Abstract
Background
Estrogen receptor (ER) α is a successful therapeutic target in breast cancer, but patients eventually develop resistance to antiestrogens such as tamoxifen.
Methods
To identify genes whose expression was associated with the development of tamoxifen resistance and metastasis, we used microarrays to compare gene expression in four primary tumors from tamoxifen-treated patients whose breast cancers did not recur vs five metastatic tumors from patients whose cancers progressed during adjuvant tamoxifen treatment. Because Rho guanine dissociation inhibitor (GDI) α was underexpressed in the tamoxifen-resistant group, we stably transfected ERα-positive MCF-7 breast cancer cells with a plasmid encoding a short hairpin (sh) RNA to silence Rho GDIα expression. We used immunoblots and transcription assays to examine the role of Rho GDIα in ER-related signaling and growth of cells in vitro and as xenografts in treated nude mice (n = 8–9 per group) to examine the effects of Rho GDIα blockade on hormone responsiveness and metastatic behavior. The time to tumor tripling as the time in weeks from randomization to a threefold increase in total tumor volume over baseline was examined in treated mice. The associations of Rho GDIα and MTA2 levels with tamoxifen resistance were examined in microarray data from patients. All statistical tests were two-sided.
Results
Rho GDIα was expressed at lower levels in ERα-positive tumors that recurred during tamoxifen treatment than in ERα-positive tamoxifen-sensitive primary tumors. MCF-7 breast cancer cells in which Rho GDIα expression had been silenced were tamoxifen-resistant, had increased Rho GTPase and p21-activated kinase 1 activity, increased phosphorylation of ERα at serine 305, and enhanced tamoxifen-induced ERα transcriptional activity compared with control cells. MCF-7 cells in which Rho GDIα expression was silenced metastasized with high frequency when grown as tumor xenografts. When mice were treated with estrogen or estrogen withdrawal, tripling times for xenografts from cells with Rho GDIα silencing were similar to those from vector-containing control cells; however, tripling times were statistically significantly faster than control when mice were treated with tamoxifen (median tripling time for tumors with Rho GDIα small interfering RNA = 2.34 weeks; for control tumors = not reached, hazard ratio = 4.13, 95% confidence interval = 1.07 to 15.96, P = .040 [adjusted for multiple comparisons, P = .119]). Levels of the metastasis-associated protein MTA2 were also increased upon Rho GDIα silencing, and combined Rho GDIα and MTA2 levels were associated with recurrence in 250 tamoxifen-treated patients.
Conclusion
Loss of Rho GDIα enhances metastasis and resistance to tamoxifen via effects on both ERα and MTA2 in models of ERα-positive breast cancer and in tumors of tamoxifen-treated patients.
CONTEXT AND CAVEATS
Prior knowledge
Tamoxifen is often used to treat estrogen receptor (ER)–positive breast cancers, but the mechanisms whereby some patients become resistant have been unclear.
Study design
When microarrays were used to analyze gene expression in tumors from tamoxifen-sensitive vs tamoxifen-resistant patients, Rho guanine dissociation inhibitor (GDI) α was identified as a possible mediator of tamoxifen sensitivity. MCF-7 cells containing silencing RNA were used to examine the effects of decreased Rho GDIα expression on tamoxifen-resistant cell growth, Rho signaling, and ERα activity. The same cells were used to establish tumors as xenografts in nude mice. Last, low Rho GDIα and high expression of the metastasis-associated protein MTA2 were examined as potential biomarkers of tamoxifen-resistance among 250 patient tumors.
Contribution
MCF-7 cells expressing Rho GDIα silencing RNA were tamoxifen-resistant and had higher levels of Rho signaling and tamoxifen-induced ERα activity than control cells. When implanted in nude mice, these cells grew tumors even in the presence of tamoxifen, unlike control cells, and metastasized more readily. Preliminary results indicate that low Rho GDIα and high MTA2 expression may be useful clinical markers of tamoxifen-resistance.
Implications
Increased Rho, PAK1, and ERα activity by virtue of Rho GDIα inactivation appear to confer tamoxifen resistance. Consequently, inhibitors of these pathways might be useful to reverse tamoxifen resistance.
Limitations
The mechanistic experiments here were done in a single cell line, and the clinical data were based on a single, retrospective analysis. Further work in additional cell lines and a prospective clinical study will be necessary.
From the Editors
Tamoxifen and aromatase inhibitors are used to target estrogen receptor (ER) α in breast cancer patients (1). However, hormone resistance remains a clinical problem and a major cause of recurrence and mortality in ERα-positive disease (2,3). It is unlikely that simple loss of ERα signaling is the driving force for resistance because both metastatic tumors and tamoxifen-resistant breast cancer cell lines frequently retain ERα expression (4,5) and remain responsive to steroidal antiestrogens like fulvestrant.
Several mechanisms have been proposed for tamoxifen-resistance in ERα-positive breast cancer [reviewed in (6)]. Complications arising from resistant tumor metastases are the most common cause of patient death; however, only a few genes have been shown to be essential for metastatic behavior [eg, TWIST (7) and some members of the Rho signaling pathway (8,9)]. The Rho family of GTP-binding proteins (RhoA-C, Rac-1, and Cdc42) regulates the polymerization of actin to produce stress fibers, lamellipodia, and filopodia in response to extracellular signals, proliferation, and apoptosis (10). The Rho guanine dissociation inhibitors (Rho GDIs) are negative regulators of all three Rho family proteins (11). Higher levels of Rho and lower levels of Rho GDI family members are associated with lymph node involvement and breast cancer metastasis (12); accordingly, Rho GDIβ has been reported to be a metastasis suppressor in human bladder cancer cells (8).
In this article, we addressed the role of Rho GDIα in ERα action, tamoxifen resistance, and metastatic behavior in breast cancer. Using cell line models in which we reduced Rho GDIα levels using short hairpin (sh) RNA, we also examined the molecular mechanisms by which loss of Rho GDIα affects cell motility and invasiveness and alters hormone response. Resistance and metastatic potential were evaluated using tumor xenografts in mice, and Rho GDIα’s ability to predict outcomes in tamoxifen-treated patients was tested using survival analysis of a retrospective cohort.
Methods
Plasmids and Chemicals
The Xenopus vitellogenin ERE2-tk-luciferase reporter plasmid, pCMV-β-galactosidase, and plasmids expressing yellow fluorescence protein (YFP) fused with wild-type ERα (YFP-WT) or YFP fused with a serine 305 to alanine mutant of ERα (YFP-S305A ERα), orglutathione-S-transferase (GST) fused with the ERα hinge domain have been previously described (13). pcDNA3-Rho GDIα was kindly provided by Dr Michael Garabedian (New York University, NY), and the Rho GDIα insert cDNA was cut out with BamHI and EcoRI and subcloned between the BglII and EcoRI sites of pEYFP-C1 (Clontech, Palo Alto, CA). Constructs expressing GST fused with the wild-type ERα hinge domain (residues 253–310) or to a mutant S305A ERα hinge domain were generated through polymerase chain reaction and inserted into the pGEX 4T-1 vector (Pharmacia, Piscataway, NJ) at the BamHI and EcoRI cloning sites. To silence cellular Rho GDIα expression, a shRNA was designed using Clontech software to recognize the RhoGDIα coding region from 389 to 407 (5′-GAAGCAGTCGTTTGTGCTG-3′, gi:76780068) and was cloned into the pSiren-Retro Q vector (Clontech). We also generated a rescue vector in which Rho GDIα was made resistant to the Rho GDIα shRNA vector using the QuickChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA); the sense primer sequence used was 5′-TCAGAAGCAAGGTCCTTCGTCCTGAAGGAGGAGG-3′ (mutated nucleotides that provide resistance to shRNA are underlined), the template was the YFP-Rho GDIα plasmid, and the manufacturer’s protocol was followed. A control RNAi oligonucleotide was purchased from Clontech. The sources of 17β-estradiol, 4-hydroxy-tamoxifen, and ICI 182780 were as previously described (14). A selective inhibitor of the Rho-associated protein kinase ROK (Y27632) was purchased from Calbiochem (La Jolla, CA); puromycin was purchased from Sigma (St Louis, MO).
Tumor Specimens for Expression Microarray Analysis
Frozen breast tumor specimens from a cohort of nine patients who received adjuvant tamoxifen were selected from the tumor bank of the Breast Center, Baylor College of Medicine (Houston, TX) for use in the RNA analyses. This study was approved by the Baylor College of Medicine Institutional Review Board in accordance with federal human research study guidelines. Samples were 1) metastatic tumors from five patients whose breast cancers recurred within 11 months of the start of tamoxifen treatment (tamoxifen-resistant) and 2) primary tumors, collected at the time of initial diagnosis, from four patients who were treated with tamoxifen and remained disease free for 93–123 months with a median follow-up of 106 months (tamoxifen-sensitive). The patients had received no treatments other than tamoxifen. Microarray analysis on this cohort has been previously described (14).
Cell Culture and Transfection
The ERα-positive T47D human breast cancer cell line and HeLa human cervical cancer cell line were obtained from American Type Tissue Culture Collection (Rockville, MD). ERα-positive MCF-7 human breast cancer cells were previously described (15). All cell lines were maintained in minimal essential medium (MEM; Invitrogen, Carlsbad, CA) supplemented with 5% fetal bovine serum (FBS; Summit Biotechnology, Fort Collins, CO), 0.1 nmol/L nonessential amino acids, 2 mmol/L L-glutamine, and 50 U/mL penicillin–streptomycin, at 37°C with 5% CO2 and 95% air.
Tamoxifen-resistant MCF-7 cells were generated as described previously by Knowlden et al. (5) and Herman and Katzenellenbogen (16). Briefly, we cultured parental MCF-7 cells in phenol red-containing MEM medium supplemented with 5% FBS, antibiotics, and 10-64-hydroxy-tamoxifen (TR1 cells) or in phenol red-free MEM medium supplemented with 5% charcoal-stripped FBS, antibiotics, and 10-7M 4-hydroxy-tamoxifen (TR2 cells). Cells were continuously exposed to 4-hydroxy-tamoxifenfor 6 months during which time the medium was replaced every 4–5 days. Initially, cell growth was slow, but gradually increased, at which time the cells were designated MCF-7-TR. TR1 cells were continuously maintained in 1μM tamoxifen, and TR2 cells in 100 nM tamoxifen, for longer than 1 year.
To generate MCF-7 cells with stable silencing of Rho GDIα expression, subconfluent cells in 10-cm tissue culture dishes were transfected with 5μg of the vector carrying shRNA against Rho GDIα or 5 μg of the control vector using Fugene 6 reagent and the manufacturer’s protocol (Roche, Indianapolis, IN). Stable clones of cells that contained Rho GDIα shRNA or control plasmid were selected with 1 μg/mL puromycin for 2 weeks, and positive clones in which Rho GDIα expression was silenced were identified using immunoblot analysis with a rabbit polyclonal anti-Rho GDIα antibody (1:2000; Santa Cruz Biotechnology, Inc, Santa Cruz, CA). One single-cell stable clone and a pool of stable transfectants were used in experiments. In some experiments, we used pools of T47D or tamoxifen-resistant TR1cells, both of which were stably transfected with YFP-vector and YFP-GDIα expression plasmids and selected for 1 week with G418 antibiotic (Invitrogen).
