Summary
Background
Two recent case-control studies in Italy reported that long-term exposure to particulate air pollution or living near major traffic roads was associated with an increased risk of deep vein thrombosis (DVT). No prospective evidence exists about long-term traffic-related air pollution and incident venous thromboembolism (VTE).
Objectives
To examine the association between long-term traffic exposure and incident VTE in a population-based prospective cohort study.
Methods
We studied 13,143 middle-aged men and women in the Atherosclerosis Risk in Communities Study without history of DVT or pulmonary embolus (PE) at baseline examination (1987-1989). Geographical Information System (GIS)-mapped traffic density and distance to major roads in the four study communities served as measures of traffic exposure. We examined the association between traffic exposure and incident VTE using proportional hazards regression models.
Results
405 subjects developed VTE through 2005. Traffic density was not significantly associated with VTE. Relative to those in the lowest quartile of traffic density, the adjusted hazard ratios across increasing quartiles were 1.18 (95%CI 0.88-1.57), 0.99 (95%CI 0.74-1.34) and 1.14 (95%CI 0.86-1.51) (p for trend across quartiles = 0.64). For residents living within 150 meters of major roads compared to subjects living further away, the adjusted hazard ratio was 1.16 (95%CI 0.95-1.42, p=0.14).
Conclusions
This first prospective study in the general population does not support an association between air pollution exposure or traffic proximity and risk of DVT. More data may be needed to clarify whether traffic or air pollution influences the risk of VTE.
Keywords: traffic exposure, VTE, air pollution, cohort
Introduction
Numerous studies have documented associations of long-term and short-term exposure to outdoor air pollution with cardiovascular mortality, morbidity, and subclinical atherosclerosis markers [1, 2]. Hypercoagulability and enhanced thrombosis have been suggested as mechanisms by which air pollution may impact cardiovascular health [1-3]. Hypercoagulability is a hallmark of venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolus (PE) [4]; however, little attention has been paid to the association of air pollution with VTE. Two recent case-control studies in Italy reported that long-term exposure to particulate air pollution [5] or living near major roads [6] was associated with increased risk of DVT. In support of these findings, a time series study found daily air pollution levels to be positively related to the timing of hospitalization for DVT [7]. However, a recent prospective study of women found no association between background exposure to particulate matter (PM) and VTE [8]. Most health conditions related to air pollution are also related to smoking [1], but there is not a consistent relationship between smoking and risk of VTE [9]. To our knowledge, no prospective evidence exists about the relationship between long-term traffic-related air pollution exposure and incident VTE.
Road traffic is a major contributor to outdoor air pollution in industrialized countries. Assessment of traffic exposure can enhance studies of health effects of outdoor air pollution, because local sources are important and because few people live close to air monitoring stations, which are not implemented to capture the component of air pollutions generated by traffic sources. Furthermore, cohorts established to investigate other risk factors may not provide sufficient geographic, and therefore air pollution, variability to permit effective investigations of air pollution exposure in relation to health outcomes. For example, Hoek et al. (2002) found a consistent positive association of cardiopulmonary mortality with traffic exposure, but not with estimated ambient background concentration of the traffic indicator pollutants black smoke and NO2 [10]. Similarly, in the Atherosclerosis Risk in Communities (ARIC) study, which is based in only four communities, greater exposure to traffic-related air pollution, but not the available measure of background air pollution, was associated with reduced lung function [11] and increased risk of coronary heart disease [12].
In this analysis, we examined the association between long-term traffic exposure and incidence of VTE in the ARIC Study.
Material and Methods
Participants
We studied participants in the Atherosclerosis Risk in Communities (ARIC) study, which was designed to investigate the natural history and etiology of atherosclerosis and its sequelae. Details of the design, objectives and quality control activities of the ARIC study have been previously reported [13]. A probability sample of 15,792 residents aged 45-64 yrs was recruited in 1987-1989 from four U.S. communities: Forsyth County, North Carolina; Jackson, Mississippi; northwest suburbs of Minneapolis, Minnesota; and Washington County, Maryland. Blacks were sampled exclusively in Jackson and sampled proportionately in Forsyth County to ensure race-specific estimates. The Minneapolis and Washington County sites were predominantly white. The Institutional Review Board of the 4 participating centers approved the study, and all participants gave written informed consent prior to participating in the study.
