sequence 5' to the XUY is termed helix I and the sequence 3' to the XUY helix III. Two hammerhead ribozymes targeted to the tat gene of HIV-1SF2 were designed to study target specificity and the potential effect of helix I mismatch on ribozyme efficacy both in vitro and in vivo. The first ribozyme (Rz1) targeted to the 5' splicing region of the tat gene was designed to cleave GUC*A. In HIV-1IIIB the A is changed to a G. The second ribozyme (Rz2) was targeted to the translational initiation region of the tat gene which is highly conserved among a variety of HIV-1 isolates, including both HIV-1SF2 and HIV-1IIIB. In vitro cleavage studies demonstrated that Rz1 efficiency cleaved HIV-1SF2 substrate RNA, but not HIV-1IIIB, presumably due to the base change from A to G. In contrast, Rz2 cleaved HIV-1SF2 or HIV-1IIIB substrate with equal efficiency. Both ribozymes were cloned into the 3' untranslated region of the neomycin gene (neo) within the pSV2neo vector and transfected into the SupT1 human CD4+ T cell line. Following selection, stable transfectants were challenged with either HIV-1SF2 or HIV-1IIIB virus. While Rz1-expressing cells were significantly protected from HIV-1SF2 infection, they exhibited no protection when infected with HIV-1IIIB virus. In contrast, Rz2 was effective in inhibiting the replication of both HIV-1SF2 and HIV-1IIIB in SupT1 cells. Expression of both ribozymes in these cells was demonstrated by Northern analysis. RT-PCR sequencing analysis confirmed the respective HIV-1 target sequence integrity. These data demonstrate the importance of the first base pair distal to the XUY within helix I of the hammerhead structure for both in vitro and in vivo ribozyme activities and imply that the effectiveness of the anti-HIV-1 ribozymes against appropriate target sequences is due to their catalytic activities rather than any antisense effect.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boom R., Sol C. J., Salimans M. M., Jansen C. L., Wertheim-van Dillen P. M., van der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990 Mar;28(3):495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayton A. I., Sodroski J. G., Rosen C. A., Goh W. C., Haseltine W. A. The trans-activator gene of the human T cell lymphotropic virus type III is required for replication. Cell. 1986 Mar 28;44(6):941–947. doi: 10.1016/0092-8674(86)90017-6. [DOI] [PubMed] [Google Scholar]
- Fedor M. J., Uhlenbeck O. C. Kinetics of intermolecular cleavage by hammerhead ribozymes. Biochemistry. 1992 Dec 8;31(48):12042–12054. doi: 10.1021/bi00163a012. [DOI] [PubMed] [Google Scholar]
- Fedor M. J., Uhlenbeck O. C. Substrate sequence effects on "hammerhead" RNA catalytic efficiency. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1668–1672. doi: 10.1073/pnas.87.5.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher A. G., Feinberg M. B., Josephs S. F., Harper M. E., Marselle L. M., Reyes G., Gonda M. A., Aldovini A., Debouk C., Gallo R. C. The trans-activator gene of HTLV-III is essential for virus replication. 1986 Mar 27-Apr 2Nature. 320(6060):367–371. doi: 10.1038/320367a0. [DOI] [PubMed] [Google Scholar]
- Goodchild J., Agrawal S., Civeira M. P., Sarin P. S., Sun D., Zamecnik P. C. Inhibition of human immunodeficiency virus replication by antisense oligodeoxynucleotides. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5507–5511. doi: 10.1073/pnas.85.15.5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseloff J., Gerlach W. L. Simple RNA enzymes with new and highly specific endoribonuclease activities. Nature. 1988 Aug 18;334(6183):585–591. doi: 10.1038/334585a0. [DOI] [PubMed] [Google Scholar]
