Abstract
Sphingolipids have emerged as bioeffector molecules, controlling various aspects of cell growth and proliferation in cancer, which is becoming the deadliest disease in the world. These lipid molecules have also been implicated in the mechanism of action of cancer chemotherapeutics. Ceramide, the central molecule of sphingolipid metabolism, generally mediates antiproliferative responses, such as cell growth inhibition, apoptosis induction, senescence modulation, endoplasmic reticulum stress responses and/or autophagy. Interestingly, recent studies suggest de novo-generated ceramides may have distinct and opposing roles in the promotion/suppression of tumors, and that these activities are based on their fatty acid chain lengths, subcellular localization and/or direct downstream targets. For example, in head and neck cancer cells, ceramide synthase 6/C16-ceramide addiction was revealed, and this was associated with increased tumor growth, whereas downregulation of its synthesis resulted in ER stress-induced apoptosis. By contrast, ceramide synthase 1-generated C18-ceramide has been shown to suppress tumor growth in various cancer models, both in situ and in vivo. In addition, ceramide metabolism to generate sphingosine-1-phosphate (S1P) by sphingosine kinases 1 and 2 mediates, with or without the involvement of G-protein-coupled S1P receptor signaling, prosurvival, angiogenesis, metastasis and/or resistance to drug-induced apoptosis. Importantly, recent findings regarding the mechanisms by which sphingolipid metabolism and signaling regulate tumor growth and progression, such as identifying direct intracellular protein targets of sphingolipids, have been key for the development of new chemotherapeutic strategies. Thus, in this article, we will present conclusions of recent studies that describe opposing roles of de novo-generated ceramides by ceramide synthases and/or S1P in the regulation of cancer pathogenesis, as well as the development of sphingolipid-based cancer therapeutics and drug resistance.
Keywords: apoptosis, autophagy, chemoresistance, endoplasmic reticulum stress, sphingolipid, sphingolipid, protein binding
Structure & metabolism of ceramide
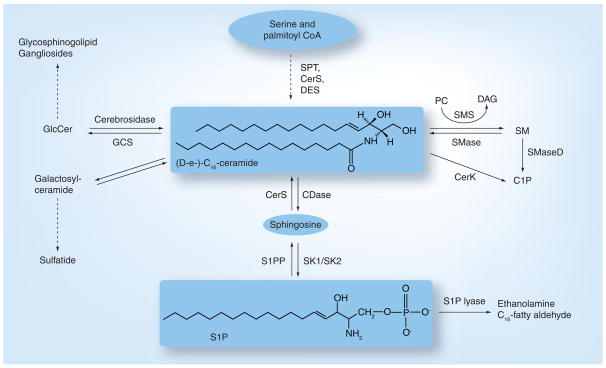
Sphingolipids are structural components of biological membranes that were first described by Thudichum, and the chemical structures of various sphingolipid molecules, including sphingosine and ceramide, were later resolved by Carter [1]. The term ‘sphingosine’ originates from a Greek word, which means ‘to bind tight’, and to denote ‘sphinx’ because of its initial elusive structure. Ceramide, a central molecule of sphingolipid metabolism, is composed of a sphingosine base and amide-linked acyl chains varying in length from C14 to C26 (C16 acyl chain is shown Figure 1). Ceramide then serves as the metabolic and structural precursor for complex sphingolipids, which are composed of hydrophilic head groups, such as sphingomyelin (SM), ceramide-1-phosphate and glucosylceramide (GlcCer), which is the precursor for glycolipids and gangliosides [2]. Endogenous ceramide is regulated by complex and integrated metabolic pathways, which direct the generation or clearance of ceramide [3,4]. Each of these pathways involves a number of specialized enzymes [2]. In addition to the activation of sphingomyelinases (SMases) [5–7], which hydrolyze SM to yield ceramide, endogenous ceramide can be generated via the de novo pathway [8,9] after condensation of serine and palmitoyl CoA by serine-palmitoyl CoA transferase (SPT) [10,11], leading to the synthesis of dihydroceramide by dihydroceramide synthases (CerS) [3]. Dihydroceramide is then converted to ceramide by desaturase (DES), which inserts the double bond between carbons 4 and 5 in the sphingosine backbone [12,13]. Ceramide is further metabolized by GlcCerS (GCS) [14] or SM synthase [15] for the generation of GlcCer or SM, respectively. Ceramide can also be hydrolyzed by ceramidases (CDases) [16,17] to yield sphingosine, which can be phosphorylated by sphingosine kinase (SK)1 or SK2, producing sphingosine-1-phosphate (S1P). Ceramide can be transported from the endoplasmic reticulum (ER) to the Golgi by a ceramide transporter (CERT) [18–20] for SM synthesis. In addtion, GlcCer, using ceramide as a substrate, is generated in the Golgi [14]; however, this process is CERT independent [21]. Importantly, nonvesicular transport of GlcCer from its site of synthesis (early Golgi) to distal Golgi compartments is carried out by four-phosphate adaptor protein (FAPP2) [22], which controls the synthesis of glycosphingolipids that are important for determining the plasma membrane lipid composition.
Figure 1. De novo generation of ceramide and its metabolism to generate sphingosine-1-phosphate.
The first step of de novo synthesis of ceramide and other complex sphingolipids is the condensation of serine and palmitoyl CoA by SPT, followed by the action of CerS and DES forming ceramide, the central molecule of sphingolipid metabolism. Sphingosine formation occurs via deacylation of ceramide by CDase. Following deacylation, sphingosine is released, which can then be phosphorylated to generate S1P by the action of SK1 or SK2. S1P is hydrolyzed by S1P lyase. Ceramide can also be formed from the degradation of SM by SMases and glycosphingolipids by cerobrosidases. Ceramide is further metabolized for the synthesis of GlcCer by GCS, which is a precursor for lactosylceramide and ganglioside generation.
C1P: Ceramide-1-phosphate; CDase: Ceramidase; CerS: Ceramide synthase; DAG: Diacylglycerol; DES: Desaturase;
GCS: Glucosylceramide synthase; GlcCer: Glucosylceramide; S1P: Sphingosine-1-phosphate; S1PP: Phosphorylated sphingosine-1 phosphate; SK: Sphingosine kinase; SM: Sphingomyelin; SMase: Sphingomyelinase; SPT: Serine-palmitoyl CoA transferase.
CerS & de novo generation of ceramide
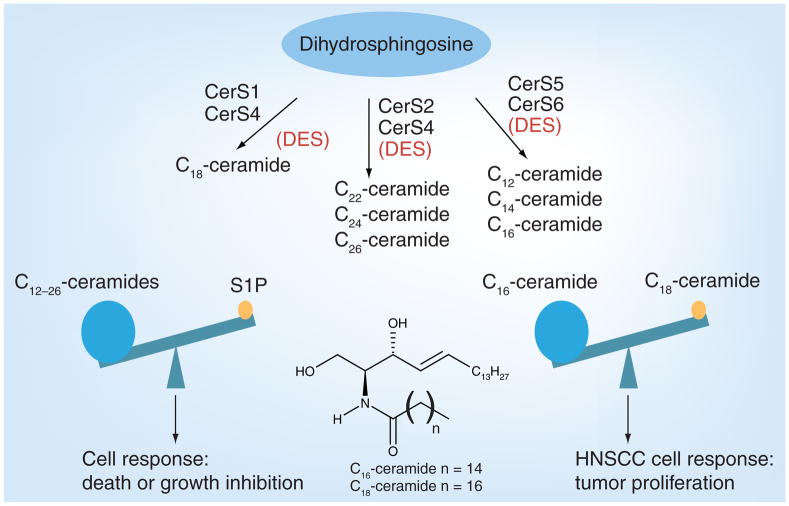
Ceramide generation by the function of SPT [11] and/or CerS [23] was implicated in inducing apoptosis in various cancer cell types. CerS, identified as the yeast longevity assurance gene 1 (LAG1), is known to regulate lifespan/longevity in Saccharomyces cerevisiae, and its deletion prolongs the replicative lifespan of yeast [24,25]. Additionally, a LAG1 homologue, LAC1, was found to be a key component of CerS [26]. The discovery of the mouse homologue of LAG1, also known as LASS1, or the upstream of growth and differentiation factor 1 (UOG1) [27,28], revealed that it specifically regulates the synthesis of C18-ceramide with a high degree of specificity for fatty acid chain length [24,29]. Additional studies confirmed six LASS proteins (LASS1–6) that were recently renamed CerS1–6 [24]. CerS1–6 are associated with the ER membrane and contain a crucial TRAM–LAG1p–CLN8 (TLC) domain [30]. The TLC domain constitutes the catalytic activity of CerS and is required for the generation of ceramide. It is well established that each CerS exerts preference for the generation of endogenous dihydroceramides with distinct fatty acid chain lengths, which are then desaturated by DES to generate ceramides [31,32]. For example, CerS1/4 mainly generate ceramide with a C18-containing fatty acid chain (C18-ceramide), whereas CerS5/6 preferentially mediates the generation of C16-ceramide and, to a lesser extent, C12- and C14-ceramides (Figure 2) [29]. Although dihydroceramides are thought to be biologically inactive molecules, recent data suggest that they, at least when generated in cells, might also be important in the regulation of cancer cell growth or survival [31].
Figure 2. Specificity of ceramide synthase(s) and the diversity of ceramide species.
The de novo synthesis of various chain length fatty acids containing ceramide are formed by the action of specific CerS. CerS1 specifically biosynthesizes C18 -ceramide; CerS2 and CerS4 mediate very-long-chain fatty acid-containing ceramide synthesis, such as C22-, C24- and C26-ceramides. Additionally, CerS5 and CerS6 are mostly responsible for the synthesis of chain lengths up to 16, such as C12-, C14- and C16-ceramides. Ceramide is the precursor for S1P. Importantly, these two molecules have opposing functions and are known to regulate each other. In addition, ceramides with different chain lengths might have distinct and sometimes opposing functions in the regulation of tumor progression and/or growth. For example, in HNSCC tumors, while CerS1-generated C18-ceramide inhibits tumor growth, CerS6-mediated C16-ceramide induces tumor growth.
CerS: Ceramide synthase; DES: Desaturase; HNSCC: Head and neck squamous cell carcinoma; S1P: Sphingosine-1-phosphate.
Regulation of CerS1–6 & distinct roles of de novo-generated ceramides in the promotion or suppression of tumor growth
Little is known about the regulation of CerS enzymes; however, recent studies are beginning to unveil the mechanisms of their expression, activation and stability in noncancerous versus cancerous cells. CerS1 is known to be responsible for generating mainly C18-ceramide, and its regulation has been shown to be dependent on proteasomal-mediated turnover [33]. Upon treatment with cisplatin, ultraviolet (UV) irradiation or doxorubicin (DOX), catalytic activity of CerS1 increases, as does its ubiquitination and degradation. The MAPK p38 positively regulates the stress-induced degradation of CerS1, whereas PKC prevents its degradation by phosphorylating CerS1, increasing its stability [33]. However, proteasomal processing of CerS1 was shown to be necessary for stress-induced translocation from the ER to the Golgi apparatus. In response to cis-platin, UV irradiation or DOX, the C-terminus of CerS1 is cleaved, moving CerS1 from the ER to the Golgi. This translocation is dependent on CerS1 in a catalytically active state, and PKC-dependent phosphorylation of CerS1 prevented its translocation [34]. Recently, the proapoptotic signaling protein, BAK, has been shown to post-translationally regulate CerS isoforms, leading to increased generation of long-chain ceramides in response to apoptotic stimuli, including cisplatin, UV irradiation and growth factor withdrawal in baby mouse kidney cells [35].
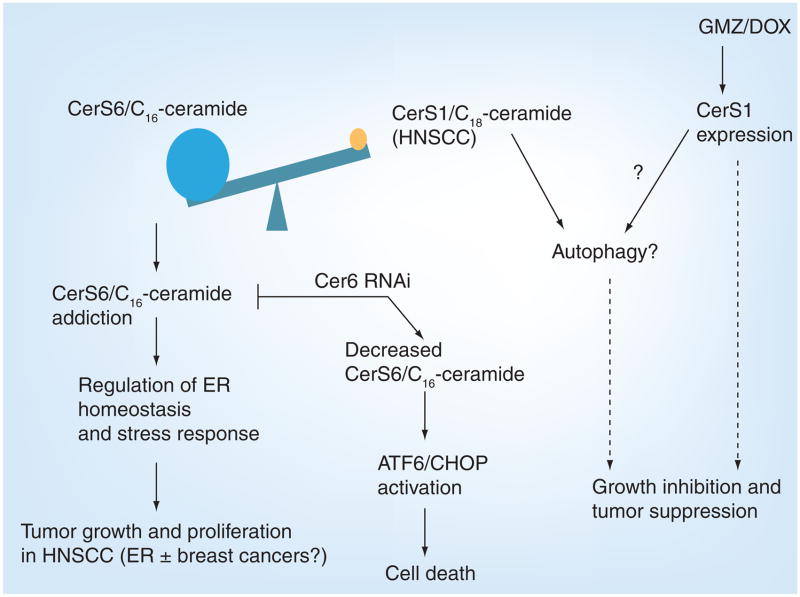
Ceramide regulation is increasingly implicated in cancer pathogenesis and prognosis. In 2004, data from our laboratory showed that total ceramide levels were elevated in the majority of head and neck squamous cell carcinoma (HNSCC) tissues compared with noncancerous adjacent head and neck tissues [36]. Interestingly, only C18-ceramide, and not other ceramide species, was significantly lower in approximately 70% of the tumor tissues of HNSCC patients sampled when compared with controls, and decreased C18-ceramide significantly correlated with lymphovascular invasion and nodal metastasis in HNSCC patients [37]. Thus, for the first time, these studies indicated the clinical significance of CerS-generated ceramide with specific fatty acid chain length, especially CerS1/C18-ceramide modulation, in the regulation of HNSCC pathogenesis and overall survival. Conversely, overexpression of CerS1 confers a decrease in HNSCC cell growth [36], concomitant with inhibition of telomerase activity, one of the downstream targets of ceramide involved in the suppression of cancer cell growth and/or proliferation. Upon chemotherapeutic treatment with gemcitabine (GMZ) and DOX in HNSCC cells, CerS1 mRNA increased, as did CerS1 enzymatic activity [38]. This was accompanied by an increase in caspase-3 and -9 activation, which was prevented by the silencing of CerS1 using siRNAs when treated with these two drugs. Importantly, GMZ/DOX treatment in vivo decreased HNSCC xenograft tumor growth and progression in severe combined immunodeficient (SCID) mice, which was associated with increased C18-ceramide and CerS1 expression [38]. Interestingly, in GMZ/DOX-treated HNSCC xenografts, downregulation of CerS6 expression and C16-ceramide were also observed. Taken together, these data suggested a novel view that perhaps not all ceramides (containing different fatty acid chain lengths) play similar roles. For example, CerS1/C18-ceramide versus CerS6/C16-ceramide might play distinct and sometimes opposing roles in the regulation of tumor growth and/or therapy in some cancer cells. This view was recently supported by data indicating that, whereas CerS1/C18-ceramide suppresses HNSCC xenografts tumor growth, CerS6/C16-ceramide induces HNSCC tumor proliferation in SCID mice (Figure 3) [39].
