Summary
Purpose
Neuroimaging studies suggest a history of febrile seizures, and depression, are associated with hippocampal volume reductions in patients with temporal lobe epilepsy (TLE).
Methods
We used radial atrophy mapping (RAM), a three-dimensional (3D) surface modeling tool, to measure hippocampal atrophy in 40 patients with unilateral TLE, with or without a history of febrile seizures and symptoms of depression. Multiple linear regression was used to single out the effects of covariates on local atrophy.
Key Findings
Subjects with a history of febrile seizures (n = 15) had atrophy in regions corresponding to the CA1 and CA3 subfields of the hippocampus contralateral to seizure focus (CHC) compared to those without a history of febrile seizures (n = 25). Subjects with Beck Depression Inventory II (BDI-II) score ≥14 (n = 11) had atrophy in the superoanterior portion of the CHC compared to subjects with BDI-II <14 (n = 29).
Significance
Contralateral hippocampal atrophy in TLE may be related to febrile seizures or depression.
Keywords: Radial mapping, Hippocampus, Febrile seizures, Depression, Temporal lobe epilepsy
Temporal lobe epilepsy (TLE) often is associated with mesial temporal sclerosis (MTS) on magnetic resonance imaging (MRI) (Cendes et al., 1993). MTS is characterized by neuron loss, gliosis, and hippocampal atrophy ipsilateral to seizure foci (Mathern et al., 1995). Because neuron loss and the extent of hippocampal atrophy vary among patients with TLE, comparing hippocampal volumes across diagnostic groups may reveal relationships among structural pathology, clinical features, and comorbidities.
MTS and decreased hippocampal volume are associated with mood disorders in some but not all studies of TLE (Quiske et al., 2000; Shamim et al., 2009; Wrench et al., 2009). Measuring whole hippocampal volumes may overlook local shape and volume differences with potential clinical relevance, since MTS may be associated with restricted neuronal loss leading to local deformations in surface morphology (Lin et al., 2005). Identifying these patterns may help detect patients most at risk for affective symptoms and elucidate the epilepsy-depression link.
Prolonged febrile seizures (FS) are associated with acute hippocampal injury (Van Landingham et al., 1998; Scott et al., 2002), increased risk for epilepsy (Verity & Golding, 1991), and MTS (Cendes et al., 1993; Van Paesschen et al., 1996; Kim et al., 1999; Theodore et al., 1999; Pittau et al., 2009). Histologic studies indicate loss of specific neuronal populations (Blüumcke et al., 1999; Crespel et al., 2002), and visualizing three-dimensional (3D) profiles of local hippocampal atrophy may elucidate pathology related to FS.
Radial atrophy mapping (RAM) computes distances from a structure's center to reconstruct neuroanatomic surfaces and visualize local changes in surface anatomy. These distances can be averaged and compared across groups or covaried with other clinical data. Hippocampal RAM on patients with TLE predicted postsurgical outcome, with seizure-free patients displaying greater presurgical hippocampal asymmetry than those not seizure free after temporal lobectomy (Lin et al., 2005). A recent RAM study found a difference in hippocampal surface anatomy between two electrographically distinct forms of epilepsy (Ogren et al., 2009).
We used RAM to investigate the effects of depressive symptoms, febrile seizure history, and epilepsy duration on hippocampal surface anatomy in 40 subjects with TLE.
Methods
Subject selection
Forty patients (16 female; mean age 34 years, range 16–56 years) who had been referred to the National Institute of Neurological Disorders and Stroke (NINDS) Clinical Epilepsy Section for uncontrollable seizures were included. All had unilateral TLE (23 left-sided) and complex partial seizures, with or without secondary generalization, as established by ictal video electroencephalography (EEG) monitoring. All subjects completed a Beck Depression Inventory II (BDI-II) and a structural MRI. A history of FS and age at onset of epilepsy (recurrent nonfebrile seizures) were established by patient and family (when available) interview, as well as medical record review. Because of the retrospective nature of the data, we were unable to collect reliable information on febrile seizure length and semiology. MTS diagnosis was made by a neuroradiologist on T2-weighted MR images. Patients were taking a wide variety of antiepileptic drugs (AEDs), but were not currently on antidepressant therapy, although past exposure was reported. The subjects had been included in a separate study measuring hippocampal volume in patients with depression (Shamim et al., 2009). The study was approved by the National Institutes of Health (NIH) Brain Internal Review Board.
Depressive symptoms
Subjects scoring ≥14 on the BDI-II were designated as presenting with symptoms of depression (Beck et al., 1996). The BDI cut-off we chose has a sensitivity of 90% and specificity of 99% for depression screening (Lasa et al., 2000).
MR imaging and processing
Thirty-five subjects underwent 1.5-Tesla (T) coronal 3D spoiled gradient recalled (SPGR) acquisition [matrix 256 × 256 with 0.9375 × 0.9375 × 1.5 mm resolution, echo time (TE): 3, repetition time (TR): 27, acquisition time (TA): 20, FOV 240 mm, number of excitations (NEX): 1] MRI scans (GE, Milwaukee, WI, U.S.A.); three subjects had axial MRI scans using the same imaging sequence and scanner. Two subjects underwent 3T sagittal 3D fast-SPGR acquisition (matrix 256 × 256 with 0.9375 × 0.9375 × 1.5 mm resolution, TE: 3, TR: 6, TA: 12, FOV 240 mm, NEX: 1) MRI scans (Phillips 5 Achieva 3T, The Netherlands). All images were loaded onto a Linux-based system and visualized using MEDx (Medical Numerics, Germantown, MD, U.S.A.). We applied a nonparametric nonuniform intensity normalization (N3) to correct for artifactual differences in intensity inhomogeneity (Sled et al., 1998). Corrected images were normalized by linear (12 parameter) transformation to a default MNI template using Statistical Parametric Mapping (SPM2) software (http://www.fil.ion. ucl.ac.uk/spm) and resliced into 1 mm × 1 mm × 1 mm voxels using the default SPM2 trilinear interpolation.
