Abstract
Increasing evidence suggested obesity, measured by body-mass index (BMI), was associated with prostate cancer-specific mortality, and its impact on biochemical recurrence was also inconclusive.
We systematically searched MEDLINE, EMBASE and bibliographies of retrieved studies up to Jan 5th, 2010. We used random-effects meta-analysis to assess the relative risks (RR) of prostate cancer-specific mortality and biochemical recurrence associated with a 5 kg/m2 increase in BMI.
Among the 6 population-based cohort studies in 1,263,483 initially cancer-free men, 6,817 prostate cancer deaths occurred; a 5kg/m2 increase in BMI was associated with 15% (RR 1.15, 95%CI 1.06–1.25, p<0.01) higher risk of dying of prostate cancer. In the 6 post-diagnosis survival studies on 18,203 patients with 932 prostate cancer deaths, a 5kg/m2 increase in BMI was associated with 20% higher prostate cancer-specific mortality (RR 1.20, 95%CI 0.99–1.46, p=0.06). In the 16 studies which followed 26,479 prostate cancer patients after primary treatment, a 5kg/m2 increase in BMI was significantly associated with 21% increased risk of biochemical recurrence (RR 1.21, 95%CI 1.11–1.31 p<0.01).
Elevated BMI is associated with risk of prostate cancer-specific mortality in prospective cohort studies and biochemical recurrence in prostate cancer patients. Its association with prostate cancer-specific mortality in diagnosed patients needs to be further evaluated.
Keywords: obesity, body-mass index, prostate cancer mortality, biochemical recurrence, meta-analysis
Introduction
Obesity, a growing epidemic in all over the world, has been linked to mortality of several cancers (1), but only in the past 5 to 10 years, body-mass index (BMI) as a surrogate of adiposity has been extensively evaluated for prostate cancer incidence and mortality.
Higher BMI in mid/late adult life is weakly associated with higher risk of incident prostate cancer (2), but recently the pattern that obesity is associated with lower risk of low grade prostate cancer and higher risk of aggressive prostate cancer emerges (2–5). Increasing evidence suggested that higher BMI is associated with poorer outcomes, i.e. higher risk of prostate cancer-specific mortality among both obese healthy adults (4, 6–8) and prostate cancer patients (9–13), and higher rates of biochemical recurrence among diagnosed patients (14–16), however, no systematic review data is available regarding the impact of obesity and overweight on prostate cancer progression.
It is crucial to review and evaluate the magnitude that obesity affects mortality and recurrence of prostate cancer as proper management of this modifiable life style factor may help improve prostate cancer outcomes. We therefore conducted a meta-analysis to quantitatively summarize the association between BMI and risk of dying of prostate cancer in initially cancer-free men, prostate cancer-specific mortality among the diagnosed, and biochemical recurrence in the treated.
Material and Methods
Search Strategy
This systematic review was conducted according to the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines (17). We searched MEDLINE and EMBASE to identify relevant articles on human subjects that were written in English from the inception of each database to January 5, 2010 using the key words related to obesity (‘obesity’, ‘overweight’, ‘body weight’, ‘body mass index’, ‘BMI’, ‘weight’, ‘body size’, ‘adiposity’) combined with specific terms on prostate cancer mortality or biochemical recurrence (‘prostate cancer’ and ‘mortality’, ‘survival’, ‘death’, ‘prognosis’, ‘progression’ or ‘recurrence’). Bibliographies of retrieved papers were also searched.
Eligibility Criteria
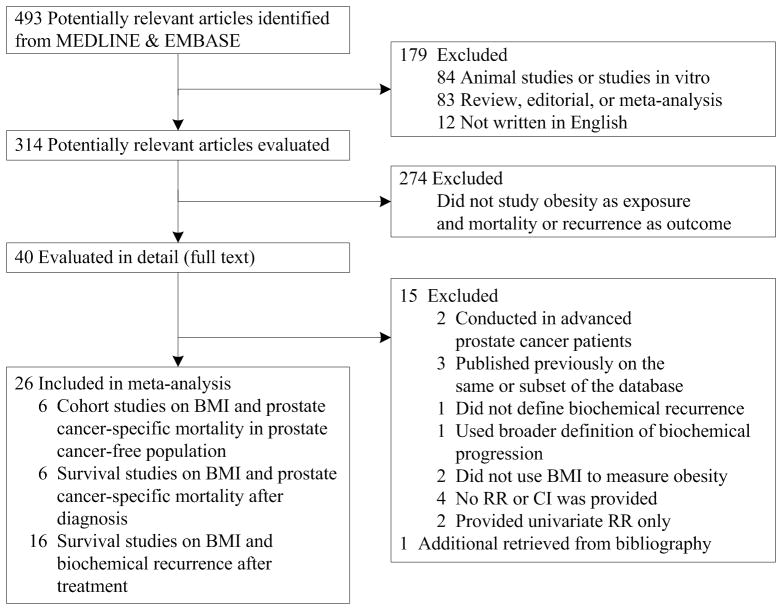
The eligibility of each study was assessed independently by two investigators (Y.C., J.M.). We included only cohort studies of mid/late life BMI and prostate cancer-specific mortality (prostate cancer as the underlying cause of death), and clinical studies of BMI and biochemical recurrence after treatment. We excluded reviews, editorials, meta-analysis, animal studies or in vitro studies, and non-English written studies. Among the 40 studies we performed a full text review on, we excluded studies conducted among patients with metastatic prostate cancer (18, 19), using a broader definition of progression rather than biochemical recurrence (20) and in which BMI was not used to measure obesity (21, 22). For studies previously published on the same database (23–25), we included only the most recent findings (16, 26).