Case classification
Study participants were followed for incident VTE (including DVT and PE) from baseline in 1987-89 until the end of 2005. VTEs were identified via the Longitudinal Investigation of Thromboembolism Etiology (LITE) study [14] and validated by physician review of hospital records. Specifically, participants were contacted annually by telephone, during which hospitalizations were identified by participant or proxy reports. Some possible VTE events were also identified by ongoing retrospective surveillance of local hospital's discharge lists. Medical records from participants with primary or secondary hospital discharge codes for possible VTE were copied and reviewed by two physicians using standardized criteria [14]. VTEs were classified as secondary if preceded within 90 days by major trauma, surgery, or marked immobility, or associated with active cancer or chemotherapy [14], or idiopathic otherwise.
Geocoding
Participant addresses were geocoded using a commercial service (Mapping Analytics LLC, Rochester NY, US) which assigned a latitude and longitude coordinate to each address. This geocoding was performed with the Centrus Enhanced Database which was primarily based on the Topologically Integrated Geographic Encoding and Referencing system (TIGER) data.
Traffic exposure
We quantified small-scale spatial variations of traffic exposure by two measurements: geographical information system (GIS)-mapped traffic density assignments at each place of residence, and the distance from the place of residence to nearest roadways of various types. The participant's address at the baseline visit (1987-1989) was used as the basis for calculating both exposure measures. Details of traffic density and distance to roads have been previously described [11, 12]. Briefly, traffic density behaves like an inverse-distance weighted traffic volume variable, except that it considers intersections and multiple roadways more accurately. Thus, these density values provide a relative indication of which residence locations are likely to be most exposed to traffic activity and, as such, are dimensionless indicators of proximity to traffic volume.
The distance-to-roadway data includes the distance [in meters (m)] from each unique residence location to the nearest roadways. Consistent with previous studies [15-17], we dichotomized distance to major roads (interstate and state highways, major arterials) at 150 m. Additionally, we conducted a sensitivity analysis and categorized distance to major roads as <100m and >=100m [18].
Background air pollution level
Data on the background ambient concentrations of particulate matter less than 10 microns (PM10) and nitrogen dioxide (NO2) during the research period were acquired from the Environmental Protection Agency (EPA) air quality data retrieval system. We abstracted annual average concentrations for PM10 and NO2 at the baseline visit. The average concentrations were spatially interpolated from air quality monitoring stations to the cohort residence locations at baseline using inverse distance weighting.
Other covariates
Anthropometric measures were determined by trained, certified technicians following a detailed, standardized protocol. Body mass index (BMI) was calculated as weight (kg)/[height (m)]2. Diabetes mellitus was defined as a fasting glucose level of 126 mg/dL (7.0 mmol/L) or greater, a nonfasting glucose level of 200 mg/dL (11.1 mmol/L) or greater, or a self-reported history of or treatment for diabetes. Trained and certified interviewers also collected information on age, ethnicity, gender, smoking, environmental tobacco smoke (ETS), alcohol intake, and medical history. Smoking variables included smoking status (never, former and current smokers) and pack years. Never smokers and former smokers were classified as exposed to ETS if they reported being in close contact with smokers for more than 1 hour per week [19]. Thus we created five strata for active and passive smoking: current smoker, former smoker with ETS, former smoker without ETS, never smoker with ETS, never smoker without ETS. Factor VIII coagulant activity was measured by a one-stage assay using VIII–deficient plasma (George King Biomedical, Overland Park, KS). Activated partial thromboplastin time (aPTT) was assayed on an automated coagulometer.
Statistical analysis
The endpoint of interest was incident VTE, so we excluded participants if they had a history of VTE at the baseline (n = 276). We also excluded persons with a race/ethnicity other than the African-American or white, and, because of their small number, African-Americans from the Minnesota and Maryland field centers (n=103) were excluded along with persons with missing data on geocode (n=1,724), smoking status (n=16), pack years (n=279), BMI (n=25), diabetes status (n=148), alcohol intake (n=72), aPTT (n=285), and factor VIII (n=281). Exclusions overlapped in some instances, leaving 13,143 subjects for analysis.