The hippocampus was traced in the coronal plane by a single-blinded rater (AF) using MultiTracer software (Laboratory of Neuroimaging, UCLA). Briefly, the first slice traced was the posterior hippocampal border where gray matter could be differentiated along the lateral ventricle medial border. The CA fields, dentate gyrus, and subiculum were included in all tracings; the protocol was modified to exclude the fimbria. The alveus served as the superior and anterior borders of the hippocampal head. Traces were verified in axial and sagittal views, and a standard neuroanatomic atlas consulted when necessary (Duvernoy, 2005). The intraclass correlation coefficient (ICC) was 0.84 for the hippocampus contralateral (CHC) and hippocampus ipsilateral (IHC) to the epileptic focus on six subjects randomly selected for retracing (Shrout & Fleiss, 1979). Subjects with right-lateralized seizure foci had hippocampal traces flipped, so that left-side was ipsilateral to the seizure focus in all subjects.
Radial atrophy mapping
Hippocampal traces were extracted in uniform contour file (UCF) format. 3D parametric surface mesh models were created from each contour, with surface points spatially uniform within and across slices for group averaging (Thompson et al., 2004). Each hippocampus central core was derived as a medial curve threading down the centroid. The hippocampus was split into dorsal and ventral halves along the medial curve. The radial distance (from central core to each surface point) was digitally recorded at each 3D boundary point on hippocampal mesh models. Subjects were divided into diagnostic groups, and radial size was averaged within each group. Averaged radial size at each point was compared across groups using analysis of variance (ANOVA). A color-coded representation of local atrophy [ratio (R)-map: showing percent atrophy in one group versus the other] and corresponding significance (p-value) maps were generated for each comparison. We also analyzed duration of epilepsy as a covariate that might influence radial size. We used multiple linear regression to identify effects of independent covariates, including FS history, BDI score, epilepsy duration, MTS, and side of seizure focus.
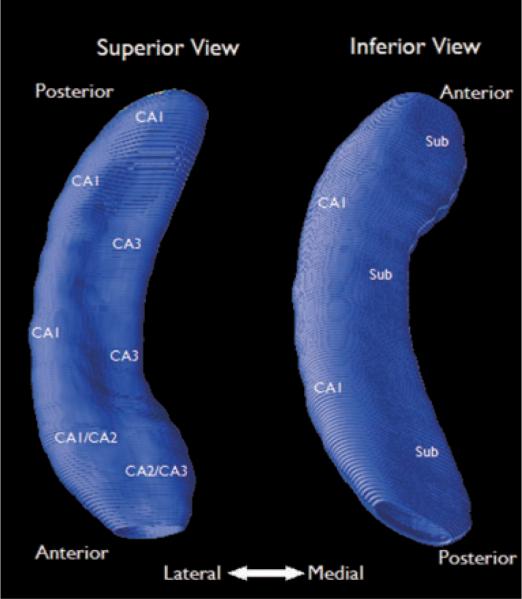
To correct for multiple comparisons and establish an overall p-value for each contour segment, we ran permutation tests simulating random subject assignments, yielding reference distributions used to determine chance probability of observing overall effect patterns (Thompson et al., 2003). Permutations can be used to assess significance of diffuse rather than focal atrophy, since all points across a 3D anatomic surface are tested (Thompson et al., 2004). We ran one million iterations at a threshold of p < 0.05 for the ipsilateral and contralateral dorsal and ventral segments, corresponding to inferior and superior segments of our template (Fig. 1). The resulting permutation test reports the likelihood of observing a greater proportion of the surface as significant (p = 0.05) by chance alone.
Figure 1.
Relative position of hippocampal subfields on the contour map. Left side (superior view) represents the dorsal segment; right side (inferior view) represents the ventral segment. The inferior view was made by flipping the superior view along the vertical axis. Subfield positions were adapted from Ogren et al. (2008) using a standard neuroanatomic atlas (Duvernoy, 2005) on a group-averaged contour map.
Statistical analysis
We used Student's t-test and Pearson's correlation coefficient to examine relationships between subject data and hippocampal volumes. Fisher's exact test was used to compare nominal data.
Results
Subject characteristics
Twenty-one subjects (53%) met criteria for MTS on MRI. Fifteen subjects (38%) reported a history of FS (FS+). Eleven (28%) had a score ≥14 on the BDI-II (range 16–43; mean 25 ± 9.8). Although, following previous studies (Lasa et al., 2000), we used the total BDI-II score in our classification (including both affective and somatic symptoms), all patients with scores ≥14 endorsed several affective symptoms; 8 of 11 had scores ≥14 based on affective symptoms alone.
Average epilepsy duration varied [mean ± standard deviation (SD) 20.0 ± 15.5, range 2–50 years]. Average IHC volume was 1,388.0 ± 536.4 mm3 (mean ± SD) (range 421.9–2,281.5 mm3), and average CHC volume was 1,776.0 ± 410.5 mm3 (mean ± SD) (range 730.3–2,735.9 mm3) on normalized MRIs. As might be expected, the CHC was significantly larger than the IHC (t = 3.63, p < 0.001).
Subject age was negatively correlated with IHC (r = −0.47, t = 3.27, p < 0.01) but not CHC volume (r = 0.08, t = 0.46). Epilepsy duration was also negatively correlated with IHC (r = −0.49, t = 3.46, p < 0.01) but not CHC volume (r = 0.0, t = 0.0). There were no statistically significant correlations between BDI scores and hippocampal volume (ipsilateral, r = −0.19, t = 1.19; contralateral, r= −0.15, t = 0.95).