We included studies that reported standardized forms of relative risk, risk ratio, hazard ratio or odds ratio, with estimates on confidence intervals (CIs), and used relative risks (RRs) to represent various effect estimates. We excluded studies failing to report the above estimates (27–30) or presenting only univariate estimates (31, 32).
Data Extraction
Data extraction was conducted independently by two investigators (Y.C., J.M.) using a standardized data extraction form. For each included article, we extracted information on the title, authors, journal and publication year, study design, study population and setting, duration of follow-up, BMI categories, definition of biochemical recurrence, number of outcomes, the most adjusted effect estimates, and covariates controlled in multivariable analysis. For studies that presented findings from more than one database, we extracted only the most recent update from each of the databases (8, 33).
Statistical Analysis
We analyzed BMI as a continuous variable by first transforming all the relative risk estimates to the corresponding RRs for every 5kg/m2 increase in BMI, with the assumption that the risk increment is constant, and also allowed for a fair comparison among studies using different BMI categories.
Whenever RR per kg/m2 increase in BMI and its 95% CI were available, we used them to estimate the RR and 95% CI for every 5kg/m2 BMI increase. Category-specific RRs were converted into RRs associated with every 5kg/m2 increase in BMI by use of generalized least-squares for trend estimation whenever person-time data were available, or weighted least square method when only counts of death were available(34, 35). The value assigned to each BMI category was the mid-point for closed categories and was adjusted for half range of the neighborhood categories when categories were open-ended. In three studies where BMI was only divided into two open-ended categories, we assumed that the RR and CI estimate for the higher BMI category was similar to estimates for a 5kg/m2 increase in BMI(15, 36, 37). We validated such methods in studies which presented RRs for both continuous and categorical BMI, and found that the RR per kg/m2 increase obtained by conversion was similar with the RR for continuous BMI shown in the paper (11).
We pooled all the RRs for a 5kg/m2 increase in BMI using DerSimonian-Laired random-effect meta-analysis(38), and assessed the heterogeneity between studies by Q and I2 statistics. Sensitivity analysis was conducted by omitting one study at a time, generating the pooled estimates and comparing with the original estimates. Stratified meta-analysis was performed by country of study, BMI measurement, definition of biochemical recurrence, and specific treatment type within patients treated with radiation therapy. Funnel plots and both Begg’s and Egger’s tests were used to evaluate publication bias. We also calculated population attributable risk percent (PAR%) among diagnosed prostate cancer patients using available category specific RRs, based on prevalence of overweight and obesity of US males aged 60 and above from the National Health and Nutrition Examination Survey (NHANES) 2007–2008 (39).
All analyses were performed using STATA version 10.0 statistical software (Stata, College Station, Texas, USA). All statistical comparisons were 2-sided, and a p-value<0.05 was considered statistically significant.
Results
Data Extracted and Quality
Twenty-six studies met the inclusion criteria. Of these studies, 12 evaluated BMI and prostate cancer-specific mortality(1, 4, 6–13, 40, 41) and 16 assessed biochemical recurrence after primary treatment (Figure 1).(12–16, 26, 33, 36, 37, 42–48) Two (12, 13) presented findings on both outcomes and therefore were included in both the meta-analyses of mortality and biochemical recurrence
Figure 1.
Selection of studies for meta-analysis
Six of the 12 studies of prostate cancer-specific mortality were population-based cohort studies conducted among volunteers in the United States and Europe(1, 4, 6–8, 40). A total of 1,263,483 initially cancer-free men were prospectively followed up for an average of over ten years except one with 5.5 years(4), and 6,817 men died of prostate cancer (Table 1). BMI was self-reported, measured or retrieved from medical records at study enrollment. All the studies had controlled for smoking status.
Table 1.
Characteristics of identified studies on body-mass index and prostate cancer-specific mortality (N=12)
| Source | Study characteristics | BMI measurement | No. of subjects | No. of PCa deaths | BMI categories and RR (95% CI) | RR per 5 kg/m2 increase of BMI (95% CI) and P-value | Confounders adjusted | |
|---|---|---|---|---|---|---|---|---|
| Population-based cohort study (N=6) | ||||||||
|
Andersson(6) (J Natl Cancer Inst, 1997) |
Period: 1971–1991 Setting: construction workers, Sweden Total person years: 2,377,960 |
Measured BMI retrieved from medical record | 135,006 | 708 | <22.1 22.1–24.1 24.2–26.2 >26.2 |
1.00 1.36 (1.03–1.79) 1.33 (1.02–1.74) 1.40 (1.09–1.81) |
1.25 (1.03–1.51) P=0.03 |
Age, smoking status, marital status |
|
Rodriguez(8) (Cancer Epidemiol Biomarkers Prev, 2001) |
Period:1959–1972 Setting: CPS I, USA Total person years: 4,120,363 |
Self reported at baseline | 381,638 | 1,590 | <25 25–30 ≥ 30 |
1.00 1.02 (0.92–1.14) 1.27 (1.04–1.56) |
1.08 (0.99–.17) P=0.09 |
Age, race, height, education, exercise, smoking status, and family history of prostate cancer |
