Abstract
Historically, examinations of the inhibition of malaria parasite growth/invasion, whether using drugs or antibodies, have relied on the use of microscopy or radioactive hypoxanthine uptake. These are considered gold standards for measuring the effectiveness of antimalarial treatments, however, these methods have well known shortcomings. With the advent of flow cytometry coupled with the use of fluorescent DNA stains allowed for increased speed, reproducibility, and qualitative estimates of the effectiveness of antibodies and drugs to limit malaria parasite growth which addresses the challenges of traditional techniques. Because materials and machines available to research facilities are so varied, different methods have been developed to investigate malaria parasites by flow cytometry. This review is intended to serve as a reference guide for advanced users and importantly, as a primer for new users, to support expanded use and improvements to malaria flow cytometry, particularly in endemic countries.
Keywords: Malaria; Cytometry; IC50; GIA, Growth Inhibition Assay; IIA, Invasion Inhibition Assay; Drug Assay
1. INTRODUCTION
Flow cytometry was originally developed by the United States Army during World War II for detection of airborne anthrax spores (Gucker et al., 1947). The original cytometer passed an air stream through the machine to attempt to detect the bacteria. Improvements since that time have reduced the amount of sample required and increased the strength and number of lasers/filters which can be used to analyze cells. While high-end cytometry equipment remains comparatively expensive (Shapiro and Perlmutter, 2008), there is an expanding understanding that to investigate infectious diseases these machines need to be used by endemic populations at or near the point of care. The information provided about cells by cytometers, cannot be discerned as easily or as quantitatively by other means. Because of the speed and amount of information it provides, cytometry is becoming particularly important for the study of malaria parasite growth and invasion because it overcomes the limitations of existing non-cytometric methods.
The study of malaria parasite infected cells historically relied on visualization of parasites in stained blood slides. It was not until the introduction of the Giemsa stain in 1904 (Giemsa, 1904), that reliable microscopic examination of blood smears for diagnosis of circulating malaria parasites could be performed (Fleischer, 2004). This rapidly became, and remains, the official gold standard for malaria diagnosis (Makler et al., 1998). However, there are shortcomings in microscopic evaluation of malaria parasite growth and invasion particularly because of subjective inter-operator error. Significant levels of misdiagnosis have been demonstrated with microscopic detection of malaria (Li et al., 2007) showing false positive rates as high as 36% and false negatives as high as 18% (Milne et al., 1994). Factors such as microscopist training are only part of the problem, methods used in the creation and staining of slides from patient samples are also an issue. Therefore, there has been a long-standing need for improved methodology.
The use of radioactive hypoxanthine (HX) was developed in an attempt to reduce the subjective nature of microscopic assays of parasite growth (Desjardins et al., 1979). This technique, which tracks the incorporation of tritiated HX into DNA as it is synthesized, could be used to perform high-throughput assays and was a large improvement compared with slide counting. Although widely used, this method also has several challenges including the need for radioactivity and it cannot differentiate when the H3- purine is incorporated by human cells or by the parasites, which can lead to a higher background. In addition, HX uptake cannot measure parasitemia because its incorporation is dependent on DNA synthesis which only occurs in the later stages of the parasite life cycle (Yayon et al., 1983). DNA synthesis in turn is dependent on the growth rate of the parasite strain being observed.
Other methods for monitoring malaria growth have used plate readers to detect the presence of indicators of DNA quantity or enzyme activity in ELISA based assays. Detecting DNA levels with these methods involved the lysis of parasite cultures after exposure to drugs of interest and then comparing the total DNA content within each sample well using fluorescent DNA stains such as PicoGreen (Corbett et al., 2004; Quashie et al., 2006). The detection of parasite enzyme activity on the other hand has focused on the parasites’ lactate dehydrogenase (pLDH) which metabolizes 3-acetyl pyridine NAD (APAD) faster than human erythrocyte native LDH. However, field studies showed a low degree of concordance between this method and standard microscopy based determinations of parasitemia (Knobloch and Henk, 1995; Jelinek et al., 1996). Alterations have been made to improve the specificity of this assay using monoclonal antibodies (which are in limited supply) against LDH (called the DELI assay : double-site enzyme-linked LDH immunodetection) which showed results similar to Hypoxanthiene uptake (Moreno et al., 2001). By switching focus to the parasite’s histidine-rich protein 2 (HRP2), researchers were able to maintain a good correlation with hypoxanthine uptake (Desakorn et al., 1997). However, this new assay, while more readily available and therefore useful in the field, requires a longer incubation time (72 hr). All of these assays have the advantage of being able to be performed in the field using patient blood samples (ex vivo) to test for drug resistance or invasion inhibition while the patient is still nearby. However, these assays also have the same shortfall swhich are that they cannot be stage specific, once the enzymes are expressed there is no way to detect parasite death, and for parasites which are quiescent, these assays are uninformative.
Flow cytometry based assays address all of the above challenges presented by microscopy, HX uptake, total DNA content detection, and both enzyme and antibody based assays and have become crucial to the study of malaria because of the objective, high content/moderate throughput assays that can be performed. As the cost of cytometers decreases, they will play a larger role in parasite evaluation in malaria endemic countries. Cytometry will also contribute to epidemiologic assessments and direct evaluation of patient’s malaria infection status, and drug resistance parasite status. Evaluation of drug/antibody efficacy and growth inhibition by flow cytometry will be crucial for the development of new antimalarial drugs and for erythrocyte stage vaccine candidates. The purpose of this review is to serve as a primer of new malaria cytometry users and as a reference to experienced users by presenting the historical background of its uses in malaria, current methods and their pitfalls, as well as future possibilities for the field.
2. BACKGROUND
Flow cytometry offers the opportunity to provide more information about malaria parasite growth and development than any other currently available method. The use of nucleic acid stains and flow cytometry is uniquely suited to study malaria. All the cytometry-based investigations of the malaria parasite erythrocyte stages take advantages of the fact that normal circulating red blood cells (RBCs) predominately lack DNA. Parasitemia in blood samples can therefore be determined by counting and comparing the ratio of RBCs which stain positive for DNA to the total number of RBCs analyzed. While most normal adult erythrocytes in the blood stream do not contain DNA or RNA, there are a few exceptions that can confound cytometric analysis of malaria if not identified and dealt with. One exception is erythrocytes which have been newly released from the bone marrow, called reticulocytes, which contain small levels of RNA but are only present at levels less than 1.5% in normal adults (Ferri, 2007) could be confused as a parasite if not using a DNA specific stain. However, remnant RNA in the circulating erythrocytes quickly degrades over time and within a few days in in vitro cultures, leading to an even smaller presence of this type of cell in the blood samples. Another exception is Howell-Jolly bodies which are remnants of DNA which may also exist in erythrocytes. Fortunately, these anomalies are rare occurrences in normal human blood (Hoffman et al., 2009). In addition, because these irregularities contain human DNA, which is thousands of times larger than the malaria parasite genome, they can be identified and eliminated from analysis of malaria-infected samples. Combining both DNA and RNA stains enables easy identification of reticulocytes and micronuclei, if present, from parasitized erythrocytes. In fact, erythrocytes infected with the mouse malaria Plasmodium berghei (P. berghei) used to be used as standards for flow cytometry, to quantify the induction of micronuclei, particularly those in in vivo assessment of toxicity of investigational drugs (Torous et al., 2006).
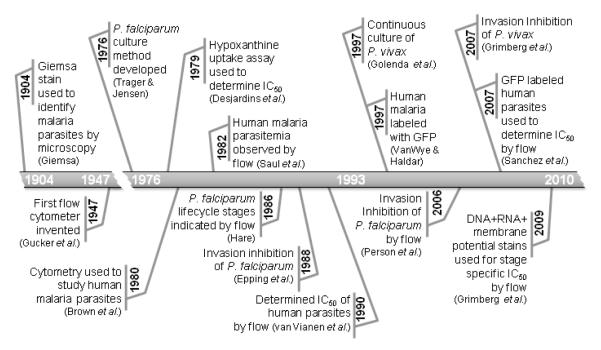
Malariologists have historically relied on studies of animal malaria parasite models, particularly rodent malarias because of their ease of use(Silverman et al., 1987). In 1977 (Jackson et al., 1977) the first experiments were performed to investigate malaria parasites using flow cytometry by staining cells with ethidium bromide and acridine orange. Other early experiments were carried out on parasites which infect other mammals such as Plasmodium vinckei with (DiOC1-3 + Hoechst 33342) (Jacobberger et al., 1984) and Plasmodium berghei (P. berghei, DRAQ5)(Billker et al., 2004). Neither of these stain/stain combinations have been used to investigate human malaria, thus far. This is, in part, because culturing methods for the human malarias were not developed until 1976 (Trager and Jensen, 1976) and the first application of flow cytometry for human malaria parasites did not occur until 1980 (Brown et al., 1980). Furthermore, an assay using flow cytometry to determine parasitemia of P. falciparum was not developed until 1982 (Saul et al., 1982) which gave studies of animal malaria historical precedent. This article provides a review of the history of malaria parasite flow cytometry methodology and the development of techniques for the assessment of malaria parasite drug and antibody sensitivity (Figure 1).
Figure 1.
History of Flow Cytometry and Analysis of Human Malaria Parasites
3. METHODS OVERVIEW
3.1 MALARIA PARASITE BLOOD STAGE LIFE CYCLE AND CULTURING
The parasites that cause malaria are transmitted to humans from the bite of an infected mosquito. The saliva of the mosquito transmits sporozoites into the human’s blood stream which enter the liver to invade hepatic cells and incubate for a couple of weeks to several months or even years in the case of P. vivax (Dronamraju, 2004). While this liver phase of the infection does not cause morbidity it is the focus of promising vaccine candidates (Higgs and Sina, 2005; Hoffman et al., 2010). After an incubation period, the parasite bursts, releasing a small erythrocyte invasion stage called merozoites which begins to invade erythrocytes. After the initial invasion, the parasite, commonly known as ring stage, because of their appearance when stained, rests before digesting the hemoglobin found in the RBC and then begins to grow. The toxic byproduct of hemoglobin breakdown, heme, is packaged into inert crystals called malaria pigment or hemozoin. The microscopic detection of hemozoin crystals along with an increase in RNA expression indicates the next stage of the parasite called trophozoites. After digestion of much of the hemoglobin, malaria parasites will begin to synthesize DNA(Inselburg and Banyal, 1984) which is the hallmark of the schizont stage. As DNA synthesis slows, the free swimming form of the parasite will begin to be created by condensing 1 copy of the parasite genome into each merozoite, just prior to lysis which is sometimes referred to as the segmenter stage. Although different strains within each species vary by several hours the time from invasion to lysis, in general this process takes ~ 24 hours for P. knowlesi, ~48 hours for P. falciparum, P. ovale and P. vivax, and ~ 72 hours for P. malariae.
The lysis of the blood stage parasite is a major contributor to a complex set of events which lead to clinical disease to which 3.2 billion humans are exposed, 500 million show clinical manifestations, of which more than 1.2 million are killed (World Health Organization, 2007). It is the erythrocyte stage of the parasite that all but one antimalarial compounds are directed towards (primaquine is the exception which is used to treat the long lived liver stages of P. vivax and P. ovale called hypnozoites), as are many of the vaccine trials. Currently, no drugs are specifically directed against the sexual stages of the parasites, called gametocytes. These gametocytes arise from some differentiated trophozoites a few weeks after the start of the blood stage infection in the case of the P. falciparum (Day et al., 1998; Eichner et al., 2001) or within the first few days for P. vivax and other human malarias parasites (Wilairatana et al., 2010). The male and female malaria gametes are then taken up by another mosquito to mate and repeat the cycle.
Trager and Jensen (Trager and Jensen, 1976) developed a relatively straightforward method to reliably culture malaria parasites which is critical for determining IC50 values which are a quantification of the amount of drug which inhibits greater than 50 % of parasite growth. While their original method is still used in some endemic countries, there have been modifications which greatly improved culturing success and reduce costs. Two major modifications included a) the use of synthetic serum (such as Albumax II) instead of the much more expensive human AB sera, and b) maintaining cultures at lower levels of oxygen (1-5% instead of a candle jar which is ~17% O2). Heparinized or EDTA-treated blood is collected from healthy adult volunteers (for immunological reasons, O+ blood is frequently used (Ljungström et al., 2004) but erythrocytic stages can be cultured in any blood group), depleted of leukocytes, and stored in a standard culture media ( RPMI-1640 )at 50% hematocrit at 4°C until ready for use. Strains of malaria parasites are cultivated at 5% hematocrit in complete malaria culture medium (CMCM; (Ljungström et al., 2004). All cultures are maintained at 37°C in an atmosphere of 5% CO2 to maintain pH, with daily medium changes. Using 1% O2, 94% N2 and 5% CO2 has been used in an attempt to improve parasite growth with good success (McNamara et al., 2006). Starting parasitemias are usually in the range of 0.1 – 1 % in culture (Persson et al., 2006). Full descriptions of different culturing methods are available from the MR4 (Malaria Research and Reference Reagent Resource Center, Manassas, VA; (Ljungström et al., 2004).
