Abstract
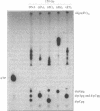
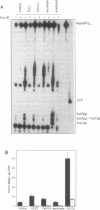
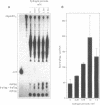
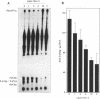
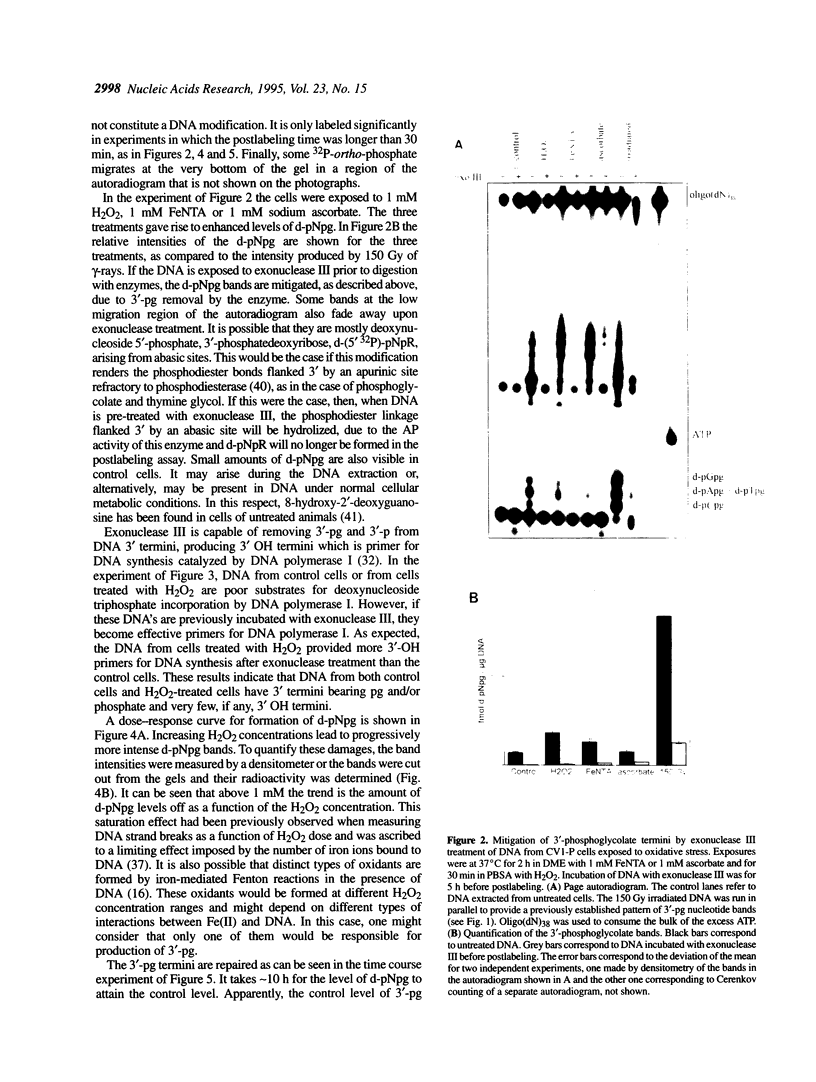
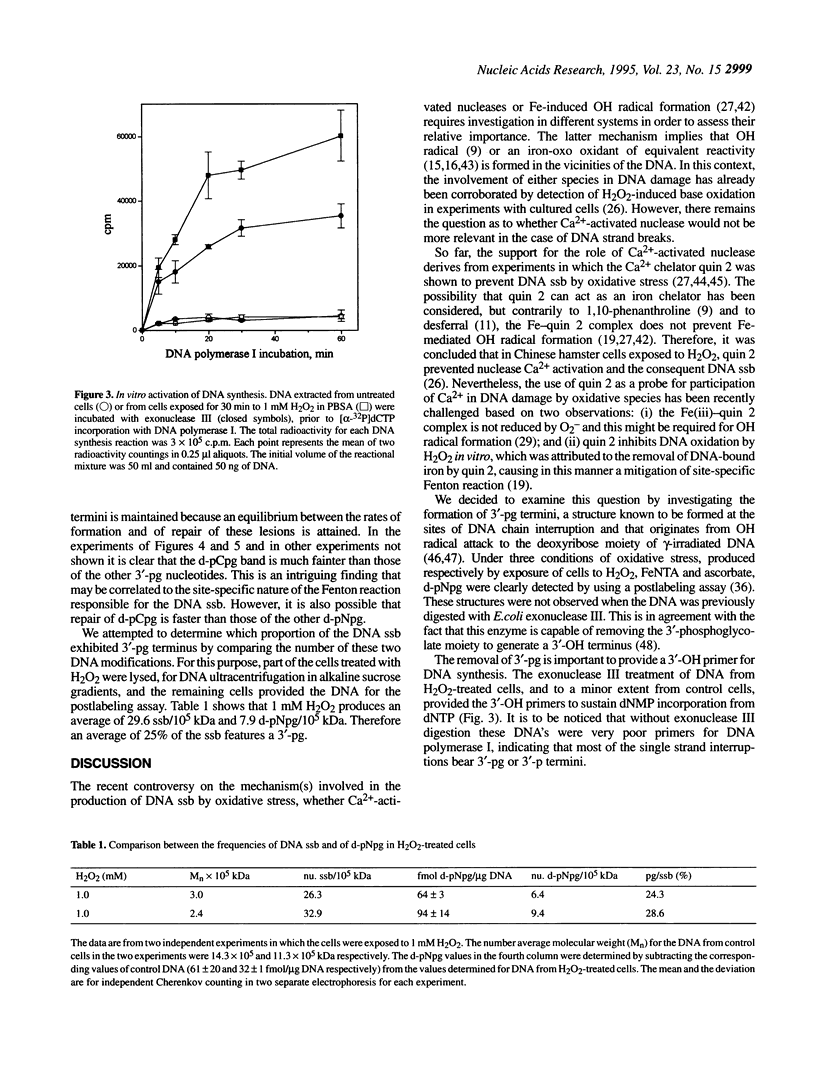
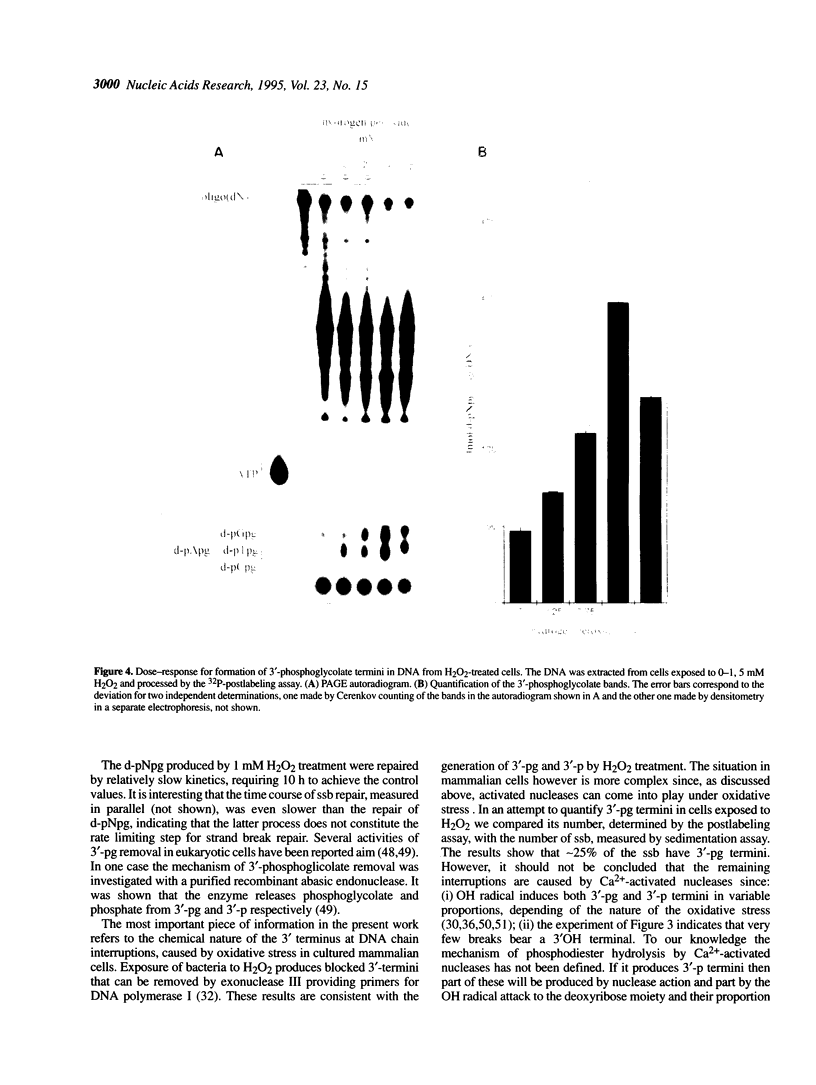
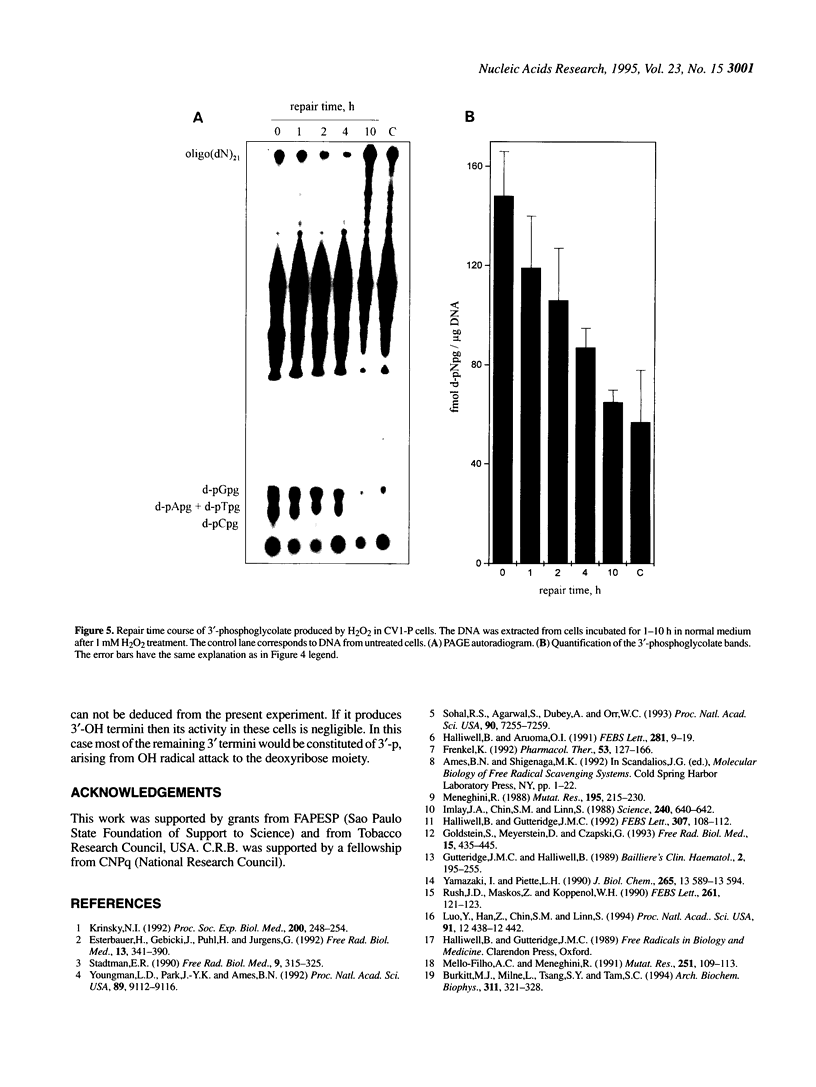
In recent years two mechanisms have been proposed for the production of DNA strand breaks in cells undergoing oxidative stress: (i) DNA attack by OH radical, produced by Fenton reaction catalyzed by DNA-bound iron; and (ii) DNA attack by calcium-activated nucleases, due to the increase of cytosolic and nuclear calcium induced by oxidative stress. We set out to investigate the participation of the former mechanism by detecting and quantifying 3'-phosphoglycolate, a 3' DNA terminus known to be formed by OH radical attack to the deoxyribose moiety, followed by sugar ring rupture and DNA strand rupture. These structures were found in DNA of monkey kidney cells exposed to hydrogen peroxide, iron nitrilotriacetate or ascorbate, all species known to favor a cellular pro-oxidant status. The method employed to measure 3' phosphoglycolate was the 32P-postlabeling assay. Repair time course experiments showed that it takes 10 h for 3'-phosphoglycolate to be removed from DNA. It was found that the DNA of both control cells and cells exposed to hydrogen peroxide had a very poor capacity of supporting in vitro DNA synthesis, catalyzed by DNA polymerase I. If the DNA was previously incubated with exonuclease III, an enzyme able to expose 3'-OH primers by removal of 3'-phosphoglycolate and 3'-phosphate termini the in vitro synthesis was substantially increased. This result shows that either of these termini are present at the break and that 3'-hydroxyl termini are virtually