Figure 3. Opposing functions of ceramide synthase 1 and 6.
CerS6/C16-ceramide accumulation regulates ER homeostasis and stress responses favoring cell proliferation and tumor growth. Conversely, deduction of CerS6/C16-ceramide by RNAi against CerS6 induces ER stress by the ATF6/CHOP arm, which leads to cell death. CerS1/C18-ceramide levels can be increased by the treatment of chemotherapeutic agents, such as GMZ and DOX, which can inhibit tumor cell growth through the induction of autophagy or by directly inducing tumor cell apoptosis.
CerS: Ceramide synthase; DOX: Doxorubicin; ER: Endoplasmic reticulum;
GMZ: Gemcitabine; HNSCC: Head and neck squamous cell carcinoma.
Although how CerS1-generated C18-ceramide inhibits HNSCC tumor growth and/or proliferation remains unknown, downregulation of CerS6 and C16-ceramide induced ER stress and apoptosis through specific activation of the ATF6/CHOP arm of the unfolded protein response pathway in HNSCC cell lines [39]. These data suggest that CerS6/C16-ceramide protects HNSCC cells from ER stress and apoptosis, and that knockdown of CerS6/C16-ceramide induces ER stress and apoptosis via selective activation of the ATF6/CHOP axis in HNSCC cells (Figure 3).
In addition, genetic knockout studies in Caenorhabditis elegans revealed that activity of CerS homologues hyl-1 and lagr-1 are necessary for radiation-induced apoptosis in germ cells [40]. By contrast, loss of hyl-2- and Hyl-2-generated C20- and C22-ceramides resulted in sensitivity to anoxia and hyperthermia-induced death in C. elegans [41]. Importantly, these data also support the theory thatthat distinct ceramides, generated de novo by specific CerS homologues, may be cytoprotective against cellular stressors. Moreover, in agreement with this notion, a published report suggested that downregulation of CerS2, which generates C24- and C24:1-ceramides, induced autophagy and the unfolded protein response [42]. Consistent with this finding, overexpression of C16-ceramide-generating CerS5, but not CerS6, was shown to significantly increase ionizing radiation (IR)-induced apoptosis overexpression of C24- and C24:1-ceramide-generating CerS2 conferred protection against IR-induced apoptosis [43]. Interestingly, schlank was recently identified as a CerS homologue in Drosophila [44], and a mutation of schlank reduced de novo ceramide generation and drastically decreased larval growth. Furthermore, a CerS2-null mouse model has been described recently [45–47], and this model has decreased C22- and C24-ceramides, whereas C16-ceramide was increased in the liver and kidneys of these animals [46]. Of note, CerS2-null animals developed hepatocellular hyperplasia and carcinomas, whereas renal pathology was not observed [47]. Collectively, these studies argue against anti-proliferative and prodeath roles of all endogenous ceramides and support the novel view that ceramides with different fatty acid chain lengths, such as C18- and C16-ceramides generated by CerS1 or CerS6, respectively, play distinct roles in the regulation of cell death, and these distinct roles might be conserved from worms to humans.
Interestingly, recent data suggest that elevated C16-ceramide associates with a positive lymph node status in breast cancer patients [48], indicating the metastatic potential of C16-ceramide in the clinic. In another published report, elevated levels of CerS2 and CerS6 mRNA were observed in breast cancer tumors [49]. Thus, these data indicate that distinct functions of ceramides with different fatty acid chain lengths, especially their unusual prosurvival roles, might not be limited to HNSCC, but can be observed in various other tumors.
Taken together, there is increasing evidence for distinct roles of ceramides generated in the de novo pathway by CerS proteins. In addition, these data suggest that certain cancers might become addicted to elevated levels of certain ceramide species, such as C16-ceramide in HNSCC, such that attenuation of C16-ceramide generation induces ER stress and apoptosis, leading to tumor suppression. Identification of the molecular mechanisms behind how these ceramides exert their distinct effects based on fatty acid composition is crucial to generating mechanism-driven novel therapeutics centered on sphingolipid metabolism modulation.
De novo-generated ceramide in chemotherapy-induced cell death &/or growth inhibition
In addition to the involvement of CerS1/C18-ceramide generation in GMZ/DOX-induced cell death in HNSCC cells and tumors, in colon cancer cells, the COX-2 inhibitor celecoxib induces de novo ceramide generation, specifically increasing C16, C24 and C24:1-dihydroceramide, which is inhibited with the addition of de novo synthesis enzyme inhibitors [50]. At higher concentrations, DES appears to be inhibited by celecoxib, leading to an accumulation of dihydroceramide, which is associated with an antiproliferative effect [50]. Treatment of mantle cell lymphoma cells with cannabinoids increased mRNA of CerS3 and CerS6 enzymes and de novo synthesis of C16, C18, C24 and C24:1-ceramides, inducing cell death [51], and treatment with inhibitors that block the CB1 receptor, SPT, CerS or DES prevented this effect. In leukemia and colon cancer cells, upregulation of p53 activated de novo ceramide generation, specifically C16-ceramide [52]. CerS5 expression was increased significantly upon upregulation of p53 in the leukemia cell line but not in the colon cancer cell line, suggesting different modes of regulation for the de novo generation of C16-ceramide upon p53 induction in these cells [52].
Recently, research into resensitizing resistant cancers suggests that upregulating enzymes in the de novo ceramide synthesis pathway is effective against drug-resistant colon cancer cells [53]. Overexpression of CerS6 in resistant cells resensitized them to TRAIL-induced apoptosis and increased C16-ceramide upon induction [53]. In prostate cancer cells resistant to radiation, activation of PKC-α radiosensitizes these cells, upregulating de novo ceramide synthesis and inducing apoptosis [54]. PKC-α downregulates ataxia telangiectasia mutated (ATM) protein, leading to CerS derepression. Thus, treating the resistant cells with TPA, a PKC-α activator, or PKC-α overexpression decreased ATM protein and elevated total ceramide significantly, thereby increasing apoptosis in these cells upon radiation treatment [54]. Min et al. reported that CerS1-overexpressing HEK293 cells had increased sensitivity to cisplatin treatment [55]. CerS4 over-expression did not confer sensitivity to any drugs tested, and CerS5-overexpressing cells became more sensitive to DOX and vincristine, but not cisplatin and carboplatin [55]. Recently, vorinostat and sorafenib treatment was shown to increase CD95 activation in gastrointestinal tumor cells via CerS6-generated ceramide, involving Ca+2, protein phosphatase 2A (PP2A) and reactive oxygen species (ROS)-dependent signaling mechanism [56].
Taken together, these results show complex and distinct signaling mechanisms regulating de novo ceramide generation via controlling mainly CerS1–6 and/or DES enzymes in chemotherapy-induced cell death in various cancer cells, including in imatinib (Gleevec®, Novartis, Switzerland)-induced apoptosis in chronic myeloid leukemia (CML) cells [57].
Although this article mainly focuses on the roles of de novo-generated ceramides by CerS1–6 in cell death, it should be noted here that there are important studies describing the importance of SMases in this process. For example, it is well known that IR induces apoptosis in several cell lines and/or tumors via activation of SMases [58–62]. Exposure of human leukemia cells (U93) to a histone deacetylase (HDAC)1 inhibitor, LAQ824, resulted in the A-SMase-dependent generation of ceramide and caused mitochondrial injury, caspase activation and apoptosis [62]. Consistent with these findings, overexpression of A-SMase in subcutaneous B16-F10 mouse melanomas, in combination with irradiation, significantly reduced tumors compared with irradiated control melanomas [63]. In addition, it was reported that overexpression of neutral SMase2 in MCF7 breast cancer cells caused a decrease in cell growth [64]. TNF-α increased N-SMase activity rapidly and mediated the translocation of the enzyme from the Golgi to plasma membrane, which was associated with the activation of PKC [65,66].
Endogenous long-chain ceramides can also be generated from hydrolysis of complex sphingolipids through the action of SMases, cerebrosidases or ceramidases. Complex sphingolipids or ceramides are broken down, eventually into sphingosine, which is then reused through reacylation by CerS to generate ceramides. This is called salvage pathway, which depends on the recycling of sphingosine for endogenous ceramide generation, and ceramides generated through this pathway also modulate cellular signaling in various cell types [67]. For example, data from our laboratory demonstrated that the sphingosine-recycling pathway is involved in the generation of endogenous long-chain ceramide in response to exogenous short-chain C6-ceramide, which plays an important biological role in controlling c-Myc function in human lung cancer cells [68].
Direct molecular targets of sphingolipids involved in the regulation of tumor growth
Over the years, our understanding of bio-active sphingolipids, in particular ceramide, has increased owing to the discovery that ceramide binds intracellular proteins (Table 1) [69]. Ceramide binding proteins were reported in the late 1990s, and these findings stemmed from the observations that ceramide activates protein kinases [70]. The ceramide-activated protein kinase was identified as the kinase suppressor of RAS (KSR) [71]. Ceramide stimulates KSR autophosphorylation, resulting in the transactivation of c-Raf, thus regulating the Ras–Raf–MAPK pathway [72]. Most recently, the CA3 domain of KSR was identified as the binding site for ceramide [73]. Interestingly, inhibition of ceramide generation prevented KSR translocation to glycosphingolipid- enriched plasma membranes and, thus, reduced its activation. Additionally, c-Raf itself was identified as a ceramide binding protein. Ceramide production via IL-1β stimulation not only bound to c-Raf, but also increased the activity of this mitogen-activated protein kinase [74]. These data signify that ceramide can modulate the Ras–Raf–MAPK pathway via specific interactions with KSR and c-Raf.
Table 1.
Sphingolipid binding proteins.
| Sphingolipid | Protein | Biological function |
|---|---|---|
| Ceramide | KSR | Transactivation of c-Raf |
| Ceramide | c-Raf | Activation of the MAPK cascade |
| Ceramide | Cathepsin D | Autocatalytic proteolysis of cathepsin D, leading to apoptosis |
| Ceramide | PKC-ζ | Interaction with stress-activated protein kinases, leading to growth suppression |
| Ceramide | I2PP2A | Increase in PP2A activity, leading to the regulation of c-Myc stability |
| Ceramide | CERT | Transports ceramide from the endoplasmic reticulum to the trans-Golgi, and it is involved in drug resistance |
| Ceramide-1-phosphate | cPLA2 | Arachidonic acid release |
| Sphingosine-1-phosphate | HDAC1 and HDAC2 | Regulation of p21 expression |
| Sphingosine-1-phosphate | TRAF2 | Stimulates TRAF2 E3 ligase activity, increasing polyubiquitination of RIP1 and regulating NF-κB |
| Sphingosine | ANP32A | Regulation of PP2A activity and COX-2 expression in human endothelial cells |
I2PP2A: Protein phosphatase 2A inhibitor 2; PP2A: Protein phosphatase 2A.
The endosomal target for ceramide was also identified as the aspartic protease, cathepsin D [75]. Procathepsin D can be secreted into the tumor microenvironment, leading to the degradation of extracellular matrix proteins and contributing to tumor metastasis and growth [76]. Interestingly, the interaction between ceramide and cathepsin D induced autocatalytic proteolysis, leading to the enzymatically active form of cathepsin D. Functionally, cathepsin D has been implicated in mediating apoptosis via IFN-γ, Fas and TNF-α [77], as well as chemotherapy agents [75]. More recently, other groups have suggested an important connection between ceramide and cathepsin D involved in apoptosis [78]. Another ceramide-activated protein kinase implicated in cancer pathogenesis is PKC-ζ, which is involved in cell survival. Studies have shown that ceramide directly activates PKC-ζ via binding to the cysteine-rich domain, a putative ceramide-binding region. This protein–lipid association induces PKC-ζ to interact with stress-activated protein kinase (SAPK), thereby resulting in growth suppression [79].
Recent studies revealed that another important ceramide binding protein, CERT, which specifically transports ceramide from the ER to the trans-Golgi for SM synthesis [80], plays a role in cancer drug resistance [81]. Downregulation of CERT sensitizes cancer cells to chemotherapy agents, such as paclitaxel [81], and this may be due to an increased accumulation of ceramide leading to ER stress and cell death (Table 1).
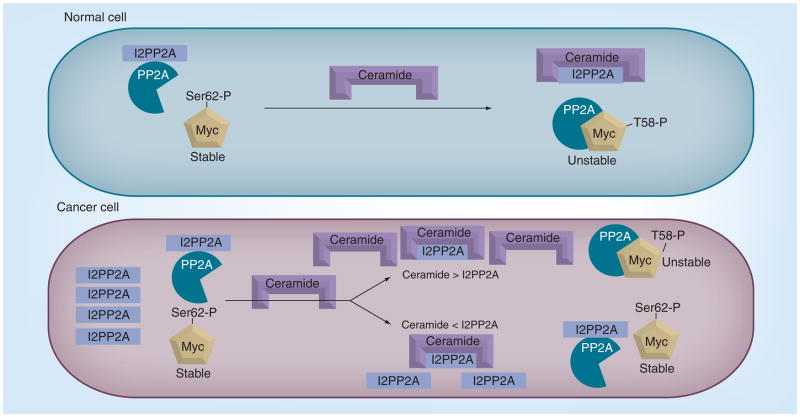
One of the well-described downstream targets of ceramide has been the protein phosphatases of the PP2A and PP1 family, also known as ceramide-activated protein phosphatases (CAPPs) [82,83]. PP2A is a tumor suppressor in cancer, and its activation regulates various downstream oncoproteins [84,85]. In particular, the naturally occurring D-erythro isoform of ceramide was the most potent at increasing PP2A activity in vitro [86,87], and this effect was demonstrated with ceramides containing short and long fatty acids chain lengths [88]. However, how ceramide mediates the activity of PP2A has been elusive. Most recently, ceramide was shown to directly bind to the oncoprotein SET/PP2A inhibitor 2 (I2PP2A) in A549 lung cancer cells. SET/I2PP2A is a nuclear protein and a known inhibitor of PP2A activity (Table 1). Thus, this discovery gave insight into the possible mechanism by which ceramide regulates PP2A activity via binding to its biological inhibitor (SET/I2PP2A), which controls PP2A activity and its downstream targets, such as protooncogene c-Myc [89], thus describing a novel mechanism for regulating PP2A-dependent antiroliferative roles of ceramide (Figure 4).
Figure 4. Regulation of oncogenic c-Myc by protein phosphatase 2A via control of ceramide in normal and cancer cells.
In normal cells, ceramide and its binding protein, I2PP2A, which is the inhibitor for PP2A, are mostly in a 1:1 ratio. Therefore, it is believed to be the binding and inactivation of ceramide by I2PP2A that liberates the active form of PP2A, which, in turn, acts upon c-Myc, leading to the dephosphorylation and degradation. In cancer cells, elevated levels of I2PP2A were observed, which inhibits most of the available PP2A and results in stable (active) oncogenic c-Myc. The stable form of c-Myc can mediate tumor growth and cancer progression by upregulating expression of several oncogenes.
I2PP2A: Protein phosphatase 2A inhibitor 2; PP2A: Protein phosphatase 2A.