All analyses were conducted using the SAS, version 9.1 (SAS Institute Inc, Cary, NC). Follow-up time was calculated as the time from baseline to an event, death, the last follow-up contact, or through the end of 2005, whichever occurred first.
Cox proportional hazards regression analyses were used to assess the associations of traffic exposure with the risk of incident VTE. Distributions of traffic density were highly skewed (Figure 1); therefore, we analyzed traffic density in quartiles and as a continuous variable after log-transformation. We estimated the hazard ratios (HRs) of incident VTE for quartiles of traffic density relative to the lowest quartile, and for one unit increase of log-transformed density values. Tests for linear trends across increasing quartiles of traffic density used the median value in each quartile. We estimated the risk for living close to major roads (≤ 150 m, or ≤ 100 m), with participants living further away serving as the referent. For comparison with the study of Baccarelli et al. [6], we also reported the risk for a decrease equal to the difference between the 90th and the 10th percentile of the distance to major roads.
Figure 1.
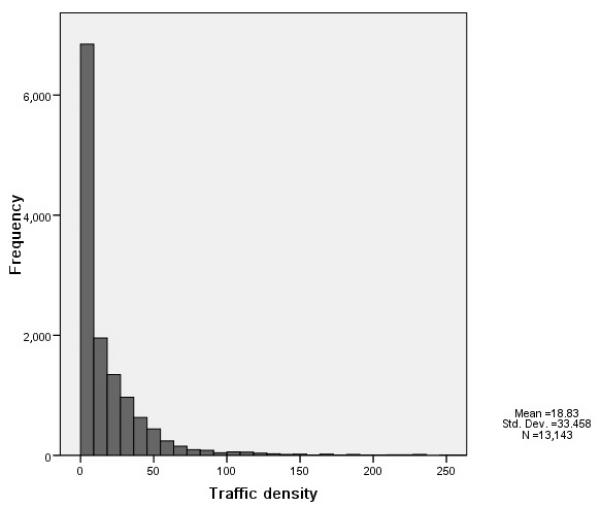
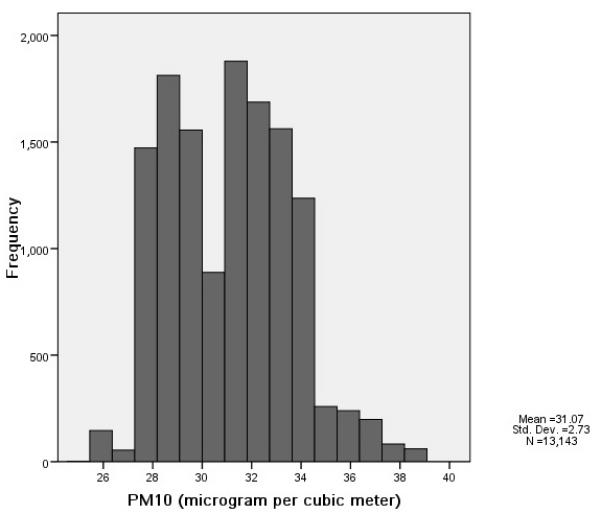
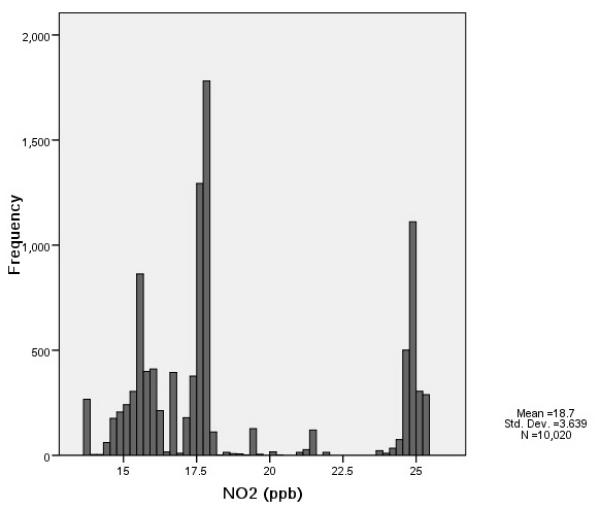
Distribution of traffic density and background air pollutant concentrations at ARIC participant residences (1987–1989). (A) Traffic density (n=13,143). (B) PM10 (μg/m3; n =13,143). (C) NO2 (ppb; n=10,020).
