Abstract
Ornithodoros hermsi Wheeler (Acari: Argasidae) is the vector of Borrelia hermsii, the primary cause of tick-borne relapsing fever in North America. This tick is one of the smallest Ornithodoros species involved with the biological transmission of spirochetes; yet, the amount of blood ingested while feeding is unknown. Therefore, we determined the amount of blood O. hermsi ingested during a bloodmeal to establish its potential for spirochete acquisition while feeding on an infected host. Ticks at different developmental stages were weighed before and after feeding and the volume of blood ingested was calculated. Females ingested the most blood, averaging ≈15 µl per meal, but late-stage nymphs took in the most blood in proportion to unfed body weight. A cohort of nymphs was weighed three more times during the 48 h after feeding, which demonstrated that O. hermsi may have excreted coxal fluid ranging from 24 –36% of the bloodmeal weight. We also developed a quantitative polymerase chain reaction method to determine the number of spirochetes ingested and maintained within the ticks after feeding. The density of spirochetes in ticks having just engorged was slightly less than in the host’s blood. In the first 5 d after feeding, the number of spirochetes within the ticks declined from the number initially ingested but then remained constant through 15 d. These observations establish a basis for future studies to determine the minimum number of spirochetes required in the host’s blood to allow O. hermsi to become persistently infected and transmit during subsequent bloodmeals.
Keywords: Borrelia hermsii, relapsing fever, ticks, quantitative PCR
Ornithodoros hermsi Wheeler (Acari: Argasidae), an argasid (“soft”) tick of the western United States and southern British Columbia, transmits the spirochete, Borrelia hermsii, the primary cause of tick-borne relapsing fever in North America (Dworkin et al. 2008). The tick feeds upon small mammals such as squirrels, chipmunks, and mice whereas humans are accidental hosts (Wheeler 1943).
Ticks acquire B. hermsii when they feed upon an infected, spirochetemic host. After tick feeding, the spirochetes disseminate through the midgut wall, invade the hemocoel, and establish a persistent infection in the salivary glands (Schwan and Hinnebusch 1998). During feeding, the tick transmits the spirochete quickly from the salivary glands to the host, possibly beginning as early as the first 30 s of attachment (Herms and Wheeler 1936b, Davis 1955), but the temporal dynamics of transmission are not known.
Unlike ixodid ticks, O. hermsi proceeds through two to five nymphal stages, molting between each stage, before maturing into either a male or female adult tick, which feed multiple times in a life span (Herms and Wheeler 1936a). O. hermsi is the smallest of the Ornithodoros species involved with relapsing fever in North America (Cooley and Kohls 1944) and feeds quickly, 15 min to 2 h, depending on the developmental stage and variation among individual ticks. The adults may feed on blood every few to several months but can survive in the laboratory for up to 7 yr (Herms and Wheeler 1936a, Wheeler 1943).
Water and ion balance is maintained in argasid ticks by the coxal glands, which are found between coxae one and two near the ventral surface of the tick (Balashov 1972). Excess water and salts are excreted from the glands during and/or shortly after feeding depending on the species of tick. O. hermsi reportedly excretes very small amounts of coxal fluid during feeding, which crystallizes rapidly upon contact with air (Herms and Wheeler 1936a).
Our study determined the amount of blood ingested by different stages of O. hermsi and how much coxal fluid may be excreted after feeding. By determining the bloodmeal size, we could predict the number of spirochetes acquired when ticks feed on an infected host with a known density of circulating spirochetes. We also developed a quantitative PCR (QPCR) method to determine the number of spirochetes the ticks ingested compared with the predicted value calculated from the size of their bloodmeal. This information will aid in determining the minimum number of spirochetes required to establish a persistent infection in ticks and elucidates some of the basic biology of O. hermsi, one of many vectors of a global disease.
Materials and Methods
Ticks
Ticks for our studies came from three uninfected colonies of O. hermsi maintained at the Rocky Mountain Laboratories that originated from ticks collected from Siskiyou County, CA (SIS), Wild Horse Island, Flathead Lake, Lake County, MT (WHI), and from an unknown geographic origin that has been at the laboratory for many years (RML). Larval, first through third stage nymphs, and adult WHI ticks were used as well as late-stage nymphs and adults of the other two colonies. Groups of ticks in each colony were kept separately in 12- or 50-ml ventilated tubes with filter paper to absorb excess fluid and stored in closed glass jars held at 22–25°C with 85% RH by using a saturated KCl solution (Winston and Bates 1960).
Tick Weighing and Feeding
Weights were determined for unfed O. hermsi ticks in various stages of their life cycle. In total, 721 nymphal and adult ticks were weighed: 475 nymphs, 129 males, and 117 females. Of those ticks, 644 ticks fed and were reweighed: 437 nymphs, 102 males, and 105 females. Ticks were weighed individually, except for the unfed larvae, on a Sartorius Semi-Micro SC-2 electronic balance (Sartorius AG, Goettingen, Germany), accurate to 0.0001 mg. The postlarval ticks were weighed in a plastic cap from a 1.5-ml tube to prevent them from walking off the balance. Ninety-six unfed larvae were weighed together in a snap-cap tube and the average weight was determined for one unfed larva. Next, the ticks were placed into separate containers each holding one 3–7-d-old neonatal RML strain mouse (a closed colony used at the RML since 1937 that originated from outbred Swiss-Webster mice). The containers were plastic jars with a lid, a fine mesh-covered air hole on the side, and plaster of Paris on the bottom (Endris et al. 1986). The containers holding a mouse and tick were placed into a dark 34°C incubator for 1–2 h, after which the fully engorged detached ticks were weighed again. The larvae that fed (n = 79) were then weighed individually and compared with the average weight of an unfed larva to estimate the amount of blood ingested. Those ticks that did not feed were excluded from further analysis.
The feeding of uninfected O. hermsi upon laboratory mice was done under protocol 2009-87. Infections of B. hermsii in O. hermsi and laboratory mice were done under protocol 2009-32. Both protocols were approved by the Institutional Animal Care and Use Committee of the Rocky Mountain Laboratories, Hamilton, MT. The laboratory work with B. hermsii was done with approval of the Institutional Biosafety Committee, Pathogen Registration HPRD 5–30.
Coxal Fluid Excretion
Multiple nymphal stages (n = 183) of each tick colony were weighed after feeding and again ≈3, 24, and 48 h later. Also, 44 female and 52 male ticks (RML and SIS) were weighed again 24 h after feeding. Between measurements, the engorged ticks were kept individually in 1.5-ml ventilated snap cap tubes with tissue paper in the bottom and stored in closed glass jars held at 22–25°C with 85% RH.