3.2 FIXATION OF ERYTHROCYTES FOR CYTOMETRIC ANALYSIS OF MALARIA
The use of certain patient samples or the rules of some facilities require that cells used in their flow cytometers be fixed to eliminate any exposure risk to the users. Fixation is particularly of use when screening unknown patient samples for malaria parasites to eliminate the chance of infecting the researcher with HIV or hepatitis by a subcutaneous inoculation (e.g. via needle stick). Mucotaneous infections by these viruses are very rare and essentially nonexistent via aerosol exposure (Gerberding, 1995). Normally, parasitized blood is analyzed under Biohazard Level 2 (BL2) conditions. Drug and growth inhibition assays performed in malaria endemic regions on samples from field isolates can also be fixed and shipped to BL2 labs for further staining and cytometric analysis.
When fixation is required because of institutional or shipping regulations, or when attempting to use a membrane-impermeant stain, such as Propidium Iodide (PI) or SYBR Green I, a few main options are available when studying human malaria parasites (Table 1). Permeablization by 15 gauge hyperdermic needle may be used but this may damage the parasites and introduced potential error in evaluating results (Shapiro, 2003). Therefore, the remaining option is to permeablize the erythrocyte membranes using chemicals. While the treatment of cells for 10 minutes with 70% ethanol or 1 minute with absolute methanol will permeablize the cells and allow staining, especially with Giemsa, in solution it aggregates RBCs into clumps which can irreparably damage many cytometers, particularly when methanol is used. Where the treatment of potential HIV infected samples is an issue, exposure to absolute methanol will kill 99.9% of HIV as will exposure to 0.5% paraformaldehyde or 1.85% formaldehyde for 30 minutes (Cory et al., 1990). Glutaraldehyde, an additional fixative, is presumed to be as effective against viruses but this has not been validated. Hopwood previously summarized the methods and action of multiple fixatives and their uses (Hopwood, 1973).
TABLE 1.
Single Fluorescent Markers Used to Study Human Malarias by Flow Cytometry
| Stain | Interacts with |
Excitation Maximum |
Emission Maximum |
Dose1 | Fixative | References |
|---|---|---|---|---|---|---|
|
Hoechst
33258 |
DNA | 345 | 478 | 2 μM, | 0.25% glutaraldehyde | (van Vianen et al., 1990)2 |
| 200 μM, | None | (Brown et al., 1980) | ||||
| 32.05 μM, | None | (Izumiyama et al., 2009) | ||||
|
| ||||||
|
Hoechst
33342 |
DNA | 355 | 465 | 4.06 μM | 0.25 % glutaraldehyde | (Dent et al., 2008)3 |
| 32.47 μM | None | (Izumiyama et al., 2009) | ||||
|
| ||||||
|
Green
Fluorescent Protein |
Varies | 3954 | 508 | NA | None | (Sanchez et al., 2007)2 |
|
| ||||||
| SYTO-9 | DNA/RNA | 485/486 | 598/501 | 2.5 μM | None | (Izumiyama et al., 2009) |
|
| ||||||
| SYBR Green I | DNA | 488 | 522 | 2X | 1% paraformaldehyde | (Izumiyama et al., 2009) |
| 10X 5 | 0.25% glutaraldehyde | (Dent et al., 2008)3 | ||||
|
| ||||||
| SYTO 16 | DNA/RNA | 488/494 | 518/525 | 100 Nm | 2% paraformaldehyde | (Dahl and Rosenthal, 2007) |
| 5 μM | 0.25% glutaraldehyde | (Jimenez-Diaz et al., 2009) | ||||
|
| ||||||
| YOYO-1 | Nucleic acids, with high affinity for DNA |
491 | 509 | 1 nM | 2% paraformaldehyde + 0.1% Triton X-100 |
(Dahl and Rosenthal, 2007)2 |
| 393.5 nM | 0.04% glutaraldehyde + 0.5 mg/ml RNAse |
(Li et al., 2007) | ||||
| 0.5 μM | 0.25% glutaraldehyde | (Angulo-Barturen et al., 2008) | ||||
|
| ||||||
|
Acridine
Orange |
DNA/RNA | 503 | 530/640 | 72.89 μM, | 1% paraformaldehyde + acid buffers |
(Hare, 1986) |
| 9.94 μM | None | (Izumiyama et al., 2009) | ||||
|
| ||||||
| SYTOX Green | Nucleic Acids |
504 | 523 | 5 μM | 4% formaldehyde + 0.0075% glutaraldehyde then 0.25% Triton X-100 |
(Chandramohanadas et al., 2009) |
|
| ||||||
|
Thiazole
Orange |
Primarily RNA |
509 | 533 | 100 nM | None | (Makler et al., 1987) |
|
| ||||||
|
Ethidium
Bromide |
Nucleic acids |
510 6 | 595 | 253.61 μM | None | (Staalsoe et al., 1999) |
| 25.4 μM | (Dent et al., 2008)3 | |||||
| 2.03 μM | (Persson et al., 2006)3 | |||||
|
| ||||||
|
Propidium
Iodide |
Nucleic Acids |
535 | 617 | 14.96 μM | 0.25% glutaraldehyde | (Pattanapanyasat et al., 1997)2 |
|
| ||||||
| Hydroethidine | Live/ DNA |
365/ 535 |
435/ 610 |
158.49 μM | None | (van der Heyde et al., 1995) |
|
| ||||||
| SYTO-61 | Nucleic Acids |
628 | 645 | 1μM | None | (Fu et al., 2010) |
Published concentrations have been converted to molar units
Used to determine IC50/90 of antimalarial compounds
Used for growth or invasion inhibition assays
GFP also has a 1/3 maximum excitation peak at 489 nm
SYBR Green is available as a 10,000 X stock from Invitrogen. The concentration is proprietary
Ethidium bromide has an additional excitation maximum at 302 nm which has yet to be employed for malaria cytometric methods
One of the issues with fixed RBC when performing assays of malaria parasite growth is that RBC are autofluorescent unlike unfixed RBC. In fact, in the era before standard beads were available, glutaraldehyde-fixed chicken erythrocytes were used for cytometer calibration because they were of such standard size after fixation and so easily seen on cytometers (Loken et al., 1979).
This RBC autofluorescence becomes an issue when attempting to observe parasitemia of fixed cells using certain DNA stains. Some stains, such as propidium iodide (PI), do not have enough fluorescent intensity to overcome autofluorescence to consistently separate infected cells, which have a single copy of DNA (including the ring stages), from uninfected cells. This lack of discrimination can potentially skew results at low parasitemias, such as may exist in certain patient samples. Many of the other stains used by malariologists in fixed samples were chosen because the stain is bright enough to distinguish between the autofluorescence and the DNA positive sample (e.g. SYBR Green I). However, in the case of YOYO-1, the combination of autofluorescence with DNA fluorescence can be used to increase the specificity of detection of parasitized cells which was first described with mouse malaria parasites (Jimenez-Diaz et al., 2005) then later in P. falciparum (Li et al., 2007). Depending on the type of analysis you wish to perform, it may be helpful to use a synchronous culture in which all parasites are expected to be late stage at the time of analysis (and therefore contain higher amounts of DNA). Additionally, the use of slightly higher starting parasitemia (2-3%), if possible, may be helpful to mitigate the loss of the analysis of some infected cells to the autofluorescent region.
3.3 IC50 DETERMINATION
Determination of the concentration of drugs which inhibit greater than 50% of parasite growth allows for the evaluation of current drugs, tracking levels of resistance, and screening for new, effective drugs. Using flow cytometry the set up for this assay is a straightforward process. The goal is to compare the presence of DNA positive cells when different concentrations of drugs are applied with controls. Because existing antimalarial drugs are effective in submicromolar ranges, samples are generally tested in log2 increments from 2 to 512 nM (World Health Organization, 2001). For screening of unknown drug samples 1/2 log steps from 0.01 nm up to 10 μM is useful. Test cultures of P. falciparum are usually grown for 48 hours (roughly one full cycle of invasion and growth depending on the strain). Some experiments call for a 72-hour incubation to improve detection (Noedl et al., 2002; Liu et al., 2008). However, sometimes after 48 hours and certainly after 72 hours, without refreshing the media, the parasites will begin to die off. Lowering the hematocrit to 1% from 4% may improve the parasite survival during these longer culture periods. Stains are applied to several million cells and 10,000 to 100,000 cells are read using a flow cytometer.
Hypoxanthine uptake and enzyme-based assays cannot provide parasitemia. Calculations of the IC50 values based on percent parasitemia found at each drug concentration can be determined using two methods. In the first method, the data is analyzed using a non-linear regression analysis on the percent inhibition at different doses (expressed in log) and then by applying a statistical analysis program such as GraphPad (La Jolla, CA). The second method preferred by some journals is to have experimenters note the concentration of the first treated well that showed less than 50% growth of the organism as the IC50. This method assures that there is consistency between and within journals and methods and that there is no ambiguity about how IC50 were determined. This method does eliminate the need for a statistical program and as we move into an era when journals are publishing raw data that graphs are based on, this method makes it easy for anyone to replicate the calculation of IC50. However, this method makes it difficult to express variability which may be misleading or impossible to determine if all replicates show < 50% growth at the same well (which frequently occurs).
3.4 GROWTH OR INVASION INHIBITORY ASSAYS
While there are some promising vaccines in development against the hepatic stages of malaria, studies of growth inhibition are a recent but important means to observe functions of the immune systems against malaria (Epping et al., 1988; O’Donnell et al., 2001; John et al., 2004) and are used to directly test the ability of potential erythrocyte stage vaccine candidates to block parasite invasion (Grimberg et al., 2007). One of the biggest advantages of using flow cytometry for this assay is that the appropriate stain can distinguish single, double, or triple invasion events. This is more difficult with microscopy and impossible by HX uptake or enzymatic assays. For invasion inhibition studies of potential vaccine candidates or assays of human sera from patients from endemic countries, the malaria parasites life cycle stages can be synchronized 24 hours before the application of the antibodies, grown for 24 hours, and then stained to observe the number of new invasion events at different dilutions of the antibody (Jensen et al., 1984; Ahlborg et al., 1996; O’Donnell et al., 2001; Moll et al., 2008). By synchronizing the parasites in this manner, experimenters can be certain that any increase in DNA is the result of a new invasion event (Dent et al., 2009) and not DNA replication. However, synchronization is not necessarily required depending on methods being used (Haynes et al., 2002) and is not recommended when using ex vivo parasites (Grimberg et al., 2007). To study growth inhibition, diluted sera from patients who live in endemic areas are applied to parasite cultures and grown for 24 to 96 hours (Persson et al., 2006) and cultures are observed to attempt to detect reductions in parasitemia compared with controls. The same caveats apply to growing parasites for longer than the traditional 48 hours without replacing the media as discussed above in section 3.3. Using SYBR Green I or Hoechst 33342 stains, Dent et al (2008), was able to show that the sera of children in malaria endemic regions had lower inhibition abilities of malaria parasite growth and invasion in culture than the adults from the same region. With the validation and comparisons of these methods, we anticipate seeing more widespread investigation of malaria immunity by cytometry.
3.5 ANTIBODIES FOR STUDYING MALARIA
There are a number (34) of antibodies available to study malaria parasite invasion inhibition available from the MR4 branch of the ATCC (Manassas, VA). These antibodies range from those directed against the merozoite surface proteins (MSP) of P. falciparum and Plasmodium vivax (P. vivax) and to the erythrocyte binding antigen (EBA; (Sim et al., 1990; Adams et al., 1992) to those directed against the Duffy Binding Protein (DBP) of P. vivax (Fraser et al., 1997) as well as others (Barr et al., 1991). Use of these antibodies to deter invasion has shown some success by microscopy (O’Donnell et al., 2001) or by flow (Dent et al., 2008) for P. falciparum. Antibodies collected from the sera of patients from malaria endemic countries showed age dependant growth inhibition assay (GIA) and invasion inhibition assay (IIA) as described later (Dent et al., 2009). Also, antibodies against the P. vivax DBP isolated from patients and inoculated animals were able to show inhibition of P. vivax invasion by microscopy (Grimberg et al., 2007) but not by cytometry so far.
