Abstract
Increased levels of EZH2, a critical regulator of cellular memory, signal the presence of metastasis and poor outcome in breast cancer patients. High levels of EZH2 are associated with nuclear pleomorphism, lack of estrogen receptor (ER) expression, and decreased nuclear levels of BRCA1 tumor suppressor protein in invasive breast carcinomas. The mechanism by which EZH2 overexpression promotes the growth of poorly-differentiated invasive carcinomas remains to be defined. Here we show that EZH2 controls the intracellular localization of BRCA1 protein. Conditional doxycycline-induced upregulation of EZH2 in benign mammary epithelial cells results in nuclear export of BRCA1 protein, aberrant mitoses with extra centrosomes and genomic instability. EZH2 inhibition in CAL51 breast cancer cells induces BRCA1 nuclear localization and rescues defects in ploidy and mitosis. Mechanistically, EZH2 overexpression is sufficient for activation of the phosphatidylinositol 3-kinase/Akt (PI3K/Akt) pathway specifically through activation of Akt isoform 1. EZH2-induced BRCA1 nuclear export, aneuploidy and mitotic defects were prevented by treatment with the PI3K inhibitors LY294002 or Wortmannin. Targeted inhibition of Akt-1, 2, and 3 isoforms revealed that the EZH2-induced phenotype requires specific activation of Akt-1. The relevance of our studies to human breast cancer is highlighted by the finding that high EZH2 protein levels are associated with upregulated expression of p-Akt1(Ser473) and decreased nuclear expression of pBRCA1 (Ser1423) in 39% of invasive breast carcinomas. These results enable us to pinpoint one mechanism by which EZH2 regulates BRCA1 expression and genomic stability mediated by the PI3K/Akt-1 pathway.
Keywords: EZH2, Polycomb, AKT, aneuploidy, tetraploidy, breast cancer, mitosis, mitotic spindle, Aurora A
INTRODUCTION
EZH2 is a Polycomb group (PcG) protein involved in the regulation of cellular memory with roles in tumorigenesis including cancer cell proliferation, stem cell maintenance, cell differentiation, and neoplastic cell transformation (1–7). In breast cancer, EZH2 protein is elevated in aggressive and metastatic tumors and it is an independent predictor of survival (8). Immunohistochemical studies of human breast tissue samples have shown that whereas EZH2 expression is low in normal epithelium, EZH2 is overexpressed in 54% of invasive carcinomas, especially in estrogen receptor (ER) negative tumors with low BRCA1 nuclear expression (8–11).
The tumor suppressor BRCA1 regulates DNA repair,activation of cell-cycle checkpoints, and has a central role in the maintenance of chromosomalstability (12). Heterozygous germ-line mutations in the BRCA1 gene predisposewomen to breast and ovarian cancer with a lifetime risk of breastcancer of up to 80% (13). Although somatic mutations of BRCA1 are not common, expression of its messenger RNA and protein are reduced in approximately 40% of sporadic (non-hereditary) breast carcinomas (14–16). Independent of the mechanism underlying the decrease in nuclear BRCA1 protein, the vast majority of breast carcinomas with reduced nuclear BRCA1 are poorly-differentiated, aneuploid, and lack expression of ER (16–18).
BRCA1 protein exerts its tumor suppressor functions in the nucleus and it can shuttle between the nucleus and the cytoplasm (19). Recent studies have provided information on the subcellular localization of BRCA1 protein during the cell cycle in normal breast cells and breast cancer cells (20–22). BRCA1 protein is exported from the nucleus transiently during the initial part of S phase. By late S phase BRCA1 resumes being a predominantly nuclear protein (20–22). Activation of the protein kinase b (Akt) has been implicated in the nuclear/cytoplasmic shuttling of BRCA1 protein in breast cells (23–26).
EZH2 has been proposed to participate in cell growth and invasion in breast cancer and it has been studied to modulate BRCA1-mediated proliferation (8, 11, 27). However, no studies have been carried out to investigate the mechanism by which EZH2 influences BRCA1 protein and the link between EZH2 and genomic stability in breast cancer. Here, we demonstrate that EZH2 regulates the intracellular localization of BRCA1 protein in benign and malignant breast cells. Conditional doxycycline-induced EZH2 overexpression in MCF10A cells leads to nuclear export of BRCA1 protein and is sufficient to trigger aberrant mitoses and numerical chromosomal alterations. EZH2 inhibition in ER negative CAL51 breast cancer cells induces BRCA1 nuclear localization and rescues their ploidy and mitotic defects. Mechanistically, our data show that EZH2-induced BRCA1 nuclear export, mitotic and ploidy abnormalities require activation of the PI3K/Akt-1 signaling pathway.
MATERIALS AND METHODS
Cell lines and Breast Tissues
CAL51 breast cancer cell line was purchased from the German Collection of Microorganism and Cell Cultures (DSMZ, Braunschweig, Germany, Cat No. DSMZ ACC 302) and grown under recommended conditions. Immortalized human mammary epithelial cells MCF10A were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and grown under recommended conditions.
Lentiviral Transductions
To conditionally overexpress EZH2 in MCF10A cells, a doxycycline inducible system was employed. EZH2 gene was isolated form pCDNA3-myc EZH2 plasmid (gift of Dr Chinnaiyan) and cloned into the pLVX-Tight-Puro, from Lenti-X Tet-On Advance Inducible Expression system (Clontech, CA). Briefly, the Lenti-X Tet-On system is based in expressing within the cells the E.coli Tet repressor protein (TetR), which negatively regulates the tetracycline operon on the Tn 10 transposon (vector: pLVX-Tet-On) together with the tetO (tet operator sequences) (vector: pLVX-Tight-Puro). In the presence of tetracycline or doxycycline, TetR dissociates from tetO and transcription of the resistance-mediating genes begins. Lentivirus bearing EZH2 conditional system and vector control were used to transduce MCF10A cells. Cells were cultured in complete media supplemented with puromycin (10μg/ml). EZH2 expression was transiently induced with Doxycycline (500 ng/ml) following the manufacturer’s instructions.
Short hairpin RNA (shRNA) targeting human EZH2 (NM_152998 NCBI) (V2LHS_17507, Open Biosystems, Huntsville, AL; Cat. No. RHS4430-99139126) was cloned into a pLKO.1-puro vector. The shRNA-containing plasmid was packaged into lentiviral particles at the Vector Core (University of Michigan, Ann Arbor, MI). Background control was Lenti-PuroEMPTY-VSVG. To generate stable CAL51 breast cancer cells with EZH2 knockdown, 1 × 106 cells per 100mm plate were transduced with the corresponding lentivirus-containing supernatant diluted 1:1 with fresh serum free medium for 48 hours. Stable clones were selected for antibiotic resistance with10μg/ml puromycin (Sigma, St. Louis, MO), at 37°C under 10% CO2 for 3 weeks (11).