Quantification of Spirochetes by QPCR and Microscopy
Three adult RML mice were inoculated by intraperitoneal injection with 3 × 105 B. hermsii DAH, a strain isolated in 1991 from a human relapsing fever patient. Each mouse was bled once a day for 12 d, while anesthetized with isoflurane by nicking the tip of the tail and expressing blood from the tail vein. Ten microliters of blood was put into 190 µl of SideStep lysis and stabilization buffer (Agilent Technologies, Santa Clara, CA) for QPCR (see below), and 2.5 µl was spread evenly within a 9.5-mm-diameter circle etched into a glass slide for microscopy. The dried blood samples on microscope slides were stained with Giemsa, and spirochetes were counted in 25 fields at 600× (one field = 0.053 µl of blood) with an Eclipse E800 microscope (Nikon, Tokyo, Japan). The QPCR samples were further diluted (10 µl of sample into 90 µl of water), and 3 µl of this preparation were used in the QPCR reaction. QPCR was performed using the Stratagene Brilliant II QPCR master mix and protocol (Stratagene, La Jolla, CA). The primers (forward, 5′-AAGTCAGCTGCTCAAAATGTAAAAAC-3′; reverse, 5′-CAGCTAGTGATGCTGGTGTGTTAAT-3′) and probe (5′-TTTGCGGGTTGCATTCCAAGCTCTT-3′) were designed to the B. hermsii flaB gene with Primer Express software, version 2.0 (Applied Biosystems, Foster City, CA) and purchased from Applied Biosystems. The probe was labeled with the VIC reporter at the 5′ end and the TAMRA quencher at the 3′ end. Amplifications and detection were performed with the ABI Prism 7700 detection system (Applied Biosystems) with one cycle of 50°C for 2 min and 95°C for 10 min followed by 55 cycles of 95°C for 15 s and 60°C for 1 min. The number of spirochetes per milliliter of blood was calculated by both methods for each day after inoculation. The correlation of the numbers of spirochetes determined by QPCR and microscopy was graphed in Excel (Microsoft, Redmond, WA), and the correlation coefficient was calculated for the results of the two methods.
Two standard curves were constructed for analysis of QPCR results, a blood standard curve and a fed tick standard curve. Spirochetes for the standard curve were grown in mBSK-c medium (Battisti et al. 2008), and concentrations were calculated with a Petroff-Hauser counting chamber (Electron Microscopy Sciences, Hatfield, PA). The cultures were centrifuged and suspended in phosphate-buffered saline (PBS)-MgCl2 in serial dilutions from zero to 1 × 109 spirochetes per ml. Uninfected, engorged second stage nymphal ticks (SIS) were flash-frozen in liquid nitrogen and crushed with pestles in microcentrifuge tubes. The uninfected, engorged ticks and uninfected mouse blood, collected from the tail vein, were diluted separately to 1:20 in the lysis and stabilization buffer. Each dilution was then split into six 28.8-µl aliquots of which 3.2µl of the appropriate spirochete dilution was added to reach the final concentrations of 1 × 108 to 1 × 104 and zero spirochetes per milliliter. These preparations were further diluted 1:10 in sterile, distilled H2O of which 3 µl were used as the template for the QPCR reaction; each sample was tested in triplicate. The standard curves were created by plotting the CT value from the QPCR reaction versus the spirochete concentration within the aliquots and a best-fit line and equation were determined in Excel (Microsoft).
Quantification of Spirochetes in Ticks
An RML mouse was intraperitoneally injected with 2 × 108 spirochetes in 200 µl of PBS with 5 mM MgCl2. The next day, an adequate spirochetemia within the mouse was obtained. Twenty second-stage nymphal ticks (SIS) were weighed and tracked individually while they fed on the infected adult mouse anesthetized by ip injection of Nembutal (pentobarbital). Blood was obtained from the mouse by tail clip while it was anesthetized, and the blood was diluted 1:20 in lysis buffer. All ticks were weighed again within 1 h of feeding. Five of the 20 ticks were immediately frozen at −80°C (day 0), and the remaining 15 ticks were stored individually in microcentrifuge tubes as outlined above. At 5, 10 and 15 d postfeeding, five ticks each were weighed again and frozen. This was performed in triplicate with three separate mice on different days, and after all 59 ticks (one tick did not feed) were frozen, QPCR was performed on them. Infected ticks and blood were prepared and analyzed for QPCR similar to the control samples, except that no aliquots were made before diluting them in water.
Calculations and Statistics
The difference between the tick’s weight before and after feeding determined the weight of the bloodmeal. To determine the volume of blood ingested by each tick, the calculated bloodmeal weight was divided by 1.057, the specific gravity of mouse blood (King 1942). For example, a weight increase of 15.000 mg is equivalent to a bloodmeal volume of 14.191 µl. The equations for percentage of increase in body weight due to feeding and the percentage of blood weight lost as coxal fluid are listed below:
The concentration of spirochetes in each tick was calculated from the equation determined from the tick standard curve and the volume of each tick’s bloodmeal. The concentrations of spirochetes in the blood of the infected mice were calculated with the equation determined from the blood standard curve. To determine significance between spirochete concentrations in ticks and the mouse host they fed upon, the following statistical test was performed. The spirochete concentration in each tick was divided by the concentration in the blood of the mouse, and the mean of the log of these fractions was calculated. This was performed for each biological replicate and a one-sample t-test was performed.
The concentrations of spirochetes in ticks immediately after they fed (day 0) were compared with the mouse blood and analyzed by the student’s unpaired t-test. The day 0- and 24-h weights as well as the 24- and 48-h weights of the 183 nymphs tracked for coxal fluid excretion also were compared by the student’s unpaired t-test. The correlation between unfed tick weight and the volume of blood ingested, and the number of spirochetes determined by QPCR and microscopy were compared using the Pearson correlation test. The other data were analyzed by the one-way analysis of variance (ANOVA) and the Newman–Keuls multiple comparison tests. All statistical tests were performed in Prism 5 (GraphPad Software, San Diego, CA).
Results
Differences in Colonies of O. hermsi
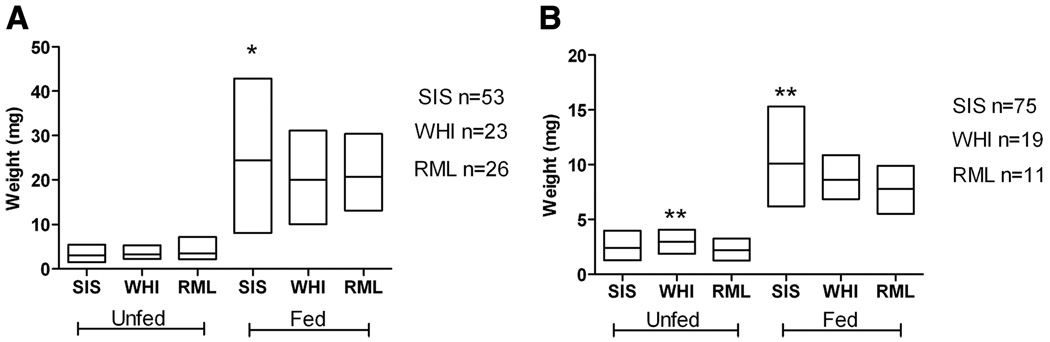
Adult ticks of the three colonies of O. hermsi varied in their fed weights (Fig. 1) and bloodmeal volume (Table 1). For example, both male (F = 17.20; df = 2, 102; P < 0.0001) and female (F = 7.808; df = 2, 99; P = 0.0007) O. hermsi SIS ingested more blood than the adult WHI and RML ticks, even though the SIS adults were not larger before feeding (Fig. 1; Table 1). This difference does not necessarily indicate inherent differences between the colonies but could be due to variation in the length of time between the last previous bloodmeal of the colony. However, for this reason comparisons among the life cycle stages that follow were done only with WHI ticks, which were weighed as larvae, first- through third-stage nymphs, and adults.