Protein phosphatase 2A inhibitor 2 is a SET domain-containing acidic protein, and it is mainly localized to the nucleus [90–92]. Chromosomal mutations of the SET/I2PP2A gene are associated with acute myelogenous leukemia (AML) [90]. The SET domain proteins function as methyltransferases that target specific lysine residues in the N-terminal tails of histones and are involved in the modulation of gene transcription or histone acetylation [91,92]. Our studies determined that SET/I2PP2A, which also plays important roles in the drug resistance of CML cells [93], preferentially associates with exogenous C6-ceramide and endogenous C18-ceramide generated in A549 cells [89]. This degree of fatty acid chain length preference of I2PP2A for ceramide binding was also observed in the CERT–ceramide interaction [94,95], which preferentially binds C16-ceramide, as well as C6- and C18-ceramides, but not very-long-chain C24-ceramide [89,96]. These data suggest that, similar to CERT, I2PP2A also preferentially interacts with C6- and C18-ceramides but, unlike CERT, it does not interact with C16-ceramide. The globular domain of I2PP2A exhibits strong structural similarities to the recently solved CERT START domain [18,20]; in addition, the central globular domain of I2PP2A, which includes VIK 207–209 residues, might be involved in ceramide interaction [89]. Our data also revealed some important biological functions of ceramide/I2PP2A binding. For example, ceramide–I2PP2A interaction prevents the inhibition of PP2A in vitro, and further induces the degradation of c-Myc via PP2A activity. However, overexpression of I2PP2A, as is observed in various cancer tissues and cells, inhibits PP2A activity, and binds ceramide as a biological sponge, leading to the protection of ceramide-mediated c-Myc degradation and resistance to ceramide-mediated antiproliferation [89,97,98]. Thus, new strategies to decrease SET/I2PP2A expression and/or ceramide/SET/I2PP2A association, in combination with increasing endogenous ceramide might be advantageous for tumor suppression.
In addition to binding to nuclear protein SET/I2PP2A, ceramide is known to play other important functional roles in the nucleus, such as the regulation of SP3-HDAC1 suppressor function for the repression of human telomerase reverse transcriptase [99,100] and inhibition of the nuclear localization of glyceraldehyde-3-phosphate dehydrogenas (GAPDH), which protects telomeres [101]. Therefore, it is important to determine the subcellular regulation of how exogenous and endogenous ceramides reach nuclear proteins such as SET/I2PP2A for binding in cells, and whether this interaction regulates only the nuclear functions of ceramide and PP2A to suppress growth and/or proliferation [102].
The connection between PP1 and ceramide in regulating the retinoblastoma (RB) protein has been reported previously [103,104]. RB plays a critical role in cell cycle regulation, and ceramide has been shown to dephosphorylate RB, leading to growth arrest in cancer cells. In addition, it was shown that the PP1-dependent mechanism of ceramide was required for the generation of proapoptotic splice variants of Bcl-x and caspase 9 in lung cancer cells. [104]. However, whether direct interaction between PP1 and ceramide is involved in the regulation of PP1 activity is unclear.
In addition to ceramide’s bioactive roles, its metabolite, ceramide-1-phosphate (C1P), has been shown to induce arachidonic acid release in lung cancer cells, and is regulated by the direct binding between C1P and cytosolic phospholipase A2 (cPLA2) (Table 1) [105]. It was later identified that the cationic b-groove of the C2 domain of cPLA2α was the interaction site for C1P [106]. Thus, it may be advantageous to produce inhibitors of ceramide kinase to block unwanted arachidonic acid formation and inflammation in cancer cells.
Importantly, endogenous protein targets of S1P in the nucleus or cytoplasm have been recently identified as HDAC1/2 or TRAF2, respectively [107,108], which might play important roles in regulating gene expression and nuclear factor NF-κB signaling in cancer cells (Table 1).
In summary, understanding the intracellular protein targets of sphingolipids that directly bind ceramide or its metabolites, such as S1P, C1P or sphingosine, which was recently shown to bind ANP32A to regulate PP2A [109], provides insight into the mechanistic details of proapoptotic versus prosurvival roles of these sphiongolipid molecules in various cancers (Table 1). With the development of new techniques to identify sphingolipid- binding proteins and their subcellular compartmentalization, the field of sphingolipid-based therapeutics will expand and provide a new avenue to combat cancer with specific molecular targeting of various important proteins, which are involved in the regulation of tumor growth and/or response to therapy.
S1P & S1P receptor signaling in the regulation of cancer growth &/or progression
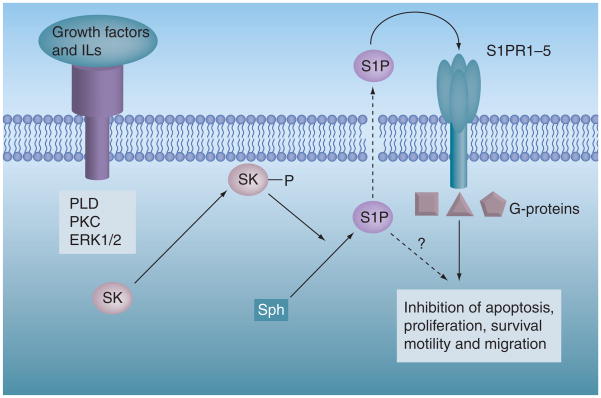
Metabolism of ceramide for the generation of S1P, a product of SK, leads to cancer cell proliferation and antiapoptosis [110,111]. Several growth factors and interleukins mediate the phosphorylation and translocation of SK1 from the cytoplasm to the plasma membrane [112]. Increased generation of S1P triggers signaling pathways that mediate these prosurvival processes mainly by engaging with S1P receptors 1–5 (S1PR1–5), usually in paracrine or autocrine modes (Figure 5) [2]. In numerous studies, SK/S1P have been shown to be tumor-promoting molecules, and elevated levels of these molecules have been observed in different cancer and tumor tissues. Overexpression of SK1 in MCF-7 cell inhibits apoptosis and blocks cell death induced by anticancer drugs, such as sphingosine and TNF-α [113]. When injected into mammary fat pads of ovariectomized nude mice implanted with estrogen pellets, MCF-7/SK1 cells formed more and larger tumors than vector transfectants with higher peripheral microvessel density. Moreover, downregulation of SK1 in MCF-7 cells reduced EGF- and serum-stimulated growth and enhanced sensitivity to DOX [113,114].
Figure 5. Sphingosine kinase/sphingosine-1-phosphate-mediated regulation of cellular functions.
Several growth factors and interleukins can activate SK by phosphorylation. Following activation, SK can translocate to the plasma membrane, where it phosporylates sphingosine to S1P. S1P is a bioactive lipid molecule, which can function as an intracellular messenger or is secreted out of the cell and coupled to its G-protein-coupled receptors (S1PRs) to mediate prosurvival, cell proliferation, tumor growth and/or metastasis.
PLD: Phospholipase D; S1P: Sphingosine-1-phosphate; S1PR: Sphingosine-1-phosphate receptor; SK: Sphingosine kinase.
The regulation of colon carcinogenesis via SK1/S1P in vivo was studied by Kawamori et al., who reported that 89% (42 out of 47) of human colon cancers had higher expression of SK1 compared with adjacent normal colon mucosa. In addition, high expression of SK1 was observed in adenomas and colon cancer metastases. Importantly, the homozygous SK1-knockout mice challenged with azoxymethane had reduced cancer incidences and had less aberrant crypt foci formation compared with wild-type mice [115].
Visentin et al. demonstrated that S1P is a potential therapeutic target itself, and a monoclonal antibody that binds to S1P with high affinity and specificity significantly slowed tumor progression and attenuated angiogenesis in several animal models of human cancer [116].
In the lung, the endothelial cells provide fluid homeostasis and adequate gas exchange by controlling vascular permeability between vascular content and the pulmonary interstitium. Increased vascular permeability is a characteristic of acute lung disease and sepsis and is an essential component of tumor metastasis and angiogenesis [117,118]. S1P protects the vascular barrier by engaging with the S1P1 receptor (at 0.5–1.0 μM) by activation of the Rho family of small GTPases, cytoskeleton reorganization, adherent- and tight-junction assembly and focal adhesion formation. However, in high concentrations (>5 μM), S1P increases the vascular barrier disruption by activating RhoA through ligation with S1P3 receptor [119–121]. In addition, Yamaguchi et al. reported that S1P differentially regulates Rac and hematogenous metastasis of melanoma cells positively or negatively via ligation through S1P1 or S1P2, respectively [122]. Activated PKC promotes cancer cell migration/invasion and inhibits apoptosis, which may exacerbate metastasis through S1PR-1 activation [123]. S1P5 receptor expression has been shown to cause proliferation and migration of human esophageal cancer cells [124]. By contrast, another published report indicated that migration of human ovarian cancer and human ovarian surface epithelial cells is mediated by S1P and not by expression of S1PR subtypes [125].
Overexpression of SK1 results in malignant transformation and tumor formation in immortalized 3T3 fibroblasts [126]. Increased S1P promotes proliferation and survival in human glioma and breast cancer cells [127]. Additionally, in endothelial and smooth muscle cells, SK1/S1P/S1PR signaling also has several specific effects on the promotion of vascular development and angiogenesis [128]. Importantly, in ovarian cancer patient samples, elevated S1P is found in the serum, and S1PR1 has been recently identified as a requirement for tumor angiogenesis in vivo [125]. In addition, SK1 mRNA and protein were significantly elevated in tumors compared with normal tissues obtained from patients with various cancers [129,130].
Sphingosine-1-phosphate generation can also be catalyzed by SK2, which is localized mainly to the nucleus [131]. The role of SK2 in the regulation of survival and apoptosis has been elusive. However, recent data suggest that, whereas endogenous SK2 also promotes survival by direct involvement of S1P for the regulation of HDAC1 and HDAC2 [107] and induces cell proliferation, overexpressed SK2 is proapoptotic, and this exogenously expressed SK-2 might not be effectively targeted to the nucleus, leading to the release of its BH3-like domain into the cytosol [131]. This might explain its proapoptotic activity in studies, in which it is exogenously introduced into the cells. In addition, Weigert et al. identified a novel mechanism of S1P production by apoptotic cells [132]. It was shown that during cell death, SK2 is cleaved by caspase 1, and a truncated active version of SK2 was released from the cell, which is associated with the exposure of phosphatidylserine [132]. Further studies analyzed the toxicity of S1P in terminally differentiated post-mitotic neurons using S1P lyase-deficient mice. Accumulation of S1P above a certain threshold induces neuronal apoptosis, which was induced only by S1P derived from exogenous S1P that was dephosphorylated and then resynthesized to S1P by SK2 [133]. These interesting data further confirm the importance of subcellular localization of S1P metabolism in the regulation of cancer cell growth and/or apoptosis.
Moreover, there are important and promising therapeutic implications for targeting SK-1 in cancer cells using small-molecule inhibitors. Several studies showed that a small-molecule inhibitor of SK1 and SK2 could slow down tumor growth, as well as sensitize cancer cells to many chemotherapeutic agents [134]. Recently, it was shown that the induction of autophagy could be a major mechanism for tumor cell killing by an SK2-specific inhibitor (ABC294640) in A-498 kidney carcinoma, PC-3 prostate and MDA-MB-231 breast adenocarcinoma cells [135]. Therefore, SK is a potentially promising therapeutic target in cancer.
Roles of ceramide & S1P in autophagy: paradox of autophagy in cancer cell survival or death
Although the molecular mechanisms by which ceramide and S1P regulates cell growth and/or proliferation have been extensively studied, recent studies indicate a role for these bioactive lipids in autophagy. Macroautophagy (referred to here as autophagy) is the intracellular lysosomal bulk degradation of long-lived proteins and damaged organelles, which can be either protective or lethal for cells. In a protective sense, the autophagic pathway is used by the cell to overcome nutrient deprivation by degrading damaged organelles, such as mitochondria, and recycling amino acids to minimize energy-requiring processes. However, autophagy has also been characterized as a functional cell death pathway, known as type II cell death, alongside apoptosis or type I cell death.
The autophagy pathway is controlled by autophagy-related genes (Atg). Atg proteins are regulated through mTOR. mTOR inhibits Atg protein induction, which can be blocked by rapamycin. mTOR regulates signaling through class I PI3K from the insulin receptor. Additionally, amino acid deprivation has been suggested to control the upregulation of Atg proteins through an unknown mechanism. Furthermore, class III PI3K and Beclin 1 seem to be important for the induction of Atg proteins [136].
Reports indicate that autophagic cell death plays a critical role in the development of certain cancers [137]. Moreover, dysfunctional autophagic pathways are an integral part of various lysosomal storage diseases, muscular disorders, neurodegeneration, pathogenesis and aging [138]. Reports from the literature suggest that bioactive sphingolipids, such as ceramide and S1P, are critical mediators of autophagy.
Several recent studies suggest that ceramide-induced autophagy is a protective mechanism, whereby cells circumvent energetic stress and maintain viability. An initial report by Guenther et al. suggested that C2-ceramide downregulates nutrient transporters, resulting in nutrient stress that led to induction of autophagy and nonapoptotic cell death, which was prevented with addition of methyl pyruvate [139]. Moreover, Guenther et al. reported that incubating cells in high levels of growth factors to increase metabolic demand sensitized cells to ceramide-induced starvation followed by cell death. Interestingly, a mouse embryonic fibroblast cell line deficient in Atg5, a crucial protein for autophagy, had increased sensitivity to ceramide-induced cell death [139]. Recently, an increase in long-chain ceramides through the downregulation of CerS2 has been shown to arrest growth with activation of PKR-like ER kinase and inositol-requiring enzyme 1 pathways, indicating that autophagy was protective in the unfolded protein response [42]. In another study, resveratrol served as a dihydroceramide desaturase inhibitor in HGC-27 cells, resulting in elevated dihydroceramide, which induced autophagy without cell death [140]. Furthermore, a study by Separovic et al. indicated the downregulation of Atg7 in MCF-7 cells responded to photodynamic therapy with both increased cell death and elevated ceramide [141]. In essence, these reports indicate autophagy induction is a protective response to ceramide-induced stress.
Findings from Beljanski et al. suggest that a SK2-selective inhibitor (ABC294649) induces nonapoptotic cell death, which is preceded by induction of autophagy [135]. Furthermore, they reported that simultaneous exposure of A-498 cells to ABC294649 and 3-methyladenine, an inhibitor of autophagy, decreased the potency of the SK-2 inhibitor and switched the mechanism of toxicity to apoptotic cell death, indicating that autophagy is a major mechanism of tumor cell killing by this compound. Results from Yacoub et al. also suggest that autophagy may be lethal in some instances [142]. These groups’ findings indicate that overexpression of melanoma differentiation-associated gene-7 (mda-7)/IL-24 induces ER stress responses, which triggers production of ceramide, Ca2+ and ROS that, in turn, promotes glioma cell autophagy and cell death [142].
Mechanistically, the regulation of autophagy by sphingolipid signaling is not completely understood. Beljansky et al. suggest that the buildup of sphingosine or ceramide induces tumor suppression by autophagic cell death in vivo via a SK2 selective inhibitor [135]. Conversely, Lavieu et al. report that upregulation of SK1 induces protective autophagy differing from lethal autophagy by a lack of Beclin-1 induction and mTOR inhibition [143].