Human Akt-1 siRNA (sense: 5′-CCAAGCACCGCGUGACC AU-3′; antisense: 5′-AUGGUCACGCGGUGCUUGG-3′), Akt-2 siRNA (sense, 5′-CAGAAUGCCAGCUGAUGAA-3′; antisense, 5′-UUCAUCAGCUGGCAUUCUG-3′), Akt-3 siRNA (sense, 5′-GAAAGAUUGUGUACCGUGA-3′; antisense, 5′-UCACGGUACACAAUCUUUC-3′), and human siRNA negative control oligonucleotides were purchased from Sigma (St. Louis, MO) (28). Cells were split into complete medium for 24h before subconfluence. siRNA oligos were transfected into subconfluent cells with Oligofectamine (Invitrogen, Carlsbad, CA) in accordance with the manufacturer’s instructions.
Western Blot Analysis
Nuclear enriched fractions were separated utilizing the NE-Per kit (Pierce, Rockford, IL, USA). Western blots were performed with 100 μg of whole cell extract, nuclear or cytoplasmic enriched fractions as indicated in the corresponding figure. Samples were boiled in 1 × SDS loading buffer, separated by SDS-PAGE gels, and transferred onto a nitrocellulose (NC) membrane. NC membranes were blocked with 5% non-fat dry milk and were incubated with corresponding primary antibodies at 4° C overnight. Immunoblot signals were visualized by a chemiluminescence system as described by the manufacturer (Amersham Bioscience, Piscataway, NJ). Blots were re-probed with α-tubulin or GAPDH to confirm the equal loading of samples, and with Laminin B1 to confirm the nuclear enrichment of the fractionated samples.
Primary antibodies including anti-EZH2 and anti-phopsho Polo-like kinase 1 (Plk1) Thr210 (BD Biosciences, San Jose, CA, USA), anti-BRCA1 (EMD Chemicals, Gibbstown, NJ, USA), anti-phospho-BRCA1(Ser1423), anti-laminin B1 (Abcam, Cambridge, MA, USA), anti-Akt, anti-Akt-1, anti-phospho-Akt (Ser473), anti-Akt-3, anti-phospho-Akt-3 (Ser472), anti-Aurora A, anti-Aurora B, anti-phosho Aurora A (Thr288) (Cell Signaling, Boston, MA), anti- phospho-Akt-1 (Ser473) (Upstate Biotechnology, Billerica, MA), anti-Akt-2, anti-phospho-Akt-2 (Ser474), and anti-Plk1 (Santa Cruz Biotechnology, Santa Cruz, CA), anti-β-actin, anti-α-tubulin (Sigma, St Louis, MO, USA), anti-phospho Aurora B (Thr232) (Biolegend, San Diego), were used at the manufacturers’ recommended dilutions. The PI3K/Akt inhibitors LY294002 and Wortmannin (Invitrogen, Carlsbad, CA) were employed to investigate the contribution of the PI3K/Akt pathway on EZH2 function following previous procedures (29).
Analysis of Mitosis and Mitotic index
Cells were grown in slides, fixed with paraformaldehyde, rotated for 45 min at RT, and incubated with anti-phospho histone H3 antibody (Ser10 mitotic maker)-FITC conjugated (Millipore, Temecula, CA). The mitotic index is the percentage of cells staining for pH3. The presence of abnormal mitosis was studied by immunofluorescence with antibodies against p-H3(Ser10), α-tubulin, and Aurora A. DAPI identified the nuclei. Slides were visualized under confocal microscopy. The number of cells with abnormal mitosis and greater than 2 Aurora A foci were recorded. A total of 300 cells were counted in triplicate. Details on cell synchronization, Nocodazole treatment, Aurora A activity assay, flow cytometry, immunofluorescence, and human tissue samples are found in the Supplementary Methods.
Metaphase Analysis
MCF10A cells were grown to 70% confluency and treated with Doxycycline (500 ng/ml) for 24 h, 3 days and 5 days. Untreated cells served as controls. Cells were treated with 50 ng/ml colcemid (Invitrogen, Carlsbad, CA) for 24 hours, then collected and resuspended in a hypotonic solution of 2% KCl and 2% Na3C6H5O7 for 7 minutes at 37°C. Metaphase spreads were prepared and stained with Giemsa as described (30). Slides were examined using an ImagingZ1 microscope (Carl Zeiss, Oberkochen, Germany) equipped with ISIS image processing software (MetaSystems, USA).100 metaphases were counted in triplicate for each sample. Tetraploidy was defined as chromosome numbers of 81–100 following established criteria (31–33).
RESULTS
EZH2 regulates the nuclear-cytoplasmic shuttling of BRCA1 in benign and in breast cancer cells
To determine the oncogenic phenotype of EZH2 overexpression in non-tumorigenic human breast epithelial cells we generated a doxycycline regulated system to overexpress EZH2 in MCF10A cells. The empty vector served as negative control (pLVX). EZH2 was detected in whole cell lysates of Dox-induced MCF10A cells transduced with EZH2 containing plasmid (pLVX-EZH2) but not in the lysates of cells transduced with the empty vector (pLVX) (Figure 1A, top). We also generated CAL51 breast cancer cells with stable downregulation of EZH2 using previously validated shRNAs (11). CAL51 breast cancer cells were chosen for EZH2 downregulation because they overexpress EZH2, are human, ER negative, and lack BRCA1 mutations (11, 34).
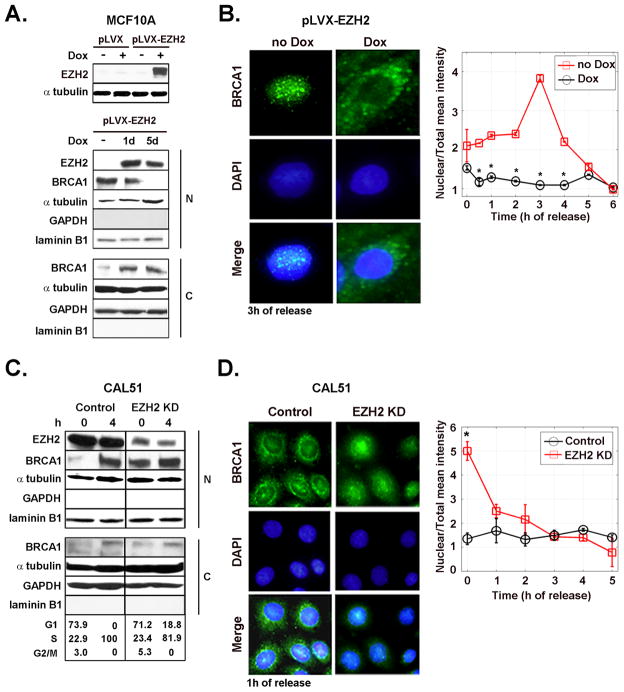
Figure 1. EZH2-dependent regulation of BRCA1 intracellular localization.
