Abstract
The International Randomized Study of Interferon vs. STI571 (IRIS) trial that investigated the use of the tyrosine kinase inhibitor (TKI) imatinib (versus interferon) changed the treatment and outcome of chronic myeloid leukemia (CML). Long-term follow-up of IRIS patients has defined response parameters and methods of tracking residual disease with cytogenetic testing of bone marrow metaphases and molecular monitoring of BCR-ABL transcripts using quantitative reverse-transcriptase polymerase chain reaction. Cytogenetic and molecular responses are now considered useful surrogates for long-term outcome. Early and robust response to imatinib predicts positive long-term outcomes. However, 15–25% of patients fail initial treatment or become intolerant of imatinib and need increased doses or alternate treatment. Second-line treatment with the second-generation TKIs nilotinib and dasatinib have resulted in favorable rates of progression-free survival (PFS) and overall survival. Data from the ENESTnd (nilotinib) and DASISION (dasatinib) trials in newly diagnosed chronic-phase CML patients demonstrated more robust and rapid complete cytogenetic (77–80%) and major molecular responses (43–46%) at 12 months compared with imatinib (65–66% and 22–28%). The relationship between a complete cytogenetic response at 12 months and long-term PFS supports a role for second-generation TKIs as first-line treatment of newly diagnosed chronic-phase CML.
Keywords: Chronic myeloid leukemia, cytogenetic response, molecular response, dasatinib, nilotinib, imatinib
Introduction
Advances in our understanding of the biologic underpinnings of chronic myeloid leukemia (CML) have led to the development of remarkably effective therapies and highly specific methods of monitoring disease response. The hallmark event responsible for the development of CML is a somatic mutation resulting in formation of the Philadelphia (Ph) chromosome. The Ph chromosome is the product of a reciprocal translocation between chromosomes 9 and 22 [t(9;22)], resulting in a new genetic sequence made up of BCR (breakpoint cluster region) from chromosome 22 and c-ABL (Abelson murine leukemia viral oncogene homolog 1) from chromosome 9. The Ph chromosome generates the BCR-ABL1 oncogene that encodes the chimeric BCR-ABL tyrosine kinase. Attachment of the BCR sequences to ABL results in 3 critical functional changes: (1) ABL becomes constitutively active as a tyrosine kinase enzyme, activating downstream kinases that prevent apoptosis; (2) the DNA-protein-binding activity of ABL is attenuated; and (3) the binding of ABL to cytoskeletal actin microfilaments is enhanced1–3.
Treatment of newly diagnosed patients with chronic-phase CML has evolved in the last decade from relatively nonspecific approaches with hydroxyurea, interferon-α, or allogeneic stem cell transplantation to highly targeted therapy with tyrosine kinase inhibitors (TKIs)1. TKIs bind to the BCR-ABL kinase, interrupting unregulated and constitutively active kinase activated downstream signaling. The first approved TKI, imatinib (previously known as STI571), revolutionized the treatment and outcome for CML patients1,4. The landmark International Randomized Study of Interferon plus cytarabine [Ara-C] vs STI571 (IRIS) demonstrated that imatinib was significantly more effective and better tolerated than the combination of interferon-α plus cytarabine as treatment for newly diagnosed chronic-phase CML. At 12 months, higher rates of progression-free survival (PFS; 97% vs 80%; P<0.001) and estimated rates of freedom from progression to accelerated- or blast-phase disease (99% vs 93%; P<0.001) were achieved in CML patients randomized to imatinib versus other combination therapy5. Long-term follow-up of participants in the IRIS trial has provided insight into the mechanisms of imatinib resistance and has generated monitoring procedures and definitions of response in CML4,6.
This review describes contemporary definitions of treatment response in CML, discusses the prognostic value of cytogenetic and molecular monitoring, and places recent trial findings of second-generation TKIs for chronic-phase CML therapy into perspective.
Monitoring response and resistance in CML
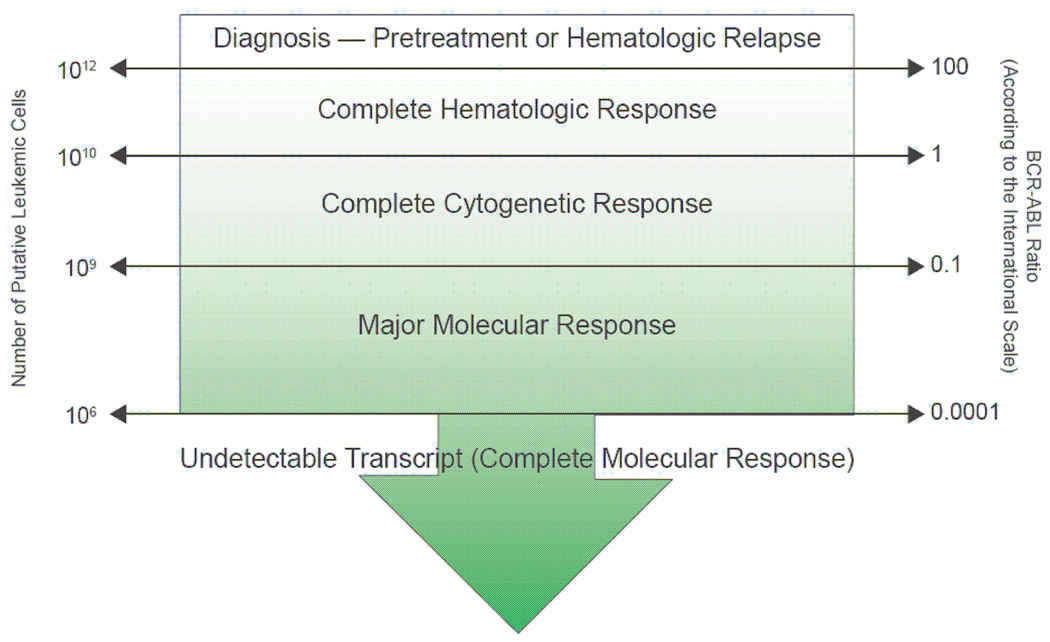
Monitoring the response to CML therapy is a continuum that begins at diagnosis and carries on serially throughout the entire course of treatment7, as detailed in recently published expert consensus guidelines4,7. Treatment monitoring (Table 1)7 initially concentrated on morphologic hematologic assessment; however, significant advances in technologies to detect BCR-ABL positive cells have now refocused therapeutic goals on cytogenetic and molecular endpoints8,9. Together, cytogenetic and molecular responses provide a measure of minimal residual disease (Fig. 1)8 and serve to both guide treatment choices and as surrogates for long-term outcome4,7. A recently published update from the National Comprehensive Cancer Network (NCCN) outlines a recommended approach to the serial monitoring of CML (Table 2)7.