Our basic models included age, sex, center and ethnicity. Center was added as a covariate to control for potential differences across study areas that might confound the results. In the adjusted models, we added reported risk factors for VTE [5, 6, 20], including BMI, diabetes status, coagulation parameters (factor VIII and aPTT), smoking status (current smoker, former smoker with ETS, former smoker without ETS, never smoker with ETS, never smoker without ETS), pack years, alcohol intake (current, former, and never drinker), and background air pollution level (PM10). NO2 data were missing in Jackson and therefore were not included in the adjusted models.
We also did separate analyses of DVT versus PE. Since a substantial proportion of VTE cases are due to cancer, trauma, immobilization, surgery, and hormone replacement therapy, we did a sensitivity analysis restricted to the idiopathic VTE cases. As postmenopausal hormone use is a risk factor for VTE [21], we also did a sensitivity analysis among female participants adding hormone use as a covariate.
Consistent with previous cohort studies of air pollution [10, 22], we used traffic exposure at baseline visit 1 (1987-1989) for our main analysis. We also did sensitively analyses using averaged exposure data throughout the follow-up period (1987-1998) and 1-year or 5-year average exposure before the VTE event.
Results
Table 1 presents selected characteristics of participants at baseline, stratified by quartiles of traffic density and distance to major roads (>= 150 m and < 150 m). Subjects in the highest quartile of traffic density and those living closest to major roads were slightly older, had slightly higher BMI and were more likely to be current smokers. There was no association of either traffic variable with factor VIII or aPTT levels.
Table 1.
Baseline characteristics of the ARIC participants (n=13,143) stratified by quartiles of traffic density and distance to major roads (>=150m, and <150m) 1987-1989 *
| Quartiles of traffic density |
Distance to major roads |
|||||
|---|---|---|---|---|---|---|
| 1 (lowest) | 2 | 3 | 4 | >=150m | <150m | |
| Age (yrs) | 53.9 (5.7) | 53.9 (5.8) | 54.1 (5.7) | 54.6 (5.8) | 53.9 (5.8) | 54.5 (5.7) |
| Female sex (%) | 54.8 | 53.8 | 55.6 | 56.5 | 54.2 | 56.8 |
| BMI (kg/m2) | 27.6 (5.3) | 27.6 (5.3) | 27.5 (5.1) | 27.8 (5.5) | 27.5 (5.2) | 27.8 (5.4) |
| Black race (%) | 30.6 | 25.6 | 25.0 | 25.7 | 26.2 | 27.5 |
| Diabetes (%) | 11.8 | 11.0 | 12.5 | 11.7 | 11.3 | 12.6 |
| Smoking status (%) † | ||||||
| Current smoker | 23.7 | 26.5 | 25.8 | 28.0 | 24.8 | 28.0 |
| Former smoker | 30.6 | 32.0 | 34.0 | 31.8 | 32.6 | 31.3 |
| Never smoker | 45.7 | 41.5 | 40.3 | 40.2 | 42.7 | 40.8 |
| Factor VIII (%) | 133 (40) | 131 (40) | 130 (39) | 132 (40) | 131 (40) | 133 (40) |
| aPTT (s) | 29 (3) | 29 (3) | 29 (3) | 29 (3) | 29 (3) | 29 (3) |
All values are mean (SD) unless specified as percentage.
Percentages add to less than 100 due to rounding.
The estimated traffic density and background air pollutant (PM10 and NO2) concentrations at the baseline home address varied greatly (Figure 1). Consistent with previous reports [23], we did not find a strong correlation between traffic density and background air pollution level; the Pearson correlation coefficients of traffic density with PM10 and NO2 were −0.12 and −0.04, respectively. Background PM10 was moderately correlated with NO2 (Pearson correlation coefficient, r = 0.61).