Immunoblot Analysis
Cell extracts or immunoprecipitated proteins were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) as described (13). Antibodies used for immunoblotting were as follows: mouse monoclonal anti-ERα clone 6F11 (1:500; Vector Labs, Newcastle, UK); rabbit polyclonal anti-SRC1 (1:200; Santa Cruz Biotechnology, Inc); mouse monoclonal anti-TIF2 (1:200; BD Transduction Lab, Los Angeles, CA); mouse monoclonal anti-p190 to RASGRF1(1:500; BD Transduction Laboratories, San Diego, CA); rabbit polyclonal anti-Rho GDIα (1:2000; Santa Cruz Biotechnology, Inc); rabbit anti-MTA2 (1:2000; Sigma); mouse monoclonal anti-β-actin AC-15 (1:10 000, Sigma); rabbit polyclonal anti-ROKα (1:1000; Upstate Biotechnology); rabbit anti-phospho-ROKα Thr249(1:1000; Abcam, Cambridge, MA); and rabbit polyclonal anti-phospho ERα Ser305 (1:100; Millipore, Billerica, MA). Rabbit polyclonal anti-ERK1/2 (1:1000) and anti-phospho-ERK1/2 (1:500), rabbit polyclonal nuclear factor κB (NF-κB) p65 (1:1000) and rabbit polyclonal pNF-κB S536 (1:1000; 93H1) were from Cell Signaling, Beverly, MA. The proteins that were transferred to nitrocellulose membranes were blocked overnight in a blocking solution (Tris-buffered saline–Tween [0.1% Tween-20; 20 mM Tris, pH 8.0; 150 mM NaCl] with 5% powdered nonfat dried milk) then incubated with primary antibodies listed above overnight at 4°C and with secondary antibodies (goat anti-mouse [1:2000] or goat anti-rabbit [1:5000] antiserum; Amersham Biosciences, Piscataway, NJ) for 1 hour at room temperature, and visualized with enhanced chemiluminescence reagents (Alpha Innotech, San Leandro, CA). Immunoblots show a single representative of at least two or three separate experiments.
Colony Formation and Soft Agar Assays
For colony formation assays, 2.5 × 105 TR1 or TR2 cells per well were placed in six-well tissue culture plates 24 hours before transfection. One microgram of DNA (as indicated in the individual figure legends) and Fugene 6 reagent (Roche) were used to transfect the cells in each well. Starting 24 hours after transfection, the cells were incubated for 14 days with 800 μg/mL G418 antibiotic in the specific medium used to initially generate the tamoxifen-resistant cells, the colonies were stained with 1% crystal violet, and photographed under a microscope.
For soft agar assays, 5000 cells per well were plated in 4 mL of 0.35% agarose, 5% charcoal-stripped FBS in phenol red–free MEM, with a 0.7% agarose base in six-well plates. Two days after plating, medium containing vehicle or treatments as indicated was added to the top of the layer and replaced every 2 days. After 14 days, 300 μL of 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT; Sigma) were added to each well and incubated at 37°C for 4 hours. Plates were then placed at 4°C overnight, and colonies larger than 50 μm in diameter were counted.
Rho GTPase Activation Assays
Cells were seeded in 10-cm plates and grown for 2 days in estrogen-deprived medium (phenol red–free MEM with 5% charcoal-stripped FBS). Then, cells were washed with ice-cold PBS and lysed in MLB buffer (25 mM HEPES, pH 7.5; 150 mM NaCl, 1% IGEPAL CA-630 [Sigma], 10% glycerol, 25 mM NaF, 10 mM MgCl2, 1 mM EDTA, 10 mg/mL leupeptin, 10 mg/mL aprotinin, and 1 mM sodium orthovanadate). Cell lysates were clarified by centrifugation at 13 000g at 4°C for 10 minutes, and 700–1000 μg of cellular proteins were incubated with 15 μg of PAK-1 p21 binding domain (PBD) or 20 μg Rhotekin Rho binding domain (RBD) agarose conjugate (Upstate, Lake Placid, NY) at 4°C for 90 minutes. The beads were centrifuged and washed four times with MLB buffer. GTP-bound activated Rho proteins were detected by immunoblotting using mouse monoclonal antibodies against Rac-1 (1:3000); cdc42 (1:100; BD Transduction Laboratories, San Jose, CA); or rabbit polyclonal antibodies to RhoA, RhoB, or RhoC (all 1:200; from Cell Signaling).
Immunoprecipitation Assays
Immunoprecipitation assays were performed as described (17) with some modifications. High salt cell lysis buffer (15) with 1:100 diluted complete proteinase inhibitor III solution (Roche) was used to prepare cellular extracts, which were then adjusted to 150 mM NaCl plus 1.0% NP-40 (binding buffer) and incubated with 1-2 μg of the appropriate antibody as indicated. After centrifugation and washing four times with the lysis buffer, the precipitated components were eluted by boiling in SDS loading buffer, resolved by SDS-PAGE, and analyzed by immunoblotting.
p21-Activated Kinase 1 (PAK1) Immunocomplex Kinase Assay
Cells (3 × 106) were plated in 10-cm dishes with MEM supplemented with 10% FBS and incubated for 24 hours at 37°C in 5% CO2.Cells were then serum-starved for 48 hours in phenol red–free MEM before lysis in 50 mM HEPES (pH 7.0), 150 mM NaCl, 1 mM MgCl2, 1 mM EGTA, 10 mM NaPO4, 1% glycerol (vol/vol), 0.1% Triton X-100 (vol/vol), 1 mM Na3VO4, and 1:100 protease inhibitor cocktail tablet. Immunocomplex kinase assays were done with PAK1 protein immunoprecipitated from 200 μg of total cellular protein using 2 μg of rabbit polyclonalanti-PAK1 antibody (Santa Cruz Biotechnology, Inc) with rotation at 4°C for 2 hours. Immunoprecipitated pellets were washed three times with immunocomplex kinase assay wash buffer (0.1% Triton X-100, 150mMNaCl, and 10mM NaPO4 [pH 7.0]). Ten micrograms of purified GST-ERα hinge domain protein, described previously (13), was added to the immunoprecipitated pellets in 20 μL kinase reaction buffer containing 20 mM HEPES (pH 7.0), 6 mM MgCl2, 20 mM Na3VO4, and 10 μCi [γ-32P]-ATP (3000 Ci/mmol; NEN, Boston, MA) and incubated for 20 minutes at 22°C. The reaction was stopped by the addition of SDS loading buffer. The samples were resolved using SDS-PAGE and transferred onto nitrocellulose membranes. The input GST-ERα hinge domain proteins were visualized with Ponceau S staining, the levels of ERα phosphorylation were determined by autoradiography, and the immunoprecipitated PAK1 was detected by immunoblot with a rabbit polyclonal anti-PAK1 antibody (1:1000; Santa Cruz Biotechnology, Inc).
ERα Transactivation Assays
Cells were maintained in phenol red–free MEM supplemented with 5% charcoal-stripped FBS (Hyclone, Logan, UT) for 5–7 days. One day before transfection, 2.5 × 105 MCF-7 or T47D cells or 0.5 × 105 HeLa cells were plated in 2 mL medium per well in six-well plates and then transfected using Fugene 6 reagent (Roche) following the manufacturer’s protocol. The cells in each well were transfected with ERE-luciferase reporter, pCMV-β-galactosidase vector, and YFP-GDIα and/or ERα expression vectors as indicated. After 24 hours, cells were treated with estrogen (10−9M), 4-hydroxy-tamoxifen (10−7M) or vehicle as indicated in the figure legend for an additional 18–24 hours. Luciferase activity was assessed using the Luciferase Assay System (Promega) following the manufacturer’s protocol.
Xenograft Studies
MCF-7 vector control cells and a Rho GDIα shRNA stably transfected clone were established as xenografts in ovariectomized 5- to 6-week-old BALB/c athymic nude mice (Harlan Sprague Dawley, Madison, WI) by inoculating the mice subcutaneously with 5× 106 cells in 100 μL of medium with 100 μL of Matrigel (BD Biosciences, San Jose, CA), as described previously (18). The mice were supplemented with 0.72 mg 60 day–release estradiol pellets that were implanted subcutaneously between the shoulder blades (Innovative Research, Sarasota, FL). Tumors were measured with calipers three times per week. When tumors reached approximately 250 mm3 (ie, in 1.5–2.5 weeks), the mice were randomly allocated to continue estradiol (n = 8–9 mice per group) or to estrogen withdrawal plus tamoxifen (n = 7–8 mice per group; estradiol pellets were removed and 500 μg/d of tamoxifen was given to each mouse subcutaneously in peanut oil, on each of 5 d/wk) for another 30 days. Tumor growth was assessed, and tumor volumes were measured as described previously (18). Mice were killed when moribund or when the tumor burden exceeded 1 cm3. Animal care was maintained in accordance with institutional guidelines.
Immunohistochemistry
Immunohistochemistry was performed by the Breast Center Pathology Core facility at Baylor College of Medicine, Houston, TX. Lung metastases arising from xenografts of Rho GDIα shRNA-containing MCF-7 cells were fixed overnight in 10% formalin buffered in methanol, followed by paraffin embedding. Thin (5 μm) sections were stained with hematoxylin and eosin (H&E). For detection of ERα and progesterone receptor, thin sections of formalin-fixed paraffin-embedded tissues were deparaffinized and rehydrated from xylene through a graded series of ethanol washes. Antigen retrieval was carried out by boiling in Tris–Cl (pH 9.0) in a standard pressure cooker for 20 minutes. Endogeneous peroxidases were blocked by treatment with 3% hydrogen peroxide in methanol. Protein blocking was performed with Avidin Solution A (A/B blocking Kit; Vector Laboratories, Burlingame, CA) according to manufacturer’s protocols. Slides were incubated with mouse anti-ERα 6F11 antibody (1:200; Vector Labs) or mouse anti-PR (1:1600, PgR1294; Dako, Inc, Carpinteria, CA) for 1 hour at room temperature, followed by a horseradish peroxidase–conjugated secondary antibody, and horseradish peroxidase was detected with diaminobenzidine tetrahydrochloride (Invitrogen), and counterstained with Gills hemotoxylin. Images were captured on a Zeiss Axioskop 2 plus microscope with a Canon Powershot G5 camera.
Invasion Assay
CTRL sh and GDIα sh cell invasion was determined using 24-well BD Biocoat invasion chambers (Bedford, MA) containing 8-μm porous membranes precoated with Matrigel. Cells were serum-starved for 2 days. The cells were removed from the plate with warm versene (Lonza, Switzerland) and 100 000 cells were seeded into the chambers in serum-free phenol red–free MEM (Invitrogen) with or without 17β-estradiol (E, 10−9 M), 4-hydroxy-tamoxifen (T, 10−7 M), or the ROK inhibitor Y-27631 (R, 10−8 M). Chemoattractant, or medium containing 10% FBS, was added to the lower compartment. After 22 hours incubation at 37°C, the noninvasive cells and Matrigel were removed from the inside of the wells with a cotton swab. The invading cells were then fixed with Diff-Quik Fixative and stained with Diff-Quik Stain Solution I and II (Siemens, Aktiengesellschaft Erlangen, Germany). Finally, the membranes were removed from the chambers using a scalpel blade and mounted onto glass microscope slides, and cell invasion was quantified by counting the number of cells per membrane at ×20 magnification. Data were then plotted as mean number of cells in triplicate. Error bars correspond to 95% confidence intervals; P values were calculated using two-sided Student t tests.