Findings in the literature regarding lethal autophagy report that ER stress is the upstream regulator of ceramide/dihydroceramide production, leading to subsequent Ca2+ induction and mitochondrial dysfunction [144]. Interestingly, increases in ceramide induce Beclin-1-dependent autophagic cell death, which is mediated by c-Jun NH2-terminal kinase (JNK) [145]. The addition of short-chain ceramides and stimulation of the de novo pathway by tamoxifen treatment have been shown to activate JNK-1, which phosphorylates Bcl-2, resulting in the dissociation of the autophagy protein Beclin-1 and Bcl-2, thereby inducing autophagy [146].
Park et al. suggest that ceramide–CD95 signaling mediates cell death through caspase-8 activation; however, the knockdown of Atg5 increased cell death, indicating that autophagy was protective [147]. Importantly, these reports suggest that sphingolipids are involved in mediating two complex autophagic pathways with opposing functions involved in cell survival or death. Nevertheless, the mechanism by which these pathways are mediated remains unclear, is of critical importance to the fate of the cell and thus need to be further elucidated.
Sphingolipid-based anticancer therapeutics
The use of ceramide analogues or mimetics could also promote apoptotic and/or autophagic pathways in cancer cells [148–150]. However, decreased solubility and bioavailability of these ceramide analogues, especially ceramides with long-chain fatty acids, present problems for their delivery as chemotherapeutic agents in vivo. To overcome these problems, varied-chain pyridinium ceramides (e.g., C6-, C16- or C18-pyridinium ceramides) have been synthesized with increased water solubility and cell membrane permeability (Table 2) [149,150]. Positively charged ceramides have been shown to increase inner membrane permeability owing to the activation of specific ion transporters [148], and cause release of cytochrome C. In HNSCC treatment with highly soluble cationic pyridinium ceramide, such as L-thereo-C6-Pyr-ceramide, causes inhibition of xenograft tumor growth in SCID mice. Combination of L-thereo-C6-Pyr-ceramide treatment with chemotherapeutic agents, such as gemcitabine, showed enhanced HNSCC tumor suppression in mice. This combination therapy resulted in a 2.5-fold increase in efficacy over 5-fluorouracil/cisplatin treatment [149]. In addition, these novel pyridinium ceramides have been shown to inhibit telomerase activity, concomitant with G0/G1 cell cycle arrest [149,150].
Table 2.
Sphingolipid-based anticancer therapeutics.
| Compound | Mechanism of action/target | Cancer type |
|---|---|---|
| Ceramidoids (Pyr-ceramides) | Mitochondrial targeting | HNSCC, lung and breast |
| 4,6,-diene-ceramide | Ceramide analog | Breast |
| C16-serinol | Ceramide analog | Neuroblastoma |
| B13 and its derivatives | A-CDase inhibitor | Prostate, colon and HNSCC |
| d-MAPP | A- and N-CDase inhibitor | Squamous cell carcinoma |
| PPMP or PPPP | GCS inhibitors | Solid tumors |
| DMS | SK inhibitor | Leukemia, colon and breast |
| Anti-S1P-mAb | Binds S1P | Solid tumors |
| PEGylated lyposomes with ceramide (nanoparticles) | Improved delivery | Breast |
| Vincristine in SM-liposomes (sphigosomes) | Improved delivery | Leukemia (ALL) |
| Safingol (l-thereo-dihydrosphingosine) | SK inhibitor | Solid tumors |
| FTY-720 | Myriocin analog | Bladder, prostate, breast and lymphoma |
ALL: Acute lymphoblastic leukemia; CDase: Ceramidase; D-MAPP: D-erythro-2-(N-myristoylamino)-1-phenyl-1-propanol; DMS: Dimethyl sphingosine; GCS: Glucosylceramide synthase; HNSCC: Head and neck squamous cell carcinoma; PPMP: 1-phenyl-2-palmitoylamino-3-morpholino-1-propanol; PPPP: 1-phenyl-2-hexadecanoylamino-3-pyrrolidino-1-propanol; S1P: Sphingosine-1-phosphate; SK: Sphingosine kinase; SM: Sphingomyelin.
Liposome-mediated delivery of C6-ceramide has been shown to be an effective drug delivery method. Liposomal delivery enhances inter-bilayer transfer upon transient liposome/cell interactions, thereby promoting efficient delivery into cells. Use of liposomal C6-ceramide targets nuclear accumulation in MDA human breast cancer cells. Moreover these targeted C6-ceramides can regulate Akt phosphorylation and activate caspase 3/7 to induce apoptosis [151].
The PEGylated form of C6-ceramide encapsulated in liposomes has been shown to be effective against murine mammary adenocarcinoma cells. Systemic delivery of liposomal C6-ceramide causes cytotoxicity in solid tumors in a murine breast adenocarcinoma model using syngenic BALB/c mice. In addition, liposomal C6-ceramides have antitumor activities in human xenograft tissues in nude mice [152]. Polymeric nanoparticles are also used to deliver ceramides against a multidrug- resistant SKOV3 ovarian cancer cell line to induce apoptotic cell killing [153].
A novel ceramide analog generated by modifying the degree and position of the unsaturation of bonds led to the development of (2S,3R)-(4E,6E)-2-octanoyl amido decadiene-1,3-diol (4,6-diene-Cer), which contains a trans double bond at C6–C7 of the sphingoid backbone. This novel 4,6-diene ceramide is effective in inducing apoptosis in MCF-7 breast cancer cell lines [154].
The cationic C8-ceramide analogue harboring a new amide functional group in the sphingosine backbone (replacing the allyl alcohol group) has been effective against endocrine-resistant MDA–MB-231 and chemoresistant MCF-7 TN-R breast cancer cell lines. This analogue inhibits clonogenic survival and induces apoptosis [155]. Other ceramide analogs, such as 5R-OH-3E-C8-ceramide, adamantyl ceramide and benzene-C4-ceramide, have been shown to be effective against SKBr3, MCF-7/Adr drug-resistant breast cancer cells [156].
An alternative strategy to induce ceramide formation is to inhibit ceramidase. Inhibition of ceramidase leads to ceramide accumulation, thereby causing apoptotic cell death in cancerous cells. B13, an inhibitor of acid ceramidase, has been shown to induce cell death in cultured prostate cancer cells. In addition, B13 increased sensitivity of androgen-insensitive xenografts to radiation therapy and was also shown to decrease tumor volume [157]. Pharmacological inhibition of acid ceramidase with N-oleoyl ethanolamine enhanced ceramide generation, thereby sensitizing hepatoma cells (HepG2, Hep3B and Hepa 1C1C7) to daunorubicin-induced cell death [16]. In addition, LC204, a lysomotropic analog inhibitor of acid ceramidase, restores ceramide levels in prostate cancer cells [158].
FTY-720, a sphingosine-based immunosuppressant, exhibiting successful outcomes against multiple sclerosis in the clinic, has been shown to alter CerS activity. FTY-720 has been shown to inhibit ceramide synthesis at high sphinganine concentrations in vivo [159], whereas it has also been shown to inhibit CerS activity competitively [160]. FTY-720 also induces ROS generation, which leads to increased PKC activation and subsequent caspase 3 activation in hepatocellular carcinomas [161]. In addition, as a combination therapy, FTY-720 is used as an add-on to dimethyl sphingosine against breast cancer cell lines (MCF-7, MDA–MB-231 and SK-Br-3). Use of FTY-720 downregulates phospho-ERK without altering p38. This effect was not observed when a phosphorylated form of FTY-720 was used, indicating that antiproliferative effects may not depend on endogenous or exogenous phosphorylaion. Similar effects were observed in colon cancer cells HCT-116 and SW620 [162]. Furthermore, pharmacologic doses of FTY-720 induced apoptosis and impaired clonogenicity in blast crisis chronic myelogenous leukemia and Philadelphia chromosome-positive acute lymphocytic leukemia, the fatal BCR/ABL-driven leukemias [163]. FTY-720 is also found to be effective against lung tumor development in BALB/c mice [164]; however, mechanisms of antitumor functions of FTY-720, which are believed to be S1P receptor-independent, remain unknown.
Roles of sphingolipids in drug resistance
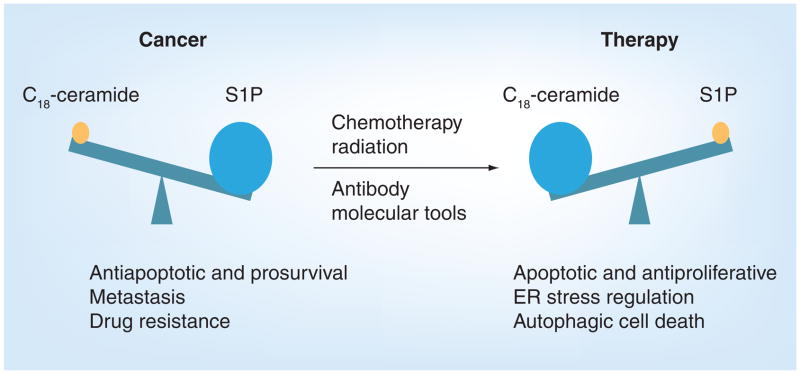
Previous studies suggest that understanding the intrinsic mechanisms of action for ceramide and S1P can open doors to new therapies to battle cancer [165,166]. It has been well established that increases in intracellular ceramides, based on their fatty acid chain lengths, will promote apoptosis [167,168]. Thus, finding ways to intrinsically elevate ceramide in cancer cells will be beneficial. Conversely, S1P has been shown to promote proliferation and prevent drug-induced apoptosis; thus, suppression of its generation and/or accumulation could inhibit tumor growth and overcome drug resistance (Figure 6).
Figure 6. Ceramide/sphingsine-1-phosphate rheostat in cancer and treatment.
In pathological conditions such as cancer, decreased levels of ceramide and elevated levels of its metabolite S1P were observed, which favors inhibition of programmed cell death, induction of metastasis and drug resistance. Upon treatment, ceramide levels increase and S1P levels decrease. These changes regulate apoptosis, ER stress and/or autophagic cell death.
ER: Endoplasmic reticulum; S1P: Sphingosine-1-phosphate.
Recent studies indicate that one of the mechanisms of resistance that cancer cells develop against chemotherapy is the alteration of ceramide accumulation. In fact, ceramide is highly metabolized into GlcCer owing to an increase in GlcCer synthase (GCS) activity and/or expression in some cancer cells [169,170]. This phenomenon has been implicated in the development of drug resistance in various cancer cell types, especially in breast cancer cells. Recent data demonstrated that knockdown of GCS expression with siRNA significantly inhibits the expression of MDR1, a gene that encodes for P-glycoprotein (P-gp) and reverses drug resistance [169,170]. Interestingly, several members of the ATP-binding cassette transporter family are implicated in the translocation of phospholipids and sphingolipids across the lipid bilayer, and P-gp has been proposed as a specific transporter for GlcCer that translocates this molecule across the Golgi to deliver it for the synthesis of neutral glycosphingolipids [171]. Thus, P-gp and GCS appear to function in the same pathway of ceramide/GlcCer metabolism, and this may provide an important link for the function of GCS in drug resistance.
These results also suggest that inhibitors of GCS may be useful in preventing resistance to chemotherapy. For example, combinations of fenretinide (4-HPR), which is known to elevate ceramide and dihydroceramide, with inhibitors of GCS or SK, such as 1-phenyl-2-palmitoylamino-3-morpholino-1-propanol or safingol (L-thereo-dihydrosphingosine), were reported to synergistically suppress the growth of various human cancer cells [172,173].
Roles of SK1/S1P & S1P receptor signaling in drug resistance
Sphingosine kinase 1/S1P signaling protects cancer cells from chemotherapy-induced apoptosis [174,175]; therefore, changes in sphingolipid metabolism play functional roles in conferring drug resistance. For example, in prostate adenocarcinoma, SK1 attenuates drug-induced apoptosis and serves as a chemotherapy sensor both in culture and in animal models [174]. In parallel with these data, increasing the expression of SK1 reduced the sensitivity of A-375 melanoma cells to Fas- and ceramide-mediated apoptosis, which could be reversed by the inhibition of SK-1 expression [175]. In addition, high expression levels of SK1 and S1P were detected in camptothecin-resistant PC-3 prostate cancer cells, and inhibition of SK1 expression or S1P signaling significantly inhibited PC-3 cell growth in response to camptothecin [175]. There are also antagonists against S1PRs, some of which also exhibit antiproliferative effects [176,177].
Interestingly, the elevated expression of SK1 and the increased levels of S1P have been observed in many types of cancers, such as colon, breast, uterus, kidney and lung, among others [115,116]. The analysis of tissue samples from healthy individuals and from patients with endometrial carcinoma revealed that SK1 activity was increased 2.5-fold in tissues from cancer patients compared with tissues from normal subjects. Moreover, S1P levels in cancer tissues were 1.6-fold higher compared with control tissues. Similarly, S1P levels in the plasma were also elevated [178]. In a microarray analysis of 1269 tumor samples from different subtypes of breast cancer, SK1 expression was found to be elevated in cancer patient tissues, and its higher expression was correlated to poor prognosis and promotion of metastasis [179]. SK1 also seems to act as an oncogene in erythroleukemia. A microarray transcriptome analysis of proerythroblasts from erythroleukemic transgenic mice showed that SK1 is transcriptionally upregulated, showing an association with the tumorigenic phenotype. Furthermore, when SK1 was overexpressed in nontumorigenic proerythroblasts, an increase in tumorigenicity and resistance to apoptosis was observed [180]. These data hint to the fact that overexpression of SK1 might be an event required for the progression of erythroleukemia.
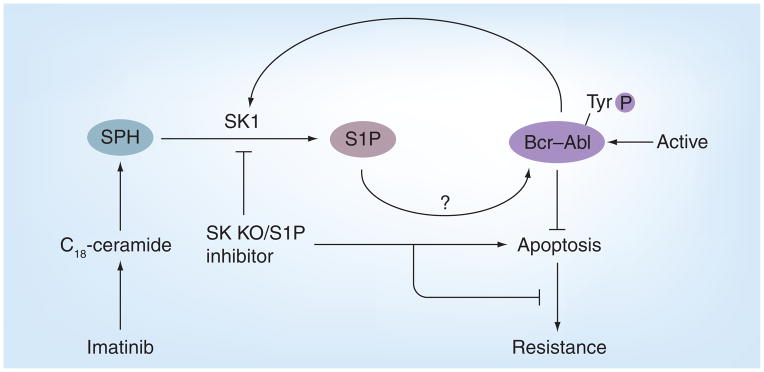
A study performed utilizing prostate cancer cells established a correlation between SK1 activity and resistance to irradiation. The SK1 activity was not affected by ionizing radiation in radiation-resistant LNCaP cells, while the activity was inhibited in radiation-sensitive human prostate cancer cells [181]. Moreover, the role of SK1 in mediating chemotherapy resistance in prostate cancer cells was also established by the use of cell lines that exhibit opposite sensitivities to chemotherapeutic agents. When docetaxel and camptothecin were utilized to treat these cell lines, SK1 was observed to be inhibited, and the ceramide:S1P ratio, known to be critical in determining the fate of the cell, was elevated only in docetaxel-sensitive cells when compared with resistant cells. By contrast, the overexpression of SK1 in both of these cell lines abolished the efficacy of the drug by decreasing the ceramide:S1P ratio/balance (rheostat) [57]. In addition, the importance of the ceramide/S1P rheostat in mediating imatinib resistance was further established in chronic myeloid leukemia (CML) cells (Figure 7). Upon imatinib treatment, K562 CML-sensitive cells showed an increased generation of endogenous ceramides, specifically C18-ceramide, while ceramide generation in K562 imatinib-resistant cells was minimal. Interestingly, SK1 mRNA and protein levels were increased in these drug-resistant cells, elevating S1P. The partial knockdown of SK1 was able to resensitize these cells to imatinib treatment by altering the ceramide/S1P rheostat, and the subsequent increase of caspase-3 activation [57]. Thus, these data support the alterations of the ceramide/S1P rheostat via overexpression of SK1 with increased S1P generation/signaling mediating imatinib-resistance in CML cells.