4. SINGLE STAINS FOR DETERMINING MALARIA PARASITEMIA
Because of the wide array of equipment available to malariologists, a large number of stains have been utilized to attempt to detect parasitemia using flow cytometry. The uses and doses applied to the parasites are listed in Table 1 along with excitation and emission information. Based on size and shape, intact RBC are easily identified (Figure 2) and uninfected cells are distinguished from DNA containing cells using stains which have differing degrees of effectiveness and fluorescent properties (Figure 3).
Figure 2. Identification of Populations from Forward Scatter/Side Scatter of Red Blood Cells.
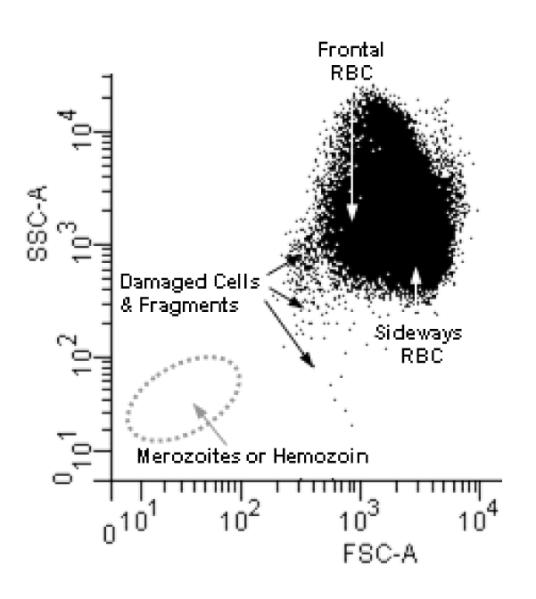
The biconcave shape of the red blood cell allows for the cell to pass through the flow core of the cytometer with the lasers passing through its front in the center of the cell which is 2 microns thick or pass through sideways where the laser passes through its’ width which is 6 microns across. This accounts for the two major populations observed. In any given sample their can also be platelets or cell fragments which can confound further analysis of the malaria parasites. Also a combination of hemozoin (of varying sizes) and free merozoites can be found in some samples. The hemozoin populations can be removed by the magnetic separation and the merozoites are positive for the presence of 1N copy of DNA (Boyle et al., 2010).
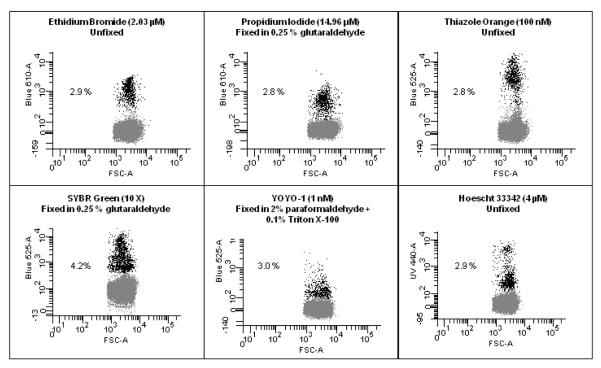
Figure 3. Comparisons of Different DNA stains.
A sample of P.falciparum (3D7 Strain, MRA-102 deposited to ATCC/MR4 by DJ Carucci) was cultured as previously indicated (McNamara et al., 2006) to a parasitemia of 2.9% as determined by Giemsa stained microscopy slide. This culture was then exposed to stains commonly used to examine malaria. Though there may be more optimal methods, staining was carried out as indicated in references; Ethidium Bromide (Persson et al., 2006), Propidium Iodide (Pattanapanyasat et al., 1997), Thiazole Orange (Makler et al., 1987), SYBR Green (Dent et al., 2008), YOYO-1 (Dahl and Rosenthal, 2007), Hoechst 33342 (Grimberg et al., 2008) and the resulting frequency of positive cells is indicated. The blood sample used for this study were leukocyte depleted using Histopaque 1119 and had been in culture for 3 days and therefore, there were few reticulocytes left at the time of analysis. In addition, the parasites had been serial transferred two times after thawing from frozen stocks which led to very clean samples, (i.e. containing very few dead parasites or cell fragments), which led to 5 out of the 6 stains tested showing the same parasitemia. When dealing with samples from patients all of the above issues can confuse a clear delineation between uninfected and infected cells. This figure shows that when using a well prepared in vitro sample with a moderate parasite load, many of the commonly used stains can determine parasitemia.
4.1 INTERCALATING CLASS STAINS
4.1.1 PROPIDIUM IODIDE (PI)
The use of propidium iodide (Nucleic acids, Ex 535/Em 617, Impermeable) for fluorescent detection of DNA dates back at least to the mid nineteen seventies (Keller, 1975; Sugden et al., 1975) and is used to distinguish between live and dead cells in the absence of fixatives similar to trypan blue (Pappenheimer, 1917; Liu and Janeway, 1990). The double positive charge of this compound results in its inability to pass through unfixed membranes (Shapiro, 2003) but gives it a high affinity for nucleic acids. Propidium iodide is in the intercalating class of nucleic acid stains, is related to ethidium bromide (ETBR) but differs in that on its heterocyclic ring the nitrogen has a n-propyltrialkyl quaternary ammonium group. Propidium iodide is commonly used in conjunction with other stains to exclude dead cells from analysis; however, its broad emission spectra constrains the useable combinations with other stains to the 525 nm range and below.
This stain is useful to study malaria parasites in part because of the broad excitation and emissions spectra (Pattanapanyasat et al., 1993) which can potentially overcome the autofluorescent nature of fixed erythrocytes at high doses. One of the potential challenges with using PI is that when the ionic strength of the fluid used to stain cells is not equal to the sheath fluid, fluctuations and shifting peaks may occur (LePecq and Paoletti, 1967; Martens et al., 1981). This stain, like the other nucleic acid stains, is most effective went used with RNAse to identify true DNA positive erythrocytes though alternatives have been considered (Krishan, 1975).
4.1.2 ETHIDIUM BROMIDE (ETBR)
This broad nucleic acid binding intercalating class stain (Ex 510/Em 595, Permeable ) has been used for decades for the purpose of detecting DNA or RNA on agarose gels. This stain has many advantages; it is cheap, it is technically permeant given enough time, and it works on most standard cytometers because it can be excited by ultraviolet (UV), violet, blue, or green lasers. Ethidium bromide shares the heterocyclic ring structure of PI but it has an ethyl group on the ring nitrogen. This structural change gives a delocalized positive charge making ETBR able to slowly pass through most membranes though it can be quickly pumped out, so it does not necessarily require the use of a fixative.
One of the major drawbacks to ETBR is that it is highly toxic to the cells and a carcinogen to the users which can require extra steps for the proper treatment and disposal of the waste sheath fluid if sufficient quantities are used (Shapiro, 2003). Because this stain interacts with both DNA and RNA alike, the presence of reticulocytes (particularly in samples from children) can also be a confounding factor. When ETBR was used for GIA, IIA, and drug assays, the number of copies of DNA within erythrocytes could not be determined making differentiation of single and multiple invasion events difficult (Staalsoe et al., 1999) (Figure 3).
4.1.3 HYDROETHIDINE (HE)
Hydroethidine (Indicates live cells, Ex 365/Em 436 & DNA Ex535/Em610, Permeable) is a reduced form of ETBR, another intercalating class stain, which has higher membrane permeability. At low concentrations ETBR is very slow to cross membranes, so much so that ETBR was long thought to be a impermeant (Shapiro, 2003). Hydroethidine was first introduced by Gallop (1984) to address this issue and has proved useful as a superoxide radical anion indicator (Zhao et al., 2003). In live cells, HE accumulates in the cytosol and appears blue when excited by UV light. This aspect of the stain has been used to demonstrate cell viability, but when HE is intercalated with DNA in a cell’s nucleus then it oxidizes and fluoresces red much like ETBR. One of the obvious issues with this stain is that when a UV laser is used the blue fluorescence of the cytosol stain can interfere with other blue color stains. Van der Hyede adapted a method used to study other intraerythrocytic parasites (Wyatt et al., 1991) for the study of P. falciparum (van der Heyde et al., 1995) to determine malaria parasitemia in the presence of leukocytes in culture using cytometry. Because HE needs to be dehydrogenated (oxidized) to become the ethidium form which intercalates into DNA, which can happen in the presence of metabolic activity, one can infer that any cells which are positive after HE staining contain live parasites. Using HE, the authors also showed that the leukocytes were alive and monitored their activity against the malaria parasite infected erythrocytes. Interestingly, they were able to observe that neither peripheral blood monocytes nor monocyte-derived macrophages showed the capacity to kill or to inhibit parasite invasion under these assay conditions. This study also showed that monocyte-derived macrophages seemed to speed parasite growth though the method of this interaction remains undetermined.
4.2 CYANINE CLASS STAINS
4.2.1 THIAZOLE ORANGE (TO)
Thiazole orange (Stains primarily RNA, Ex 509/ Em533, Permeable) is a cyanine stain, based on thioflavin T, that was purposely designed to identify reticulocytes in blood samples (Lee et al., 1986). This stain preferentially stains the AU nucleic acid doublets of RNA and when bound to RNA it fluoresces 3,000 fold more than free dye. Thiazole orange works because it has a delocalized positive charge that makes it permeant and does not require the use of fixatives or RNAse. Thiazole orange can measure some of the DNA content of cells much like thioflavin T; however, this stain predominantly detects RNA content. As described by Grimberg et al (2008), and discussed later, binding of TO to DNA can be out competed by the DNA stain Hoechst 33342. This small DNA binding affinity when TO is used alone makes it difficult to detect new infections and ring stage parasites and only detects late stage parasites (Makler et al., 1987). There is difficulty in detecting new invasions with TO because of its propensity for RNA which is not expressed in sufficient quantities until many hours after invasion. However, because non-reticulocytes generally do not contain DNA and adults contain a low recticulocytemia (Ferri, 2007), this is not generally an issue.
4.2.2 SYTOX Green
SYTOX Green (Nucleic acids, Ex 504/Em 523, Impermeable) is a cyanine derivative of TO which binds to nucleic acids and can be useful to quantify DNA content provided that the that the cells are fixed and permeablized (Chandramohanadas et al., 2009). This stain is highly fluorescent when bound to double stranded DNA but remains un-fluorescent when unbound in solution. Generally, this stain has been used as a live/dead indicator. The excitation peak is not particularly broad (though it does have a shoulder that allows > 50% fluorescence to be read in a 575 channel. Because of the lengthy preparation time and effort and because it occupies a widely used section of the fluorescence spectrum this stain has not been widely adapted to study malaria. But with a 1000X increase in fluorescence when bound to DNA it is extremely bright which allows for good discrimination of parasitized erythrocytes.
4.2.3 YOYO-1
YOYO-1 (Nucleic acids, Ex 491/Em 509, Impermeable) was first synthesized by Rye et al. (1991), a dimeric cyanine stain, is comprised of is two molecules of oxazole yellow joined together and was created to have increased binding intensity over its relative TO. The binding affinity of YOYO-1 is high enough to even out compete ETBR. YOYO-1 is one of the most sensitive stains for nucleic acids particularly among those which have a propensity for DNA (Figure 3) and under some conditions its specificity make it an improvement over than Hoechst 33258 (HO258) in human (Whaun et al., 1983) and mouse (Barkan et al., 2000) samples. In order to get the most reliable results and to not be confused by the presence of reticulocytes, the use of RNAse after fixation is highly recommended (Barkan et al., 2000; Li et al., 2007). The use of optimized staining, RNAse, and analysis techniques (through identification of autofluorescent populations) have reportedly allowed detection levels down to 0.01% parasites in mouse models (Jimenez-Diaz et al., 2005).
4.2.4 SYTO SERIES
The SYTO family of stains are relatively new stains which are asymmetric cyanine nucleic acid stains that are in the cell-permeant class (Frey, 1995). These stains opened many new color options to malaria cytometrists who are sometimes limited when it comes to permeant stains. Also, because of the variety of colors available, these stains are suitable for combination with other common fluorochrome labeled antibodies. Three different colors of the 85 SYTO stains have been used to study malaria parasites in the past two years since their release. The first two are SYTO-9 (DNA, Ex485/Em598 & RNA Ex 486/501, Permeable) (Izumiyama et al., 2009) and SYTO-16 (DNA Ex 488/Em 518 & RNA Ex 494/Em 525, Permeable)(Dahl and Rosenthal, 2007; Angulo-Barturen et al., 2008). Two years ago versions which were excited by red lasers were introduced (Wlodkowic et al., 2008) and in February of this year SYTO-61 (Nucleic acids, Ex 628/Em 645, Permeable) was applied to malaria studies (Fu et al., 2010). Additional SYTO stains have different binding affinities for DNA and RNA which increases their utility in malaria studies (Tarnok, 2008).
