Abstract
The influence of mast cells upon aberrant wound repair and excessive fibrosis has supportive evidence, but the mechanism for these mast cell activities is unclear. It is proposed that heterocellular gap junctional intercellular communication (GJIC) between fibroblasts and mast cells directs some fibroblast activities. An in vitro model was used employing a rodent derived peritoneal mast cell line (RMC-1) and human dermal derived fibroblasts. The influence of the expression of the gap junction channel structural protein, connexin 43 (Cx-43) on heterocellular GJIC, the expression of microtubule β-tubulin and microfilament α smooth muscle actin (SMA) were investigated. The knockdown of Cx-43 by siRNA in RMC-1 cells completely blocked GJIC between RMC-1 cells. SiRNA knockdown of Cx-43 within fibroblasts only dampened GJIC between fibroblasts. It appears Cx-43 is the only expressed connexin in RMC-1 cells. Fibroblasts express other connexins that participate in GJIC between fibroblasts in the absence of Cx-43 expression. Heterocellular GJIC between RMC-1 cells and fibroblasts transformed fibroblasts into myofibroblasts, expressing α SMA within cytoplasmic stress fibers. The knockdown of Cx-43 in RMC-1 cells increased β-tubulin expression, but its knockdown in fibroblasts reduced β-tubulin expression. Knocking down the expression of Cx-43 in fibroblasts limited α SMA expression. Cx-43 participation is critical for heterocellular GJIC between mast cells and fibroblasts, which may herald a novel direction for controlling fibrosis.
Keywords: Wound healing, Hypertrophic scar, Keloid, Fibrosis
INTRODUCTION
Gap junction intercellular communication (GJIC) plays a key role in numerous biological processes, including wound repair and fibrosis. During wound closure by reepithelialization, there is a temporary loss of GJIC between keratinocytes, which is required for optimizing their migration over the open wound surface [Mori et al., 2006]. Fibroblasts within wound granulation tissue have gap junction hemichannels on their surface [Gabbiani et al., 1978]. Gap junctions are intercellular, gated membrane channels which facilitate the passage of molecules less than 1,000 MW between cells [Kumar and Gilula, 1996]. The trans-membrane channel is composed of hemichannel structures made up of 6 connexin (Cx) proteins embedded within the plasma membrane. The intercellular channel is made up of half the hemichannel structure contributed by each coupled cell. The connexin family of proteins has more than 20 members, where connexin 43 (Cx-43) is the most commonly expressed. GJIC usually occurs between like cells, but can occur between unlike cells. Hemichannels with Cx-43 are located on the surface of both mouse mast cells [Vliagoftis et al., 1999] and fibroblasts. By transmission electron microscopy gap junctions are visualized between mast cells and fibroblasts in the developing avian eye [Oliani et al., 1995]. Human mast cells make GJIC with fibroblasts in populated collagen lattices [Moyer et al., 2004].
A relatively ignored cellular player in wound healing is the mast cell. Although most often studied in the context of allergic and general inflammatory responses, mast cell involvement in wound healing and fibrosis has not been commonly investigated, however, a better understanding of their role in repair may prove to direct new therapeutic approaches in promoting repair and/or controlling fibrosis. Mast cells are known to exist in mucous membranes and connective tissues, including skin [Kube et al., 1998; Kuther et al., 1998]. The best known consequence of mast cell activation, through a variety of stimuli, is their degranulation, where a number of biological mediators are released into the extracellular space [Bradding, 1996].
The mast cell appears in all phases of the repair process. In the lag or inflammatory phase of repair, following injury, a five-fold increase in mast cell numbers around the border of the wound is correlated with the upregulation of monocyte chemoattractant protein-1 [Trautmann et al., 2000] and TGF-β [Gruber et al., 1994]. Mast cells aid in platelet activation and aggregation and extravascular deposition of fibrin by releasing platelet activating factor and IL-1 as well as IL-8 [Kauhanen et al., 1998; Mekori and Galli, 1990]. During the proliferative phase of repair, mast cells influence fibroblast deposition of the new connective tissue matrix that makes up granulation tissue. They stimulate chemotaxis, migration, phenotypic differentiation, and synthetic activities of fibroblasts through release of substances like histamine [Kupietzky and Levi-Schaffer, 1996; Russel et al., 1977], fibrogenic cytokines [Kovacs, 1991], proteinases tryptase and chymase [Abe et al., 1998; Gruber et al., 1997], and growth factors [Bressler et al., 1997; Qu et al., 1998]. The growth factors FGF and TGF-β, along with mast cell-released cytokines (IL-1, IL-4, and IL-6), influence fibroblast phenotype that promotes their transformation into myofibroblasts [Hebda et al., 1993; Moulin et al., 1998]. Mast cells are involved in the remodeling phase of repair by promoting maturation of granulation tissue into scar tissue [Nishikori et al., 1998]. They are also a common feature of excessive scaring conditions, such as hypertrophic scar [Kischer and Bailey, 1972]. In the other side of impaired wound healing, the continually activated chronic degranulation of mast cell populations has the potential to contribute to chronic wounds and aberrant scarring [Noli and Miolo, 2001]. A recent twist to the efficient synergism between mast cells and fibroblasts is the discovery of the formation of heterocellular GJIC developing between mast cells and fibroblasts [Moyer et al., 2004].
An established human mast cell line (HMC-1) [Butterfield et al., 1988] co-cultured with human fibroblasts in collagen lattices promotes lattice contraction through GJIC [Moyer et al., 2004]. Unfortunately, HMC-1 cells fail to communicate with fibroblasts in monolayer culture via GJIC [Au et al., 2007]. When fibroblasts in monolayer culture are co-cultured with a newly established rat mast cell line, altered fibroblast behavioral and functional changes occur, involving heterocellular GJIC. The importance of Cx-43 expression in GJIC between mast cells and fibroblasts is the focus of this study using an in vitro tissue culture system to investigate the expression of Cx-43 in fibroblasts and cultured mast cells. The influence of heterocellular GJIC between these cell types on fibroblast physiology is documented. If specific gap junctional proteins can be altered in a targeted manner, a potentially new approach for the therapeutic intervention in modulating aberrant wound healing and scarring is possible.