absent. At least 25% of the strand breaks exhibited 3'-phosphoglycolate termini as determined by the 32P-postlabeling assay, but due to the characteristic of the method this percentage is likely to be higher. These results favor the hypothesis that an oxidative agent generated by Fenton reaction is responsible for DNA strand breakage in cells undergoing oxidative stress.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Kanabus-Kaminska M. The production of DNA strand breaks in human leukocytes by superoxide anion may involve a metabolic process. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6820–6824. doi: 10.1073/pnas.82.20.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkitt M. J., Mason R. P. Direct evidence for in vivo hydroxyl-radical generation in experimental iron overload: an ESR spin-trapping investigation. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8440–8444. doi: 10.1073/pnas.88.19.8440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkitt M. J., Milne L., Tsang S. Y., Tam S. C. Calcium indicator dye Quin2 inhibits hydrogen peroxide-induced DNA strand break formation via chelation of iron. Arch Biochem Biophys. 1994 Jun;311(2):321–328. doi: 10.1006/abbi.1994.1244. [DOI] [PubMed] [Google Scholar]
- Cantoni O., Sestili P., Cattabeni F., Bellomo G., Pou S., Cohen M., Cerutti P. Calcium chelator Quin 2 prevents hydrogen-peroxide-induced DNA breakage and cytotoxicity. Eur J Biochem. 1989 Jun 15;182(2):209–212. doi: 10.1111/j.1432-1033.1989.tb14819.x. [DOI] [PubMed] [Google Scholar]
- Carrier W. L., Setlow R. B. Paper strip method for assaying gradient fractions containing radioactive macromolecules. Anal Biochem. 1971 Oct;43(2):427–432. doi: 10.1016/0003-2697(71)90272-7. [DOI] [PubMed] [Google Scholar]