Table 1.
Definitions of hematologic, cytogenetic, and molecular response in chronic myeloid leukemia.
| Hematologic Response | |
|---|---|
Complete hematologic response
| |
Partial hematologic response (identical to complete response with the following exceptions)
| |
| Cytogenetic Response | |
| |
| Molecular Response | |
|
Ph+, Philadelphia chromosome-positive; RT-PCR, reverse-transcriptase polymerase chain reaction.
Modified by permission of National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Chronic Myelogenous Leukemia. V2.2010. Available at: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed December 15, 2010.
Figure 1.
Therapeutic responses as a function of the number of leukemic cells and BCR-ABL transcript levels.
Reproduced by permission of Aguayo A, Couban S. State-of-the-art in the management of chronic myelogenous leukemia in the era of the tyrosine kinase inhibitors: evolutionary trends in diagnosis, monitoring and treatment. Leuk Lymphoma 2009;50(Suppl 2):1–8.
Table 2.
Recommendations for serial cytogenetic and molecular monitoring of tyrosine kinase inhibitor therapy in patients with chronic myeloid leukemia.
| Cytogenetic and Quantitative RT-PCR Analysesa | |
|---|---|
| At diagnosis, prior to beginning treatment |
|
| While patient appears to be responding |
|
| Upon reaching CCyR |
|
| When BCR-ABL transcript levels increase (≥1-log increase) |
|
| Consider ABL Kinase Domain Mutation Analysis | |
| Chronic-phase CML | |
| Accelerated- or blast-phase CML |
|
qRT-PCR, quantitative reverse-transcriptase polymerase chain reaction from peripheral blood; FISH, fluorescence in situ hybridization; CCyR, complete cytogenetic response; MMR, major molecular response.
Inadequate initial response = failure to achieve complete hematologic response at 3 months, minimal cytogenetic response at 6 months, or major cytogenetic response at 12 months.
Loss of response = hematologic relapse, relapse to Philadelphia chromosome positivity (Ph+), or increase in BCR-ABL transcript ratio/1-log increase and loss of MMR.
Modified by permission of National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Chronic Myelogenous Leukemia. V2.2010. Available at: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed December 15, 2010.
Hematologic and morphologic responses
Integral parts of the initial diagnostic work-up and subsequent monitoring of CML include a complete blood cell count with differential and bone marrow aspiration and biopsy for morphologic review4,7. The absence of immature cells and normalization of leukocyte and platelet counts in peripheral blood in conjunction with reversal of splenomegaly define a complete hematologic response (CHR) (Table 1). Achievement of a CHR is not equivalent to the absence of leukemic cells, because as many as 1010 cells undetectable cells by microscopy may still be present. Therefore, assessment of CHR is only the first step in measuring treatment efficacy, and more sensitive tests are necessary to quantitate the burden of occult CML disease8,10,11 (Fig. 1).
Cytogenetic responses
Cytogenetic testing is a more sensitive measure of leukemic burden by determining the number of cells carrying the Ph chromosome. Cytogenetic response (CyR) is defined by the reduction in the percentage of Ph+ cells. The absence of any Ph+ cells is considered a complete cytogenetic response (CCyR) (Table 1). Current guidelines recommend the use of conventional karyotyping with chromosome banding in a bone marrow sample to determine a pretreatment baseline of metaphase cells4,7. Conventional cytogenetic analysis uses light microscopy to analyze mitotic cells arrested during metaphase in a minimum of 20 metaphases. Visualization is enhanced with Giemsa dye-staining (G-banding) to detect characteristic chromosome banding. Conventional cytogenetic testing has an estimated sensitivity of 1–5%11. This method will also identify any chromosomal aberration in addition to t(9;22) (see below) including complex (or variant) translocations involving 3, 4, or more chromosomes. Following the initiation of TKI therapy, current guidelines recommend that cytogenetic analysis be performed at 3- to 6-month intervals after treatment initiation (see below).
Fluorescence in situ hybridization (FISH) is another method of monitoring CyR to treatment in CML. FISH measures the proportion of nuclei with the characteristic BCR-ABL fusion and can be performed even on nondividing cells from the peripheral blood or bone marrow. Labeled DNA probes are hybridized to interphase nuclei. Dual-color, double-fusion FISH (D-FISH) results in distinct signals: green for BCR and red/orange for ABL. BCR-ABL translocation fusions are yellow due to the summation of the 2 colors under a microscope with dual filters (green and red/orange). The sensitivity of D-FISH ranges from 0.1–1%11,12.
The ability to monitor treatment response and disease progression using peripheral blood is clearly an attractive alternative to bone marrow sampling13. Some oncologists consider FISH testing of peripheral blood to be interchangeable with and preferred over conventional bone marrow cytogenetic testing due to the relative ease of sampling12. Advances in the instruments used to obtain bone marrow samples, such as needle design, have increased the latter procedure’s efficiency while minimizing patient discomfort14. Nevertheless, marrow sampling is invasive, costly, and labor intensive14. Compared with bone marrow testing, peripheral blood sampling is technically straightforward and not associated with the same degree of risk and pain.