Febrile seizures
FS+ patients (n = 15) were significantly older, had longer epilepsy duration, smaller IHC volume, and more frequently had MTS than those without FS history (FS−) (Table 1).
Table 1.
Characteristics of subjects with a history of febrile and subjects with no history of febrile seizures
| Febrile (n = 15) | Afebrile (n = 25) | Sig. | |
|---|---|---|---|
| Age | 30.2 ± 10.7 | 30.2 ± 10.7 | ** |
| Epilepsy duration | 29.7 ± 14.6 | 14.4 ± 13.2 | ** |
| Female | 7 (47%) | 9 (36%) | NS |
| BDI-II | 12.7 ± 8.4 | 10.7 ± 12.0 | NS |
| Depressed | 5 (33%) | 6 (24%) | NS |
| Mesial temporal sclerosis | 13 (87%) | 8 (32%) | ** |
| Left seizure focus | 8 (53%) | 15 (60%) | NS |
| IHC volume | 1,068.7 ± 337.2 | 1,579.6 ± 546.9 | ** |
| CHC volume | 1,641.9 ± 336.9 | 1,856.4 ± 435.5 | p = 0.11 |
Data presented as mean ± SD or total (%).
NS, not significant;
p < 0.01.
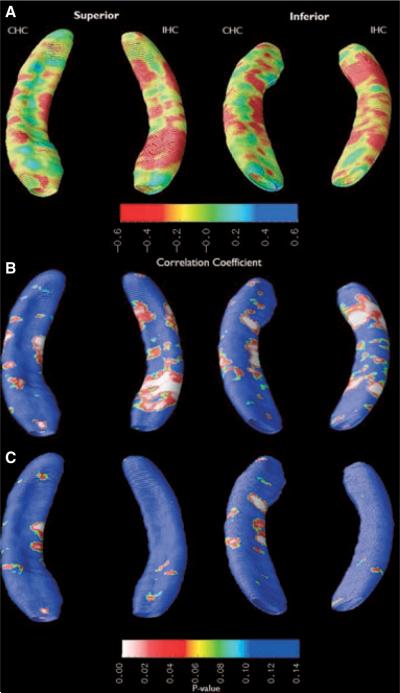
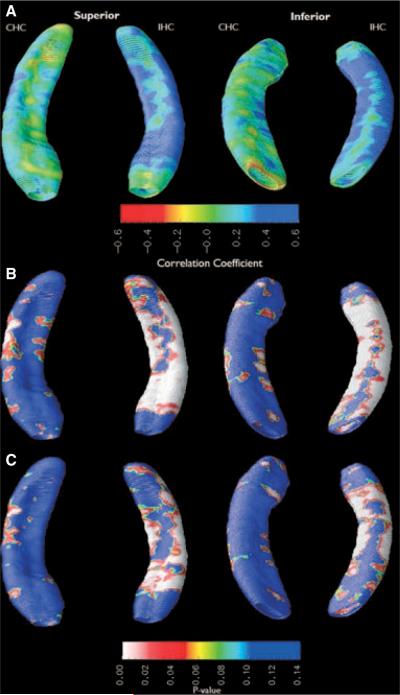
A qualitative assessment of the atrophy maps shows that FS+ patients had increased hippocampal atrophy in areas corresponding to CA3 and the CA1-subiculum border in the CHC compared to FS− (Fig. 2). IHC atrophy was more diffuse, with a large deformation across superior and inferior portions of hippocampal head and anterior hippocampal body as well as along inferior portions of hippocampal tail. After the effects of epilepsy duration, gender, seizure focus hemisphere, and BDI score were regressed out as covariates (Fig. 2C), statistical significance of atrophy in CHC changed minimally, whereas the statistical significance of IHC atrophy decreased.
Figure 2.
Subjects with a history of FS have increased atrophy in the CHC. Hippocampal segments were compared between febrile and nonfebrile subjects. (A) Ratio-map showing relative atrophy before multiple regression. (B) Probability (p) map showing statistical significance of atrophy before multiple regression. (C) p-map showing statistical significance of atrophy after the effects of BDI score, duration of epilepsy, side of seizure focus, and MTS were regressed out as covariates.
After multiple regression, permutation tests for FS+ versus FS− subject volume differences failed to reach statistical significance in dorsal CHC (p = 0.50), ventral CHC (p = 0.12), dorsal IHC (p = 0.92), and ventral IHC (p = 0.86).
Depression
Subjects classified as depressed by the BDI-II (n = 11) were not significantly different by any reported measure and did not have an increased incidence of FS compared to nondepressed subjects (Table 2).
Table 2.
Characteristics of subjects classified as depressed and subjects classified as not depressed
| BDI > 14 (n = 11) | BDI < 13 (n = 29) | Sig. | |
|---|---|---|---|
| Age | 35.4 ± 12.4 | 33.8 ± 12.3 | NS |
| Epilepsy duration | 23.2 ± 17.2 | 18.8 ± 15.0 | NS |
| Female | 5 (45%) | 11 (38%) | NS |
| Febrile seizure history | 5 (45%) | 10(34%) | NS |
| Mesial temporal sclerosis | 8 (72%) | 13 (45%) | p = 0.11 |
| Left seizure focus | 7 (64%) | 16(55%) | NS |
| IHC volume | 1,208.5 ± 539.85 | 1,456.12 ± 508.33 | NS |
| CHC volume | 1,610.75 ± 472.54 | 1,838.63 ± 370.22 | NS |
Data presented as mean ± SD or total (%).