RESULTS
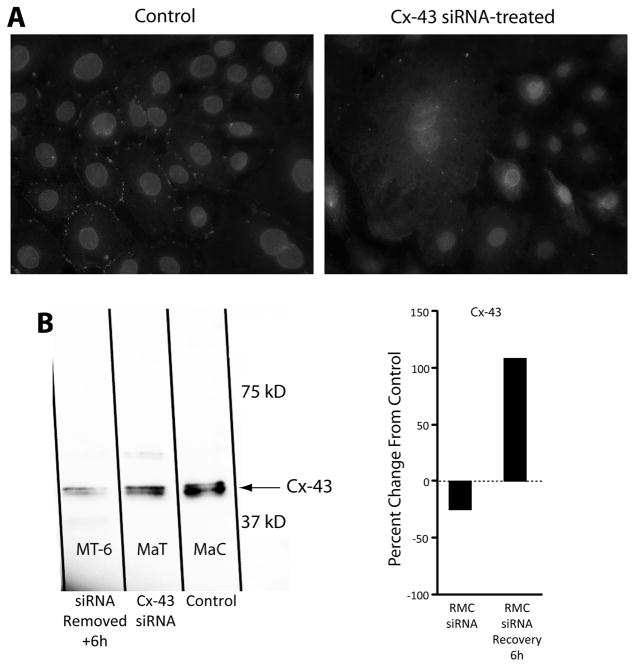
GJIC between RMC-1 cells derived from a rat peritoneal cavity mast cell line grown in monolayer was documented by the scrape loading technique. RMC-1 cells pretreated with siRNA against Cx-43 showed impaired GJIC as compared to untreated, control RMC-1 cells. Cx-43 was expressed in control RMC-1 cells and Cx-43 was successfully knocked down in RMC-1 cells, by siRNA treatment (Fig. 1A). As expected, the majority of Cx-43 expressed in untreated, control RMC-1 cells was localized on the cell’s plasma membrane between cells. It was assumed that the staining represents functioning hemichannel gap junctional complexes (Fig. 1A, left). With RMC-1 cells immuno-histology investigation of the presence of other members of the gap junction protein family, Cx-32 and Cx-26 was negative (data not shown). Treatment of RMC-1 cells with siRNA directed to Cx-43 effectively eliminated membrane-localized Cx-43 expression, which represented the potential abolition of GJIC between RMC-1 cells (Fig. 1A, right). Furthermore, residual native Cx-43 was noted to be sequestered within the cells’ cytosol, suggesting that knocking down Cx-43 expression caused a defect in localization of Cx-43 to the cells’ plasma membrane, and an accumulation of residual Cx-43 within the cell’s cytoplasm. It appears that in the absence of new Cx-43 synthesis, a pool of Cx-43 which was not transported to the cell’s surface is retained within the cytoplasm. A Western blot of cell lysates from siRNA treated RMC-1 cells demonstrated a diminished protein band density for Cx-43 compared to the protein band of untreated control cell lysate (Fig. 1B). As expected like RMC-1 cells, monolayer cultured fibroblasts, reaching confluence on glass cover slips, also showed Cx-43 localized between cells (data not shown). A Western blot of siRNA treated fibroblast lysates demonstrated a diminished protein band density for Cx-43 compared to the protein band to untreated control cell lysate (data not shown). From differences in protein band densities, it was calculated that siRNA treated fibroblasts had a 70% reduction in Cx-43 protein levels.
Figure 1.
Cx-43 siRNA on its expression in mast cells. Panel A shows immune-histology of Cx-43 localization in RMC-1 cells, showing in the control (left) staining limited to the periphery of the cells, where Cx-43 staining is sequestered in the intercellular space. RMC-1 cells treated with Cx-43 siRNA (right) have Cx-43 localization within the cytoplasm of some cells. Staining is absent from the cell’s plasma membrane. Panel B shows shows a Western blot of RMC-1 lysate from untreated controls and RMC-1 cells treated with Cx-43 siRNA for 3 days. Cx-43 was successfully knocked down by siRNA and subsequently recovered and was upregulated following siRNA removal, as measured by percent change in density from the control (right).
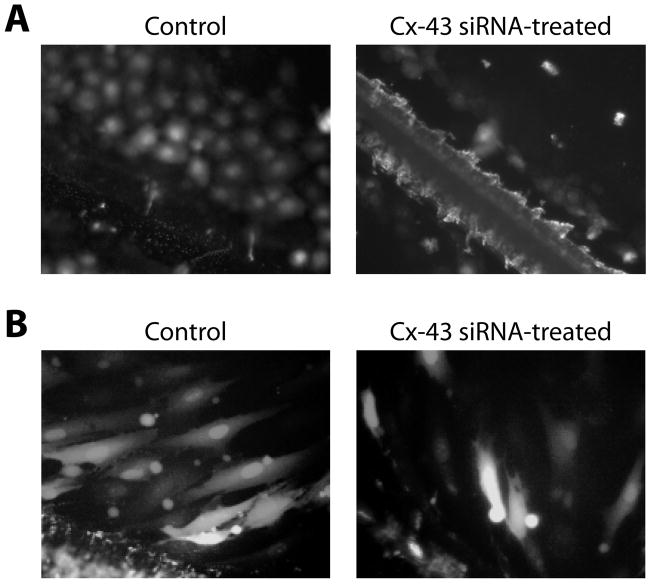
The scrape-loading technique confirmed Cx-43 significance in GJIC within monolayer cultures of RMC-1 cells and human dermal fibroblasts. A robust passage of dye via GJIC between untreated RMC-1 cells was demonstrated (Fig. 2A, left). The calculated coupling index for control RMC-1 cells was 8.9 (fluorescent green cells/ fluorescent red cells). Dye passage was absent in siRNA-treated RMC-1 cells (Fig. 2A, right) with a calculated coupling index of 0.7. Compared to controls the statistical significant was p > 0.0001 and a mean difference of 8.2. It supports the notion that GJIC between RMC-1 cells was dependent on hemichannels with the exclusive expression of Cx-43 protein. Determining gap junction communications between human dermal fibroblasts by scrape loading, presented in Figure 2B, showed a reduction in GJIC between fibroblasts treated with Cx-43 siRNA. GJIC was largely but, unlike RMC-1 cells, not completely dependent upon Cx-43 expression. Note that approximately 3 rows of cells out from the scrape line in the control group accumulated dye (Fig. 2B left). The accumulation of dye in siRNA treated fibroblasts was minimal (Fig. 2B right). By the scrape loading assay the coupling index for siRNA-treated fibroblasts was 3.1, while the coupling index for control fibroblasts was 7.2, that difference was significant with p > 0.0001 and a mean difference = 4.1, which was half of the mean difference compared to RMC-1 cells. Because of the incomplete loss of GJIC in Cx-43 siRNA-treated fibroblasts, other members of the Cx protein family appeared to be involved in generating GJIC between fibroblasts. By immuno-histology these CRL-2522, human dermal fibroblasts have Cx-32 (data not shown), which is in agreement with previous reported findings [Hinz et al., 2007].