Among the 13,143 study participants who were free of VTE at baseline, 405 subjects developed VTE (149 idiopathic and 256 secondary VTE). As expected, subjects with incident VTE were older, more likely to be male or black; and had higher BMI.
Traffic density was not significantly associated with VTE in either base or adjusted models (Table 2). Relative to those in the lowest quartile of traffic density, the adjusted hazard ratios across increasing quartiles were 1.18 (95%CI 0.88-1.57), 0.99 (95%CI 0.74-1.34) and 1.14 (95%CI 0.86-1.51) (p for trend across quartiles = 0.64). When traffic density was treated as a continuous variable, the adjusted hazard ratio per one unit increase of log-transformed density was 1.01 (95%CI 0.98-1.03, p = 0.51).
Table 2.
Hazard rate ratios (and 95% CIs) for incident venous thromboembolism (VTE) associated with traffic density, ARIC, 1987-2005 (n=13,143)
| Quartiles |
Continuous variable (log-transformed) |
||||||
|---|---|---|---|---|---|---|---|
| 1 (lowest) | 2 | 3 | 4 | p value for trend * |
One unit increase | p value | |
| Median of quartiles | 0 | 2.92 | 15.00 | 41.91 | |||
| No. of cases/subjects | 97/3,285 | 107/3,286 | 90/3,287 | 111/3,285 | |||
| Basic model † | 1.00 | 1.18 (0.89, 1.57) |
0.98 (0.72, 1.32) |
1.16 (0.88, 1.54) |
0.53 | 1.01 (0.98, 1.03) |
0.49 |
| Adjusted model ‡ | 1.00 | 1.18 (0.88, 1.57) |
0.99 (0.74, 1.34) |
1.14 (0.86, 1.51) |
0.64 | 1.01 (0.98, 1.03) |
0.51 |
p values for trend based on quartiles scaled by the quartile medians.
Covariates included age, sex, center and ethnicity.
Covariates included age, sex, center, ethnicity, BMI, diabetes status, factor VIII, aPTT, active and passive smoking, alcohol intake and background air pollution level.
For residents living within 150 m of major roads compared to subjects living further away, the hazard ratios were 1.18 (95%CI 0.97-1.44, p=0.10) in the base model and 1.16 (95%CI 0.95-1.42, p=0.14) in the adjusted model (Table 3). In the analysis with an alternative cut-point of distance to major roads (100 m), the adjusted hazard ratio was 1.12 (95%CI 0.90-1.38, p=0.32). For residents living in the 10th centile of the distance to major roads compared with those living in the 90th distance, the adjusted hazard ratio was 1.18 (95%CI 0.97-1.44, p = 0.10).
Table 3.
Hazard rate ratios (and 95% CIs) for incident venous thromboembolism (VTE) by distance to major roads, ARIC, 1987-2005 (n=13,143)
| Dichotomized at 150m |
Dichotomized at 100m |
Continuous variable |
||||||
|---|---|---|---|---|---|---|---|---|
| < 150 m | >= 150 m | p | < 100 m | >= 100 m | p | Difference between the 90th and 10th percentile ‡ |
p | |
| No. of cases/subjects | 176/5,095 | 229/8,048 | 123/3,567 | 282/9,576 | ||||
| Basic model * | 1.18 (0.97, 1.44) |
1.00 | 0.10 | 1.15 (0.93, 1.42) |
1.00 | 0.20 | 1.19 (0.98, 1.45) |
0.09 |
| Adjusted model † | 1.16 (0.95, 1.42) |
1.00 | 0.14 | 1.12 (0.90, 1.38) |
1.00 | 0.32 | 1.18 (0.97, 1.44) |
0.10 |
Covariates included age, sex, center and ethnicity.
Covariates included age, sex, center, ethnicity, BMI, diabetes status, factor VIII, aPTT, active and passive smoking, alcohol intake and background air pollution level.
10th percentile of distance to major road: 22.5 meters; 90th percentile of distance from the nearest major traffic road: 588.8 meters.
We did not observe a significant association of background air pollution with incident VTE; the hazard ratio of incident VTE per 10 μg/m3 increase of PM10 was 0.87 (95%CI 0.50-1.51, p=0.61).