Figure 7. Sphingosine kinase/sphingosine-1-phosphate-mediated imantinib resistance of chronic myeloid leukemia cells.
The overexpression of SK1 in Bcr–Abl-positive cells leads to alteration of the levels of S1P and ceramide, which induces the stabilization/activation of Bcr–Abl, leading to the inhibition of apoptosis and drug resistance in chronic myeloid leukemia.
KO: Knockout; S1P: Sphingosine-1-phosphate; SK: Sphingosine kinase;
SPH: Sphingosine.
The involvement of SK1 in mediating drug resistance was also established in MCF-7 breast cancer cells. The forced overexpression of SK1 in MCF-7 cells caused promotion of cell proliferation and resistance to tamoxifen-induced apoptosis. After prolonged exposure of MCF-7 to tamoxifen, tamoxifen-resistant clones were selected. Interestingly, resistant cells showed increased levels of SK1 expression compared with sensitive cells. The inhibition of SK1 by a specific inhibitor was able to resensitize MCF-7 cells to tamoxifen-induced apoptosis [182].
Sphingosine-1-phosphate elicits most of its prosurvival effects by acting in an autocrine and/or paracrine fashion through a family of five G-protein-coupled receptors, termed S1PR1–5 [128]. Three receptors, S1PR2, 4 and 5, are located on chromosome 19, while S1PR1 and S1PR3 are found on chromosome 1 and 9, respectively [183]. These receptors are coupled to different heterotrimeric subsets of G-proteins (Gi, Gq and G12/13), therefore mediating a variety of cellular functions. Interestingly, the involvement of specific receptors in many cancer types has also been shown, suggesting the use of specific S1PR antagonists as a novel therapeutic approach. For instance, S1PR2 was shown to be involved in hypoxia-triggered pathological angiogenesis of the mouse retina. S1PR2−/− mice exposed to ischemia-induced retinopathy demonstrated decreased neovascularization in the vitreous chamber compared with S1PR2+/+ mice. In addition, S1PR2−/− mice showed decreased levels of cyclooxygenase-2 (COX-2) proinflammatory enzyme and elevated levels of endothelial nitric oxide synthase, suggesting a role for S1PR2 in mediating inflammatory processes that are important for retinal angiogenesis [184].
Sphingosine-1-phosphate receptor 2 was shown to have an important role in esophageal cancer, as well by modulating TGF-induced migration and invasion via signaling of SK1-generated S1P [185]. Moreover, signaling by S1PR2 was shown to be important in Wilms tumor, the most common malignant renal tumor in children. Studies demonstrated that S1P induced COX-2 mRNA and protein expression in WiT49 Wilms tumor cells in a concentration- dependent manner. The mRNA levels of S1PR1, 2, 3 and 5, were expressed in Wilms tumor specimens. Importantly, S1P modulation of COX-2 was shown to be S1PR2-dependent only, since overexpression or downregulation of S1PR2 increased or decreased COX-2 mRNA and protein expression, respectively. Moreover, treatment with the specific S1PR2 antagonist JTE-013 completely blocked S1P-induced COX-2 expression in WiT49 cells overexpressing S1PR2 [186]. In glioblastoma multiforme, a CNS tumor, the S1P receptors, specifically S1PR1, 2 and 3, were involved in inducing glioma proliferation. S1PR1 and S1PR3 were shown to promote glioma cell migration and invasion. S1PR2 was demonstrated to enhance glioma invasion through the increased expression of CCN1/Cyr61, a matri-cellular protein shown to be involved in tumor cell adhesion, invasion and angiogenesis [187].
Interestingly, the inhibition of SK1 by the use of an inhibitor, SK1-I, was able to reduce growth and suppress migration and invasion through the inhibition of signaling pathways involving Akt and its targets p70S6K and GSK3-α [188,189]. Thus, these studies highlight the roles of SK1/S1P/S1PRs in promoting cancer and suggest novel therapeutic approaches that include the use of specific SK1 and S1PRs inhibitors and antagonists.
Conclusion & future perspective
Although recent advances that provide important molecular and analytical tools to study sphingolipids add another layer of complexity to the field regarding the importance of fatty acid chain lengths, subcellular localization and/or protein targets of sphingolipids, it has become more evident now that these bioactive sphingolipid molecules play important roles in cancer pathogenesis and therapeutics. Reverse translational studies indicate that regulation of ceramide generation, or expression of enzymes of sphingolipid metabolism in several types of tumor tissues, are altered. Reconstitution of ceramides using small-molecule ceramide mimetics or analogues, or inhibitors of enzymes of ceramide metabolism, has been successful in terms of the development of novel targets for anticancer therapeutics. By contrast, SK and S1P are important players in antiapoptosis, neovascularization and inflammation. Recent studies also identified Spns2 as a novel S1P transporter [190], which might help define mechanisms of S1P transport in and out of cancer cells. In addition, animal studies suggest possible functions for sphingolipids in chemo-prevention, which are well studied in colon cancer models [191]. Development of knockout models for sphingolipid-metabolizing enzymes, such as S1P-lyase(−/−) mice [192], will also be extremely helpful to delineate their roles in development, as well as in cancer pathogenesis and/or therapy.
There are also some limitations remain in this exciting field. For example, our understanding of individual cancer subtypes and their complex molecular circuitry, including networks of sphingolipid metabolism and their subcellular compartmentalization, coupled with inherent experimental difficulties in studying membrane-bound enzymes of lipid metabolism, is still limited and needs further development.
Nevertheless, an enormous growth of sphingo-lipid research will be anticipated in the coming years, which will produce numerous advances in dissecting the roles and mechanisms of action of bioactive sphingolipids in the regulation of cancer pathogenesis, progression and/or metastasis. It is also anticipated that further advances in sphingolipidomics, in terms of both analytical and synthetic lipidomics, will define diagnostic or prognostic sphingolipid cancer markers and develop novel therapeutic and prevention strategies against various human cancers.
Acknowledgments
We apologize to those investigators whose important studies could not be cited here because of space limitations. We would like to thank Jennifer Schnellmann for editorial review of this manuscript.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
This work was supported by research grants (CA088932, DE016572 and CA097132) from the NIH. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Yu RK, Law JH. Herbert Edmund Carter 1910–2007 – A Biographical Memoir. National Academy of Sciences; Washington, DC, USA: 2009. [Google Scholar]
- 2.Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer. 2004;4:604–616. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- 3.Futerman AH, Hannun YA. The complex life of simple sphingolipids. EMBO Rep. 2004;5:777–782. doi: 10.1038/sj.embor.7400208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hannun YA, Obeid LM. The ceramidecentric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. J Biol Chem. 2002;277:25847–25850. doi: 10.1074/jbc.R200008200. [DOI] [PubMed] [Google Scholar]
- 5.Andrieu-Abadie N, Levade T. Sphingomyelin hydrolysis during apoptosis. Biochim Biophys Acta. 2002;1585:126–134. doi: 10.1016/s1388-1981(02)00332-3. [DOI] [PubMed] [Google Scholar]
- 6.Clarke CJ, Hannun YA. Neutral sphingomyelinases and nSMase2: bridging the gaps. Biochim Biophys Acta. 2006;1758:1893–1901. doi: 10.1016/j.bbamem.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 7.Clarke CJ, Snook CF, Tani M, et al. The extended family of neutral sphingomyelinases. Biochemistry. 2006;45:11247–11256. doi: 10.1021/bi061307z. [DOI] [PubMed] [Google Scholar]
- 8.Dolgachev V, Farooqui MS, Kulaeva OI, et al. De novo ceramide accumulation due to inhibition of its conversion to complex sphingolipids in apoptotic photosensitized cells. J Biol Chem. 2004;279:23238–23249. doi: 10.1074/jbc.M311974200. [DOI] [PubMed] [Google Scholar]
- 9.Reynolds CP, Maurer BJ, Kolesnick RN. Ceramide synthesis and metabolism as a target for cancer therapy. Cancer Lett. 2004;206:169–180. doi: 10.1016/j.canlet.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 10.Merrill AH, Jr, Wang E, Mullins RE. Kinetics of long-chain (sphingoid) base biosynthesis in intact LM cells: effects of varying the extracellular concentrations of serine and fatty acid precursors of this pathway. Biochemistry. 1988;27:340–345. doi: 10.1021/bi00401a051. [DOI] [PubMed] [Google Scholar]
- 11.Kang MS, Ahn KH, Kim SK, et al. Hypoxia-induced neuronal apoptosis is mediated by de novo synthesis of ceramide through activation of serine palmitoyltransferase. Cell Signal. 2010;22:610–618. doi: 10.1016/j.cellsig.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 12.Michel C, van Echten-Deckert G, Rother J, et al. Characterization of ceramide synthesis A dihydroceramide desaturase introduces the 4, 5-trans-double bond of sphingosine at the level of dihydroceramide. J Biol Chem. 1997;272:22432–22437. doi: 10.1074/jbc.272.36.22432. [DOI] [PubMed] [Google Scholar]
- 13▪.Kraveka JM, Li L, Szulc ZM, et al. Involvement of dihydroceramide desaturase in cell cycle progression in human neuroblastoma cells. J Biol Chem. 2007;282:16718–16728. doi: 10.1074/jbc.M700647200. Demonstrates a functional role of dihydroceramide in the inhibition of growth in cancer cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stefanic S, Spycher C, Morf L, et al. Inhibition of glucosylceramide synthesis affects cell cycle progression, membrane trafficking and stage differentiation in the minimized protozoan Giardia lamblia. J Lipid Res. 2010;51(9):2527–2545. doi: 10.1194/jlr.M003392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tafesse FG, Ternes P, Holthuis JC. The multigenic sphingomyelin synthase family. J Biol Chem. 2006;281:29421–29425. doi: 10.1074/jbc.R600021200. [DOI] [PubMed] [Google Scholar]
- 16.Morales A, Paris R, Villanueva A, et al. Pharmacological inhibition or small interfering RNA targeting acid ceramidase sensitizes hepatoma cells to chemotherapy and reduces tumor growth in vivo. Oncogene. 2007;26:905–916. doi: 10.1038/sj.onc.1209834. [DOI] [PubMed] [Google Scholar]
- 17.Park JH, Schuchman EH. Acid ceramidase and human disease. Biochim Biophys Acta. 2006;1758:2133–2138. doi: 10.1016/j.bbamem.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 18▪▪.Hanada K, Kumagai K, Yasuda S, et al. Molecular machinery for non-vesicular trafficking of ceramide. Nature. 2003;426:803–809. doi: 10.1038/nature02188. Demostrates the trafficking of ceramide from the endoplastic reticulum (ER) to the Golgi by nonvesicular-mediated transporters. [DOI] [PubMed] [Google Scholar]
- 19.Kumagai K, Yasuda S, Okemoto K, et al. CERT mediates intermembrane transfer of various molecular species of ceramides. J Biol Chem. 2005;280:6488–6495. doi: 10.1074/jbc.M409290200. [DOI] [PubMed] [Google Scholar]
- 20.Kudo N, Kumagai K, Tomishige N, et al. Structural basis for specific lipid recognition by CERT responsible for nonvesicular trafficking of ceramide. Proc Natl Acad Sci USA. 2008;105:488–493. doi: 10.1073/pnas.0709191105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nylund M, Kjellberg MA, Molotkovsky JG, et al. Molecular features of phospholipids that affect glycolipid transfer protein-mediated galactosylceramide transfer between vesicles. Biochim Biophys Acta. 2006;1758:807–812. doi: 10.1016/j.bbamem.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 22.D’Angelo G, Polishchuk E, Di Tullio G, et al. Glycosphingolipid synthesis requires FAPP2 transfer of glucosylceramide. Nature. 2007;449:62–67. doi: 10.1038/nature06097. [DOI] [PubMed] [Google Scholar]
- 23.Bose R, Verheij M, Haimovitz-Friedman A, et al. Ceramide synthase mediates daunorubicin induced apoptosis: an alternative mechanism for generating death signals. Cell. 1995;82:405–414. doi: 10.1016/0092-8674(95)90429-8. [DOI] [PubMed] [Google Scholar]
- 24.Pewzner-Jung Y, Ben-Dor S, Futerman AH. When do lasses (longevity assurance genes) become CerS (ceramide synthases)?: insights into the regulation of ceramide synthesis. J Biol Chem. 2006;281:25001–25005. doi: 10.1074/jbc.R600010200. [DOI] [PubMed] [Google Scholar]
- 25.Jazwinski SM, Conzelmann A. LAG1 puts the focus on ceramide signaling. Int J Biochem Cell Biol. 2002;34:1491–1495. doi: 10.1016/s1357-2725(02)00044-4. [DOI] [PubMed] [Google Scholar]
- 26.Obeid LM, Hannun YA. Ceramide, stress, and a “LAG” in aging. Sci Aging Knowledge Environ. 2003;2003(39):PE27. doi: 10.1126/sageke.2003.39.pe27. [DOI] [PubMed] [Google Scholar]
- 27.Lee SJ. Expression of growth/differentiation factor 1 in the nervous system: conservation of a bicistronic structure. Proc Natl Acad Sci USA. 1991;88:4250–4254. doi: 10.1073/pnas.88.10.4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28▪▪.Venkataraman K, Riebeling C, Bodennec J, et al. Upstream of growth and differentiation factor 1 (UOG1), a mammalian homolog of the yeast longevity assurance gene 1 (LAG1), regulates N-stearoylsphinganine (C18-(dihydro)ceramide) synthesis in a fumonisin B1-independent manner in mammalian cells. J Biol Chem. 2002;277:35642–35649. doi: 10.1074/jbc.M205211200. First study that defined the fatty acid chain length specificity of ceramide synthesase (CerS)1 for the generation of C18-ceramide. [DOI] [PubMed] [Google Scholar]
- 29.Mizutani Y, Kihara A, Igarashi Y. Mammalian Lass6 and its related family members regulate synthesis of specific ceramides. Biochem J. 2005;390:263–271. doi: 10.1042/BJ20050291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kageyama-Yahara N, Riezman H. Transmembrane topology of ceramide synthase in yeast. Biochem J. 2006;398:585–593. doi: 10.1042/BJ20060697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schulz A, Mousallem T, Venkataramani M, et al. The CLN9 protein, a regulator of dihydroceramide synthase. J Biol Chem. 2006;281:2784–2794. doi: 10.1074/jbc.M509483200. [DOI] [PubMed] [Google Scholar]
