Abstract
The arcuate fasciculus (AF) connects cortical regions important in language processing, but how fiber coherence and organization relates to gray matter macrostructure remains uncharacterized. We used high‐resolution structural and 30‐direction diffusion imaging data from 36 healthy adults (24 male/12 female; mean age, 30.5 ± 9.8 years) to establish the relationships between AF microstructure and regional variations in cortical gray matter within language networks. Cortical pattern‐matching algorithms were used to measure gray matter thickness at high‐spatial density, and a validated diffusion tractography method was used to reconstruct the AF in the left and right hemisphere of each subject. Relationships between imaging measures and neuropsychological scores of verbal fluency were additionally assessed. Results revealed positive and highly topographical associations between arcuate fractional anisotropy (FA) and cortical thickness within anterior and posterior language regions and surrounding cortices, more prominently in the left hemisphere. These regional cortical thickness/FA relationships were primarily attributable to variations in radial diffusivity. Associations between cortical thickness and verbal fluency were observed in perisylvian language‐related regions. Language scores were associated with left‐hemisphere AF axial diffusivity, but not with AF FA or radial diffusivity. These findings thus suggest that particular components of white matter microstructure and regional increases in cortical thickness benefit aspects of language processing. Furthermore, the topographical relationships between independent measures of white matter and gray matter integrity suggest that rich developmental or environmental interactions influence brain structure and function where the presence and strength of such associations may elucidate pathophysiological processes influencing language systems. Hum Brain Mapp, 2011. © 2010 Wiley‐Liss, Inc.
Keywords: diffusion tensor imaging, magnetic resonance imaging, white matter, gray matter, language, neuroanatomy, tractography, superior longitudinal fasciculus, fractional anisotropy
INTRODUCTION
Although both white matter connectivity and neural integrity constitute critical components of brain function, how white matter fiber coherence and organization relates to cortical gray matter structure remains under‐explored with in vivo imaging methods. Cortical thickness varies across the cortical mantle in accordance with the size, density and arrangement of neurons, glial cells, and nerve fibers. Regional variations in cortical gray matter thickness identified with computational structural magnetic imaging (sMRI) analysis methods are shown to occur during normal brain maturation and aging and to associate with individual differences such as sex, cognitive ability, and disease status, for example [Luders et al.,2006; Narr et al.,2007; Thompson et al.,2004]. White matter microstructure measured noninvasively using diffusion tensor imaging (DTI) is most frequently estimated through the measurement of fractional anisotropy (FA), which reflects the degree to which water diffusion is directionally biased in brain tissue. FA is also shown to vary regionally in accordance with demographic and clinical characteristics and measures of brain function in human subjects, for example, [Lebel et al.,2008; Powell et al.,2006; White et al.,2008] and may be estimated along discrete fibers linking cortical networks using methods that assemble the local diffusion tensor data into particular white matter pathways. Measures of axial and radial diffusivity, which represent water diffusion parallel to the major axis of fiber orientation and along the two minor axes respectively, may also be determined within these pathways to help establish the basis for variations in tissue architecture [Song et al.,2002].
The arcuate fasciculus (AF), which is considered a component of the superior longitudinal fasciculus (SLF) [Makris et al.,2005], is a major white matter tract that has received particular attention for its role in language [Geschwind,1970]. Some recent DTI data suggest that the AF may be further subdivided according to the termination points of fibers [Bernal and Ardila,2009; Catani and Mesulam,2008; Catani et al.,2005; Frey et al.,2008; Lawes et al.,2008]. Notwithstanding, in accordance with early postmortem studies, the long continuous fibers of the AF are described to represent the temporofrontal arching white matter pathway linking posterior and anterior language‐related regions. Specifically, the AF projects from the superior temporal gyrus and Wernicke's area [Brodmann's area (BA) 22, involved in speech comprehension] and curves around the Sylvian fissure to connect with Broca's area (BA 44, 45, involved in speech production) in the inferior frontal cortex. AF fibers also link association cortices in the caudal dorsolateral prefrontal cortex (BA 6, 8) and the inferior parietal lobule (BA 39, 40) [Catani et al.,2005; Frey et al.,2008]. These connections with multimodal neural sites may facilitate the processing of more complex aspects of spoken or written words as surveyed in verbal intelligence tasks [Hoeft et al.,2007; Jung and Haier,2007; Lebel and Beaulieu,2009; Schmithorst et al.,2005; Turken et al.,2008]. AF microstructure reflecting leftward language dominance [Catani et al.,2007; Glasser and Rilling,2008; Lebel and Beaulieu,2009] is shown to relate to verbal recall in adults [Catani et al.,2007] and to some cognitive abilities in children [Lebel and Beaulieu,2009]. Associations between functional lateralization and FA within pathways connecting inferior frontal and superior temporal brain regions activated by language tasks (verb generation and reading comprehension) have also been demonstrated [Powell et al.,2006].
Language skills acquired during development associate with increased cortical thickness in the left‐inferior frontal cortex [Lu et al.,2007]. Moreover, cortical thickness in Broca's area demonstrates strong anatomical correlations (>0.8) with thickness in Wernicke's area, the superior temporal gyrus, inferior parietal lobule, and neighboring frontal regions. These anatomical correlations correspond closely with tractographic maps of the AF and strengthen as language function develops during brain maturation [Lerch et al.,2006]. However, very few studies have examined associations between white matter microstructure and cortical thickness directly. One prior investigation showed that FA and cortical thickness are positively associated at the level of the whole brain and hemispheres [Kochunov et al.,2007]. Although not substantiated statistically, another study has documented positive FA‐cortical thickness relationships within the temporal cortices in elderly control subjects but not in individuals with mild cognitive impairment [Wang et al.,2009]. To address the hypothesis that associations between white matter microstructure and cortical thickness occur in language networks, we used cortical pattern‐matching methods applied to sMRI data and tractography methods applied to DTI data. As a secondary goal, we explored the presence of relationships between measures of AF integrity and cortical thickness with language function by examining associations of brain‐imaging measures with verbal fluency. Verbal fluency, which requires both language expression and comprehension, is shown to engage left‐lateral prefrontal (including Broca's area) and posterior temporal language‐related regions in functional imaging tasks [Powell et al.,2006]. Performance for this task also associates with gray matter density in left‐lateral prefrontal regions in healthy adults [Newman et al.,2007]. Furthermore, associations between FA for left‐frontal connections and verbal fluency‐related brain activation have been demonstrated [Powell et al.,2006]. Thus, verbal fluency may represent one aspect of language function that associates with cortical thickness and white matter microstructure in language networks.
MATERIALS AND METHODS
Subjects
Thirty‐six (24 male/12 female) normal adult subjects were recruited for participation through direct calls using a survey sampling methodology and advertisements placed in newspapers and fliers. Two subjects were identified as left‐handed (laterality quotient scores ≤−0.70) as determined using a modified version of the Edinburgh Handedness Inventory [Oldfield,1971]. Table I provides demographic details of subjects within each sex. Exclusion criteria for all subjects included mental retardation, neurological disorder (e.g., temporal lobe epilepsy), and recent or past history of significant and habitual drug abuse or alcoholism. A radiologist reviewed any suspected abnormalities in the high‐resolution sMRI data. Subjects were additionally screened by clinical interview using the SCID‐NP [First et al.,1994] to exclude the presence of schizophrenia spectrum or other psychiatric disorder. All participants provided informed consent as approved by the University of California, Los Angeles (UCLA) Institutional Review Board.
Table I.