However, FISH testing should not be considered to be a replacement for conventional cytogenetic testing for a number of reasons. Although findings from case series have shown some degree of correlation between FISH and conventional cytogenetic testing15–18, there are no data from rigorously conducted prospective studies that compare FISH with conventional cytogenetics as predictors of long-term outcome1,8,12. Furthermore, the existing evidence for response to TKI treatment is composed of randomized, controlled studies utilizing marrow cytogenetic testing to determine CCyR5,19–24. Indeed, the definition of CyR is based on analysis of bone marrow metaphases4,7. Further, procedures for FISH testing are not consistent across all commercial laboratories, with some relying on older probes (different colors, background, false-positive rates of 1–10%) that hinder interpretation of findings1,12.
Another significant limitation of FISH technology is its inability to detect clonal chromosome abnormalities in Ph+ or Ph- cells, which are present at diagnosis in 5–10% of CML patients4. This is of prime importance as clonal evolution places the patient at a more advanced (accelerated) stage and changes the treatment paradigm (see below). In clinical settings where bone marrow sampling is not practical, FISH testing of peripheral blood is an acceptable alternative for the diagnostic work-up of CML only if paired with dual probes for BCR-ABL genes4,7. Metaphase karyotyping of bone marrow samples remains the preferred choice for cytogenetic testing4,7.
Molecular responses
Patients who achieve CCyR can still carry as many as 109 leukemic cells (Fig. 1). Therefore, the most sensitive method of testing minimal residual disease in CML patients is quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR). This technique measures the level of BCR-ABL transcript following treatment (Table 1) and is an indicator of the number of circulating leukemic cells10. qRT-PCR constitutes a highly precise method of measuring the level of BCR-ABL transcripts in real time using fluorescent probes11. Patients may have one or both of two transcripts – e13a2 (formerly b2a2) or e14a2 (formerly b3a2)25. Molecular response is reported as the ratio of BCR-ABL transcripts to a control gene (most commonly ABL). A major molecular response (MMR) is defined as a ≥3 log-reduction of BCR-ABL mRNA transcripts (Table 1). Measurement of residual leukemic burden using qRT-PCR can be conducted using either peripheral blood or bone marrow samples, but the interchangeable use of these sampling methods in individual patients may hinder interpretation of results and is not recommended26. The ability to quantitate BCR-ABL transcripts in CML patients has enabled a more accurate measure of residual disease, particularly in patients who achieve a CCyR9. In addition, reduction in BCR-ABL transcripts correlates with PFS26.
Despite its value as a prognostic tool, there are several important concerns about molecular response testing that came to the forefront as a result of the IRIS trial27. For example, although a single validated method of testing treatment response is ideal, not all laboratories use standardized methods of qRT-PCR to measure levels of BCR-ABL transcripts in CML patients. In the IRIS trial, 3 laboratories conducted qRT-PCR testing, and their results were not identical. In order to compare results from the different laboratories, samples from 30 patients who had not yet been treated were tested at each site, and the median value was used to standardize results from individual laboratories27. In recognition of the problems inherent with nonstandardized methodologies and data reporting, Hughes and colleagues made recommendations for harmonizing methods of molecular testing and suggested the use of a conversion factor so that results are reported using a standardized international scale (expressed as % or IS units)26. A score of 100% on the IS corresponds with baseline levels of BCR-ABL in newly diagnosed patients, and a score of 0.1% indicates a MMR25,26. The use of 1 of 3 appropriate control genes (BCR, ABL, β-glucuronidase [GUSB]) and quality control samples from commercial or federally funded laboratories were recommended26. Collaborative work determined a BCR-ABL/ABL percent value in one laboratory that was equivalent to a MMR in another laboratory25. According to currently published guidelines, a MMR is now been redefined as a ratio of BCR-ABL/ABL (or other housekeeping genes) of ≤0.1 on the IS4 or a 3-log or greater reduction in BCR-ABL mRNA7. Although such molecular analyses were highly specialized tools primarily performed in academic settings, they are increasingly being used in nonresearch settings due to their sensitivity and ease of use.
Using cytogenetic and molecular analyses to guide TKI therapy
The concomitant complementary role of cytogenetic and molecular testing in monitoring treatment response was further explored in the IRIS trial. Subsequent long-term follow-up of the original patient cohort has firmly established these analyses as surrogate markers of clinical outcome, residual disease monitoring, and treatment resistance. Indeed, cytogenetic and molecular testing are now considered to be fundamental endpoints in contemporary clinical trial design for CML therapy and are essential tools guiding the choice of initial therapy in CML patients.
Imatinib responses
Yearly sequential follow-up of the IRIS trial5 cohort originally randomized to imatinib treatment demonstrated that early, robust CyRs predict better long-term outcome. For example, 72% of patients who attained partial cytogenetic response (PCyR) after the first 3 months of treatment maintained stable CCyR thereafter as compared with only 63% of patients who achieved that level of response at 6 months (Table 3)28. Findings from a single-center, open-label study of 204 patients with chronic-phase CML also found that achievement of a CCyR at 12 months corresponded with higher rates of PFS and overall survival (OS) at 5 years compared with patients who did not attain a CCyR29.
Table 3.
Cumulative incidence of complete cytogenetic response versus estimated event rates 8 years after beginning imatinib treatment in the IRIS trial (95% confidence intervals).
| Cytogenetic Response (% Ph+) |
Time on Therapy at Cytogenetic Testing | |||||||
|---|---|---|---|---|---|---|---|---|
| 3 Months | 6 Months | 12 Months | 18 Months | |||||
| Stable CCyR (%) |
Est. Event Rate (%) |
Stable CCyR (%) |
Est. Event Rate (%) |
Stable CCyR (%) |
Est. Event Rate (%) |
Stable CCyR (%) |
Est. Event Rate (%) |
|
| Complete (0) | -- | 6 [1,11] | -- | 7 [3,11] | -- | 6 [2,9] | -- | 3 [1,6] |
| Partial (>0–35) | 72 [65,80] | 10 [5,15] | 63 [53,73] | 17 [8,26] | 57 [42,72] | 20 [7,33] | 29a [16,42] | 31a [15,46] |
| Minor (>35–65) | 55 [39,71] | 30 [15,45] | 35 [15,55] | 38 [16,60] | 14a [2,25] | 62a [42,81] | ||
| Minimal (>65–95) | 37 [19,54] | 32 [13,51] | 25 [3,47] | 45 [20,71] | ||||
| None (>95) | 32 [16,47] | 40 [22,58] | 32a [10,53] | 36a [12,60] | ||||
Cytogenetic response at the indicated time points is considered "failure" according to European LeukemiaNet (ELN) guidelines. Reproduced by permission of Deininger M, O'Brien SG, Guilhot F, et al. International Randomized Study of Interferon vs STI571 (IRIS) 8-year follow up: sustained survival and low risk for progression or events in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib. ASH Annual Meeting Abstracts 2009;114:abstr 1126.