- 32.Spassieva S, Seo JG, Jiang JC, et al. Necessary role for the Lag1p motif in (dihydro)ceramide synthase activity. J Biol Chem. 2006;281:33931–33938. doi: 10.1074/jbc.M608092200. [DOI] [PubMed] [Google Scholar]
- 33.Sridevi P, Alexander H, Laviad EL, et al. Ceramide synthase 1 is regulated by proteasomal mediated turnover. Biochim Biophys Acta. 2009;1793:1218–1227. doi: 10.1016/j.bbamcr.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sridevi P, Alexander H, Laviad EL, et al. Stress-induced ER to Golgi translocation of ceramide synthase 1 is dependent on proteasomal processing. Exp Cell Res. 2010;316:78–91. doi: 10.1016/j.yexcr.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siskind LJ, Mullen TD, Romero Rosales K, et al. The BCL-2 protein BAK is required for long-chain ceramide generation during apoptosis. J Biol Chem. 2010;285:11818–11826. doi: 10.1074/jbc.M109.078121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36▪▪.Koybasi S, Senkal CE, Sundararaj K, et al. Defects in cell growth regulation by C18,0-ceramide in longevity assurance gene 1 human head and neck squamous cell carcinomas. J Biol Chem. 2004;279:44311–44319. doi: 10.1074/jbc.M406920200. First study to demonstrate that downregulation of C18-ceramide is associated with human head and neck squamous cell carcinoma (HNSCC) pathogenesis and that reconstitution of CerS1/C18-ceramide inhibits HNSCC growth. [DOI] [PubMed] [Google Scholar]
- 37.Karahatay S, Thomas K, Koybasi S, et al. Clinical relevance of ceramide metabolism in the pathogenesis of human head and neck squamous cell carcinoma (HNSCC): attenuation of C18-ceramide in HNSCC tumors correlates with lymphovascular invasion and nodal metastasis. Cancer Lett. 2007;256:101–111. doi: 10.1016/j.canlet.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Senkal CE, Ponnusamy S, Rossi MJ, et al. Role of human longevity assurance gene 1 and C18, 0-ceramide in chemotherapy-induced cell death in human head and neck squamous cell carcinomas. Mol Cancer Ther. 2007;6:712–722. doi: 10.1158/1535-7163.MCT-06-0558. [DOI] [PubMed] [Google Scholar]
- 39▪▪.Senkal CE, Ponnusamy S, Bielawski J, et al. Antiapoptotic roles of ceramide-synthase-6-generated C16-ceramide via selective regulation of the ATF6/CHOP arm of ER-stress-response pathways. FASEB J. 2010;24:296–308. doi: 10.1096/fj.09-135087. Describes, for the first time, the involvement of CerS6/C16-ceramide in ER stress responses and prosurvival roles of CerS6-generated C16-ceramide in HNSCC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40▪▪.Deng X, Yin X, Allan R, et al. Ceramide biogenesis is required for radiation-induced apoptosis in the germ line of C. elegans. Science. 2008;322:110–115. doi: 10.1126/science.1158111. Describes the roles of Caenorhabditis elegans CerS and ceramides in the regulation of radiation-induced apoptosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Menuz V, Howell KS, Gentina S, et al. Protection of C elegans from anoxia by HYL-2 ceramide synthase. Science. 2009;324:381–384. doi: 10.1126/science.1168532. [DOI] [PubMed] [Google Scholar]
- 42.Spassieva SD, Mullen TD, Townsend DM, et al. Disruption of ceramide synthesis by CerS2 downregulation leads to autophagy and the unfolded protein response. Biochem J. 2009;424:273–283. doi: 10.1042/BJ20090699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mesicek J, Lee H, Feldman T, et al. Ceramide synthases 2, 5, and 6 confer distinct roles in radiation-induced apoptosis in HeLa cells. Cell Signal. 2010;22(9):1300–1307. doi: 10.1016/j.cellsig.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bauer R, Voelzmann A, Breiden B, et al. Schlank, a member of the ceramide synthase family controls growth and body fat in Drosophila. EMBO J. 2009;28:3706–3716. doi: 10.1038/emboj.2009.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Imgrund S, Hartmann D, Farwanah H, et al. Adult ceramide synthase 2 (CerS2)-deficient mice exhibit myelin sheath defects, cerebellar degeneration, and hepatocarcinomas. J Biol Chem. 2009;284:33549–33560. doi: 10.1074/jbc.M109.031971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pewzner-Jung Y, Brenner O, Braun S, et al. A critical role for ceramide synthase 2 in liver homeostasis: II Insights into molecular changes leading to hepatopathy. J Biol Chem. 2010;285:10911–23. doi: 10.1074/jbc.M109.077610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pewzner-Jung Y, Park H, Laviad EL, et al. A critical role for ceramide synthase 2 in liver homeostasis: I Alterations in lipid metabolic pathways. J Biol Chem. 2010;285:10902–10910. doi: 10.1074/jbc.M109.077594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schiffmann S, Sandner J, Birod K, et al. Ceramide synthases and ceramide levels are increased in breast cancer tissue. Carcinogenesis. 2009;30:745–752. doi: 10.1093/carcin/bgp061. [DOI] [PubMed] [Google Scholar]
- 49.Erez-Roman R, Pienik R, Futerman AH. Increased ceramide synthase 2 and 6 mRNA levels in breast cancer tissues and correlation with sphingosine kinase expression. Biochem Biophys Res Commun. 2009;391:219–223. doi: 10.1016/j.bbrc.2009.11.035. [DOI] [PubMed] [Google Scholar]
- 50.Schiffmann S, Sandner J, Schmidt R, et al. The selective COX-2 inihibitor celecoxib modulates sphingolipid synthesis. J Lipid Res. 2009;50:32–40. doi: 10.1194/jlr.M800122-JLR200. [DOI] [PubMed] [Google Scholar]
- 51.Gustafsson K, Sander B, Bielawski J, et al. Potentiation of cannabinoid-induced cytotoxicity in mantle cell lymphoma through modulation of ceramide metabolism. Mol Cancer Res. 2009;7:1086–1098. doi: 10.1158/1541-7786.MCR-08-0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Panjarian S, Kozhaya L, Arayssi S, et al. De novo N-palmitoylsphingosine synthesis is the major biochemical mechanism of ceramide accumulation following p53 upregulation. Prostaglandins Other Lipid Mediat. 2008;86:41–48. doi: 10.1016/j.prostaglandins.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 53.White-Gilbertson S, Mullen T, Senkal C, et al. Ceramide synthase 6 modulates TRAIL sensitivity and nuclear translocation of active caspase 3 in colon cancer cells. Oncogene. 2009;28:1132–1141. doi: 10.1038/onc.2008.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Truman JP, Rotenberg SA, Kang JH, et al. PKCα activation downregulates ATM and radio-sensitizes androgen-sensitive human prostate cancer cells in vitro and in vivo. Cancer Biol Ther. 2009;8:1–10. doi: 10.4161/cbt.8.1.7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Min J, Mesika A, Sivaguru M, et al. (Dihydro)ceramide synthase 1-regulated sensitivity to cisplatin is associated with the activation of p38 mitogen-activated protein kinase and is abrogated by sphingosine kinase 1. Mol Cancer Res. 2007;5:801–812. doi: 10.1158/1541-7786.MCR-07-0100. [DOI] [PubMed] [Google Scholar]
- 56.Park MA, Mitchell C, Zhang G, et al. Vorinostat and sorafenib increase CD95 activation in gastrointestinal tumor cells through a Ca2+- de novo ceramide-PP2A-reactive oxygen species-dependent signaling pathway. Cancer Res. 2010;70(15):6313–6324. doi: 10.1158/0008-5472.CAN-10-0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57▪.Baran Y, Salas A, Senkal CE, et al. Alterations of ceramide/sphingosine-1-phosphate rheostat involved in the regulation of resistance to imatinib-induced apoptosis in K562 human chronic myeloid leukemia cells. J Biol Chem. 2007;282:10922–10934. doi: 10.1074/jbc.M610157200. Demonstrates, for the first time, that the alterations of the ceramide/sphingosine-1-phosphate (S1P) rheostat mediates imatinib resistance in chronic myeloid leukemia cells. [DOI] [PubMed] [Google Scholar]
- 58.Santana P, Peña LA, Haimovitz-Friedman A, et al. Acid sphingomyelinase-deficient human lymphoblasts and mice are defective in radiation-induced apoptosis. Cell. 86:189–199. doi: 10.1016/s0092-8674(00)80091-4. [DOI] [PubMed] [Google Scholar]
- 59.Paris F, Fuks Z, Kang A, Capodieci P, et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293:293–297. doi: 10.1126/science.1060191. [DOI] [PubMed] [Google Scholar]
- 60.Garcia-Barros M, Paris F, Cordon-Cardo C, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155–1159. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 61.Bezombes C, Grazide S, Garret C, et al. Rituximab antiproliferative effect in B-lymphoma cells is associated with acid-sphingomyelinase activation in raft microdomains. Blood. 2004;104:1166–1173. doi: 10.1182/blood-2004-01-0277. [DOI] [PubMed] [Google Scholar]
- 62.Rosato RR, Maggio SC, Almenara JA, et al. The histone deacetylase inhibitor LAQ824 induces human leukemia cell death through a process involving XIAP downregulation, oxidative injury, and the acid sphingomyelinase-dependent generation of ceramide. Mol Pharmacol. 2006;69:216–225. doi: 10.1124/mol.105.017145. [DOI] [PubMed] [Google Scholar]
- 63.Smith EL, Schuchman EH. Acid sphingomyelinase overexpression enhances the antineoplastic effects of irradiation in vitro and in vivo. Mol Ther. 2008;16:1565–1571. doi: 10.1038/mt.2008.145. [DOI] [PubMed] [Google Scholar]
- 64.Marchesini N, Luberto C, Hannun YA. Biochemical properties of mammalian neutral sphingomyelinase 2 and its role in sphingolipid metabolism. J Biol Chem. 2003;278:13775–13783. doi: 10.1074/jbc.M212262200. [DOI] [PubMed] [Google Scholar]
- 65.Clarke CJ, Truong TG, Hannun YA. Role for neutral sphingomyelinase-2 in tumor necrosis factor α-stimulated expression of vascular cell adhesion molecule-1 (VCAM) and intercellular adhesion molecule-1 (ICAM) in lung epithelial cells: p38 MAPK is an upstream regulator of nSMase2. J Biol Chem. 2007;282:1384–1396. doi: 10.1074/jbc.M609216200. [DOI] [PubMed] [Google Scholar]
- 66.Clarke CJ, Guthrie JM, Hannun YA. Regulation of neutral sphingomyelinase-2 (nSMase2) by tumor necrosis factor-α involves protein kinase C-δ in lung epithelial cells. Mol Pharmacol. 2008;74:1022–1032. doi: 10.1124/mol.108.046250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kitatani K, Idkowiak-Baldys J, Hannun YA. The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell Signal. 2008;20:1010–1018. doi: 10.1016/j.cellsig.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sultan I, Senkal CE, Ponnusamy S, et al. Regulation of the sphingosine-recycling pathway for ceramide generation by oxidative stress, and its role in controlling c-Myc/Max function. Biochem J. 2006;393:513–521. doi: 10.1042/BJ20051083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Snook CF, Jones JA, Hannun YA. Sphingolipid-binding proteins. Biochim Biophys Acta. 2006;1761:927–946. doi: 10.1016/j.bbalip.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 70.Pettus BJ, Chalfant CE, Hannun YA. Ceramide in apoptosis: an overview and current perspectives. Biochim Biophys Acta. 2002;1585:114–125. doi: 10.1016/s1388-1981(02)00331-1. [DOI] [PubMed] [Google Scholar]
- 71.Zhang Y, Yao B, Delikat S, et al. Kinase suppressor of Ras is ceramide-activated protein kinase. Cell. 1997;89:63–72. doi: 10.1016/s0092-8674(00)80183-x. [DOI] [PubMed] [Google Scholar]
- 72.Kolch W. Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem J. 2000;351:289–305. [PMC free article] [PubMed] [Google Scholar]
- 73.Yin X, Zafrullah M, Lee H, et al. A ceramide-binding C1 domain mediates kinase suppressor of ras membrane translocation. Cell Physiol Biochem. 2009;24:219–230. doi: 10.1159/000233248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huwiler A, Brunner J, Hummel R, et al. Ceramide-binding and activation defines protein kinase c-Raf as a ceramide-activated protein kinase. Proc Natl Acad Sci USA. 1996;93:6959–6963. doi: 10.1073/pnas.93.14.6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Heinrich M, Wickel M, Schneider-Brachert W, et al. Cathepsin D targeted by acid sphingomyelinase-derived ceramide. EMBO J. 1999;18:5252–5263. doi: 10.1093/emboj/18.19.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abbott DE, Margaryan NV, Jeruss JS, et al. Reevaluating cathepsin D as a biomarker for breast cancer: serum activity levels versus histopathology. Cancer Biol Ther. 2010;1:23–30. doi: 10.4161/cbt.9.1.10378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Deiss LP, Galinka H, Berissi H, et al. Cathepsin D protease mediates programmed cell death induced by interferon-γ, Fas/APO-1 and TNF-α. EMBO J. 1996;15:3861–3870. [PMC free article] [PubMed] [Google Scholar]
- 78.De Stefanis D, Reffo P, Bonelli G, et al. Increase in ceramide level alters the lysosomal targeting of cathepsin D prior to onset of apoptosis in HT-29 colon cancer cells. Biol Chem. 2002;6:989–999. doi: 10.1515/BC.2002.106. [DOI] [PubMed] [Google Scholar]
- 79.Bourbon N, Yun J, Kester M. Ceramide directly activates protein kinase C ζ to regulate a stress-activated protein kinase signaling complex. J Biol Chem. 2000;275:35617–35623. doi: 10.1074/jbc.M007346200. [DOI] [PubMed] [Google Scholar]
- 80.Hanada K, Kumagai K, Tomishige N, et al. CERT-mediated trafficking of ceramide. Biochim Biophys Acta. 2009;1791:684–691. doi: 10.1016/j.bbalip.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 81.Swanton C, Marani M, Pardo O, et al. Regulators of mitotic arrest and ceramide metabolism are determinants of sensitivity to paclitaxel and other chemotherapeutic drugs. Cancer Cell. 2007;11:498–512. doi: 10.1016/j.ccr.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 82.Chalfant CE, Ogretmen B, Galadari S, et al. FAS activation induces dephosphorylation of SR proteins; dependence on the de novo generation of ceramide and activation of protein phosphatase 1. J Biol Chem. 2001;30:44848–44855. doi: 10.1074/jbc.M106291200. [DOI] [PubMed] [Google Scholar]