Subject characteristics and means and standard deviations for arcuate fasciculus (AF) measurements
| All subjects (n = 36) | Females (n = 12) | Males (n = 24) | |
|---|---|---|---|
| Age (mean ± SD) | 31.3 ± 9.7 | 34.4 ± 10.7 | 29.7 ± 10.7 |
| Handedness (right/left)a | 34/2 | 12/0 | 22/2 |
| Years of educationb | 15.0 ± 2.6 | 14.8 ± 2.4 | 15.0 ± 2.8 |
| Total brain volume (cc) | 1415.5 ± 155.8 | 1265.7 ± 80.7 | 1487.4 ± 129.8 |
| White matter volume (cc) | 540.4 ± 76.6 | 484.7 ± 44.0 | 567.1 ± 75.0 |
| Gray matter volume (cc) | 703.8 ± 78.8 | 619.2 ± 44.8 | 744.5 ± 55.6 |
| Verbal fluency total scorea | 11.38 ± 3.14 | 10.45 ± 3.24 | 11.83 ± 3.07 |
| Hemisphere | Left | Right | Left | Right | Left | Right |
|---|---|---|---|---|---|---|
| Arcuate FA | 0.470 ± 0.029 | 0.470 ± 0.038 | 0.470 ± 0.037 | 0.474 ± 0.052 | 0.470 ± 0.026 | 0.469 ± 0.043 |
| Arcuate tract volume (cc) | 4.48 ± 2.82 | 4.03 ± 2.86 | 3.39 ± 2.80 | 2.84 ± 2.36 | 5.01 ± 2.74 | 4.63 ± 2.94 |
| Arcuate axial diff (×104 mm2/s) | 10.79 ± 0.35 | 10.27 ± 0.41 | 10.78 ± 0.40 | 10.32 ± 0.37 | 10.79 ± 0.33 | 10.24 ± 0.44 |
| Arcuate radial diff (×104 mm2/s) | 5.46 ± 0.20 | 5.21 ± 0.42 | 5.48 ± 0.19 | 5.22 ± 0.18 | 5.45 ± 0.21 | 5.21 ± 0.50 |
| Overall cortical thickness (mm) | 4.61 ± 0.14 | 4.58 ± 0.15 | 4.62 ± 0.14 | 4.62 ± 0.15 | 4.55 ± 0.13 | 4.64 ± 0.15 |
| Middle temporal gyrus (mm) | 5.18 ± 0.61 | 5.17 ± 0.38 | 5.18 ± 0.28 | 5.19 ± 0.74 | 5.10 ± 0.43 | 5.22 ± 0.35 |
| Superior temporal gyrus (mm) | 5.37 ± 0.53 | 5.35 ± 0.27 | 5.39 ± 0.27 | 5.43 ± 0.59 | 5.33 ± 0.31 | 5.48 ± 0.30 |
| Supramarginal gyrus (mm) | 4.85 ± 0.84 | 4.90 ± 0.56 | 4.84 ± 0.36 | 4.78 ± 0.84 | 4.75 ± 0.47 | 4.79 ± 0.42 |
| Precentral gyrus (mm) | 4.53 ± 0.39 | 4.50 ± 0.22 | 4.53 ± 0.20 | 4.50 ± 0.48 | 4.42 ± 0.23 | 4.52 ± 0.26 |
| Postcentral gyrus (mm) | 4.62 ± 0.69 | 4.59 ± 0.40 | 4.64 ± 0.33 | 4.47 ± 0.72 | 4.41 ± 0.39 | 4.50 ± 0.37 |
| Inferior frontal gyrus (mm) | 4.42 ± 0.17 | 4.45 ± 0.18 | 4.40 ± 0.17 | 4.59 ± 0.17 | 4.58 ± 0.17 | 4.59 ± 0.17 |
| Superior occipital gyrus (mm)c | 3.97 ± 0.27 | 3.97 ± 0.27 | 3.97 ± 0.27 | 3.86 ± 0.24 | 3.88 ± 0.25 | 3.85 ± 0.25 |
Verbal fluency scores averaged across letters (range, 5–19), which are similar to reported norms, for example, [Loonstra et al., 2001], were unavailable for one male and one female subject. Means represent raw values (uncorrected for sex or age).
Education data were unavailable for one male subject.
Control region: the superior occipital gyrus is a region of interest through which the AF does not pass.
Verbal fluency scores were obtained using the Controlled Oral Word Association Test. This test, also known as the phonemic or letter‐fluency test, requires subjects to name as many words as possible beginning with a single letter (F, A, and S) during a 1‐min time period [Lezak,1997]. Total scaled scores for verbal fluency (averaged across letters) were used to examine correlations with brain‐imaging measures. Neuropsychological data were unavailable for two subjects.
Image Acquisition
DTI and sMRI data were acquired on a 1.5T Siemens Sonata scanner (Erlangen, Germany) at the UCLA Ahmanson‐Lovelace Brain Mapping Center using an eight‐channel head coil. The DTI acquisition protocol included three averages of a whole‐brain sequence with 30 noncollinear diffusion directions, 5 b0's, and 55 brain slices oriented perpendicular to the anterior/posterior commissure line (TR = 6,400 ms, TE = 83 ms, b = 0, 1,000 s/mm2, FOV: 240 × 240 mm, matrix: 96 × 96, voxel size: 2.5 mm3, and acquisition time: 3.52 min per average). The DTI sequence was designed to have minimal eddy current‐induced distortions [Reese et al.,2003], and parallel imaging using GRAPPA reconstruction with an acceleration factor of two was used to reduce EPI distortions [Heidemann et al.,2003]. High‐resolution T1‐weighted sMRI data were acquired using a 3D MP‐RAGE sequence with four averages (NEX) (TR = 1,900 ms, TE = 4.38, FOV: 256 × 256 mm; voxel size: 1 mm3, Flip angle: 15°, acquisition time: 8.08 min per average).
DTI Data Processing
After image reconstruction, the diffusion gradient table was corrected for slice prescription, and images were corrected for eddy current distortions and motion artifacts using a nonlinear 2D registration [Jezzard et al.,1998] and a 3D rigid body registration [Woods et al.,1998a,b], where a combined transformation file was used to minimize interpolation. The diffusion tensor was estimated at each voxel using a linear least squares algorithm applied to the log‐transformed signal intensities. Each diffusion tensor was diagonalized to obtain the eigenvalues that were then used to compute FA [Pierpaoli and Basser,1996]. Axial diffusivity, representing the principal axis of diffusion, and radial diffusivity, reflecting the two minor axes of diffusion, were computed from the principal eigenvalue and the average of the two minor eigenvalues, respectively.
Three‐dimensional tract reconstruction of the AF was performed in DTIstudio [Jiang et al.,2006] using the Fiber Assignment by Continuous Tracking algorithm, which has been demonstrated as a reliable method for identifying major white matter pathways with a high degree of reproducibility [Mori and van Zijl,2002; Mori et al.,1999; Okada et al.,2006; Wakana et al.,2004]. Previously described protocols were used to isolate the AF [Phillips et al.,2009; Wakana et al.,2007] using regions of interest identified in the FA‐weighted color maps of each subject (Fig. 1). Average intravoxel FA and axial and radial diffusivity were computed in each subject for the left and right AF separately. These diffusion metrics were then used in statistical analyses to assess relationships with regional variations in cortical thickness within the respective hemisphere. Intraclass correlation coefficients used to determine intra and interrater reliability for tractography seed region placement ranged between r I = 0.96 and r I = 0.99 for measures of tract volume, voxel count, and mean FA between and within two independent raters.
Figure 1.