Statistical Analyses
Three major types of statistical analysis were used to analyze data for this study: those for the cell line experiments, for xenograft data, and for analysis of a breast tumor dataset that was previously described by others (see below). In all cases, P values less than .05 were considered to denote statistical significance, and all statistical tests were two-sided. For cell line experiments, statistical differences (P values) among groups were obtained using a two-sided Student t test with GraphPad Prism 5 software (GraphPad Software, San Diego, CA).
The xenograft experiments were analyzed using survival analysis methods to address the main question of whether, in the absence of tamoxifen, the tumors that arose from cells with Rho GDIα shRNA grew faster than those that arose from cells containing only the empty vector and whether they were resistant to tamoxifen. We tested the hypothesis that decreased Rho GDIα expression would render cells resistant to the growth inhibitory effects of tamoxifen. We calculated the time to tumor tripling as the time in weeks from randomization to a threefold increase in total tumor volume over baseline. Time to tumor tripling is a direct way to assess differences in tumor growth rate, without explicitly modeling tumor growth (which requires a common model structure across all groups). Survival curves were computed by the Kaplan–Meier method and compared with the two-sided log-rank test. Wald 95% confidence intervals for the proportion of mice that were tumor-tripling free at 4 and 8 weeks were computed using the standard error of the survival function at the maximum event time less than or equal to 4 (or 8) weeks. Individual comparisons, after rejection of the global null hypothesis, were tested using contrasts in the context of a Cox regression. We used visual inspection of a graphical examination of log-log(s) plots to feel comfortable that the assumptions of proportionality were confirmed for the Cox analysis. Exponentiation was used to calculate the upper and lower limits of Wald 95% confidence intervals of the corresponding regression coefficients. P values were adjusted by the method of Holm (19). Analyses were carried out using SAS (V9.2; Cary, NC) and R (Version 2.9.2) (20).
To test the effects of Rho GDIα and MTA2 in retrospective clinical samples, we used the dataset previously described by Loi et al. (21), available at the Gene Expression Omnibus (GEO) repository (http://www.ncbi.nlm.nih.gov/geo), accession number GSE6532. The probe sets 213606_s_at, 201168_x_at, 201167_x_at, and 211716_x_at were averaged to represent the expression of Rho GDIα, and 203444_s_at was used to represent the expression of MTA2. Clinical data were matched to the expression data for all 255 tamoxifen-treated samples in the Loi et al. training dataset in the GEO database and samples that were not ERα positive or that were missing expression data were excluded, resulting in the inclusion of samples from 250 ER-positive tamoxifen-treated patients. Optimum cut points for the analyses were calculated using a bootstrapping approach wherein the χ2 statistic was calculated using the two-sided log-rank test for each of a range of cut points on each of 500 bootstrapped samples. From these χ2 statistics, the median χ2 statistic was obtained for each cut point and used to select the cut points presented here. Because this dataset contains samples from many institutions and studies (21), we compared the proportion of samples that were RhoGDIα low and MTA2 high in each study with that of all other patients using the two-sided χ2 test and found no difference in study proportions in these populations, indicating that the observed survival difference was not caused by a study bias. The statistical program R with the package Survival was used to analyze the Loi dataset (20). Survival curves were compared using log-rank tests.
Results
Rho GDIα Loss and the Acquisition of a Tamoxifen-Resistant Phenotype
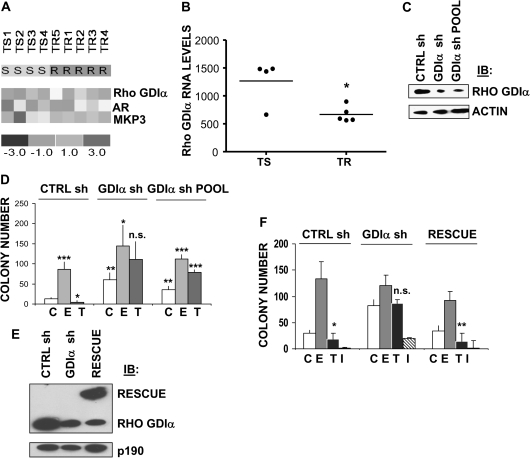
To identify genes whose expression is associated with the development of tamoxifen resistance, we used microarrays to compare gene expression in four primary tumors from patients treated with tamoxifen whose breast cancers did not recur during extended follow-up (in >93 months), with that in five metastatic tumors from patients whose breast cancers progressed during tamoxifen treatment (in <11 months).We have already reported finding novel mechanisms of tamoxifen resistance in this cohort that involve mitogen-associated protein kinase phosphatase 3 (MKP3) and tamoxifen-induced oxidative stress (14) and overexpression of the androgen receptor (AR; 22). Accordingly, cluster analysis showed that both MKP3 and AR were overexpressed in these tamoxifen-resistant tumors (Figure 1, A).
Figure 1.
Rho guanine dissociation inhibitor (GDI) α expression and resistance to tamoxifen. A) Clustering analysis of selected gene expression patterns in tamoxifen-resistant vs tamoxifen-sensitive breast cancers. Rho GDIα, AR, and MKP3 expression were measured by microarray analysis of mRNA from primary breast cancer tissue from patients whose tumors were tamoxifen-sensitive (TS, n = 4) and from metastatic breast cancer tissue from patients whose tumors were tamoxifen-resistant (TR, n = 5). Relative gene expression between the two groups is represented by color, with red indicating high and blue indicating low levels of expression. B) Statistical analysis of Rho GDIα RNA expression in the four tamoxifen-sensitive and five tamoxifen-resistant breast tumors shown in (A). There was a statistically significant difference in Rho GDIα expression between tamoxifen-resistant and tamoxifen-sensitive cells (P = .017, two-sided t test). C) Silencing of Rho GDIα expression in MCF-7 cells. Immunoblot (IB) of whole-cell lysates from MCF-7 cells stably transfected with either a scrambled shRNA (CTRL sh) or Rho GDIα shRNA (GDIα sh), or from a pool of MCF-7 cells stably expressing the Rho GDIα shRNA (GDIα sh POOL). Blots were cut and rabbit polyclonal antibodies recognizing Rho GDIα were used to probe one portion of the membrane, and a mouse monoclonal antibody to β-actin (ACTIN), as a loading control, on the other. D) Anchorage-independent growth and tamoxifen sensitivity of MCF-7 cells with and without Rho GDIα silencing. CTRL sh, GDIα sh, and GDIα sh POOL cells were seeded (5000/well) in 0.35% agarose and then treated with 17β-estradiol (E, 10-9 M), 4-hydroxy-tamoxifen (T, 10-7 M), or vehicle (C). Cells were allowed to grow for 14 days and the numbers of colonies that were larger than 50 μm were counted. The graphs represent the mean colony number on three plates from each of three independent experiments. Error bars correspond to 95% confidence intervals. P values were calculated using two-sided Student t tests. For C treatment between CTRL sh and GDIα sh, **P = .004 and between CTRL sh and GDIα sh POOL, **P = .006. For C vs E and C vs T in CTRL sh cells, ***P = .001 and *P = .020, respectively. For C vs E and C vs T in GDIα sh cells, *P = .027 and n.s. (not significant), respectively. For C vs E and C vs T in GDIα sh POOL, ***P < .001 and ***P < .001, respectively. E) Rescue of Rho GDIα expression. GDIα sh-containing MCF-7 cells were transfected with a silencing-resistant version of Rho GDIα, YFP-GDIα. Whole-cell lysates from CTRL sh, GDIα sh, and the RESCUE clone were immunoblotted. Rabbit polyclonal antibodies recognizing Rho GDIα were used to evaluate its expression on one portion of the blot, and a mouse monoclonal antibody to p190 RASGRF1was used to probe the other portion to ensure equal loading. F) Anchorage-independent growth and tamoxifen and fulvestrant sensitivity of GDIα sh vs rescue cells. CTRL sh, GDIα sh and RESCUE clones were plated in soft agar and then treated with 17β-estradiol (E, 10−9 M), 4-hydroxy tamoxifen (T, 10−7 M), fulvestrant (ICI 182780, I, 10−8 M), or vehicle (C). Data are the mean colony number from three plates and are representative of two independent experiments. Error bars correspond to 95% confidence intervals. P values were calculated using two-sided Student t tests. For C vs T in CTRL sh, GDIα sh, and RESCUE cells, *P = .01, P = n.s. (statistically nonsignificant), and **P = .002, respectively.
Multiple resistance mechanisms could be operating in tamoxifen-resistant patients. Levels of Rho GDIα were statistically significantly reduced in tamoxifen-resistant tumors as compared with tamoxifen-sensitive tumors (P = .017; Figure 1, B). Because lower levels of the Rho GDIs have been associated with metastasis (12), we established clones of ERα-positive MCF-7 cells, which have low metastatic potential (23) that were stably transfected with a plasmid encoding a Rho GDIα shRNA (GDIα sh) or a scrambled control shRNA (CTRL sh).We also generated a pool of MCF-7 cells that stably expressed the Rho GDIα shRNA (GDIα sh POOL). Expression of GDIα protein was efficiently silenced in both the cloned and pooled GDIα sh–transfected cells (Figure 1, C). We examined anchorage-independent growth using soft agar assays (Figure 1, D); the growth of cells transfected with vector alone was enhanced with estrogen and inhibited with tamoxifen, whereas pooled or cloned cells containing GDIα shRNA grew better under basal hormone-independent conditions and were further growth-stimulated with either estrogen or tamoxifen, thus demonstrating tamoxifen insensitivity. Similar results were seen in cells grown in culture using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) growth assays (Supplementary Figure 1, available online).
We next generated a stable pool of cells in which we “rescued” GDIα expression by transfecting cloned GDIα sh–containing cells with a mutant version of Rho GDIα that was resistant to small interfering RNA interference (Figure 1, E). CTRL sh cells and rescued cells were growth-inhibited with tamoxifen, whereas GDIα sh cells were insensitive to tamoxifen-mediated growth inhibition (Figure 1, F), but still sensitive to growth inhibition by the anti-estrogen ICI 182780, also called fulvestrant. We hypothesized that the requirement of Rho GDIα expression for tamoxifen sensitivity may be dependent on sustained ERα expression because fulvestrant, which inhibits growth, degrades the ERα protein (24). Tamoxifen-resistant growth of the GDIα sh clone was blocked in the RESCUE cells, confirming the role of Rho GDIα in tamoxifen action.
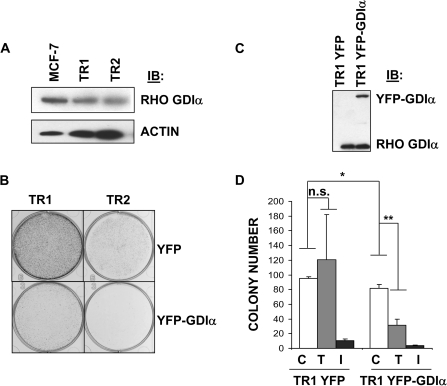
To explore the relationship between Rho GDIα levels and tamoxifen resistance, we generated two tamoxifen-resistant MCF-7 sublines (TR1 and TR2) using long-term exposure to tamoxifen. Rho GDIα levels were lower in both sublines compared with parental cells (Figure 2, A). We transfected both cell lines with YFP-vector or YFP-Rho GDIα plasmids and examined colony formation efficiency (Figure 2, B). Both tamoxifen-resistant sublines formed colonies in the presence of tamoxifen, but when these cell lines were transfected to express YFP-Rho GDIα, the colonies were greatly reduced in size and numbers. We used growth assays to examine the properties of tamoxifen-resistant cells with stable reexpression of YFP-GDIα (Figure 2, C and D). TR1 cell growth was not inhibited with tamoxifen. However, when Rho GDIα was stably reexpressed, the basal growth of TR1 cells was decreased and they became tamoxifen-sensitive. These results suggested that Rho GDIα levels may be a major determinant of tamoxifen resistance in ERα-positive cells.
