Summary
Purpose
To study the development of epilepsy following hypoxia-induced neonatal seizures in Long Evans rats and to establish the presence of spontaneous seizures in this model of early life seizures.
Methods
Long-Evans rat pups were subjected to hypoxia-induced neonatal seizures at postnatal day 10 (P10). Epidural cortical electroencephalography (EEG) and hippocampal depth electrodes were used to detect the presence of seizures in later adulthood (>P60). In addition, subdermal wire electrode recordings were used to monitor age at onset and progression of seizures in the juvenile period, at intervals between P10–P60. Timm staining was performed to evaluate mossy fiber sprouting in the hippocampi of P100 adult rats that had experienced neonatal seizures.
Key Findings
In recordings made from adult rats (P60–P180), the prevalence of epilepsy in cortical and hippocampal EEG recordings was 94.4% following early life hypoxic seizures. These spontaneous seizures were identified by characteristic spike and wave activity on EEG accompanied by behavioral arrest and facial automatisms (electroclinical seizures). Phenobarbital injection transiently abolished spontaneous seizures. EEG in the juvenile period (P10–60) showed that spontaneous seizures first occurred approximately 2 weeks after the initial episode of hypoxic seizures. Following this period, spontaneous seizure frequency and duration progressively increased with time. Furthermore, significantly increased sprouting of mossy fibers was observed in the CA3 pyramidal cell layer of the hippocampus in adult animals following hypoxia-induced neonatal seizures. Notably, Fluoro-Jade B staining confirmed that hypoxic seizures at P10 did not induce acute neuronal death.
Significance
The rodent model of hypoxia-induced neonatal seizures leads to the development of epilepsy in later life, accompanied by increased mossy fiber sprouting. In addition, this model appears to exhibit a seizure-free latent period, following which there is a progressive increase in the frequency of electroclinical seizures.
Keywords: Neonatal Seizures, electroencephalogram, epilepsy, infant, animal model
Introduction
Seizures are a common neurological disorder in the neonatal period, occurring in 1.8–5 per thousand live births in the United States and Canada (Hauser, et al., 1993, Ronen, et al., 2007). Hypoxic/ischemic encephalopathy (HIE) is the most common cause, and occurs in approximately 1–2/1000 live births, accounting for two-thirds of neonatal seizure cases (Tekgul, et al., 2006, Ronen, et al., 2007). Neonatal seizures can be clinically difficult to diagnose and may be exclusively electrographic (Mizrahi 1987, Volpe 2008). Furthermore, neonatal seizures are frequently refractory to currently available antiepileptic drugs (AEDs) (Sankar and Painter 2005).
HIE-associated neonatal seizures usually occur within the first 1–2 days of life and often remit after a few days (Volpe 2008), but are associated with later life epilepsy, neurological and/or cognitive deficits (Tekgul, et al., 2006, Ronen, et al., 2007). Despite advances in neonatal care, recent prospective studies in North America have reported a high incidence of epilepsy (28% and 31%) as well as cognitive disabilities (30–43%) in infants who experienced neonatal seizures (Tekgul, et al., 2006, Ronen, et al., 2007).
We previously developed an experimental model of neonatal seizures that utilizes graded global hypoxia to induce seizures in postnatal day (P) 10–12 rat pups (Jensen, et al., 1991, Jensen, et al., 1995). This enhanced susceptibility to seizures during the second postnatal week (P10–12) in Long-Evans rats coincides with a developmental stage of enhanced excitability and synaptic plasticity, and is analogous to the human neonatal period between 32–40 gestational weeks (Talos, et al., 2006, Rakhade and Jensen 2009). We have demonstrated that hypoxia-induced neonatal seizures involve the hippocampus and cortex, induce hippocampal hyperexcitability (Jensen, et al., 1998, Rakhade, et al., 2008), and produce long-term cognitive deficits (Jensen, et al., 1992, Mikati, et al., 2005). Importantly, these acute and chronic effects following these early life seizures occur in the absence of cell death (Jensen, et al., 1991, Koh and Jensen 2001). Similar to humans, seizures in the neonatal rodent can be resistant to conventional AEDs such as lorazepam and phenobarbital (Jensen, et al., 1995, Dzhala, et al., 2005, Dzhala, et al., 2008). The refractoriness of these seizures as well as their consequences are likely to result from unique age-specific mechanisms (Silverstein and Jensen 2007). For example, these early life seizures are associated with transient dysregulation of expression and function of the α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate glutamate receptor (AMPAR) subunits (Sanchez, et al., 2001, Sanchez, et al., 2005a, Rakhade, et al., 2008), and AMPAR antagonists have superior anticonvulsant efficacy in this model (Jensen, et al., 1995, Koh, et al., 2004).
It has been shown that hypoxia-induced early life seizures have long-term cognitive consequences (Jensen, et al., 1998, Koh, et al., 2004), but yet to be determined is whether this model generates spontaneous seizures in later life. Other models of infant and childhood epilepsy syndromes that exhibit the development of epilepsy include those induced by chemoconvulsants (Stafstrom, et al., 1997, Smith, et al., 1998, Holmes 2005), hyperthermia (Dube, et al., 2006), and hypoxia-ischemia (Kadam, et al., 2010).
Despite an absence of neuronal death in this model of hypoxia-induced neonatal seizures, we showed increased susceptibility to seizures and seizure-induced neuronal injury in later life (Jensen, et al., 1992, Jensen 1999, Koh and Jensen 2001). We hypothesized that hypoxia-induced neonatal seizures also promote the development of epilepsy in later life. In the present study, hypoxia-induced seizures initially result in a brief period (24–48 hours) of continuing behavioral seizures. Histopathologic analysis of brain at 24 and 48 hours following these early life seizures fail to show any significant increase in neuronal death. The acute seizures are followed by a 7–15 day latent period with subsequent development of increasingly frequent behavioral and electrographic seizures at juvenile and adult ages. In addition, these brief hypoxia-induced neonatal seizures also were associated with mossy fiber sprouting in stratum oriens of hippocampal area CA3. Taken together, these data suggest that neonatal seizures can result in development of long-term epilepsy and structural alterations in the hippocampal network.
Methods
Animals
Litters of male Long-Evans hooded rats (10 pups per litter, Charles River Laboratories, Wilmington, MA) were housed in a facility with a 12/hr light-dark cycle with unlimited food and water. P10 rat pups (18–22g) were subjected to hypoxia and returned to their dams until P21, and at weaning placed in shared cages. All procedures were approved by and in accordance with the guidelines of the Animal Care and Use Committee at Children's Hospital (Boston, MA) and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize animal suffering and the number of animals used.
Hypoxia-induced seizures
P10 rats were exposed to graded global hypoxia for 15 minutes in an airtight chamber, as described previously (Jensen et al., 1991). Briefly, oxygen concentration was maintained at 7% for 8 minutes, 5% for 6 minutes and 4% for 1 minute before termination of hypoxia. Litter mate controls were kept at room air. The entire rat litter was returned to their dams within one hour after the experiment (additional information in supplemental materials).
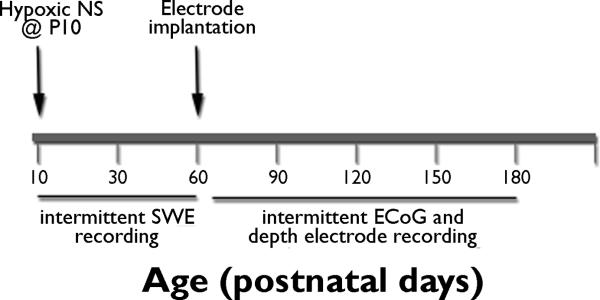
Rat pups exposed to hypoxia were kept alive for up to 6 months, and video-EEG recordings were performed either during infancy and young adolescence or during adulthood (Figure 1 and Supplemental Materials). Additional animals were subjected to hypoxia-induced seizures to assess cell death and comparisons were made to littermate controls (n=11/group).