NS, not significant;
p < 0.01.
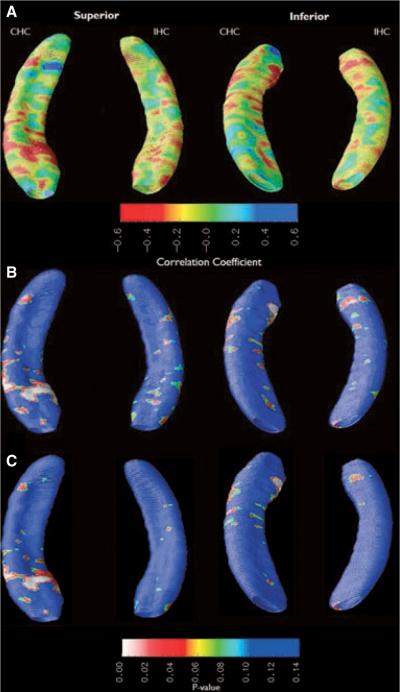
A qualitative assessment of the atrophy maps shows that depressed subjects had increased hippocampal atrophy in the CHC and IHC compared to nondepressed subjects (Fig. 3). The most significant hippocampal atrophy was confined to the superoanterior portion of the CHC, in the area corresponding to the superior aspect of the hippocampal head, running along the entire mediolateral extent of the hippocampus, and possibly representing a deformation in all CA fields. There is also atrophy in the anterolateral aspect of the CHC, along an area corresponding to CA1-subiculum border. Although the R-map shows a diffuse decrease in IHC radial distance, few regions reached statistical significance. There was no change in statistical significance after epilepsy duration, gender, seizure focus hemisphere, and FS history were regressed out as covariates.
Figure 3.
Subjects with symptoms suggestive of depression (BDI >14) have a large surface deformation in the superior head of the CHC when compared to nondepressed subjects. (A) Ratiomap showing relative atrophy before multiple regression. (B) Probability (p) map showing statistical significance of atrophy before multiple regression. (C) p-map showing statistical significance of atrophy after the effects of febrile seizure history, duration of epilepsy, side of seizure focus, and MTS were regressed out as covariates.
After multiple regression, permutation tests for depressed versus nondepressed subjects showed a trend toward significance in the dorsal CHC segment (p = 0.054). The ventral CHC (p = 0.26), dorsal IHC (p = 0.94), and ventral IHC (p = 0.82) segments were not statistically significant.
Epilepsy duration
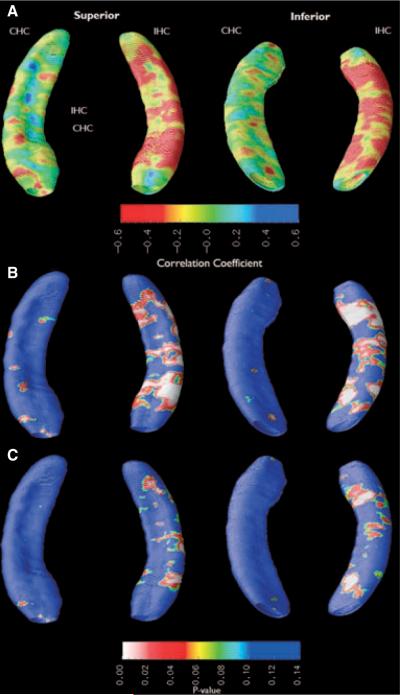
We used epilepsy duration as a covariate of local hippocampal atrophy to assess a linear relationship between the two variables. A qualitative assessment of the atrophy maps shows that epilepsy duration was negatively correlated with hippocampal radial distance across the entire IHC extent, with little deformation of the CHC (Fig. 4). The negative correlation was statistically significant along the inferior aspect of the IHC corresponding to subiculum, as well as the lateral aspect of the IHC corresponding to CA1. Only FS history and BDI-II ≥14 diagnosis were regressed out as covariates. The extent of statistically significant atrophy in IHC decreased after multiple regression.
Figure 4.
Duration of epilepsy is negatively correlated with radial distance along the entire extent of the IHC before but not after multiple regression. (A) Ratio-map showing relative atrophy before multiple regression. (B) Probability (p) map showing statistical significance of atrophy before multiple regression. (C) p-map showing statistical significance of atrophy after the effects of febrile seizure history and BDI score were regressed out as covariates.
Before multiple regression, p-values were significant in both the dorsal (p = 0.004) and ventral (p = 0.004) segments of the IHC, but neither segment of the CHC (dorsal: p = 0.55; ventral: p = 0.83). After multiple regression, p-values were no longer significant for the ventral IHC (p = 0.88) or dorsal IHC (p = 0.50), as well as the ventral CHC (p = 0.74) and dorsal CHC (p = 0.57).
Mesial temporal sclerosis
Subjects with MTS identified on MRI had significantly decreased radial distance across the entire extent of the IHC before and after multiple regression (Fig. 5). The extent of statistically significant atrophy across the CHC appeared to be reduced after multiple regression. After multiple regression, p-values were significant in the dorsal IHC (p = 0.0003) and ventral IHC (p = 0.0003), but not the dorsal CHC (p = 0.13) and ventral CHC (p = 0.25).
Figure 5.
Subjects with MTS have significantly smaller radial distances across the entire IHC but not CHC. (A) Ratio-map showing relative atrophy before multiple regression. (B) Probability (p) map showing statistical significance of atrophy before multiple regression. (C) p-map showing statistical significance of atrophy after the effects of febrile seizure history, BDI score, side of seizure focus, and epilepsy duration were regressed out as covariates.