Cytogenetic and molecular definitions of imatinib failure and resistance
Despite the clinical success of imatinib as the first TKI therapy for CML (and cancer therapy overall), approximately 15–25% of patients do not respond to initial imatinib treatment (termed primary resistant) or are intolerant of imatinib7,29, suggesting the need for increased doses or alternate TKI treatment. In one retrospective analysis, 35% of 594 CML patients required dose escalation30. Similarly, other investigators report that over a 5-year follow-up period, 9% of 159 patients had a loss of CCyR29. Finally, in the 8-year follow-up of the IRIS trial, 45% of the original cohort (249 of 553 patients) discontinued treatment. Of these patients, 16% discontinued therapy due to suboptimal treatment responses and 6% due to intolerance or safety issues28, suggesting the need for other therapies. Data from the imatinib clinical trial program indicate that the timing and degree of CCyR and MMR are both reliable surrogate markers for a favorable CML prognosis4. For example, attaining a CCyR or a MMR within the first 12 months of imatinib treatment predicts a low risk of disease progression4,28,31. Nevertheless, a substantial number of patients (30–40%) do not achieve a CCyR to imatinib during the first year29,31. A MMR achieved in the first 12 months of treatment corresponds with a long-lasting CCyR32,33.
When it occurs, the loss of response to imatinib treatment and progression to accelerated- or blast-phase CML happens primarily during the first 3 years of treatment31. In one 5-year follow-up study of 224 patients treated with imatinib, the probability of losing the CCyR was 0% for patients who attained a MMR by 18 months, whereas 25% of patients who did not reach a MMR by 18 months lost their CCyR (P=0.008)34. Recent data suggest that CyR to second-line TKI therapy with dasatinib at 12 months is also predictive of long-term survival35.
Recent guidelines have been developed to both identify and address the clinical scenario of CML patients who have failed imatinib. The NCCN guidelines recommend imatinib dose escalation (to 800 mg/day) or change to another TKI in patients who do not achieve a CHR within 3 months, any CyR at 6 months, a CCyR or PCyR at 12 months, or a CCyR at 18 months7. The somewhat more stringent guidelines from the European Leukemia Net stipulate that suboptimal responses (ie, no CyR at 3 months, less than PCyR at 6 months, PCyR at 12 months, less than MMR at 18 months) to primary treatment with imatinib warrants either imatinib dose escalation or a trial of another TKI4.
Second-generation TKIs
Nilotinib and dasatinib are second-generation TKIs which are Food and Drug Administration (FDA) approved in the United States for treatment of CML. Both agents are more potent in vitro inhibitors of BCR-ABL than imatinib. Nilotinib is 10- to 50-times more potent and dasatinib is 325-times more potent36,37. The efficacy of these TKIs in patients who have previously failed or were intolerant of imatinib is well-established. In a 2-year follow-up of 321 CML patients, nilotinib (400 mg twice daily) resulted in an estimated PFS of 67% at 18 months and an estimated OS of 88% at 24 months38. Four-year follow-up of 167 CML patients treated with dasatinib 100 mg once daily resulted in a PFS of 66%, an estimated OS of 82%, and a rate of progression to accelerated- or blast-phase of 4%35. CyR to second-line dasatinib at 6 and 12 months predicted 2-year PFS35.
BCR-ABL mutations as a guide to TKI therapy
Mutations in the BCR-ABL oncogene are a common cause of resistance to imatinib therapy. Therefore, the presence of specific BCR-ABL mutations may inform the choice of TKI therapy. Direct sequencing of DNA after qRT-PCR is most often used by clinicians to identify specific mutations in the BCR-ABL kinase domain12. Clinically relevant mutations generally increase in frequency with disease progression. In one report, 18% of patients with chronic-phase CML had evidence of imatinib-resistant mutations with much higher incidence in accelerated- or blast-phase CML patients39. Since the T315I mutation confers resistance to imatinib, nilotinib, and dasatinib1, experimental therapies other than the currently approved TKIs are recommended in those patients whose disease is characterized by this mutation39. In a survey of BCR-ABL mutations in 386 CML patients, Branford and colleagues identified specific mutations, which conferred clinically significant resistance to nilotinib (E255K/V, Y253H and F359C/V) and dasatinib (V299L and F317L)39. The presence of these specific mutations warrants consideration of alternate TKI therapy39.
Second-generation TKIs for front-line CML therapy
The results of phase 2/3 clinical trials as well as the recent accelerated FDA approval of nilotinib40 and dasatinib19 support the evolving use of second-generation TKI agents for upfront treatment of newly diagnosed chronic-phase CML patients. Two recently published phase 3 trials comparing imatinib with front-line nilotinib (Evaluating Nilotinib Efficacy and Safety in Clinical Trials–Newly Diagnosed Patients [ENESTnd])23 or dasatinib (Dasatinib versus Imatinib Study in Treatment-Naive CML Patients [DASISION])21 have confirmed the phase 2 results (Table 4–Table 7)21,23. Findings of both the ENESTnd and DASISION trials support the notion that early robust cytogenetic and molecular responses are valid treatment goals of CML TKI therapy21,23.