- 83.Kitatani K, Idkowiak-Baldys J, Bielawski J, et al. Protein kinase C-induced activation of a ceramide/protein phosphatase 1 pathway leading to dephosphorylation of p38 MAPK. J Biol Chem. 2006;281:36793–36802. doi: 10.1074/jbc.M608137200. [DOI] [PubMed] [Google Scholar]
- 84.Janssens V, Goris J, Van Hoof C. PP2A: the expected tumor suppressor. Curr Opin Genet Dev. 2005;15:34–41. doi: 10.1016/j.gde.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 85.Westermarck J, Hahn W. Multiple pathways regulated by the tumor suppressor PP2A in transformation. Trends Mol Med. 2008;14:152–160. doi: 10.1016/j.molmed.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 86▪.Dobrowsky RT, Kamibayashi C, Mumby M, et al. Ceramide activates heterotrimeric protein phosphatase 2A. J Biol Chem. 1993;268:15523–15530. Describes the activation of protein phosphatase 2A (PP2A) by ceramide. [PubMed] [Google Scholar]
- 87.Wolff RA, Dobrowsky RT, Bielawska A, et al. Role of ceramide-activated protein phosphatase in ceramide-mediated signal transduction. J Biol Chem. 1994;269:19605–19609. [PubMed] [Google Scholar]
- 88.Chalfant CE, Kishikawa K, Mumby MC, et al. Long chain ceramides activate protein phosphatase-1 and protein phosphatase-2A. J Biol Chem. 1999;274:20313–20317. doi: 10.1074/jbc.274.29.20313. [DOI] [PubMed] [Google Scholar]
- 89▪▪.Mukhopadhyay A, Saddoughi SA, Song P, et al. Direct interaction between the inhibitor 2 and ceramide via sphingolipid-protein binding is involved in the regulation of protein phosphatase 2A activity and signaling. FASEB J. 2009;23:751–763. doi: 10.1096/fj.08-120550. First study to describe a mechanism by which ceramide regulates PP2A activity via directly binding to its biological inhibitor SET/PP2A inhibitor 2 oncoprotein in human lung cancer cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang GG, Cai L, Pasillas MP, et al. NUP98-NSD1 links H3K36 methylation to Hox-A gene activation and leukaemogenesis. Nat Cell Biol. 2007;9:804–812. doi: 10.1038/ncb1608. [DOI] [PubMed] [Google Scholar]
- 91.Guo HB, Guo H. Mechanism of histone methylation catalyzed by protein lysine methyltransferase SET7/9 and origin of product specificity. Proc Natl Acad Sci USA. 2007;104:8797–8802. doi: 10.1073/pnas.0702981104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Firestein R, Cui X, Huie P, et al. Set domain-dependent regulation of transcriptional silencing and growth control by SUV39H1, a mammalian ortholog of Drosophila Su(var)3–9. Mol Cell Biol. 2000;20:4900–4909. doi: 10.1128/mcb.20.13.4900-4909.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93▪▪.Neviani P, Santhanam R, Trotta R, et al. The tumor suppressor PP2A is functionally inactivated in blast crisis CML through the inhibitory activity of the Bcr/Abl-regulated SET protein. Cancer Cell. 2005;8:355–368. doi: 10.1016/j.ccr.2005.10.015. First study to describe the role for PP2A in the regulation of Bcr–Abl degradation and drug resistance in chronic myeloid leukemia. [DOI] [PubMed] [Google Scholar]
- 94.Kawano M, Kumagai K, Nishijima, et al. Efficient trafficking of ceramide from the endoplasmic reticulum to the golgi apparatus requires a VAMP-associated protein-interacting FFAT motif of CERT. J Biol Chem. 2006;281:30279–30288. doi: 10.1074/jbc.M605032200. [DOI] [PubMed] [Google Scholar]
- 95.Muto S, Senda M, Akai Y, et al. Relationship between the structure of SET/TAF-Iβ/INHAT and its histone chaperone activity. Proc Natl Acad Sci USA. 2007;104:4285–4290. doi: 10.1073/pnas.0603762104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ledeen RW, Wu G. Sphingolipids of the nucleus and their role in nuclear signaling. Biochim Biophys Acta. 2006;1761:588–598. doi: 10.1016/j.bbalip.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 97.Ledeen RW, Wu G. Nuclear sphingolipids: metabolism and signaling. J Lipid Res. 2008;49:1176–1186. doi: 10.1194/jlr.R800009-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wooten LG, Ogretmen B. Sp1/Sp3-dependent regulation of human telomerase reverse transcriptase promoter activity by the bioactive sphingolipid ceramide. J Biol Chem. 2005;280:28867–28876. doi: 10.1074/jbc.M413444200. [DOI] [PubMed] [Google Scholar]
- 99▪▪.Wooten-Blanks LG, Song P, Senkal CE, et al. Mechanisms of ceramide-mediated repression of the human telomerase reverse transcriptase promoter via deacetylation of Sp3 by histone deacetylase 1. FASEB J. 2007;21:3386–3397. doi: 10.1096/fj.07-8621com. First study to link ceramide signaling with histone deacetylase 1-dependent deacetylation of Sp3 for the repression of human telomerase reverse transcriptase transcription in lung cancer cells. [DOI] [PubMed] [Google Scholar]
- 100▪▪.Sundararaj KP, Wood RE, Ponnusamy S, et al. Rapid shortening of telomere length in response to ceramide involves the inhibition of telomere binding activity of nuclear glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem. 2004;279:6152–6162. doi: 10.1074/jbc.M310549200. First study to show that nuclear glyceraldehyde-3-phosphate dehydrogenase binds and protects telomeres. [DOI] [PubMed] [Google Scholar]
- 101.Arnold HK, Sears RC. A tumor suppressor role of PP2A-B56α through negative regulation of c-Myc and other key oncoproteins. Cancer Metastasis Rev. 2008;27:147–158. doi: 10.1007/s10555-008-9128-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yan L, Lavin VA, Moser LR, et al. PP2A regulates the proapoptotic activity of FoxO1. J Biol Chem. 2008;283:7411–7420. doi: 10.1074/jbc.M708083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee JY, Bielawska AE, Obeid LM. Regulation of cyclin-dependent kinase 2 activity by ceramide. Exp Cell Res. 2000;261:303–311. doi: 10.1006/excr.2000.5028. [DOI] [PubMed] [Google Scholar]
- 104.Chalfant CE, Rathman K, Pinkerman RL, et al. De novo ceramide regulates the alternative splicing of caspase 9 and Bcl-x in A549 lung adenocarcinoma cells Dependence on protein phosphatase-1. J Biol Chem. 2002;277:12587–12595. doi: 10.1074/jbc.M112010200. [DOI] [PubMed] [Google Scholar]
- 105▪.Pettus BJ, Bielawska A, Subramanian P, et al. Ceramide-1-phosphate is a direct activator of cytosolic phospholipase A2. J Biol Chem. 2004;12:11320–11326. doi: 10.1074/jbc.M309262200. Describes the role for ceramide-1-phosphate in the regulation of cytosolic and inflammation. phospholipase A2. [DOI] [PubMed] [Google Scholar]
- 106.Stahelin RV, Subramanian P, Vora M, et al. Ceramide-1-phosphate binds group IVA cytosolic phospholipase A2 via a novel site in the C2 domain. J Biol Chem. 2007;282:20467–20474. doi: 10.1074/jbc.M701396200. [DOI] [PubMed] [Google Scholar]
- 107▪.Hait N, Allegood J, Maceyka, et al. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 2009;325:1254–1257. doi: 10.1126/science.1176709. Describes histone deacetylase 1 and 2 as nuclear targets of sphingosine kinase 2-generated S1P in cancer cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108▪▪.Alvarez S, Harikumar K, Hait N, et al. Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature. 2010;465:1084–1088. doi: 10.1038/nature09128. Describes TRAF2 as a direct cellular target of S1P for the regulation of NF-κB signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Habrukowich C, Han DK, Le A, et al. Sphingosine interaction with acidic leucine-rich nuclear phosphoprotein-32A (ANP32A) regulates PP2A activity and cyclooxygenase (COX)-2 expression in human endothelial cells. J Biol Chem. 2010;285:26825–26831. doi: 10.1074/jbc.M110.147058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zheng W, Kollmeyer J, Symolon H, et al. Ceramides and other bioactive sphingolipid backbones in health and disease: lipidomic analysis, metabolism and roles in membrane structure, dynamics, signaling and autophagy. Biochim Biophys Acta. 2006;1758:1864–1884. doi: 10.1016/j.bbamem.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 111.Pruett ST, Bushnev A, Hagedorn K, et al. Biodiversity of sphingoid bases (“sphingosines”) and related amino alcohols. J Lipid Res. 2008;49:1621–1639. doi: 10.1194/jlr.R800012-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pitson SM, Moretti PA, Zebol JR, et al. Activation of sphingosine kinase 1 by ERK1/2-mediated phosphorylation. EMBO J. 2003;22:5491–5500. doi: 10.1093/emboj/cdg540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nava VE, Hobson JP, Murthy S, et al. Sphingosine kinase type 1 promotes estrogen-dependent tumorigenesis of breast cancer MCF-7 cells. Exp Cell Res. 2004;281:115–127. doi: 10.1006/excr.2002.5658. [DOI] [PubMed] [Google Scholar]
- 114.Sarkar S, Maceyka M, Hait NC, et al. Sphingosine kinase 1 is required for migration, proliferation and survival of MCF-7 human breast cancer cells. FEBS Lett. 2005;579:5313–5317. doi: 10.1016/j.febslet.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 115.Kawamori T, Kaneshiro T, Okumura M, et al. Role for sphingosine kinase 1 in colon carcinogenesis. FASEB J. 2009;23:405–414. doi: 10.1096/fj.08-117572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116▪▪.Visentin B, Vekich JA, Sibbald BJ, et al. Validation of an anti-sphingosine-1-phosphate antibody as a potential therapeutic in reducing growth, invasion, and angiogenesis in multiple tumor lineages. Cancer Cell. 2006;9:225–238. doi: 10.1016/j.ccr.2006.02.023. First study to describe the anti-cancer roles of S1P-specific monoclonal antibodies. [DOI] [PubMed] [Google Scholar]
- 117.Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol. 2001;91:487–500. doi: 10.1152/jappl.2001.91.4.1487. [DOI] [PubMed] [Google Scholar]
- 118.Jacobson JR, Garcia JG. Novel therapies for microvascular permeability in sepsis. Curr Drug Targets. 2007;8:509–514. doi: 10.2174/138945007780362719. [DOI] [PubMed] [Google Scholar]
- 119.Wang L, Dudek SM. Regulation of vascular permeability by sphingosine-1-phosphate. Microvasc Res. 2008;77:39–45. doi: 10.1016/j.mvr.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Komarova YA, Mehta D, Malik AB. Dual regulation of endothelial junctional permeability. Sci STKE. 2007;412:RE8. doi: 10.1126/stke.4122007re8. [DOI] [PubMed] [Google Scholar]
- 121.Shikata Y, Birukov KG, Birukova AA, et al. Involvement of site-specific FAK phosphorylation in sphingosine-1-phosphate- and thrombin-induced focal adhesion remodeling: role of Src and GIT. FASEB J. 2003;17:2240–2249. doi: 10.1096/fj.03-0198com. [DOI] [PubMed] [Google Scholar]
- 122.Yamaguchi H, Kitayama J, Takuwa N, et al. Sphingosine-1-phosphate receptor subtype-specific positive and negative regulation of Rac and haematogenous metastasis of melanoma cells. Biochem J. 2003;374:715–722. doi: 10.1042/BJ20030381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Van Sluis GL, Niers TM, Esmon CT, et al. Endogenous activated protein C limits cancer cell extravasation through sphingosine-1-phosphate receptor 1-mediated vascular endothelial barrier enhancement. Blood. 2009;114:1968–1973. doi: 10.1182/blood-2009-04-217679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hu WM, Li L, Jing BQ, et al. Effect of S1P5 on proliferation and migration of human esophageal cancer cells. World J Gastroenterol. 2010;16:1859–1866. doi: 10.3748/wjg.v16.i15.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang D. S1P differentially regulates migration of human ovarian cancer and human ovarian surface epithelial cells. Mol Cancer Ther. 2008;7:1993–2002. doi: 10.1158/1535-7163.MCT-08-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xia P, Gamble JR, Wang L, et al. An oncogenic role of sphingosine kinase. Curr Biol. 2000;10:1527–1530. doi: 10.1016/s0960-9822(00)00834-4. [DOI] [PubMed] [Google Scholar]
- 127.Mizugishi K, Yamashita T, Olivera A, et al. Essential role for sphingosine kinases in neural and vascular development. Mol Cell Biol. 2005;25:11113–11121. doi: 10.1128/MCB.25.24.11113-11121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rosen H, Goetzl EJ. Sphingosine-1-phosphate and its receptors: an autocrine and paracrine network. Nat Rev Immunol. 2005;5:560–570. doi: 10.1038/nri1650. [DOI] [PubMed] [Google Scholar]
- 129.Johnson KR, Johnson KY, Crellin HG, et al. Immunohistochemical distribution of sphingosine kinase 1 in normal and tumor lung tissue. J Histochem Cytochem. 2005;53:1159–1166. doi: 10.1369/jhc.4A6606.2005. [DOI] [PubMed] [Google Scholar]
- 130.Li J, Guan HY, Gong LY, et al. Clinical significance of sphingosine kinase-1 expression in human astrocytomas progression and overall patient survival. Clin Cancer Res. 2008;14:6996–7003. doi: 10.1158/1078-0432.CCR-08-0754. [DOI] [PubMed] [Google Scholar]
- 131.Sankala HM, Hait NC, Paugh SW, et al. Involvement of sphingosine kinase 2 in p53-independent induction of p21 by the chemotherapeutic drug doxorubicin. Cancer Res. 2007;67:10466–10474. doi: 10.1158/0008-5472.CAN-07-2090. [DOI] [PubMed] [Google Scholar]
- 132.Weigert A, Cremer S, Schmidt MV, et al. Cleavage of sphingosine kinase 2 by caspase-1 provokes its release from apoptotic cells. Blood. 2010;115:3531–3540. doi: 10.1182/blood-2009-10-243444. [DOI] [PubMed] [Google Scholar]
- 133.Hagen N, Van Veldhoven PP, Proia RL, et al. Subcellular origin of sphingosine-1-phosphate is essential for its toxic effect in lyase-deficient neurons. Biol Chem. 2009;284:11346–11353. doi: 10.1074/jbc.M807336200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.French KJ, Upson JJ, Keller SN, et al. Antitumor activity of sphingosine kinase inhibitors. J Pharmacol Exp Ther. 2006;318:596–603. doi: 10.1124/jpet.106.101345. [DOI] [PubMed] [Google Scholar]
- 135.Beljanski V, Knaak C, Smith CD. A novel sphingosine kinase inhibitor induces autophagy in tumor cells. J Pharmacol Exp Ther. 2010;333:454–464. doi: 10.1124/jpet.109.163337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kondo Y, Kondo S. Autophagy and cancer therapy. Autophagy. 2006;2:85–90. doi: 10.4161/auto.2.2.2463. [DOI] [PubMed] [Google Scholar]