Figure 2.
The demonstration of GJIC in RMC-1 mast cells and fibroblasts treated with Cx-43 siRNA. Panel A shows scrape injured RMC-1 cells with the scrape line on the lower left corner panels. On the left LY dye has passed through more than 4 rows of cells. On the right the accumulation of dye is limited to the cells in the scrape injured line. No dye has passed to cells beyond the scrape injury line. Panel B shows fibroblasts scrape loading experiments with scrape line on the bottom left of the figures. The panel on the left of untreated fibroblasts shows many fluorescent fibroblasts far removed from the scrape line that received dye from cells by GJIC. On the right are scrape injured cells from Cx-43 siRNA treated fibroblasts demonstrating reduced GJIC compared to the control fibroblasts but some dye transfer through GJIC as compared to RMC-1 cells.
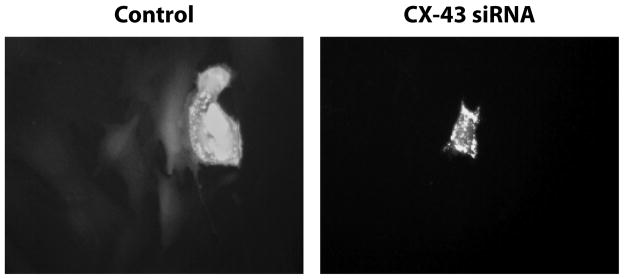
In prior studies, the incorporation of human mast cells (HMC-1 cells) with human fibroblasts in co-cultured collagen lattices showed enhancement of lattice contraction, which was dependent upon heterocellular GJIC [Moyer et al., 2004]. However, in monolayer culture HMC-1 cells failed to form heterocellular gap junctions with fibroblasts [Au et al., 2007]. Freshly isolated rat peritoneal cavity derived mast cells formed gap junctions with human dermal fibroblasts in monolayer culture [Au et al., 2007]. The action of eliminating Cx-43 expression in RMC-1 cells and their ability to form GJIC with fibroblasts was studied with RMC-1 cell paratroopers. RMC-1 paratroopers, dye loaded mast cells, were shown to communicate through GJIC with human fibroblasts in a Cx-43-dependent manner (Fig. 3). Untreated (control) RMC-1 paratrooper cells, which are smaller, appearing fluorescently brighter; passed dye to underlying fibroblasts, which were larger, spindle shaped cells generating less dye fluorescence intensity (Fig. 3, left). SiRNA-treated RMC-1 paratroopers, having a similar morphology with untreated RMC-1 paratroopers, showed intense fluorescence, but were unable to pass detectable dye to underlying fibroblasts (Fig. 3, right). Cx-43 siRNA pretreated RMC-1 paratroopers were unable to form heterocellular GJIC with underlying fibroblasts, confirming that the absence of Cx-43 expression in RMC-1 cells prevented the formation of Cx-43 hemichannels (Fig. 1A).
Figure 3.
Demonstration of RMC-1 dye loaded paratroopers GJIC with fibroblasts is dependent upon Cx-43 expression. Untreated RMC-1 cells or RMC-1 cells pretreated with Cx-43 siRNA loaded with Calcein AM (RMC-1 paratroopers) were released by trypsinization and a suspension of these cells added to a confluent monolayer of fibroblasts. On the left, control, an untreated RMC-1 cell appearing as a brightly-stained paratrooper in the upper half of the panel has passed dye to spindle shaped fibroblasts below. A fibroblast coupled to a RMC-1 paratrooper that receives dye can subsequently pass that dye to coupled fibroblast via GJIC. In the same paratrooper experiment RMC-1 cells pretreated with Cx-43 siRNA prior to dye-loading (right) did not pass dye to fibroblasts. Fluorescent dye is only localized in the RMC-1 paratrooper.
Next, alterations in the expression of selected proteins within fibroblasts in the absence of heterocellular GJIC with RMC-1 cells were investigated. Changes in fibroblast activities in response to heterocellular GJIC with HMC-1 cells in collagen lattices [Moyer et al., 2004] suggested changes in protein synthesis within fibroblasts in response to forming heterocellular GJIC with RMC-1 cells. An icon of fibrosis is the myofibroblast, identified by the expression of the α SMA isoform of actin within cytoplasmic stress fibers [Laird et al., 1991]. Untreated RMC-1 cells co-cultured with fibroblasts were evaluated for αSMA expression in fibroblasts by immuno-fluorescent microscopy. RMC-1 cells were found not to express αSMA. CRL 2522 fibroblasts co-cultured with untreated RMC-1 cells for 4 hours showed 16 out of 44 cells (36%) expressed αSMA within cytoplasmic stress fibers; but when the co-culture incubation was continued for an additional 20 hours, all fibroblasts expressed αSMA (100%) (Fig. 4, left). It suggested that heterocellular GJIC between fibroblasts and RMC-1 cells promoted αSMA expression in fibroblasts. At 4 hours the co-culture of fibroblasts with Cx-43-siRNA treated RMC-1 cells showed 14 of 39 fibroblasts (35%) expressing αSMA, which was identical to controls (Fig. 4, right). At 24 hours the co-culture of fibroblasts with pretreated Cx-43-siRNA RMC-1 cells showed 12 of 41 cells (29%) expressing αSMA, demonstrating no significant change in αSMA expression over a 24 hour period in the absence of heterocellular GJIC with RMC-1 cells (Fig. 4, right). Changes in protein synthesis by cultured human fibroblasts in response to reduction in Cx-43 expression were investigated. Cell lysates from confluent human dermal fibroblast monocultures show increased αSMA synthesis, the hallmark of the myofibroblast (Fig. 5). When Cx-43 expression was knocked down by Cx-43-siRNA treatment of human dermal fibroblasts, αSMA expression was decreased in treated fibroblast lysates (Fig. 5). By Western blot analysis a 40% reduction in αSMA expression was calculated in Cx-43-siRNA treated fibroblasts.
Figure 4.