A. Inducible synthesis of EZH2 in MCF10A cells and its effect on BRCA1 protein. Western blot analysis of MCF10A cells transduced with the empty vector (pLVX) and with EZH2-containing vector (pLVX-EZH2). Cells were untreated or treated with Dox (500 ng/ml) to induce EZH2 expression. Underneath is a Western blot of EZH2 and BRCA1 proteins in nuclear and cytoplasmic enriched fractions of pLVX-EZH2 cells untreated or treated with Dox. Laminin B1 and GAPDH confirm nuclear and cytoplasmic enrichment of the fractionated samples, respectively. B. Effect of EZH2 overexpression on the intracellular localization of BRCA1 protein. Representative immunofluorescence images of BRCA1 protein at 3 h after release of G1/S cell cycle block (double thymidine block). The graph shows the mean intensity of BRCA1 protein expression in the nucleus normalized to the total intensity at different times after release from cell cycle block. The percentage of cells in each cell cycle phase after release of cell cycle block for untreated and Dox treated cells was at 0 h: G1 94.84%, S 5.15%, G2/M 0%, and G1 94.09%; S 4.97%, G2/M 0.94%, respectively. At 4 h: G1 78.52%, S 21.02%, G2/M 0.46%, and G1 74.27%, S 25.7%, G2/M 0.03%, respectively. C. Western blot for EZH2 and BRCA1 proteins in CAL51/EZH2 KD and controls at 0 and 4 h after release of cell cycle block. The number of cells in G1, S and M cell cycle phases was obtained by flow cytometry. D. Representative immunofluorescence images of BRCA1 protein at 1 h after release of cell cycle block and quantification of results, as described above. The experiment was repeated for three independent times. Error bars are the standard deviation and * represents p<0.05.
Western blot analyses showed that Dox treatment of MCF10A-pLVX-EZH2 cells decreased nuclear BRCA1 protein and increased BRCA1 in the cytoplasm (Figure 1A, middle and bottom). To investigate the effect of EZH2 on the kinetics of BRCA1 shuttling between the nucleus and cytoplasm during the cell cycle, MCF10A-pLVX-EZH2 cells with or without Dox treatment were synchronized at G1/S using double thymidine block, released and analyzed at the specified time points of early S phase. By immunofluorescence BRCA1 localized to the nucleus of untreated MCF10A-pLVX-EZH2 cells. In contrast, Dox-induced EZH2 upregulation led to cytoplasmic localization of BRCA1 (Figure 1B). Fluorescence signals of individual cells in the nucleus and cytoplasm were quantified using the ImageJ NIH software (Figure 1B). Confirming the specificity of these results, no effect on BRCA1 intracellular localization was observed when MCF10A-pLVX cells (empty vector) were treated with Dox (Supplementary figure 1).
EZH2 KD on CAL51 breast cancer cells increased BRCA1 protein in the nuclear-enriched fraction immediately after release from cell cycle block at G1/S (Figure 1C). While CAL51 controls exhibited predominantly cytoplasmic and perinuclear BRCA1 protein as previously reported (20), EZH2 KD cells accumulated BRCA1 in the nucleus (Figure 1C and D). We conclude that EZH2 influences the intracellular localization of BRCA1 protein in non-tumorigenic breast cells and in breast cancer cells.
Overexpression of EZH2 Protein Induces Extra Centrosomes and Abnormal Mitosis
Immunofluorescence studies showed that Dox-induced EZH2 overexpression led to mitotic defects including multiple mitotic spindles which contrasted with the absence of mitotic defects in untreated controls (Figure 2A, left). To determine the effect of EZH2 overexpression on centrosome number we detected Aurora A by immunofluorescence. Early in mitosis, Aurora A localizes to the centrosomes to mediate their maturation, separation, and spindle formation (35). As expected, Aurora A localized to the centrosomes during metaphase of untreated MCF10A-pLVX-EZH2 cells as evidenced by the two distinct foci that colocalized to the spindle poles (Figure 2A, right). Dox -induced EZH2 overexpression led to a 6-fold increase in the percentage of mitotic cells with more than two Aurora A foci (Figure 2A, right).
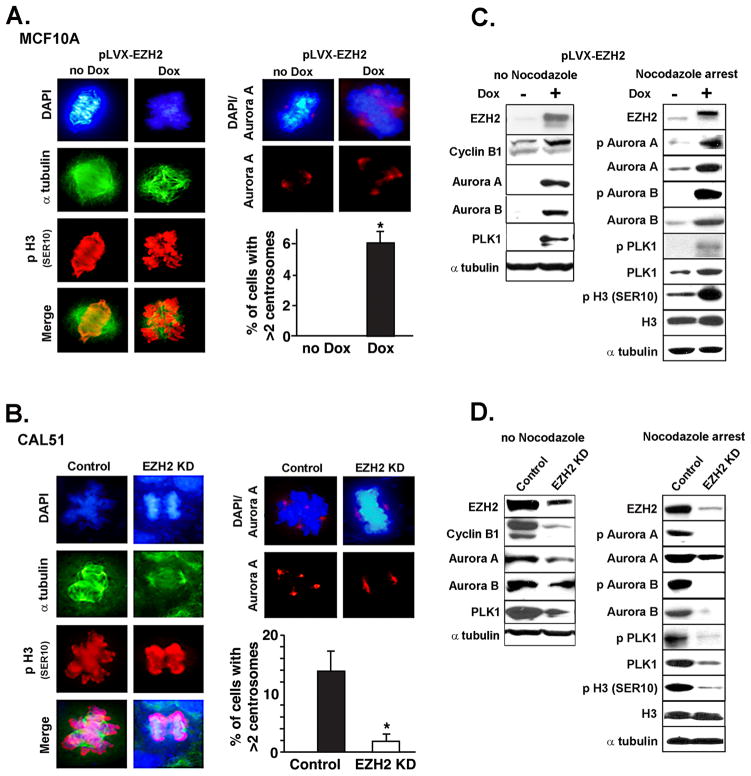
Figure 2. EZH2-mediated induction of abnormal mitosis and Aurora kinase activity.
A and B. Immunofluorescence analysis of mitotic MCF10A- pLVX-EZH2 cells untreated or treated with Dox for 3 days (A), and in CAL51/KD and controls (B). p-H3 marks mitotic DNA; Aurora A marks the centrosomes. Bar graphs show the percentage of cells with > 2 centrosomes ± SD. The number of mitotic cells per 300 cells was 59 (19%) and 78 (26%) for untreated and Dox treated cells, respectively. The number of mitotic cells per 300 cells was 198 (66%) and 78 (26%) for control and EZH2 KD cells, respectively. C. Western blot analysis of MCF10A-pLVX-EZH2 cells untreated or treated with Nocodazole (50 ng/ml for 20 h at 80% confluence) to induce mitotic arrest. EZH2 overexpression induces Aurora A and B levels in asynchronized cultures, and upregulates p Aurora A and B, p Plk1, and the Aurora kinase substrate p H3 Ser10 in Nocodazole treated cells. D. Western blot of CAL51 cells untreated or treated with Nocodazole. EZH2 KD in CAL51 cells show the opposite effects than EZH2 overexpression.
Because CAL51 cells contain a tetraploid population with centrosome amplification and multiple mitotic spindles they constitute a good model to test the effect of EZH2 KD on centrosome number, mitotic spindle and mitotic defects (34). EZH2 KD on CAL51 cancer cells significantly reduced the number of aberrant mitosis and the number of cells with more than two Aurora A foci (Figure 2B).