|
Calle(1) (NEJM,2003) |
Period:1982–1998 Setting: CPS II, USA Total person years: not shown |
Self reported at baseline | 404,576 | 4,004 | 18.5–24.9 25–29.9 30–34.9 ≥ 35 |
1.00 1.08 (1.01–1.15) 1.20 (1.06–1.36) 1.34 (0.98–1.83) |
1.08 (1.04–1.12) P<0.001 |
Age, education, smoking status, number of cigarettes smoked, physical activity, alcohol use, marital status, race, aspirin use, fat consumption and vegetable consumption |
|
Eichholzer(40) (Swiss Med Wkly, 2005) |
Period: 1971–1990 Setting: employees from chemical/pharmaceutical companies, Switzerland Total person years: not shown |
Examination at study entry | 2,974 | 30 | Continuous | 0.95 (0.93–1.18) | 0.77 (0.70–2.29) P=0.42 |
Age, smoking |
|
Wright(4) (Cancer, 2007) |
Period:1995–2000 Setting: NIII-AARP Diet and Health Study, USA Total person years: 1,578,732 |
Self reported at baseline | 287,760 | 173 | <25 25–29.9 30–34.9 ≥ 35 |
1.0 1.25(0.87–1.80) 1.46(0.92–2.33) 2.12(1.08–4.15) |
1.25 (1.04–1.50) P=0.02 |
Age, race, smoking status, education, personal history of diabetes, and family history of prostate cancer |
|
Giovannucci(7) (Int J Cancer, 2007) |
Period: 1986–2002 Setting: Health Professionals Follow-up Study, USA Total person years: not shown |
Self reported at baseline | 51,529 | 312 | <23 23–24.9 25–27.4 27.5–29.9 ≥ 30 |
1.00 1.44 (1.01–2.05) 1.30 (0.91–1.87) 1.43 (0.93–2.22) 1.80 (1.10–2.93) |
1.38 (1.16–1.66) P<0.001 |
Age, time period, cigarette pack years, physical activity, family history of prostate cancer, BMI at 21, history of diabetes, race, intake of total calories, processed meat fish, α linolenic acid, tomato sauce, vitamine E supplement |
| Source | Study characteristics | BMI measurement | No. of Subjects | No. of PCa deaths (%) | No. of Others Deaths (%) | BMI categories and RR (95% CI) | RR per 5 kg/m2 increase of BMI (95% CI) and P-value | Confounders adjusted | |
|---|---|---|---|---|---|---|---|---|---|
| Post-diagnosis survival study (N=6) | |||||||||
|
Siddiqui(12) (Cancer, 2006) |
Period:1990–1999 Setting: 1 center, USA Treatment: RP Median follow-up: 10.1 yrs |
Measured at surgery | 5,313 | 151 (3%) | 967 (18%) | Continuous | 1.02 (0.97–1.07) P=0.372 |
1.10 (0.86–1.40) P=0.37 |
Gleason Score, preoperative serum PSA, surgical margin status, seminal vesicle invasion, adjuvant treatment. |
|
Efstathiou(9) (Cancer, 2007) |
Period: 1987–1992 Setting: multicenter trial, USA Treatment: i)RT and immediate ADT; ii)RT and ADT at recurrence Median follow-up: 8.1 yrs |
Measured at trial enrollment | 788 | 169 (21%) | 307 (39%) | <25 25–29.9 ≥30 |
1.00 1.52(1.02–2.28) 1.64(1.01–2.66) |
1.34 (1.09–1.65) P=0.01 |
Age, race, treatment arm, history of prostatectomy, nodal involvement, Gleason score, stage, |
|
Gong(10) (Cancer, 2007) |
Period: 1993–2004 Setting: participants for a population-based case-control study, USA Treatment: all types Median follow-up: 9.5 yrs |
Self reported BMI 1 yr prior to prostate cancer diagnosis | 752 | 50 (7%) | 64 (9%) | <25 25–30 ≥ 30 |
1.00 1.11 (0.55–2.25) 2.64(1.18–5.92) |
1.63 (1.09–2.44) P=0.02 |
Age, race, smoking status, Gleason score, stage, primary treatment |
|
Ma(11) (Lancet Oncology, 2008) |
Period: 1982–2007 Setting: Physicians’ Health Study, USA Treatment: all types Median follow-up: 7 yrs |
Self reported at baseline | 2,546 | 281 (11%) | 485 (19%) | Continuous <25 25–29.9 ≥ 30 |
1.07(1.02–1.12) 1.00 1.26(0.98–1.62) 1.95(1.17–3.23) |
1.40 (1.10–1.76) P<0.001 |
Age, baseline smoking status(1982), time interval from BMI measurement to prostate-cancer diagnosis, stage, Gleason grade |
|
van Roermund(13) (BJU Int, 2009) |
Period: 1991–2008 Setting: 1 center, Netherlands Treatment: Brachytherapy Median follow-up: 3.9 yrs |
At surgery (Anaesthesia record) | 1,530 | 61 (4%) | 132 (9%) | <25 25–30 ≥ 30 |
1.00 1.07 (0.60–1.88) 1.46 (0.50–4.28) |
1.14 (0.77–1.69) P=0.51 |
Age, risk group(stage, grade and preoperative PSA), preoperative PLND, treatment period, number of seeds |
|
Davies(41) (J Urol, 2009) |
Period: 1995–2007 Setting: CaPSURE, USA Treatment: all types Average follow-up: 4.3 yrs |
After diagnosis, available info From CaPSURE | 7,274 | 220 (3%) | 824 (11.3%) | <25 25–29.9 30–34.9 ≥ 35 |
1.0 0.9 (0.7–1.2) 0.9 (0.6–1.3) 0.5 (0.2–1.2) |
0.90 (0.78–1.03) P=0.14 |
Age, clinical risk, diabetes, treatment |
Abbreviations: BMI, body-mass index; RR, relative risk; CI: confidence interval; PCa: prostate cancer; RP, prostatectomy, RT, radiation therapy; ADT, androgen deprivation therapy
The other 6 studies (9–13, 41) followed the survival of 18,203 diagnosed prostate cancer patients in the United States and the Netherlands; 932 prostate cancer deaths were found. Prostate cancer patients were identified from population-based case-control or cohort studies(10, 11) or various clinical settings(9, 12, 13, 41). Four studies had an average follow-up of over 7 years but the two largest studies had only 4 years of follow-up(13, 41). The two population-based studies assessed BMI by self-report either one year before diagnosis(10) or at the study entrance years before diagnosis(11). Three clinical studies measured BMI at the time of first treatment(9, 12, 13), and one study retrieved post-diagnostic BMI from urologists at the time of entering the CaPSURE database(41). The two population-based studies controlled for smoking status but none of the 4 clinical studies did so. All the studies controlled for clinical risk, mostly through Gleason score.