Figure 2.
Rho guanine dissociation inhibitor (GDI) α expression in and growth properties of tamoxifen-resistant MCF-7 cells. A) Rho GDIα expression in two tamoxifen-resistant MCF-7–derived cell lines, TR1 and TR2. Cells were growth in the presence of 4-hydroxy-tamoxifen (1 μM for TR1 and 100 nM for TR2) for longer than 1 year to generate pools of resistant cells. Whole-cell lysates from these and parental MCF-7 cells were immunoblotted and blots were cut and probed with rabbit polyclonal antibodies recognizing Rho GDIα or with mouse monoclonal antibodies to β-actin (ACTIN) to ensure equal loading. B) Effect of stable RhoGDIα expression on tamoxifen-resistant growth of TR1 and TR1 cells. TR1 and TR2 cells transfected with yellow fluorescence protein-vector (YFP) or YFP-Rho GDIα (YFP-GDIα) plasmids were used in colony formation assays as described in “Materials and Methods”. Fourteen days after plating of the cells at low density, the colonies were stained with 1% crystal violet and then photographed under a microscope. The pictures are representative of two independent experiments. C) Stable transfection of TR1 cells with YFP-vector (TR1 YFP) or YFP-Rho GDIα (TR1 YFP-GDIα) for Rho GDIα expression. An immunoblot (IB) of whole-cell lysates from pools of transfected TR1 cells was probed with a rabbit polyclonal antibody against Rho GDIα. D) Anchorage-independent growth, tamoxifen and fulvestrant sensitivity of tamoxifen-resistant MCF-7 cells transfected with YFP-Rho GDIα. Pools of TR1 cells stably transfected with YFP-vector (TR1 YFP) or YFP-Rho GDIα (TR1 YFP-GDIα) were plated in soft agar and then treated with 4-hydroxy-tamoxifen (T, 10−6 M), fulvestrant (I, 10−8 M), or vehicle (C). Data are the mean colony number from three plates and are representative of three independent experiments. Error bars correspond to 95% confidence intervals. P values were calculated using two-sided Student t tests. For vehicle-treated TR1 YFP-GDIα vs vehicle-treated TR1 YFP cells, * P = .04. For C vs T in TR1 YFP and TR1 YFP-GDIα, n.s. = statistically nonsignificant, and **P = .007, respectively.
Effect of Rho GDIα Silencing on Rho and ERα Signaling
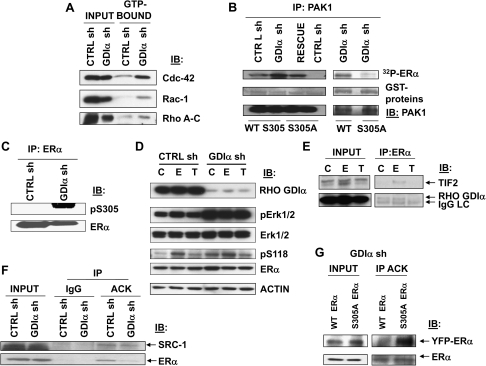
To study mechanism, we first examined the activation of Rho family of GTPases using GST-pulldown assays (Figure 3, A and Supplementary Figure 2, available online). Increased levels of activated Cdc-42, Rac-1, and Rho A, B, and C were detected in cloned GDIα sh cells compared with CTRL sh cells, indicating increased Rho-GTPase activity in cells containing Rho GDI shRNA.
Figure 3.
Effects of Rho guanine dissociation inhibitor (GDI) α silencing on Rho GTPase and estrogen receptor (ER) α signaling. A) Activation of cdc-42, Rac-1, and Rho (A–C) GTPases in cells with reduced Rho GDIα expression. Between 700 and 1000 μg of cellular proteins from cloned CTRL sh and GDIα sh cells were incubated with 15 μg of PAK-1 p21 binding domain (PBD) or 20 μg Rhotekin Rho binding domain (RBD) agarose conjugate at 4°C for 90 minutes and separated by centrifugation. GTP-bound activated Rho proteins were detected by immunoblotting using mouse monoclonal antibodies against Rac-1 and cdc42; or rabbit polyclonal antibodies to RhoA, RhoB, or RhoC. Whole-cell lysates were used as input controls (INPUT). Blots are representative of three independent experiments. B) Activation of PAK1 in cells with reduced Rho GDIα expression. Immunocomplex kinase assays were performed using purified GST-ERα fragments (aa 253-310) as the substrate and using PAK1 that was immunoprecipitated (IP) from CTRL sh, GDIα sh, and RESCUE clones as the enzyme. An ERα GST-fragment in which the serine 305 phosphorylation site was converted to an alanine (S305A) was used as negative control. Phosphorylated ERα levels (32P-ERα) were determined by autoradiography. The input GST-ERα hinge domain proteins (GST-proteins) were visualized with Ponceau S staining, and the immunoprecipitated PAK1 was detected by immunoblotting (IB) with a rabbit polyclonal anti-PAK1 antibody. C) Phosphorylation of ERα S305 in cells with reduced Rho GDIα expression. Cellular lysates from CTRL sh and GDIα sh cells were immunoprecipitated with a mouse monoclonal anti-ERα antibody and subsequently immunoblotted (IB) for detection of phosphorylated ERα S305 (rabbit polyclonal antibody to pS305 ERα) and total ERα protein. D) Phosphorylation of Erk 1 and 2 and ERα S118 in cells with reduced Rho GDIα expression. CTRL sh and GDIα sh cells were treated with 17β-estradiol (E, 10−9 M), 4-hydroxy-tamoxifen (T, 10−7 M), or vehicle (C) for 5 minutes before lysis. Levels of Rho GDIα, phosphorylated (p) Erk1 and 2 (rabbit polyclonal antibody to Thr202 and Tyr204 of Erk1 and Thr185 and Tyr187 of Erk2), total Erk 1 and 2 (rabbit polyclonal antibody to Erk 1 and Erk2), phosphorylated ERα (mouse monoclonal antibody to pS118 ERα), and total ERα (mouse monoclonal antibody to ERα), proteins in cellular extracts were analyzed by immunoblot analysis (IB). Mouse monoclonal antibody to β-actin (ACTIN) was used as a control for equal loading and transfer. E) Binding of Rho GDIα to ERα in estrogen- and tamoxifen-treated MCF-7 cells. Cellular lysates from parental MCF-7 cells that were treated with 17β-estradiol (E, 10−9 M), 4-hydroxy-tamoxifen (T, 10−7 M), or vehicle (C) for 2 hours were immunoprecipitated with a mouse monoclonal anti-ERα antibody, and subsequently immunoblotted (IB) for detection of Rho GDIα (using a rabbit polyclonal antibody) or of the ERα coactivator TIF2, a positive control (mouse monoclonal anti-TIF2 antibody). Whole-cell lysates were used as input controls (INPUT). LC = light chain. F) Acetylation of ERα in cells with reduced Rho GDIα expression. Cellular lysates from CTRL sh and GDIα sh cells were immunoprecipitated with a rabbit polyclonal anti-acetylated lysine antibody (ACK) or IgG (negative control) and subsequently immunoblotted (IB) for detection of ERα (mouse monoclonal antibody to ERα) or its coactivator protein, SRC-1 (as a positive control, rabbit polyclonal antibody to SRC-1). Whole-cell lysates were used as input controls (INPUT). G) Acetylation of ERα in cells with the ERα S305A phosphorylation site mutation. GDIα sh cells were transfected with the yellow fluorescence protein (YFP)-ER WT or YFP-ER S305A plasmid constructs (encoding 96 kDa YFP-receptor fusion proteins) to evaluate changes in the acetylation status of ERα in the absence of phosphorylation (S305A). Cellular lysates were immunoprecipitated (IP) with a rabbit polyclonal anti-acetylated lysine antibody (ACK) and subsequently immunoblotted (IB) for detection of the ERα. Whole-cell lysates were used as input controls (INPUT). For all panels shown (A–G), immunoblots are representative of two or three separate experiments.
The Rho GTPases activate downstream effectors (25). Either activation or increased expression of one effector, PAK1, can render breast cancer cells tamoxifen-resistant (26,27). Protein kinase A and PAK1 kinase signal to and phosphorylate ERα at serine (S) 305 (13,28,29). Therefore, we examined PAK1 activation by immunoprecipitating PAK1 from cells with and without Rho GDIα expression and using purified GST-ERα protein (residues 253–310) as substrate (Figure 3, B). We found higher levels of phosphorylated ERα in Rho GDIα shRNA-containing cells. When we mutated the serine 305 residue of ERα to an alanine (S305A), we found no phosphorylation by PAK1. Expression of Rho GDIα shRNA also led to enhanced phosphorylation of ERα at S305 (Figure 3, C). Phosphorylation of ERα at S305 can enhance sensitivity to estrogen treatment (13,29) and predicts poor response to tamoxifen in breast cancer patients (30). Thus, increased PAK1 activity in cells with decreased Rho GDIα expression may lead to enhanced phosphorylation of a site on ERα that is critical for resistance to tamoxifen.
Phosphorylation of ERα S305 by PAK1 and phosphorylation of ERα S118 by MAPK may be linked (28). We observed constitutive activation of pMAPK in GDIα sh cells, but not elevated levels of ERαpS118 with tamoxifen (Figure 3, D). We observed elevated pS118 levels in both cells with estrogen as expected (31). Thus, phosphorylation of ERα S305 and S118 are not coupled with tamoxifen treatment in our model, and elevated pMAPK levels might be a consequence of increased activation of PAK1 in cells with decreased GDIα expression.
Rho GDIα and ERα interact in MCF-7 cells (32). We next examined how tamoxifen affected their interaction by immunoprecipitation of ERα and immunoblotting for Rho GDIα in the presence of estrogen or tamoxifen (Figure 3, E). We observed an estrogen-dependent interaction between ERα and the transcription intermediary factor 2 (TIF2, an ERα coactivator protein used as a control) as reported previously (15). We observed an interaction between Rho GDIα and ERα under control and estrogen treatments but not with tamoxifen. We conclude that Rho GDIα probably does not enable tamoxifen sensitivity by direct binding to ERα in tamoxifen-treated cells.
Because phosphorylation of ERα at S305 has been reported to block acetylation of ERα at lysines (K) 302 and 303 (13), we also used immunoblots to check whether ERα could be immunoprecipitated with an anti-acetyllysine antibody from lysates of cells with decreased Rho GDIα expression. We found lower levels of acetylated ERα in GDIα sh cells (Figure 3, F), whereas the acetylation of the ERα coactivator SRC-1 (a positive control) was unchanged. When Rho GDIα sh cells expressing ERα S305A were used in these experiments, acetylation of ERα was restored in the S305A mutant (Figure 3, G). These results suggest that the hinge region of ERα, which contains the important regulatory residues, K302, K303, and S305, is altered concomitant with Rho GDIα silencing.