We found that EZH2 expression in MCF10A and CAL51 cells regulates the levels and activity of Aurora A and Aurora B kinases, essential for mitotic entry and progression. Corresponding with the increase in Aurora A and B proteins observed in asynchronized cultures (Figure 2C, left), EZH2 overexpression increased their enzymatic activity in nocodazole treated samples. EZH2 overexpression induced phosphorylation of Aurora A on Thr288, Aurora B on Thr232, Aurora A interacting protein Polo-like kinase 1 (Plk1) on Thr210, and Aurora kinase substrate p-H3 Ser10, as well as Aurora A in vitro kinase activity (Figure 2C right and Supplementary Figure 2A) (36). EZH2 KD in CAL51 cells had the opposite effect (Figure 2D). Further strengthening these data, EZH2 protein regulated Aurora A and B protein levels during cell cycle progression and their messenger RNA levels (Supplementary Figure 2C–D). Collectively, these data implicate EZH2 in mitosis and demonstrate a novel regulatory role for EZH2 on Aurora A and B kinases expression and activity, and on centrosome number in benign and breast cancer cells.
EZH2 regulates genomic stability
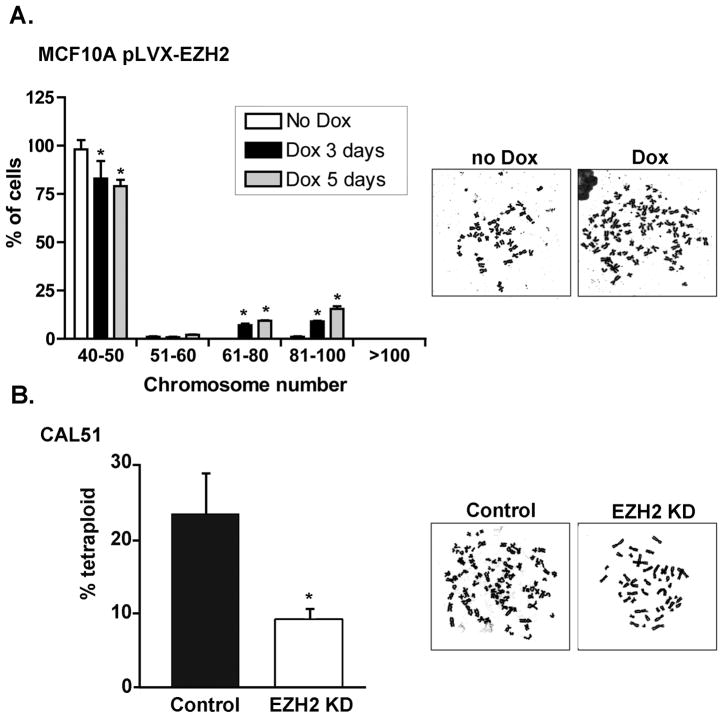
Errors in mitosis can lead to genomic instability. In contrast to the diploid chromosome number of untreated MCF10A cells, EZH2 overexpression resulted in 16.8% and 26.8% polyploidy (≥51 chromosomes) after 72 and 120 hours of Dox treatment, respectively. Chromosome counting indicated that 57% of cells within the polyploid population were near-tetraploid (51–100 chromosomes) at 5 days of Dox treatment (Figure 3A) (31–33). In contrast, EZH2 KD decreased the percentage of tetraploid CAL51 cells from 23.2% to 9.2% (Figure 3B). These data reveal that besides its ability to regulate the number of centrosomes EZH2 plays a role in the maintenance of genomic stability.
Figure 3. EZH2 regulates genomic stability in benign and breast cancer cells.
A. MCF10A-pLVX-EZH2 cells untreated or treated with Dox for 3 and 5 days were subjected to Giemsa-stained metaphase spread analysis. While untreated cells are diploid, EZH2 overexpression induced polyploidy (≥51 chromosomes) and increased near tetraploid cells (81–100 chromosomes). Bar graph shows the mean percentage of cells ± SD. B. Metaphase counts in CAL51 control and EZH2 KD cells. For both experiments, 100 metaphases per sample were counted and the assay was performed in triplicate. * p<0.05.
EZH2-induced BRCA1 nuclear export, mitotic and ploidy defects require activation of PI3K/Akt isoform 1
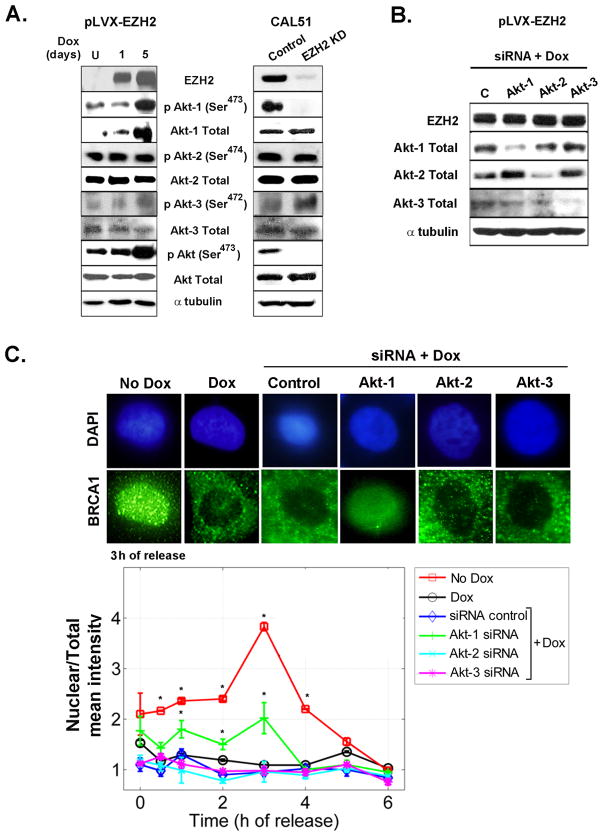
We found that Dox treatment of MCF10A-pLVX-EZH2 cells increased the levels of Akt phosphorylated at Ser473, required to promote its maximal activation (Figure 4A, left). As expected, Dox treatment of MCF10A-pLVX cells did not alter pAkt expression (Supplementary figure 3).To pinpoint which Akt isoform is necessary for the EZH2-induced phenotype we investigated the effect of EZH2 on the expression and phosphorylation of Akt isoforms 1, 2, and 3 on benign and breast cancer cells. EZH2 overexpression in MCF10A cells increased Akt-1 protein but did not influence Akt-2 or Akt-3 expression or phosphorylation, compared to controls (Figure 4A, left). Consistently, CAL51/EZH2 KD cells exhibited decreased Akt-1 phosphorylation at Ser473 compared to scrambled controls (Figure 4A, right). Reciprocal co-immunoprecipitation showed that EZH2 was able to directly interact with Akt-1 in MCF10A cells (Supplementary figure 4). These data led us to hypothesize that Akt-1 may mediate the observed EZH2-induced phenotype.