4.3 MISCELLANEOUS CLASS STAINS
4.3.1 ACRIDINE ORANGE (AO)
Acridine orange (DNA Ex 503/Em 530 & RNA Ex 503/Em 640, Impermeable) dual color stain introduced by Darzynkiewicz et al.(1976) is a miscellaneous class nucleic acid stain which can function as a DNA/RNA indicator stain not because of differences in its binding affinities for these nucleic acids but rather because of the localization of different stain molecules. For DNA, the stain intercalates to the double helix which allows for fluorescence but prevents the stain molecules from interacting with each other and thus emits a green fluorescence (Shapiro, 2003). When the stain binds to single stranded RNA, the stain molecules can bind to the nucleic acid more closely to one another and form a metachromatic aggregate which fluoresces red. While this is a good general indication of DNA and RNA, when single-stranded or denatured DNA is present, the stain will also bind and fluoresce red. Therefore, the levels of each nucleic acid are not absolute. Based on the fluorescence of this stain the different levels of nucleic acids expressed can be identified and the G1, S, and G2 cell-cycle stages can be distinguished (Darzynkiewicz et al., 1980a; Darzynkiewicz et al., 1980b).
Several groups have tried to infer the life cycle stage of the malaria parasite based on their cytometry data resulting from AO staining (Hare, 1986; Hare and Bahler, 1986; Shapiro and Mandy, 2007). Theoretically, this is possible because the ring stages generally do not express much RNA and have no DNA synthesis so they should fluoresce exclusively green. As RNA increases there will be more red staining indicating the trophozoite stage and as DNA synthesis increases there will be more green and red staining. The issues with AO in particular is that it is not generally a user-friendly stain and requires precise timing of acid treatment or fixatives to effectively get the stain in to the cells. The stain also quenches on binding to nucleic acids (Shapiro and Mandy, 2007) which makes reproducibility difficult thus decreasing its reliability. These technical issues in addition to AO staining of vesicles that form during ring stage development which interferes with measurements of DNA have made precise quantification of malaria parasite life cycle stages difficult (Jacobberger et al., 1992). These issues, the cellular toxicity of the stain, and the incompatibility with surface antigens studies, (Shapiro, 1981) make it less than ideal for current malaria studies. However, AO is the progenitor of the SYTO families of stains which are becoming more widely used.
4.9 SYBR GREEN I
SYBR Green I (DNA, Ex 488/Em 522, Impermeable) was initially developed as a nontoxic and more quantifiable replacement for ETBR on agarose gels (Shapiro, 2003). One of the biggest advantages of SYBR Green I is that it can be used on most standard cytometers including those being developed and adapted for fieldwork. This stain is reasonably selective for double stranded DNA and can be using to detect DNA copy numbers (up to 3N) in synchronized parasite cultures (Figure 3). However, this stain can also detect some double stranded RNA therefore, to obtain optimum results the cells may need to be treated with RNAse. However, because of the brightness of the stain (higher quantum efficiently than the Hoechst stains) this has not been required (Dent et al., 2008). For malaria studies, SYBR Green I is used at 2-10X final concentration from a 10,000X stock (Molecular Probes, Eugene, OR) to provide the best results. Based on a head to head comparison with other DNA or nucleic acid stains conducted for this review, SYBR GI shows a larger number of positive cells than other stains and is not well correlated with the actual parasitemia in this example (Figure 3) though alternative methods may improve accuracy.
4.10 HOECHST 33258 (HO258)
The Hoechst series of stains synthesized by Loewe (1974) were initially developed to be used as treatments for malaria and other diseases caused by protozoans. While their utility as drugs was short lived, Latt (1973) showed that they could be very useful to the burgeoning field of flow cytometry. Hoechst stains are bisbenzimidazole derivatives which are supravital, and bind to the minor groove of DNA (minor groove class) with AT selectivity (Muller and Gautier, 1975). Hoechst 33258 (DNA only, Ex 345/Em 478, Permeable) is an ideal stain for studying malaria, P. falciparum in particular because the parasite’s genome is more than 80% AT rich (Gardner et al., 2002). HO258 has reversible nonintercalating binding that has no effect on parasite growth, and allows for surface staining (Howard et al., 1979) with other fluorochromes without fixation or damage to surface antigens. Initial cytometric studies of any malaria parasite were carried out on the mouse malaria parasite P. berghei (Howard et al., 1979) and HO258 was quickly adapted to study human malaria parasites (Brown et al., 1980). It wasn’t until 1990, however, that this stain was used to directly measure intracellular DNA levels of erythrocytes and the parasite productivity related to determine IC50 values (van Vianen et al., 1990). In order to increase the speed of the technique, lysed parasites in the presence and absence of antimalarial compounds were used to determine the IC50 values of antimalarial compounds on total amount of DNA in samples (Smeijsters et al., 1996). One of the criticisms of this family of stains is that they require the use of a cost-prohibitive UV laser which does not have widespread application, particularly in malaria endemic regions (Shapiro and Perlmutter, 2008; Izumiyama et al., 2009).
4.11 HOECHST 33342 (HO342)
Hoechst 33342 (DNA only, Ex 355/Em 465, Permeable) is a stain related to HO258 with an additional ethyl group that results in increased lipophilicity. This ultra low background stain otherwise acts in a similar manner to its cousin, HO258. Other than DRAQ5 this has been the only stain which has been able to make precise measurements of DNA content (Shapiro, 2003) which is very useful when attempting to determine the number of invasion events (Dent et al., 2008). In 1977, it was shown that this stain could be used to examine and sort cells and more importantly, it was shown that the stain did not damage the cells. After treatment with the stain, cells continued to live and grow in culture (Arndt-Jovin and Jovin, 1977). This technique was successfully applied to the sorting of human malaria parasites in 1980 (Brown et al., 1980). Early examinations of P. falciparum staining with HO342 were performed with 1.6 μM (Franklin et al., 1986) but effective use of this stain generally requires a minimum of 5 μM stain for 30 minutes (Shapiro, 2003) to allow enough time for the stain to pervade the cells. Saturation of the DNA is needed if accurate quantification of DNA content is desired. Because this stain emits in the lower wavelengths it is a likely candidate to be combined with antibody labeled fluorochromes as well as membrane potential stains such as DiOC1-3 (Jacobberger et al., 1984), DiIC1-5 (Grimberg et al., 2009), and intracellular stains such as thiazole orange (Jouin et al., 1995; Grimberg et al., 2008) and Pyronin Y(Shapiro, 1981).
5. MULTIPLE STAINS
As cytometers have improved and the ability to add more lasers has developed, combinations of stains have been used to examine more aspects of human malaria parasite growth and development. The variety of different nucleic acid stains described above as well as membrane potential stains have been combined together (summarized in Table 2) with additional stains to learn more about malaria parasite biology. Also, in addition to stain combinations there are an ever expanding array of fluorescent colors which could be combined with the various nucleic acid stains discussed here. Fluorochromes could be added in conjunction with malaria parasite antibodies or against red cell surface antigens related to malaria parasite invasion or growth, but thus far few have been utilized in cytometry (PE, (Jouin et al., 1995; Woolley et al., 2000; Angulo-Barturen et al., 2008)); FITC, (Jouin et al., 1995; Piper et al., 1999; Staalsoe et al., 1999; Riquelme et al., 2006)).
TABLE 2.
Stain Combinations to Study Human Malaria Parasite Growth and Invasion
| Stain | Interacts with | Excitation Maximum |
Emission Maximum |
Dose | Fixative | Reference |
|---|---|---|---|---|---|---|
|
Hydroethidine
+ Thiazole Orange |
DNA | 536 | 610 | 158.48 μM | None | (Jouin et al., 2004) |
| Predominately RNA |
509 | 533 | 100 nM1 | |||
|
| ||||||
|
Hoechst 33342
+ Thiazole Orange |
DNA Only | 355 | 465 | 4 μM | None | (Grimberg et al., 2008)2, 4 |
| Predominately RNA |
509 | 533 | 100 nM | |||
|
| ||||||
|
Hoechst 33342 + Thiazole Orange + DiIC 1-5 |
DNA Only | 355 | 465 | 5 μM | None | (Grimberg et al., 2009)3, 4 |
| Predominately RNA |
509 | 533 | 100 nM | |||
| Membrane Potential |
638 | 658 | 25 nM | |||
Retic-Count Solution was later determined to contain 100 nM Thiazole Orange.
Also used by Jouin et al.(1995) using 32 μM Hoechst and 1 mL of BD Retic-Count solution 1.
Used to determine IC50 of antimalarial compounds.
Allows for differentiation of parasite erythrocytic stages (rings, trophozoites, schizonts).
Multicolored staining of P. falciparum began with Acridine Orange, but it has been complicated to use effectively therefore it was unlikely to be used in combination with other stains. Shapiro described the use of an easier stain combination to differentiate nucleic acids by combining HO342 with a more RNA selective stain (Shapiro, 1981) and used this to increase sensitivity for cell cycle detection using flow cytometry. Pyronin Y, a homolog of AO, was an obvious choice because it is more selective for double stranded RNA, does not fluoresce when attached to DNA (Darzynkiewicz et al., 1986; Kapuscinski and Darzynkiewicz, 1987), and can detect phases of cell growth cycle (Crissman et al., 1985). Pyronin Y at low concentrations (below 3.3 pM) localizes in the mitochondria while at concentrations higher than 5 pM it interacts with RNA but is toxic to the cells (Darzynkiewicz et al., 1986; Kapuscinski and Darzynkiewicz, 1987). It is in part for this reason that this method was not employed but the idea of combining DNA and RNA selective stains is gaining a lot of ground in the malaria community for use in differentiation different stages of the malaria parasite erythrocyte growth cycle.
5.1 HOECHST 33342 + THIAZOLE ORANGE
Shapiro’s methods were modified by Jouin et al. (Jouin et al., 1995) who used HO342 plus the RNA selective stain, TO (Lee et al., 1986; Makler et al., 1987). When P. falciparum erythrocyte life cycle stages were isolated on density gradients, Jouin et al. (Jouin et al., 1995) showed a large difference in the staining patterns of the parasite stages using HO342 and TO much like AO studies of Jacobberger (Jacobberger et al., 1992). However, these studies used a large number of parasites, a lethal dose of HO342 which was 6 times more than previously described (Jacobberger et al., 1992), and they could not identify life cycle stages from an asynchronous culture. Grimberg et al. expanded and improved this technique (2008) as is explained in more detail below.
5.2 HYDROETHIDINE + THIAZOLE ORANGE
To improve over existing stains, in particular AO, without the use of an UV laser such as with HO342 or use of fixatives Jouin et al, (Jouin et al., 2004) altered their previous strategy using the permeant stain HE, which has the added advantage of being a pseudo membrane live/dead indicator when it accumulates in the cytosol (van der Heyde et al., 1995) but it was not used in this manner in this paper. The additional advantage to this staining strategy is that it allows for the addition of fluorescent antibody. Clear delineation of the trophozoite/schizont group from the ring group was identified from a mixed asynchronous culture based on RNA staining (Figure 4). However, this led to the idea that with appropriate staining differentiation of the ring from the trophozoites could be made based on a marked increase in the amount of RNA. In addition, the use of a better or more effective DNA stain which identifies malaria parasite DNA copy number, could distinguish trophozoites from schizonts.
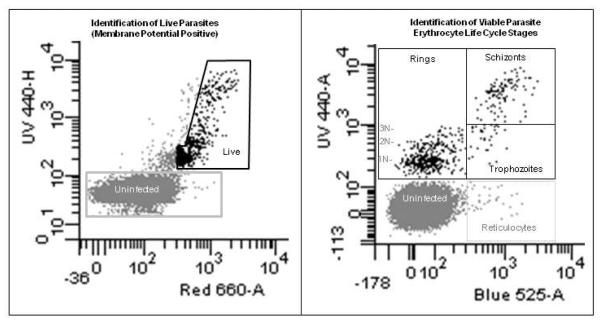
Figure 4. Identification of Erythrocyte Lifecycle Stages of P. falciparum.