- Jeffries A. C., Symons R. H. A catalytic 13-mer ribozyme. Nucleic Acids Res. 1989 Feb 25;17(4):1371–1377. doi: 10.1093/nar/17.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi M., Iwai S., Ohtsuka E. Construction of a series of several self-cleaving RNA duplexes using synthetic 21-mers. FEBS Lett. 1988 Feb 15;228(2):228–230. doi: 10.1016/0014-5793(88)80004-8. [DOI] [PubMed] [Google Scholar]
- Lo K. M., Biasolo M. A., Dehni G., Palú G., Haseltine W. A. Inhibition of replication of HIV-1 by retroviral vectors expressing tat-antisense and anti-tat ribozyme RNA. Virology. 1992 Sep;190(1):176–183. doi: 10.1016/0042-6822(92)91203-7. [DOI] [PubMed] [Google Scholar]
- Nichols R. C., Raben N. Hints for direct sequencing of PCR-generated single-stranded DNA. Biotechniques. 1994 Sep;17(3):412–414. [PubMed] [Google Scholar]
- Rossi J. J. Ribozymes. Curr Opin Biotechnol. 1992 Feb;3(1):3–7. doi: 10.1016/0958-1669(92)90117-2. [DOI] [PubMed] [Google Scholar]
- Sarver N., Cantin E. M., Chang P. S., Zaia J. A., Ladne P. A., Stephens D. A., Rossi J. J. Ribozymes as potential anti-HIV-1 therapeutic agents. Science. 1990 Mar 9;247(4947):1222–1225. doi: 10.1126/science.2107573. [DOI] [PubMed] [Google Scholar]
- Schwartz S., Felber B. K., Pavlakis G. N. Mechanism of translation of monocistronic and multicistronic human immunodeficiency virus type 1 mRNAs. Mol Cell Biol. 1992 Jan;12(1):207–219. doi: 10.1128/mcb.12.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon C. C., Symons R. H. Mutagenesis analysis of a self-cleaving RNA. Nucleic Acids Res. 1989 Jul 25;17(14):5679–5685. doi: 10.1093/nar/17.14.5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L. Q., Warrilow D., Wang L., Witherington C., Macpherson J., Symonds G. Ribozyme-mediated suppression of Moloney murine leukemia virus and human immunodeficiency virus type I replication in permissive cell lines. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):9715–9719. doi: 10.1073/pnas.91.21.9715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons R. H. Small catalytic RNAs. Annu Rev Biochem. 1992;61:641–671. doi: 10.1146/annurev.bi.61.070192.003233. [DOI] [PubMed] [Google Scholar]
- Uhlenbeck O. C. A small catalytic oligoribonucleotide. Nature. 1987 Aug 13;328(6131):596–600. doi: 10.1038/328596a0. [DOI] [PubMed] [Google Scholar]
- Weerasinghe M., Liem S. E., Asad S., Read S. E., Joshi S. Resistance to human immunodeficiency virus type 1 (HIV-1) infection in human CD4+ lymphocyte-derived cell lines conferred by using retroviral vectors expressing an HIV-1 RNA-specific ribozyme. J Virol. 1991 Oct;65(10):5531–5534. doi: 10.1128/jvi.65.10.5531-5534.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada O., Kraus G., Leavitt M. C., Yu M., Wong-Staal F. Activity and cleavage site specificity of an anti-HIV-1 hairpin ribozyme in human T cells. Virology. 1994 Nov 15;205(1):121–126. doi: 10.1006/viro.1994.1626. [DOI] [PubMed] [Google Scholar]
- Yu M., Ojwang J., Yamada O., Hampel A., Rapapport J., Looney D., Wong-Staal F. A hairpin ribozyme inhibits expression of diverse strains of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6340–6344. doi: 10.1073/pnas.90.13.6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamecnik P. C., Goodchild J., Taguchi Y., Sarin P. S. Inhibition of replication and expression of human T-cell lymphotropic virus type III in cultured cells by exogenous synthetic oligonucleotides complementary to viral RNA. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4143–4146. doi: 10.1073/pnas.83.12.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoumadakis M., Neubert W. J., Tabler M. The influence of imperfectly paired helices I and III on the catalytic activity of hammerhead ribozymes. Nucleic Acids Res. 1994 Dec 11;22(24):5271–5278. doi: 10.1093/nar/22.24.5271. [DOI] [PMC free article] [PubMed] [Google Scholar]