Fig. 1.
Weights of O. hermsi female (A) and male (B) ticks before and immediately after feeding. The three colonies used were O. hermsi Siskiyou County (SIS), Wild Horse Island (WHI), and Rocky Mountain Laboratories (RML). The asterisks (*, P < 0.05; **, P < 0.01) signify a statistically significant difference compared with the other colonies within the fed or unfed weights (Newman–Keuls multiple comparison test). The length of the box signifies the range in weight and the horizontal line within the box is the mean value; n, number of ticks.
Table 1.
Unfed, fed, and ingested blood weights and bloodmeal volumes (means ± SE) of O. hermsi by developmental stage
| Stagea | Colony | No. | Unfed tick wt (mg), mean ± SE |
Fed tick wt (mg), mean ± SE |
Ingested blood wt (mg), mean ± SE |
Bloodmeal vol (µl), mean ± SE |
|---|---|---|---|---|---|---|
| Larvae | WHI | 79 | 0.067 ± NAb | 0.308 ± 0.006 | 0.241 ± n/a | 0.228 ± NA |
| N1 | WHI | 43 | 0.190 ± 0.004 | 1.150 ± 0.037 | 0.961 ± 0.035 | 0.909 ± 0.033 |
| N2 | WHI | 121 | 0.498 ± 0.009 | 3.485 ± 0.082 | 2.988 ± 0.075 | 2.827 ± 0.071 |
| N3 | WHI | 67 | 1.405 ± 0.037 | 9.738 ± 0.282 | 8.333 ± 0.257 | 7.884 ± 0.243 |
| NL | RML, SIS | 127 | 1.663 ± 0.059 | 11.378 ± 0.392 | 9.714 ± 0.378 | 9.191 ± 0.358 |
| Male | WHI | 19 | 2.964 ± 0.144 | 8.615 ± 0.274 | 5.651 ± 0.280 | 5.346 ± 0.265 |
| Male | RML | 11 | 2.193 ± 0.179 | 7.793 ± 0.438 | 5.600 ± 0.315 | 5.298 ± 0.298 |
| Male | SIS | 75 | 2.408 ± 0.073 | 10.086 ± 0.220 | 7.679 ± 0.203 | 7.265 ± 0.192 |
| Female | WHI | 23 | 3.255 ± 0.157 | 20.009 ± 1.008 | 16.754 ± 0.942 | 15.850 ± 0.891 |
| Female | RML | 26 | 3.495 ± 0.280 | 20.728 ± 0.735 | 17.233 ± 0.767 | 16.300 ± 0.725 |
| Female | SIS | 53 | 3.007 ± 0.110 | 24.496 ± 1.021 | 21.489 ± 0.939 | 20.330 ± 0.888 |
N1, first nymphal stage; N2, second nymphal stage; N3, third nymphal stage; NL, late nymphal stage.
NA, not applicable.
Bloodmeal Size of WHI O. hermsi
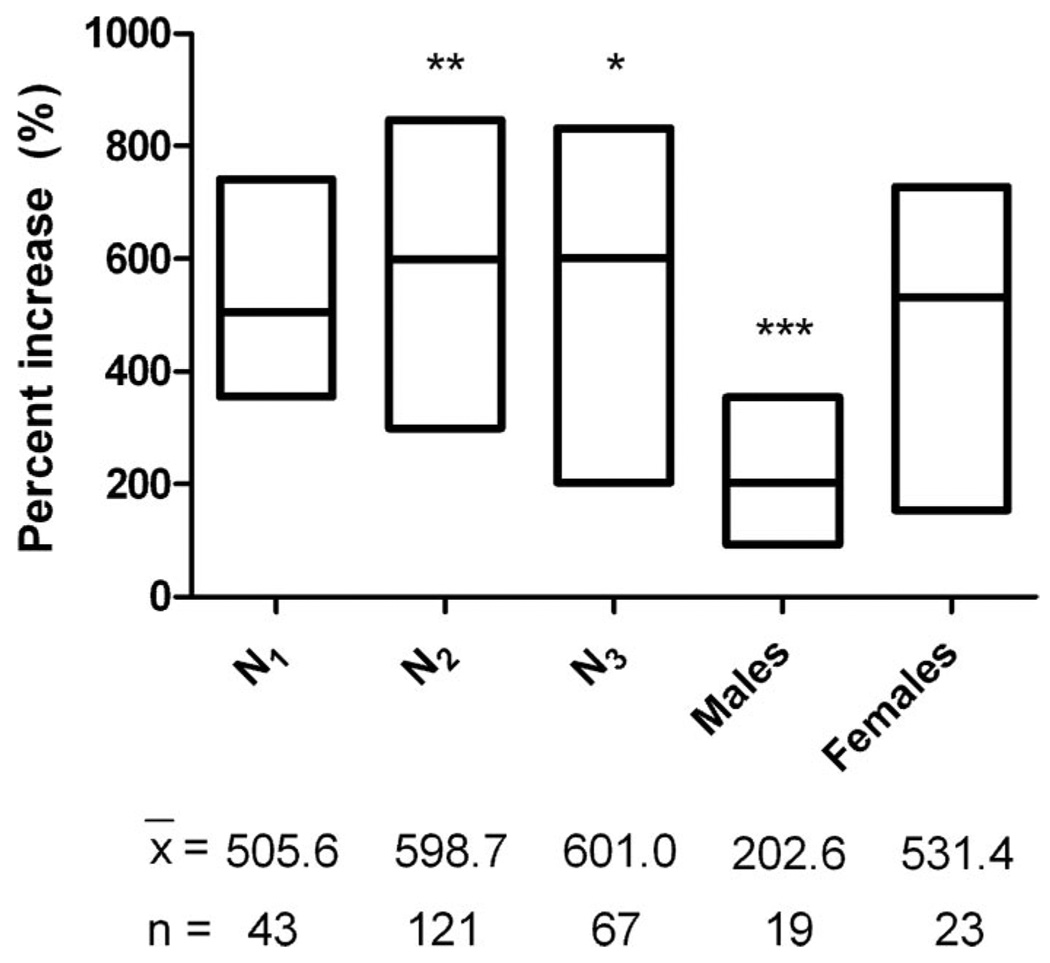
Female ticks ingested more blood than did the other stages, whereas third-stage nymphs ingested more blood on average than did males (Table 1). Second and third stage nymphs ingested the most blood in proportion to their unfed weight, and males took in the least (Fig. 2). The percent increase in body weight due to the bloodmeal for males was 202.6% whereas the first three nymphal stages and females increased in weight by >500% (Fig. 2).