The same sample of 2.9% parasitemia culture of P. falciparum used in Figure 3 was stained with HO342, TO, and DiIC1-5 as described in Grimberg et al. (2009). Gates positions were determined previously (Grimberg et al., 2008; Grimberg et al., 2009). In brief, DNA positive cells (Y-axis, UV 440, HO342) in the left hand panel which showed membrane potential (X axis, Red 660, DiIC1-5) greater than that of the red blood cells were considered to be alive. The slanted area of this gate was determined by the application of a cyanide derivative (carbonyl cyanide 3-chlorophenylhydrazone) which disrupts membrane potential within the cells. In the right hand panel, the DNA negative uninfected cells (from the gray square) are shown along with the Live DNA positive cells (black polygon). By showing the relative amount of RNA in these cells one can clearly identify the different erythrocyte life cycle stages of the parasites. Ring stages do not express very much RNA, trophozoites express RNA but do not replicate DNA, and schizonts have increases in both RNA and DNA. The delineation between schizonts and trophozoites has been made between 3 and 4 copies of DNA (Grimberg et al., 2008) in general because of the lack of 4N rings (therefore 4N DNA/RNA positive cells almost exclusively arise through DNA synthesis).
5.3 HOECHST 33342 + THIAZOLE ORANGE + DiIC1-5
The high dose of HO342 used in previous combinations of TO + HO342 prevented binding of the TO stain to DNA similar to Pyronin Y as described earlier (Shapiro, 2003). It has recently been theorized that despite their lack of apoptotic mechanisms, particularly in culture, a number of parasites within live RBC may actually be dead (Meslin et al., 2007). Use of membrane potential stains have been important also for the study of apoptosis in human and mammalian cell lines (Shapiro et al., 1979; Cossarizza et al., 1994; Cossarizza et al., 2001). This idea arose because Grimberg et al. (2009) showed that the effects of experimental drugs compounds not only inhibit cell growth and subsequent invasion but also induce parasite death. This effect could not be seen previously because erythrocytes remain intact and therefore standard live/dead stains (such as PI) would not be taken up by the parasite; and HE would not work because although it enters the cell through the live membrane, it still interacts with the DNA of the parasties (live or dead).
The Grimberg et al. method (HO342 + TO + DiIC1-5 (Indicates membrane potential, Ex 638/Em 658, Permeable) allows for objective determinations of the malaria parasite life cycle stage and the effects of drugs on the various parasite stages. The addition of a membrane potential stain to DNA+RNA stains begins to reveal another picture of malaria parasite: frequently in culture and presumably in vivo, the malaria parasites die but maintain intact DNA and more importantly remain within live erythrocytes. This idea calls into question the validity of all DNA only assays, which are certainly skewed by the presences of these crisis forms (Meslin et al., 2007) of the parasite. The identification of active parasite death and not just halting of parasite invasion opens the door for true determinations of drug (lethal dose for 50% of the cells (LD50 )) values instead of just IC50 values for drugs and also possibly by invasive antibodies.
With this stain combination we can also begin to identify what is believed to be a prelysis stage called segmenters (Moore et al., 2006) which are metabolically shut down, have decreased membrane potential, and RNA levels, but have peaked their DNA copy number (Figure 4). This stage is particularly impervious to most common antimalarials which are focused on interruption of hemozoin formation which is completed by the time the parasites reach the segmented phase. If this stage could be targeted it would block continuing disease by halting further infection of naive RBCs even though dead parasites may have pathogenic properties even within a live parasites (Hughes et al., 2010).
6. FLUORESCENT PROTEINS
In addition to staining malaria parasites with stains for nucleic acids and membrane potential the parasites can also be manipulated to express green fluorescent protein (GFP Ex 395/ Em 508) (VanWye and Haldar, 1997). Thus far, few labs have successfully created stable transfections of P. falciparum and none have transfected P. vivax. Crabb et al. provided an excellent overview and description of vectors and transfection methodology for P. falciparum (2004). The use of GFP-labeled parasites to investigate malaria growth has its advantages and recently a number of knockout/knock-in transfectants have been developed to track organelles and surface proteins (Tilley et al., 2007). This fluorescent protein has been used to investigate the influence of drug resistance genes such as DHFR (Crabb et al., 2004) and to identify IC50 values (Sanchez et al., 2007). The difficulty in performing successful transfections to label parasites however, severely limits the number of strains of malaria parasites which can be studied and thus far eliminates the possibility of studying wild parasites. Also there are only a few stably transfected strains available to researchers from the ATCC/MR4, one of which is a GFP labeled strain of 3D7 (PfHDGFP ) (Kadekoppala et al., 2000). A drawback to performing drug or growth inhibition assays by flow using GFP-labeled parasites is that once expressed, the fluorescent protein can remain active in a cell long after the parasite has died which confuses any results observed.
7. DETECTION OF HEMOZOIN WITHIN CELLS
When hemoglobin is digested, it releases free heme (in the form of ferriprotoporphyrin IX also called α-hematin) which is a highly reactive particle that if not neutralized, it will kill the parasite. Lacking a mechanism to extrude the toxin the parasite combines it together to the inert form, hemozoin. When fully formed the hemozoin is birefringent and can reflect light. Therefore, one can observe the presence of this substance via microscopy even in the absence of Giemsa stain (Lawrence and Olson, 1986). Several papers have used Cell Dyn cytometers (Hanscheid et al., 2000a; Hanscheid et al., 2000b; Grobusch et al., 2003) to detect the presence of hemozoin crystals as an indication of a malaria infection. Thus far this method has had sensitivity issues which can be lower than 50% (Grobusch et al., 2003). The difficultly in correlating with microscopy is in part because hemozoin crystals do not begin to form until the trophozoite stage, which means that this method is completely missing treating early stage parasites. So far, this method has not been used to attempt to observe the malaria parasite’s response to antimalarial drugs or antibodies. However, for making clinical diagnosis of malaria the sensitivity of this method has been reported to be improving up to 95% when focused on the presence of pigment not in the RBCs but in the WBCs of patient samples (Hanscheid et al., 2001).
8. FUTURE DIRECTIONS
The future of malaria cytometry is moving beyond simple identifications of DNA content of cultures and is further parsing apart the parasite life cycle and the life cycle stage specific response to drugs and antibodies. Malaria cytometry is advancing in three major directions. The first of which is the identification and validation of simple DNA stains which can be analyzed on simpler or cheaper machines for use in malaria endemic regions on patient samples. Success in portability and reliability of a basic one or two color laser cytometer means that regional clinic may be able to perform cytometric assays on patient samples and potentially identify treatments appropriate for each individual. The second direction is the utilization of advanced cytometry methods using 4 to 5 lasers and increasing numbers of fluorochromes to learn more about the malaria parasite biology and to, perform high throughput screening of new compounds for malaria treatment. We are moving into an era where we can know not only the IC50 of the given drugs are but also the LD50 of drugs which will help us to design and deliver safer, more effective treatments. The final direction of this area of study is the mainstreaming of antibody assays as they relate to human malaria parasite invasion. The labeling of antimalarial antibodies coupled with the previously discussed nucleic acid binding fluorochromes will improve our understanding of parasite binding and invasion. The improvement and further use of GIA/IIA and studies of erythrocyte surface antigens increases the likelihood that we will be successful in the creation of a useful vaccine candidate.
In addition to the myriad of stains described above, malaria investigations may also be expanded using new stains and stain combinations. Several new stains are being explored out of necessity. Most malaria laboratories in endemic countries do not have access to UV lasers needed to use the Hoechst stains in particular. On machines with isolinear lasers, HO342 is difficult to use in combination with other stains because of its broad emission spectrum that interferes with the 525 nm channel where so many other stains are read. The vibrant dye cycle stains are a useful alternative for these types of machines even though they lack the huge stokes shift seen in Hoechst stains. This family of stains come in several colors options which allows for multiplex staining of malaria parasites and the use of other stains or fluorochrome conjugated antibodies. Vibrant dye cycle green and related colors are also highly selective for DNA and may be used to instead of Hoechst. In addition, new RNA styryl stains are being developed which can further open the spectra for standard conjugates (Cervantes et al., 2009). Picogreen also shows a great deal of promise because of the high specificity for parasite DNA, but requires the complete lysis of the cells and so has therefore not yet been used in cytometric assays (Corbett et al., 2004). The search remains for simple stains which can perform this of reliable detection of DNA content, preferably without requiring fixation, which is excited by either a red or blue laser.
Several older stains have been used in microscopy or cytometric studies of nonhuman malarias. These stains such as DAPI (Baniecki et al., 2007) and DRAQ5 (Billker et al., 2004), may have potential for the examination of human malaria cytometric studies. The lack of reported use of DRAQ5 in the study of human malaria flow cytometer is interesting since it is the only membrane permeable stain other than HO342 which can create DNA content histograms (Smith et al., 2000). However, perhaps in part because of the expense, or difficulty detecting ring stage parasites, or that it can be excited by both red and blue lasers, this stain remains primarily used for the microscopic visualization of nuclei. SYTO-13 and SYTO-17 are two additional stains which have not been tried even though both label DNA and RNA similar to the other SYTO stains. Finally, there are several other Hoechst stain derivatives (34580, 33378, 33662, and nuclear yellow) which have not been used to study malaria. However, it is unknown if any of these stains offer marked improvement over existing staining strategies.
As drug resistance becomes more wide spread it has becomes important to not only identify new drugs which halt parasite growth but also to further identify those which directly kill the parasites and lessen the likelihood for the development of resistance. Malaria parasite apoptosis induction is a new and expanding area of study (Meslin et al., 2007) which will become the future of drug studies and the source for effective new antimalarial compounds. The use of flow cytometry could serve as an excellent platform to make these evaluations. In addition, as malaria parasite drug resistance spreads an additional goal would be to treat patients and take a small amount of their blood for screening against available drugs at the point of care to provide an indication for the most effective treatments. The ability for malaria endemic countries to perform cytometric assays “in house” will greatly improve diagnosis and aid our understanding of the epidemiology of the disease particularly as WHO and the Global Fund prepare to implement malaria eradication efforts once again. As smaller and more portable cytometers, made by Accuri (C6), Guava/Millipore (easyCyte), Stratedigm (S1000), and Partec (CyFlow SL) become more reliable, their presence at the site of malaria treatment will increase. The ability to determine parasitemia, quickly and accurately, will aid in our understanding of the effectiveness of interventional efforts such as mass drug treatment, bed nets, education, and indoor spraying, etc. Moving forward on all of these fronts to improve malaria cytometric investigation will help to minimize the global effect of malaria.
9. ACKNOWLEDGMENTS
The author thanks Kerry O. Grimberg for helpful discussions and critical evaluation of this manuscript. The author thanks the Flow Cytometry Core Facility of the Comprehensive Cancer Center of Case Western Reserve University and University Hospitals of Cleveland (P30 CA43703) and Harvey Motulsky, for their generous technical support. BTG is supported by the Case Western Reserve University School of Medicine Vision Fund and the NIH AI079388.