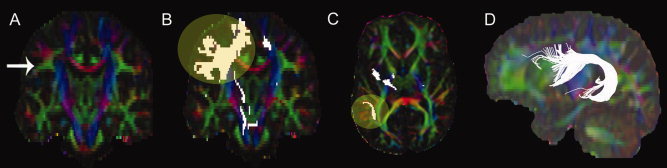
Locations of the seed regions used for extracting the arcuate fasciculus. (A) The coronal section showing the middle of the internal capsule posterior limb used to identify the core of the arcuate fasciculus (intense triangular shaped area), (B) fiber tracts projecting from A, (C) fiber tracts selected from the level of the anterior commissure in the axial plane that project laterally to the sagittal striatum, and (D) fiber bundles crossing both A and C.
sMRI Data Processing
Preprocessing of the T1‐weighted MPRAGE images included the correction of scanner field inhomogeneities [Sled and Pike,1998], automated the removal of extra‐cortical tissue (http://www.fmrib.ox.ac.uk/fsl/bet2/index.html), where errors in scalp editing were manually corrected on a slice‐by slice basis, correction for head tilt and alignment using a three‐translation and three‐rotation rigid‐body transformation [Woods et al.,1998a,b], and the automated classification of voxels into tissue types including gray matter, white matter, and CSF [Shattuck et al.,2001]. Previously detailed cortical pattern‐matching methods [Narr et al.,2007; Thompson et al.,2004] were then used to spatially relate homologous cortical surface locations between individuals to allow relationships between tract FA or verbal fluency test scores and cortical thickness to be assessed at high‐spatial density across subjects. Briefly, parametric models of the left and right hemisphere consisting of 65,536 vertices were first extracted from each image volume and used to manually identify 29 sulcal/gyral landmarks for which inter‐ and intrarater reliability protocols have been previously established [Narr et al.,2007]. The sulcal landmarks were then used as anchors to drive the surrounding cortical surface anatomy of each individual into spatial correspondence by applying surface‐warping algorithms that regrid the hemispheric surfaces to associate the same anatomical locations across hemispheres and individuals without scaling. Finally, cortical thickness, defined as the shortest 3D distance from the white‐gray matter interface to the hemispheric surface without crossing CSF voxels, was estimated at each of the 65,536 spatially matched coordinate locations in each subject, applying an 8‐mm smoothing filter.
Cortical regions of interest were additionally generated to describe and confirm the presence of relationships between tract FA and cortical thickness for gyral regions through which the AF traverses. These regions, including the superior and middle temporal gyri, the supramarginal gyrus, the pre and postcentral gyri, and the inferior frontal gyrus, were generated using the Laboratory of NeuroImaging Probabilistic Brain Atlas (LPBA40) [Shattuck et al.,2008] (http://www.loni.ucla.edu/Atlases/LPBA40). The superior occipital gyrus was included as a control ROI, because the AF does not project to this region.
Statistical Analysis
Because the deformation fields serve to match cortical anatomy across subjects, variations in gray matter thickness that occur in association with indices of white matter connectivity may be determined at high‐spatial resolution. The general linear model incorporating multiple linear regression and implemented in R (http://www.r-project.org/) was used to map statistically significant relationships between arcuate FA and cortical thickness at each vertex while controlling for sex and age. Regional relationships were assessed within each hemisphere separately. That is, relationships between mean FA values from the arcuate measured in the left hemisphere and left‐hemisphere cortical thickness were assessed, and, likewise, relationships between right hemisphere measures were examined. Differences in the slopes of these relationships across sex were also examined. False discovery rate (FDR) methods, which estimate the proportion of false positive statistical tests (rejections of the null hypothesis when the null hypothesis is actually true) among all positive statistical tests, were used to control for multiple spatially correlated comparisons [Storey,2002]. The same linear regression model was used to test for associations between neuropsychological test scores of verbal fluency and cortical thickness at each vertex and to test for associations between verbal fluency and mean FA from the AF in each hemisphere.
To further confirm the significance of results and determine the strength of associations within specific gyral regions, the six regions of interest generated from the LPBA40 atlas were used for both permutation testing and for establishing relationships between arcuate FA (or verbal fluency) and cortical thickness averaged within regions and across each hemisphere using the regression model above. For permutation testing [Anderson and Braak,2003], the number of surface points within each gyral region showing significant associations at a threshold of P < 0.05 using the reduced model (controlling for sex and age) were compared to the number of significant surface points within the same gyral region that occurred by chance when the residuals were randomly permuted in 10,000 new analyses.
Post hoc analyses were performed, again using the same regression model, to address whether tract volume of the AF also associates with regional variations in cortical thickness and verbal fluency. In addition, analyses were conducted to assess whether significant regional relationships between cortical thickness and FA may be more attributable to differences in axial or radial diffusivity. For these analyses, relationships between cortical thickness and AF axial or radial diffusivity were compared at each vertex and also within the six gyral regions of interest. Finally, because several prior studies have reported hemispheric differences in AF microstructure, for example [Catani et al.,2007; Glasser and Rilling,2008; Lebel and Beaulieu,2009], asymmetries for arcuate FA, axial and radial diffusivity, and tract volume were assessed using paired t‐tests.
RESULTS
Male and female subjects did not differ significantly in age [F(1,34) = 0.16, P > 0.68] or years of education [F(1,33) = 0.19, P > 0.66]. Sex effects were also absent for left or right arcuate FA, radial and axial diffusivity [F(1,33) = 0.01–0.63, all P > 0.50], verbal fluency [F(1,31) = 1.61, P < 0.21], and cortical thickness measurements averaged across the hemispheres or within gyral regions of interest [F(1,33) = 0.02–2.07, all P > 0.15]. As expected, males exhibited greater overall brain [F(1,33) = 32.19, P < 0.001], and brain tissue volumes [F(1,33) = 39.17, P < 0.001] for gray matter, [F(1,33) = 14.33, P < 0.001] for white matter, and [F(1,33) = 6.81, P < 0.01 for CSF] than females. AF tract volumes did not differ between males and females {left: [F(1,33) = 2.40, P < 0.13, right: [F(1,33) = 3.41, P < 0.07]} though trended larger in males. Means and standard deviations for age, arcuate FA, axial and radial diffusivity, brain tissue volumes, cortical thickness measurements, and verbal fluency scores are provided in Table I. Paired t‐tests showed significantly greater left compared to right‐hemisphere AF axial, t(1,35) = 8.10, P < 0.001, and radial diffusivity t(1,35) = 3.85, P < 0.001. Although mean tract volume was larger in the left hemisphere, asymmetry effects were not significant for tract volume or for arcuate FA (all P > 0.05). Because brain volume was not shown to correlate with average cortical thickness, left or right arcuate FA or with verbal fluency scores, all P > 0.10, brain volume was not included as an additional covariate in any subsequent statistical analysis.
Associations Between AF Microstructure and Cortical Thickness
Arcuate FA and hemisphere averaged cortical thickness values did not deviate from normality (Kolmogorov–Smirnov tests, all P > 0.05). Relationships between left and right arcuate FA and cortical thickness assessed at each vertex are mapped to the average surface representation of the cortex shown in Figure 2. Uncorrected probability values (left panel) reflect positive relationships; no significant negative relationships were identified. Corresponding partial correlation coefficients (right panel) map the strength of regional relationships in both directions. These statistical maps show highly significant associations between FA and cortical thickness along the trajectory of the AF encompassing Broca's area (BA 44, 45) and proximal prefrontal cortex (BA 6), Wernicke's area (BA 22), and inferior parietal (BA 39 and 40), superior and middle temporal (BA 41, 42), and inferior temporal cortices (BA 37). Effects were pronounced for the left hemisphere particularly in primary language areas (BA 44, 45, and 22). FDR correction indicated that 90% of the uncorrected P values thresholded at P < 0.01 (orange, red, and magenta) are expected to reflect true positive findings. Differences in the slopes of FA‐cortical thickness relationships were compared across sex, but did not survive FDR correction at any cortical location. Table II shows corrected P‐values from permutation analyses confirming relationships between arcuate FA and cortical thickness in frontal, temporal, and parietal gyral regions in the left hemisphere. Results were similar for the right hemisphere, although relationships were not significant in the inferior frontal gyrus.
Figure 2.