Fig. 2.
Percentage increase in body weight for O. hermsi WHI ticks due to feeding. The asterisks (*, P < 0.05; **, P < 0.01; ***, P < 0.001) signify a statistically significant difference in those cohorts compared with females (Newman–Keuls multiple comparison test). The length of the box signifies the range in percentage weight increase and the horizontal line within the box is the mean value. x̄, mean percentage of increase; n, number of ticks.
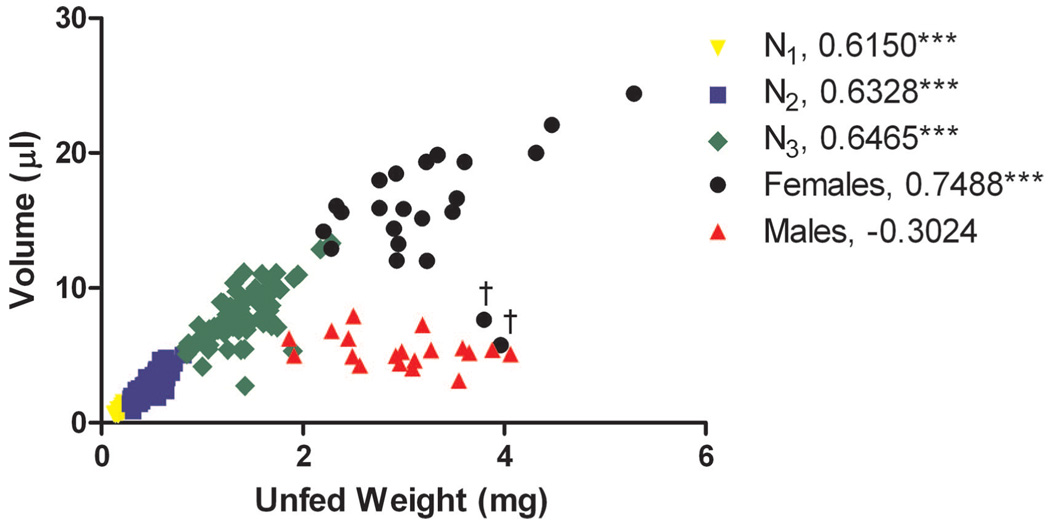
All nymphal stages and females, when excluding the two outliers, had a significant correlation between unfed weight and bloodmeal volume (Fig. 3). Larger nymphs ingested more blood than the smaller nymphs. In contrast, males had no significant correlation between their unfed weight and the amount of blood ingested (Fig. 3). Regardless of the size of the male, these ticks ingested about the same amount of blood.
Fig. 3.
Volume of blood ingested in relation to unfed body weight for multiple nymphal stages and adults of O. hermsi WHI. The asterisks (***, P < 0.001) signify a significant statistical correlation between unfed body weight and volume of blood (Pearson’s correlation coefficient). The number listed in the legend is the correlation coefficient. †, outliers.
Coxal Fluid Excretion
During the course of the study, O. hermsi seemed to excrete larger amounts of coxal fluid than previously reported (Herms and Wheeler 1936a,b). Ticks lost weight during the first 24 h after feeding (t = 4.277, df = 364, P < 0.0001) but not during the next 24 h (t = 0.5052, df = 364, P = 0.614). The loss of weight due to the excretion of coxal fluid ranged from 24.1 to 35.8% of the blood ingested (Table 2). No sign of defecation was found in the tubes or on the tissue paper kept with the ticks overnight, supporting our conclusion that the weight loss was due primarily to the excretion of coxal fluid.
Table 2.
Percentage of blood weight (mean ± SE) apparently lost as coxal fluid from O. hermsi by developmental stage during 24 h after feeding
| Stage | Colony | No. | % of blood wt being coxal fluid, mean ± SE |
|---|---|---|---|
| N1 | WHI | 36 | 24.1 ± 1.490 |
| N2 | WHI | 106 | 32.9 ± 0.795 |
| N3 | WHI | 28 | 35.8 ± 0.931 |
| NL | RML | 13 | 35.5 ± 2.169 |
| Males | RML and SIS | 52 | 34.7 ± 0.961 |
| Females | RML and SIS | 44 | 29.9 ± 1.111 |
N1, first nymphal stage; N2, second nymphal stage; N3, third nymphal stage; NL, late nymphal stage.
Quantification of Spirochetes by QPCR and Microscopy
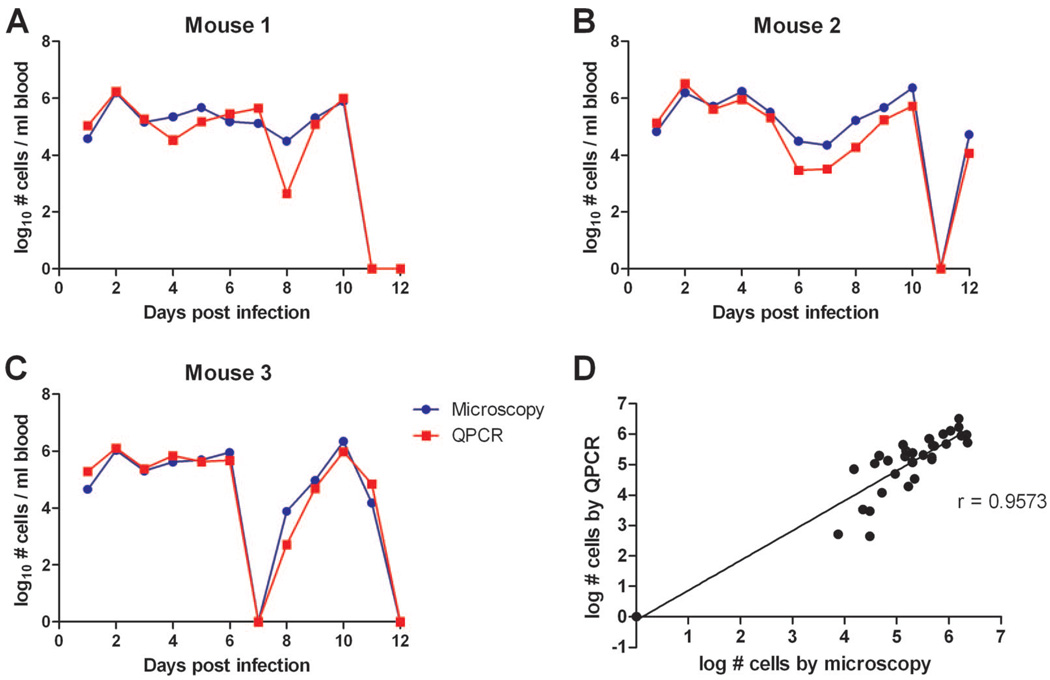
The numbers of spirochetes per milliliter of mouse blood examined during the 12 d after infection were plotted for QPCR and microscopy. The spirochetemias were observed by both methods and yielded strikingly similar estimates of the spirochete densities in blood (Fig. 4A and B). Because QPCR was much more efficient and less labor intensive than microscopy, we used QPCR to quantify spirochete concentrations in mouse blood and in the tick’s bloodmeal.