Discussion
Using RAM, a relatively new approach to hippocampal structural analysis, we found that patients with a history of FS, and BDI-II ≥14, had evidence for atrophy contralateral as well as ipsilateral to the seizure focus, suggesting that both FS and depressive symptoms have widespread effects.
Although RAM is a reliable method for detecting differences in surface morphology across groups, interindividual variation in substructures complicates histologic interpretation of these differences. Surface deformations may reflect inner subfield as well as hippocampal surface atrophy. Moreover, use of different tracing protocols may affect the surface position of hippocampal substructures across reported studies using RAM. Our study's amended tracing procedure produced volumes about half as large as reported in healthy volunteers (Pruessner et al., 2000); part of this difference can be explained by exclusion of the fimbria (Hsu et al., 2002) and possible bilateral hippocampal atrophy associated with TLE. Our results are comparable to those reported in some previous RAM studies in TLE (Lin et al., 2005), but lower than in others (Ogren et al., 2009). The absolute volume measurements would not affect detection of bilateral effects of FS or depressive symptoms.
After multiple linear regression to correct for the effects of epilepsy duration, MTS, depressive symptoms, and side of seizure focus on hippocampal radial distance, FS+ subjects had statistically significant decreases in radial distance in the CHC but not IHC. IHC volume loss in FS+ relative to FS− subjects has been reported (Bernasconi et al., 2005), although this relationship was not significant in another study (Bower et al., 2000). A history of FS may not affect IHC surface morphology in TLE.
The pattern of CHC surface deformation in FS+ subjects in our study is indicative of MTS, with atrophy in areas corresponding to CA1 and CA3 and relative sparing of the area between the two subfields. Previous studies have documented the relation of FS to MTS (Cendes et al., 1993; Van Landingham et al., 1998; Theodore et al., 1999). Because only a minority of our patients with MTS had no history of FS, we used multiple regression to isolate factors contributing to CHC atrophy. Qualitatively, the extent of statistically significant decreases in radial distance along the CHC was not appreciably reduced by multiple linear regression.
Voxel-based morphometry (VBM) studies in TLE show gray matter volume reductions in brain regions contralateral to seizure focus, although usually not in the CHC (Keller & Roberts, 2008). A 4T MRI study of subfield volumes in patients with TLE found no difference in CHC subfield volumes between subjects with and without MTS (Mueller et al., 2009). However, whether volumetric studies generalize to surface mapping is unclear, since each measures a different property of the hippocampus.
A trend toward CHC in FS+ patients with TLE has been reported (Theodore et al., 1999), although the study did not control for MTS; it was suggested that an early global insult caused by FS may predispose FS+ patients to CHC atrophy (Theodore et al., 1999). There was also evidence of endfolium sclerosis identified by postmortem histology in CHC of patients with unilateral TLE, suggesting that unilateral TLE may be associated with bilateral hippocampal changes not identifiable using MRI or volumetry (Margeris & Corsellis, 1966; King et al., 1995).
We found focal atrophy in the CHC in subjects with BDI ≥14. A large surface deformation across the superoanterior aspect of the CHC is in the same anatomic position as atrophy identified in a previous RAM study comparing elderly depressed subjects with controls (Ballmaier et al., 2008). This study suggested that increased hypothalamic input to CA3 and CA2 may make these neurons more susceptible to damage from corticosteroids (Herman et al., 1989; Ballmaier et al., 2008). The idea is further supported because the internal digitations of the hippocampal head expose these hippocampal subfields, so that this area may provide a more sensitive measure of neuron loss. In our study, volume loss associated with TLE may have masked this deformation in the IHC. Previous studies in patients with TLE indicate that left hippocampal volume reduction is associated with depression (Baxendale et al., 2005; Shamim et al., 2009). Our study is complicated by comparison of hippocampi based on side of seizure focus. We did not find a difference in seizure focus lateralization between subjects with symptoms suggestive of depression and those with fewer symptoms. Our data suggest a bilateral effect of depression on hippocampal surface anatomy in TLE, but comparing left and right hippocampi explicitly may reveal unilateral effects of depression.
We also report that decreased IHC radial distance is associated with increased duration of epilepsy and MTS. Our finding is consistent with cross-sectional imaging studies in patients with MTS and long epilepsy duration that show progressive hippocampal atrophy and hypometabolism in the seizure focus as well as areas outside the focus (Tasch et al., 1999; Theodore et al., 1999). Ipsilateral hippocampal volume loss over 3–4 years in patients with TLE was associated with generalized tonic-clonic (Briellmann et al., 2002) or partial (Fuerst et al., 2003) seizure frequency. Thirteen percent of patients with newly diagnosed epilepsy developed ipsilateral hippocampal volume decrease over 2–3 years, associated with longer epilepsy duration and higher seizure number before treatment (Salmenperä et al., 2005). A longitudinal study of outpatients with a variety of epilepsy syndromes over 3.5 years found significant atrophy of hippocampus, neocortex, or cerebellum in 17% of patients and 6.7% of controls; there was no effect of seizure frequency or epilepsy duration (Liu et al., 2005). A cross-sectional study using VBM found no evidence for CHC atrophy, or an association of IHC atrophy with FS, epilepsy duration, or onset age (Keller et al., 2002).
Our study showed that CHC surface morphology is not significantly different between TLE patients with and without MTS. Although a negative correlation between CHC volume and epilepsy duration has been reported (Jokeit et al., 1999), we failed to find an association between duration of epilepsy and CHC volume or surface morphology. VBM and volumetric studies have not found a definitive association between MTS and CHC volume (Keller & Roberts, 2008; Mueller et al., 2009).