- Cunningham M. L., Peak J. G., Peak M. J. Single-strand DNA breaks in rodent and human cells produced by superoxide anion or its reduction products. Mutat Res. 1987 Nov;184(3):217–222. doi: 10.1016/0167-8817(87)90019-8. [DOI] [PubMed] [Google Scholar]
- Demple B., Harrison L. Repair of oxidative damage to DNA: enzymology and biology. Annu Rev Biochem. 1994;63:915–948. doi: 10.1146/annurev.bi.63.070194.004411. [DOI] [PubMed] [Google Scholar]
- Demple B., Johnson A., Fung D. Exonuclease III and endonuclease IV remove 3' blocks from DNA synthesis primers in H2O2-damaged Escherichia coli. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7731–7735. doi: 10.1073/pnas.83.20.7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dizdaroglu M., Nackerdien Z., Chao B. C., Gajewski E., Rao G. Chemical nature of in vivo DNA base damage in hydrogen peroxide-treated mammalian cells. Arch Biochem Biophys. 1991 Mar;285(2):388–390. doi: 10.1016/0003-9861(91)90378-v. [DOI] [PubMed] [Google Scholar]
- Esterbauer H., Gebicki J., Puhl H., Jürgens G. The role of lipid peroxidation and antioxidants in oxidative modification of LDL. Free Radic Biol Med. 1992 Oct;13(4):341–390. doi: 10.1016/0891-5849(92)90181-f. [DOI] [PubMed] [Google Scholar]
- Feingold J. M., Masch J., Maio J., Mendez F., Bases R. Base sequence damage in DNA from X-irradiated monkey CV-1 cells. Int J Radiat Biol Relat Stud Phys Chem Med. 1988 Feb;53(2):217–235. doi: 10.1080/09553008814550581. [DOI] [PubMed] [Google Scholar]
- Fraga C. G., Shigenaga M. K., Park J. W., Degan P., Ames B. N. Oxidative damage to DNA during aging: 8-hydroxy-2'-deoxyguanosine in rat organ DNA and urine. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4533–4537. doi: 10.1073/pnas.87.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel K. Carcinogen-mediated oxidant formation and oxidative DNA damage. Pharmacol Ther. 1992;53(1):127–166. doi: 10.1016/0163-7258(92)90047-4. [DOI] [PubMed] [Google Scholar]