Fig. 4.
Quantification of the number of B. hermsii in the blood of three mice for 12 d after inoculation with 3 × 105 spirochetes (A–C). The quantities of spirochetes per milliliter of blood were determined by microscopy (blue) and by QPCR (red). Correlation between the numbers of spirochetes in mouse blood determined by microscopy and QPCR (D). r, correlation coefficient.
Spirochete Acquisition during Tick Feeding
Based on the amount of blood ingested by O. hermsi, we predicted the number of spirochetes that should be acquired by ticks when they feed on an infected animal with a known spirochete density in the blood (Table 3). For example, a female WHI O. hermsi that ingested an average bloodmeal (mean = 15.85 µl; n = 23; SD = 18.27) from a mouse with 1 × 106 spirochetes per ml in the blood should ingest 1.59 × 104 spirochetes.
Table 3.
Theoretical calculation of spirochete numbers acquired by O. hermsi WHI if fed upon a mouse with a spirochete concn within the blood of 1 × 106 spirochetes/ml
| Stage | Avg. bloodmeal vol (µl) |
Spirochete no. in tick |
|---|---|---|
| Larva | 0.228 | 2.28 × 102 |
| N1 | 0.909 | 9.09 × 102 |
| N2 | 2.827 | 2.83 × 103 |
| N3 | 7.884 | 7.88 × 103 |
| Male | 5.346 | 5.35 × 103 |
| Female | 15.850 | 1.59 × 104 |
N1, first nymphal stage; N2, second nymphal stage; N3, third nymphal stage.
Next, we applied QPCR to determine the number of spirochetes actually acquired by ticks that fed on spirochetemic mice. Three groups of ticks (n = 19, 20, and 20) were each fed on a different infected mouse with every tick being weighed before and immediately after feeding. Some ticks were then immediately frozen (day 0), whereas the others were kept alive and frozen on days 5, 10, or 15 after infection. Blood from the infected mice and all the ticks were then tested by QPCR. On day 0, spirochete concentrations within the ticks were lower, although marginally not significant (t = 3.855, df = 2, P = 0.0612), compared with the concentration of spirochetes in the blood of the mice they fed upon (Fig. 5). The concentration of spirochetes within the ticks initially dropped during the first 5 d after infection but not during the next 10 d (F = 16.30; df = 3, 55; P < 0.0001) (Fig. 6). These results suggested that the concentration of spirochetes may be diluted during the feeding process, and that the population of B. hermsii is not increasing in its vector, at least during the first 2 wk after acquisition by ticks.
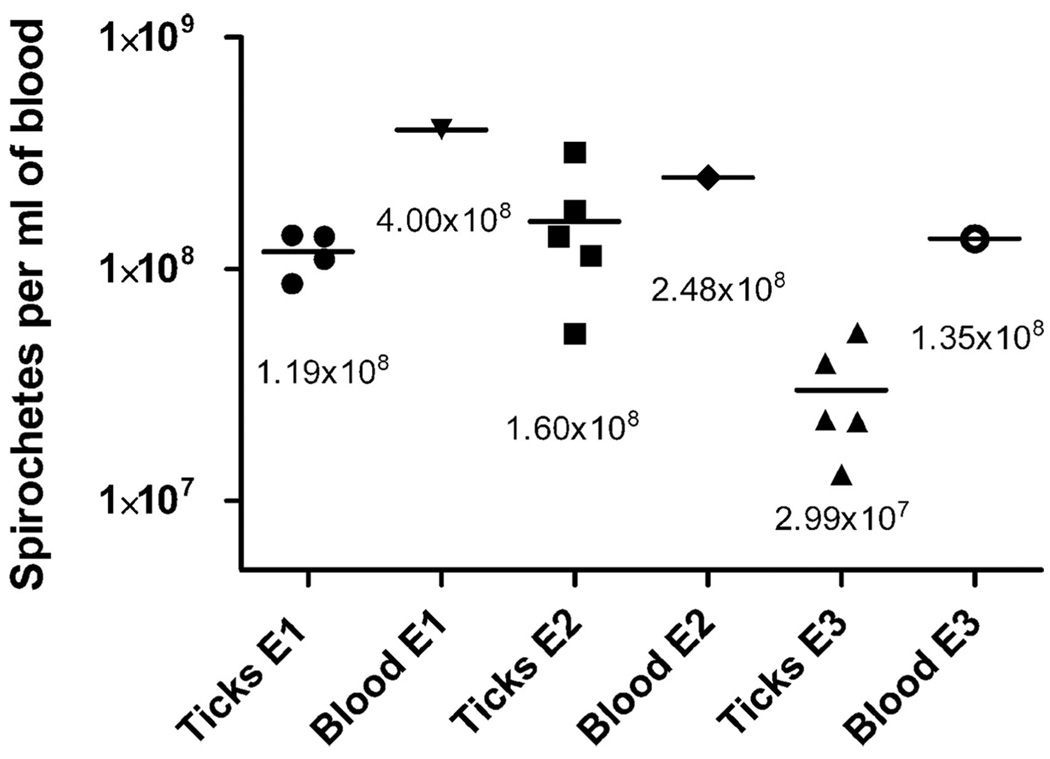
Fig. 5.
Concentrations of B. hermsii in ticks and blood from the mice upon which the ticks had just fed determined by QPCR. The mean spirochete concentrations (signified by the bar) are shown below the data points. E1, mouse experiment 1; E2, mouse experiment 2; E3, mouse experiment 3.