Our study had several limitations. We were unable to determine whether FS were simple, complex, or prolonged, which limited our ability to predict a causal relationship between FS and CHC atrophy. The relationship between FS and MTS needs additional investigation. Fourteen children who had large hippocampal volumes and prolonged T2 relaxation time after prolonged FS showed return toward normal values 4–8 months later, but hippocampal volume asymmetry increased significantly, consistent either with resolution of acute edema but development of MTS, or reappearance of a preexisting hippocampal abnormality (Scott et al., 2003). A recent study in eight healthy adults suggested an association between a history of simple FS and reduced hippocampal volume compared to controls (Auer et al., 2008).
Although the BDI-II is a reliable indicator of depressed mood, we were not able to obtain a more detailed assessment of depression for all of our subjects. The BDI-II allowed us to characterize subjects' current depressive state and examine a linear relationship between depressive symptoms and hippocampal volume and shape. However, the test may have missed past depressive symptoms or a history of depression that could contribute hippocampal atrophy. Assessing current and past depression using a Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) would improve our classification of depressed and nondepressed subjects and may strengthen our results.
Quantitative statistical significance was lost after a permutation procedure. Previous studies of the effects of depression and febrile seizures on hippocampus in TLE have not used this rigorous approach. Permutation methods are widely used in statistical brain mapping (Nichols & Holmes, 2002) and have been used extensively to assess the overall significance of surface-based maps of statistics (Thompson et al., 2004). Advantages include the fact that they are nonparametric, and do not make assumptions that the residuals of the statistical model are Gaussian—the model is built from the null distribution of the empirical data. Possible explanations for loss of significance in our study include relatively small sample size (a problem common in imaging studies), as well as interactions among the variables we wished to test. Like FS, depression is a risk factor for epilepsy, and can contribute to bilateral hippocampal atrophy, as well as the neurotransmitter and metabolic alterations found in patients with TLE (Hesdorffer et al., 2006; Gilliam et al., 2007; Hasler et al., 2007). Possible links between febrile seizures and depression have not been examined.
In conclusion, our RAM study shows that FS, and depression, are associated with specific patterns of bilateral hippocampal atrophy in patients with TLE.
Acknowledgments
Supported by the NINDS Division of Intramural Research.
Footnotes
Disclosure We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. None of the authors has anything to disclose
References
- Auer T, Barsi P, Bone B, Angyalosi A, Aradi M, Szalay C, Horvath RA, Kovacs N, Kotek G, Fogarasi A, Komoly S, Janszky I, Schwarcz A, Janszky J. History of simple febrile seizures is associated with hippocampal abnormalities in adults. Epilepsia. 2008;49:1562–1569. doi: 10.1111/j.1528-1167.2008.01679.x. [DOI] [PubMed] [Google Scholar]
- Barr WB, Ashtari M, Schaul N. Bilateral reductions in hippocampal volume in adults with epilepsy and a history of febrile seizures. J Neurol Neurosurg Psychiatry. 1997;63:461–467. doi: 10.1136/jnnp.63.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxendale SA, Thompson PJ, Duncan JS. Epilepsy & depression: the effects of comorbidity on hippocampal volume – a pilot study. Seizure. 2005;14:435–438. doi: 10.1016/j.seizure.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Beck AT, Brown G, Steer RA. Beck Depression Inventory II manual. The Psychological Corporation; 1996. [Google Scholar]
- Bernasconi N, Natsume J, Bernasconi A. Progression in temporal lobe epilepsy - Differential atrophy in mesial temporal structures. Neurology. 2005;65:223–228. doi: 10.1212/01.wnl.0000169066.46912.fa. [DOI] [PubMed] [Google Scholar]
- Blüumcke I, Beck H, Suter B, Hoffmann D, FodischFödisch HJ, Wolf HK, Schramm J, Elger CE, Wiestler OD. An increase of hippocampal calretinin-immunoreactive neurons correlates with early febrile seizures in temporal lobe epilepsy. Acta Neuropathol. 1999;97:31–39. doi: 10.1007/s004010050952. [DOI] [PubMed] [Google Scholar]
- Bower SPC, Kilpatrick CJ, Vogrin SJ, Morris K, Cook MJ. Degree of hippocampal atrophy is not related to a history of febrile seizures in patients with proved hippocampal sclerosis. J Neurol Neurosurg Psychiatry. 2000;69:733–738. doi: 10.1136/jnnp.69.6.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyes RG, Gunter JL, Frost C, Janke AL, Yeatman T, Hill DL, Bernstein MA, Thompson PM, Weiner MW, Schuff N, Alexander GE, Killiany RJ, DeCarli C, Jack CR, Fox NC; ADNI Study Intensity non-uniformity correction using N3 on 3-T scanners with mutichannel phased array coils. Neuroimage. 2008;39:1752–1762. doi: 10.1016/j.neuroimage.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briellmann RS, Berkovic SF, Syngeniotis A, King MA, Jackson GD. Seizure-associated hippocampal volume loss: a longitudinal magnetic resonance study of temporal lobe epilepsy. Ann Neurol. 2002;51:641–644. doi: 10.1002/ana.10171. [DOI] [PubMed] [Google Scholar]