Figure 4. EZH2 requires Akt-1 to regulate the intracellular localization of BRCA1.
A. Immunoblots of MCF10A and CAL51 cells show that EZH2 expression regulates Akt-1 protein, while it has no effect on Akt-2 or Akt-3 protein levels. B. Western blot of MCF10A-pLVX-EZH2 cells treated with siRNA control or siRNA-Akt-1, siRNA-Akt-2, or siRNA-Akt-3 and then subjected to Dox for 3 days to induce EZH2 overexpression. Immunoblot shows specific inhibition of Akt isoforms. C. Cells described in B as well as pLVX-EZH2 untreated or treated with Dox were subjected to immunofluorescence analyses of BRCA1 protein at the indicated time points after release from cell cycle block at G1/S. Representative confocal images at 3 h after release from cell cycle block.Greater than 500 cells were counted per slide, in triplicate. Mean intensity of BRCA1 protein in the nucleus was normalized to the total intensity utilizing the ImageJ NIH program. Error bars are the standard deviation. * p<0.05.
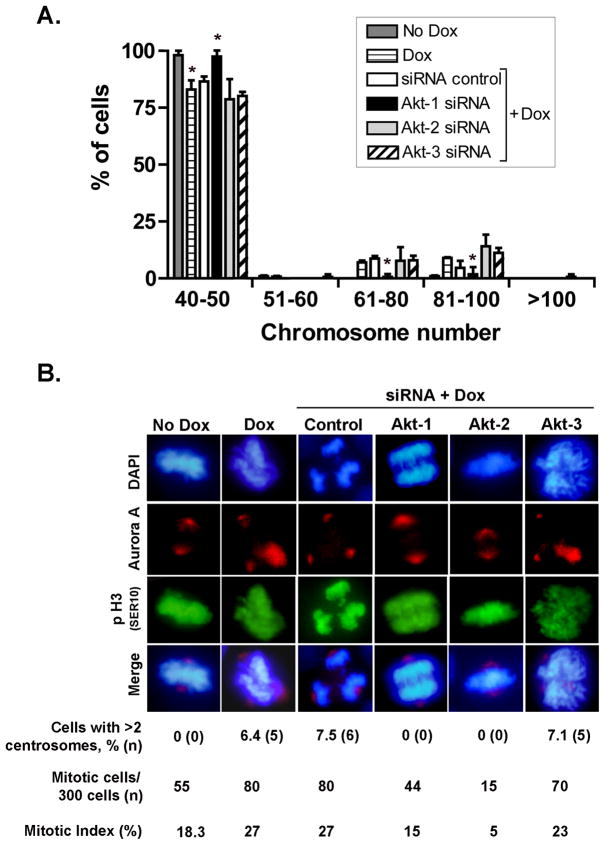
We next investigated the specific contribution of each Akt isoform to EZH2-induced functions by independent siRNA knockdown of Akt-1, Akt-2 and Akt-3 followed by Dox treatment to induce EZH2 overexpression (Figure 4B). Specific inhibition of Akt-1 decreased EZH2-induced BRCA1 nuclear export. In contrast, knockdown of Akt-2 or Akt-3 had no effect (Figure 4C). Akt-1 isoform was required for EZH2-induced genomic instability and abnormal mitosis. siRNA inhibition of Akt-1 completely prevented EZH2-induced polyploidy and mitotic defects (Figure 5A and B). Akt-2 and Akt-3 proteins were dispensable for EZH2-induced polyploidy (Figure 5A). Likewise, Akt3 expression was not required for EZH2 effect on abnormal mitosis (Figure 5B). Interestingly, Akt-2 KD blunted mitosis in MCF10A cells independent of EZH2 expression (mitotic index for siRNA control/no Dox = 18.3% and for siRNA control/Dox = 27%; compared to mitotic index for siRNA Akt-2/no Dox = 5.6% and for siRNA Akt-2/Dox = 5%). Further supporting the role of Akt pathway on BRCA1 localization and genomic instability, pharmacological inhibition of PI3K/Akt using LY294002 or Wortmannin prevented the EZH2-induced phenotype (Supplementary Figure 5 and Supplementary Table 1). Altogether, these results directly demonstrate that activation of PI3K/Akt-1 pathway is essential for EZH2-induced BRCA1 nuclear export, aneuploidy, and mitotic defects in benign breast cells.
Figure 5. Akt-1 is required for EZH2-dependent regulation of mitosis and genomic stability.
A. MCF10A-pLVX-EZH2 cells treated with siRNA control or siRNA-Akt-1, siRNA-Akt-2, or siRNA-Akt-3 were subjected to Dox for 3 days to induce EZH2 overexpression. Giemsa-stained metaphase spread analyses show that siRNA-Akt-1 is sufficient to prevent EZH2-induced polyploidy (≥51 chromosomes). In contrast, EZH2 overexpression increased numerical chromosomal alterations in cells treated with siRNA control, siRNA Akt-2, or siRNA Akt-3. Bar graph shows the mean percentage of cells ± SD. B. Immunofluorescence analysis of mitotic MCF10A- pLVX-EZH2 cells treated with siRNA control or siRNA-Akt-1, siRNA-Akt-2, or siRNA-Akt-3 and were then subjected to Dox for 3 days. p-H3 (Ser10) marks mitotic DNA; Aurora A marks the centrosomes, DAPI marks the nuclei. Note that Akt-1 siRNA prevented EZH2-induced mitotic aberrations and the number of cells with extra centrosomes. Akt-2 siRNA caused a 3-fold decrease in the number of mitotic cells independent of EZH2 overexpression (mitotic index for siRNA control/no Dox = 18.3% and for siRNA control/Dox = 27%; compared to mitotic index for siRNA Akt-2/no Dox = 5.6% and for siRNA Akt-2/Dox = 5%). Akt-3 KD was not able to prevent EZH2-induced chromosomal alterations.
EZH2 overexpression is associated with increased Akt-1 phosphorylation and decreased pBRCA1 nuclear localization in human invasive breast carcinomas
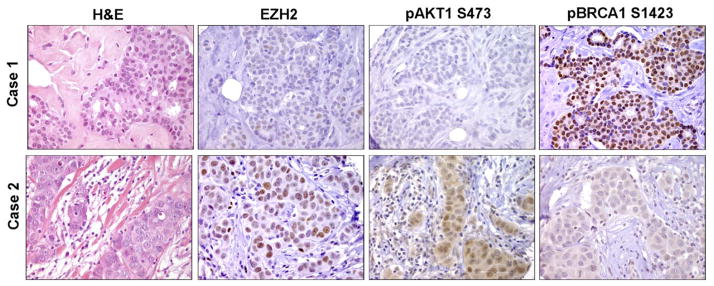
To examine whether this regulation also exists in tumor tissues, we compared the levels of EZH2, pAkt-1, and the expression and localization of pBRCA1 in 138 tumors by immunostaining. Consistent with our observations in cell cultures, upregulation of EZH2 was significantly associated with upregulation of pAkt-1 and decreased nuclear levels of pBRCA1 protein (Figure 6, Table 1). Of the 138 tumors 86 (62.3%) exhibited reciprocal expression of EZH2 and pBRCA1 proteins (49 (35.5%) had high EZH2 and low nuclear pBRCA1, and 37 (26.8%) had low EZH2 and high nuclear pBRCA1), Fisher’s exact test, p<0.005 (Table 1). Invasive breast carcinomas with high EZH2 and high pAkt-1 significantly showed low nuclear pBRCA1 expression, while those tumors with low EZH2 and low pAkt-1 exhibited high pBRCA1 expression, Fisher’s exact test, p=0.03 (Table 1). Concomitant high EZH2/high pAkt-1/low nuclear pBRCA1 is associated with ER negative status and high histological grade compared to low EZH2/low pAkt-1/high nuclear pBRCA1, Fisher’s exact test, p=0.005 (Supplementary Table 2).