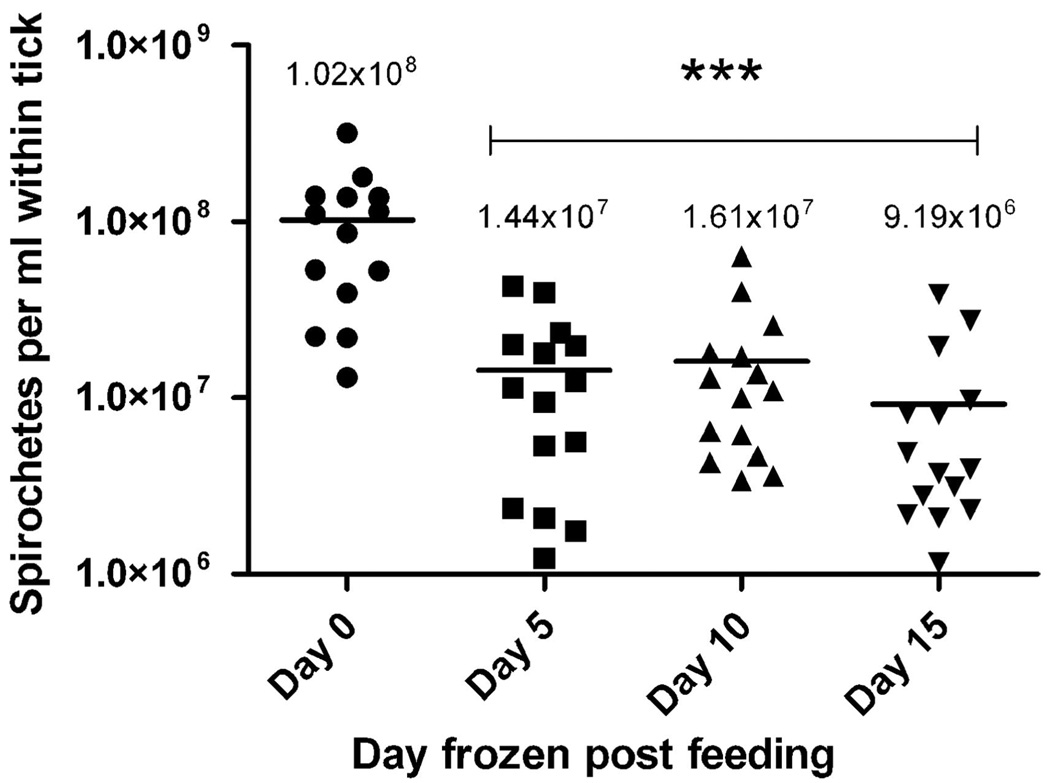
Fig. 6.
Concentration of B. hermsii in ticks on days 0, 5, 10, and 15 after infection quantified by QPCR. Spirochete concentrations (spirochetes per milliliter of blood) were normalized for comparison between ticks based on the volume of blood within the tick on day 0. The asterisk (***, P < 0.001) signifies a statistically significant difference between day 0 compared with the latter days (Newman–Keuls multiple comparison test). The mean spirochete concentrations (signified by the bar) are shown above the data points.
Discussion
Several studies have reported the size of the bloodmeal for Old World argasid ticks compared with the unfed body weight of the species examined. However, comparing our results to data in the published literature is complicated by several factors, which include the unknown or very small sample sizes, unknown stages and sex of the ticks that were fed, when ticks were weighed after feeding in relation to when the coxal fluid was excreted, and reporting weights after engorgement but with no weights measured before feeding. Lavoipierre and Riek (1955) examined the size of the bloodmeal and coxal fluid excretion in nine species of soft ticks, but for all but one species only one to seven individuals were observed and the stage and sex were not identified. Hefnawy et al. (1979) examined the bloodmeal of nymphal and adult Ornithodoros savignyi (Audouin), although the number of ticks used was not reported and all ticks were weighed after coxal fluid had been excreted. Isaac (1977) also waited until coxal fluid was excreted before weighing Argas arboreus Kaiser, Hoogstraal & Kohls that fed on domestic pigeons.
In his seminal monograph on ticks, Balashov (1972) presented the weight of blood ingested by different nymphal stages and adults of Ornithodoros papillipes [=O. tholozani (Laboulbène and Mégnin)] (25 ticks in each group) before their excretion of coxal fluid. The average bloodmeals ranged from a low of 2.54 mg for first-stage nymphs to a high of 68.09 mg for the females. Khalil et al. (1986) measured the weight of the bloodmeal for only female Ornithodoros erraticus (Lucas), a tick much smaller than O. tholozani, during three successive bloodmeals and found that the average amount of blood ingested varied from 26.9 to 32.7 mg (the third bloodmeal was the largest). Hafez et al. (1971) examined bloodmeals for three nymphal stages of A. arboreus, which averaged 2.87, 6.87, and 14.96 mg of blood for first to third nymphs, respectively. Based on the unfed weights of these ticks, all three species are larger than O. hermsi and they ingested considerably more blood, as would be expected on size alone. In addition, the data presented above are average weights of blood for the cohort examined. For both O. tholozani and A. arboreus, many individual ticks consumed far less or considerably more blood than the average amount calculated.
O. hermsi females, as in other species of argasid ticks, are larger on average than the males and nymphs (Table 1) (Wheeler 1935). Therefore, as one might have predicted, female O. hermsi imbibed the most blood compared with the other stages, which also was shown for female O. papillipes and O. erraticus (Balashov 1972, Khalil et al. 1986). However, these species are larger than O. hermsi and the females had average bloodmeal weights of 68.09 mg (O. papillipes) and 26.9–31.7 mg (O. erraticus) compared with 16.75–21.49 mg for the females of the three colonies of O. hermsi we examined (Table 1). Overall, we found a strong positive correlation between the weight of the unfed ticks and the amount of blood ingested; larger individuals consumed more blood (Table 1; Fig. 3); however, this was not true for males. Regardless of their unfed weight, the males ingested similar amounts of blood (Fig. 3), which were considerably less than the amounts of blood ingested by females of comparable size. Males also consumed less blood compared with many of the third-stage nymphs that were smaller (Table 1; Fig. 3). In fact, all nymphal stages ingested more blood in proportion to their unfed weights (percentage of weight gain) than did the adults and the males ingested the least amount of blood in proportion to their unfed body weight (Fig. 2). Balashov (1972) also found that male O. papillipes ingested the least amount of blood in proportion to their body weight (123% gain) compared with the other stages of this species.
Argasid nymphs require a large energy source to support their development through their molt to the next life stage. If a sufficient amount of blood is not ingested, nymphs may not molt or they will be smaller, resulting in more nymphal stages before they become adults (Balashov 1972). Female argasid ticks, as for many other female blood-feeding arthropods, have been referred to as “egg factories” that use the large bloodmeal to produce yolk-rich eggs during each gonotrophic cycle. Generally, female argasid ticks that ingest more blood will produce more eggs (Diehl et al. 1982), although there is a threshold for the amount of blood ingested, below which no eggs are laid. The fact that O. hermsi males ingested the least amount of blood relative to their size probably reflects their lower nutritional requirements compared with the nymphs and females.
All postlarval stages of argasid ticks excrete coxal fluid during or relatively soon after their rapid engorgement to eliminate excess water and salts from the bloodmeal and to concentrate the blood nutrients for more efficient digestion (Kaufman and Sauer 1982). Some ticks, such as Ornithodoros moubata (Murray) and Argas monolakensis Schwan, Corwin & Brown, may begin excreting copious amounts of coxal fluid while still attached to the host (Kaufman et al. 1982, Schwan et al. 1992). However, when O. hermsi begins to excrete coxal fluid is unclear. Early investigators of O. hermsi reported that these ticks excrete only minimal amounts of coxal fluid that crystallize immediately upon contact with air (Herms and Wheeler 1936a,b). We found only part of this observation is true; O. hermsi excreted very little coxal fluid at one time. We witnessed this microscopically by placing ticks that had fed 2–4 h previously with their ventral side up. By doing so, we observed extremely small droplets of coxal fluid that crystallized within a few seconds after appearing at the openings of the pores of the coxal glands. However, these ticks must have excreted coxal fluid repeatedly over 24 h after engorgement because during this time the fully fed ticks lost 24.1–35.8% of their bloodmeal weight without any evidence of other excretory products being eliminated.