Table 4.
| ENESTnd (300 mg BID) | DASISION | ||||
|---|---|---|---|---|---|
| Sokal | Nilotinib | Imatinib | Hasford | Dasatinib | Imatinib |
| Low | 37% | 37% | Low | 33% | 33% |
| Intermediate | 36% | 36% | Intermediate | 48% | 47% |
| High | 28% | 28% | High | 19% | 19% |
Table 7.
| ENESTnd (300 mg BID) | DASISION | ||||
|---|---|---|---|---|---|
| Event | Nilotinib, % | Imatinib, % | Event | Dasatinib, % | Imatinib, % |
| Peripheral edema |
0 | 0 | Fluid retention | 1 | 1 |
| Eyelid edema | 0 | <1 | Superficial edema | 0 | <1 |
| Periorbital edema |
0 | 0 | Pleural effusion | 0 | 0 |
| Other | 1 | <1 | |||
| Diarrhea | 1 | 1 | Diarrhea | <1 | 1 |
| Nausea | <1 | 0 | Nausea | 0 | 0 |
| Myalgia | <1 | 0 | Myalgia | 0 | 0 |
| Fatigue | 0 | <1 | Fatigue | <1 | 0 |
| Muscle spasm | 0 | 1 | Muscle inflammation |
0 | <1 |
| Musculoskeletal pain |
0 | <1 | |||
| Rash | <1 | 1 | Rash | 0 | 1 |
| Pruritus | <1 | 0 | |||
| Headache | 1 | 0 | Headache | 0 | 0 |
| Cardiac a | Prolongs QT interval |
Cardiac | Prolongs QT interval; 1- <10% arrhythmia, palpitations |
||
| Patients >65 years of age a |
No increased toxicity |
Patients >65 years of age |
Likely to experience toxicity |
||
| Discontinuation due to AEs a |
7% | Discontinuation due to AEs |
6% | ||
AEs, adverse events.
Data from drug prescribing information.
Nilotinib
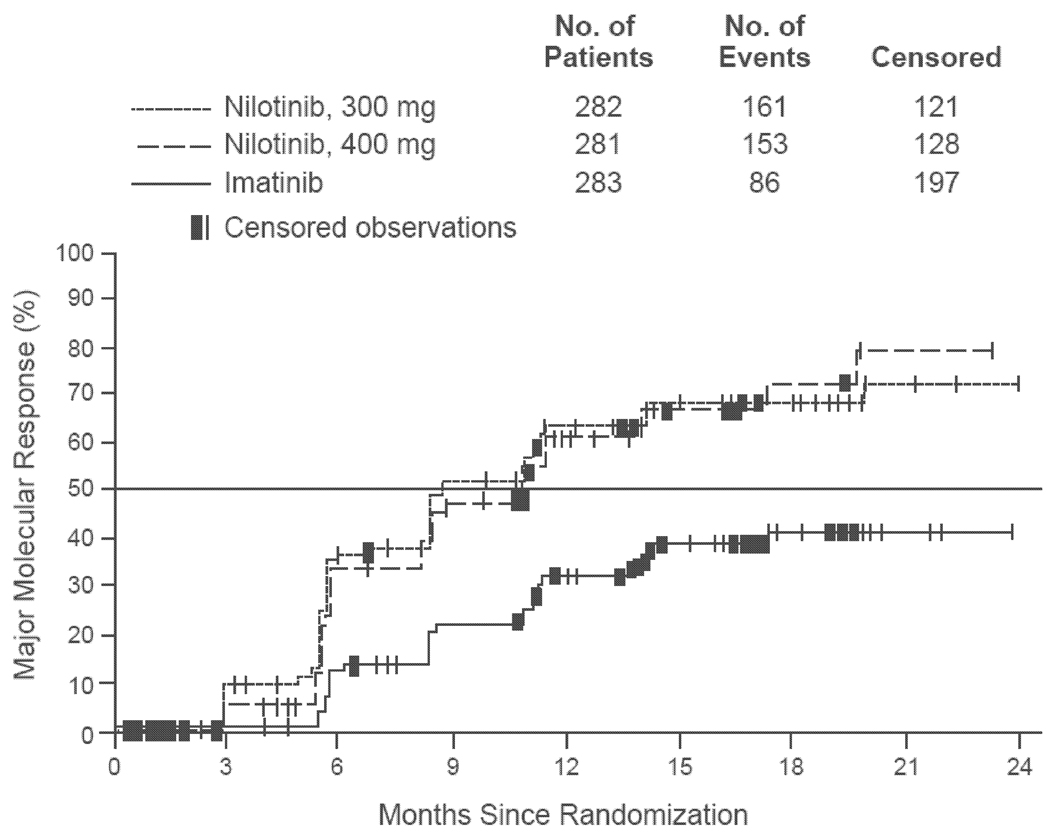
The ENESTnd trial was a phase 3 comparison between nilotinib (300 or 400 mg twice daily) and imatinib (400 mg once daily) in 846 patients with newly diagnosed chronic-phase CML23. Treatment groups were well-matched with regard to Sokal risk scores (Table 4). The primary endpoint was MMR at 12 months as defined by a BCR-ABL transcript level ≤0.1% (IS) on peripheral blood qRT-PCR. Rates of MMR at 12 months were significantly higher in patients treated with nilotinib 300 mg (44%) compared to imatinib (22%; P<0.001) (Table 5). Similarly, rates of CCyR at 12 months were greater in the nilotinib groups (80% for 300 mg, 78% for 400 mg) than in the imatinib group (65%; P<0.001 for both nilotinib doses) (Table 5). According to Kaplan-Meier estimates, the median time to reach MMR for all patients was 8.6 months (nilotinib 300 mg) and 11.0 months (nilotinib 400 mg); the median time to MMR had not yet been reached for the imatinib cohort at the time of publication (Fig. 2)23. Rates of progression from the chronic phase to the accelerated or blast phase were <1% in the nilotinib groups (300 and 400 mg), and 4% in the imatinib group. Although none of the patients in any treatment group who achieved a MMR had disease progression during follow-up, disease progression occurred in 3 patients who had a CCyR on imatinib (Table 5). Rates of grade 3–4 neutropenia and anemia were higher in the imatinib group (Table 6). The most common nonhematologic adverse events were diarrhea and headache (nilotinib) and gastrointestinal disturbances, muscle spasm, and peripheral edema (imatinib) (Table 7).