Figure 1. Study design.
Long-Evans rat pups were exposed to hypoxia-induced neonatal seizures at P10. Short-term video-EEG recordings were performed using subdermal wire electrodes during neonatal and adolescent period. Hippocampal depth electrodes and cortical electrodes were implanted at P55–60 and long-term sequential EEG recordings were obtained from P60–180.
Long-term video-EEG recordings with implanted cranial electrodes
Rat pups (n=11) experiencing hypoxia-induced neonatal seizures survived into adulthood and were implanted with hippocampal and cortical electrodes to analyze seizure onset and progression and compared to littermate rat pups without hypoxia (n=10).
At P55, rats were implanted unilaterally with cranial electrodes (Schreiber, et al., 1992). Intracranial electrodes were implanted in hippocampus (AP −3.7, ML 2.3 from bregma, depth −2.3) as well as the cortical surface using epidural contact electrodes (Plastics One, Roanoke VA, USA). Starting 1 week after electrode implantation and continuing up to P180 (6 months), video-EEG recordings were obtained at predetermined intervals for longitudinal studies (described in Supplemental Materials). A subgroup of adult rats was treated with phenobarbital (50 mg/kg i.p.) to confirm the epileptic nature of the observed EEG abnormalities (n=7). EEG power analysis was performed on 4 hour epochs prior to and after injection of phenobarbital. EEG power was computed per 10 second interval by FFT of the EEG spectrum within 1–32 Hz.
Short Video-EEG using sub-dermal wire electrodes
In the initial period of 7 weeks following P10 hypoxic seizures (P10–P60), EEG recordings were acquired with Teflon-coated silver/silver chloride subdermal wire electrodes (SWEs) (Ives 2005, Rotenberg, et al., 2008). SWE implantation is minimally invasive and well tolerated by the young rat pups. Furthermore, SWEs could be removed following the EEG recordings so that pups could be returned to their dams, allowing multiple recording. Video-EEG recordings in pups were 2.5–3 hours in duration, freely-moving with a low torque commutator (Dragonfly Inc, VA) and connector assembly (John Ives, Manitoba, Canada, Supplemental Materials).
EEGs were analyzed by two individuals (T.H or P.K) and all analyses were confirmed by study-blinded secondary review, including a board certified clinical neurophysiologist (SNR and AR). Seizures were defined by the appearance of sustained polyspike activity, significantly different than background rhythm, longer than 3 seconds and associated with a behavioral correlate on video. Behavioral automatisms associated with abnormal EEG activity were used as benchmark for identifying electroclinical seizures. Average seizure frequency was calculated per hour of video-EEG recording, and seizure duration was measured as the time from first spike to last spike.
Timm Staining
Timm staining was performed in brain tissue from P100 rats after hypoxia-induced neonatal seizures or age-matched littermate controls (Holmes, et al., 1999, Huang, et al., 1999). Slides from control and experimental animals were stained simultaneously and imaged with light intensity and filter settings maintained at constant level (details described in supplemental methods).
Fluoro-Jade B immunostaining
Fluoro-Jade B immunostaining was performed in brain collected 24 and 48 hours after hypoxic neonatal seizures in P10 rats to identify neuronal injury (Fluoro-Jade B, AB310, Millipore, Billerica, MA) (Schmued, et al., 2005, Meikle, et al., 2007). Photomicrographs were obtained using Nikon 80i microscope and analyzed for Fluoro-Jade B staining in dying neuronal cells (details of staining procedures in supplemental materials).
Statistical Analysis
Comparisons between the groups experiencing hypoxic seizures versus age-matched controls were performed using paired t-test and ANOVA with post-hoc t-test for multiple group comparisons (SigmaPlot, Systat Inc., Chicago, IL).
Results
Acute behavioral and EEG seizures during hypoxia
Consistent with prior results, exposure to graded global hypoxia for 15 minutes induced acute seizures in 93% of the rats (58/61 rat pups, more than 5 seizures during hypoxia). The semiology of these seizures consisted of myoclonic jerks of trunk and limbs, chewing, wet dog shakes, head bobbing and tonic-clonic movements of head and limbs (Jensen, et al., 1991, Koh, et al., 2004). These behavioral changes were associated with electrographically recorded trains of polyspikes and sharp waves (Supplemental Video 1 and Supplemental Figure 1). In a representative population of pups during hypoxia (n=20), the average latency to the first behavioral seizure was 4.3±0.7 minutes, and the average seizure number was 8.7±1.2. The rat pups continued to exhibit short unprovoked behavioral seizures over 48 hours following the initial hypoxic insult. Brain sections obtained 24 and 48 hours after exposure to graded global hypoxia and coincident neonatal seizures did not show the presence of neuronal death and degeneration when stained with Fluoro-Jade B, a high affinity fluorescent marker for staining neurons undergoing degeneration (Supplemental Figure 2).
Depth electrode recordings from adult rats reveal spontaneous ictal activity following early life seizures
At 45 days following neonatal seizures, intracranial electrodes were successfully implanted in rats in right hippocampus and overlying cortex (n=11) and in the same locations in normoxic littermate controls (n=10). Video-EEG recordings were obtained from 8–16 hour epochs on multiple days from P60 to P175. Seizures were defined as electrographic seizures recorded from the unilateral bipolar hippocampal electrodes and epidural cortical electrodes (Figure 2.A) only when they consisted of spikes and sharp wave trains with amplitude 2 times greater than background and duration greater than 3 seconds, and had an associated behavioral correlate, such as sudden behavioral arrest, staring episodes, head-jerking and facial automatisms (Supplemental Video 2). When epileptiform activity was observed in the hippocampal electrodes, there were accompanying widespread changes in the electrographic activity in the cortical leads as well (Figure 2.A). The seizure epochs, shown in 10 second/page representation reveal the established patterns associated with epileptic seizures(Kadam, et al., 2010), including incremental increase in frequency and amplitude of spike discharges, tonic spike wave discharges and post-ictal slowing (Figure 2). Following any one seizure, the EEG returned to baseline within seconds and coincided with resumption of baseline behavioral activity observed prior to the seizure.
Figure 2. Typical Spontaneous electrographic seizure recorded from hippocampal electrodes in adult rats that have experienced hypoxic seizures during neonatal period.
Seizures were defined as typical events that met electrographic and behavioral criteria (A) Hippocampal and cortical EEG recordings show baseline activity prior to onset of seizures. Arrow points to the onset of epileptiform discharges. These events were typically associated with behavioral arrest, accompanied by staring, facial automatisms and head jerking/pawing. Inset represents the EEG trace with an expanded time scale showing the seizure initiation with spikes, progression to high frequency, large amplitude spikes during tonic phase of seizure and termination phase of seizure with smaller amplitude spike post-ictal slowing.
Electroclinical seizures (behavioral automatisms with electrographic epileptiform activity) were observed in 11 rats experiencing early life seizures (one rat was excluded due to electrode-induced cerebral lesion - see Supplemental Methods). Spontaneous seizures were observed in the first recording performed after electrode implantation in 9 rats following neonatal seizures at P10. Furthermore, at least one seizure occurred in 48/51 sessions of video-EEG recordings (94.4% of total) recorded for up to 8 hours. In contrast, 12.5 % of recordings from the normoxic controls showed abnormal activity on EEG. The presence of rare low frequency epileptiform discharges in control rats is consistent with previous reports in normal Long Evans rats (Shaw 2007).