- Cendes F, Andermann F, Dubeau F, Gloor P, Evans A, Jones-Gotman M, Olivier A, Andermann E, Robitaille Y, Lopes-Cendes I, Peters T, Melanson D. Early-onset childhood prolonged febrile convulsions, atrophy and sclerosis of mesial structures and temporal lobe epilepsy: an MRI volumetric study. Neurology. 1993;43:1083–1087. doi: 10.1212/wnl.43.6.1083. [DOI] [PubMed] [Google Scholar]
- Crespel A, Coubes P, Rousset MC, Brana C, Rougier A, Rondouin G, Bockaert J, Baldy-Moulinier M, Lerner-Natoli M. Inflammatory reactions in human medial temporal lobe epilepsy with hippocampal sclerosis. Brain Res. 2002;952:159–169. doi: 10.1016/s0006-8993(02)03050-0. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM. The Human Hippocampus, Functional Anatomy, Vascularization and Serial Sections with MRI. 3rd edn. Springer Verlag; New York: 2005. [Google Scholar]
- Fuerst D, Shah J, Shah A, Watson C. Hippocampal sclerosis is a progressive disorder: a longitudinal volumetric MRI study. Ann Neurol. 2003;53:413–416. doi: 10.1002/ana.10509. [DOI] [PubMed] [Google Scholar]
- Gilliam FG, Maton BM, Martin RC, Sawrie SM, Faught RE, Hugg JW, Viikinsalo M, Kuzniecky RI. Hippocampal 1H-MRSI correlates with severity of depression symptoms in temporal lobe epilepsy. Neurology. 2007;68:364–368. doi: 10.1212/01.wnl.0000252813.86812.81. [DOI] [PubMed] [Google Scholar]
- Hasler G, Bonwetsch R, Giovacchini G, Toczek MT, Bagic A, Luckenbaugh DA, Drevets WC, Theodore WH. 5-HT1A receptor binding in temporal lobe epilepsy patients with and without major depression. Biol Psychiatry. 2007;62:1258–1264. doi: 10.1016/j.biopsych.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Schafer MKH, Young EA, Thompson R, Douglass J, Akil H, Watson SJ. Evidence for hippocampal regulation of neuroendocrine neurons of the hypothalamo-pituitary-adrenocortical area. J Neurosci. 1989;9:3072–3082. doi: 10.1523/JNEUROSCI.09-09-03072.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesdorffer DC, Hauser WA, Olafsson E, Ludvigsson P, Kjartansson O. Depression and suicide attempt as risk factors for incident unprovoked seizures. Ann Neurol. 2006;59:35–41. doi: 10.1002/ana.20685. [DOI] [PubMed] [Google Scholar]
- Hsu Y-Y, Schuff N, An-Tao D, Mark K, Zhu X, Hardin D, Weiner MW. Comparison of Automated and Manual MRI Volumetry of Hippocampus in Normal Aging and Dementia. J Magn Reson Imaging. 2002;16:305–310. doi: 10.1002/jmri.10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokeit H, Ebner A, Arnold S, Schüller M, Antke C, Huang Y, Steinmetz H, Seitz RJ, Witte OW. Bilateral reduction of hippocampal volume, glucose metabolism, and WADA hemispheric memory performance are related to the duration of mesial temporal lobe epilepsy. J Neurol. 1999;246:926–933. doi: 10.1007/s004150050484. [DOI] [PubMed] [Google Scholar]
- Keller SS, Roberts N. Voxel-based morphometry of temporal lobe epilepsy: an introduction and review of the literature. Epilepsia. 2008;49:741–757. doi: 10.1111/j.1528-1167.2007.01485.x. [DOI] [PubMed] [Google Scholar]
- Keller SS, Wieshmann UC, Mackay CE, Denby CE, Webb J, Roberts N. Voxel based morphometry of grey matter abnormalities in patients with medically intractable temporal lobe epilepsy: effects of side of seizure onset and epilepsy duration. J Neurol Neurosurg Psychiatry. 2002;73:648–655. doi: 10.1136/jnnp.73.6.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WJ, Park SC, Lee SJ, Lee JH, Kim JY, Lee BI, Kim DI. The prognosis for control of seizures with medications in patients with MRI evidence for mesial temporal sclerosis. Epilepsia. 1999;40:290–293. doi: 10.1111/j.1528-1157.1999.tb00706.x. [DOI] [PubMed] [Google Scholar]
- King D, Spencer SS, McCarthy G, Luby M, Spencer DD. Bilateral Hippocampal Atrophy in Medial Temporal Lobe Epilepsy. Epilepsia. 1995;36:905–910. doi: 10.1111/j.1528-1157.1995.tb01634.x. [DOI] [PubMed] [Google Scholar]
- Lasa L, Ayuso-Mateos JL, Va'zquez-Barquero JL, D'lez-Manrique FJ, Dowrick CF. The use of the Beck Depression Inventory to screen for depression in the general population: a preliminary analysis. J Affect Disord. 2000;57:261–265. doi: 10.1016/s0165-0327(99)00088-9. [DOI] [PubMed] [Google Scholar]
- Lin JJ, Salamon N, Dutton RA, Lee AD, Geaga JA, Hayashi KM, Toga AW, Engel J, Jr, Thompson PM. Three-dimensional preoperative maps of hippocampal atrophy predict surgical outcomes in temporal lobe epilepsy. Neurology. 2005;65:1094–1097. doi: 10.1212/01.wnl.0000179003.95838.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RS, Lemieux L, Bell GS, Sisodiya SM, Bartlett PA, Shorvon SD, Sander JW, Duncan JS. Cerebral damage in epilepsy: a population-based longitudinal quantitative MRI study. Epilepsia. 2005;46:1482–1494. doi: 10.1111/j.1528-1167.2005.51603.x. [DOI] [PubMed] [Google Scholar]
- Margeris JH, Corsellis JA. Epilepsy and temporal lobes: a clinical electroencephalographic and neuropathological study of brain in epilepsy with particular reference to temporal lobes. Brain. 1966;89:499–537. doi: 10.1093/brain/89.3.499. [DOI] [PubMed] [Google Scholar]