Table 5.
| ENESTnd (300 mg BID) | DASISION | |||||
|---|---|---|---|---|---|---|
| Nilotinib | Imatinib | P | Dasatinib | Imatinib | P | |
| MMR | 44% | 22% | <0.001 | 46% | 28% | <0.0001 |
| CCyR | 80% | 65% | <0.001 | 83%a | 72%a | <0.001 |
| Disease transformation |
<1% | 4% | 0.01 | 1.9% | 3.5% | NS |
MMR, major molecular response; CCyR, complete cytogenetic remission; NS, not stated.
Observed on at least one assessment.
Figure 2.
Time to first major molecular response in the ENESTnd trial of nilotinib versus imatinib in newly diagnosed patients with chronic-phase chronic myelogenous leukemia. All time-to-event comparisons in the intention-to-treat population were performed with the use of the log-rank test, stratified according to Sokal risk group.
Reproduced by permission of Saglio G, Kim DW, Issaragrisil S, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med 2010;362:2251–9.
Table 6.
| ENESTnd (300 mg BID) | DASISION | |||
|---|---|---|---|---|
| Nilotinib, % | Imatinib, % | Dasatinib, % | Imatinib, % | |
| Neutropenia | 12 | 20 | 21 | 20 |
| Thrombocytopenia | 10 | 9 | 19 | 10 |
| Anemia | 3 | 5 | 10 | 7 |
Dasatinib
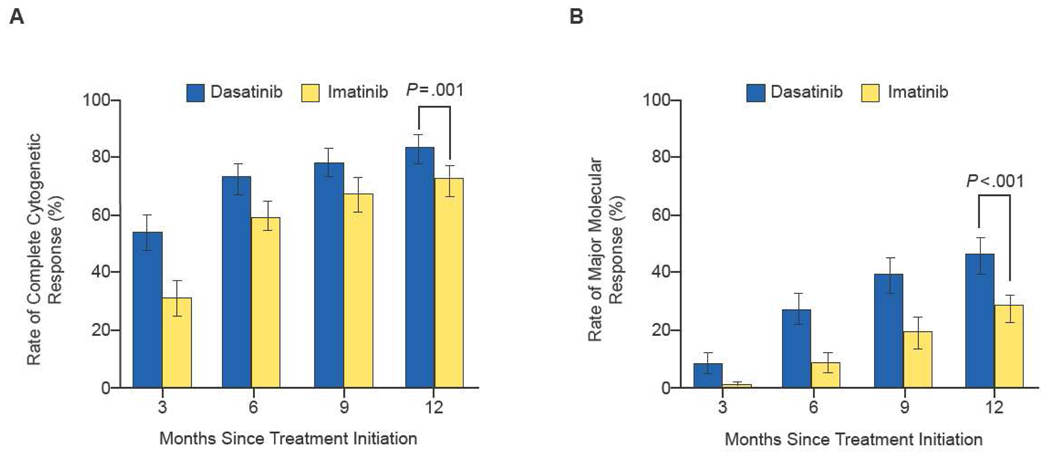
The DASISION phase 3 trial compared a 12-month course of dasatinib (100 mg once daily) with imatinib (400 mg once daily) in 547 patients with newly diagnosed chronic-phase CML21. Patients were well-matched with regard to Hasford risk scores (Table 4). The primary endpoint was CCyR at 12 months21. Compared with imatinib, dasatinib resulted in significantly higher 12-month rates of CCyR (83% vs 72%), confirmed CCyR (77% vs 66%; P=0.007), MMR (46% vs 28%; P<0.0001), and MMR in patients with CCyR at 12 months (54% vs 39%; P=0.002) (Table 5). Times to CCyR and MMR were significantly shorter with dasatinib than imatinib (hazard ratio for shorter time to response with dasatinib = 1.5 and 2.0, respectively; P<0.0001) (Fig. 3)21. No patients who achieved a MMR had disease progression during 12-month follow-up21. Rates of progression to blastic phase were 1.9% for dasatinib and 3.5% for imatinib (Table 5). Grade 3–4 neutropenia occurred in similar proportions of patients in both groups; grade 3–4 thrombocytopenia and anemia were more common with dasatinib (Table 6). The most common nonhematologic adverse events were fluid retention (19% dasatinib, 42% imatinib) and gastrointestinal disturbances (30% dasatinib, 47% imatinib). Grade 3 or 4 nonhematologic adverse events were infrequent (Table 7).
Figure 3.
Rates of complete cytogenetic response (panel A) and major molecular response (panel B) in the DASISION trial of dasatinib versus imatinib.
Reproduced by permission of Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 2010;362:2260–70.
Conclusions
The advent of TKI therapy, as demonstrated in the IRIS trial, redefined the treatment of chronic-phase CML and has irrevocably altered the natural course of the disease in the majority of CML patients. The IRIS trial also firmly established the utility of cytogenetic and molecular analyses as both requisite tools and endpoints for CML therapy. Expert consensus panels now agree that the optimal methods for determining cytogenetic and molecular responses in CML are bone marrow karyotype and peripheral blood BCR-ABL qRT-PCR testing, respectively. It must be emphasized that misinterpreting the results of minimal residual disease testing because of nonstandardized methods or poor correlations with recommended sampling methods poses the risk of making unnecessary or unfounded changes in treatment strategy for individual patients. Recommendations for harmonized methods of molecular BCR-ABL testing with conversion of results to an international standard are being increasingly used by academic and commercial laboratories.
Cytogenetic and molecular responses are now considered standard surrogates for long-term outcome. Longitudinal data from the IRIS trial, now available for 8 years of follow-up, show that achievement of CCyR and MMR within the first 12 months of TKI initiation are associated with high rates of event-free survival, PFS, and OS.