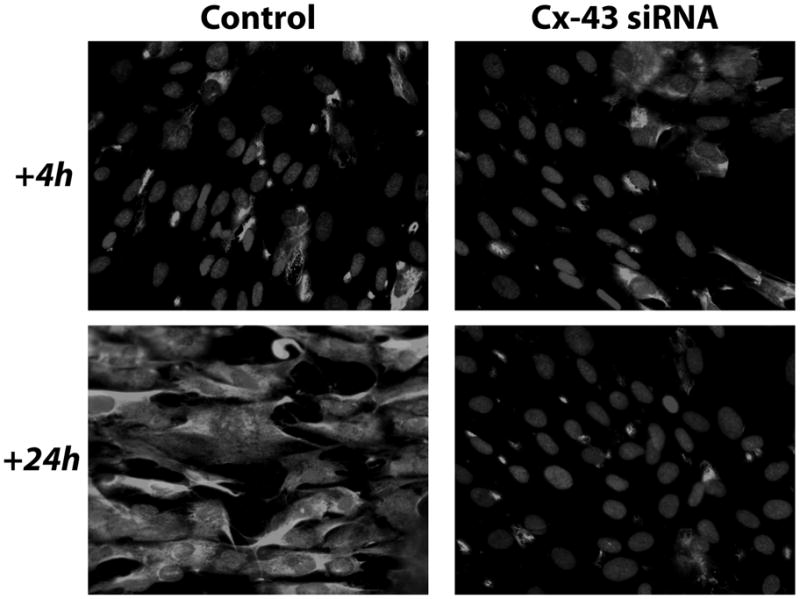
Fibroblast expression of α-smooth muscle actin. RMC-1 cells influence, through GJIC, the expression of αSMA in human fibroblasts. When human dermal fibroblasts are co-cultured with untreated control RMC-1 cells for 4 hrs, 16 out of 44 cells express cytoplasmic αSMA (left top). When fibroblasts are co-cultured with RMC-1 cells for 24 hrs (bottom left), 31 of 31 control fibroblasts expressed cytoplasmic αSMA. The 2 panels on the right show the results of the co-culture of Cx-43 siRNA treated RMC-1 cells with human dermal fibroblasts. At 4 hrs the co-culture of fibroblasts with Cx-43 siRNA treated RMC-1 cells show 14 of 39 fibroblasts expressing cytoplasmic αSMA (top right). At 24 hrs the co-culture of fibroblasts with Cx-43 siRNA treated RMC-1 cells showed 12 of 41 cells expressing cytoplasmic αSMA (bottom right).
Figure 5.
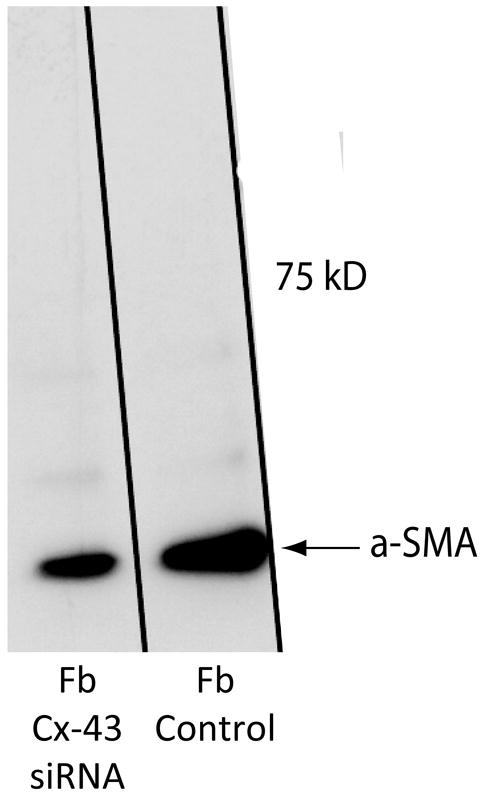
Western blot showing αSMA expression in fibroblasts. Fibroblasts in monoculture treated with Cx-43 siRNA for 24 h were compared to untreated fibroblasts (control). Untreated fibroblasts expressed αSMA. Treating fibroblasts for 24 hrs with Cx-43 siRNA significantly reduced this expression.
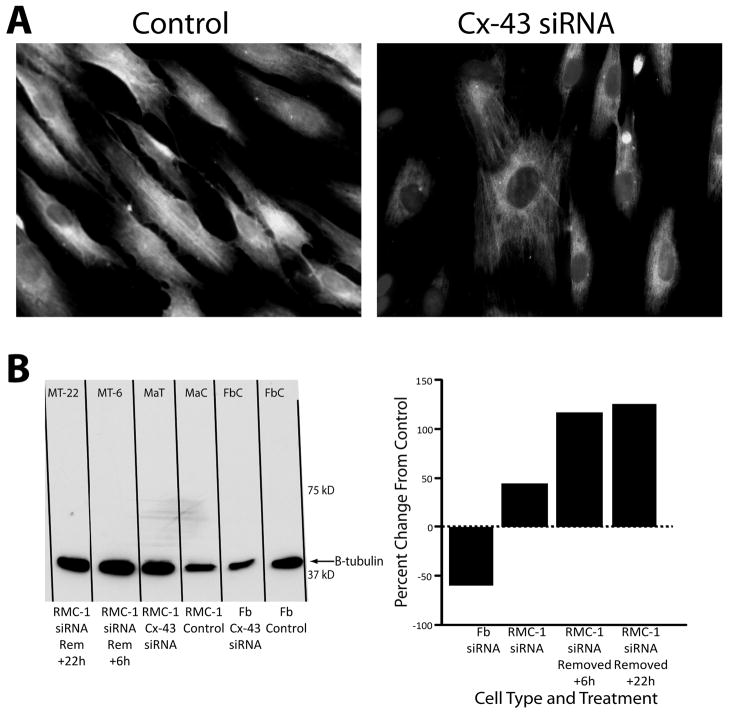
The knockdown of Cx-43 produced differing responses in levels of tubulin, a cytoskeleton structural protein, in fibroblast and mast cell microtubules. By immuno-histology the co-culture of untreated RMC-1 cells with fibroblasts increased the intensity of β-tubulin expression (Fig. 6A, left). By Western blot analysis the expression of β-tubulin in fibroblasts and RMC-1 cells in monoculture was investigated. Treating RMC-1 cells with siRNA increased the quantity of β-tubulin in cell lysates (Fig. 6B). In contrast, treating fibroblasts with siRNA decreased β-tubulin quantity in fibroblast lysates (Fig. 6B). The knockdown of Cx-43 expression decreased β-tubulin expression in fibroblasts, while increasing expression of β-tubulin in RMC-1 cells.
Figure 6.