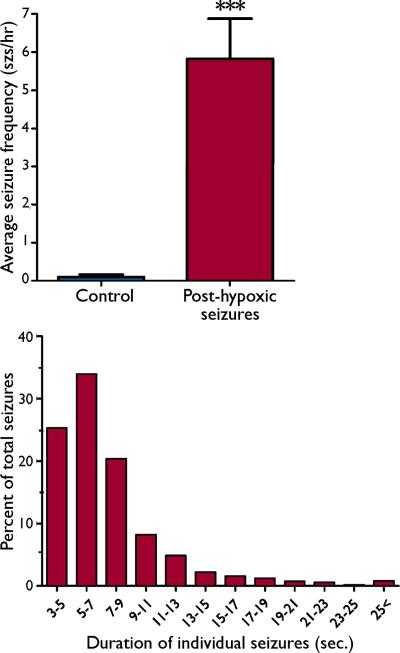
Averaging data recordings over 2–6 months of age, the average seizure frequency of 5.82±1.05 seizures/hour in post hypoxic neonatal seizure rats was significantly higher than the frequency of 0.10±0.06 seizures/hour observed in the normoxic controls (n=10 /group, p <0.001; Figure 3.A). Evaluation of the 1215 seizures recorded from all animals showed that seizure duration ranged from 3–36.5 seconds, with a mean duration of 7.32±0.12 seconds. 25.3% of recorded seizures were 3–5 seconds in duration, while 58.2% of the recorded seizures were between 5–11 seconds, and all events were accompanied by previously described behavioral alterations. Only 4.9% of the seizures recorded across all age groups were longer than 15 seconds (Figure 3.B). Seizure durations in this range are similar to those in other rat models of acquired epilepsy (Cha, et al., 2004, D'Ambrosio, et al., 2009). Using graded thresholds for defining seizure duration, we observed a significant increase in the number of epileptic seizures observed after hypoxia-induced neonatal seizures compared to normoxic littermate controls for ranges of 3–10 seconds (Supplemental Figure 3).
Figure 3. Frequency and duration of seizure events in rats experiencing neonatal seizures and age-matched controls.
(A) The frequency of seizures observed per hour of video-EEG recording analyzed from rats exposed to hypoxia-induced neonatal seizures at P10, and age-matched control rats is plotted. There is a significant increase in the number of seizure events observed in the rats exposed to neonatal seizures at P10, an average of 5.82±1.0 seizures/hour were recorded in rats exposed to neonatal seizures, compared with normoxic littermate controls (0.10±0.06 seizures/hour, n=11 hypoxic seizure rats, n = 10 controls, p< 0.001). (B) Cumulative histogram of the duration of hippocampal EEG-recorded seizures in adult rats that had been subjected to graded global hypoxia at P 10. Approximately 58.2% of all seizures recorded from the rats exposed to hypoxic neonatal seizures were in the 5–11 seconds duration time bin (n=1215 seizures from 11 rats). The rats also exhibited some seizures with significantly longer durations, but the long epileptic seizures were infrequent.
Analysis of the progression of seizure frequency and duration in a subset of animals exposed to hypoxia-induced neonatal seizures revealed that average seizure frequency increased by 234±94% (n=7, p<0.05) between the first and last recorded video-EEG session per animal, while there was a trend but no significant increase in the seizure duration (Figure 4). The increase in seizure frequency was consistently observed in a majority of animals that had experienced neonatal seizures (representative data, Fig 4C). Similarly the average seizure duration in this experimental subject increased from 4.6±0.9 seconds to 12.9±1.5 seconds (Fig 4D).
Figure 4. Increase in severity of seizure frequency and duration with increasing age in Long Evans rats exposed to neonatal seizures.
Analysis of long-term EEG recording data from each individual rat that had been recorded sequentially for up to 6 months following initiation of hypoxic neonatal seizures, we observed a trend of increasing seizure frequency and duration with increasing age of the rats. The average frequency of seizures increased 234.2±94% (n=7, p<0.05) in the last video-EEG recording compared to the first recording from the same individual subject (A), the seizure duration however did not show a significant difference (B). Representative data from one subject shows that the average frequency of seizures increased from 1.8±0.5 seizures/hour to 5.9±1.2 seizures/hour (C), the seizure duration in the subject increased from 4.6±0.9 seconds to 12.9±1.5 seconds (D).
Epileptiform EEG abnormalities in adult rats can be suppressed by phenobarbital
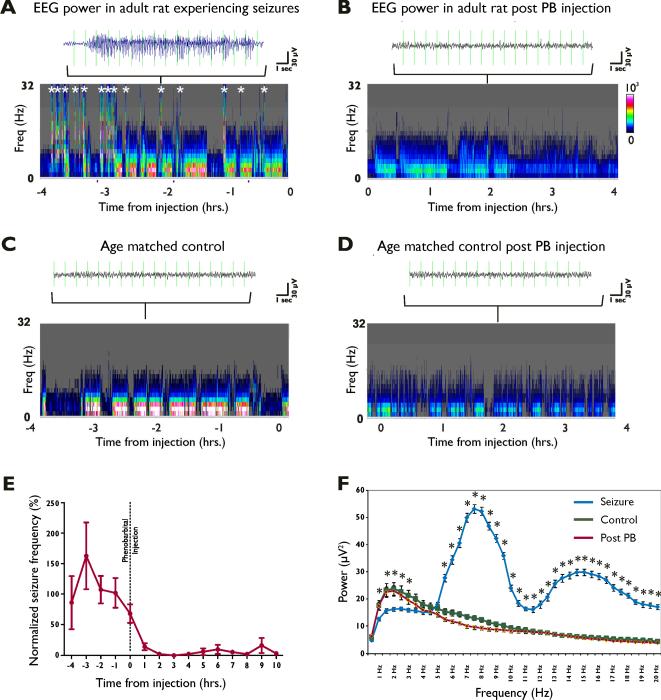
To validate the ictal nature of the events defined as seizures, we examined the effect of the anticonvulsant phenobarbital on the activity. Prior to injection of phenobarbital, spontaneous seizures and ictal EEG changes were correlated with increased EEG power in both the lower and higher frequency bins (Figure 5.A). A single phenobarbital dose (50 mg/kg i.p.) decreased total power over 15–30 minutes sustained over more than 6 hours, consistent with other models of ictal activity (Figure 5.B) (Dzhala, et al., 2008, Raol, et al., 2009). Spectral analysis demonstrated persistently increased power at higher frequencies (12–32 Hz, Fig 5 A and 5B). In contrast, EEG power plots from age-matched control rats prior to and following phenobarbital administration did not show increased EEG power in the high frequency bins (Figure 5.C and 5.D). Compared to baseline seizure frequency, phenobarbital dramatically reduced seizure activity within the first hour to 14.0±5.6% of baseline from the preceding 4 hours, and to 2.4±1.8% within 2 hours in adults with previous neonatal seizures (Figure 5E). Fast Fourier transform (FFT) analysis revealed that the spectral power in the 7–20Hz range was significantly greater during the seizure epochs relative to random EEG baseline epochs from the same individual (Figure 5.F). These results suggest that the abnormal, high frequency spikes in these rats are indeed epileptic seizures that can be controlled by anticonvulsant administration.
Figure 5. Increase in electrocorticogram (EEG) power parallels seizure occurrence and is reversed by phenobarbital.