Separate analysis by DVT or PE alone found no significant association with traffic exposure (data not shown). In the sensitivity analysis limited to the 159 idiopathic VTE cases, results above were similar. Among female participants, inclusion of a term for hormone use did not appreciably change our findings (Table S1 in the online supplement). We found no evidence of effect modification by hormone use (p for interaction > 0.05).
We found similar null associations of traffic exposure with VTE when we used averaged exposure data throughout the follow-up period (1987-1998) (data not shown). In sensitivity analysis using 1-year or 5-year average exposure before the VTE event, we found similar null associations (Tables S2-3).
Discussion
We found no significant association between long-term traffic exposure, assessed by traffic density and distance to major roads, and risk of VTE. To our knowledge, this is the first prospective study of the association between traffic-related air pollution exposure and incident VTE in the general population. Distance to roads was related inversely to DVT in a recent case-control study in Italy [6]; however, we did not find a comparable association. The same group reported that long-term exposure to PM10 was positively related to DVT [5]. A recent time series study reported associations between daily air pollution levels and timing of hospitalization for DVT [7]. However, our study is consistent with a recent large prospective cohort study reporting a null association between background PM exposure and VTE [8].
We did not observe a significant association of background air pollution (PM10) with incident VTE. This is not surprising given that the ARIC study was not designed to examine air pollution. The four communities were not well supplied with air pollution monitors during the baseline period which limits the ability to detect within community variation in exposure.
Some limitations of our analysis should be noted. We did not predict the air pollutant concentration based on traffic density data, and we could not validate our exposure assessment with actual measurements given that the exposure period was 1987-1989. Likewise, our traffic density metric does not reflect local meteorological conditions that could influence the emission, mixing and transport of air pollutants. Some studies have suggested a stronger association of wheezing in early infancy with stop-and-go traffic than moving traffic and with truck traffic compared to car traffic [24]. However, in most studies, including ours and the study by Baccarelli et al. [6], it was not possible to separate traffic types. Similar to the study of Baccarelli et al. [6], our exposure assessment was limited to residential address. We did not have updated residential addresses after 1998 available for the analysis. We lacked exposure information on the approximately 10.9% of subjects that could not be assigned a geocode at the level of an exact address match. Most of the missing geocodes were due to problems such as missing state (most often military addresses), apartment name without address, or only a post office box address. However, in order for missing geocode data to have created a spurious null association between higher traffic exposure and incident VTE, subjects with and without geocodes would need to differ in both traffic exposure and incident VTE. Although we have no data on their traffic exposure, they were socio-demographically similar to subjects with non-missing geocodes and had a similar incidence of VTE (data not shown). Thus, it is unlikely that the missing geocode data could have created the observed null associations. We only used inverse distance weighting to estimate background air pollution levels. Advanced methods (such as Kriging or land use regression model) are more relevant for mapping hourly or daily air quality where larger gradients are common. It is unlikely that the advanced methods would make significant differences in annual air quality assignments for our subjects.
A major strength of our study is the use of an objective measure of traffic-related air pollution at residential addresses (such as GIS-based assessment of traffic density and distance to major roads) to capture exposure relevant for subjects living in close proximity to busy roads. A further strength is the overall residential stability of the cohort; 84% of the ARIC participants had stayed in the same census tract at visit 1 and visit 3. An earlier study of ARIC study participants reported very high concordance between county and state of residence in past decades to that at visit 1 [25]. Of course, there might be exposure misclassification if there was sizeable movement within or out of the census tract. Moreover, our analysis was based on carefully collected incidence data in a large cohort from four U.S. communities. Validation of VTE events was standardized and thorough, and data on exposure, outcome and a wide range of potential confounders were prospectively collected at the individual levels using standardized protocols and extensive quality assurance.