The 16 studies on BMI and biochemical recurrence (12–16, 26, 33, 36, 37, 42–48) followed 26,479 prostate cancer patients after primary treatment for 2 to 10 years (Table 2). Majority were conducted in the United States, two in the Netherlands, one in Japan, and mostly in a single clinic or medical center. In most studies, BMI was measured or self-reported at study enrollment, either at diagnosis or right before surgery, while some did not indicate the timing for BMI measurement. Most studies controlled for preoperative clinical and/or pathologic characteristics, i.e. preoperative PSA, Gleason score, and surgical margin status but none of the studies controlled for smoking status.
Table 2.
Characteristics of identified studies on body-mass index and biochemical recurrence after prostate cancer treatment (N=16)
| Source | Study characteristics | BMI measurement | Definition of recurrence | No. of subjects | No. of recurrence (%) | BMI categories and RR (95% CI) | RR per 5 kg/m2 increase of BMI (95% CI) and P-value | Confounders adjusted | |
|---|---|---|---|---|---|---|---|---|---|
| Primary Treatment: Radical prostatectomy (N=11) | |||||||||
|
Bassett(42) (Urology, 2005) |
Period: 1989–2002 Setting: 31 cites, USA Treatment: RP Median follow-up: 1.9 yrs |
Not indicated | First PSA≥0.2ng/mL or if a second treatment after RP was needed | 2,131 | 251 (12%) | Continuous | 1.20 (1.02–1.41) | 2.46 (1.10–5.48) P=0.03 |
Stage, PSA, biopsy Gleason score, age, ethnicity, comorbidities (hyptertension, diabetes, heart disease) |
|
Strom(43) (Clin Cancer Res, 2005) |
Period: 1991–2001 Setting: 1 center, USA Treatment: RP Average follow-up: 4.5 yrs |
Self reported at diagnosis | PSA≥0.1ng/mL | 526 | 97 (18%) | Continuous <30 ≥30 <30 ≥30 <30 ≥30 |
At diagnosis 1.07 (1.02–1.13) 1.00 1.41(0.91–2.18) At age 25(univariate) 1.00 2.31 (1.01–5.30) At age 40 (univariate) 1.00 2.35 (1.43–3.86) |
1.40 (1.10–1.84) P=0.01 |
Preoperative PSA, Gleason score, pathologic stage, extraprostatic extension, seminal vesicle invasions, surgical margins, lymph node metastasis |
|
Freedland(36) (Clin Cancer Res, 2005) |
Period: 1985–2004 Setting: 1 surgeon at 1 center, USA Treatment: RP Median follow-up: 5 yrs |
Preoperative, retrieved from medical records | PSA≥0.2ng/mL | 2,832 | 374 (14%) | <30 ≥30 |
1.00 1.36 (0.98–1.89) |
1.36 (0.98–1.89) P=0.07 |
Age, race, height, year of surgery, clinical stage, biopsy Gleason sum, preoperative PSA; prostate weight, pathologic Gleason sum, positive surgical margins, extraprostatic extension, seminal vesicle invasion and lymph node metastasis |
|
Siddiqui(12) (Cancer, 2006) |
Period:1990–1999 Setting: 1 center, USA Treatment: RP Median follow-up: 10.1 yrs |
Measured at surgery | PSA≥0.4 ng/mL | 5,313 | 1,687 (32%) | Continuous | 1.00 (0.99–1.01) | 1.00 (0.95–1.05) P=0.86 |
Gleason Score, preoperative serum PSA, surgical margin status, seminal vesicle invasion, adjuvant treatment. |
|
Spangler(37) (J Urol, 2007) |
Period: 1995–2004 Setting: 1 center, USA Treatment: RP Median follow-up: 3 yrs |
Self reported at baseline | PSA≥0.2ng/dL | 924 140 784 |
153 (17%) 29 (21%) 124 (16%) |
<30 ≥30 <30 ≥30 <30 ≥30 |
Overall 1.00 1.76 (1.26–2.47) African American 1.00 5.49 (2.16–13.9) European American 1.00 1.41 (0.96–2.08) |
1.76 (1.26–2.47) | Age, race, stage, pathology Gleason grade and seminal vesicle invasion |
|
Hisasue(15) (Jpn J Clin Oncology, 2008) |
Period: 1998–2006 Setting: 1 center, Japan Treatment: RP Median follow-up: 1.4 yrs |
Pre-operative data | PSA elevation≥0.2 ng/mL | 126 | 30 (23%) | <26.4 ≥26.4 |
1.00 3.53 (1.29–9.68) |
3.53 (1.29–9.68) P=0.01 |
Age, surgical period, total testerone, PSA, T1c, Gleason sum, surgical margin |