- Mathern GW, Babb TL, Vickrey BG, Melendez M, Pretorius JK. The clinical-pathogenic mechanisms of hippocampal neuron loss and surgical outcomes in temporal lobe epilepsy. Brain. 1995;118:105–118. doi: 10.1093/brain/118.1.105. [DOI] [PubMed] [Google Scholar]
- Mueller SG, Laxer KD, Barakos J, Cheong I, Garcia P, Weiner MW. Subfield atrophy pattern in temporal lobe epilepsy with and without mesial sclerosis detected by high-resolution MRI at 4-Tesla: preliminary results. Epilepsia. 2009;50:1474–1483. doi: 10.1111/j.1528-1167.2009.02010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogren JA, Bragin A, Wilson CL, Hoftman GD, Lin JJ, Dutton RA, Fields TA, Toga AW, Thompson PM, Engel J, Jr, Staba RJ. Three-dimensional hippocampal atrophy maps distinguish two common temporal lobe seizure-onset patterns. Epilepsia. 2009;50:1361–1370. doi: 10.1111/j.1528-1167.2008.01881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittau F, Bisulli F, Mai R, Fares JE, Vignatelli L, Labate A, Naldi I, Avoni P, Parmeggiani A, Santucci M, Capannelli D, Di Vito L, Gambardella A, Baruzzi A, Tinuper P. Prognostic factors in patients with mesial temporal lobe epilepsy. Epilepsia. 2009;50:41–44. doi: 10.1111/j.1528-1167.2008.01969.x. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Li LM, Serles W, Pruessner M, Collins DL, Kabani N, Lupien S, Evans AC. Volumetry of hippocampus and amygdala with high-resolution MRI and three-dimensional analysis software: minimizing the discrepancies between laboratories. Cereb Cortex. 2000;10:433–442. doi: 10.1093/cercor/10.4.433. [DOI] [PubMed] [Google Scholar]
- Quiske A, Helmstaedter C, Lux S, Elger CE. Depression in patients with temporal lobe epilepsy is related to mesial temporal sclerosis. Epilepsy Res. 2000;39:121–125. doi: 10.1016/s0920-1211(99)00117-5. [DOI] [PubMed] [Google Scholar]
- Salmenperä T, Könönen M, Roberts N, Vanninen R, Pitkänen A, Kälviäinen R. Hippocampal damage in newly diagnosed focal epilepsy: a prospective MRI study. Neurology. 2005;64:62–68. doi: 10.1212/01.WNL.0000148643.36513.2A. [DOI] [PubMed] [Google Scholar]
- Scott RC, Gadian DG, King MD, Chong WK, Cox TC, Neville BG, Connelly A. Magnetic resonance imaging findings within 5 days of status epilepticus in childhood. Brain. 2002;125:1951–1959. doi: 10.1093/brain/awf202. [DOI] [PubMed] [Google Scholar]
- Scott RC, King MD, Gadian DG, Neville BG, Connelly A. Hippocampal abnormalities after prolonged febrile convulsion: a longitudinal MRI study. Brain. 2003;126:2551–2557. doi: 10.1093/brain/awg262. [DOI] [PubMed] [Google Scholar]
- Shamim S, Hasler G, Liew C, Sato S, Theodore WH. Temporal lobe epilepsy, depression, and hippocampal volume. Epilepsia. 2009;50:1067–1071. doi: 10.1111/j.1528-1167.2008.01883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrout P, Fleiss JL. Intraclass correlation: uses in assessing rater reliability. Psychol Bull. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A non-parametric method for automatic correction of intensity non-uniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Tasch E, Cendes F, Li LM, Dubeau F, Andermann F, Arnold DL. Neuroimaging evidence of progressive neuronal loss and dysfunction in temporal lobe epilepsy. Ann Neurol. 1999;45:568–576. doi: 10.1002/1531-8249(199905)45:5<568::aid-ana4>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Theodore WH, Bhatia S, Hatta J, Fazilat S, DeCarli C, Bookheimer SY, Gaillard WD. Hippocampal Atrophy, Epilepsy Duration, and Febrile Seizures in Patients with Partial Seizures. Neurology. 1999;52:132–136. doi: 10.1212/wnl.52.1.132. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, de Zubicaray G, Janke AL, Rose SE, Semple J, Herman D, Hong MS, Dittmer SS, Doddrell DM, Toga AW. Dynamics of gray matter loss in Alzheimer's disease. J Neurosci. 2003;23:994–1005. doi: 10.1523/JNEUROSCI.23-03-00994.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, De Zubicaray GI, Janke AL, Rose SE, Semple J, Hong MS, Herman DH, Gravano D, Doddrell DM, Toga AW. Mapping hippocampal and ventricular change in Alzheimer disease. Neuroimage. 2004;22:1754–1766. doi: 10.1016/j.neuroimage.2004.03.040. [DOI] [PubMed] [Google Scholar]
- Van Landingham KE, Heinz ER, Cavazos JE, Lewis DV. Magnetic resonance imaging evidence of hippocampal injury after prolonged focal febrile convulsions. Ann Neurol. 1998;43:413–426. doi: 10.1002/ana.410430403. [DOI] [PubMed] [Google Scholar]
- Van Paesschen W, Connelly A, Johnson CL, Duncan JS. The amygdala and intractable temporal lobe epilepsy: a quantitative magnetic resonance imaging study. Neurology. 1996;47:1021–1031. doi: 10.1212/wnl.47.4.1021. [DOI] [PubMed] [Google Scholar]
- Verity CM, Golding J. Risk of epilepsy after febrile convulsions: a national cohort study. Br Med J. 1991;303:1373–1376. doi: 10.1136/bmj.303.6814.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrench JM, Wilson SJ, Bladin PF, Reutens DC. Hippocampal volume and depression: insights from epilepsy surgery. J Neurol Neurosurg Psychiatr. 2009;80:539–544. doi: 10.1136/jnnp.2008.152165. [DOI] [PubMed] [Google Scholar]