If the previously reported association between VTE and distance to roads [6] is causal, several factors may account for why we were not able to replicate it. The number of incident cases in our analysis (n=405) was smaller than the number of prevalent cases (n=663) used in their analysis leading to lower power. However, based on the exposure-response relationship reported by Baccarelli et al [6] and overall event rate in our study, we conducted Cox Regression power analysis. We achieved 97% power at a 0.05 significance level, suggesting that we had sufficient power to detect an association between distance and VTE of the same magnitude. Exposure variability was greater in the Italian study which included a very busy urban area (Milan), which may also lead to greater power to detect associations. The studies in Italy [5, 6] and Chile [7] examined recent exposure in relation to VTE; in comparison, our study examined the association between long-term exposure to traffic and incident VTE. In support of our finding with traffic exposure, a recent large cohort study found no association between particulate matter exposure and VTE up to one year prior to the event [8].
Because of the enormous number of people affected, the relationship between air pollution exposure and VTE has potentially important public health and medical implications. If there is a causal association between air pollution and DVT, it would be expected to be very small in magnitude and a larger body of data would be needed to rule it out. Although two recent analyses from a case control study in Italy [5, 6] and a time series study [7] support an association between air pollution and VTE, our prospective findings with traffic exposure support recent prospective data on particulate matter in providing evidence against this association [8].
Supplementary Material
Acknowledgments
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute (NHLBI) contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022. This work was also supported by NHLBI grant R01-HL59367 and the Intramural Research Program, National Institute of Environmental Health Sciences, National Institutes of Health, DHHS ZO1 ES043012. Haidong Kan was supported by the National Basic Research Program (973 program) of China (2011CB503802).
Footnotes
Disclosure of Conflict of Interests
The authors declare they have no competing financial interests.
References
- 1.Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, Luepker R, Mittleman M, Samet J, Smith SC, Jr., Tager I. Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation. 2004;109:2655–71. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- 2.Brook RD, Rajagopalan S, Pope CA, 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC, Jr., Whitsel L, Kaufman JD. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–78. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 3.Bonzini M, Tripodi A, Artoni A, Tarantini L, Marinelli B, Bertazzi PA, Apostoli P, Baccarelli A. Effects of inhalable particulate matter on blood coagulation. J Thromb Haemost. 8:662–8. doi: 10.1111/j.1538-7836.2009.03694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schafer AI. Venous thrombosis as a chronic disease. N Engl J Med. 1999;340:955–6. doi: 10.1056/NEJM199903253401209. [DOI] [PubMed] [Google Scholar]
- 5.Baccarelli A, Martinelli I, Zanobetti A, Grillo P, Hou LF, Bertazzi PA, Mannucci PM, Schwartz J. Exposure to particulate air pollution and risk of deep vein thrombosis. Arch Intern Med. 2008;168:920–7. doi: 10.1001/archinte.168.9.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baccarelli A, Martinelli I, Pegoraro V, Melly S, Grillo P, Zanobetti A, Hou L, Bertazzi PA, Mannucci PM, Schwartz J. Living near major traffic roads and risk of deep vein thrombosis. Circulation. 2009;119:3118–24. doi: 10.1161/CIRCULATIONAHA.108.836163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dales RE, Cakmak S, Vidal CB. Air pollution and hospitalization for venous thromboembolic disease in Chile. J Thromb Haemost. 2010;8:669–74. doi: 10.1111/j.1538-7836.2010.03760.x. [DOI] [PubMed] [Google Scholar]
- 8.Shih RA, Griffin BA, Salkowski N, Jewell A, Eibner C, Bird CE, Liao D, Cushman M, Margolis HG, Eaton CB, Whitsel EA. Ambient particulate matter air pollution and venous thromboembolism in the Women's Health Initiative Hormone Therapy Trials. Environ Health Perspect. 2010 doi: 10.1289/ehp.1002256. DOI: 10.1289/ehp.1002256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ageno W, Becattini C, Brighton T, Selby R, Kamphuisen PW. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation. 2008;117:93–102. doi: 10.1161/CIRCULATIONAHA.107.709204. [DOI] [PubMed] [Google Scholar]