- 138.Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science. 2004;306:990–995. doi: 10.1126/science.1099993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Guenther GG, Peralta ER, Rosales KR, et al. Ceramide starves cells to death by downregulating nutrient transporter proteins. Proc Natl Acad Sci USA. 2008;105:17402–17407. doi: 10.1073/pnas.0802781105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Signorelli P, Munoz-Olaya JM, Gagliostro V, et al. Dihydroceramide intracellular increase in response to resveratrol treatment mediates autophagy in gastric cancer cells. Cancer Lett. 2009;282:238–243. doi: 10.1016/j.canlet.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 141.Separovic D, Kelekar A, Nayak AK, et al. Increased ceramide accumulation correlates with downregulation of the autophagy protein ATG-7 in MCF-7 cells sensitized to photodamage. Arch Biochem Biophys. 2010;494:101–105. doi: 10.1016/j.abb.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yacoub A, Hamed HA, Allegood J, et al. PERK-dependent regulation of ceramide synthase 6 and thioredoxin play a key role in MDA-7/IL-24-induced killing of primary human glioblastoma multiforme cells. Cancer Res. 2010;70:1120–1129. doi: 10.1158/0008-5472.CAN-09-4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lavieu G, Scarlatti F, Sala G, et al. Regulation of autophagy by sphingosine kinase 1 and its role in cell survival during nutrient starvation. J Biol Chem. 2006;281:8518–8527. doi: 10.1074/jbc.M506182200. [DOI] [PubMed] [Google Scholar]
- 144.Sakaki K, Wu J, Kaufman RJ. Protein kinase Cθ is required for autophagy in response to stress in the endoplasmic reticulum. J Biol Chem. 2008;283:15370–15380. doi: 10.1074/jbc.M710209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Li DD, Wang LL, Deng R, et al. The pivotal role of c-Jun NH2-terminal kinase mediated Beclin 1 expression during anticancer agents-induced autophagy in cancer cells. Oncogene. 2009;28:886–898. doi: 10.1038/onc.2008.441. [DOI] [PubMed] [Google Scholar]
- 146.Pattingre S, Bauvy C, Carpentier S, et al. Role of JNK1-dependent Bcl-2 phosphorylation in ceramide-induced macroautophagy. J Biol Chem. 2009;284:2719–2728. doi: 10.1074/jbc.M805920200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Park MA, Zhang G, Norris J. Regulation of autophagy by ceramide–CD95–PERK signaling. Autophagy. 2008;4:929–931. doi: 10.4161/auto.6732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Novgorodov SA, Szulc ZM, Luberto C, et al. Positively charged ceramide is a potent inducer of mitochondrial permeabilization. J Biol Chem. 2005;280:16096–16105. doi: 10.1074/jbc.M411707200. [DOI] [PubMed] [Google Scholar]
- 149.Senkal CE, Ponnusamy S, Rossi MJ, et al. Potent antitumor activity of a novel cationic pyridinium ceramide alone or in combination with gemcitabine against human head and neck squamous cell carcinomas in vitro and in vivo. J Pharmacol Exp Ther. 2006;317:1188–1199. doi: 10.1124/jpet.106.101949. [DOI] [PubMed] [Google Scholar]
- 150.Rossi MJ, Sundararaj K, Koybasi S, et al. Inhibition of growth and telomerase activity by novel cationic ceramide analogs with high solubility in human head and neck squamous cell carcinoma cells. Otolaryngol Head Neck Surg. 2005;132:55–62. doi: 10.1016/j.otohns.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 151.Stover T, Kester M. Liposomal delivery enhances short-chain ceramide-induced apoptosis of breast cancer cells. J Pharmacol Exp Ther. 2003;307:68–75. doi: 10.1124/jpet.103.054056. [DOI] [PubMed] [Google Scholar]
- 152.Stover TC, Sharma A, Robertson GP, et al. Systemic delivery of lysosomal short-chain ceramide limits tumor growth in murine models of breast adenocarcinoma. Clin Cancer Res. 2005;11(9):3465–3474. doi: 10.1158/1078-0432.CCR-04-1770. [DOI] [PubMed] [Google Scholar]
- 153.Vlerken LE, Duan Z, Seiden MV, et al. Modulation of intracellular ceramide using polymeric nanoparticles to overcome multidrug resistance in cancer. Cancer Res. 2007;67:4843–4850. doi: 10.1158/0008-5472.CAN-06-1648. [DOI] [PubMed] [Google Scholar]
- 154.Struckhoff AP, Bittman R, Burow ME, et al. Novel ceramide analogs as potential chemotherapeutic agents in breast cancer. J Pharmacol Exp Ther. 2004;309:523–532. doi: 10.1124/jpet.103.062760. [DOI] [PubMed] [Google Scholar]
- 155.Antoon JW, Liu J, Gestaut MM, et al. Design, synthesis, and biological activity of a family of novel ceramide analogues in chemoresistant breast cancer cells. J Med Chem. 2009;24(52):5748–5752. doi: 10.1021/jm9009668. [DOI] [PubMed] [Google Scholar]
- 156.Crawford KW, Bittman R, Chun J, et al. Novel ceramide analogues display selective cytotoxicity in drug-resistant breast tumor cell lines compared to normal breast epithelial cells. Cell Mol Biol (Noisy-le-grand) 2003;49:1017–1023. [PubMed] [Google Scholar]
- 157.Samsel L, Zaidel G, Drumgoole HM, et al. The ceramide analog, B13, induces apoptosis in prostate cancer cell lines and inhibits tumor growth in prostate cancer xenografts. Prostate. 2004;58:382–393. doi: 10.1002/pros.10350. [DOI] [PubMed] [Google Scholar]
- 158.Holman DH, Turner LS, El-Zawahry A, et al. Lysosomotropic acid ceramidase inhibitor induces apoptosis in prostate cancer cells. Cancer Chemother Pharmacol. 2008;61:231–242. doi: 10.1007/s00280-007-0465-0. [DOI] [PubMed] [Google Scholar]
- 159.Lahiri S, Park H, Laviad EL, et al. Ceramide synthesis is modulated by the sphingosine analog FTY720 via a mixture of uncompetitive and noncompetitive inhibition in an acyl-CoA chain length-dependent manner. J Biol Chem. 2009;284:16090–16098. doi: 10.1074/jbc.M807438200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Berdyshev EV, Gorshkova I, Skobeleva A, et al. FTY720 inhibits ceramide synthases and upregulates dihydrosphingosine-1-phosphate formation in human lung endothelial cells. J Biol Chem. 2009;284:5467–5477. doi: 10.1074/jbc.M805186200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hung JH, Lu YS, Wang YC, et al. FTY720 induces apoptosis in hepatocellular carcinoma cells through activation of protein kinase C δ signaling. Cancer Res. 2008;68:1204–1212. doi: 10.1158/0008-5472.CAN-07-2621. [DOI] [PubMed] [Google Scholar]
- 162.Nagaoka Y, Otsuki K, Fujita T, et al. Effects of phosphorylation of immunomodulatory agent FTY720 (fingolimod) on antiproliferative activity against breast and colon cancer cells. Biol Pharm Bull. 2008;31:1177–1181. doi: 10.1248/bpb.31.1177. [DOI] [PubMed] [Google Scholar]
- 163▪▪.Neviani P, Santhanam R, Oaks JJ, et al. FTY720, a new alternative for treating blast crisis chronic myelogenous leukemia and Philadelphia chromosome-positive acute lymphocytic leukemia. J Clin Invest. 2007;117:2408–2421. doi: 10.1172/JCI31095. Demonstrates a potential drug for sensitizing the blast crisis leukemic cancer cells by treating these cancer cells with FTY-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lucas da Silva LB, Ribeiro DA, Curt PM, et al. FTY720 treatment in experimentally urethane-induced lung tumors. J Exp Ther Oncol. 2008;7:9–15. [PubMed] [Google Scholar]
- 165.Ogretmen B, Hannun YA. Updates on functions of ceramide in chemotherapy-induced cell death and in multidrug resistance. Drug Resist Updat. 2001;4:368–377. doi: 10.1054/drup.2001.0225. [DOI] [PubMed] [Google Scholar]
- 166▪▪.Ogretmen B, Kraveka JM, Schady D, et al. Molecular mechanisms of ceramide-mediated telomerase inhibition in the A549 human lung adenocarcinoma cell line. J Biol Chem. 2001;276:32506–32514. doi: 10.1074/jbc.M101350200. Describes the role of ceramide in the ubiquitination and degradation of c-Myc, leading to inhibition of hTERT transcription. [DOI] [PubMed] [Google Scholar]
- 167.Senchenkov A, Litvak DA, Cabot MC. Targeting ceramide metabolism – a strategy for overcoming drug resistance. J Natl Cancer Inst. 2001;93:347–357. doi: 10.1093/jnci/93.5.347. [DOI] [PubMed] [Google Scholar]
- 168.Radin NS. The development of aggressive cancer: a possible role for sphingolipids. Cancer Invest. 2002;20:779–786. doi: 10.1081/cnv-120002495. [DOI] [PubMed] [Google Scholar]
- 169.Gouaze V, Liu YY, Prickett CS, et al. Glucosylceramide synthase blockade downregulates P-glycoprotein and resensitizes multidrug-resistant breast cancer cells to anticancer drugs. Cancer Res. 2005;65:3861–3867. doi: 10.1158/0008-5472.CAN-04-2329. [DOI] [PubMed] [Google Scholar]
- 170.Gouaze V, Yu JY, Bleicher RJ, et al. Overexpression of glucosylceramide synthase and P-glycoprotein in cancer cells selected for resistance to natural product chemotherapy. Mol Cancer Ther. 2004;3:633–639. [PubMed] [Google Scholar]
- 171.De Rosa MF, Sillence D, Ackerley C, et al. Role of multiple drug resistance protein 1 in neutral but not acidic glycosphingolipid biosynthesis. J Biol Chem. 2004;279:7867–7876. doi: 10.1074/jbc.M305645200. [DOI] [PubMed] [Google Scholar]
- 172.Maurer BJ, Metelitsa LS, Seeger RC, et al. Increase of ceramide and induction of mixed apoptosis/necrosis by N-(4-hydroxyphenyl)- retinamide in neuroblastoma cell lines. J Natl Cancer Inst. 1999;91:1138–1114. doi: 10.1093/jnci/91.13.1138. [DOI] [PubMed] [Google Scholar]
- 173.Maurer BJ, Melton L, Billups C, et al. Synergistic cytotoxicity in solid tumor cell lines between N-(4-hydroxyphenyl)retinamide and modulators of ceramide metabolism. J Natl Cancer Inst. 2000;92:1897–1909. doi: 10.1093/jnci/92.23.1897. [DOI] [PubMed] [Google Scholar]
- 174.Pchejetski D, Golzio M, Bonhoure E, et al. Sphingosine kinase-1 as a chemotherapy sensor in prostate adenocarcinoma cell and mouse models. Cancer Res. 2005;65:11667–11675. doi: 10.1158/0008-5472.CAN-05-2702. [DOI] [PubMed] [Google Scholar]
- 175.Bektas M, Jolly PS, Muller C, et al. Sphingosine kinase activity counteracts ceramide-mediated cell death in human melanoma cells: role of Bcl-2 expression. Oncogene. 2005;24(1):178–187. doi: 10.1038/sj.onc.1208019. [DOI] [PubMed] [Google Scholar]
- 176.Akao Y, Banno Y, Nakagawa Y, et al. High expression of sphingosine kinase 1 and S1P receptors in chemotherapy-resistant prostate cancer PC3 cells and their camptothecin-induced upregulation. Biochem Biophys Res Commun. 2006;342:1284–1290. doi: 10.1016/j.bbrc.2006.02.070. [DOI] [PubMed] [Google Scholar]
- 177.Davis MD, Clemens JJ, Macdonald TL, et al. Sphingosine-1-phosphate analogs as receptor antagonists. J Biol Chem. 2005;280:9833–9841. doi: 10.1074/jbc.M412356200. [DOI] [PubMed] [Google Scholar]
- 178.Knapp P, Baranowski M, Knapp M, et al. Altered sphingolipid metabolism in human endometrial cancer. Prostaglandins Other Lipid Mediat. 2010;3:10. doi: 10.1016/j.prostaglandins.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 179.Ruckhäberle E, Rody A, Engels K, et al. Microarray analysis of altered sphingolipid metabolism reveals prognostic significance of sphingosine kinase 1 in breast cancer. Breast Cancer Res Treat. 2008;112:41–52. doi: 10.1007/s10549-007-9836-9. [DOI] [PubMed] [Google Scholar]
- 180.Le Scolan E, Pchejetski D, Banno Y, et al. Overexpression of sphingosine kinase 1 is an oncogenic event in erythroleukemic progression. Blood. 2005;106:1808–1816. doi: 10.1182/blood-2004-12-4832. [DOI] [PubMed] [Google Scholar]
- 181.Nava VE, Cuvillier O, Edsall LC, et al. Sphingosine enhances apoptosis of radiation-resistant prostate cancer cells. Cancer Res. 2000;60:4468–4474. [PubMed] [Google Scholar]
- 182.Sukocheva O, Wang L, Verrier E, et al. Restoring endocrine response in breast cancer cells by inhibition of the sphingosine kinase-1 signaling pathway. Endocrinology. 2009;150:4484–4492. doi: 10.1210/en.2009-0391. [DOI] [PubMed] [Google Scholar]
- 183.Liu Y, Wada R, Yamashita T, et al. EDG-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Investig. 2000;106:951–961. doi: 10.1172/JCI10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Skoura A, Sanchez T, Claffey K, et al. Essential role of sphingosine-1-phosphate receptor 2 in pathological angiogenesis of the mouse retina. J Clin Invest. 2007;117(9):2506–2516. doi: 10.1172/JCI31123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Miller AV, Alvarez SE, Spiegel S, et al. Sphingosine kinases and sphingosine-1-phosphate are critical for transforming growth factor β-induced extracellular signal-regulated kinase 1 and 2 activation and promotion of migration and invasion of esophageal cancer cells. Mol Cell Biol. 2008;28:4142–4151. doi: 10.1128/MCB.01465-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Li MH, Sanchez T, Milne GL, et al. S1P/S1P2 signaling induces cyclooxygenase-2 expression in Wilms tumor. J Urol. 2009;181:1347–1352. doi: 10.1016/j.juro.2008.10.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Young N, Van Brocklyn JR. Roles of sphingosine-1-phosphate (S1P) receptors in malignant behavior of glioma cells. Exp Cell Res. 2007;313:1615–1627. doi: 10.1016/j.yexcr.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kapitonov D, Allegood JC, Mitchell C, et al. Targeting sphingosine kinase 1 inhibits Akt signaling, induces apoptosis, and suppresses growth of human glioblastoma cells and xenografts. Cancer Res. 2009;69:6915–6923. doi: 10.1158/0008-5472.CAN-09-0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hla T. Physiological and pathological actions of sphingosine-1-phosphate. Semin Cell Dev Biol. 2004;15:513–520. doi: 10.1016/j.semcdb.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 190.Kawahara A, Nishi T, Hisano Y, Fukui H, Yamaguchi A, Mochizuki N. The sphingolipid transporter spns2 functions in migration of zebrafish myocardial precursors. Science. 2009;323:524–527. doi: 10.1126/science.1167449. [DOI] [PubMed] [Google Scholar]
- 191.Schmelz EM, Dillehay DL, Webb SK, Reiter A, Adams J, Merrill AH., Jr Sphingomyelin consumption suppresses aberrant colonic crypt foci and increases the proportion of adenomas versus adenocarcinomas in CF1 mice treated with 1, 2-dimethylhydrazine: implications for dietary sphingolipids and colon carcinogenesis. Cancer Res. 1996;56:4936–4941. [PubMed] [Google Scholar]
- 192.Bektas M, Allende ML, Lee BG, et al. Sphingosine-1-phosphate lyase deficiency disrupts lipid homeostasis in liver. J Biol Chem. 2010;285:10880–10889. doi: 10.1074/jbc.M109.081489. [DOI] [PMC free article] [PubMed] [Google Scholar]