RMC-1 cells co-cultured with fibroblasts and β-tubulin expression. RMC-1 paratrooper cells were added to confluent fibroblasts in monolayer culture. Immuno-histology shows β-tubulin fluorescence in fibroblasts with added control RMC-1 cells (panel A, left) and a decreased amount of β-tubulin in Cx-43 siRNA treated RMC-1 cells (panel A, right). Western blot illustrating changes in β-tubulin expression from RMC-1 cells and fibroblasts in monoculture are shown in panel B. The 2 lanes on the left show β-tubulin expression increased in RMC-1 cells following Cx-43 knockdown by siRNA treatment. In contrast, β-tubulin expression in monocultured fibroblasts, the 2 right lanes, show β-tubulin expression was decreased by the knockdown of Cx-43 by siRNA treatment. Percent change in expressionfrom the control group in each cell type, as measured by densitometry, is shown on the right.
DISCUSSION
These results support a role for heterocellular GJIC between fibroblasts and mast cells for mast cell promotion of fibroblast pro-fibrotic activities. Here the significance of expressing a specific Cx protein, Cx-43, was investigated in cultured mast cells and fibroblasts. It appears that cultured rat mast cells, RMC-1 cells, contain only Cx-43 as the Cx protein generating hemichannels on their plasma membrane surface. Therefore, the absence of Cx-43 from RMC-1 cells prevents GJIC between RMC-1 cells as well as heterocellular GJIC between mast cells and fibroblasts. The effect of knocking down Cx-43 in fibroblasts was not as robust as knocking down Cx-43 in RMC-1 cells, which completely blocks GJIC between cells. There is evidence that this may be related to fibroblasts capacity to express other Cx such as Cx-32 and Cx-26, which may compensate for the absence of Cx-43 in GJIC between fibroblasts. The sequestration of Cx-43 within the cytoplasm of Cx-43-siRNA knockdown RMC-1 cells suggests that the Cx-43-siRNA was not as effective in eliminating Cx-43 expression in RMC-1 cells as it was in human dermal fibroblasts. Another possibility in RMC-1 cells the translocation of Cx-43 from its site of synthesis to a hemichannel location on the plasma membrane is impaired, causing an accumulation of Cx-43 in the cytoplasmic compartment. Normally the turnover of Cx in hemichannels is rapid with a half life of less than 4 hours [Dbouk et al., 2009; Yamaguchi and Ma, 2003]. The expectation is Cx-43 should be cleared rapidly from Cx-43-siRNA treated cells. It suggests that the turnover of Cx in RMC-1 cells is different than that in cultured dermal fibroblasts.
From the synthesis of Cx-43 on ribosomes to its transport to the cell surface and incorporation into hemichannels, Cx-43 associates with a variety of proteins including: 1) cytoskeletal proteins like: tubulin of microtubules, actin of microfilaments and actin-binding proteins such as α-spectrin and drebrin; 2) junctional molecules including adherens junction components such as cadherins, α-catenin, and β-catenin, as well as tight junction components such as ZO-1 and ZO-2; 3) enzymes such as kinases and phosphatases, which regulate their assembly, function and degradation; and 4) other proteins such as caveolin [Noli and Miolo, 2001]. The sequestration of Cx-43 in fibroblasts to numerous cytoplasmic proteins may explain the residual accumulation of Cx-43 within the cytoplasmic compartment of siRNA treated fibroblasts. The apparent aggregation of cytoplasmic Cx-43 within Cx-43-siRNA-treated RMC-1 cells warrants further investigation to determine if pretreated-Cx-43 or newly-synthesized malformed Cx-43 due to siRNA is sequestered in the cytoplasm.
The C-terminal tail of Cx-43 binds directly to tubulin [Plaut et al., 1989]. The binding of Cx-43 to tubulin directs Cx-43 transport to the plasma membrane, where it is incorporated into plasma membrane hemichannels. Gap junction hemichannels also anchor microtubules to the cell membrane. The co-culture of fibroblasts with Cx-43 knockdown RMC-1 cells inhibits β-tubulin expression in fibroblasts, but the surprising finding was Cx-43-siRNA therapy promotes tubulin accumulation in mast cells. These differences in tubulin expression suggest a different mechanism for controlling tubulin synthesis in fibroblasts compared to mast cells. The relationship between tubulin expression and Cx-43 synthesis in cultured fibroblasts and mast cells is unclear.
Published findings report mast cells release multiple cytokines, chemokines, and growth factors [Artuc et al., 2002; Wodnar-Filipowicz et al., 1989]. Hemichannels can release small soluble cytoplasmic molecules, such as cytokines directly into the extracellular compartment. Further investigation needs to confirm the presence or absence of connexin family members other than Cx-43 in RMC-1 cells and whether these other Cxs generate hemichannels that are uninvolved in GJIC, but may play a role in the release of small molecules through hemichannels. The release of small cytosol soluble molecules from mast cells into the extracellular environment via hemichannels may be another avenue for influencing fibroblasts and/or other cells. Mast cells accumulate in excessive scaring conditions, such as keloids and hypertrophic scar. Keratinocyte growth factors produced and released from mast cells induce the release angiogenic and matrix remodeling factors from fibroblasts [Noli and Miolo, 2001]. The details of hemichannel and release of these factors are currently unclear. If Cx-43 or other mast cell connexins are involved in promoting profibrotic fibroblast activities, then regulating the release of these factors through induced alterations in mast cell Cxs is a potential therapeutic target for optimizing normal wound healing and preventing excessive fibrosis.
Mast cells and fibroblasts are known to participate in most phases of wound healing and fibrosis, where GJIC interactions are partially influenced by the expression of Cx-43. In these in vitro experiments, eliminating Cx-43 expression in RMC-1 cells completely blocked heterocellular GJIC with fibroblasts. Apparently RMC-1 cells with Cx-43 knocked down by siRNA therapy do not form heterocellular GJIC with fibroblasts in culture. It supports the notion that, if this same knockdown of Cx-43 within mast cells in healing wounds occurs, then alterations in the repair process may follow. One effect of GJIC between mast cells and fibroblasts in cell culture is the upregulation of αSMA and the transformation of fibroblasts into myofibroblasts. Whether the interactions between fibroblasts and mast cells through GJIC in granulation tissue influence the generation of myofibroblasts needs to be investigated. Here, the knockdown of Cx-43 within RMC-1 cells retards that transformation. Further studies to elucidate the effect on fibrosis by blocking the generation of myofibroblasts using Cx-43 siRNA therapy within granulation tissue of healing wounds in vivo are warranted.