|
Freedland(33) (BJU Int, 2008) |
Period: 1988–2006 Setting: 1 center (DPC), USA Treatment: RP Mean follow-up: 4.5 yrs |
Not indicated | Single PSA >0.2ng/mL, two of 0.2ng/mL, or secondary treatment for a high PSA level after RP | 2,014 | 483 (24%) | <25 25–29.9 30–34.9 ≥35 |
1.00 1.16 (0.92–1.46) 1.43 (1.08–1.88) 1.29 (0.87–1.92) |
1.14 (1.05–1.25) P<0.01 |
Age, race, biopsy Gleason sum, clinical stage, preoperative PSA, year at surgery |
|
Magheli(26) (Urology, 2008) |
Period: 1984–2006 Setting: 1 center, USA Treatment: RP Mean follow-up: 4.5 yrs |
Not indicated | PSA≥0.2 ng/mL | 5,631 | Not indicated | <25 25–30 ≥30 |
1.00 1.15 (0.89–1.49) 1.53 (1.20–1.96) |
1.22 (1.09–1.36) P<0.001 |
Age, race, Gleason score, preoperative PSA, clinical stage, year of surgery |
|
van Roermund(48) (BJU Int, 2009) |
Period: 1992–2005& 1998–2007 Setting: 2 centers, Netherlands Treatment: RP Median follow-up: 4.9 yrs |
Preoperative, from anaesthesia records | Two subsequent PSA>0.1 ng/mL or if a second treatment after RP was needed | 1,302 | 297 (22.8%) | <25 25–30 ≥30 |
1.00 0.98 (0.74–1.29) 0.72 (0.40–1.30) |
0.92 (0.75–1.12) P=0.4 |
Not clearly indicated, Gleason score, pathological stage |
|
King(47) (Int J Radiat Oncol Biol Phys, 2009) |
Period: 1984–2004 Setting: USA Treatment: RP+salvage RT Median follow-up: 3.7 yrs |
Clinical information, retrieved at the time of salvage RT | PSA≥0.2ng/mL with repeated measures | 90 | 40 (36%) | Continuous | 1.2 (1.04–1.4) | 2.49 (1.22–5.38) P=0.01 |
Preoperative PSA, Gleason, seminal vesicle, extracapsular extension, surgical margin, dose, pre-RT PSA, whole pelvic RT |
|
Jayachandran (16) (Cancer, 2009) |
Period: Not indicated Setting: multi-centers, USA Treatment: RP Median follow-up: 3.3 yrs |
At surgery | Single PSA>0.2ng/mL, or 2 PSA at 0.2ng/mL, or if a second treatment after RP was needed | Total 1,415 Black 662 White 753 |
Total 452 (31.9%) Black 222 (33.5%) White 230 (30.5%) |
Continuous | Black 1.04 (1.01–1.07) White 1.06 (1.03–1.10) |
Black 1.22 (1.05–1.40) P=0.01 White 1.34 (1.16–1.61) P<0.001 |
Age, pathological Gleason score, prostate weight, extracapsular extension, positive surgical margins, seminal vesicale invasion, lymph node involvement, preoperative PSA, year of surgery and center |
| Source | Study characteristics | BMI measurement | Definition of recurrence | No. of subjects | No. of recurrence (%) | BMI categories and RR (95% CI) | RR per 5 kg/m2 increase of BMI (95% CI) and P-value | Confounders adjusted | Source |
|---|---|---|---|---|---|---|---|---|---|
| Primary Treatment: Radiation therapy (N=5) | |||||||||
|
Strom(44) (Cancer, 2006) |
Period: 1988–2001 Setting: 1 center, USA Treatment: EBRT Average follow-up: 8 yrs |
Retrieved from chart at initial examination of EBRT | ASTRO, 3 consecutive increases in post-treatment PSA after achievement of a nadir | 873 | 168 (19%) | Continuous | 1.04 (1.02–1.07) | 1.22 (1.10–1.40) P<0.01 |
Clinical stage, Gleason score, PSA, dose, year of diagnosis |
|
Efstathiou(14) (Cancer, 2007) |
Period:1995–2001 Setting: multi-center trial, USA Treatment: RT+ADT Median follow-up: 6.9 yrs |
Available at baseline | PSA≥1.0 ng/mL and increased by more than 0.2ng/mL on 2 consecutive measurements | 99 | 25 (25%) | Continuous | 1.10 (1.01–1.19) | 1.61 (1.05–2.39) P=0.03 |
PSA, age, Gleason, stage |
|
Stroup(45) (Cancer, 2007) |
Period: 1989–2003 Setting: multi-center database, USA Treatment: EBRT Median follow-up: 3.6 yrs |
Retrieved from medical record | PSA nadir+ ≥2ng/mL | 1,320 | 554 (42%) | Continuous | 1.03 (1.01–1.05) P<0.01 |
1.13 (1.03–1.25) P<0.01 |
PSA, dose, ethnicity, stage, Gleason score, Neoadjuvant ADT, PSA nadir after EBRT, year of diagnosis |