EEG data were analyzed for total power and power at various frequencies over extended time period in a rat exposed to hypoxic seizures during the neonatal period. For each experimental condition the panel shows the power in 1 Hz frequency bins per 10 second interval, calculated by Fast Fourier Transform (FFT) (A) Seizure activity causes a increase in EEG power as evidenced by the intensity of the color and increase in high-frequency power (Each segment marked with * corresponds to a similar increase in the power that is associated with the occurrence of a epileptiform event). (B) Administration of phenobarbital abolishes the occurrence of these epileptiform discharges. Power spectrograms from EEG recordings obtained from age-matched normoxic controls prior to administration of phenobarbital (C) and following phenobarbital administration (D) reveal the absence of these high intensity epileptiform events. (E) Representative data from 7 different subjects that underwent these long-term recordings with phenobarbital injections administered i.p. during the course of the recording. Normalized seizure frequency reduced to 14.0% of baseline seizure frequency within 1 hour, and 2.4% of baseline seizure frequency within 2 hours of phenobarbital administration. (F) Fast Fourier transform (FFT) analysis revealed that the spectral power in the 7–20Hz range was significantly greater during the seizure epochs relative to random EEG baseline epochs, and that the spectral power in EEG epochs following phenobarbital administration was attenuated to levels observed in baseline EEG epochs.
Neonatal seizures lead to increased mossy fiber sprouting in area CA3 of hippocampus in later life
Increased and aberrant mossy fiber sprouting is observed in cases of human temporal lobe epilepsy and many experimental models of epilepsy and has been associated with epileptogenesis in animal models. The Timm staining in P100 rats after hypoxia-induced neonatal seizures revealed a significantly increased distribution of Timm granules in the CA3 region compared to controls (Figure 6.A, 6.B), as well as significantly higher average Timm scores (2.71±0.11), compared to littermate control scores (1.92±0.14, n=7,7, p<0.001). Consistently, densitometric analysis of the relative intensities of Timm staining showed that animals experiencing hypoxia-induced seizures had a significantly stronger staining intensity (228±19.0%) compared to littermate controls (100±10.8, n=7, 7; p<0.001) (Figure 6.D). This staining was most prominent in the septal regions of the hippocampus.
Figure 6. Neonatal seizures lead to increased mossy fiber sprouting from CA3 pyramidal cells.
(A) Photomicrographs of Timm staining in hippocampi of adult rats that had experienced hypoxia-induced neonatal seizures at P10 show a marked increase in mossy fiber sprouting in the stratum pyramidale and stratum oriens layers near the hippocampal CA3 region, as compared to age-matched littermate controls (B). There is slight staining of pyramidal cells in the septal region of the hippocampus observed in control animals. Inset shows higher magnification views of the distribution of Timm granules in the stratum pyramidale and stratum oriens (scale bar = 500 μm). (C) Average Timm scores, calculated using a semi-quantitative scoring scale previously described, from hippocampal sections from animals experiencing neonatal seizures were significantly higher (2.71±0.11), as compared scores in the littermate controls (1.92±0.14, n=7,7, p<0.001) (D) Mean density measurements of Timm staining as measured in the stratum pyramidale-oriens, confirmed the increase in Timm granules, showing a 228±19% increase as compared to littermate controls (100±10.8, n=7,7 ; p < 0.001).
Evidence for a latent period following acute and subacute ictal activity induced by early life seizures
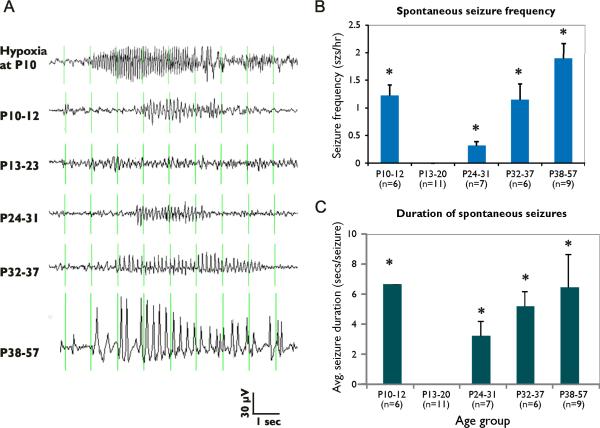
In order to examine the process of seizure development and progression, video-EEG monitoring was performed in rat pups starting immediately after and extending up to 50 days from the hypoxic seizures. Five age windows were examined: the immediate 1–48 hours post seizure (P10–12), the early juvenile period of 2–12 days (P13–P22), mid-juvenile age P23–P31, late juvenile period of P32–P37, and early adult (P38–P56) (Figure 7.A).
Figure 7. EEG recordings with representations of ictal discharges.
(A) during hypoxia, (B) 0–2 days, (C) 3–10 days, (D) 14–21 days, (E) 22–27 days, and (F) 28–47 days following hypoxia. Abnormal activity were classified as seizures when activity was paroxysmal, rhythmic, increasing in amplitude and >3 seconds in duration. Electrographic seizures associated with abnormal behavioral automatisms were observed within the first 48 hours following hypoxia-induced seizures at P10. There was a decrease in the electrographic seizure activity between the ages of P13–20. The frequency of electrographic seizures associated with abnormal behavior increased again in the animals at P24–31 and continued to increase into adulthood.
In the immediate 48 hours following hypoxia-induced seizures, 92% of the pups (10 of 11 pups) exhibited behavioral seizures accompanied by EEG changes, with an average duration of 5.4±1.4 seconds (Figure 7). The average frequency was 0.66 seizures/hour, significantly less than during hypoxia (8.14 seizures/hour, n=11, p<0.05). Following these subacute seizures, there was a relative cessation of spontaneous seizures in the early juvenile period at 2–12 days after hypoxic seizures, suggesting a latent period of epileptogenesis. During this time (P13–P21), none of the video-EEG recordings from 11 rats revealed any abnormal behaviors. EEG recordings did reveal rare and short polyspike discharges lasting <1 second (data not shown), without behavioral correlate, and thus not defined as seizures.
Electroclinical seizures were observed again as early as P25 in 19% of the rats recorded from during the age-window from P25–P31 (2/9 rats). The average seizure duration at this age was brief (3.2±0.4 seconds), while no activity was seen in controls (n=5, p<0.05) (Table 1). The incidence of electroclinical seizures steadily increased up to 50% (n=6, p<0.05) by P32–37, and 55% by P37–P55 (n=9, p<0.05). Behavioral correlates included cessation of activity and behavioral arrest, whisker movements and head nodding/shaking in some cases. Seizure duration and frequency also increased at these time points; the average seizure duration and frequency at P32–37 was 5.2±0.2_sec and 2.26±0.8 seizures/hour, respectively, and at P38–P55 was 6.42±1.1_sec and 2.8±0.8 seizures/hour, respectively (Figures 7.B, 7.C). These data indicate that the incidence, frequency, and duration of seizures observed in individual animals after hypoxia-induced seizures increased with age following the hypoxic insult.
Table 1.
Emergence of spontaneous seizures following hypoxia-induced neonatal seizures: EEG recordings were performed in rat pups exposed to neonatal seizures and the percentage of pups exhibiting spontaneous recurrent seizures during specified time windows was calculated. The average number of seizures observed during the 3 hour video-EEG recording epoch is tabulated.
| Age Window (postnatal days) | Percentage of animals with spontaneous seizures | Average number of seizures recorded/animal |
|---|---|---|
| P 10–12 (n = 6) | 92% | 4.2 seizures |
| P12–24 (n = 11) | 0% | 0 |
| P25–31 (n = 9) | 21% | 8.3 seizures |
| P32–37 (n = 6) | 50% | 9 seizures |
| P38–58 (n = 9) | 50% | 11.2 seizures |
| P 58 – 180 (n = 11) | 100% | 22 seizures |
Discussion
The major focus of this study is the emergence of spontaneous seizure activity following early life hypoxia-induced seizures in the immature rat. We and others have reported acute seizures in this model (Rakhade and Jensen 2009) and increased seizure susceptibility to “second hit” seizures in later life (Koh, et al., 2004). Enhanced susceptibility to provoked seizures demonstrates increased network excitability.