Another aspect of fibrosis is scar contracture, which is not to be confused with wound contraction. Scar contracture is the compaction of scar tissue after the completion of wound closure. If a scar contracture occurs over a joint, then the motility of that joint is compromised. Preventing scar contractures would be a major clinical benefit in reducing the time and pain required for the complete recovery and restoration of normal functions. Cultured human mast cells (HMC-I cells) co-cultured with fibroblasts in collagen lattices enhances lattice contraction through GJIC [Moyer et al., 2004]. Targeting the elimination of Cx-43 expression within mast cells in hypertrophic scars may be an approach in preventing scar contractures and eliminating the need for multiple surgical interventions and prolonged physical therapy.
MATERIALS AND METHODS
Cell cultures
Human dermal fibroblasts number CRL-2522 derived from human foreskin were purchased from ATCC. The mast cell line was developed from isolated Sprague Dawley rat peritoneal cavity fluid and called RMC-1 cells (manuscript accepted in Plast Reconstr Surg). They grow in Dulbecco’s modification of Eagle’s medium (DMEM) with 10% bovine serum, where both RMC-1 cells and fibroblasts are maintained. To create RMC-1 cells the established human mast cell line HMC-1 cells [Butterfield et al., 1988] were maintained in Iscove’s modification of Eagle’s medium (Lonza group Ltd, Basel, Switzerland), with 10% bovine serum. Media collected from HMC-1 changes were pooled, filter sterilized and stored. Mast cells were isolated by the technique of Caulfield and coworkers [Caulfield JP, 1986]. Briefly a Sprague Dawley rat was euthanized, a midline abdominal incision made, sterile lavage solution instilled into the cavity, followed by suction harvesting of the peritoneal lavage fluid. Cells were collected by centrifugation and layered onto an Accudenz (Accurate Chemical & Scientific Group, Westbury, NY) separation gradient of 40%, 26% and 13%, which was spun for 1 hour. The collected buffy coat was concentrated by centrifugation and the cells resuspended in growth medium, composed of 60% spent HMC-1 media and 40% DMEM with 10% bovine serum, called Growth Medium. Cells were incubated at 370 with 5% CO2. Lymphocytes, macrophages, and other cells in the peritoneal lavage fluid did not survive. Surviving mast cells were sustained for 1 month with 3 weekly media changes of Growth Medium and at 4 weeks Growth Medium was replaced with DMEM with 10% bovine serum. The rat RMC-1 cells have remained viable after more than 40 passages. RMC-1 cells have unique mast cell characteristics of Stem Cell Factor (SCF), using the C-kit monoclonal antibody (Santa Cruz Biotechnology, Inc.), on their cell surface and the proteinase chymase, using monoclonal antibody (NeoMarker; Fremont, CA), contained in cytoplasmic granules.
SiRNA-treatment
To knockdown Cx-43 expression in both fibroblasts and RMC-1 cells, complete DMEM was replaced with 1μM Accell SMART pool siRNA against Cx-43 (Rat GJA1, NM_012567, Thermo Scientific, Rockford, IL) in Accell Delivery Media. For control cells, Accell Delivery Media without siRNA was added to confluent cells in monolayer. Cells were returned to the incubator for 72 hours. At that time assays could be performed or, alternately, cells could be maintained in Cx-43-knockdown / control by changing to fresh Accell Delivery Media with or without siRNA.
Scrape loading
GJIC between confluent cells (RMC-1 and fibroblasts) was evaluated by the scrape-loading technique [Moyer et al., 2004]. When RMC-1 cells or fibroblasts in 35-mm dishes approached confluence, their medium was discarded, the cells were rinsed with phosphate buffered saline (PBS) and 1.0 ml of a dye solution, consisting of 20 mg Lucifer yellow (LY) and 5 mg rhodamine (Rh)-dextran (Invitrogen; Carlsbad, CA) in PBS, was added. The cells covered in the dye solution were scratched with a commercial glasscutter. The culture dishes were returned to the incubator for 2 min. The dye solution was removed; the cells rinsed with warmed PBS, the cell layer fixed in buffered 4% paraformaldehyde (PFA) and cells in the scratched line viewed with a fluorescent microscope. Both LY and Rh-dextran accumulated within the scrape-injured cells. If a scrape-injured cell was coupled to a neighboring cell by an open gap junction channel, LY dye would pass into the uninjured coupled cell. Dye could continue to pass from coupled cells into other coupled cells. The Rh-dextran particles were too large to pass through gap junction channels and they are retained in scrape-injured cells. The ratio of yellow–green (LY) fluorescent cells to red fluorescent cells (Rh-Dextran) was reported as the “coupling index”, which was the ratio of counted green/yellow over red fluorescent stained cells within ten randomly selected microscope fields containing scrape lines in two dishes (total 20 fields from treated and 20 fields from control RMC-1 cells and fibroblasts. A student’s two-tailed t-test was performed on the data to determine the mean difference and significance between treatment groups with a p value less than 0.05.
Mast Cell Paratroopers
Using the method of Goldberg [Goldberg et al., 1995], RMC-1 paratrooper cells were generated. Briefly, confluent 35 mm dishes of Cx-43 siRNA-treated or untreated control RMC-1 cells had complete DMEM medium replaced with 1.0 ml of 300 mM glucose in 30 mM HEPES buffer pH 7.4 (Glucose-HEPES buffer) (31) [Hinz et al., 2007]. The glucose-HEPES buffer was replaced with 1 ml of the same buffer containing 10 μg of Calcein AM (Invitrogen). Calcein AM is a fluorescent dye that once incorporated into the cell’s cytoplasm passes dye exclusively between coupled cells via GJIC.22 The dishes were incubated for 10 min at 37o, the medium discarded and cells rinsed 3 times with complete DMEM to eliminate any residual Calcein AM dye. The Calcein AM treated cells were suspended by trypsinization and resuspended in 1ml of DMEM with 10% NBBS and the cell number determined with a hemacytometer. The fluorescent tagged RMC-1 cells referred to as MC-paratroopers were briefly visualized with a fluorescent stereo-microscope to confirm fluorescent labeling. When a MC-paratrooper formed gap junctions with another cells, Calcein AM dye would pass into the coupled cells. MC-paratroopers are easily distinguished from fibroblasts by their small size, MC-paratroopers are small, round cells and fibroblasts are large spindle shaped cells. Suspended MC-paratroopers were pipetted onto a confluent monolayer of fibroblasts and dye passage into fibroblasts monitored over time with an inverted fluorescent microscope.