- Goldstein S., Meyerstein D., Czapski G. The Fenton reagents. Free Radic Biol Med. 1993 Oct;15(4):435–445. doi: 10.1016/0891-5849(93)90043-t. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Aruoma O. I. DNA damage by oxygen-derived species. Its mechanism and measurement in mammalian systems. FEBS Lett. 1991 Apr 9;281(1-2):9–19. doi: 10.1016/0014-5793(91)80347-6. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Biologically relevant metal ion-dependent hydroxyl radical generation. An update. FEBS Lett. 1992 Jul 27;307(1):108–112. doi: 10.1016/0014-5793(92)80911-y. [DOI] [PubMed] [Google Scholar]
- Henner W. D., Grunberg S. M., Haseltine W. A. Enzyme action at 3' termini of ionizing radiation-induced DNA strand breaks. J Biol Chem. 1983 Dec 25;258(24):15198–15205. [PubMed] [Google Scholar]
- Henner W. D., Rodriguez L. O., Hecht S. M., Haseltine W. A. gamma Ray induced deoxyribonucleic acid strand breaks. 3' Glycolate termini. J Biol Chem. 1983 Jan 25;258(2):711–713. [PubMed] [Google Scholar]
- Hoffmann M. E., Mello-Filho A. C., Meneghini R. Correlation between cytotoxic effect of hydrogen peroxide and the yield of DNA strand breaks in cells of different species. Biochim Biophys Acta. 1984 Apr 5;781(3):234–238. doi: 10.1016/0167-4781(84)90088-5. [DOI] [PubMed] [Google Scholar]
- Imlay J. A., Chin S. M., Linn S. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science. 1988 Apr 29;240(4852):640–642. doi: 10.1126/science.2834821. [DOI] [PubMed] [Google Scholar]
- Imlay J. A., Linn S. DNA damage and oxygen radical toxicity. Science. 1988 Jun 3;240(4857):1302–1309. doi: 10.1126/science.3287616. [DOI] [PubMed] [Google Scholar]