ABBREVIATIONS
- AO
Acridine Orange
- DBP
Duffy Binding Protein
- EBA
Erythrocyte Binding Antigen
- ETBR
Ethidium Bromide
- GIA
Growth Inhibitory Assay
- HO258
Hoechst 33258
- HO342
Hoechst 33342
- HE
Hydroethidine
- HX
Hypoxanthine
- IC50
50% Inhibitory Concentration
- IIA
Invasion Inhibitory Assay
- MSP
Merozoite Surface Proteins
- PI
Propidium Iodide
- RBC
Red Blood Cells
- SYBR GI
SYBR Green I
- TO
Thiazole Orange
- WBC
White Blood Cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
10. REFERENCES
- Adams JH, Sim BK, Dolan SA, Fang X, Kaslow DC, Miller LH. A family of erythrocyte binding proteins of malaria parasites. Proc Natl Acad Sci USA. 1992;89:7085–9. doi: 10.1073/pnas.89.15.7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlborg N, Iqbal J, Bjork L, Stahl S, Perlmann P, Berzins K. Plasmodium falciparum: differential parasite growth inhibition mediated by antibodies to the antigens Pf332 and Pf155/RESA. Exp Parasitol. 1996;82:155–63. doi: 10.1006/expr.1996.0020. [DOI] [PubMed] [Google Scholar]
- Angulo-Barturen I, Jimenez-Diaz MB, Mulet T, Rullas J, Herreros E, Ferrer S, Jimenez E, Mendoza A, Regadera J, Rosenthal PJ, Bathurst I, Pompliano DL, de las Heras F. Gomez, Gargallo-Viola D. A murine model of falciparum-malaria by in vivo selection of competent strains in non-myelodepleted mice engrafted with human erythrocytes. PLoS One. 2008;3:e2252. doi: 10.1371/journal.pone.0002252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt-Jovin DJ, Jovin TM. Analysis and sorting of living cells according to deoxyribonucleic acid content. J Histochem Cytochem. 1977;25:585–9. doi: 10.1177/25.7.70450. [DOI] [PubMed] [Google Scholar]
- Baniecki ML, Wirth DF, Clardy J. High-throughput Plasmodium falciparum growth assay for malaria drug discovery. Antimicrob Agents Chemother. 2007;51:716–23. doi: 10.1128/AAC.01144-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan D, Ginsburg H, Golenser J. Optimisation of flow cytometric measurement of parasitaemia in plasmodium-infected mice. Int J Parasitol. 2000;30:649–53. doi: 10.1016/s0020-7519(00)00035-7. [DOI] [PubMed] [Google Scholar]
- Barr PJ, Green KM, Gibson HL, Bathurst IC, Quakyi IA, Kaslow DC. Recombinant Pfs25 protein of Plasmodium falciparum elicits malaria transmission-blocking immunity in experimental animals. J Exp Med. 1991;174:1203–8. doi: 10.1084/jem.174.5.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billker O, Dechamps S, Tewari R, Wenig G, Franke-Fayard B, Brinkmann V. Calcium and a calcium-dependent protein kinase regulate gamete formation and mosquito transmission in a malaria parasite. Cell. 2004;117:503–14. doi: 10.1016/s0092-8674(04)00449-0. [DOI] [PubMed] [Google Scholar]
- Boyle MJ, Wilson DW, Richards JS, Riglar DT, Tetteh KK, Conway DJ, Ralph SA, Baum J, Beeson JG. Isolation of viable Plasmodium falciparum merozoites to define erythrocyte invasion events and advance vaccine and drug development. Proc Natl Acad Sci U S A. 2010;107:14378–83. doi: 10.1073/pnas.1009198107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GV, Battye FL, Howard RJ. Separation of stages of Plasmodium falciparum-infected cells by means of a fluorescence-activated cell sorter. Am J Trop Med Hyg. 1980;29:1147–9. doi: 10.4269/ajtmh.1980.29.1147. [DOI] [PubMed] [Google Scholar]
- Cervantes S, Prudhomme J, Carter D, Gopi KG, Li Q, Chang YT, Le Roch KG. High-content live cell imaging with RNA probes: advancements in high-throughput antimalarial drug discovery. BMC Cell Biol. 2009;10:45. doi: 10.1186/1471-2121-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandramohanadas R, Davis PH, Beiting DP, Harbut MB, Darling C, Velmourougane G, Lee MY, Greer PA, Roos DS, Greenbaum DC. Apicomplexan parasites co-opt host calpains to facilitate their escape from infected cells. Science. 2009;324:794–7. doi: 10.1126/science.1171085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett Y, Herrera L, Gonzalez J, Cubilla L, Capson TL, Coley PD, Kursar TA, Romero LI, Ortega-Barria E. A novel DNA-based microfluorimetric method to evaluate antimalarial drug activity. Am J Trop Med Hyg. 2004;70:119–24. [PubMed] [Google Scholar]
- Cory JM, Rapp F, Ohlsson-Wilhelm BM. Effects of cellular fixatives on human immunodeficiency virus production. Cytometry. 1990;11:647–51. doi: 10.1002/cyto.990110514. [DOI] [PubMed] [Google Scholar]
- Cossarizza A, Kalashnikova G, Grassilli E, Chiappelli F, Salvioli S, Capri M, Barbieri D, Troiano L, Monti D, Franceschi C. Mitochondrial modifications during rat thymocyte apoptosis: a study at the single cell level. Exp Cell Res. 1994;214:323–30. doi: 10.1006/excr.1994.1264. [DOI] [PubMed] [Google Scholar]
- Cossarizza A, Salviolit S, Darzynkiewicz H.A.C.J.P.R. Zbigniew. Methods in Cell Biology. Part 1. Volume 63. Academic Press; 2001. Chapter 21 Analysis of mitochondria during cell death; pp. 467–486. [DOI] [PubMed] [Google Scholar]
- Crabb BS, Rug M, Gilberger TW, Thompson JK, Triglia T, Maier AG, Cowman AF. Transfection of the human malaria parasite Plasmodium falciparum. Methods Mol Biol. 2004;270:263–76. doi: 10.1385/1-59259-793-9:263. [DOI] [PubMed] [Google Scholar]
- Crissman HA, Darzynkiewicz Z, Tobey RA, Steinkamp JA. Correlated measurements of DNA, RNA, and protein in individual cells by flow cytometry. Science. 1985;228:1321–4. doi: 10.1126/science.2408339. [DOI] [PubMed] [Google Scholar]
- Dahl EL, Rosenthal PJ. Multiple antibiotics exert delayed effects against the Plasmodium falciparum apicoplast. Antimicrob Agents Chemother. 2007;51:3485–90. doi: 10.1128/AAC.00527-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darzynkiewicz Z, Kapuscinski J, Carter SP, Schmid FA, Melamed MR. Cytostatic and cytotoxic properties of pyronin Y: relation to mitochondrial localization of the dye and its interaction with RNA. Cancer Res. 1986;46:5760–6. [PubMed] [Google Scholar]
- Darzynkiewicz Z, Sharpless T, Staiano-Coico L, Melamed MR. Subcompartments of the G1 phase of cell cycle detected by flow cytometry. Proc Natl Acad Sci U S A. 1980a;77:6696–9. doi: 10.1073/pnas.77.11.6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darzynkiewicz Z, Traganos F, Melamed MR. New cell cycle compartments identified by multiparameter flow cytometry. Cytometry. 1980b;1:98–108. doi: 10.1002/cyto.990010203. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z, Traganos F, Sharpless T, Melamed MR. Lymphocyte stimulation: a rapid multiparameter analysis. Proc Natl Acad Sci U S A. 1976;73:2881–4. doi: 10.1073/pnas.73.8.2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day KP, Hayward RE, Dyer M. The biology of Plasmodium falciparum transmission stages. Parasitology. 1998;116:S95–S109. doi: 10.1017/s0031182000084985. [DOI] [PubMed] [Google Scholar]
- Dent AE, Bergmann-Leitner ES, Wilson DW, Tisch DJ, Kimmel R, Vulule J, Sumba PO, Beeson JG, Angov E, Moormann AM, Kazura JW. Antibody-mediated growth inhibition of Plasmodium falciparum: relationship to age and protection from parasitemia in Kenyan children and adults. PLoS One. 2008;3:e3557. doi: 10.1371/journal.pone.0003557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent AE, Chelimo K, Sumba PO, Spring MD, Crabb BS, Moormann AM, Tisch DJ, Kazura JW. Temporal stability of naturally acquired immunity to Merozoite Surface Protein-1 in Kenyan adults. Malar J. 2009;8:162. doi: 10.1186/1475-2875-8-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desakorn V, Silamut K, Angus B, Sahassananda D, Chotivanich K, Suntharasamai P, Simpson J, White NJ. Semi-quantitative measurement of Plasmodium falciparum antigen PfHRP2 in blood and plasma. Trans R Soc Trop Med Hyg. 1997;91:479–83. doi: 10.1016/s0035-9203(97)90292-3. [DOI] [PubMed] [Google Scholar]
- Desjardins RE, Canfield CJ, Haynes JD, Chulay JD. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob Agents Chemother. 1979;16:710–8. doi: 10.1128/aac.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dronamraju KR. Infectious Disease and Host-Pathogen Evolution. Cambridge University Press; New York: 2004. [Google Scholar]
- Eichner M, Diebner HH, Molineaux L, Collins WE, Jeffery GM, Dietz K. Genesis, sequestration and survival of Plasmodium falciparum gametocytes: parameter estimates from fitting a model to malariatherapy data. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2001;95:497–501. doi: 10.1016/s0035-9203(01)90016-1. [DOI] [PubMed] [Google Scholar]
- Epping RJ, Goldstone SD, Ingram LT, Upcroft JA, Ramasamy R, Cooper JA, Bushell GR, Geysen HM. An epitope recognised by inhibitory monoclonal antibodies that react with a 51 kilodalton merozoite surface antigen in Plasmodium falciparum. Mol Biochem Parasitol. 1988;28:1–10. doi: 10.1016/0166-6851(88)90173-9. [DOI] [PubMed] [Google Scholar]
- Ferri FF. Practical Guide to the Care of the Medical Patient. Elsevier Mosby; Philadelphia: 2007. [Google Scholar]
- Fleischer B. Editorial: 100 years ago: Giemsa’s solution for staining of plasmodia. Trop Med Int Health. 2004;9:755–6. doi: 10.1111/j.1365-3156.2004.01278.x. [DOI] [PubMed] [Google Scholar]
- Franklin RM, Brun R, Grieder A. Microscopic and flow cytophotometric analysis of parasitemia in cultures of Plasmodium falciparum vitally stained with Hoechst 33342--application to studies of antimalarial agents. Z Parasitenkd. 1986;72:201–12. doi: 10.1007/BF00931147. [DOI] [PubMed] [Google Scholar]
- Fraser T, Michon P, Barnwell JW, Noe AR, Al-Yaman F, Kaslow DC, Adams JH. Expression and serologic activity of a soluble recombinant Plasmodium vivax Duffy binding protein. Infect Immun. 1997;65:2772–7. doi: 10.1128/iai.65.7.2772-2777.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey T. Nucleic acid dyes for detection of apoptosis in live cells. Cytometry. 1995;21:265–74. doi: 10.1002/cyto.990210307. [DOI] [PubMed] [Google Scholar]
- Fu Y, Tilley L, Kenny S, Klonis N. Dual labeling with a far red probe permits analysis of growth and oxidative stress in P. falciparum-infected erythrocytes. Cytometry A. 2010. epub ahead of print. [DOI] [PubMed]
- Gallop PM, Paz MA, Henson E, Latt SA. Dynamic approaches to the delivery of reporter reagents into living cells. Biotechniques. 1984;2:32–36. [Google Scholar]
- Gardner MJ, Hall N, Fung E, White O, Berriman M, Hyman RW, Carlton JM, Pain A, Nelson KE, Bowman S, Paulsen IT, James K, Eisen JA, Rutherford K, Salzberg SL, Craig A, Kyes S, Chan MS, Nene V, Shallom SJ, Suh B, Peterson J, Angiuoli S, Pertea M, Allen J, Selengut J, Haft D, Mather MW, Vaidya AB, Martin DM, Fairlamb AH, Fraunholz MJ, Roos DS, Ralph SA, McFadden GI, Cummings LM, Subramanian GM, Mungall C, Venter JC, Carucci DJ, Hoffman SL, Newbold C, Davis RW, Fraser CM, Barrell B. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 2002;419:498–511. doi: 10.1038/nature01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerberding JL. Management of occupational exposures to blood-borne viruses. N Engl J Med. 1995;332:444–51. doi: 10.1056/NEJM199502163320707. [DOI] [PubMed] [Google Scholar]
- Giemsa G. Eine Vereinfachung und Vervollkommnung meiner Methylenblau-Eosin-Färbemethode zur Erzielung der Romanowsky-Nocht’schen Chromatinfärbung. Centralblatt fü r Bakteriologie. 1904;32:307–313. [Google Scholar]