Figure 6. EZH2 overexpression is associated with upregulation of pAkt1 and decreased nuclear BRCA1 expression.
Human breast cancer tissue samples (n=138) were immunostained for EZH2, pAkt1, and pBRCA1. Representative images of invasive ductal carcinomas.
Table 1.
Relationships between EZH2 expression and pAkt1, and pBRCA1 in human invasive carcinomas of the breast.
| EZH2 expression | EZH2 low/pAKT1 low | EZH2 high/pAKT1 high | p value | |||
|---|---|---|---|---|---|---|
| Low | High | p value | ||||
| pBRCA1 | ||||||
| Low nuclear | 31 | 49 | 19 | 30 | ||
| High nuclear | 37 | 21 | <0.005 | 18 | 9 | <0.03 |
| pAKT1 | ||||||
| Low | 37 | 8 | ||||
| High | 14 | 46 | <0.0001 | |||
DISCUSSION
A salient feature of EZH2 overexpressing human invasive breast carcinomas is their high histological grade and poorly-differentiated cells with pleomorphic nuclei (8–10). EZH2 overexpressing invasive carcinomas are largely ER negative and exhibit BRCA1 downregulation (8–11). We discovered that EZH2 regulates the intracellular distribution of BRCA1 protein in benign breast cells and in ER negative breast cancer cells. To draw these conclusions we investigated the effect of EZH2 on the intracellular localization of BRCA1 protein utilizing independent and complementary gain- and loss-of function approaches. By measuring the nuclear and cytoplasmic expression of BRCA1 protein at different time points after release from G1/S cell cycle block, it was concluded that EZH2 overexpression in MCF10A induced nuclear export with cytoplasmic retention of BRCA1 protein. Consistent with this observation, while BRCA1 was mainly localized to the cytoplasm of CAL51 breast cancer cells, it was translocated to the nucleus upon lentiviral-mediated EZH2 KD.
The mechanisms governing the nuclear-cytoplasmic shuttling of BRCA1 protein are not fully elucidated but recent studies implicate the membrane serine/threonine protein kinase B, Akt (23–26). A tumorigenic mechanism of Akt upon its phosphorylation is the induction of cytoplasmic localization of tumor suppressor proteins including p21 Cip1/WAF1 and FOXO3a (37, 38). The functional relationship between Akt and BRCA1 is complex and contextual (23). The PI3K/Akt pathway promoted nuclear translocation of BRCA1 and reciprocally, BRCA1 deficiency was able to activate the PI3K/Akt signaling (26, 39). Akt–1 activation was shown to induce cytoplasmic retention of BRCA1 protein in breast cancer cells (24). By using pharmacologic pathway inhibition and transient specific siRNA interference of Akt isoforms, we provide direct evidence that the effect of EZH2 on BRCA1 intracellular localization necessitates the activation of Akt-1, while Akt-2 and Akt-3 are dispensable for this function. Immunostaining of surgical samples highlights the relevance of our mechanistic studies to human breast cancer as EZH2 overexpression is significantly associated with increased pAkt-1 and with decreased pBRCA1 nuclear protein.
The stepwise progression from an aypical lesion to full-blown malignancy with metastatic capacity is associated with increases in genomic instability (40). BRCA1 deficiency can cause tetraploidy and aneuploidy (41). However, whether EZH2 regulates genomic stability is not known. Conditional EZH2 upregulation induced numerical chromosomal alterations in MCF10A cells as early as 72 hours after addition of doxycycline. Of note, over 50% of polyploid cells were near-tetraploid. These results are intriguing as several lines of evidence show that tetraploidy can be an initiator of chromosomal instability and tumorigenesis in vivo, and has been detected in human tissues before aneuploidy occurs (42–44). Our data on CAL51 breast cancer cells support the possible therapeutic role of EZH2 blockade in breast cancer, as EZH2 KD was sufficient to significantly decrease the percentage of tetraploid breast cancer cells. Thus, preventing or reverting tetraploidization through EZH2 inhibition may halt breast cancer development.
Although multiple mechanisms can lead to aneuploidy (40), alterations in mitosis play an important role. Overexpression of Aurora kinases A and B are required for centrosome maturation, bipolar spindle assembly and mitotic entry, and their overexpression in human cells results in abnormal mitosis and aneuploidy (40, 45, 46). We demonstrate that transient EZH2 overexpression in benign breast cells was sufficient to induce aberrant mitosis with extra centrosomes. The effect of EZH2 on mitosis was also evident in CAL51 breast cancer cells. While CAL51 controls exhibited aberrant mitosis with supernumerary centrosomes and multiple mitotic spindles, EZH2 KD abrogated these abnormalities. Mechanistically, EZH2 overexpression increased the messenger RNA and protein levels of Aurora kinase A and B and enhanced their kinase activity. These data implicate EZH2 in mitosis and in the regulation of Aurora kinase function in benign and in breast cancer cells.
Although Akt has been reported to play a role in mitosis and aneuploidy, the specific mechanisms have not been fully defined. Likewise, the specific role of each Akt isoform in the maintenance of genomic stability is unknown. Akt was shown to mediate abnormal checkpoint control and aneuploidy in PTEN-deficient cells by impairing CHK1 through phosphorylation, ubiquitination, and reduced nuclear localization (47). Especially intriguing in light of our data are results from a recent study demonstrating that Akt-1 activation induced supernumerary centrosomes and genomic instability through cytoplasmic retention of BRCA1 in a hamster ovary cell line (25). Here, we demonstrate that the effects of EZH2 overexpression on mitosis and genomic instability require specific activation of Akt-1. Interestingly, our data suggest a novel role for Akt-2 during mitosis unrelated to EZH2 expression. We observed that Akt-2 siRNA inhibition caused a 3-fold decrease in the number of cells undergoing mitosis in an EZH2-independent manner. Based on our data, we hypothesize that the blunting of mitoses may explain the absence of mitotic defects in Akt-2 KD cells after induction of EZH2 overexpression, as was observed with Akt-3 KD. The function of Akt-2 in mitosis warrants further study.