- 10.Hoek G, Brunekreef B, Goldbohm S, Fischer P, van den Brandt PA. Association between mortality and indicators of traffic-related air pollution in the Netherlands: a cohort study. Lancet. 2002;360:1203–9. doi: 10.1016/S0140-6736(02)11280-3. [DOI] [PubMed] [Google Scholar]
- 11.Kan H, Heiss G, Rose KM, Whitsel E, Lurmann F, London SJ. Traffic exposure and lung function in adults: the Atherosclerosis Risk in Communities study. Thorax. 2007;62:873–9. doi: 10.1136/thx.2006.073015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kan H, Heiss G, Rose KM, Whitsel EA, Lurmann F, London SJ. Prospective Analysis of Traffic Exposure as a Risk Factor for Incident Coronary Heart Disease: The Atherosclerosis Risk in Communities (ARIC) Study. Environ Health Perspect. 2008;116:1463–8. doi: 10.1289/ehp.11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The ARIC investigators The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 14.Cushman M, Tsai AW, White RH, Heckbert SR, Rosamond WD, Enright P, Folsom AR. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004;117:19–25. doi: 10.1016/j.amjmed.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann B, Moebus S, Stang A, Beck EM, Dragano N, Mohlenkamp S, Schmermund A, Memmesheimer M, Mann K, Erbel R, Jockel KH. Residence close to high traffic and prevalence of coronary heart disease. Eur Heart J. 2006;27:2696–702. doi: 10.1093/eurheartj/ehl278. [DOI] [PubMed] [Google Scholar]
- 16.Venn AJ, Lewis SA, Cooper M, Hubbard R, Britton J. Living near a main road and the risk of wheezing illness in children. Am J Respir Crit Care Med. 2001;164:2177–80. doi: 10.1164/ajrccm.164.12.2106126. [DOI] [PubMed] [Google Scholar]
- 17.Wilkinson P, Elliott P, Grundy C, Shaddick G, Thakrar B, Walls P, Falconer S. Case-control study of hospital admission with asthma in children aged 5-14 years: relation with road traffic in north west London. Thorax. 1999;54:1070–4. doi: 10.1136/thx.54.12.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schikowski T, Sugiri D, Ranft U, Gehring U, Heinrich J, Wichmann HE, Kramer U. Long-term air pollution exposure and living close to busy roads are associated with COPD in women. Respir Res. 2005;6:152. doi: 10.1186/1465-9921-6-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howard G, Wagenknecht LE, Burke GL, Diez-Roux A, Evans GW, McGovern P, Nieto FJ, Tell GS. Cigarette smoking and progression of atherosclerosis: The Atherosclerosis Risk in Communities (ARIC) Study. Jama. 1998;279:119–24. doi: 10.1001/jama.279.2.119. [DOI] [PubMed] [Google Scholar]
- 20.Tsai AW, Cushman M, Rosamond WD, Heckbert SR, Polak JF, Folsom AR. Cardiovascular risk factors and venous thromboembolism incidence: the longitudinal investigation of thromboembolism etiology. Arch Intern Med. 2002;162:1182–9. doi: 10.1001/archinte.162.10.1182. [DOI] [PubMed] [Google Scholar]
- 21.Daly E, Vessey MP, Hawkins MM, Carson JL, Gough P, Marsh S. Risk of venous thromboembolism in users of hormone replacement therapy. Lancet. 1996;348:977–80. doi: 10.1016/S0140-6736(96)07113-9. [DOI] [PubMed] [Google Scholar]
- 22.Pope CA, 3rd, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K, Thurston GD. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287:1132–41. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoek G, Fischer P, Van Den Brandt P, Goldbohm S, Brunekreef B. Estimation of long-term average exposure to outdoor air pollution for a cohort study on mortality. J Expo Anal Environ Epidemiol. 2001;11:459–69. doi: 10.1038/sj.jea.7500189. [DOI] [PubMed] [Google Scholar]
- 24.Ryan PH, LeMasters G, Biagini J, Bernstein D, Grinshpun SA, Shukla R, Wilson K, Villareal M, Burkle J, Lockey J. Is it traffic type, volume, or distance? Wheezing in infants living near truck and bus traffic. J Allergy Clin Immunol. 2005;116:279–84. doi: 10.1016/j.jaci.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 25.Rose KM, Wood JL, Knowles S, Pollitt RA, Whitsel EA, Diez Roux AV, Yoon D, Heiss G. Historical measures of social context in life course studies: retrospective linkage of addresses to decennial censuses. Int J Health Geogr. 2004;3:27. doi: 10.1186/1476-072X-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