- Grimberg BT, Erickson JJ, Sramkoski RM, Jacobberger JW, Zimmerman PA. Monitoring Plasmodium falciparum growth and development by UV flow cytometry using an optimized Hoechst-thiazole orange staining strategy. Cytometry A. 2008;73:546–54. doi: 10.1002/cyto.a.20541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimberg BT, Jaworska MM, Hough LB, Zimmerman PA, Phillips JG. Addressing the malaria drug resistance challenge using flow cytometry to discover new antimalarials. Bioorg Med Chem Lett. 2009;19:5452–7. doi: 10.1016/j.bmcl.2009.07.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimberg BT, Udomsangpetch R, Xainli J, McHenry A, Panichakul T, Sattabongkot J, Cui L, Bockarie M, Chitnis C, Adams J, Zimmerman P, King C. Plasmodium vivax Invasion of Human Erythrocytes Inhibited by Antibodies Directed Against the Duffy Binding Protein. PLoS Medicine. 2007;4:1940–1948. doi: 10.1371/journal.pmed.0040337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobusch MP, Hanscheid T, Kramer B, Neukammer J, May J, Seybold J, Kun JF, Suttorp N. Sensitivity of hemozoin detection by automated flow cytometry in non- and semi-immune malaria patients. Cytometry B Clin Cytom. 2003;55:46–51. doi: 10.1002/cyto.b.10039. [DOI] [PubMed] [Google Scholar]
- Gucker F, O’Konski CO, Pickard HB, Pitts J. A photoelectric counter for colloidal particles. American Journal of Chemistry. 1947;69:2422–2431. doi: 10.1021/ja01202a053. [DOI] [PubMed] [Google Scholar]
- Hanscheid T, Melo-Cristino J, Pinto BG. Automated detection of malaria pigment in white blood cells for the diagnosis of malaria in Portugal. Am J Trop Med Hyg. 2001;64:290–2. doi: 10.4269/ajtmh.2001.64.290. [DOI] [PubMed] [Google Scholar]
- Hanscheid T, Pinto BG, Cristino JM, Grobusch MP. Malaria diagnosis with the haematology analyser Cell-Dyn 3500: What does the instrument detect? Clin Lab Haematol. 2000a;22:259–61. doi: 10.1046/j.1365-2257.2000.00327.x. [DOI] [PubMed] [Google Scholar]
- Hanscheid T, Valadas E, Grobusch MP. Automated malaria diagnosis using pigment detection. Parasitol Today. 2000b;16:549–51. doi: 10.1016/s0169-4758(00)01742-7. [DOI] [PubMed] [Google Scholar]
- Hare JD. Two-color flow-cytometric analysis of the growth cycle of Plasmodium falciparum in vitro: identification of cell cycle compartments. J Histochem Cytochem. 1986;34:1651–8. doi: 10.1177/34.12.2431031. [DOI] [PubMed] [Google Scholar]
- Hare JD, Bahler DW. Analysis of Plasmodium falciparum growth in culture using acridine orange and flow cytometry. J Histochem Cytochem. 1986;34:215–20. doi: 10.1177/34.2.2418101. [DOI] [PubMed] [Google Scholar]
- Haynes JD, Moch JK, Smoot DS. Erythrocytic malaria growth or invasion inhibition assays with emphasis on suspension culture GIA. Methods in Molecular Medicine. 2002;72:535–554. doi: 10.1385/1-59259-271-6:535. [DOI] [PubMed] [Google Scholar]
- Higgs ES, Sina B. Plasmodium vivax vaccine research: steps in the right direction. Am J Trop Med Hyg. 2005;73:1–2. doi: 10.4269/ajtmh.2005.73.5_suppl.0730001. [DOI] [PubMed] [Google Scholar]
- Hoffman R, Benz EJ, Shattil SJ, Furie B, Silberstein LE, McGlave P, Heslop HE. Hematology: Basic Principles and Practice. Churchill Livingstone Elsevier; Philadelphia: 2009. [Google Scholar]
- Hoffman SL, Billingsley PF, James E, Richman A, Loyevsky M, Li T, Chakravarty S, Gunasekera A, Chattopadhyay R, Li M, Stafford R, Ahumada A, Epstein JE, Sedegah M, Reyes S, Richie TL, Lyke KE, Edelman R, Laurens MB, Plowe CV, Sim BK. Development of a metabolically active, non-replicating sporozoite vaccine to prevent Plasmodium falciparum malaria. Hum Vaccin. 2010;6:97–106. doi: 10.4161/hv.6.1.10396. [DOI] [PubMed] [Google Scholar]
- Hopwood D. Recent advances in fixation of tissues. In: Wisse E, Daems WT, Molenaar I, van Duijn P, editors. Electron Microrcopy and Cytochemistry. North-Holland Publishing Company; North Holland, Amsterdam: 1973. p. 367. [Google Scholar]
- Howard RJ, Battye FL, Mitchell GF. Plasmodium-infected blood cells analyzed and sorted by flow fluorimetry with the deoxyribonucleic acid binding dye 33258 Hoechst. J Histochem Cytochem. 1979;27:803–13. doi: 10.1177/27.4.87413. [DOI] [PubMed] [Google Scholar]
- Hughes KR, Biagini GA, Craig AG. Continued cytoadherence of Plasmodium falciparum infected red blood cells after antimalarial treatment. Mol Biochem Parasitol. 2010;169:71–8. doi: 10.1016/j.molbiopara.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inselburg J, Banyal HS. Synthesis of DNA during the asexual cycle of Plasmodium falciparum in culture. Mol Biochem Parasitol. 1984;10:79–87. doi: 10.1016/0166-6851(84)90020-3. [DOI] [PubMed] [Google Scholar]
- Izumiyama S, Omura M, Takasaki T, Ohmae H, Asahi H. Plasmodium falciparum: development and validation of a measure of intraerythrocytic growth using SYBR Green I in a flow cytometer. Exp Parasitol. 2009;121:144–50. doi: 10.1016/j.exppara.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Jackson PR, Winkler DG, Kimzey SL, Fisher FM., Jr. Cytofluorograf detection of Plasmodium yoelii, Trypanosoma gambiense, and Trypanosoma equiperdum by laser excited fluorescence of stained rodent blood. J Parasitol. 1977;63:593–8. [PubMed] [Google Scholar]
- Jacobberger JW, Horan PK, Hare JD. Flow cytometric analysis of blood cells stained with the cyanine dye DiOC1[3]: reticulocyte quantification. Cytometry. 1984;5:589–600. doi: 10.1002/cyto.990050607. [DOI] [PubMed] [Google Scholar]
- Jacobberger JW, Horan PK, Hare JD. Cell cycle analysis of asexual stages of erythrocytic malaria parasites. Cell Prolif. 1992;25:431–45. doi: 10.1111/j.1365-2184.1992.tb01452.x. [DOI] [PubMed] [Google Scholar]
- Jelinek T, Kilian AH, Henk M, Mughusu EB, Nothdurft HD, Loscher T, Knobloch J, Van Sonnenburg F. Parasite-specific lactate dehydrogenase for the diagnosis of Plasmodium falciparum infection in an endemic area in west Uganda. Trop Med Int Health. 1996;1:227–30. doi: 10.1111/j.1365-3156.1996.tb00031.x. [DOI] [PubMed] [Google Scholar]
- Jensen JB, Hoffman SL, Boland MT, Akood MA, Laughlin LW, Kurniawan L, Marwoto HA. Comparison of immunity to malaria in Sudan and Indonesia: crisis-form versus merozoite-invasion inhibition. Proc Natl Acad Sci U S A. 1984;81:922–5. doi: 10.1073/pnas.81.3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Diaz MB, Mulet T, Gomez V, Viera S, Alvarez A, Garuti H, Vazquez Y, Fernandez A, Ibanez J, Jimenez M, Gargallo-Viola D, Angulo-Barturen I. Quantitative measurement of Plasmodium-infected erythrocytes in murine models of malaria by flow cytometry using bidimensional assessment of SYTO-16 fluorescence. Cytometry A. 2009;75:225–35. doi: 10.1002/cyto.a.20647. [DOI] [PubMed] [Google Scholar]
- Jimenez-Diaz MB, Rullas J, Mulet T, Fernandez L, Bravo C, Gargallo-Viola D, Angulo-Barturen I. Improvement of detection specificity of Plasmodium-infected murine erythrocytes by flow cytometry using autofluorescence and YOYO-1. Cytometry A. 2005;67:27–36. doi: 10.1002/cyto.a.20169. [DOI] [PubMed] [Google Scholar]
- John CC, O’Donnell RA, Sumba PO, Moormann AM, de Koning-Ward TF, King CL, Kazura JW, Crabb BS. Evidence that invasion-inhibitory antibodies specific for the 19-kDa fragment of merozoite surface protein-1 (MSP-1 19) can play a protective role against blood-stage Plasmodium falciparum infection in individuals in a malaria endemic area of Africa. J Immunol. 2004;173:666–72. doi: 10.4049/jimmunol.173.1.666. [DOI] [PubMed] [Google Scholar]
- Jouin H, Daher W, Khalife J, Ricard I, Puijalon OM, Capron M, Dive D. Double staining of Plasmodium falciparum nucleic acids with hydroethidine and thiazole orange for cell cycle stage analysis by flow cytometry. Cytometry A. 2004;57:34–8. doi: 10.1002/cyto.a.10110. [DOI] [PubMed] [Google Scholar]
- Jouin H, de la Salmoniere Y. Goguet, Behr C, Dat M. Huyin Qan, Michel J, Sarthou J, da Silva L. Pereira, Dubois P. Flow cytometry detection of surface antigens on fresh, unfixed red blood cells infected by Plasmodium falciparum. Journal of Immunological Methods. 1995;179:11–12. doi: 10.1016/0022-1759(94)00265-x. [DOI] [PubMed] [Google Scholar]
- Kadekoppala M, Kline K, Akompong T, Haldar K. Stable expression of a new chimeric fluorescent reporter in the human malaria parasite Plasmodium falciparum. Infect Immun. 2000;68:2328–32. doi: 10.1128/iai.68.4.2328-2332.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapuscinski J, Darzynkiewicz Z. Interactions of pyronin Y(G) with nucleic acids. Cytometry. 1987;8:129–37. doi: 10.1002/cyto.990080205. [DOI] [PubMed] [Google Scholar]
- Keller W. Characterization of purified DNA-relaxing enzyme from human tissue culture cells. Proc. Nat. Acad. Sci. USA. 1975;72:2550–2554. doi: 10.1073/pnas.72.7.2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch J, Henk M. Screening for malaria by determination of parasite-specific lactate dehydrogenase. Trans R Soc Trop Med Hyg. 1995;89:269–70. doi: 10.1016/0035-9203(95)90533-2. [DOI] [PubMed] [Google Scholar]
- Krishan A. Rapid flow cytofluorometric analysis of mammalian cell cycle by propidium iodide staining. J Cell Biol. 1975;66:188–93. doi: 10.1083/jcb.66.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latt SA. Microfluorometric detection of deoxyribonucleic acid replication in human metaphase chromosomes. Proc Natl Acad Sci U S A. 1973;70:3395–9. doi: 10.1073/pnas.70.12.3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence C, Olson JA. Birefringent hemozoin identifies malaria. Am J Clin Pathol. 1986;86:360–3. doi: 10.1093/ajcp/86.3.360. [DOI] [PubMed] [Google Scholar]
- Lee LG, Chen CH, Chiu LA. Thiazole orange: a new dye for reticulocyte analysis. Cytometry. 1986;7:508–17. doi: 10.1002/cyto.990070603. [DOI] [PubMed] [Google Scholar]
- LePecq JB, Paoletti C. A fluorescent complex between ethidium bromide and nucleic acids. Physical-chemical characterization. J Mol Biol. 1967;27:87–106. doi: 10.1016/0022-2836(67)90353-1. [DOI] [PubMed] [Google Scholar]
- Li Q, Gerena L, Xie L, Zhang J, Kyle D, Milhous W. Development and validation of flow cytometric measurement for parasitemia in cultures of P. falciparum vitally stained with YOYO-1. Cytometry A. 2007;71:297–307. doi: 10.1002/cyto.a.20380. [DOI] [PubMed] [Google Scholar]
- Liu S, Mu J, Jiang H, Su XZ. Effects of Plasmodium falciparum mixed infections on in vitro antimalarial drug tests and genotyping. Am J Trop Med Hyg. 2008;79:178–84. [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Janeway CA., Jr. Interferon gamma plays a critical role in induced cell death of effector T cell: a possible third mechanism of self-tolerance. J Exp Med. 1990;172:1735–9. doi: 10.1084/jem.172.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungström I, Perlmann H, Schlichtherle M, Scherf A, Wahlgren M. Malaria Research and Reference Reagent Resource Center. American Type Culture Collection; Manassas: 2004. Methods in Malaria Research. [Google Scholar]
- Loewe H, Urbanietz J. Basic-substituted 2,6-bisbenzimidazole derivates, a novel class of substances with chemotherapeutic activity. Arzneimittelforschung. 1974;24:1927–33. [PubMed] [Google Scholar]
- Loken MR, Stout RD, Herzenberg LA. Lymphoid cell analysis and sorting. In: Melamed MR, Mullaney PF, Mendelsohn ML, editors. Flow Cytometry and Sorting. Wiley; New York: 1979. [Google Scholar]
- Makler MT, Lee LG, Recktenwald D. Thiazole orange: a new dye for Plasmodium species analysis. Cytometry. 1987;8:568–70. doi: 10.1002/cyto.990080606. [DOI] [PubMed] [Google Scholar]
- Makler MT, Palmer CJ, Ager AL. A review of practical techniques for the diagnosis of malaria. Ann Trop Med Parasitol. 1998;92:419–33. doi: 10.1080/00034989859401. [DOI] [PubMed] [Google Scholar]
- Martens AC, van den Engh GJ, Hagenbeek A. The fluorescence intensity of propidium iodide bound to DNA depends on the concentration of sodium chloride. Cytometry. 1981;2:24–5. doi: 10.1002/cyto.990020105. [DOI] [PubMed] [Google Scholar]