Our estimates of the amount of coxal fluid excreted by O. hermsi as a percentage of the blood ingested are in line with values determined for several other species of argasid ticks: O. papillipes, 17–48% (Balashov 1972); O. erraticus, 19.7–23.0% (Khalil et al. 1986), and 37.4% (Lavoipierre and Riek 1955); O. moubata, 35% (Kaufman et al. 1981); and A. arboreus, 25.2% (Hafez et al. 1971). However, given that we weighed ticks within a few minutes to >1 h after they completed feeding, our estimates of the amount of blood ingested may be slightly less than what these ticks actually imbibed. Some weight loss due to the excretion of coxal fluid before them being weighed is possible. Some metabolic or evaporative water loss may have occurred also, but the fed nymphal ticks showed no indication of such loss between days 5 and 15 after engorgement. Although early observations regarding the excretion of coxal fluid by O. hermsi were partially correct, our data demonstrate that this species does excrete a significant amount of coxal fluid similar to values presented by other investigators for other species of ticks. The primary difference is that O. hermsi does not excrete copious amounts of fluid in only one or a few releases but probably does so with many repeated releases of tiny amounts for many hours after engorgement.
Our interest in how much blood O. hermsi ingested was driven by our broader goal to determine the potential these ticks have to acquire relapsing fever spirochetes when feeding on infected vertebrate hosts. O. hermsi is one of the smallest species of Ornithodoros known to be a vector of relapsing fever-causing borreliae, possibly second only to Ornithodoros sonrai Sautet and Witkowski (Wheeler 1935, Sautet and Witkowski 1944). Yet, all stages have high potential to acquire B. hermsii in spite of their small bloodmeal volumes, relative to other ticks, because of the high densities this bacterium can achieve in the peripheral blood (Table 3) (Coffey and Eveland 1967a, b).
We found that the concentrations of spirochetes in ticks immediately after they had fed were lower than the concentrations of spirochetes in the blood from the mice upon which they had fed (Fig. 5). These results were only marginally not significant with the P value close to the P < 0.05 cutoff. The experiment lacked an adequate number of blood samples, which might have obscured the significance of these data. However, the numbers of spirochetes predicted to be acquired during feeding were higher than what we observed. The three cohorts of second stage nymphs used in the acquisition experiments (Fig. 5) contained 22–65% fewer spirochetes than we would predict based on the size of their bloodmeals. This difference could be due to several factors. The blood samples from the infected mice were collected from the tail vein by nicking and expressing the blood by hand from the tip of the tail. Spirochete concentrations could potentially vary between the distal part of the tail vein and the capillaries in the skin of the belly where the ticks fed. Such differences in the concentration of spirochetes also may result from regional differences in blood flow induced by the pentobarbital anesthesia the mice were under when the ticks fed on them (Altura and Altura 1984). A drug-induced vasodilation could reduce the concentration of spirochetes in the blood vessels. O. hermsi, like all ticks, is a pool feeder, creating a hemorrhage in the dermis at their site of attachment (Sonenshine 1991). Possibly, spirochetes that escaped from the capillaries into the feeding lesions were less concentrated than the spirochetes in the peripheral circulation. Furthermore, O. hermsi feed quickly like other species of argasid ticks. Although these ticks are thought to ingest little or no lymph while feeding compared with the hard ticks that feed for many days (Sonenshine 1991), some lymph fluid may have been ingested, diluting the bloodmeals and decreasing the spirochete concentrations in the ticks.
Another goal for our future research is to determine the fate of B. hermsii numbers after ticks ingest the spirochetes. In our first attempt presented here (Fig. 6), we found that the concentration of spirochetes decreased by nearly 86% within the first 5 d after their acquisition by feeding, and then numbers remained stable over the next 10 d. We cannot explain this rather immediate decrease in the numbers of spirochetes early during infection in ticks, but might signify a drop in the number of genome equivalents detectable by PCR. We would anticipate an immediate decrease in the rate of replication of the spirochetes as they switch from a 37 to 21°C environment as the spirochetes move from the peripheral blood of the mammal to a tick no longer attached to its host and in a cooler ambient temperature. Although these engorged, second-stage nymphs defecate little this soon after feeding (we saw no evidence during the first 48 h after feeding), some spirochetes might be lost through fecal elimination. Also, some processes associated with digestion of blood in ticks that begin soon after engorgement might produce substances that are partially inhibitory for the survival of spirochetes or for the amplification of spirochete DNA by PCR.
One of the spirochetal agents of Lyme disease, B. burgdorferi, goes through significant replication in larval and nymphal Ixodes scapularis Say during and after these ticks feed (Piesman et al. 1990, de Silva and Fikrig 1995). In this tick–spirochete association, the bacteria reside in the interstitial spaces of the mammalian host’s skin (Sinsky and Piesman 1989) and are then acquired by these slow feeding ticks, inside which the bacteria become mixed with the nutrient-rich bloodmeal in the lumen of the midgut, then replicate and decline in numbers during the tick’s molt (Piesman et al. 1990). This transition of a relatively small number of spirochetes from skin to blood-engorged tick contrasts with the rapid acquisition of hundreds to thousands of B. hermsii along with the host’s blood in which these spirochetes already reside.
In future studies, we will follow the numbers of B. hermsii in O. hermsi for much longer periods, through the tick’s molt, and for months beyond. We also will follow spirochete numbers in specific locations to determine the quantity of spirochetes in midgut, hemolymph, and the salivary glands, where in the latter tissues the spirochetes establish a persistent infection to expedite transmission by these fast-feeding ticks (Schwan and Hinnebusch 1998). We also will use the methods described here to determine the minimum infectious doses that are required for B. hermsii to colonize O. hermsi to allow for transmission during subsequent bloodmeals.