Immuno-histology
Media was removed from either RMC-1 cells and fibroblasts maintained either on glass coverslips or plastic culture dishes. The cells were fixed with 4% PFA in cytoskeletal stabilizing buffer (CSB; 137mM NaCl, 5mM KCl, 1.1mM Na2HPO4, 0.4mM KH2PO4, 4mM NaHCO3, 2mM MgCl2, 5.5mM glucose, 2mM EGTA, and 5mM PIPES in distilled water to pH 6.1), washed, permeabilized with 0.1% Triton X-100 in CSB, and incubated in primary antibody with a solution containing 10 mg/mL bovine serum albumin in CSB for 1 hour (except for anti-Cx-43, which was incubated at 4° C overnight). Primary antibodies concentrations for immune staining were: 1:100 monoclonal anti-Cx-43 (#3067) anti-Cx-32 (#3069) and anti-Cx-26 (#8143) [Millipore–Chemicon; Billerca, MA], 1:100 polyclonal anti-β-tubulin (#2146, Cell Signaling, Danvers, MA), 1:100 monoclonal anti-αSMA (# A5228 Sigma Chemical Co. St Louis, MO). After washing, cells were incubated in secondary (1:200 donkey anti-mouse – rhodamine or 1:200 goat anti-rabbit – Alexa) for 1 hour. When necessary, cells were treated with 1:200 Alexa phalloidin (Invitrogen) to fluorescently stain actin microfilaments. Cells were washed and then incubated for 5 min with DAPI (4′,6-diamidino-2-phenylindole) at 2 μg/ml, which stains nuclei fluorescently blue. After the last wash, the cell layer received a drop of gel mounting media, covered with a glass cover slip and then viewed with a fluorescent microscope equipped with a digital CCD camera.
Western Blotting
Proteins were resolved by electrophoresis in precast 4–15% gradient SDS-PAGE gels and transferred to Immobilon-FL PVDF membranes (Millipore). Membranes were blocked with milk and incubated overnight at 4°C with 1:1000 dilution of monoclonal anti-Cx-43 (# C8093; Sigma), polyclonal anti-β-tubulin (Cell Signaling), or monoclonal anti-αSMA (Sigma) antibodies. Blots were washed and incubated in either 1:100,000 dilution of goat anti-mouse or goat anti-rabbit horseradish peroxidase-conjugated secondary antibodies. Bound secondary antibody was detected by SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific) and then visualized by exposing the membrane with blots to X-ray film. Protein bands were analyzed, using Adobe Photoshop to determine blot intensity for densitometry by selecting the area of each protein band and recording the intensity of each pixel as a histogram. Histogram data were quantified based on intensity and band size (providing a value for absolute intensity). Relative values for each experimental group were then calculated by comparison of absolute intensity against that of their control and reported as a percent change ((experimental – control) / control X 100).
Acknowledgments
We would like to thank Christopher Schank, Gregory Saggers, and Gretchen Allison for their excellent technical assistance. This research was supported by NIH grant GM56851.
Footnotes
Work was performed at the Milton S. Hershey Medical Center, Hershey, PA 17033.
References
- Abe M, Kurosawa M, Ishikawa O, Miyachi Y, Kido H. Mast cell tryptase stimulates both human dermal fibroblast proliferation and type I collagen production. Clin Exp Allergy. 1998;28:1509–17. doi: 10.1046/j.1365-2222.1998.00360.x. [DOI] [PubMed] [Google Scholar]
- Artuc M, Steckelings UM, Henz BM. Mast cell-fibroblast interactions: human mast cells as source and inducers of fibroblast and epithelial growth factors. J Invest Dermatol. 2002;118:391–5. doi: 10.1046/j.0022-202x.2001.01705.x. [DOI] [PubMed] [Google Scholar]
- Au SR, Au K, Saggers GC, Karne N, Ehrlich HP. Rat mast cells communicate with fibroblasts via gap junction intercellular communications. J Cell Biochem. 2007;100:1170–7. doi: 10.1002/jcb.21107. [DOI] [PubMed] [Google Scholar]
- Bradding P. Human mast cell cytokines. Clin Exp Allergy. 1996;26:13–9. doi: 10.1111/j.1365-2222.1996.tb00051.x. [DOI] [PubMed] [Google Scholar]
- Bressler RB, Lesko J, Jones ML, Wasserman M, Dickason RR, Huston MM, Cook SW, Huston DP. Production of IL-5 and granulocyte-macrophage colony-stimulating factor by naive human mast cells activated by high-affinity IgE receptor ligation. J Allergy Clin Immunol. 1997;99:508–14. doi: 10.1016/s0091-6749(97)70078-2. [DOI] [PubMed] [Google Scholar]
- Butterfield JH, Weiler D, Dewald G, Gleich GJ. Establishment of an immature mast cell line from a patient with mast cell leukemia. Leuk Res. 1988;12:345–55. doi: 10.1016/0145-2126(88)90050-1. [DOI] [PubMed] [Google Scholar]
- Caulfield JPHA, Tsunoda Ki, Shapiro R. Detection of sulfur in fixed and embedded rat peritoneal mast cell granules by x-ray energy dispersive spectroscopy. J Elec Micro Tech. 1986;3:347–356. [Google Scholar]
- Dbouk HA, Mroue RM, El-Sabban ME, Talhouk RS. Connexins: a myriad of functions extending beyond assembly of gap junction channels. Cell Commun Signal. 2009;7:4. doi: 10.1186/1478-811X-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G, Chaponnier C, Huttner I. Cytoplasmic filaments and gap junctions in epithelial cells and myofibroblasts during wound healing. J Cell Biol. 1978;76:561–8. doi: 10.1083/jcb.76.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg GS, Bechberger JF, Naus CC. A pre-loading method of evaluating gap junctional communication by fluorescent dye transfer. Biotechniques. 1995;18:490–7. [PubMed] [Google Scholar]
- Gruber BL, Kew RR, Jelaska A, Marchese MJ, Garlick J, Ren S, Schwartz LB, Korn JH. Human mast cells activate fibroblasts: tryptase is a fibrogenic factor stimulating collagen messenger ribonucleic acid synthesis and fibroblast chemotaxis. J Immunol. 1997;158:2310–7. [PubMed] [Google Scholar]