In conclusion, these data show a novel function of EZH2 in breast tumorigenesis: its ability to promote the nuclear export of BRCA1, induce aberrant mitosis and genomic instability. Our results enable us to pinpoint one mechanism by which EZH2 controls BRCA1 intracellular localization and genomic stability by activating Akt-1. In breast cancer cells, EZH2 downregulation induces nuclear localization of BRCA1, decreased mitotic aberrations and reverses tetraploidy. We propose that modulation of EZH2 expression may be a valid strategy to prevent or halt neoplastic progression in the breast.
Supplementary Material
Acknowledgments
We thank all members of the Kleer laboratory for helpful suggestions and critical reading of the manuscript, Dr. Nallasivam Palanisamy from the Center of Translational Pathology at the University of Michigan for help with the chromosomal analyses. We thank Drs. David Ferguson and Jeffrey DuBois for their help with the kinase assays, and Robin Kunkel for assistance with art work.
Financial Support: This work was supported by NIH grants R01 CA107469, R01 CA125577 and U01CA154224 (to CGK), the Fashion Charitable Foundation of New York/QVC, and the National Institutes of Health through the University of Michigan’s Cancer Center Support Grant (5 P30 CA46592). ACV was supported by grants from the Department of Defense Breast Cancer Research Program and the Center for Computational Medicine and Bioinformatics.
References
- 1.Laible G, Wolf A, Dorn R, et al. Mammalian homologues of the Polycomb-group gene Enhancer of zeste mediate gene silencing in Drosophila heterochromatin and at S. cerevisiae telomeres. Embo J. 1997;16:3219–32. doi: 10.1093/emboj/16.11.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Satijn DP, Otte AP. Polycomb group protein complexes: do different complexes regulate distinct target genes? Biochim Biophys Acta. 1999;1447:1–16. doi: 10.1016/s0167-4781(99)00130-x. [DOI] [PubMed] [Google Scholar]
- 3.Ringrose L, Paro R. Epigenetic regulation of cellular memory by the Polycomb and Trithorax group proteins. Annu Rev Genet. 2004;38:413–43. doi: 10.1146/annurev.genet.38.072902.091907. [DOI] [PubMed] [Google Scholar]
- 4.Haupt Y, Alexander WS, Barri G, Klinken SP, Adams JM. Novel zinc finger gene implicated as myc collaborator by retrovirally accelerated lymphomagenesis in E mu-myc transgenic mice. Cell. 1991;65:753–63. doi: 10.1016/0092-8674(91)90383-a. [DOI] [PubMed] [Google Scholar]
- 5.Hess JL. MLL: a histone methyltransferase disrupted in leukemia. Trends Mol Med. 2004;10:500–7. doi: 10.1016/j.molmed.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Raaphorst FM, van Kemenade FJ, Blokzijl T, et al. Coexpression of BMI-1 and EZH2 polycomb group genes in Reed-Sternberg cells of Hodgkin's disease. Am J Pathol. 2000;157:709–15. doi: 10.1016/S0002-9440(10)64583-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Visser HP, Gunster MJ, Kluin-Nelemans HC, et al. The Polycomb group protein EZH2 is upregulated in proliferating, cultured human mantle cell lymphoma. Br J Haematol. 2001;112:950–8. doi: 10.1046/j.1365-2141.2001.02641.x. [DOI] [PubMed] [Google Scholar]
- 8.Kleer CG, Cao Q, Varambally S, et al. EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proc Natl Acad Sci U S A. 2003;100:11606–11. doi: 10.1073/pnas.1933744100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collett K, Eide GE, Arnes J, et al. Expression of enhancer of zeste homologue 2 is significantly associated with increased tumor cell proliferation and is a marker of aggressive breast cancer. Clin Cancer Res. 2006;12:1168–74. doi: 10.1158/1078-0432.CCR-05-1533. [DOI] [PubMed] [Google Scholar]
- 10.Bachmann IM, Halvorsen OJ, Collett K, et al. EZH2 expression is associated with high proliferation rate and aggressive tumor subgroups in cutaneous melanoma and cancers of the endometrium, prostate, and breast. J Clin Oncol. 2006;24:268–73. doi: 10.1200/JCO.2005.01.5180. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez ME, Li X, Toy K, et al. Downregulation of EZH2 decreases growth of estrogen receptor–negative invasive breast carcinoma and requires BRCA1. Oncogene. 2009;28:843–53. doi: 10.1038/onc.2008.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Venkitaraman AR. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell. 2002;108:171–82. doi: 10.1016/s0092-8674(02)00615-3. [DOI] [PubMed] [Google Scholar]
- 13.Narod SA, Foulkes WD. BRCA1 and BRCA2: 1994 and beyond. Nat Rev Cancer. 2004;4:665–76. doi: 10.1038/nrc1431. [DOI] [PubMed] [Google Scholar]
- 14.Yoshikawa K, Ogawa T, Baer R, et al. Abnormal expression of BRCA1 and BRCA1-interactive DNA-repair proteins in breast carcinomas. Int J Cancer. 2000;88:28–36. [PubMed] [Google Scholar]
- 15.Wilson CA, Ramos L, Villasenor MR, et al. Localization of human BRCA1 and its loss in high-grade, non-inherited breast carcinomas. Nat Genet. 1999;21:236–40. doi: 10.1038/6029. [DOI] [PubMed] [Google Scholar]
- 16.Turner NC, Reis-Filho JS, Russell AM, et al. BRCA1 dysfunction in sporadic basal-like breast cancer. Oncogene. 2007;26:2126–32. doi: 10.1038/sj.onc.1210014. [DOI] [PubMed] [Google Scholar]
- 17.Foulkes WD, Stefansson IM, Chappuis PO, et al. Germline BRCA1 mutations and a basal epithelial phenotype in breast cancer. J Natl Cancer Inst. 2003;95:1482–5. doi: 10.1093/jnci/djg050. [DOI] [PubMed] [Google Scholar]
- 18.Lakhani SR, Reis-Filho JS, Fulford L, et al. Prediction of BRCA1 status in patients with breast cancer using estrogen receptor and basal phenotype. Clin Cancer Res. 2005;11:5175–80. doi: 10.1158/1078-0432.CCR-04-2424. [DOI] [PubMed] [Google Scholar]
- 19.Chen CF, Li S, Chen Y, Chen PL, Sharp ZD, Lee WH. The nuclear localization sequences of the BRCA1 protein interact with the importin–alpha subunit of the nuclear transport signal receptor. J Biol Chem. 1996;271:32863–8. doi: 10.1074/jbc.271.51.32863. [DOI] [PubMed] [Google Scholar]
- 20.Glover-Collins K, Thompson ME. Nuclear export of BRCA1 occurs during early S phase and is calcium-dependent. Cell Signal. 2008;20:958–68. doi: 10.1016/j.cellsig.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lotti LV, Ottini L, D'Amico C, et al. Subcellular localization of the BRCA1 gene product in mitotic cells. Genes Chromosomes Cancer. 2002;35:193–203. doi: 10.1002/gcc.10105. [DOI] [PubMed] [Google Scholar]