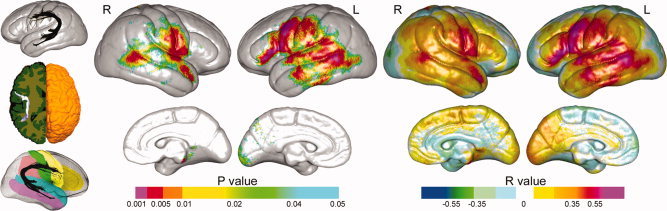
Statistical maps showing topographic associations between arcuate fasciculus fractional anisotropy, and cortical thickness encoded in color. Uncorrected probability values and partial correlation coefficients (age and gender controlled) are shown in the left and right panels, respectively. Far left insets: the AF mapped in different orientations. The bottom left inset shows the gyral regions of interest from the LPBA40 atlas mapped onto the average representation of the cortex.
Table II.
Permutation results (obtained using the reduced model) for cortical thickness associations within gyral regions of interest for arcuate fasiculus (AF) fractional anisotropy (FA) and verbal fluency measures
| Gyral region of interest | Thickness/arcuate FA associations | Thickness/verbal fluency associations | ||
|---|---|---|---|---|
| Left corrected P value | Right corrected P value | Left corrected P value | Right corrected P value | |
| Middle temporal gyrus | 0.003 | 0.03 | 0.38 | 1.0 |
| Superior temporal gyrus | 0.004 | 0.004 | 0.03 | 0.40 |
| Supramarginal gyrus | 0.01 | 0.03 | 0.14 | 0.27 |
| Postcentral gyrus | 0.0009 | 0.008 | 0.02 | 0.53 |
| Precentral gyrus | 0.0008 | 0.02 | 0.02 | 0.33 |
| Inferior frontal gyrus | 0.01 | 0.14 | 0.24 | 1.0 |
Bold italics mark significant P‐values
Results from analyses performed to assess relationships between FA and cortical thickness averaged within each gyral ROI were in agreement with the statistical mapping results where significant positive associations were observed in gyral regions through which the AF projects including the superior and middle temporal gyri, supramarginal gyrus, pre‐ and postcentral gyri, and the inferior frontal gyrus in the left hemisphere and in all regions with the exception of the inferior frontal gyrus in the right hemisphere (r and P values are provided in Fig. 3). Associations were not significant for cortical thickness values averaged across the entire left hemisphere: r(1,33) = 0.15, P > 0.40 or the entire right hemisphere: r(1,33) = 0.07, P > 0.63, nor for the superior occipital gyrus control region, left: r(1,33) = 0.16, P > 0.65 and right: r(1,33) = −0.01, P > 0.96. The slopes of the relationships for all gyral regions of interest did not differ statistically between males and females, F(1,33) = 0.001–3.01, all P > 0.09. Follow‐up analyses performed to examine FA‐cortical thickness relationships after excluding the two left‐handed subjects from the sample did not alter the direction, strength, or pattern of results (results not shown).
Figure 3.
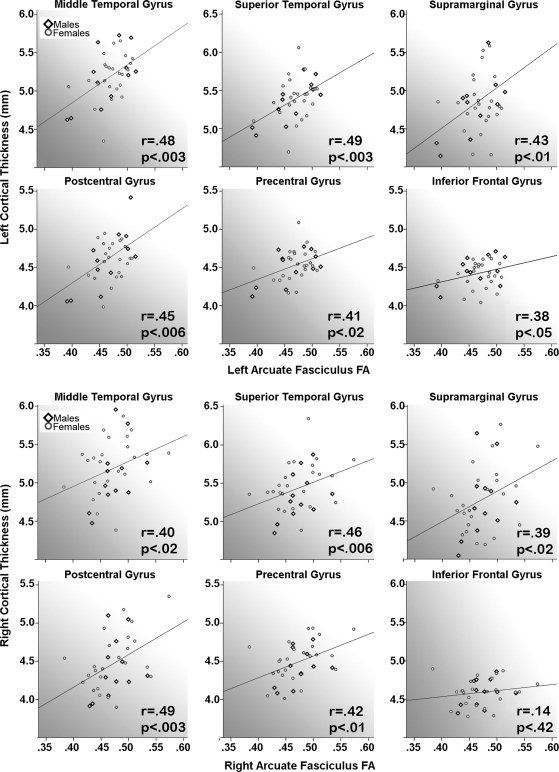
Relationships between arcuate fasciculus (AF) fractional anisotropy (FA) and cortical thickness averaged within gyral regions of interest for the left (top panels) and right (bottom panels) hemispheres, respectively.
Follow‐up testing of relationships between left and right AF axial and radial diffusivity and cortical thickness at each vertex mapped to the average surface representation of the cortex are shown in Figure 4. Positive associations between cortical thickness and axial diffusivity (top panel) and negative associations between cortical thickness and radial diffusivity (shaded bottom panel) are seen in a similar spatial pattern to the regional associations observed for arcuate FA and cortical thickness shown in Figure 2. However, only left hemisphere radial diffusivity exceeded FDR thresholding to indicate that >90% of uncorrected findings at 0.01 represent true positives. Associations between axial and radial diffusivity and cortical thickness averaged across each hemisphere and within each gyral region showed significant effects for radial diffusivity in the bilateral postcentral and right supramarginal gyrus and middle temporal gyrus only (partial correlations and probability values are provided in Table III), although trends were observed for other regions.
Figure 4.
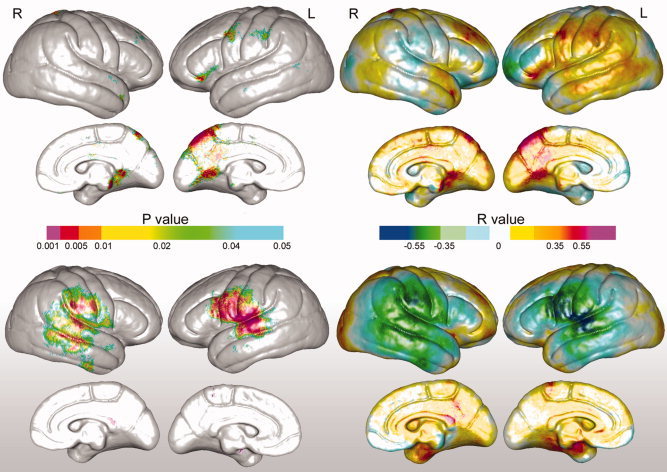
Statistical maps showing associations between arcuate fasciculus axial diffusivity (top panel) and radial diffusivity (shaded bottom panel) and cortical thickness encoded in color. Uncorrected probability values and partial correlation coefficients (age and gender controlled) are shown on the left and right panels, respectively.
Table III.
Partial correlations (controlling for age and gender) between arcuate fasciculus tract volume, axial, and radial diffusivity and cortical thickness averaged across the cortex and within gyral regions of interest for each hemisphere
| Region | Left hemisphere | Right hemisphere | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tract volume | Axial diffusivity | Radial diffusivity | Tract volume | Axial diffusivity | Radial diffusivity | |||||||
| r | P | r | P | r | P | r | P | r | P | r | P | |
| Overall cortex | −0.124 | 0.485 | 0.237 | 0.176 | −0.122 | 0.498 | 0.027 | 0.878 | 0.102 | 0.568 | 0.091 | 0.668 |
| Middle temporal gyrus | 0.172 | 0.330 | 0.258 | 0.141 | −0.221 | 0.217 | −0.267 | 0.127 | 0.118 | 0.507 | −0.346 | 0.045 |
| Superior temporal gyrus | 0.114 | 0.416 | 0.226 | 0.200 | −0.330 | 0.057 | −0.313 | 0.073 | 0.076 | 0.670 | −0.335 | 0.053 |
| Supramarginal gyrus | 0.036 | 0.899 | 0.285 | 0.102 | −0.246 | 0.161 | −0.334 | 0.053 | 0.049 | 0.784 | −0.407 | 0.017 |
| Precentral gyrus | 0.061 | 0.730 | 0.291 | 0.095 | −0.323 | 0.063 | −0.080 | 0.652 | 0.059 | 0.740 | −0.242 | 0.167 |
| Postcentral gyrus | 0.045 | 0.799 | 0.252 | 0.150 | −0.429 | 0.011 | −0.248 | 0.158 | 0.027 | 0.881 | −0.382 | 0.026 |
| Inferior frontal gyrus | −0.003 | 0.986 | 0.018 | 0.918 | −0.225 | 0.201 | −0.043 | 0.809 | 0.022 | 0.903 | −0.054 | 0.760 |
| Superior occipital gyrus | −0.050 | 0.779 | 0.242 | 0.168 | 0.110 | 0.537 | 0.251 | 0.152 | 0.171 | 0.335 | 0.249 | 0.155 |
Control region: the superior occipital gyrus is a region of interest through which the AF does not pass. Means and standard deviations for left and right arcuate tract volume, axial and radial diffusivity, and cortical thickness averaged across each hemisphere and within each gyral region are provided in Table I.