Here we report for the first time that hypoxia-induced seizures during the neonatal period in rats lead to recurrent, unprovoked, and spontaneous behavioral and electrographic (electroclinical) seizures in adulthood. In addition, this is the first report of a 2–3 week latent period in this model. Following this latent phase, there was a progressive intensification of seizure activity as evidenced by the increased seizure frequency and duration, as well an increase in the cumulative number of individual rats exhibiting spontaneous epileptiform events. Furthermore, similar to other immature seizure models, we observed increased mossy fiber sprouting in hippocampal area CA3 in the adult brains after hypoxia-induced neonatal seizures at P10. Taken together, these data indicate that the hypoxia-induced early life seizures result in the development of epilepsy in rats and thus may represent a useful model to study the epileptogenic process, as well as interventional strategies to prevent the long-term sequelae of this form of neonatal seizures.
Emergence of epilepsy following neonatal seizures in human infants
Hypoxic encephalopathy is one of the most common causes of neonatal seizures (Volpe 2008), and can result in later life epilepsy and other neurodevelopmental sequelae (Berg and Shinnar 1997, Ronen, et al., 2007). The age of onset of seizures influences the long-term developmental challenges such as intellectual impairment (Hermann, et al., 2002), learning disabilities (Soria, et al., 2007) and medical refractoriness (Berg, et al., 1996, Camfield and Camfield 2002). Neonatal seizures can lead to development of both partial and generalized epilepsy in later life in 23–40 % of cases (Ronen, et al., 2007). Furthermore, children experiencing neonatal seizures have lower scores on measures of intellectual ability (Toet, et al., 2005, Glass, et al., 2009).
Development of an animal model of neonatal seizures with later life epilepsy
Here we present a model suitable for the study of molecular mechanisms and therapeutic efficacy trials for both the previously reported neurobehavioral outcomes as well as the presently described epileptogenic effects. Depth electrode recordings of spontaneous recurrent seizures arising over 8 weeks and into adulthood demonstrated involvement of both hippocampal and cortical structures. The accompanying seizure semiology was primarily behavioral arrest with head jerking and wet dog shakes, rather than episodes of generalized tonic clonic seizures or status epilepticus. Cortical ictal EEG patterns were present synchronously in both hemispheres, consistent with the fact that the original insult was global hypoxia. In addition, this model induced later life spontaneous electroclinical events predominantly 15 seconds or less in duration as opposed to recurrent episodes of prolonged status or generalized seizure activity. Other models of acquired epilepsy have similarly reported outcomes of either partial or brief seizures, including models involving prior traumatic brain injury (D'Ambrosio, et al., 2009), hypoxia/ischemia and stroke (Kadam, et al., 2010), as well as febrile seizures (Dube, et al., 2006). In particular, D'Ambrosio et al. have described the presence of short electroclinical epileptiform events with duration of 0.8–2 seconds being associated with behavioral arrest in both rodents exposed to rostral parasagital fluid percussion injury. We showed that administration of phenobarbital temporarily abolished the seizure activity observed in the adult rats with previously exposure to hypoxia-induced seizures.
Our prior in vitro experiments performed in hippocampal slices during the subacute period have shown enhanced network excitability and changes in neurotransmitter function following these early life seizures (Sanchez, et al., 2001, Sanchez, et al., 2005a, Rakhade, et al., 2008). Depth electrode recordings confirmed involvement of the hippocampus in initiating and/or maintaining epileptic seizures, the seizure semiology was similar to behavioral changes typically observed in seizures involving limbic areas (Ben Ari, et al., 1981, D'Ambrosio, et al., 2009).
Neonatal seizures lead to increased mossy fiber sprouting in hippocampus
Sprouting of axon terminals after prolonged or recurrent seizures has been described in a number of models including kainic acid (Tauck and Nadler 1985, Cronin and Dudek 1988), electrical stimulation (Sutula, et al., 1988) and PTZ kindling (Golarai, et al., 1992). Mossy fiber sprouting has been observed in tissue from patients with temporal lobe epilepsy (Sutula, et al., 1989, Babb 1991). Indirect evidence from several studies suggest that these new neurites establish functional synaptic connections and they may contribute to the state of hyperexcitability that either provokes or facilitates abnormal discharges (Wuarin and Dudek 1996). Alterations in the CA3 pyramidal region have been observed in other models of seizures in the immature brain, including seizures caused by PTZ kindling (Holmes, et al., 1999), kainic acid (Huang, et al., 1999), amygdala kindling (Represa and Ben-Ari 1992), corticotrophin releasing hormone (CRH) induced status epilepticus (Ribak and Baram 1996) and intrahippocampal injections of kainic acid at P7. This altered mossy fiber distribution may contribute to the enhanced synaptic activity seen at CA1:CA3 synapses following hypoxic neonatal seizures (Sanchez, et al., 2005b, Rakhade, et al., 2008) and may correlate with disturbance in cognitive function seen in later life (Crusio, et al., 1987, Schwegler, et al., 1988). The axons of the granule cells are elongating during the first 2 weeks of postnatal development (Amaral 1979, Rahimi and Claiborne 2007), and neonatal seizures during this window of development may alter the hippocampal circuitry that is being actively modulated.
Neonatal hypoxia is followed by latent period of epileptogenesis and spontaneous seizures progressively increase with age
Most models of acquired epilepsy in adult brain suggest that epileptogenesis is progressive and that there is a `latent period' between the initial insult and the appearance of the epileptic seizures (D'Ambrosio, et al., 2004) (Cherubini, et al., 1983, Cronin and Dudek 1988, Dudek, et al., 2002). While an increasing number of studies in immature rats have shown that early life insults can lead to the development of epilepsy in later life (Holmes, et al., 1998, Dube, et al., 2006, Kadam, et al., 2010), these studies have not focused on characterizing an intervening latent period. Our experimental model of hypoxia-induced seizures reveals a progression of epileptic activity following early life seizures and we did not observe spontaneous electroclinical seizures between 2–15 days following the original hypoxia-induced seizures. Due to the age of the pups and their inability to be separated from their dam for more than 2–3 hours, continuous EEG recordings were not possible, and hence we can only conclude that there was relatively less seizure activity in this period. The earliest electroclinical seizures, starting approximately around fourth postnatal week, were manifested by a behavioral arrest and showed that animals progressed to develop electroclinical events associated with repetitive motor activity/pawing and head-nodding/head shakes. The potential presence of the latent phase suggests that there may be a possibility of intervening during this phase to prevent the development of clinical epilepsy. Indeed, we have previously shown that the administration of specific anticonvulsant agents can attenuate the hippocampal hyperexcitability and induction of signaling cascades in this rodent model (Sanchez, et al., 2005a, Rakhade, et al., 2008).