- 22.Ruffner H, Verma IM. BRCA1 is a cell cycle-regulated nuclear phosphoprotein. Proc Natl Acad Sci U S A. 1997;94:7138–43. doi: 10.1073/pnas.94.14.7138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nelson AC, Lyons TR, Young CD, Hansen KC, Anderson SM, Holt JT. AKT regulates BRCA1 stability in response to hormone signaling. Mol Cell Endocrinol. 319:129–42. doi: 10.1016/j.mce.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plo I, Laulier C, Gauthier L, Lebrun F, Calvo F, Lopez BS. AKT1 inhibits homologous recombination by inducing cytoplasmic retention of BRCA1 and RAD51. Cancer Res. 2008;68:9404–12. doi: 10.1158/0008-5472.CAN-08-0861. [DOI] [PubMed] [Google Scholar]
- 25.Plo I, Lopez B. AKT1 represses gene conversion induced by different genotoxic stresses and induces supernumerary centrosomes and aneuploidy in hamster ovary cells. Oncogene. 2009;28:2231–7. doi: 10.1038/onc.2009.85. [DOI] [PubMed] [Google Scholar]
- 26.Xiang T, Ohashi A, Huang Y, et al. Negative Regulation of AKT Activation by BRCA1. Cancer Res. 2008;68:10040–4. doi: 10.1158/0008-5472.CAN-08-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Puppe J, Drost R, Liu X, et al. BRCA1-deficient mammary tumor cells are dependent on EZH2 expression and sensitive to Polycomb Repressive Complex 2-inhibitor 3-deazaneplanocin A. Breast Cancer Res. 2009;11:R63. doi: 10.1186/bcr2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang W, Gonzalez ME, Toy KA, Banerjee M, Kleer CG. Blockade of CCN6 (WISP3) activates growth factor-independent survival and resistance to anoikis in human mammary epithelial cells. Cancer Res. 2010;70:3340–50. doi: 10.1158/0008-5472.CAN-09-4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roca H, Varsos Z, Pienta KJ. CCL2 protects prostate cancer PC3 cells from autophagic death via phosphatidylinositol 3-kinase/AKT-dependent survivin up-regulation. J Biol Chem. 2008;283:25057–73. doi: 10.1074/jbc.M801073200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Privette LM, Weier JF, Nguyen HN, Yu X, Petty EM. Loss of CHFR in human mammary epithelial cells causes genomic instability by disrupting the mitotic spindle assembly checkpoint. Neoplasia. 2008;10:643–52. doi: 10.1593/neo.08176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heerema NA, Sather HN, Sensel MG, et al. Prognostic impact of trisomies of chromosomes 10, 17, and 5 among children with acute lymphoblastic leukemia and high hyperdiploidy (> 50 chromosomes) J Clin Oncol. 2000;18:1876–87. doi: 10.1200/JCO.2000.18.9.1876. [DOI] [PubMed] [Google Scholar]
- 32.Raimondi SC, Pui CH, Hancock ML, Behm FG, Filatov L, Rivera GK. Heterogeneity of hyperdiploid (51–67) childhood acute lymphoblastic leukemia. Leukemia. 1996;10:213–24. [PubMed] [Google Scholar]
- 33.Raimondi SC, Zhou Y, Shurtleff SA, Rubnitz JE, Pui CH, Behm FG. Near-triploidy and near-tetraploidy in childhood acute lymphoblastic leukemia: association with B-lineage blast cells carrying the ETV6-RUNX1 fusion, T-lineage immunophenotype, and favorable outcome. Cancer Genet Cytogenet. 2006;169:50–7. doi: 10.1016/j.cancergencyto.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 34.Gioanni J, Le Francois D, Zanghellini E, et al. Establishment and characterisation of a new tumorigenic cell line with a normal karyotype derived from a human breast adenocarcinoma. Br J Cancer. 1990;62:8–13. doi: 10.1038/bjc.1990.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marumoto T, Zhang D, Saya H. Aurora-A - a guardian of poles. Nat Rev Cancer. 2005;5:42–50. doi: 10.1038/nrc1526. [DOI] [PubMed] [Google Scholar]
- 36.Hirota T, Kunitoku N, Sasayama T, et al. Aurora-A and an interacting activator, the LIM protein Ajuba, are required for mitotic commitment in human cells. Cell. 2003;114:585–98. doi: 10.1016/s0092-8674(03)00642-1. [DOI] [PubMed] [Google Scholar]
- 37.Yang JY, Hung MC. A new fork for clinical application: targeting forkhead transcription factors in cancer. Clin Cancer Res. 2009;15:752–7. doi: 10.1158/1078-0432.CCR-08-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou BP, Liao Y, Xia W, Spohn B, Lee MH, Hung MC. Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu–overexpressing cells. Nat Cell Biol. 2001;3:245–52. doi: 10.1038/35060032. [DOI] [PubMed] [Google Scholar]
- 39.Hinton CV, Fitzgerald LD, Thompson ME. Phosphatidylinositol 3-kinase/Akt signaling enhances nuclear localization and transcriptional activity of BRCA1. Exp Cell Res. 2007;313:1735–44. doi: 10.1016/j.yexcr.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schvartzman JM, Sotillo R, Benezra R. Mitotic chromosomal instability and cancer: mouse modelling of the human disease. Nat Rev Cancer. 10:102–15. doi: 10.1038/nrc2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schlegel BP, Starita LM, Parvin JD. Overexpression of a protein fragment of RNA helicase A causes inhibition of endogenous BRCA1 function and defects in ploidy and cytokinesis in mammary epithelial cells. Oncogene. 2003;22:983–91. doi: 10.1038/sj.onc.1206195. [DOI] [PubMed] [Google Scholar]
- 42.Barrett MT, Pritchard D, Palanca-Wessels C, Anderson J, Reid BJ, Rabinovitch PS. Molecular phenotype of spontaneously arising 4N (G2-tetraploid) intermediates of neoplastic progression in Barrett's esophagus. Cancer Res. 2003;63:4211–7. [PubMed] [Google Scholar]
- 43.Maley CC. Multistage carcinogenesis in Barrett's esophagus. Cancer Lett. 2007;245:22–32. doi: 10.1016/j.canlet.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 44.Storchova Z, Kuffer C. The consequences of tetraploidy and aneuploidy. J Cell Sci. 2008;121:3859–66. doi: 10.1242/jcs.039537. [DOI] [PubMed] [Google Scholar]
- 45.Vader G, Lens SM. The Aurora kinase family in cell division and cancer. Biochim Biophys Acta. 2008;1786:60–72. doi: 10.1016/j.bbcan.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 46.Zhang D, Hirota T, Marumoto T, et al. CreloxP-controlled periodic Aurora-A overexpression induces mitotic abnormalities and hyperplasia in mammary glands of mouse models. Oncogene. 2004;23:8720–30. doi: 10.1038/sj.onc.1208153. [DOI] [PubMed] [Google Scholar]
- 47.Puc J, Keniry M, Li HS, et al. Lack of PTEN sequesters CHK1 and initiates genetic instability. Cancer Cell. 2005;7:193–204. doi: 10.1016/j.ccr.2005.01.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.