Bold italics mark significant P‐values.
Associations Between Verbal Fluency and Cortical Thickness
Verbal fluency scores showed significant correlations with regional variations in cortical thickness predominantly in left‐perisylvian regions. Uncorrected probability values and corresponding partial correlation coefficients showing regional associations between cortical thickness and verbal fluency measures are encoded in color in the left and right panels of Figure 5. Significant associations that survived permutation testing were observed within the superior temporal, pre‐, and postcentral gyri in the left hemisphere (corrected P values are provided in Table II). Analyses examining relationships between verbal fluency and cortical thickness averaged within each of the six gyral regions of interest were in agreement with the statistical mapping results, showing significant correlations for the left‐superior temporal gyrus, r(1,33) = 0.41, P < 0.02, the precentral, gyrus, r(1,33) = 0.38, P < 0.03, and postcentral gyrus, r(1,33) = 0.43, P < 0.02.
Figure 5.
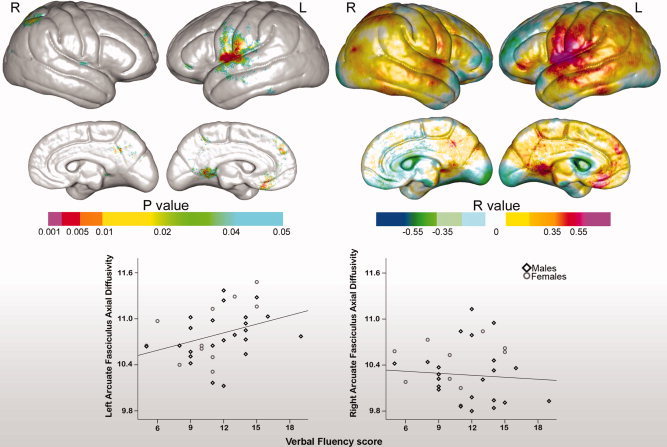
Top: Statistical maps showing relationships between verbal fluency and cortical thickness. Uncorrected probability values and partial correlation coefficients (age and gender controlled) encoded in color are shown in the left and right panels, respectively. Bottom: Relationships between verbal fluency scores and left and right hemisphere arcuate fasciculus axial diffusivity (×104 mm2/s).
Associations Between Verbal Fluency and AF Microstructure
Associations between arcuate FA and verbal fluency did not reach the threshold of statistical significance, r(1,31) = 0.27, P > 0.20 and r(1,31) = 0.05, P > 0.78 for the left and right arcuate, respectively. However, verbal fluency scores were significantly correlated with axial diffusivity in the left, r(1,31) = 0.37, P < 0.03, but not in the right hemisphere, r(1,31) = 0.02, P > 0.92 (Fig. 5, bottom panel). Radial diffusivity was not shown to associate with verbal fluency in either hemisphere, both P > 0.20.
Associations Between AF Tract Volume With Cortical Thickness and Verbal Fluency
Post hoc analyses performed to assess relationships between AF tract volume and cortical thickness sampled at each vertex were not significant at cortical location even without FDR correction (results not shown). Furthermore, AF tract volume did not show significant associations with cortical thickness averaged across each hemisphere or within each gyral ROI [partial correlation coefficients and probability values are provided in Table III]. Similarly, AF tract volume did not show significant associations with measures of verbal fluency, r(1,33) = −0.01, P > 0.97 and r(1,33) = −0.08, P > 0.62 for the left and right hemispheres, respectively.
DISCUSSION
White matter tracts transmit information between functionally connected cortical regions, although how measures of white matter connectivity relate to regional cortical gray matter structure within language networks have not been a focus of prior research. The AF is a major white matter tract with extensive projections that plays a role in language processing. Using in vivo structural and diffusion imaging data, we showed strong and highly localized associations between indices of AF white matter microstructure and gray matter macrostructure within cortical regions encompassing Broca's and Wernicke's area, ventral premotor/motor, primary auditory, and inferior parietal cortex and surrounding areas that were pronounced in the left hemisphere, particularly in the inferior frontal cortex. In principle, variations in FA may reflect differences in the degree of angular distribution of fibers, differences in the number of densely packed fibers, or differences in the degree myelination of fibers [Madler et al.,2008; Song et al.,2002]. Caution has been advised in interpreting axial and radial diffusivities in terms of underlying tissue characteristics [Wheeler‐Kingshott and Cercignani,2009], particularly in the context of crossing fibers, partial volume effects, or pathology. Nonetheless, given the large size of the AF and the inclusion only of normal subjects in this study, the more pronounced regional associations between cortical thickness and radial diffusivity may support variations in the degree of myelination as the basis for the observed relationships between cortical thickness and FA. Furthermore, increased cortical thickness may reflect microstructural features of the neuropil that are beneficial for neural processing [Lu et al.,2007; Narr et al.,2007]. Thus, positive associations between measures of white matter connectivity and cortical thickness in regions widely acknowledged to contribute to language function could prove useful as a biological marker for determining the functional integrity of language networks.
Language circuitry in the human brain is complex, and processing nodes appear to be both highly specialized to reflect a large degree of interactivity and interdependence and to involve multiple linguistic stages involving central brain systems as well as those in the sensory and motor periphery [Bookheimer,2002; Hickok,2009]. In the current study, verbal fluency, which requires both language comprehension and expression, did not correlate with arcuate FA. However, positive associations with AF axial diffusivity were observed in the left hemisphere, suggesting that some aspects of AF structure relate to this aspect of language function specifically. Again, with caution [Wheeler‐Kingshott and Cercignani,2009], these results might suggest that fiber number/density may more closely relate with task performance. In independent contrasts, results further suggest that regional increases in cortical thickness benefit language function. Specifically, as consistent with functional imaging studies showing the involvement of posterior prefrontal and temporal language‐related regions as well as premotor cortex in word‐generation tasks [Vigneau et al.,2006], significant associations between verbal fluency scores and cortical thickness were observed in left‐hemisphere perisylvian regions including the superior temporal and ventral pre‐ and postcentral gyrus, although associations in the left‐inferior frontal cortices did not survive statistical correction procedures.
Although this investigation is the first to our knowledge to map associations between indices of AF fiber integrity and gray matter structure in cortical regions relevant for language processing at high‐spatial resolution, one prior study has described positive relationships between cortical thickness and FA sampled arbitrarily from temporal lobe regions in an older adult sample [Wang et al.,2009]. Our findings showing positive associations between cortical thickness and AF microstructure in young adults thus complement and extend this prior research. Although our observations underscore that the AF serves as a major pathway linking anterior and posterior language regions in line with classic postmortem and prior DTI tractography studies, some recent diffusion data suggests that this fiber pathway may be separated into more specialized components. For example, though noting that ground truths from human postmortem studies remain lacking, some studies define the AF to include fibers of the SLF, while others describe the AF to be a functionally and anatomically distinct segment of the SLF [Makris et al.,2005] and/or indicate that fibers of the AF may subdivided into direct and indirect parallel pathways [Catani et al.,2005]. Using sophisticated methods, one recent investigation has also demonstrated that connections from BA 44 and 45 (Broca's area) may be differentiated to represent dorsal and ventral language‐related white matter pathways [Frey et al.,2008]. In particular, investigators have shown that the ventral language pathway, connecting posterior and anterior language regions, occurs via the extreme and/or external capsule and uncinate fasciculus [Frey et al.,2008]. These pathways may contribute to distinct aspects of language processing; the AF “dorsal pathway” for mapping sound to articulation and the “ventral pathway” for higher‐level language comprehension [Hickok and Poeppel,2004; Saur et al.,2008]. However, while prior studies suggest that language pathways may be anatomically differentiated when examined on a finer scale [Catani et al.,2005; Frey et al.,2008; Hong et al.,2009; Lawes et al.,2008; Makris et al.,2005], even those mapping more distinct connections of the AF show the majority of fibers overlap with the arching pathway of the AF linking anterior prefrontal and posterior superior temporal language regions as well as proximal cortical association areas [Bernal and Altman,2009].