Effect of Rho GDIα on ERα Transactivation
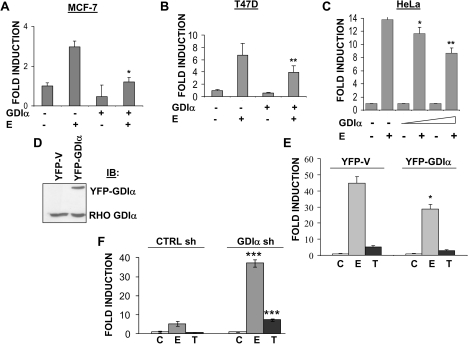
Because Rho GDIα overexpression and PAK1 activation had been reported to enhance ERα transcriptional activity (27,33), we transfected three human cell lines (MCF-7 and T47D breast cancer cells, and HeLa cervical cancer cells) with YFP-GDIα and a luciferase reporter plasmid from which transcription could be activated through an estrogen response element. Because HeLa cells were ER-negative, they were also transfected with an ERα expression vector. In all cells tested, Rho GDIα overexpression decreased estrogen-induced transactivation (Figure 4, A–C). We also tested the effect of Rho GDIα expression on ERα activity in stable pools of T47D cells overexpressing YFP-Rho GDIα. ERα activity was decreased with estrogen but not affected by tamoxifen in these cells (Figure 4, D and E). In keeping with these results, estrogen- and tamoxifen-induced ERα activity was enhanced with Rho GDIα silencing (Figure 4, F). These data demonstrate that Rho GDIα expression is inversely related to ERα activity. The reason for the differences in our results compared with those of some others (32,33) is not presently understood; however, the cell lines were different and it is possible that the data were normalized differently.
Figure 4.
Effect of Rho guanine dissociation inhibitor (GDI) α expression on estrogen receptor (ER) α transcriptional activity. A–C) Effect of transient Rho GDIα expression on estrogen-stimulated luciferase reporter activity. MCF-7 (A) and T47D (B) cells were cotransfected with an estrogen response element (ERE)-luciferase reporter, a pCMV-β-galactosidase vector, and the yellow fluorescence protein (YFP)-GDIα vector. The ERα-negative HeLa cells (C) were also cotransfected with a wild-type ERα expression vector. Cells were treated with estrogen (E, 10−9 M) and after 18–24 hours, cell lysates were harvested. ERE-luciferase activity was measured and normalized by dividing by the β-galactosidase activity to give relative luciferase units. The data are representative of three independent experiments, each performed in triplicate, and are reported as fold induction. For E treatment between GDIα and YFP-expressing MCF-7 cells (A), T47D cells (B) and Hela cells (C), *P = .004, **P = .041, and *P = .041 and *P = .003, respectively. D) Creation of stable pools of T47D cells overexpressing the YFP-vector (YFP-V) or YFP-Rho GDIα (YFP-GDIα). Whole-cell lysates were immunoblotted and probed with a rabbit polyclonal anti-Rho GDIα antibody. E) Estrogen-stimulated luciferase reporter activity in T47D cells with and without YFP-Rho GDIα expression. ERE-luciferase activity was assayed in stable pools of T47D cells overexpressing the YFP-vector (YFP-V) or YFP-Rho GDIα (YFP-GDIα) and treated with 17β-estradiol (E, 10−9 M), 4-hydroxy-tamoxifen (T, 10-−7 M), or vehicle (C). Data are representative of three independent experiments, each performed in triplicate, and reported as fold induction. For E treatment between YFP-V and YFP-GDIα stable pools, *P = .012. F) Effect of Rho GDIα silencing on estrogen and tamoxifen-induced ERα transcriptional activity. ERE-luciferase activity was measured in stable CTRL sh and GDIα sh clones treated with 17β-estradiol (E, 10−9 M), 4-hydroxy-tamoxifen (T, 10−7 M), or vehicle (C). Data are representative of three independent experiments, each performed in triplicate, and are reported as fold induction. For E and T treatments between CTRL sh and GDIα sh,***P < .001. For panels (A–C), (E), and (F), error bars correspond to 95% confidence intervals and P values were calculated using two-sided Student t tests.
Effect of Rho GDIα Silencing on Tamoxifen-Resistant Growth and Metastasis of Xenografts
To examine the effects of decreased Rho GDIα expression on tumor growth in vivo, we next injected MCF-7 cells containing Rho GDIα shRNA or the empty vector into athymic nude mice. Mice were implanted with 60-day release estrogen pellets, and at approximately 1.5–2.5 weeks, when tumors reached approximately 250 mm3, the mice were randomized to continue estrogen (E), to have estrogen withdrawn (ie, the pellet removed; N), or to have estrogen withdrawn and to begin tamoxifen treatment (T). Xenograft tumor growth is shown in Supplementary Table 1 (available online).
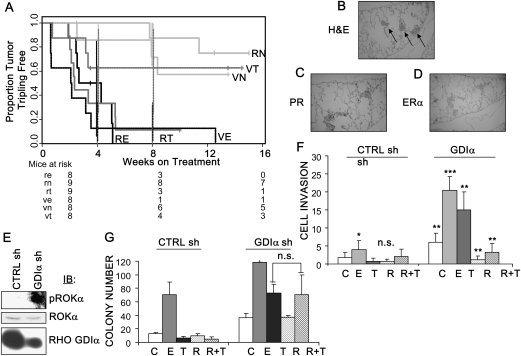
Our results were consistent with tamoxifen-resistance among tumors from cells with Rho GDIα silencing. Among mice that carried tumors derived from CTRL sh cells, the time to tumor tripling in the presence of tamoxifen treatment (VT) was not statistically significantly different from that in the presence of estrogen withdrawal (VN) (median tripling time of VT tumors = not reached, of VN tumors = not reached, hazard ratio [HR] = 1.36, 95% confidence interval [CI] = 0.274 to 6.80, P = .704 [with adjustment for multiple comparisons, P = 1]; Figure 5, A; Table 1). However, among mice that carried tumors derived from Rho GDIα shRNA-expressing cells, the time to tumor tripling in the presence of tamoxifen (RT) was not statistically different from that in mice treated with estrogen (RE), suggesting that tamoxifen was acting like estrogen to stimulate growth (tripling time of RT tumors = 2.34 weeks, of RE tumors = 3.48 weeks, HR = 0.953, 95% CI = 0.338 to 2.688, P = .928 [adjusted, 1]).Comparison of the two tamoxifen-treated groups, mice with tumors derived from vector vs GDIα sh-containing cells (RT vs VT), demonstrated a statistically significant difference in time to tumor tripling (tripling time of RT tumors = 2.34 weeks, of VT tumors = not reached, HR = 4.133, 95% CI = 1.07 to 15.96, P = .040 [adjusted, 0.119]).
Figure 5.
Loss of Rho guanine dissociation inhibitor (GDI) α expression and tamoxifen resistance in vivo. A) Overall survival in mice with MCF-7 xenografts expressing Rho GDIα shRNA. Stably transfected CTRL shRNA (V, dashed lines) and Rho GDIα shRNA (R, solid lines) containing cells were injected into the mammary fat pad of athymic nude mice. Mice were implanted with 0.72 mg 60-day release estradiol pellets at the time of xenograft injection. At 1.5–2.5 weeks, when tumors reached approximately 250 mm3, the mice were randomly allocated to continue estrogen treatment (VE [n = 8, tumor tripling events = 8] and RE [n = 8, events = 8], black lines) or to estrogen withdrawal (VN [n = 8, events = 3] and RN [n = 9, events = 2], green lines) or to estrogen withdrawal plus tamoxifen administration (VT [n = 8, events = 3] and RT [n = 9, events = 8], red lines) for another 30 days. Survival curves (shown as the proportion of mice in which tumors had not tripled in size) are graphed as the time in weeks from randomization to a threefold increase in total tumor volume over baseline (time to tumor tripling). Mice at risk in each group at 0, 4, and 8 weeks are shown beneath the survival curves, 95% confidence intervals for survival proportion are shown as vertical lines at 4 and 8 weeks. Curves were computed by the Kaplan–Meier method and compared using two-sided log-rank tests. B) Lung metastases from mice carrying xenografts of MCF-7 cells containing Rho GDIα shRNA. Histological analysis of hematoxylin and eosin (H&E)-stained sections from spontaneous lung metastases that arose in estrogen-treated mice carrying tumors derived from GDIαsh cells. Arrows point to metastatic lesions. C and D) Immunohistochemical detection of the progesterone receptor (PR, C) and estrogen receptor (ER) α (D) in the metastatic lesions; ×10 magnification. E) ROKα expression in cells with Rho GDIα silencing. Immunoblot (IB) of whole-cell lysates from CTRL sh and GDIα sh cells probed with rabbit polyclonal antibodies to phosphorylated (p) or total ROKα protein, and to Rho GDIα. Immunoblots are representative of three separate experiments. F) Invasiveness of cells containing Rho GDIα shRNA. In vitro invasion chambers were used with CTRL sh and cloned GDIα sh cells treated with 17β-estradiol (E, 10−9 M), 4-hydroxy-tamoxifen (T, 10−7 M), vehicle (C) and/or the ROK inhibitor Y-27632 (R, 10−8 M). Data are representative of three independent experiments, each performed in triplicate. Error bars correspond to 95% confidence intervals. P values were calculated using two-sided Student t tests. For C treatment between CTRL sh and GDIα sh, **P = .002. For C vs E in CTRL sh cells, *P = .03. For C vs T, C vs R, and T vs R+T in CTRL sh cells, n.s. = statistically nonsignificant. For C vs E in GDIα sh cells, ***P < .001. For C vs T, C vs R, and T vs R+T in GDIα sh cells, **P < .001. G) Anchorage-independent growth, tamoxifen and Y-27632 sensitivity of GDIα sh vs CTRL sh cells. CTRL sh and GDIα sh clones were plated in soft agar and then treated with 17β-estradiol (E, 10−9 M), 4-hydroxy- tamoxifen (T, 10−7 M), vehicle (C), and/or Y-27632 (R, 10−8 M). Data are the mean colony number of two wells. Error bars correspond to 95% confidence intervals. P values were calculated using two-sided Student t tests. For T vs R+T in GDIα sh cells, n.s. = statistically nonsignificant.
Table 1.
Statistical analysis of xenograft tumor growth
| Group comparison* | P value† | Adjusted P value‡ |
| VT vs VN | .704 | 1 |
| RT vs RE | .928 | 1 |
| RT vs VT | .039 | .119 |
| VT vs VE | .008 | .032 |
| RT vs RN | .003 | .018 |
| RE vs RN | .004 | 019 |
Vector control tumors (V); Rho GDIα sh tumors (R); treatment groups: no treatment (N), tamoxifen (T), estrogen (E).
Wald χ2 tests using contrasts in a Cox proportional hazards regression.
P values adjusted for multiplicity by the Holm method (19).
To summarize, when mice were treated with estrogen or estrogen withdrawal, xenografts derived from Rho GDIα shRNA-expressing cells (RE or RN) tripled in size at about the same rate as those derived from vector control cells (VE or VN). However, when the mice were treated with tamoxifen, xenografts from cells with Rho GDIα silencing (RT) tripled in size at a statistically significantly faster rate compared with those derived from vector-containing cells (VT).These data provide strong evidence that Rho GDIα silencing can confer tamoxifen resistance in vivo.