However, resistance, as determined by cytogenetic and molecular parameters, and clinical intolerance to imatinib therapy in 15–25% of patients has necessitated the development of other treatment approaches. The second-generation TKIs, nilotinib and dasatinib, are established effective alternatives to imatinib in these patients in the second-line setting. Moreover, recent data from the ENESTnd and DASISION trials have demonstrated that front-line treatment with nilotinib or dasatinib, respectively, in newly diagnosed chronic-phase CML patients’ results in more robust and more rapid cytogenetic and molecular responses at 12 months as compared with imatinib. The relationship between achieving a CCyR in the first 12 months of treatment and PFS over the long-term provides an argument for the use of the second-generation TKIs as front-line treatment of newly diagnosed chronic-phase CML. In 2010, the FDA approved nilotinib (June 17) and dasatinib (October 28) for front-line therapy of chronic-phase CML. Further studies are needed to better delineate the role of the TKIs in this patient population and to inform decisions regarding long-term response, PFS, resistance profiles, and adverse events of TKI therapy in individual CML patients.
Acknowledgements
The author takes full responsibility for the content of this publication, and confirms that it reflects his viewpoint and medical expertise. He also wishes to acknowledge StemScientific, funded by Bristol-Myers Squibb, for providing writing and editing support. Bristol-Myers Squibb did not influence the content of the manuscript, nor did the author receive financial compensation for authoring the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
Meir Wetzler is on the speakers bureaus of Novartis and Bristol-Myers Squibb. The other coauthors have no conflict of interests.
References
- 1.Deininger MW. Milestones and monitoring in patients with CML treated with imatinib. Hematology Am Soc Hematol Educ Program. 2008:419–426. doi: 10.1182/asheducation-2008.1.419. [DOI] [PubMed] [Google Scholar]
- 2.Druker BJ, Tamura S, Buchdunger E, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med. 1996;2:561–566. doi: 10.1038/nm0596-561. [DOI] [PubMed] [Google Scholar]
- 3.Quintás-Cardama A, Cortes J. Molecular biology of bcr-abl1-positive chronic myeloid leukemia. Blood. 2009;113:1619–1630. doi: 10.1182/blood-2008-03-144790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baccarani M, Cortes J, Pane F, et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009;27:6041–6051. doi: 10.1200/JCO.2009.25.0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 6.Hochhaus A, O'Brien SG, Guilhot F, et al. Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia. Leukemia. 2009;23:1054–1061. doi: 10.1038/leu.2009.38. [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. [Accessed December 15, 2010];Chronic Myelogenous Leukemia. doi: 10.6004/jnccn.2009.0065. V2.2010. Available at: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. [DOI] [PubMed]
- 8.Aguayo A, Couban S. State-of-the-art in the management of chronic myelogenous leukemia in the era of the tyrosine kinase inhibitors: evolutionary trends in diagnosis, monitoring and treatment. Leuk Lymphoma. 2009;50 Suppl 2:1–8. doi: 10.3109/10428190903370387. [DOI] [PubMed] [Google Scholar]
- 9.Branford S. Chronic myeloid leukemia: molecular monitoring in clinical practice. Hematology Am Soc Hematol Educ Program. 2007:376–383. doi: 10.1182/asheducation-2007.1.376. [DOI] [PubMed] [Google Scholar]
- 10.Löwenberg B. Minimal residual disease in chronic myeloid leukemia. N Engl J Med. 2003;349:1399–1401. doi: 10.1056/NEJMp038130. [DOI] [PubMed] [Google Scholar]
- 11.Tefferi A, Dewald GW, Litzow ML, et al. Chronic myeloid leukemia: current application of cytogenetics and molecular testing for diagnosis and treatment. Mayo Clin Proc. 2005;80:390–402. doi: 10.4065/80.3.390. [DOI] [PubMed] [Google Scholar]
- 12.Kantarjian H, Schiffer C, Jones D, Cortes J. Monitoring the response and course of chronic myeloid leukemia in the modern era of BCR-ABL tyrosine kinase inhibitors: practical advice on the use and interpretation of monitoring methods. Blood. 2008;111:1774–1780. doi: 10.1182/blood-2007-09-110189. [DOI] [PubMed] [Google Scholar]
- 13.Radich JP. How I monitor residual disease in chronic myeloid leukemia. Blood. 2009;114:3376–3381. doi: 10.1182/blood-2009-02-163485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riley RS, Hogan TF, Pavot DR, et al. A pathologist's perspective on bone marrow aspiration and biopsy: Performing a bone marrow examination. J Clin Lab Anal. 2004;18:70–90. doi: 10.1002/jcla.20008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fugazza G, Miglino M, Bruzzone R, et al. Cytogenetic and fluorescence in situ hybridization monitoring in Ph+ chronic myeloid leukemia patients treated with imatinib mesylate. J Exp Clin Cancer Res. 2004;23:295–299. [PubMed] [Google Scholar]
- 16.Lesser ML, Dewald GW, Sison CP, Silver RT. Correlation of three methods of measuring cytogenetic response in chronic myelocytic leukemia. Cancer Genet Cytogenet. 2002;137:79–84. doi: 10.1016/s0165-4608(02)00558-7. [DOI] [PubMed] [Google Scholar]
- 17.Reinhold U, Hennig E, Leiblein S, Niederwieser D, Deininger MW. FISH for BCR-ABL on interphases of peripheral blood neutrophils but not of unselected white cells correlates with bone marrow cytogenetics in CML patients treated with imatinib. Leukemia. 2003;17:1925–1929. doi: 10.1038/sj.leu.2403077. [DOI] [PubMed] [Google Scholar]
- 18.Testoni N, Marzocchi G, Luatti S, et al. Chronic myeloid leukemia: a prospective comparison of interphase fluorescence in situ hybridization and chromosome banding analysis for the definition of complete cytogenetic response: a study of the GIMEMA CML WP. Blood. 2009;114:4939–4943. doi: 10.1182/blood-2009-07-229864. [DOI] [PubMed] [Google Scholar]