The precise role of the AF towards language and other aspects of cognitive function remain under active investigation. However, much empirical evidence from complementary areas of research point to functional circuits for which projections of the AF can be linked [Hickok,2009]. For example, research on auditory‐motor integration in speech underscores the relation between posterior auditory‐related language areas (Wernicke's area) and anterior–inferior frontal areas related to speech production. In particular, the posterior left‐superior temporal gyrus has been implicated in phonological access during speech production, for example, [Hickok et al.,2000; Indefrey and Levelt,2004; Okada and Hickok,2006; Okada et al.,2003], and a left‐dominant auditory‐motor integration circuit for speech and associated functions has been shown to encompass the superior temporal sulcus, the pars opercularis portion of Broca's area, and an area close to the termination point of the SYLVIAN fissure at the parietal–temporal boundary (termed area Spt) [Hickok,2009; Hickok et al.,2003]. From a functional perspective, this network that includes the inferior parietal cortex, links with aspects of speech production in addition to phonological short‐term memory, vocabulary development, and auditory‐feedback control of speech. These functions are relevant for understanding pathological conditions such as stuttering, auditory hallucinations, and conduction aphasia, a syndrome associated with speech‐repetition deficits [Hickok,2009; Hickok and Poeppel,2000,2004,2007]. In clinical studies, lesions of the AF are associated with conduction aphasia specifically. However, speech‐repetition deficits may also be attributable to damage of the overlaying cortex [Anderson et al.,1999; Bernal and Ardila,2009], highlighting that the integrity of both gray matter and white matter is critical for language function. AF lesions are also associated with specific naming, reading, and writing impairments as well as other neurological abnormalities such as ideomotor apraxia [Bernal and Ardila,2009]. Thus, clinical/pathological data support the involvement of the AF in many features of language function as well as in other information processes consistent with findings from functional imaging and electrocortical studies that suggest complex circuitry and parallel processing within language networks [Catani and Mesulam,2008; Hickok,2009; Matsumoto et al.,2004].
Our findings of associations between verbal fluency scores with cortical thickness in perisylvian regions, but only with the axial diffusivity aspect of AF microstructure may reflect duplicity of function in language networks [Bernal and Ardila,2009]. Furthermore, specific language functions such as word generation may better associate with anatomically distinct components of the AF or with overlapping and/or ancillary language pathways as have been identified in recent diffusion studies [Catani et al.,2005; Frey et al.,2008; Hong et al.,2009; Lawes et al.,2008; Makris et al.,2005]. For example, when comparing AF tractography results with peak activation coordinates from prior functional imaging studies of phonology and lexical‐semantics, Glasser and Rilling (2008) showed that AF connections between the superior temporal gyrus and the frontal lobe overlapped with left‐lateralized phonological activations, whereas left‐lateralized lexical‐semantic activations overlapped terminations of the AF in the middle temporal gyrus.
Hemispheric dominance is an important characteristic of language function. Existing evidence suggests that AF connections between auditory association cortex and frontal regions are less pronounced in the right hemisphere [Glasser and Rilling,2008; Powell et al.,2006] findings that are consistent with our statistical mapping results showing stronger and more spatially diffuse relationships between AF fiber integrity and cortical thickness in the left with respect to the right hemisphere (Fig. 2). Several prior studies have demonstrated asymmetries of AF microstructure such as tract size, fiber density FA, and/or with regard to fiber trajectories, for example [Buchel et al.,2004; Catani et al.,2007; Glasser and Rilling,2008; Lebel and Beaulieu,2009; Nucifora et al.,2005; Parker et al.,2005]. Our results showing significant leftward asymmetries of AF axial and radial diffusivity and trends for larger mean tract volumes in the left hemisphere are compatible with these observations. As mentioned earlier, AF pathways in the left and right hemisphere may be responsive to distinct aspects of language, where, for example, right hemisphere AF connections may be more involved in processing prosodic information [Glasser and Rilling,2008]. Others have also shown increased FA in fibers arising from Broca's area (left BA 44/45) in the left‐inferior prefrontal cortex, but not from its right‐hemisphere homologue in association with better grammar learning in healthy subjects [Floel et al.,2009]. In the present study, significant arcuate FA‐cortical thickness correlations in the inferior prefrontal cortex were only observed in the left hemisphere adding further support for differences in the connectivity patterns of the AF between hemispheres. Findings also resonate with recent evidence suggesting that the evolution of the AF has specific relevance to language specialization [Glasser and Rilling,2008].
In spite of using sophisticated imaging analysis methods, several limitations that are relevant to the current research should be noted. First, although diffusion imaging has proved to be extremely useful tool for characterizing the anatomy of white matter pathways in the human brain, the lower spatial resolution of DTI data may increase the potential for partial volume effects. Second, although more sensitive methods have been developed that may allow the determination of primary and secondary fiber directions that may better resolve the anatomy of specific white matter tracts [Behrens et al.,2007], it is still not possible to fully resolve crossing fibers at the voxel level. Third, variations in tractography results between studies may arise from the different strategies for fiber tracking and/or from operator bias. Although several studies have suggested that the AF is more differentiated that originally thought, the present study was focused on mapping the classical and more prominent arching pathway of the AF, which has also been described as the temporal component of the SLF [Wakana et al.,2004,2007], with high reproducibility to determine correlations with cortical gray matter structure in language networks. However, it is possible that subcomponents of the AF that differ according to their end points in frontal (Broca's area versus BA 6), premotor, parietal (supramarginal and angular gyral areas), and superior and middle temporal cortices may better associate with particular aspects of language processing [Bernal and Altman,2009; Makris et al.,2005]. Finally, although males and females did not show differences in the slopes of the relationships between arcuate FA and cortical thickness, studies including larger samples may be required to detect more subtle differences in the spatial pattern or magnitude of these relationships across sex.
In conclusion, the presence of strong topographical associations between indices of FA connectivity with gray matter thickness in frontal, temporal, and parietal language‐related regions supports that these pathways are functionally interrelated and are influenced by structural integrity of gray and white matter across language networks. Furthermore, associations between cortical thickness and aspects of AF microstructure with word generation are in line with separate observations showing that increased cortical thickness as well as greater AF connectivity relate to language acquisition and skill [Floel et al.,2009; Lu et al.,2007]. Understanding the relationships between measures of white matter fiber and gray matter integrity may provide new insight toward understanding the underlying disturbances of neurodevelopmental disorders such as dyslexia or schizophrenia for which language dysfunction represents a primary feature.