|
Efstathiou(46) (Int J Radiat Oncol Biol Phys, 2008) |
Period: 1996–2001 Setting: 2 centers, USA Treatment: Brachytherapy Median follow-up: 6 yrs |
Available at baseline | PSA nadir+ ≥2ng/mL | 353 | 76 (21%) | <25 25–30 ≥30 |
1.00 0.76 (0.45–1.29) 0.56 (0.29–1.10) |
0.75 (0.43–1.32) P=0.32 |
Age, race, preimplantation PSA, Gleason score, T stage, percent of prescription dose to 90% of the prostate, use of supplemental EBRT and ADT |
|
van Roermund(13) (BJU Int, 2009) |
Period: 1991–2008 Setting: 1center, Netherlands Treatment: Brachytherapy Median follow-up: 3.9 yrs |
Preoperative, from anaesthesia records | PSA nadir+ ≥2ng/mL | 1,530 | 249 (16.3%) | <25 25–30 ≥30 |
1.00 1.00 (0.77–1.31) 1.15 (0.72–1.85) |
1.04 (0.87–1.24) P=0.7 |
Age, risk group(stage, grade and preoperative PSA), preoperative PLND, treatment period, number of seeds |
Abbreviations: BMI, body-mass index; RR, relative risk; CI: confidence interval; PCa: prostate cancer; RP, prostatectomy, RT, radiation therapy; ADT, androgen deprivation therapy; EBRT, external beam radiation therapy
Main Findings
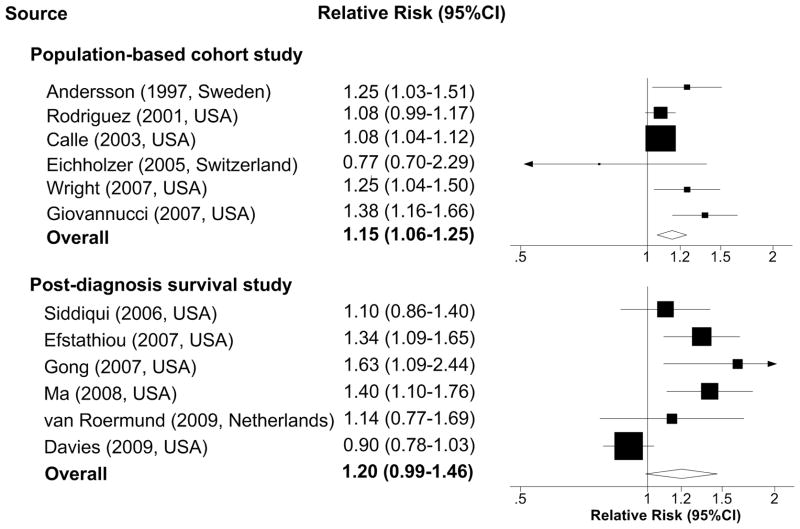
The pooled estimates for the 6 cohort studies showed a significant 15% (RR 1.15, 95% CI 1.06–1.25, p<0.01) higher risk of prostate cancer mortality associated with each 5kg/m2 increase in BMI (Figure 2a). The P value for heterogeneity from the Cochran Q test (Q=12.23) was 0.03 and I2 was 59%, suggesting a moderate heterogeneity between studies.
Figure 2.
Figure 2a. Relative risks per 5kg/m2 increase of body-mass index and prostate cancer-specific mortality
Abbreviation: CI, confidence interval
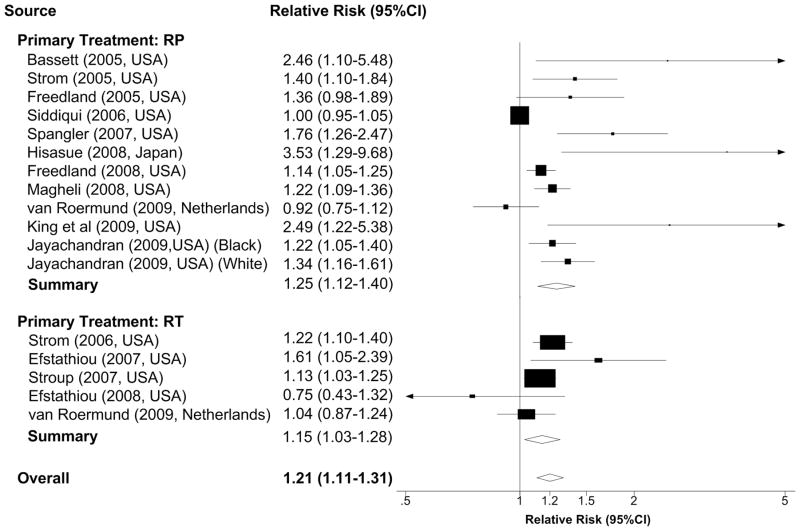
Figure 2b. Relative risks per 5kg/m2 increase of body-mass index and biochemical recurrence after treatment
Abbreviation: RP, radical prostatectomy; RT, radiation therapy, including brachytherapy, external beam radiation therapy and radiation therapy with androgen suppression therapy; CI, confidence interval.
Pooling the 6 post-diagnosis survival studies showed a 20% higher risk of prostate cancer-specific mortality (RR 1.20, 95% CI 0.99–1.46, p=0.06) associated with each 5kg/m2 increase in BMI (Figure 2a). The P value for heterogeneity from Cochran Q test (Q=19.18) was less than 0.01 and I2 was 74%, indicating high heterogeneity among studies. The high heterogeneity explains the borderline non-significance of the overall estimation, and was mainly driven by non-significant inverse association reported from the CaPSURE study,(41) which counted for more than a third of the pooled population.
A 5kg/m2 increase in BMI was associated with a 21% increased risk of biochemical recurrence (RR 1.21, 95% CI 1.11–1.31, P<0.01) (Figure 2b). The P value for heterogeneity was less than 0.01(Q=63.15) and I2 was 75%, suggesting a high degree of heterogeneity among studies. We also evaluated the association by types of treatment because severe obesity might prohibit patients from receiving surgical treatment and thus bias the overall estimate. Among the eleven (12 data points) studies of radical prostatectomy, ten (11 data points) showed positive associations and nine were statistically significant. The pooled estimate showed that a 5kg/m2 BMI increase was associated with a significant 25% higher risk of biochemical recurrence (RR 1.25, 95% CI 1.12–1.40, P<0.01; Q=54.63 I2=79%). Among patients treated with radiation therapy with or without ADT, four of the 5 studies showed positive associations and three were statistically significant. The pooled estimate showed that a 5kg/m2 BMI increase was associated with a significant 15% higher risk of biochemical recurrence (RR 1.15, 95% CI 1.03–1.28, P=0.01; Q=7.09 I2=44%).