- Gruber BL, Marchese MJ, Kew RR. Transforming growth factor-beta 1 mediates mast cell chemotaxis. J Immunol. 1994;152:5860–7. [PubMed] [Google Scholar]
- Hebda PA, Collins MA, Tharp MD. Mast cell and myofibroblast in wound healing. Dermatol Clin. 1993;11:685–96. [PubMed] [Google Scholar]
- Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807–16. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauhanen P, Kovanen PT, Reunala T, Lassila R. Effects of skin mast cells on bleeding time and coagulation activation at the site of platelet plug formation. Thromb Haemost. 1998;79:843–7. [PubMed] [Google Scholar]
- Kischer CW, Bailey JF. The mast cell in hypertrophic scars. Tex Rep Biol Med. 1972;30:327–38. [PubMed] [Google Scholar]
- Kovacs EJ. Fibrogenic cytokines: the role of immune mediators in the development of scar tissue. Immunol Today. 1991;12:17–23. doi: 10.1016/0167-5699(91)90107-5. [DOI] [PubMed] [Google Scholar]
- Kube P, Audige L, Kuther K, Welle M. Distribution, density and heterogeneity of canine mast cells and influence of fixation techniques. Histochem Cell Biol. 1998;110:129–35. doi: 10.1007/s004180050274. [DOI] [PubMed] [Google Scholar]
- Kumar NM, Gilula NB. The gap junction communication channel. Cell. 1996;84:381–8. doi: 10.1016/s0092-8674(00)81282-9. [DOI] [PubMed] [Google Scholar]
- Kupietzky A, Levi-Schaffer F. The role of mast cell-derived histamine in the closure of an in vitro wound. Inflamm Res. 1996;45:176–80. doi: 10.1007/BF02285158. [DOI] [PubMed] [Google Scholar]
- Kuther K, Audige L, Kube P, Welle M. Bovine mast cells: distribution, density, heterogeneity, and influence of fixation techniques. Cell Tissue Res. 1998;293:111–9. doi: 10.1007/s004410051103. [DOI] [PubMed] [Google Scholar]
- Laird DW, Puranam KL, Revel JP. Turnover and phosphorylation dynamics of connexin43 gap junction protein in cultured cardiac myocytes. Biochem J. 1991;273(Pt 1):67–72. doi: 10.1042/bj2730067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekori YA, Galli SJ. [125I]fibrin deposition occurs at both early and late intervals of IgE-dependent or contact sensitivity reactions elicited in mouse skin. Mast cell-dependent augmentation of fibrin deposition at early intervals in combined IgE-dependent and contact sensitivity reactions. J Immunol. 1990;145:3719–27. [PubMed] [Google Scholar]
- Mori R, Power KT, Wang CM, Martin P, Becker DL. Acute downregulation of connexin43 at wound sites leads to a reduced inflammatory response, enhanced keratinocyte proliferation and wound fibroblast migration. J Cell Sci. 2006;119:5193–203. doi: 10.1242/jcs.03320. [DOI] [PubMed] [Google Scholar]
- Moulin V, Castilloux G, Auger FA, Garrel D, O’Connor-McCourt MD, Germain L. Modulated response to cytokines of human wound healing myofibroblasts compared to dermal fibroblasts. Exp Cell Res. 1998;238:283–93. doi: 10.1006/excr.1997.3827. [DOI] [PubMed] [Google Scholar]
- Moyer KE, Saggers GC, Ehrlich HP. Mast cells promote fibroblast populated collagen lattice contraction through gap junction intercellular communication. Wound Repair Regen. 2004;12:269–75. doi: 10.1111/j.1067-1927.2004.012310.x. [DOI] [PubMed] [Google Scholar]
- Nishikori Y, Kakizoe E, Kobayashi Y, Shimoura K, Okunishi H, Dekio S. Skin mast cell promotion of matrix remodeling in burn wound healing in mice: relevance of chymase. Arch Dermatol Res. 1998;290:553–60. doi: 10.1007/s004030050351. [DOI] [PubMed] [Google Scholar]
- Noli C, Miolo A. The mast cell in wound healing. Vet Dermatol. 2001;12:303–13. doi: 10.1046/j.0959-4493.2001.00272.x. [DOI] [PubMed] [Google Scholar]
- Oliani SM, Girol AP, Smith RL. Gap junctions between mast cells and fibroblasts in the developing avian eye. Acta Anat (Basel) 1995;154:267–71. doi: 10.1159/000147778. [DOI] [PubMed] [Google Scholar]
- Plaut M, Pierce JH, Watson CJ, Hanley-Hyde J, Nordan RP, Paul WE. Mast cell lines produce lymphokines in response to cross-linkage of Fc epsilon RI or to calcium ionophores. Nature. 1989;339:64–7. doi: 10.1038/339064a0. [DOI] [PubMed] [Google Scholar]
- Qu Z, Kayton RJ, Ahmadi P, Liebler JM, Powers MR, Planck SR, Rosenbaum JT. Ultrastructural immunolocalization of basic fibroblast growth factor in mast cell secretory granules. Morphological evidence for bfgf release through degranulation. J Histochem Cytochem. 1998;46:1119–28. doi: 10.1177/002215549804601004. [DOI] [PubMed] [Google Scholar]
- Russel JD, Russell SB, Trupin KM. The effect of histamine on the growth of cultured fibroblasts isolated from normal and keloid tissue. J Cell Physiol. 1977;93:389–93. doi: 10.1002/jcp.1040930310. [DOI] [PubMed] [Google Scholar]
- Trautmann A, Toksoy A, Engelhardt E, Brocker EB, Gillitzer R. Mast cell involvement in normal human skin wound healing: expression of monocyte chemoattractant protein-1 is correlated with recruitment of mast cells which synthesize interleukin-4 in vivo. J Pathol. 2000;190:100–6. doi: 10.1002/(SICI)1096-9896(200001)190:1<100::AID-PATH496>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Vliagoftis H, Hutson AM, Mahmudi-Azer S, Kim H, Rumsaeng V, Oh CK, Moqbel R, Metcalfe DD. Mast cells express connexins on their cytoplasmic membrane. J Allergy Clin Immunol. 1999;103:656–62. doi: 10.1016/s0091-6749(99)70239-3. [DOI] [PubMed] [Google Scholar]
- Wodnar-Filipowicz A, Heusser CH, Moroni C. Production of the haemopoietic growth factors GM-CSF and interleukin-3 by mast cells in response to IgE receptor-mediated activation. Nature. 1989;339:150–2. doi: 10.1038/339150a0. [DOI] [PubMed] [Google Scholar]
- Yamaguchi DT, Ma D. Mechanism of pH regulation of connexin 43 expression in MC3T3-E1 cells. Biochem Biophys Res Commun. 2003;304:736–9. doi: 10.1016/s0006-291x(03)00633-8. [DOI] [PubMed] [Google Scholar]