Most strikingly, two of eight (25%) examined lungs from the tamoxifen-treated vs three of five (60%) estrogen-treated mice that carried tumors derived from GDIα sh cells carried metastases within 3 months. For example, in a representative H&E slide from the lung of an estrogen-treated mouse carrying a GDIα sh cell–derived tumor, three metastases can be seen in a single section (Figure 5, B, arrows). MCF-7 infrequently metastasizes to distant organs (23), and none of the mice carrying tumors derived from vector-only cells developed metastases during this experiment. Thus, Rho GDIα silencing enhanced metastasis. The metastases probably have an intact hormone response pathway, not only because their growth was stimulated by both estrogen and tamoxifen but also because tumors in the estrogen-treated group were positive for the estrogen-responsive progesterone receptor (Figure 5, C). Because estrogen treatment results in decreased ERα protein levels (34), we did not see ERα expression in the metastases (Figure 5, D). These results are important because there are few preclinical models of hormone-responsive metastases, and breast cancer metastasizes to the lung more frequently than to any other site but bone. About half of lung metastases are ERα-positive (35).
Among other downstream effectors of Rho signaling, the Rho-associated kinases (ROKs) and NF-κB, are important for cell motility and invasion (36–39). Although we did not observe NF-κB activation (Supplementary Figure 3, A and B, available online), we detected elevated levels of activated ROKα in cells containing Rho GDIα shRNA (Figure 5, E). It has been suggested that estrogen promotes an epithelial-to-mesenchymal-like transition that would foster cellular invasion (40). Consistent with this idea, when we measured cell invasion in modified Boyden chamber assays, we saw enhanced invasion by estrogen-treated CTRL sh cells compared with untreated cells (Figure 5, F). In cells with decreased Rho GDIα expression, we saw enhanced constitutive and estrogen-stimulated invasion. Tamoxifen also enhanced invasion, suggesting that tamoxifen is acting as an ERα agonist. ROK inhibition by treatment with Y-27632 reduced basal growth and blocked tamoxifen-stimulated invasion only in cells in which Rho GDIα expression was decreased. It is known that many metastasis suppressor genes do not affect primary tumor growth, but only metastatic processes such as motility and invasion (9). Therefore, we tested the ROK inhibitor in anchorage-independent assays (Figure 5, G).Treatment with the ROK inhibitor Y-27632 did not affect basal growth cells with decreased Rho GDIα expression, and it did not restore tamoxifen-sensitive growth. This suggests that the ROK inhibitor’s activities might be restricted to the invasive potential of these cells but not useful to block tamoxifen’s agonist-like effects on growth.
Association of Combined Rho GDIα and MTA2 Status With Clinical Outcomes
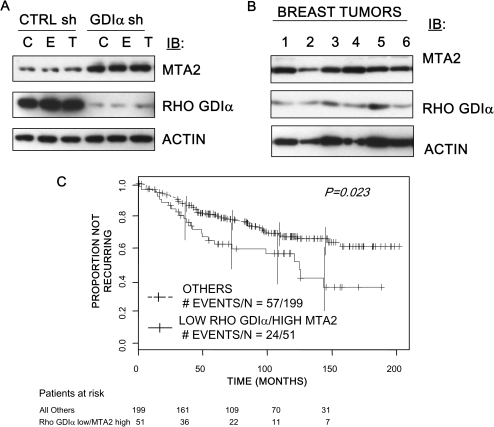
The metastasis-associated protein (MTA) family is associated with breast tumor progression and ERα activity (41). We demonstrated that MTA2 overexpression in ERα-positive cells generated a tamoxifen-resistant phenotype (42). To explore the metastatic phenotype associated with Rho GDIα silencing, we performed immunoblot analysis and found an increase in MTA2 protein levels in cells with decreased Rho GDIα expression compared with CTRL sh cells (Figure 6, A). The increase in MTA2 levels was independent of estrogen and tamoxifen treatment. An immunoblot analysis of six primary breast tumors showed that levels of both proteins varied among the tumors (Figure 6, B).
Figure 6.
Rho guanine dissociation inhibitor (GDI) α and MTA2 expression and clinical tamoxifen resistance. A) Rho GDIα and MTA2 expression in MCF-7 cells expressing short hairpin (sh) RNA to Rho GDIα (GDIα sh cells) or control shRNA (CTRL sh cells).CTRL sh and GDIα sh cells were treated with 17β-estradiol (E, 10−9 M), 4-hydroxy- tamoxifen (T, 10−7 M), or vehicle (C) for 24 hours before lysis. Levels of Rho GDIα (rabbit polyclonal antibody to Rho GDIα) and rabbit polyclonal antibody to MTA2 were analyzed in cellular extracts by immunoblot analysis (IB). Mouse monoclonal antibody to β-actin (ACTIN) was used as a control for equal loading and transfer. The immunoblots are representative of three separate experiments. B) Rho GDIα and MTA2 expression in six breast tumors. Protein extracts from six breast tumors were analyzed for Rho GDIα and MTA2 expression using immunoblot analysis (IB). β-actin (ACTIN) was used as a control for equal loading and transfer. C) Rho GDIα and MTA2 expression and clinical outcome in tamoxifen-treated patients. The proportion of patients remaining disease-free is shown using Kaplan–Meier plots of survival data from tamoxifen-treated estrogen receptor α–positive patients from the Loi et al study (21) stratified by low Rho GDIα and high MTA2 RNA levels. The number of events and patients in each group are shown (P = .011, log-rank test, Cox-proportional hazards, hazard ratio = 5.84, 95% confidence interval = 1.149 to 2.988).
To assess the clinical significance of Rho GDIα and MTA2 expression levels, we analyzed a microarray dataset described by Loi et al. (21), GSE6532. To determine the effects of Rho GDIα and MTA2 on the response to tamoxifen, only data from the 250 patients who were classified as ER+ and who received tamoxifen monotherapy were considered. On the basis of the microarray data, we subdivided patients in the tamoxifen-treated group into a Rho GDIα low and MTA2 high (“low/high”) group vs all other groups using a bootstrapping approach to select optimum cut points. Of the 250 patients, 51 were classified as “Rho GDIα low and MTA2 high” and 199 were classified as “Others”. The patients were similar in terms of nodal status, histological grade, age, and tumor size (Supplementary Table 2, available online). In the tamoxifen-treated group, the low/high patients (n = 51) had a statistically significantly poorer recurrence-free survival compared with all other patients (Figure 6, C, P = .023 [log-rank test, Cox-proportional hazards, HR = 5.84, 95% CI = 1.15 to 2.99]). No difference in survival was observed when patients were subdivided based on expression of Rho GDIα or MTA2 alone in the treated group. We performed the same analysis on the samples classified as tamoxifen-untreated also in the Loi dataset and found no difference in recurrence-free survival; however, the number of untreated patients was small precluding a definitive conclusion about prognosis (data not shown). We hypothesize that low levels of Rho GDIα expression may predict poor outcome among tamoxifen-treated patients, potentially through its effects on metastatic pathways such as MTA2.
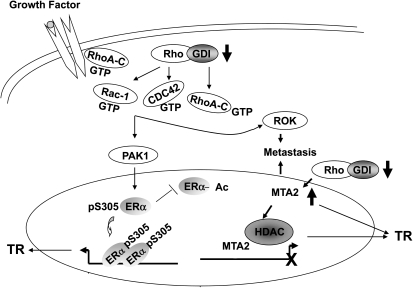
Our working model (Figure 7) is that loss of Rho GDIα expression enhances metastasis and tamoxifen resistance via effects on both ERα and MTA2. Decreased expression or loss of Rho GDIα subsequently causes release of bound Rho family members (RhoA-C, Cdc42, and Rac-1), leading to the subsequent activation of its downstream effectors, including PAK1 and ROK. Activated PAK1, either directly or indirectly, leads to ERα phosphorylation at S305, which inhibits ERα acetylation by shifting the conformation of ERα thus altering hormone response. ROK activation enhances cell motility, invasiveness, and metastasis. Rho GDIα loss also enhances MTA2, which can participate both in metastasis and tamoxifen resistance.
Figure 7.
Suggested model for induction of tamoxifen resistance and metastasis by Rho guanine dissociation inhibitor (GDI) α silencing via effects on estrogen receptor (ER) α and MTA2. Loss of Rho GDIα leads to the release of bound Rho family members (Rho A, Cdc-42, and Rac-1) with the subsequent activation of downstream effectors PAK1 and ROK. Activated ROK may enhance cell motility and invasion. Activated PAK1 may phosphorylate ERα at serine (S) 305 and inhibit its acetylation, thus inducing tamoxifen resistance (TR). Loss of Rho GDIα is also associated with enhanced MTA2 expression, which can induce both metastasis and TR.
Discussion
We demonstrate that Rho GDIα silencing affects pathways that affect both the metastatic phenotype and hormone responsiveness of breast cancer cells. Despite recent advances with antihormonal therapies, the median survival of patients with metastatic breast cancer is very poor—only 2–3 years (43). Thus, resistance to hormonal therapies continues to be a major clinical problem. The ERα status of metastases does not always correlate with that of the primary tumor (4,44), but it has been shown that ERα expression was lost in only 17% of tamoxifen-resistant tumors as compared with expression in the primary tumor of each patient (45), so that sustained ERα expression and function are probably important for hormone resistance.
One major mechanism that has been proposed for resistance is enhanced bidirectional cross talk between ERα and growth factor receptor pathways, as ERBB2 (46). Another emerging mechanism involves the activation of intracellular signaling molecules that can either circumvent growth dependence on ERα (47–49) or augment ER’s function (49). Therefore, selectively inhibition of intracellular signal transduction is a promising new method to target tamoxifen-resistance in patients (50). In our pilot cohort of tamoxifen-resistant patients, it appeared that multiple mechanisms of resistance could occur simultaneously. Our identification of a role for Rho GDIα in ERα action, tamoxifen resistance, and metastatic behavior further underscores the potential for inhibitors of signal transduction to overcome tamoxifen resistance in breast cancer. If multiple mechanisms of resistance occur in patients, then a combination of therapeutics targeted to multiple pathways may be required for effective treatment.
The acquisition of a remodeled cytoskeleton and a motile invasive phenotype are important steps in metastasis, and the Rho GTPases mediate these processes [reviewed in (51)]. Several reports have demonstrated a connection between pathways that regulate the cytoskeleton and hormone resistance in breast cancer. Tamoxifen treatment has been reported to alter the localization of actin and fibronectin receptors, and actin skeleton remodeling in endometrial cells (52). Furthermore, overexpression of the Ras-GDP exchange factor AND-34/BCAR3, which associates with the focal adhesion protein p130Cas, has been reported to alter actin distribution, and to confer tamoxifen-resistant growth (53).
Here, we examined whether loss of a negative regulator of the Rho GTPases could affect hormone response. By comparing tamoxifen-sensitive primary tumors with metastatic tumors that recurred while the patients were undergoing tamoxifen treatment, we discovered lower levels of Rho GDIα RNA in the resistant tumors. By contrast to other laboratories that used tumors that developed before tamoxifen treatment (54,55), we used tumors that developed during tamoxifen treatment, with the hypothesis that mechanisms of resistance might differ under tamoxifen selection. Indeed, our study of one tumor from this cohort revealed that overexpression of the MAPK phosphatase MKP3 conferred tamoxifen resistance in breast cancer cells (14). We might not have identified MKP3 as a determinant of resistance if we had not screened tumors that appeared during tamoxifen treatment because tamoxifen induced altered MKP3 expression via induction of reactive oxygen species and altered MKP3 expression was among the genes associated with tamoxifen resistance in primary breast tumors (56,57).