Sensitivity Analysis
We conducted sensitivity analysis by omitting one study at a time, generating the pooled estimates and comparing with the original estimates. Omitting any one of 6 population-based cohort studies had no dramatic influence on the original pooled RRs, with newly pooled RR ranging from 1.11 (95% CI 1.04–1.18) to 1.20 (95% CI 1.05–1.35). In the 6 post-diagnosis survival studies, omitting Davies et al generated a significant RR 1.30 (95% CI 1.15–1.47), while none of the other had a huge influence on the original estimates with RRs ranging from 1.17 (95% CI 0.95–1.39) to 1.26 (95% CI 0.99–1.54). Of the 16 studies on BMI and biochemical recurrence, none of the studies altered significance of the original estimate with newly pooled RRs from 1.24 (95% CI 1.14–1.34) to 1.30 (95% CI 1.19–1.41).
Subsequent sensitivity analysis by stratification suggested greater RR in studies conducted in the United States compared to Europe, self-reported BMI compared to measured BMI in population-based cohort study, post diagnosis survival study and study of biochemical recurrence among patients treated with radical prostatectomy (Table 3). RR was slighter lower and non-significant (RR 1.24; 95% CI (0.98–1.58) in studies (12, 37, 42, 43, 48) that used definition of biochemical recurrence other than single PSA ≥0.2ng/mL (15, 26, 36, 47) or single PSA >0.2ng/mL, two of 0.2ng/mL, or secondary treatment for a high PSA level after radical prostatectomy (16, 33). Among patients treated with radiation therapy, no association between BMI and biochemical recurrence was detected in patients receiving brachytherapy (RR 0.99; 95% CI 0.78–1.25) (13, 46).
Table 3.
Stratified meta-analysis of the assocation of BMI and prostate cancer-specific mortality and biochemical recurrence
| Study Type | No. of Studies | RR per 5kg/m2 increase in BMI | 95% CI |
|---|---|---|---|
| BMI and prostate cancer-specific mortality | |||
| Population-based cohort study | |||
| Country (BMI measurement) | |||
| USA (Self-reported BMI) | 4 | 1.15 | (1.05–1.25) |
| Europe (Measured BMI) | 2 | 1.07 | (0.68–1.67) |
| Post-diagnosis survival study | |||
| Country | |||
| USA | 5 | 1.21 | (0.97–1.51) |
| Europe | 1 | 1.14 | (0.77–1.69) |
| BMI measurement | |||
| Self-reported | 2 | 1.46 | (1.19–1.78) |
| Measured | 4 | 1.10 | (0.89–1.35) |
| BMI and biochemical recurrence after treatment | |||
| Primary treatment: RP | |||
| Country | |||
| USA | 9 | 1.27 | (1.13–1.43) |
| Europe | 1 | 0.92 | (0.75–1.12) |
| Asia | 1 | 3.53 | (1.29–9.68) |
| BMI measurement | |||
| Self-reported at/around diagnosis | 2 | 1.53 | (1.23–1.90) |
| Measured preoperative BMI | 7 | 1.21 | (1.02–1.44) |
| Definition of biochemical recurrence | |||
| PSA ≥ 0.2ng/mL | 4 | 1.32 | (1.13–1.52) |
| Single PSA >0.2ng/mL, two of 0.2ng/mL, or secondary treatment for a high PSA level after RP | 2 | 1.22 | (1.04–1.42) |
| Others | 5 | 1.24 | (0.98–1.58) |
| Primary treatment: RT | |||
| Treatmentment | |||
| EBRT | 2 | 1.16 | (1.08–1.26) |
| Brachytherapy | 2 | 0.99 | (0.78–1.25) |
Abbreviations: BMI, body-mass index; RR, relative risk; CI: confidence interval; RP, prostatectomy, RT, radiation therapy; EBRT, external beam radiation therapy
Publication Bias
Publication bias was not observed among the 6 population-based cohort studies and the 6 post-diagnosis survival studies on BMI and prostate cancer-specific mortality. Significant publication bias as indicated by an asymmetric funnel plot among the 16 studies on BMI and biochemical recurrence revealed the possibility of selective publication of positive findings.
Population attributable risk percent (PAR%) in diagnosed patients
In total, 20% of the prostate cancer deaths were attributable to overweight (10.9%) and obesity (9.1%) without the study by Davies et al. With this study, the PAR% was 11.7% in total, 6.1% from overweight and 5.6% from obesity.
Discussion
We found that higher BMI in initially cancer-free population was significantly associated with higher risk of future prostate cancer mortality. Among diagnosed patients, higher BMI was associated with a significantly higher risk of biochemical recurrence after primary treatment, and a borderline non-significantly elevated risk of prostate cancer-specific mortality. To our knowledge, this is the first meta-analysis that comprehensively summarized and quantitatively analyzed the current findings on obesity and outcomes of prostate cancer.
Previously, two meta-analyses on BMI and risk of prostate cancer were published, but each addressed different hypothesis compared to our study. Robinson et al summarized findings on the association of childhood and young adulthood BMI and risk of advanced prostate cancer and fatal prostate cancer (only 1 study on fatal outcome), and the RR was close to the null (RR 1.01, 95% CI: 0.89–1.14) for each 5-unit increase in BMI (49), indicating little impact of young adulthood BMI on risk of advanced prostate cancer. MacInnis et al meta-analyzed both cohort and case controls studies on BMI and risk of advanced prostate cancer, and found that BMI was associated with 12% higher risk of advanced prostate cancer (RR 1.12, 95% CI: 1.01–1.23) for each 5-unit increase (2). However, the association of BMI and fatal prostate cancer was not addressed in that study. In the present study, we assessed endpoints of disease progression such as prostate cancer mortality and biochemical recurrence among healthy population as well as among the diagnosed patients to specifically evaluate the role of adiposity on prostate cancer progression.