- Krinsky N. I. Mechanism of action of biological antioxidants. Proc Soc Exp Biol Med. 1992 Jun;200(2):248–254. doi: 10.3181/00379727-200-43429. [DOI] [PubMed] [Google Scholar]
- Mello Filho A. C., Hoffmann M. E., Meneghini R. Cell killing and DNA damage by hydrogen peroxide are mediated by intracellular iron. Biochem J. 1984 Feb 15;218(1):273–275. doi: 10.1042/bj2180273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello Filho A. C., Meneghini R. In vivo formation of single-strand breaks in DNA by hydrogen peroxide is mediated by the Haber-Weiss reaction. Biochim Biophys Acta. 1984 Feb 24;781(1-2):56–63. doi: 10.1016/0167-4781(84)90123-4. [DOI] [PubMed] [Google Scholar]
- Mello-Filho A. C., Meneghini R. Iron is the intracellular metal involved in the production of DNA damage by oxygen radicals. Mutat Res. 1991 Nov;251(1):109–113. doi: 10.1016/0027-5107(91)90220-i. [DOI] [PubMed] [Google Scholar]
- Menghini R. Genotoxicity of active oxygen species in mammalian cells. Mutat Res. 1988 May;195(3):215–230. [PubMed] [Google Scholar]
- Muehlematter D., Larsson R., Cerutti P. Active oxygen induced DNA strand breakage and poly ADP-ribosylation in promotable and non-promotable JB6 mouse epidermal cells. Carcinogenesis. 1988 Feb;9(2):239–245. doi: 10.1093/carcin/9.2.239. [DOI] [PubMed] [Google Scholar]
- Ochi T., Cerutti P. A. Clastogenic action of hydroperoxy-5,8,11,13-icosatetraenoic acids on the mouse embryo fibroblasts C3H/10T1/2. Proc Natl Acad Sci U S A. 1987 Feb;84(4):990–994. doi: 10.1073/pnas.84.4.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Sandström B. E., Granström M., Marklund S. L. New roles for quin2: powerful transition-metal ion chelator that inhibits copper-, but potentiates iron-driven, Fenton-type reactions. Free Radic Biol Med. 1994 Feb;16(2):177–185. doi: 10.1016/0891-5849(94)90141-4. [DOI] [PubMed] [Google Scholar]