Spontaneous recurrent seizures arise in the absence of neuronal death
Models of adult epileptogenesis, including those initiated by kainic acid or pilocarpine-induced status epilepticus, result in acute neuronal death (Holmes 2009). However, seizure-induced neuronal death is not a consistent feature in immature rodents. Previous studies performed in immature rats exposed to hyperthermia (Dube, et al., 2006) and flurothyl-induced seizures (Schmid, et al., 1999) have reported the development of spontaneous seizures despite the lack of cell death. In contrast, a recent study in an experimental model of perinatal stroke and hypoxic ischemic encephalopathy suggests that neuronal death (i.e., a clear infarct following neonatal stroke) is required for the development of epilepsy (Dudek, et al., 2010, Kadam, et al., 2010). We have previously shown that hypoxia-induced neonatal seizures do not result in neuronal death (Koh, et al., 2004), although they evoke synaptic dysplasticity in the surviving neuronal network (Sanchez, et al., 2005a, Rakhade, et al., 2008). The second postnatal week is a critical period of development in the rat brain (Silverstein and Jensen 2007). Insults such as pilocarpine injection (Liu, et al., 1994), flurothyl inhalation (Sogawa, et al., 2001), and hyperthermia (Dube, et al., 2006) during this critical period can induce recurrent seizures in rats despite the lack of neuronal cell death. Furthermore, most of these models have shown considerable impairment in spatial learning as well as tests of social interaction when studied during young adolescence and adulthood (Baram, et al., 2002, Karnam, et al., 2009). The present data suggest that cellular death may not be an essential component for the development of cognitive, developmental and epileptogenic effects of early life seizures. Another important observation is that the spontaneous seizures observed in later life in immature seizure models that lack cell death (Holmes, et al., 1999, Dube, et al., 2005) are generally of shorter duration and lower frequency than in those immature models with neuronal death (Kadam, et al., 2010). It is possible that a relatively intact neuronal network may restrain excessive seizure activity, and may explain this difference. In addition, the absence of cell death allows study of the cellular and molecular alterations during a period of relative seizure silence (P12–24), without the confounds of cell death mechanisms.
Conclusions
The hypoxia-induced neonatal seizure model recapitulates a number of salient clinical features of hypoxic encephalopathy and neonatal seizures. In this model, seizures during the neonatal period appear to be sufficient to initiate epileptogenic processes and development of later life epilepsy. Furthermore, while there is a lack of cell death in this experimental model, there are structural alterations including increased mossy fiber sprouting in the hippocampal CA3 region. Our prior work has shown that the administration of AMPAR antagonists can attenuate the hippocampal hyperexcitability and induction of signaling cascades in this rodent model (Koh, et al., 2004, Sanchez, et al., 2005a, Rakhade, et al., 2008). Given the present demonstration that this model results in spontaneous seizures in later life, future work can now address whether intervention following seizures can modify epileptogenesis.
Supplementary Material
Acknowledgements
The authors wish to thank Neil Marya, Andrew Murphy and Victor Lan for help with animal handling. This work was supported by funding from National Institutes of Health NINDS NS 31718 (FEJ), Office of the Director, National Institutes of Health GMS DP1 OD003347 (FEJ), Parents Against Childhood Epilepsy (SNR and FEJ) and core support from the Mental Retardation Developmental Disorders Research Center Grant NIH NICHHD P30 HD18655.
Footnotes
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
None of the authors has any conflict of interest to disclose.
References
- Amaral DG. Synaptic extensions from the mossy fibers of the fascia dentata. Anat Embryol (Berl) 1979;155:241–251. doi: 10.1007/BF00317638. [DOI] [PubMed] [Google Scholar]
- Babb TL. Bilateral pathological damage in temporal lobe epilepsy. Can J Neurol Sci. 1991;18:645–648. doi: 10.1017/s031716710003287x. [DOI] [PubMed] [Google Scholar]
- Baram TZ, Eghbal-Ahmadi M, Bender RA. Is neuronal death required for seizure-induced epileptogenesis in the immature brain? Prog.Brain Res. 2002;135:365–375. doi: 10.1016/S0079-6123(02)35033-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Ari Y, Tremblay E, Riche D, Ghilini G, Naquet R. Electrographic, clinical and pathological alterations following systemic administration of kainic acid, bicuculline or pentetrazole: metabolic mapping using the deoxyglucose method with special reference to the pathology of epilepsy. Neuroscience. 1981;6:1361–1391. doi: 10.1016/0306-4522(81)90193-7. [DOI] [PubMed] [Google Scholar]
- Berg AT, Levy SR, Novotny EJ, Shinnar S. Predictors of intractable epilepsy in childhood: a case-control study. Epilepsia. 1996;37:24–30. doi: 10.1111/j.1528-1157.1996.tb00507.x. [DOI] [PubMed] [Google Scholar]
- Berg AT, Shinnar S. Do seizures beget seizures? An assessment of the clinical evidence in humans. Clinical Neurophysiology. 1997;14:102–110. doi: 10.1097/00004691-199703000-00003. [DOI] [PubMed] [Google Scholar]
- Camfield P, Camfield C. Epileptic syndromes in childhood: clinical features, outcomes, and treatment. Epilepsia. 2002;43(Suppl 3):27–32. doi: 10.1046/j.1528-1157.43.s.3.3.x. [DOI] [PubMed] [Google Scholar]
- Cha BH, Akman C, Silveira DC, Liu X, Holmes GL. Spontaneous recurrent seizure following status epilepticus enhances dentate gyrus neurogenesis. Brain Dev. 2004;26:394–397. doi: 10.1016/j.braindev.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Cherubini E, DeFeo MR, Mecarelli O, Ricci GF. Behavioral and electrographic patterns induced by systemic administration of kainic acid in developing rats. Dev.Brain Res. 1983;9:69–77. doi: 10.1016/0165-3806(83)90110-4. [DOI] [PubMed] [Google Scholar]
- Cronin J, Dudek FE. Chronic seizures and collateral sprouting of dentate mossy fibers after kainic acid treatment in rats. Brain Res. 1988;474:181–184. doi: 10.1016/0006-8993(88)90681-6. [DOI] [PubMed] [Google Scholar]
- Crusio WE, Schwegler H, Lipp HP. Radial-maze performance and structural variation of the hippocampus in mice: a correlation with mossy fibre distribution. Brain Res. 1987;425:182–185. doi: 10.1016/0006-8993(87)90498-7. [DOI] [PubMed] [Google Scholar]
- D'Ambrosio R, Fairbanks JP, Fender JS, Born DE, Doyle DL, Miller JW. Post-traumatic epilepsy following fluid percussion injury in the rat. Brain. 2004;127:304–314. doi: 10.1093/brain/awh038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ambrosio R, Hakimian S, Stewart T, Verley DR, Fender JS, Eastman CL, Sheerin AH, Gupta P, Diaz-Arrastia R, Ojemann J, Miller JW. Functional definition of seizure provides new insight into post-traumatic epileptogenesis. Brain. 2009;132:2805–2821. doi: 10.1093/brain/awp217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube C, Baram TZ, Pitkanen A, Schwartzkroin PA. Models of Seizures and Epilepsy. Elsevier; San Diego: 2005. Complex febrile seizures-an experimental model in immature rodent; pp. 333–340. [Google Scholar]