- McNamara DT, Kasehagen LJ, Grimberg BT, Cole-Tobian J, Collins WE, Zimmerman PA. Diagnosing infection levels of four human malaria parasite species by a polymerase chain reaction/ligase detection reaction fluorescent microsphere-based assay. Am J Trop Med Hyg. 2006;74:413–21. [PMC free article] [PubMed] [Google Scholar]
- Meslin B, Barnadas C, Boni V, Latour C, Monbrison FD, Kaiser K, Picot S. Features of Apoptosis in Plasmodium falciparum Erythrocytic Stage through a Putative Role of PfMCA1 Metacaspase-Like Protein. The Journal of Infectious Diseases. 2007;195:1852–1859. doi: 10.1086/518253. [DOI] [PubMed] [Google Scholar]
- Milne LM, Kyi MS, Chiodini PL, Warhurst DC. Accuracy of routine laboratory diagnosis of malaria in the United Kingdom. J Clin Pathol. 1994;47:740–2. doi: 10.1136/jcp.47.8.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll K, Ljungstrom I, Perlmann H, Scherf A, Wahlgren M. Malaria Research and Reference Reagent Resource Center. American Type Culture Collection; Manassas: 2008. Methods in Malaria Research. [Google Scholar]
- Moore LR, Fujioka H, Williams PS, Chalmers JJ, Grimberg B, Zimmerman PA, Zborowski M. Hemoglobin degradation in malaria-infected erythrocytes determined from live cell magnetophoresis. Faseb J. 2006;20:747–9. doi: 10.1096/fj.05-5122fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno A, Brasseur P, Cuzin-Ouattara N, Blanc C, Druilhe P. Evaluation under field conditions of the colourimetric DELI-microtest for the assessment of Plasmodium falciparum drug resistance. Trans R Soc Trop Med Hyg. 2001;95:100–3. doi: 10.1016/s0035-9203(01)90351-7. [DOI] [PubMed] [Google Scholar]
- Muller W, Gautier F. Interactions of heteroaromatic compounds with nucleic acids. A - T-specific non-intercalating DNA ligands. Eur J Biochem. 1975;54:385–94. doi: 10.1111/j.1432-1033.1975.tb04149.x. [DOI] [PubMed] [Google Scholar]
- Noedl H, Wernsdorfer WH, Miller RS, Wongsrichanalai C. Histidine-rich protein II: a novel approach to malaria drug sensitivity testing. Antimicrob Agents Chemother. 2002;46:1658–64. doi: 10.1128/AAC.46.6.1658-1664.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell R, de Koning-Ward T, Burt R, Bockarie M, Reeder J, Cowman A, Crabb B. Antibodies against merozoite surface protein (MSP)-1(19) are a major component of the invasion-inhibitory response in individuals immune to malaria. J Exp Med. 2001;193:1403–12. doi: 10.1084/jem.193.12.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappenheimer AM. Experimental Studies Upon Lymphocytes : I. the Reactions of Lymphocytes under Various Experimental Conditions. J Exp Med. 1917;25:633–650. doi: 10.1084/jem.25.5.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattanapanyasat K, Thaithong S, Kyle DE, Udomsangpetch R, Yongvanitchit K, Hider RC, Webster HK. Flow cytometric assessment of hydroxypyridinone iron chelators on in vitro growth of drug-resistant malaria. Cytometry. 1997;27:84–91. doi: 10.1002/(sici)1097-0320(19970101)27:1<84::aid-cyto11>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Pattanapanyasat K, Udomsangpetch R, Webster HK. Two-color flow cytometric analysis of intraerythrocytic malaria parasite DNA and surface membrane-associated antigen in erythrocytes infected with Plasmodium falciparum. Cytometry. 1993;14:449–54. doi: 10.1002/cyto.990140415. [DOI] [PubMed] [Google Scholar]
- Persson KE, Lee CT, Marsh K, Beeson JG. Development and optimization of high-throughput methods to measure Plasmodium falciparum-specific growth inhibitory antibodies. J Clin Microbiol. 2006;44:1665–73. doi: 10.1128/JCM.44.5.1665-1673.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper KP, Roberts DJ, Day KP. Plasmodium falciparum: analysis of the antibody specificity to the surface of the trophozoite-infected erythrocyte. Exp Parasitol. 1999;91:161–9. doi: 10.1006/expr.1998.4368. [DOI] [PubMed] [Google Scholar]
- Quashie NB, de Koning HP, Ranford-Cartwright LC. An improved and highly sensitive microfluorimetric method for assessing susceptibility of Plasmodium falciparum to antimalarial drugs in vitro. Malar J. 2006;5:95. doi: 10.1186/1475-2875-5-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riquelme BD, de Isla NG, Valverde JR, Stoltz JF. A simple method for quantifying high density antigens in erythrocyte membrane by flow cytometry. J Biochem Biophys Methods. 2006;68:31–42. doi: 10.1016/j.jbbm.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Rye HS, Quesada MA, Peck K, Mathies RA, Glazer AN. High-sensitivity two-color detection of double-stranded DNA with a confocal fluorescence gel scanner using ethidium homodimer and thiazole orange. Nucleic Acids Res. 1991;19:327–33. doi: 10.1093/nar/19.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez BA, Varotti FP, Rodrigues FG, Carvalho LH. Validation of a Plasmodium falciparum parasite transformed with green fluorescent protein for antimalarial drug screening. J Microbiol Methods. 2007;69:518–22. doi: 10.1016/j.mimet.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Saul A, Myler P, Mangan T, Kidson C. Plasmodium falciparum: automated assay of erythrocyte invasion using flow cytofluorometry. Exp Parasitol. 1982;54:64–71. doi: 10.1016/0014-4894(82)90111-4. [DOI] [PubMed] [Google Scholar]
- Shapiro H. Practical Flow Cytometry. John Wiley & Sons, Inc.; San Francisco: 2003. [Google Scholar]
- Shapiro HM. Flow cytometric estimation of DNA and RNA content in intact cells stained with Hoechst 33342 and pyronin Y. Cytometry. 1981;2:143–50. doi: 10.1002/cyto.990020302. [DOI] [PubMed] [Google Scholar]
- Shapiro HM, Mandy F. Cytometry in malaria: moving beyond Giemsa. Cytometry A. 2007;71:643–5. doi: 10.1002/cyto.a.20453. [DOI] [PubMed] [Google Scholar]
- Shapiro HM, Natale PJ, Kamentsky LA. Estimation of membrane potentials of individual lymphocytes by flow cytometry. Proc Natl Acad Sci U S A. 1979;76:5728–30. doi: 10.1073/pnas.76.11.5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro HM, Perlmutter NG. Killer applications: toward affordable rapid cell-based diagnostics for malaria and tuberculosis. Cytometry B Clin Cytom. 2008;74(Suppl 1):S152–64. doi: 10.1002/cyto.b.20401. [DOI] [PubMed] [Google Scholar]
- Silverman P, Schooley J, Mahlmann L. Murine malaria decreases hemopoietic stem cells. Blood. 1987;69:408. [PubMed] [Google Scholar]
- Sim BK, Orlandi PA, Haynes JD, Klotz FW, Carter JM, Camus D, Zegans ME, Chulay JD. Primary structure of the 175K Plasmodium falciparum erythrocyte binding antigen and identification of a peptide which elicits antibodies that inhibit malaria merozoite invasion. J Cell Biol. 1990;111:1877–84. doi: 10.1083/jcb.111.5.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeijsters LJ, Zijlstra NM, Franssen FF, Overdulve JP. Simple, fast, and accurate fluorometric method to determine drug susceptibility of Plasmodium falciparum in 24-well suspension cultures. Antimicrob Agents Chemother. 1996;40:835–8. doi: 10.1128/aac.40.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PJ, Blunt N, Wiltshire M, Hoy T, Teesdale-Spittle P, Craven MR, Watson JV, Amos WB, Errington RJ, Patterson LH. Characteristics of a novel deep red/infrared fluorescent cell-permeant DNA probe, DRAQ5, in intact human cells analyzed by flow cytometry, confocal and multiphoton microscopy. Cytometry. 2000;40:280–91. doi: 10.1002/1097-0320(20000801)40:4<280::aid-cyto4>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Staalsoe T, Giha HA, Dodoo D, Theander TG, Hviid L. Detection of antibodies to variant antigens on Plasmodium falciparum-infected erythrocytes by flow cytometry. Cytometry. 1999;35:329–36. doi: 10.1002/(sici)1097-0320(19990401)35:4<329::aid-cyto5>3.3.co;2-p. [DOI] [PubMed] [Google Scholar]
- Sugden B, De Troy B, Roberts RJ, Sambrook J. Agarose slab-gel electrophoresis equipment. Anal Biochem. 1975;68:36–46. doi: 10.1016/0003-2697(75)90676-4. [DOI] [PubMed] [Google Scholar]
- Tarnok A. SYTO dyes and histoproteins--myriad of applications. Cytometry A. 2008;73:477–9. doi: 10.1002/cyto.a.20588. [DOI] [PubMed] [Google Scholar]
- Tilley L, McFadden G, Cowman A, Klonis N. Illuminating Plasmodium falciparum-infected red blood cells. Trends Parasitol. 2007;23:268–77. doi: 10.1016/j.pt.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Torous D, Asano N, Tometsko C, Sugunan S, Dertinger S, Morita T, Hayashi M. Performance of flow cytometric analysis for the micronucleus assay--a reconstruction model using serial dilutions of malaria-infected cells with normal mouse peripheral blood. Mutagenesis. 2006;21:11–3. doi: 10.1093/mutage/gei053. [DOI] [PubMed] [Google Scholar]
- Trager W, Jensen J. Human Malaria Parasites in Continuous Culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- van der Heyde HC, Elloso MM, vande Waa J, Schell K, Weidanz WP. Use of hydroethidine and flow cytometry to assess the effects of leukocytes on the malarial parasite Plasmodium falciparum. Clin Diagn Lab Immunol. 1995;2:417–25. doi: 10.1128/cdli.2.4.417-425.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vianen PH, Thaithong S, Reinders PP, van Engen A, van der Keur M, Tanke HJ, van der Kaay HJ, Mons B. Automated flow cytometric analysis of drug susceptibility of malaria parasites. Am J Trop Med Hyg. 1990;43:602–7. doi: 10.4269/ajtmh.1990.43.602. [DOI] [PubMed] [Google Scholar]
- VanWye JD, Haldar K. Expression of green fluorescent protein in Plasmodium falciparum. Mol Biochem Parasitol. 1997;87:225–9. doi: 10.1016/s0166-6851(97)00059-5. [DOI] [PubMed] [Google Scholar]
- Whaun JM, Rittershaus C, Ip SH. Rapid identification and detection of parasitized human red cells by automated flow cytometry. Cytometry. 1983;4:117–22. doi: 10.1002/cyto.990040204. [DOI] [PubMed] [Google Scholar]
- Wilairatana P, Krudsood S, Tangpukdee N. Appropriate Time for Primaquine Treatment to Reduce Plasmodium falciparum Transmission in Hypoendemic Areas. Korean J Parasitol. 2010;48:179–182. doi: 10.3347/kjp.2010.48.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlodkowic D, Skommer J, Hillier C, Darzynkiewicz Z. Multiparameter detection of apoptosis using red-excitable SYTO probes. Cytometry A. 2008;73:563–9. doi: 10.1002/cyto.a.20564. [DOI] [PubMed] [Google Scholar]
- Woolley IJ, Hotmire KA, Sramkoski RM, Zimmerman PA, Kazura JW. Differential expression of the duffy antigen receptor for chemokines according to RBC age and FY genotype. Transfusion. 2000;40:949–53. doi: 10.1046/j.1537-2995.2000.40080949.x. [DOI] [PubMed] [Google Scholar]
- World Health Organization World Health Organization; Geneva: In vitro micro-test (mark III) for the assessment of the response of Plasmodium falciparum to chloroquine, mefloquine, quinine, amodiaquine, sufadoxine/pyrimethamine and artemisinin. (Rev. 2) 2001;Vol. CTD/MAL/97.20
- World Health Organization World Health Organization; Geneva: World Malaria Report: 2005. 2007
- Wyatt CR, Goff W, Davis WC. A flow cytometric method for assessing viability of intraerythrocytic hemoparasites. J Immunol Methods. 1991;140:23–30. doi: 10.1016/0022-1759(91)90122-v. [DOI] [PubMed] [Google Scholar]
- Yayon A, Vande Waa JA, Yayon M, Geary TG, Jensen JB. Stage-dependent effects of chloroquine on Plasmodium falciparum in vitro. J Protozool. 1983;30:642–7. doi: 10.1111/j.1550-7408.1983.tb05336.x. [DOI] [PubMed] [Google Scholar]
- Zhao H, Kalivendi S, Zhang H, Joseph J, Nithipatikom K, Vasquez-Vivar J, Kalyanaraman B. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic Biol Med. 2003;34:1359–68. doi: 10.1016/s0891-5849(03)00142-4. [DOI] [PubMed] [Google Scholar]