It has been shown that the kinase PAK1 phosphorylates ERα and that nuclear PAK1 localization is associated with tamoxifen resistance in breast cancer patients (27,58). PAK1 expression might be intricately interwoven with the metastasis resistance phenotype associated with Rho GDIα shRNA expression. It is also possible that the ERα S305 site, a target of PAK1 phosphorylation, is a major determinant of tamoxifen resistance as has been suggested by Michalides et al. (29,58). The S305 site is a factor in resistance to both tamoxifen and aromatase inhibitors via cross talk with growth factor signaling pathways in cells expressing somatic ERα K303R mutations (59–61).This mutation was associated with poor outcomes in univariate analyses of tumors from untreated breast cancer patients, and its presence was correlated with older age, larger tumor size, and lymph node–positive disease. Molecular analyses of the K303R ERα mutation have shown that the mutated arginine at the 303 position allows ERα to be more highly phosphorylated by protein kinase A (PKA) and Akt kinase signaling (61,62) and alters the dynamic recruitment of coactivators and corepressors, such as BRCA-1 (63). Overexpression of the K303R mutation in ERα-positive MCF-7 breast cancer cells conferred increased sensitivity to subphysiological levels of estrogen and decreased sensitivity to tamoxifen treatment when engaged in cross talk with growth factor receptor signaling pathways (60). Expression of the K303R ERα mutation also conferred resistance to the nonsteroidal aromatase inhibitor anastrozole in ERα-positive cells (59,61). In these studies, blocking ERα S305 phosphorylation ablated tamoxifen resistance and cross talk between the ERαK303R mutant and the ERBB2 and IGFR signaling pathways (59,61). Our results suggest that the development of specific PAK1 (64) and ROK inhibitors might be useful to reverse tamoxifen resistance.
Low Rho GDIβ is associated with increased metastasis risk and decreased survival in patients with locally advanced bladder cancer (65). Both endothelin-1 and neuromedin U are mediators of Rho GDIβ effects on metastasis and tumor progression (66,67). Here, we report that MTA2 may be a mediator of Rho GDIα’s effects on metastasis and tumor progression in patients. The MTA family is important to ER function and ER-associated pathology [for a review, see (68)]. MTA2 can act as a negative corepressor of ERα action leading to hormone-independent growth (42). Expression of GDIα shRNA leads to a dramatic increase in MTA2 levels, and tamoxifen-treated breast cancer patients with low Rho GDIα and high MTA2 protein levels appear to exhibit a shorter disease-free survival time compared with all other patients. These are the first data to suggest a clinically important connection between the Rho GDIα and MTA2 pathways. Our data also suggest a possible mechanism, in which the loss of Rho GDIα function promotes distant progression of breast tumors by triggering downstream molecules, such as MTA2, with metastasis-promoting activities. If Rho GDIα works as a suppressor of prometastatic genes, then genes such as MTA2 and ROK would be activated and present as druggable targets for treatment of breast cancer patients with metastatic or tamoxifen-resistant disease. The association of combined Rho GDIα and MTA2 status with clinical outcome supports our hypothesis that Rho GDIα loss can cause resistance to tamoxifen.
There are limitations to this study. First, the hormone-resistant phenotype conferred via Rho GDIα silencing has been shown in only one human breast cancer cell line. For this finding to be generalizable, it will be important to test the effects of Rho GDIα in other ER-positive models. Second, the presented clinical data are based on a retrospective analysis, which may suffer from bias. Future prospective studies could clarify the role of Rho GDIα and MTA2 in breast cancer.
Funding
This work was supported by NIH/NCI R01 CA72038, P01 CA30195, SPORE developmental project funding from P50 CA58183 to S.A.W.F., DOD BC073237 to K.R.C., and generous funding from the Cancer Prevention and Research Institute of Texas.
Supplementary Material
Footnotes
The study sponsors had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the article, and the decision to submit the article for publication.
The authors would like to thank Mrs Robin Sample for excellent administrative assistance, Dr Rakesh Kumar for helpful discussions, and QinxiGuo for cloning assistance.
References
- 1.Hortobagyi GN, de la Garza Salazar J, Pritchard K, et al. The global breast cancer burden: variations in epidemiology and survival. Clin Breast Cancer. 2005;6(5):391–401. doi: 10.3816/cbc.2005.n.043. [DOI] [PubMed] [Google Scholar]
- 2.Dowsett M, Martin LA, Smith I, Johnston S. Mechanisms of resistance to aromatase inhibitors. J Steroid Biochem Mol Biol. 2005;95(1–5):167–172. doi: 10.1016/j.jsbmb.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 3.Saphner T, Tormey DC, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol. 1996;14(10):2738–2746. doi: 10.1200/JCO.1996.14.10.2738. [DOI] [PubMed] [Google Scholar]
- 4.Hull DF, Clark GM, Osborne CK, Chamness GC, Knight WAI, McGuire WL. Multiple estrogen receptor assays in human breast cancer. Cancer Res. 1983;43(1):413–416. [PubMed] [Google Scholar]
- 5.Knowlden JM, Hutcheson IR, Jones HE, et al. Elevated levels of epidermal growth factor receptor/c-erbB2 heterodimers mediate an autocrine growth regulatory pathway in tamoxifen-resistant MCF-7 cells. Endocrinology. 2003;144(3):1032–1044. doi: 10.1210/en.2002-220620. [DOI] [PubMed] [Google Scholar]
- 6.Herynk MH, Fuqua SA. Estrogen receptors in resistance to hormone therapy. Adv Exp Med Biol. 2007;608:130–143. doi: 10.1007/978-0-387-74039-3_10. [DOI] [PubMed] [Google Scholar]
- 7.Yang J, Mani SA, Donaher JL, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117(7):927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Gildea JJ, Seraj MJ, Oxford G, et al. RhoGDI2 is an invasion and metastasis suppressor gene in human cancer. Cancer Res. 2002;62(22):6418–6423. [PubMed] [Google Scholar]
- 9.Vaidya KS, Welch DR. Metastasis suppressors and their roles in breast carcinoma. J Mammary Gland Biol Neoplasia. 2007;12(2–3):175–190. doi: 10.1007/s10911-007-9049-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420(6916):629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 11.Lin M, van Golen KL. Rho-regulatory proteins in breast cancer cell motility and invasion. Breast Cancer Res Treat. 2004;84(1):49–60. doi: 10.1023/B:BREA.0000018424.43445.f3. [DOI] [PubMed] [Google Scholar]
- 12.Jiang WG, Watkins G, Lane J, et al. Prognostic value of rho GTPases and rho guanine nucleotide dissociation inhibitors in human breast cancers. Clin Cancer Res. 2003;9(17):6432–6440. [PubMed] [Google Scholar]
- 13.Cui Y, Zhang M, Pestell R, Curran EM, Welshons WV, Fuqua SAW. Phosphorylation of estrogen receptor α blocks its acetylation and regulates estrogen sensitivity. Cancer Res. 2004;64(24):9199–9208. doi: 10.1158/0008-5472.CAN-04-2126. [DOI] [PubMed] [Google Scholar]
- 14.Cui Y, Parra I, Zhang M, et al. Elevated expression of mitogen-activated protein kinase phosphatase 3 in breast tumors: a mechanism of tamoxifen resistance. Cancer Res. 2006;66(11):5950–5959. doi: 10.1158/0008-5472.CAN-05-3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuqua SAW, Wiltschke C, Zhang QX, et al. A hypersensitive estrogen receptor-α mutation in premalignant breast lesions. Cancer Res. 2000;60(15):4026–4029. [PubMed] [Google Scholar]
- 16.Herman ME, Katzenellenbogen BS. Response-specific antiestrogen resistance in a newly characterized MCF-7 human breast cancer cell line resulting from long-term exposure to trans-hydroxytamoxifen. J Steroid Biochem Mol Biol. 1996;59(2):121–134. doi: 10.1016/s0960-0760(96)00114-8. [DOI] [PubMed] [Google Scholar]
- 17.Nishitani J, Nishinaka T, Cheng CH, Rong W, Yokoyama KK, Chiu R. Recruitment of the retinoblastoma protein to c-Jun enhances transcription activity mediated through the AP-1 binding site. J Biol Chem. 1999;274(9):5454–5461. doi: 10.1074/jbc.274.9.5454. [DOI] [PubMed] [Google Scholar]
- 18.Osborne CK, Coronado-Heinsohn EB, Hilsenbeck SG, McCue BL, Wakeling AE, McClelland RA. Comparison of the effects of a pure steroidal antiestrogen with those of tamoxifen in a model of human breast cancer. J Natl Cancer Inst. 1995;87(10):746–750. doi: 10.1093/jnci/87.10.746. [DOI] [PubMed] [Google Scholar]
- 19.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6(2):65–70. [Google Scholar]
- 20.Team RDC. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2008. [Google Scholar]
- 21.Loi S, Haibe-Kains B, Desmedt C, et al. Definition of clinically distinct molecular subtypes in estrogen receptor-positive breast carcinomas through genomic grade. J Clin Oncol. 2007;25(10):1239–1246. doi: 10.1200/JCO.2006.07.1522. [DOI] [PubMed] [Google Scholar]
- 22.De Amicis F, Thirugnansampanthan J, Cui Y, et al. Androgen receptor overexpression induces tamoxifen resistance in human breast cancer cells. Breast Cancer Res Treat. 2009;89(9):4037–4041. doi: 10.1007/s10549-009-0436-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrell JC, Dye WW, Allred DC, et al. Estrogen receptor positive breast cancer metastasis: altered hormonal sensitivity and tumor aggressiveness in lymphatic vessels and lymph nodes. Cancer Res. 2006;66(18):9308–9315. doi: 10.1158/0008-5472.CAN-06-1769. [DOI] [PubMed] [Google Scholar]
- 24.Dauvois S, Danielian PS, White R, Parker MG. Antiestrogen ICI 164,384 reduces cellular estrogen receptor content by increasing its turnover. Proc Natl Acad Sci U S A. 1992;89(9):4037–4041. doi: 10.1073/pnas.89.9.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karnoub AE, Symons M, Campbell SL, Der CJ. Molecular basis for Rho GTPase signaling specificity. Breast Cancer Res Treat. 2004;84(1):61–71. doi: 10.1023/B:BREA.0000018427.84929.5c. [DOI] [PubMed] [Google Scholar]
- 26.Rayala SK, Molli PR, Kumar R. Nuclear p21-activated kinase 1 in breast cancer packs off tamoxifen sensitivity. Cancer Res. 2006;66(12):5985–5988. doi: 10.1158/0008-5472.CAN-06-0978. [DOI] [PubMed] [Google Scholar]
- 27.Wang RA, Mazumdar A, Vadlamudi RK, Kumar R. P21-activated kinase-1 phosphorylates and transactivates estrogen receptor-alpha and promotes hyperplasia in mammary epithelium. EMBO J. 2002;21(20):5437–5447. doi: 10.1093/emboj/cdf543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rayala SK, Talukder AH, Balasenthil S, et al. P21-activated kinase 1 regulation of estrogen receptor-alpha activation involves serine 305 activation linked with serine 118 phosphorylation. Cancer Res. 2006;66(3):1694–1701. doi: 10.1158/0008-5472.CAN-05-2922. [DOI] [PubMed] [Google Scholar]