- Dube C, Richichi C, Bender RA, Chung G, Litt B, Baram TZ. Temporal lobe epilepsy after experimental prolonged febrile seizures: prospective analysis. Brain. 2006;129:911–922. doi: 10.1093/brain/awl018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek FE, Ekstrand JJ, Staley KJ. Is neuronal death necessary for acquired epileptogenesis in the immature brain? Epilepsy Curr. 2010;10:95–99. doi: 10.1111/j.1535-7511.2010.01369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek FE, Hellier JL, Williams PA, Ferraro DJ, Staley KJ. The course of cellular alterations associated with the development of spontaneous seizures after status epilepticus. Prog.Brain Res. 2002;135:53–65. doi: 10.1016/S0079-6123(02)35007-6. [DOI] [PubMed] [Google Scholar]
- Dzhala VI, Brumback AC, Staley KJ. Bumetanide enhances phenobarbital efficacy in a neonatal seizure model. Ann.Neurol. 2008;63:222–235. doi: 10.1002/ana.21229. [DOI] [PubMed] [Google Scholar]
- Dzhala VI, Talos DM, Sdrulla DA, Brumback AC, Mathews GC, Benke TA, Delpire E, Jensen FE, Staley KJ. NKCC1 transporter facilitates seizures in the developing brain. Nat.Med. 2005;11:1205–1213. doi: 10.1038/nm1301. [DOI] [PubMed] [Google Scholar]
- Glass HC, Glidden D, Jeremy RJ, Barkovich AJ, Ferriero DM, Miller SP. Clinical Neonatal Seizures are Independently Associated with Outcome in Infants at Risk for Hypoxic-Ischemic Brain Injury. J Pediatr. 2009;155(3):318–323. doi: 10.1016/j.jpeds.2009.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golarai G, Cavazos JE, Sutula TP. Activation of the dentate gyrus by pentylenetetrazol evoked seizures induces mossy fiber synaptic reorganization. Brain Res. 1992;593:257–264. doi: 10.1016/0006-8993(92)91316-7. [DOI] [PubMed] [Google Scholar]
- Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota:1935–1984. Epilepsia. 1993;34:453–468. doi: 10.1111/j.1528-1157.1993.tb02586.x. [DOI] [PubMed] [Google Scholar]
- Hermann BP, Seidenberg M, Bell B. The neurodevelopmental impact of childhood onset temporal lobe epilepsy on brain structure and function and the risk of progressive cognitive effects. Prog.Brain Res. 2002;135:429–438. doi: 10.1016/S0079-6123(02)35040-4. [DOI] [PubMed] [Google Scholar]
- Holmes GL. Effects of seizures on brain development: lessons from the laboratory. Pediatr.Neurol. 2005;33:1–11. doi: 10.1016/j.pediatrneurol.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Holmes GL. The long-term effects of neonatal seizures. Clin Perinatol. 2009;36:901–914. vii–viii. doi: 10.1016/j.clp.2009.07.012. [DOI] [PubMed] [Google Scholar]
- Holmes GL, Gaiarsa JL, Chevassus-Au-Louis N, Ben-Ari Y. Consequences of neonatal seizures in the rat: morphological and behavioral effects. Ann.Neurol. 1998;44:845–857. doi: 10.1002/ana.410440602. [DOI] [PubMed] [Google Scholar]
- Holmes GL, Sarkisian M, Ben-Ari Y, Chevassus-Au-Louis N. Mossy fiber sprouting after recurrent seizures during early development in rats. Journal of Comparative Neurology. 1999;404(4):537–553. doi: 10.1002/(sici)1096-9861(19990222)404:4<537::aid-cne9>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Huang L, Cilio MR, Silveira DC, McCabe BK, Sogawa Y, Stafstrom CE, Holmes GL. Long-term effects of neonatal seizures: a behavioral, electrophysiological, and histological study. Brain Research Developmental Brain Research. 1999;118(1–2):99–107. doi: 10.1016/s0165-3806(99)00135-2. [DOI] [PubMed] [Google Scholar]
- Ives JR. New chronic EEG electrode for critical/intensive care unit monitoring. J.Clin.Neurophysiol. 2005;22:119–123. doi: 10.1097/01.wnp.0000152659.30753.47. [DOI] [PubMed] [Google Scholar]
- Jensen FE. Acute and chronic effects of seizures in the developing brain: experimental models. Epilepsia. 1999;40(Suppl 1):S51–S58. doi: 10.1111/j.1528-1157.1999.tb00879.x. [DOI] [PubMed] [Google Scholar]
- Jensen FE, Alvarado S, Firkusny IR, Geary C. NBQX blocks the acute and late epileptogenic effects of perinatal hypoxia. Epilepsia. 1995;36(10):966–972. doi: 10.1111/j.1528-1157.1995.tb00954.x. [DOI] [PubMed] [Google Scholar]
- Jensen FE, Applegate CD, Holtzman D, Belin T, Burchfiel J. Epileptogenic effect of hypoxia in the immature rodent brain. Ann.of Neurol. 1991;29:629–637. doi: 10.1002/ana.410290610. [DOI] [PubMed] [Google Scholar]
- Jensen FE, Holmes GL, Lombroso CT, Blume HK, Firkusny IR. Age dependent changes in long term seizure susceptibility and behavior after hypoxia in rats. Epilepsia. 1992;33:971–980. doi: 10.1111/j.1528-1157.1992.tb01746.x. [DOI] [PubMed] [Google Scholar]
- Jensen FE, Wang C, Stafstrom CE, Liu Z, Geary C, Stevens MC. Acute and chronic increases in excitability in rat hippocampal slices after perinatal hypoxia in vivo. J.Neurophysiol. 1998;79:73–81. doi: 10.1152/jn.1998.79.1.73. [DOI] [PubMed] [Google Scholar]
- Kadam SD, White AM, Staley KJ, Dudek FE. Continuous electroencephalographic monitoring with radio-telemetry in a rat model of perinatal hypoxia-ischemia reveals progressive post-stroke epilepsy. J Neurosci. 2010;30:404–415. doi: 10.1523/JNEUROSCI.4093-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnam HB, Zhou JL, Huang LT, Zhao Q, Shatskikh T, Holmes GL. Early life seizures cause long-standing impairment of the hippocampal map. Exp Neurol. 2009;217:378–387. doi: 10.1016/j.expneurol.2009.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh S, Jensen FE. Topiramate blocks perinatal hypoxia-induced seizures in rat pups. Ann.Neurol. 2001;50:366–372. doi: 10.1002/ana.1122. [DOI] [PubMed] [Google Scholar]
- Koh S, Tibayan FD, Simpson J, Jensen FE. NBQX or topiramate treatment following perinatal hypoxia-induced seizures prevents later increases in seizure-induced neuronal injury. Epilepsia. 2004;45:569–575. doi: 10.1111/j.0013-9580.2004.69103.x. [DOI] [PubMed] [Google Scholar]
- Liu Z, Gatt A, Werner SJ, Mikati MA, Holmes GL. Long term behavioral defects following pilocarpine seizures in immature rats. Epilepsy Res. 1994;19:191–204. doi: 10.1016/0920-1211(94)90062-0. [DOI] [PubMed] [Google Scholar]
- Meikle L, Talos DM, Onda H, Pollizzi K, Rotenberg A, Sahin M, Jensen FE, Kwiatkowski DJ. A mouse model of tuberous sclerosis: neuronal loss of Tsc1 causes dysplastic and ectopic neurons, reduced myelination, seizure activity, and limited survival. J Neurosci. 2007;27:5546–5558. doi: 10.1523/JNEUROSCI.5540-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikati MA, Zeinieh MP, Kurdi RM, Harb SA, El Hokayem JA, Daderian RH, Shamseddine A, Obeid M, Bitar FF, El Sabban M. Long-term effects of acute and of chronic hypoxia on behavior and on hippocampal histology in the developing brain. Brain Res.Dev.Brain Res. 2005;157:98–102. doi: 10.1016/j.devbrainres.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Mizrahi EM. Neonatal seizures: problems in diagnosis and classification. Epilepsia. 1987;28(Suppl 1):S46–55. doi: 10.1111/j.1528-1157.1987.tb05757.x. [DOI] [PubMed] [Google Scholar]
- Rahimi O, Claiborne BJ. Morphological development and maturation of granule neuron dendrites in the rat dentate gyrus. Prog Brain Res. 2007;163:167–181. doi: 10.1016/S0079-6123(07)63010-6. [DOI] [PubMed] [Google Scholar]