- Sohal R. S., Agarwal S., Dubey A., Orr W. C. Protein oxidative damage is associated with life expectancy of houseflies. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):7255–7259. doi: 10.1073/pnas.90.15.7255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman E. R. Metal ion-catalyzed oxidation of proteins: biochemical mechanism and biological consequences. Free Radic Biol Med. 1990;9(4):315–325. doi: 10.1016/0891-5849(90)90006-5. [DOI] [PubMed] [Google Scholar]
- Weinfeld M., Liuzzi M., Paterson M. C. Selective hydrolysis by exo- and endonucleases of phosphodiester bonds adjacent to an apurinic site. Nucleic Acids Res. 1989 May 25;17(10):3735–3745. doi: 10.1093/nar/17.10.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinfeld M., Soderlind K. J. 32P-postlabeling detection of radiation-induced DNA damage: identification and estimation of thymine glycols and phosphoglycolate termini. Biochemistry. 1991 Jan 29;30(4):1091–1097. doi: 10.1021/bi00218a031. [DOI] [PubMed] [Google Scholar]
- Winters T. A., Henner W. D., Russell P. S., McCullough A., Jorgensen T. J. Removal of 3'-phosphoglycolate from DNA strand-break damage in an oligonucleotide substrate by recombinant human apurinic/apyrimidinic endonuclease 1. Nucleic Acids Res. 1994 May 25;22(10):1866–1873. doi: 10.1093/nar/22.10.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngman L. D., Park J. Y., Ames B. N. Protein oxidation associated with aging is reduced by dietary restriction of protein or calories. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9112–9116. doi: 10.1073/pnas.89.19.9112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Sonntag C. Carbohydrate radicals: from ethylene glycol to DNA strand breakage. Int J Radiat Biol Relat Stud Phys Chem Med. 1984 Nov;46(5):507–519. doi: 10.1080/09553008414551721. [DOI] [PubMed] [Google Scholar]