- 19.Cortes JE, Jones D, O'Brien S, et al. Results of dasatinib therapy in patients with early chronic-phase chronic myeloid leukemia. J Clin Oncol. 2010;28:398–404. doi: 10.1200/JCO.2009.25.4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hochhaus A, Baccarani M, Deininger M, et al. Dasatinib induces durable cytogenetic responses in patients with chronic myelogenous leukemia in chronic phase with resistance or intolerance to imatinib. Leukemia. 2008;22:1200–1206. doi: 10.1038/leu.2008.84. [DOI] [PubMed] [Google Scholar]
- 21.Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362:2260–2270. doi: 10.1056/NEJMoa1002315. [DOI] [PubMed] [Google Scholar]
- 22.Kantarjian HM, Giles F, Gattermann N, et al. Nilotinib (formerly AMN107), a highly selective BCR-ABL tyrosine kinase inhibitor, is effective in patients with Philadelphia chromosome-positive chronic myelogenous leukemia in chronic phase following imatinib resistance and intolerance. Blood. 2007;110:3540–3546. doi: 10.1182/blood-2007-03-080689. [DOI] [PubMed] [Google Scholar]
- 23.Saglio G, Kim DW, Issaragrisil S, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362:2251–2259. doi: 10.1056/NEJMoa0912614. [DOI] [PubMed] [Google Scholar]
- 24.Shah NP, Kantarjian HM, Kim DW, et al. Intermittent target inhibition with dasatinib 100 mg once daily preserves efficacy and improves tolerability in imatinib-resistant and -intolerant chronic-phase chronic myeloid leukemia. J Clin Oncol. 2008;26:3204–3212. doi: 10.1200/JCO.2007.14.9260. [DOI] [PubMed] [Google Scholar]
- 25.Branford S, Fletcher L, Cross NC, et al. Desirable performance characteristics for BCR-ABL measurement on an international reporting scale to allow consistent interpretation of individual patient response and comparison of response rates between clinical trials. Blood. 2008;112:3330–3338. doi: 10.1182/blood-2008-04-150680. [DOI] [PubMed] [Google Scholar]
- 26.Hughes T, Deininger M, Hochhaus A, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006;108:28–37. doi: 10.1182/blood-2006-01-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hughes TP, Kaeda J, Branford S, et al. Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia. N Engl J Med. 2003;349:1423–1432. doi: 10.1056/NEJMoa030513. [DOI] [PubMed] [Google Scholar]
- 28.Deininger M, O'Brien SG, Guilhot F, et al. International Randomized Study of Interferon vs STI571 (IRIS) 8-year follow up: sustained survival and low risk for progression or events in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib. ASH Annual Meeting Abstracts. 2009;114 abstr 1126. [Google Scholar]
- 29.de Lavallade H, Apperley JF, Khorashad JS, et al. Imatinib for newly diagnosed patients with chronic myeloid leukemia: incidence of sustained responses in an intention-to-treat analysis. J Clin Oncol. 2008;26:3358–3363. doi: 10.1200/JCO.2007.15.8154. [DOI] [PubMed] [Google Scholar]
- 30.Michallet M, Tulliez M, Corm S, et al. Management of chronic myeloid leukaemia in clinical practice in France: results of the French subset of patients from the UNIC study. Curr Med Res Opin. 2010;26:307–317. doi: 10.1185/03007990903479299. [DOI] [PubMed] [Google Scholar]
- 31.Druker BJ, Guilhot F, O'Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 32.Cortes J, Talpaz M, O'Brien S, et al. Molecular responses in patients with chronic myelogenous leukemia in chronic phase treated with imatinib mesylate. Clin Cancer Res. 2005;11:3425–3432. doi: 10.1158/1078-0432.CCR-04-2139. [DOI] [PubMed] [Google Scholar]
- 33.Iacobucci I, Saglio G, Rosti G, et al. Achieving a major molecular response at the time of a complete cytogenetic response (CCgR) predicts a better duration of CCgR in imatinib-treated chronic myeloid leukemia patients. Clin Cancer Res. 2006;12:3037–3042. doi: 10.1158/1078-0432.CCR-05-2574. [DOI] [PubMed] [Google Scholar]
- 34.Marin D, Milojkovic D, Olavarria E, et al. European LeukemiaNet criteria for failure or suboptimal response reliably identify patients with CML in early chronic phase treated with imatinib whose eventual outcome is poor. Blood. 2008;112:4437–4444. doi: 10.1182/blood-2008-06-162388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shah NP, Cortes JE, Schiffer CA, et al. Four-year follow-up of patients with chronic-phase chronic myeloid leukemia (CP-CML) receiving dasatinib 100 mg once daily. ASCO Annual Meeting Abstracts. 2009;28 abstr 6512. [Google Scholar]
- 36.O'Hare T, Walters DK, Stoffregen EP, et al. In vitro activity of Bcr-Abl inhibitors AMN107 and BMS-354825 against clinically relevant imatinib-resistant Abl kinase domain mutants. Cancer Res. 2005;65:4500–4505. doi: 10.1158/0008-5472.CAN-05-0259. [DOI] [PubMed] [Google Scholar]
- 37.Weisberg E, Manley PW, Breitenstein W, et al. Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer Cell. 2005;7:129–141. doi: 10.1016/j.ccr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 38.Kantarjian H, Giles F, Bhalla K, et al. Nilotinib in chronic myeloid leukemia patients in chronic phase (CML-CP) with imatinib (IM) resistance or intolerance: longer follow-up results of a phase II study. ASCO Annual Meeting Abstracts. 2009;27 abstr 7029. [Google Scholar]
- 39.Branford S, Melo JV, Hughes TP. Selecting optimal second-line tyrosine kinase inhibitor therapy for chronic myeloid leukemia patients after imatinib failure: does the BCR-ABL mutation status really matter? Blood. 2009;114:5426–5435. doi: 10.1182/blood-2009-08-215939. [DOI] [PubMed] [Google Scholar]
- 40.Rosti G, Palandri F, Castagnetti F, et al. Nilotinib for the frontline treatment of Ph(+) chronic myeloid leukemia. Blood. 2009;114:4933–4938. doi: 10.1182/blood-2009-07-232595. [DOI] [PubMed] [Google Scholar]