- 29.Michalides R, Griekspoor A, Balkenende A, et al. Tamoxifen resistance by a conformational arrest of the estrogen receptor alpha after PKA activation in breast cancer. Cancer Cell. 2004;5(6):597–605. doi: 10.1016/j.ccr.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 30.Holm C, Kok M, Michalides R, et al. Phosphorylation of the oestrogen receptor alpha at serine 305 and prediction of tamoxifen resistance in breast cancer. J Pathol. 2009;217(3):372–379. doi: 10.1002/path.2455. [DOI] [PubMed] [Google Scholar]
- 31.Chen D, Washbrook E, Sarwar N, et al. Phosphorylation of human estrogen receptor alpha at serine 118 by two distinct signal transduction pathways revealed by phosphorylation-specific antisera. Oncogene. 2002;21(32):4921–4931. doi: 10.1038/sj.onc.1205420. [DOI] [PubMed] [Google Scholar]
- 32.El Marzouk S, Schultz-Norton JR, Likhite VS, McLeod IX, Yates JR, Nardulli AM. Rho GDP dissociation inhibitor alpha interacts with estrogen receptor alpha and influences estrogen responsiveness. J Mol Endocrinol. 2007;39(4):249–259. doi: 10.1677/JME-07-0055. [DOI] [PubMed] [Google Scholar]
- 33.Su LF, Knoblauch R, Garabedian MJ. Rho GTPases as modulators of the estrogen receptor transcriptional response. J Biol Chem. 2001;276(5):3231–3237. doi: 10.1074/jbc.M005547200. [DOI] [PubMed] [Google Scholar]
- 34.Nawaz Z, Lonard DM, Dennis AP, Smith CL, O’Malley BW. Proteasome-dependent degradation of the human estrogen receptor. Biochemistry. 1999;96(5):1858–1862. doi: 10.1073/pnas.96.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kayser K, Biechele U, Kayser G, et al. Pulmonary metastases of breast carcinomas: ligandohistochemical, nuclear, and structural analysis of primary and metastatic tumors with emphasis on period of occurrence of metastases and survival. J Surg Oncol. 1998;69(3):137–146. doi: 10.1002/(sici)1096-9098(199811)69:3<137::aid-jso4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 36.Lin M, van Golen KL. Rho-regulatory proteins in breast cancer cell motility and invasion. Breast Cancer Res Treat. 2004;84(1):49–60. doi: 10.1023/B:BREA.0000018424.43445.f3. [DOI] [PubMed] [Google Scholar]
- 37.Jo M, Thomas KS, Somlyo AV, Somlyo AP, Gonias SL. Cooperativity between the Ras-ERK and Rho-Rho kinase pathways in urokinase-type plasminogen activator-stimulated cell migration. J Biol Chem. 2002;277(14):12479–12485. doi: 10.1074/jbc.M111147200. [DOI] [PubMed] [Google Scholar]
- 38.Nakshatri H, Bhat-Nakshatri P, Martin DA, Goulet RJ, Sledge GW. Constitutive activation of NF-kappaB during progression of breast cancer to hormone-independent growth. Mol Cell Biol. 1997;17(7):3629–3639. doi: 10.1128/mcb.17.7.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burbelo P, Wellstein A, Pestell RG. Altered Rho GTPase signaling pathways in breast cancer cells. Breast Cancer Res Treat. 2004;84(1):43–48. doi: 10.1023/B:BREA.0000018422.02237.f9. [DOI] [PubMed] [Google Scholar]
- 40.Planas-Silva MD, Waltz PK. Estrogen promotes reversible epithelial-to-mesenchymal-like transition and collective motility in MCF-7 breast cancer cells. J Steroid Biochem Mol Biol. 2007;104(1–2):11–21. doi: 10.1016/j.jsbmb.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 41.Kumar R. Another tie that binds the MTA family to breast cancer. Cell. 2003;113(2):142–143. doi: 10.1016/s0092-8674(03)00274-5. [DOI] [PubMed] [Google Scholar]
- 42.Cui Y, Niu A, Pestell R, et al. Metastasis-associated protein 2 is a repressor of estrogen receptor alpha whose overexpression leads to estrogen-independent growth of human breast cancer cells. Mol Endocrinol. 2006;20(9):2020–2035. doi: 10.1210/me.2005-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nicolini A, Giardino R, Carpi A, et al. Metastatic breast cancer: an updating. Biomed Pharmacother. 2006;60(9):548–556. doi: 10.1016/j.biopha.2006.07.086. [DOI] [PubMed] [Google Scholar]
- 44.Robertson J. Oestrogen receptor: a stable phenotype in breast cancer. Br J Cancer. 1996;73(1):5–12. doi: 10.1038/bjc.1996.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gutierrez MC, Detre S, Johnston S, et al. Molecular changes in tamoxifen-resistant breast cancer: relationship between estrogen receptor, HER-2, and p38 mitogen-activated protein kinase. J Clin Oncol. 2005;23(11):2469–2476. doi: 10.1200/JCO.2005.01.172. [DOI] [PubMed] [Google Scholar]
- 46.Shou J, Massarweh S, Osborne CK, et al. Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst. 2004;96(12):926–935. doi: 10.1093/jnci/djh166. [DOI] [PubMed] [Google Scholar]
- 47.Creighton CJ, Hilger AM, Murthy S, Rae JM, Chinnaiyan AM, El-Ashry D. Activation of mitogen-activated protein kinase in estrogen receptor alpha-positive breast cancer cells in vitro induces an in vivo molecular phenotype of estrogen receptor alpha-negative human breast tumors. Cancer Res. 2006;66(7):3903–3911. doi: 10.1158/0008-5472.CAN-05-4363. [DOI] [PubMed] [Google Scholar]
- 48.El-Ashry D, Miller D, Kharbanda S, Lippman M, Kern F. Constitutive Raf-1 kinase activity in breast cancer cells induces both estrogen-independent growth and apoptosis. Oncogene. 1997;15(4):423–435. doi: 10.1038/sj.onc.1201198. [DOI] [PubMed] [Google Scholar]
- 49.Iorns E, Turner NC, Elliott R, et al. Identification of CDK10 as an important determinant of resistance to endocrine therapy for breast cancer. Cancer Cell. 2008;13(2):91–104. doi: 10.1016/j.ccr.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 50.Bedard PL, Freedman OC, Howell A, Clemons M. Overcoming endocrine resistance in breast cancer: are signal transduction inhibitors the answer? Breast Cancer Res Treat. 2008;108(3):307–317. doi: 10.1007/s10549-007-9606-8. [DOI] [PubMed] [Google Scholar]
- 51.Schmitz AA, Govek EE, Bottner B, Van Aelst L. Rho GTPases: signaling, migration, and invasion. Exp Cell Res. 2000;261(1):1–12. doi: 10.1006/excr.2000.5049. [DOI] [PubMed] [Google Scholar]
- 52.Albright CD, Carter CA, Kaufman DG. Tamoxifen alters the localization of F-actin and alpha 5/beta 1-integrin fibronectin receptors in human endometrial stromal cells and carcinoma cells. Pathobiology. 1997;65(4):177–183. doi: 10.1159/000164120. [DOI] [PubMed] [Google Scholar]
- 53.Cai D, Iyer A, Felekkis KN, et al. AND-34/BCAR3, a GDP exchange factor whose overexpression confers antiestrogen resistance, activates Rac, PAK1, and the cyclin D1 promoter. Cancer Res. 2003;63(20):6802–6808. [PubMed] [Google Scholar]
- 54.Ma XJ, Wang Z, Ryan PD, et al. A two-gene expression ratio predicts clinical outcome in breast cancer patients treated with tamoxifen. Cancer Cell. 2004;5(6):607–616. doi: 10.1016/j.ccr.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 55.Jansen MP, Foekens JA, van Staveren IL, et al. Molecular classification of tamoxifen-resistant breast carcinomas by gene expression profiling. J Clin Oncol. 2005;23(4):732–740. doi: 10.1200/JCO.2005.05.145. [DOI] [PubMed] [Google Scholar]
- 56.Chanrion M, Negre V, Fontaine H, et al. A gene expression signature that can predict the recurrence of tamoxifen-treated primary breast cancer. Clin Cancer Res. 2008;14(6):1744–1752. doi: 10.1158/1078-0432.CCR-07-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kok M, Linn SC, Van Laar RK, et al. Comparison of gene expression profiles predicting progression in breast cancer patients treated with tamoxifen. Breast Cancer Res Treat. 2009;113(2):275–283. doi: 10.1007/s10549-008-9939-y. [DOI] [PubMed] [Google Scholar]
- 58.Holm C, Rayala S, Jirstrom K, Stal O, Kumar R, Landberg G. Association between Pak1 expression and subcellular localization and tamoxifen resistance in breast cancer patients. J Natl Cancer Inst. 2006;98(10):671–680. doi: 10.1093/jnci/djj185. [DOI] [PubMed] [Google Scholar]
- 59.Barone I, Cui Y, Herynk MH, et al. Expression of the K303R estrogen receptor-alpha breast cancer mutation induces resistance to an aromatase inhibitor via addiction to the PI3K/Akt kinase pathway. Cancer Res. 2009;69(11):4724–4732. doi: 10.1158/0008-5472.CAN-08-4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Giordano C, Cui Y, Barone I, et al. Growth factor-induced resistance to tamoxifen is associated with a mutation of estrogen receptor alpha and its phosphorylation at serine 305. Breast Cancer Res Treat. 2010;19(1):71–85. doi: 10.1007/s10549-009-0334-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barone I, Iacopetta D, Covington KR, et al. Phosphorylation of the mutant K303R estrogen receptor alpha at serine 305 affects aromatase inhibitor sensitivity. Oncogene. 2010;29(16):2404–2414. doi: 10.1038/onc.2009.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cui Y, Zhang M, Pestell R, Fuqua SAW. Phosphorylation of estrogen receptor α blocks its acetylation and regulates estrogen sensitivity. Cancer Res. 2004;64(24):9199–9208. doi: 10.1158/0008-5472.CAN-04-2126. [DOI] [PubMed] [Google Scholar]
- 63.Ma Y, Fan S, Hu C, et al. BRCA1 regulates acetylation and ubiquitination of estrogen receptor alpha. Mol Endocrinol. 2010;24(1):76–90. doi: 10.1210/me.2009-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Talukder AH, Meng Q, Kumar R. CRIPak, a novel endogenous Pak1 inhibitor. Oncogene. 2006;25(9):1311–1319. doi: 10.1038/sj.onc.1209172. [DOI] [PubMed] [Google Scholar]
- 65.Theodorescu D, Sapinoso LM, Conaway MR, Oxford G, Hampton GM, Frierson HF., Jr. Reduced expression of metastasis suppressor RhoGDI2 is associated with decreased survival for patients with bladder cancer. Clin Cancer Res. 2004;10(11):3800–3806. doi: 10.1158/1078-0432.CCR-03-0653. [DOI] [PubMed] [Google Scholar]
- 66.Titus B, Frierson HF, Jr, Conaway M, et al. Endothelin axis is a target of the lung metastasis suppressor gene RhoGDI2. Cancer Res. 2005;65(16):7320–7327. doi: 10.1158/0008-5472.CAN-05-1403. [DOI] [PubMed] [Google Scholar]
- 67.Wu Y, McRoberts K, Berr SS, Frierson HF, Jr, Conaway M, Theodorescu D. Neuromedin U is regulated by the metastasis suppressor RhoGDI2 and is a novel promoter of tumor formation, lung metastasis and cancer cachexia. Oncogene. 2007;26(5):765–773. doi: 10.1038/sj.onc.1209835. [DOI] [PubMed] [Google Scholar]
- 68.Manavathi B, Singh K, Kumar R. MTA family of coregulators in nuclear receptor biology and pathology. Nucl Recept Signal. 2007;5:e010. doi: 10.1621/nrs.05010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.