REFERENCES
- Anderson MJ, Braak CJ ( 2003): Permutation tests for multi‐factorial analysis of variance. J Stat Comput Simul 73: 85–113. [Google Scholar]
- Anderson JM, Gilmore R, Roper S, Crosson B, Bauer RM, Nadeau S, Beversdorf DQ, Cibula J, Rogish M III, Kortencamp S, Hughes JD, Gonzalez Rothi LJ, Heilman KM ( 1999): Conduction aphasia and the arcuate fasciculus: A reexamination of the Wernicke‐Geschwind model. Brain Lang 70: 1–12. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW ( 2007): Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage 34: 144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal B, Altman N ( 2009): The connectivity of the superior longitudinal fasciculus: A tractography DTI study. Magn Reson Imaging 2: 217–225. [DOI] [PubMed] [Google Scholar]
- Bernal B, Ardila A ( 2009): The role of the arcuate fasciculus in conduction aphasia. Brain 132: 2309–2316. [DOI] [PubMed] [Google Scholar]
- Bookheimer S ( 2002): Functional MRI of language: New approaches to understanding the cortical organization of semantic processing. Annu Rev Neurosci 25: 151–188. [DOI] [PubMed] [Google Scholar]
- Buchel C, Raedler T, Sommer M, Sach M, Weiller C, Koch MA ( 2004): White matter asymmetry in the human brain: A diffusion tensor MRI study. Cereb Cortex 14: 945–951. [DOI] [PubMed] [Google Scholar]
- Catani M, Mesulam M ( 2008): The arcuate fasciculus and the disconnection theme in language and aphasia: History and current state. Cortex 44: 953–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Jones DK, Ffytche DH ( 2005): Perisylvian language networks of the human brain. Ann Neurol 57: 8–16. [DOI] [PubMed] [Google Scholar]
- Catani M, Allin MP, Husain M, Pugliese L, Mesulam MM, Murray RM, Jones DK ( 2007): Symmetries in human brain language pathways correlate with verbal recall. Proc Natl Acad Sci USA 104: 17163–17168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW ( 1994): Structured Clinical Interview for DSM‐IV Avis I Disorders. New York: New York State Psychiatric Institute, Biometrics Research. [Google Scholar]
- Floel A, de Vries MH, Scholz J, Breitenstein C, Johansen‐Berg H ( 2009): White matter integrity in the vicinity of Broca's area predicts grammar learning success. Neuroimage 4: 1974–1981. [DOI] [PubMed] [Google Scholar]
- Frey S, Campbell JS, Pike GB, Petrides M ( 2008): Dissociating the human language pathways with high angular resolution diffusion fiber tractography. J Neurosci 28: 11435–11444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind N ( 1970): The organization of language and the brain. Science 170: 940–944. [DOI] [PubMed] [Google Scholar]
- Glasser MF, Rilling JK ( 2008): DTI tractography of the human brain's language pathways. Cereb Cortex 18: 2471–2482. [DOI] [PubMed] [Google Scholar]
- Heidemann RM, Griswold MA, Kiefer B, Nittka M, Wang J, Jellus V, Jakob PM ( 2003): Resolution enhancement in lung 1H imaging using parallel imaging methods. Magn Reson Med 49: 391–394. [DOI] [PubMed] [Google Scholar]
- Hickok G ( 2009): The functional neuroanatomy of language. Phys Life Rev 6: 121–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, Poeppel D ( 2000): Towards a functional neuroanatomy of speech perception. Trends Cogn Sci 4: 131–138. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D ( 2004): Dorsal and ventral streams: A framework for understanding aspects of the functional anatomy of language. Cognition 92: 67–99. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D ( 2007): The cortical organization of speech processing. Nat Rev Neurosci 8: 393–402. [DOI] [PubMed] [Google Scholar]
- Hickok G, Erhard P, Kassubek J, Helms‐Tillery AK, Naeve‐Velguth S, Strupp JP, Strick PL, Ugurbil K ( 2000): A functional magnetic resonance imaging study of the role of left posterior superior temporal gyrus in speech production: Implications for the explanation of conduction aphasia. Neurosci Lett 287: 156–160. [DOI] [PubMed] [Google Scholar]
- Hickok G, Buchsbaum B, Humphries C, Muftuler T ( 2003): Auditory‐motor interaction revealed by fMRI: Speech, music, and working memory in area Spt. J Cogn Neurosci 15: 673–682. [DOI] [PubMed] [Google Scholar]
- Hoeft F, Ueno T, Reiss AL, Meyler A, Whitfield‐Gabrieli S, Glover GH, Keller TA, Kobayashi N, Mazaika P, Jo B, Just MA, Gabrieli JD ( 2007): Prediction of children's reading skills using behavioral, functional, and structural neuroimaging measures. Behav Neurosci 121: 602–613. [DOI] [PubMed] [Google Scholar]
- Hong JH, Kim SH, Ahn SH, Jang SH ( 2009): The anatomical location of the arcuate fasciculus in the human brain: A diffusion tensor tractography study. Brain Res Bull 80: 52–55. [DOI] [PubMed] [Google Scholar]
- Indefrey P, Levelt WJ ( 2004): The spatial and temporal signatures of word production components. Cognition 92: 101–144. [DOI] [PubMed] [Google Scholar]
- Jezzard P, Barnett AS, Pierpaoli C ( 1998): Characterization of and correction for eddy current artifacts in echo planar diffusion imaging. Magn Reson Med 39: 801–812. [DOI] [PubMed] [Google Scholar]
- Jiang H, van Zijl PC, Kim J, Pearlson GD, Mori S ( 2006): DtiStudio: Resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed 81: 106–116. [DOI] [PubMed] [Google Scholar]
- Jung RE, Haier RJ ( 2007): The Parieto‐Frontal Integration Theory (P‐FIT) of intelligence: Converging neuroimaging evidence. Behav Brain Sci 30: 135–154; discussion 154–187. [DOI] [PubMed] [Google Scholar]
- Kochunov P, Thompson PM, Lancaster JL, Bartzokis G, Smith S, Coyle T, Royall DR, Laird A, Fox PT ( 2007): Relationship between white matter fractional anisotropy and other indices of cerebral health in normal aging: Tract‐based spatial statistics study of aging. Neuroimage 35: 478–487. [DOI] [PubMed] [Google Scholar]
- Lawes IN, Barrick TR, Murugam V, Spierings N, Evans DR, Song M, Clark CA ( 2008): Atlas‐based segmentation of white matter tracts of the human brain using diffusion tensor tractography and comparison with classical dissection. Neuroimage 39: 62–79. [DOI] [PubMed] [Google Scholar]
- Lebel C, Beaulieu C ( 2009): Lateralization of the arcuate fasciculus from childhood to adulthood and its relation to cognitive abilities in children. Hum Brain Mapp 11: 3563–3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C ( 2008): Microstructural maturation of the human brain from childhood to adulthood. Neuroimage 40: 1044–1055. [DOI] [PubMed] [Google Scholar]
- Lezak M ( 1997): Neuropsychological Assessment, 3rd ed. New York: Oxford University Press. [Google Scholar]
- Lerch JP, Worsley K, Shaw WP, Greenstein DK, Lenroot RK, Giedd J, Evans AC ( 2006): Mapping anatomical correlations across cerebral cortex (MACACC) using cortical thickness from MRI. Neuroimage 31: 993–1003. [DOI] [PubMed] [Google Scholar]
- Loonstra AS, Tarlow AR, Sellers AH ( 2001): COWAT metanorms across age, education, and gender. Appl Neuropsychol 8: 161–166. [DOI] [PubMed] [Google Scholar]
- Lu L, Leonard C, Thompson P, Kan E, Jolley J, Welcome S, Toga A, Sowell E ( 2007): Normal developmental changes in inferior frontal gray matter are associated with improvement in phonological processing: A longitudinal MRI analysis. Cereb Cortex 17: 1092–1099. [DOI] [PubMed] [Google Scholar]