- Rakhade SN, Jensen FE. Epileptogenesis in the immature brain: emerging mechanisms. Nat Rev Neurol. 2009;5:380–391. doi: 10.1038/nrneurol.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakhade SN, Zhou C, Aujla PK, Fishman R, Sucher NJ, Jensen FE. Early Alterations of AMPA Receptors Mediate Synaptic Potentiation Induced by Neonatal Seizures. J. Neurosci. 2008;28:7979–7990. doi: 10.1523/JNEUROSCI.1734-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raol YH, Lapides DA, Keating JG, Brooks-Kayal AR, Cooper EC. A KCNQ channel opener for experimental neonatal seizures and status epilepticus. Ann Neurol. 2009;65:326–336. doi: 10.1002/ana.21593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Represa A, Ben-Ari Y. Kindling is associated with the formation of novel mossy fibre synapses in the CA3 region. Exp Brain Res. 1992;92:69–78. doi: 10.1007/BF00230384. [DOI] [PubMed] [Google Scholar]
- Ribak CE, Baram TZ. Selective death of hippocampal CA3 pyramidal cells with mossy fiber afferents after CRH-induced status epilepticus in infant rats. Brain Res Dev Brain Res. 1996;91:245–251. doi: 10.1016/0165-3806(95)00183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronen GM, Buckley D, Penney S, Streiner DL. Long-term prognosis in children with neonatal seizures: a population-based study. Neurology. 2007;69:1816–1822. doi: 10.1212/01.wnl.0000279335.85797.2c. [DOI] [PubMed] [Google Scholar]
- Rotenberg A, Muller P, Birnbaum D, Harrington M, Riviello JJ, Pascual-Leone A, Jensen FE. Seizure suppression by EEG-guided repetitive transcranial magnetic stimulation in the rat. Clin Neurophysiol. 2008;119:2697–2702. doi: 10.1016/j.clinph.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez RM, Dai W, Levada RE, Lippman JJ, Jensen FE. AMPA/kainate receptor-mediated downregulation of GABAergic synaptic transmission by calcineurin after seizures in the developing rat brain. J.Neurosci. 2005a;25:3442–3451. doi: 10.1523/JNEUROSCI.0204-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez RM, Jensen FE, Pitanken A, Schwartzkroin PA, Moshe SL. Models of Seizures and Epilepsy. Elsevier Press; San Diego: 2005b. Modeling hypoxia-induced seizures and hypoxic encephalopathy in the neonatal period. [Google Scholar]
- Sanchez RM, Koh S, Rio C, Wang C, Lamperti ED, Sharma D, Corfas G, Jensen FE. Decreased glutamate receptor 2 expression and enhanced epileptogenesis in immature rat hippocampus after perinatal hypoxia-induced seizures. J.Neurosci. 2001;21:8154–8163. doi: 10.1523/JNEUROSCI.21-20-08154.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankar R, Painter MJ. Neonatal seizures: After all these years we still love what doesn't work. Neurology. 2005;64:776–777. doi: 10.1212/01.WNL.0000157320.78071.6D. [DOI] [PubMed] [Google Scholar]
- Schmid R, Tandon P, Stafstrom CE, Holmes GL. Effects of neonatal seizures on subsequent seizure-induced brain injury. Neurology. 1999;53:1754–1761. doi: 10.1212/wnl.53.8.1754. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Stowers CC, Scallet AC, Xu L. Fluoro-Jade C results in ultra high resolution and contrast labeling of degenerating neurons. Brain Res. 2005;1035:24–31. doi: 10.1016/j.brainres.2004.11.054. [DOI] [PubMed] [Google Scholar]
- Schreiber SS, Tocco G, Najm I, Finch CE, Johnson SA, Baudry M. Absence of c-fos induction in neonatal rat brain after seizures. Neurosci Lett. 1992;136:31–35. doi: 10.1016/0304-3940(92)90640-s. [DOI] [PubMed] [Google Scholar]
- Schwegler H, Crusio WE, Lipp HP, Heimrich B. Water-maze learning in the mouse correlates with variation in hippocampal morphology. Behav Genet. 1988;18:153–165. doi: 10.1007/BF01067837. [DOI] [PubMed] [Google Scholar]
- Shaw FZ. 7–12 Hz high-voltage rhythmic spike discharges in rats evaluated by antiepileptic drugs and flicker stimulation. J Neurophysiol. 2007;97:238–247. doi: 10.1152/jn.00340.2006. [DOI] [PubMed] [Google Scholar]
- Silverstein FS, Jensen FE. Neonatal seizures. Ann.Neurol. 2007;62:112–120. doi: 10.1002/ana.21167. [DOI] [PubMed] [Google Scholar]
- Smith KL, Lee CL, Swann JW. Local cicuit abnormalities in chronically epileptic rats after intrahippocampal tetanus toxin injection in infancy. J Neurophysiol. 1998;79:106–116. doi: 10.1152/jn.1998.79.1.106. [DOI] [PubMed] [Google Scholar]
- Sogawa Y, Monokoshi M, Silveira DC, Cha BH, Cilio MR, McCabe BK, Liu X, Hu Y, Holmes GL. Timing of cognitive deficits following neonatal seizures: relationship to histological changes in the hippocampus. Brain Res.Dev.Brain Res. 2001;131:73–83. doi: 10.1016/s0165-3806(01)00265-6. [DOI] [PubMed] [Google Scholar]
- Soria C, El Sabbagh S, Escolano S, Bobet R, Bulteau C, Dellatolas G. Quality of life in children with epilepsy and cognitive impairment: a review and a pilot study. Dev Neurorehabil. 2007;10:213–221. doi: 10.1080/13638490601111129. [DOI] [PubMed] [Google Scholar]
- Stafstrom CE, Tandon P, Hori A, Liu Z, Mikati MA, Holmes GL. Acute effects of MK801 on kainic acid-induced seizures in neonatal rats. Epilepsy Res. 1997;26:335–344. doi: 10.1016/s0920-1211(96)00904-7. [DOI] [PubMed] [Google Scholar]
- Sutula T, Cascino G, Cavazos J, Parada I, Ramirez L. Mossy fiber synaptic reorganization in the epileptic human temporal lobe. Ann Neurol. 1989;26:321–330. doi: 10.1002/ana.410260303. [DOI] [PubMed] [Google Scholar]
- Sutula T, He XX, Cavazos J, Scott G. Synaptic reorganization in the hippocampus induced by abnormal functional activity. Science. 1988;239:1147–1150. doi: 10.1126/science.2449733. [DOI] [PubMed] [Google Scholar]
- Talos DM, Fishman RE, Park H, Folkerth RD, Follett PL, Volpe JJ, Jensen FE. Developmental regulation of alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor subunit expression in forebrain and relationship to regional susceptibility to hypoxic/ischemic injury. I. Rodent cerebral white matter and cortex. J Comp Neurol. 2006;497:42–60. doi: 10.1002/cne.20972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauck DL, Nadler JV. Evidence of functional mossy fiber sprouting in hippocampal formation of kainic acid-treated rats. J Neurosci. 1985;5:1016–1022. doi: 10.1523/JNEUROSCI.05-04-01016.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekgul H, Gauvreau K, Soul J, Stewart J, Volpe JJ, Bourgeois B, du Plessis A. The current etiologic profile and neurodevelopmental outcome of seizures in term newborn infants. Pediatrics. 2006;117:1270–1280. doi: 10.1542/peds.2005-1178. [DOI] [PubMed] [Google Scholar]
- Toet MC, Groenendaal F, Osredkar D, van Huffelen AC, de Vries LS. Postneonatal epilepsy following amplitude-integrated EEG-detected neonatal seizures. Pediatr Neurol. 2005;32:241–247. doi: 10.1016/j.pediatrneurol.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. Neurology of the newborn. Saunders/Elsevier; Philadelphia: 2008. [Google Scholar]
- Wuarin JP, Dudek FE. Electrographic seizures and new recurrent excitatory circuits in the dentate gyrus of hippocampal slices from kainate-treated epileptic rats. J.Neurosci. 1996;16:4438–4448. doi: 10.1523/JNEUROSCI.16-14-04438.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.