Acknowledgments
We thank Paul Policastro for providing the RML colony ticks used in this study, Michael Fay for statistical help, and Philip Stewart and Joseph Hinnebusch for review of the manuscript. This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
References Cited
- Altura BT, Altura BM. Effects of barbiturates, phencyclidine, ketamine and analogs on cerebral circulation and cerebrovascular muscle. Microcirc. Endothelium Lymphatics. 1984;1:169–184. [PubMed] [Google Scholar]
- Balashov YS. Bloodsucking ticks (Ixodoidea)—vectors of diseases of man and animals. Misc. Publ. Entomol. Soc. Am. 1972;8:161–376. [Google Scholar]
- Battisti JM, Raffel SJ, Schwan TG. A system for site-specific genetic manipulation of the relapsing fever spirochete Borrelia hermsii. In: DeLeo FR, Otto M, editors. Methods in molecular biology 431: bacterial pathogenesis methods and protocols. Totowa, NJ: Humana Press; 2008. pp. 69–84. [DOI] [PubMed] [Google Scholar]
- Coffey EM, Eveland WC. Experimental relapsing fever initiated by Borrelia hermsi. I. Identification of major serotypes by immunofluorescence. J. Infect. Dis. 1967a;117:23–28. doi: 10.1093/infdis/117.1.23. [DOI] [PubMed] [Google Scholar]
- Coffey EM, Eveland WC. Experimental relapsing fever initiated by Borrelia hermsi. II. Sequential appearance of major serotypes in the rat. J. Infect. Dis. 1967b;117:29–34. doi: 10.1093/infdis/117.1.29. [DOI] [PubMed] [Google Scholar]
- Cooley RA, Kohls GM. The Argasidae of North America, Central America and Cuba. Am. Midl. Nat. Monogr. 1944;1:1–152. [Google Scholar]
- Davis GE. The endemic relapsing fevers. In: Hull TG, editor. Diseases transmitted from animals to man. Springfield, IL: Charles C. Thomas; 1955. pp. 552–565. [Google Scholar]
- de Silva AM, Fikrig E. Growth and migration of Borrelia burgdorferi in Ixodes ticks during blood feeding. Am. J. Trop. Med. Hyg. 1995;53:397–404. doi: 10.4269/ajtmh.1995.53.397. [DOI] [PubMed] [Google Scholar]
- Diehl PA, Aeschlimann A, Obenchain FD. Tick reproduction: oogenesis and oviposition. In: Obenchain FD, Galun R, editors. Physiology of ticks. Oxford, United Kingdom: Pergamon; 1982. pp. 277–350. [Google Scholar]
- Dworkin MS, Schwan TG, Anderson DE, Borchardt SM. Tick-borne relapsing fever. Infect. Dis. Clin. North Am. 2008;22:449–468. doi: 10.1016/j.idc.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endris RG, Haslett TM, Monahan MJ, Hess WS, Rutledge LC. Techniques for mass rearing soft ticks (Acari: Argasidae) J. Med. Entomol. 1986;23:225–229. doi: 10.1093/jmedent/23.3.225. [DOI] [PubMed] [Google Scholar]
- Hafez M, Abdel-Malek AA, Guirgis SS. The subgenus Persicargas (Ixodoidea: Argasidae, Argas). 12. Biological studies on the immature stages of A. (P.) arboreus Kaiser, Hoogstraal & Kohls in Egypt. J. Med. Entomol. 1971;8:421–429. doi: 10.1093/jmedent/8.4.421. [DOI] [PubMed] [Google Scholar]
- Hefnawy T, Khalil GM, Sidrak W. Blood meal weight and heme content during development of Ornithodoros (O.) savignyi. J. Med. Entomol. 1979;15:445–451. doi: 10.1093/jmedent/15.5-6.445. [DOI] [PubMed] [Google Scholar]
- Herms WB, Wheeler CM. The tick vector of the infection. Relapsing fever. California State Department of Public Health Special Bull. 1936a;61:24–28. [Google Scholar]
- Herms WB, Wheeler CM. Ornithodoros hermsi Wheeler as a vector of relapsing fever in California. J. Parasitol. 1936b;22:276–282. [Google Scholar]
- Isaac IS. The subgenus Persicargas (Ixodoidea: Argaside: Argas). 28. Argas (P.) arboreus: effect of blood meal weight on nymphal instar numbers. J. Med. Entomol. 1977;13:609–611. doi: 10.1093/jmedent/13.4-5.609. [DOI] [PubMed] [Google Scholar]
- Kaufman SE, Kaufman WR, Phillips JE. Fluid balance in the argasid tick, Ornithodorus moubata, fed on modified blood meals. J. Exp. Biol. 1981;93:225–242. [Google Scholar]
- Kaufman SE, Kaufman WR, Phillips JE. Mechansim and characteristics of coxal fluid excretion in the argasid tick Ornithodorus moubata. J. Exp. Biol. 1982;98:343–352. [Google Scholar]
- Kaufman WR, Sauer JR. Ion and water balance in feeding ticks: mechanisms of tick excretion. In: Obenchain FD, Galun R, editors. Physiology of ticks. Oxford, United Kingdom: Pergamon; 1982. pp. 213–244. [Google Scholar]
- Khalil GM, Helmy N, Hoogstraal H, El-Said A. Female Ornithodoros (Pavlovskyella) erraticus (Acari: Ixodoidea: Argasidae): effect of feeding. J. Med. Entomol. 1986;23:380–383. doi: 10.1093/jmedent/23.4.380. [DOI] [PubMed] [Google Scholar]
- King LS. Studies on eastern equince encephalomyelitis. J. Exp. Med. 1942;76:324–334. doi: 10.1084/jem.76.4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoipierre MMJ, Riek RF. Observations on the feeding habits of argasid ticks and on the effect of their bites on laboratory animals, together with a note on the productions of coxal fluid by several of the species. Ann. Trop. Med. Parasitol. 1955;49:96–113. doi: 10.1080/00034983.1955.11685655. [DOI] [PubMed] [Google Scholar]
- Piesman J, Oliver JR, Sinsky RJ. Growth kinetics of the Lyme disease spirochete (Borrelia burgdorferi) in vector ticks (Ixodes dammini) Am. J. Trop. Med. Hyg. 1990;42:352–357. doi: 10.4269/ajtmh.1990.42.352. [DOI] [PubMed] [Google Scholar]
- Sautet J, Witkowski M. A propos d’un Ornithodorus trouvé a Gao. Bull. Soc. Path. Exot. 1944;36:182–188. [Google Scholar]
- Schwan TG, Hinnebusch BJ. Bloodstream-versus tick-associated variants of a relapsing fever bacterium. Science. 1998;280:1938–1940. doi: 10.1126/science.280.5371.1938. [DOI] [PubMed] [Google Scholar]
- Schwan TG, Corwin MD, Brown SJ. Argas (Argas) monolakensis, new species (Acari: Ixodoidea: Argasidae), a parasite of California gulls on islands in Mono Lake, California: description, biology, and life cycle. J. Med. Entomol. 1992;29:78–97. doi: 10.1093/jmedent/29.1.78. [DOI] [PubMed] [Google Scholar]
- Sinsky RJ, Piesman J. Ear punch biopsy method for detection and isolation of Borrelia burgdorferi from rodents. J. Clin. Microbiol. 1989;27:1723–1727. doi: 10.1128/jcm.27.8.1723-1727.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenshine DE. Biology of ticks. New York: Oxford University Press; 1991. [Google Scholar]
- Wheeler CM. Anew species of tick which is a vector of relapsing fever in California. Am. J. Trop. Med. 1935;15:435–438. [Google Scholar]
- Wheeler CM. A contribution to the biology of Ornithodoros hermsi Wheeler, Herms and Meyer. J. Parasitol. 1943;29:33–41. [Google Scholar]
- Winston PW, Bates DH. Saturated solutions for the control of humidity in biological research. Ecology. 1960;41:232–237. [Google Scholar]