- Luders E, Narr KL, Thompson PM, Rex DE, Woods RP, Deluca H, Jancke L, Toga AW ( 2006): Gender effects on cortical thickness and the influence of scaling. Hum Brain Mapp 27: 314–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madler B, Drabycz SA, Kolind SH, Whittall KP, MacKay AL ( 2008): Is diffusion anisotropy an accurate monitor of myelination? Correlation of multicomponent T2 relaxation and diffusion tensor anisotropy in human brain. Magn Reson Imaging 26: 874–888. [DOI] [PubMed] [Google Scholar]
- Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS Jr., Pandya DN ( 2005): Segmentation of subcomponents within the superior longitudinal fascicle in humans: A quantitative, in vivo, DT‐MRI study. Cereb Cortex 15: 854–869. [DOI] [PubMed] [Google Scholar]
- Matsumoto R, Nair DR, LaPresto E, Najm I, Bingaman W, Shibasaki H, Luders HO ( 2004): Functional connectivity in the human language system: A cortico‐cortical evoked potential study. Brain 127: 2316–2330. [DOI] [PubMed] [Google Scholar]
- Mori S, van Zijl PC ( 2002): Fiber tracking: Principles and strategies—A technical review. NMR Biomed 15: 468–480. [DOI] [PubMed] [Google Scholar]
- Mori S, Crain BJ, Chacko VP, van Zijl PC ( 1999): Three‐dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol 45: 265–269. [DOI] [PubMed] [Google Scholar]
- Narr KL, Woods RP, Thompson PM, Szeszko P, Robinson D, Dimtcheva T, Gurbani M, Toga AW, Bilder RM ( 2007): Relationships between IQ and regional cortical gray matter thickness in healthy adults. Cereb Cortex 17: 2163–2171. [DOI] [PubMed] [Google Scholar]
- Newman LM, Trivedi MA, Bendlin BB, Ries ML, Johnson SC ( 2007): The relationship between gray matter morphometry and neuropsychological performance in a large sample of cognitively healthy adults. Brain Imaging Behav 1: 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nucifora PG, Verma R, Melhem ER, Gur RE, Gur RC ( 2005): Leftward asymmetry in relative fiber density of the arcuate fasciculus. Neuroreport 16: 791–794. [DOI] [PubMed] [Google Scholar]
- Okada K, Hickok G ( 2006): Left posterior auditory‐related cortices participate both in speech perception and speech production: Neural overlap revealed by fMRI. Brain Lang 98: 112–117. [DOI] [PubMed] [Google Scholar]
- Okada K, Smith KR, Humphries C, Hickok G ( 2003): Word length modulates neural activity in auditory cortex during covert object naming. Neuroreport 14: 2323–2326. [DOI] [PubMed] [Google Scholar]
- Okada T, Miki Y, Fushimi Y, Hanakawa T, Kanagaki M, Yamamoto A, Urayama S, Fukuyama H, Hiraoka M, Togashi K ( 2006): Diffusion‐tensor fiber tractography: Intraindividual comparison of 3.0‐T and 1.5‐T MR imaging. Radiology 238: 668–678. [DOI] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologica 9: 91–113. [DOI] [PubMed] [Google Scholar]
- Parker GJ, Luzzi S, Alexander DC, Wheeler‐Kingshott CA, Ciccarelli O, Lambon Ralph MA ( 2005): Lateralization of ventral and dorsal auditory‐language pathways in the human brain. Neuroimage 24: 656–666. [DOI] [PubMed] [Google Scholar]
- Phillips OR, Nuechterlein KH, Clark KA, Hamilton LS, Asarnow RF, Hageman NS, Toga AW, Narr KL ( 2009): Fiber tractography reveals disruption of temporal lobe white matter tracts in schizophrenia. Schizophr Res 107: 30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli C, Basser PJ ( 1996): Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med 36: 893–906. [DOI] [PubMed] [Google Scholar]
- Powell HW, Parker GJ, Alexander DC, Symms MR, Boulby PA, Wheeler‐Kingshott CA, Barker GJ, Noppeney U, Koepp MJ, Duncan JS ( 2006): Hemispheric asymmetries in language‐related pathways: A combined functional MRI and tractography study. Neuroimage 32: 388–399. [DOI] [PubMed] [Google Scholar]
- Reese TG, Heid O, Weisskoff RM, Wedeen VJ ( 2003): Reduction of eddy‐current‐induced distortion in diffusion MRI using a twice‐refocused spin echo. Magn Reson Med 49: 177–182. [DOI] [PubMed] [Google Scholar]
- Saur D, Kreher BW, Schnell S, Kummerer D, Kellmeyer P, Vry MS, Umarova R, Musso M, Glauche V, Abel S, Huber W, Rijntjes M, Hennig J, Weiller C ( 2008): Ventral and dorsal pathways for language. Proc Natl Acad Sci USA 105: 18035–18040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK ( 2005): Cognitive functions correlate with white matter architecture in a normal pediatric population: A diffusion tensor MRI study. Hum Brain Mapp 26: 139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattuck DW, Sandor‐Leahy SR, Schaper KA, Rottenberg DA, Leahy RM ( 2001): Magnetic resonance image tissue classification using a partial volume model. Neuroimage 13: 856–876. [DOI] [PubMed] [Google Scholar]
- Shattuck DW, Mirza M, Adisetiyo V, Hojatkashani C, Salamon G, Narr KL, Poldrack RA, Bilder RM, Toga AW ( 2008): Construction of a 3D probabilistic atlas of human cortical structures. Neuroimage 39: 1064–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sled JG, Pike GB ( 1998): Standing‐wave and RF penetration artifacts caused by elliptic geometry: An electrodynamic analysis of MRI. IEEE Trans Med Imaging 17: 653–662. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH ( 2002): Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 17: 1429–1436. [DOI] [PubMed] [Google Scholar]
- Storey J ( 2002): A direct approach to false discovery rates. R Stat Soc Ser B 64: 479–496. [Google Scholar]
- Thompson PM, Hayashi KM, Sowell ER, Gogtay N, Giedd JN, Rapoport JL, de Zubicaray GI, Janke AL, Rose SE, Semple J, Doddrell DM, Wang Y, van Erp TG, Cannon TD, Toga AW ( 2004): Mapping cortical change in Alzheimer's disease, brain development, and schizophrenia. Neuroimage 23 ( Suppl 1): S2–S18. [DOI] [PubMed] [Google Scholar]
- Turken A, Whitfield‐Gabrieli S, Bammer R, Baldo JV, Dronkers NF, Gabrieli JD ( 2008): Cognitive processing speed and the structure of white matter pathways: Convergent evidence from normal variation and lesion studies. Neuroimage 42: 1032–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Herve PY, Duffau H, Crivello F, Houde O, Mazoyer B, Tzourio‐Mazoyer N ( 2006): Meta‐analyzing left hemisphere language areas: Phonology, semantics, and sentence processing. Neuroimage 30: 1414–1432. [DOI] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae‐Poetscher LM, van Zijl PC, Mori S ( 2004): Fiber tract‐based atlas of human white matter anatomy. Radiology 230: 77–87. [DOI] [PubMed] [Google Scholar]
- Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, Hua K, Zhang J, Jiang H, Dubey P, Blitz A, van Zijl P, Mori S ( 2007): Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage 36: 630–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Goldstein FC, Veledar E, Levey AI, Lah JJ, Meltzer CC, Holder CA, Mao H ( 2009): Alterations in cortical thickness and white matter integrity in mild cognitive impairment measured by whole‐brain cortical thickness mapping and diffusion tensor imaging. AJNR Am J Neuroradiol 30: 893–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler‐Kingshott CA, Cercignani M ( 2009): About “axial” and “radial” diffusivities. Magn Reson Med 61: 1255–1260. [DOI] [PubMed] [Google Scholar]
- White T, Nelson M, Lim KO ( 2008): Diffusion tensor imaging in psychiatric disorders. Top Magn Reson Imaging 19: 97–109. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC ( 1998a) Automated image registration. I. General methods and intrasubject, intramodality validation. J Comput Assist Tomogr 22: 139–152. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Watson JD, Sicotte NL, Mazziotta JC ( 1998b): Automated image registration. II. Intersubject validation of linear and nonlinear models. J Comput Assist Tomogr 22, 153–165. [DOI] [PubMed] [Google Scholar]