Overall, we found that the magnitude of the pooled effect estimates were quite similar with 15%–21% increased risk for each 5 kg/m2 increase in BMI, despite different study designs (cohort or survival studies), study settings (cohort from healthy group or clinical studies), outcome assessments (prostate cancer-specific mortality or recurrence), or from multiple countries with different social economic or racial (Caucasians, African Americans, and Asian men) backgrounds. The similar pooled estimates across different types of clinical treatments further suggest the robust association between obesity and prostate cancer progression.
Several possible explanations have been proposed. First, such association could be due to delayed diagnosis and more advanced stage at diagnosis in obese men. It has been suggested that obesity makes prostate cancer early detection more difficult due to less PSA screening, lower accuracy of digital rectal examination in obese men and lower PSA values caused by obesity-related hemodilution (33, 50). Obese individual has higher chance to be missed as the cancer detected by PSA screening is so small and larger prostate gland (51) makes the detection of existent cancer less likely (52). Although the existence of such detection bias could not be fully ruled out, studies by Wright et al and Ma et al suggested that elevated BMI was significantly associated with higher risk of prostate cancer-specific mortality in those without PSA screening (4) and in both pre and PSA screening era (11). Alternatively, difficulties in treatment, such as increased risk of positive surgical margins (12, 23, 31), and the greater day-to-day variation of prostate location that leads to lower dose and less effective radiation (53), could also contribute to the poorer outcome observed in diagnosed patients. However, the association with recurrence is still strong and significant after adjusting for margin status in many of the studies included in our analysis (Table 2).
Potential biological mechanisms of adiposity and prostate cancer progression have been proposed and under investigation. Hormonal and metabolic changes in obese men are the primary concern. One hypothesis is that certain obesity-related metabolic dysregulation such as hyperinsulinemia and/or hypoadiponectinemia favors aggressive neoplastic behavior (11, 54). It was also found that lower levels of testosterone in obese men may be linked to poorly differentiated and hormone insensitive tumors (55, 56). Obesity is also associated with increased levels of free IGF-1, which is found to stimulate growth of prostate cell lines in vitro and be more closely related to advanced stage prostate cancer in human (57).
High heterogeneity was detected among the studies reviewed in the present analysis. The stratified meta-analyses suggested strong and consistent association between BMI and higher prostate cancer mortality and biochemical recurrence in studies conducted in the United States. Smaller relative risks in the few available studies from Europe could be attributable to large variability in the linear transformed RRs under a lower prevalence of obesity in European countries. We also found that studies using self-reported BMI presented stronger association than studies utilizing measure BMI, and different magnitudes of association between BMI and biochemical recurrence among patients on different radiation therapies were also observed. These evidence reflected the need of investigations in different countries and among different subgroup of patients.
In further reviewing the heterogeneity between cohort studies and clinical studies, several issues are worth noting. First, missing data of BMI and shorter period of follow-up in clinical studies could bias the estimate and limit the findings. For example, in the study by Siddiqui et al, 23% of the patients had missing BMI and in Davies et al, only 53% of the patients in the CaPSURE database were included. Both studies and the study by van Roermund et al had lower prostate cancer-specific mortality (3–4%) compared to other studies either due to short follow-up of 3–4 years or selection of much healthier individuals. Secondly, clinical studies have detailed treatment information but many of these studies lack of data for major confounding factors such as cigarette smoking. The J-shape association of BMI with total mortality confounded by cigarette smoking (58, 59) may apply similarly to BMI and prostate cancer mortality, as current smokers may have increased risk of dying of prostate cancer (60, 61). However, none of the clinical studies included in our analysis controlled for smoking. In contrast, although large prospective cohort studies tend to have a more valid measurement of exposure and covariates, as well as complete follow-up, these studies usually lack detailed clinical treatment information. Therefore, the totality of the evidence obtained from different population, study settings, and outcome assessments in our meta-analysis provide a more objective conclusion.
Over the past two decades, widespread PSA screening significantly increased the number of prostate cancers detected at very early stage, whereas cancer-specific mortality remains relatively constant over time (62). Many men with localized tumors, especially those obese or overweight men are likely to have diabetes and cardiovascular disease and are more likely to die of diseases other than prostate cancer. Since the majority of the studies reviewed in this meta-analysis did not control for competing causes of death, the pooled RR could be an attenuated estimate.
Timing of the BMI assessment is important to evaluate the possibility of reverse causation, i.e. weight change influenced by disease severity or treatments (i.e. ADT causes weight gain even after a short period of treatment (63), and is crucial to the design of intervention strategy. In our study, all of the 6 cohort studies and study by Ma et al assessed BMI years in mid-life and found stronger association, suggesting that adiposity precede cancer progression. This observation provides encouraging evidence for using weight management as a long term strategy to prevent death from prostate cancer at the population level. Although whether weight control will help improve outcomes among overweight and obese patient remains unknown, our findings from BMI measured at diagnosis or before surgery suggest additional clinical benefit to improve outcome from prostate cancer. These interventions may include increasing self-awareness, more early detection efforts by health professionals, more counseling on healthy lifestyle (i.e. exercise) after diagnosis and appropriate individualized treatment for overweight or obese patients.
The strengths of our study include the use of generalized least square methods for relative risk transformations associated with a standard per 5 kg/m2 increase in BMI to allow for comparisons among different studies using different BMI categories, the use of the random-effect model to incorporate heterogeneity, separated analysis on BMI and fatal prostate cancer by different study design and different outcomes, sensitivity analysis, and estimation of population attributable risk.
Meta-analysis of observational studies cannot avoid undetected biases and confounding factors inherent in the original studies. Analyzing BMI as a continuous variable by first transforming all the relative risk estimates to the corresponding RRs for every 5kg/m2 increase in BMI was a way to allow for comparisons among studies but it also assumed the risk increment was constant. We validated such methods in studies that presented RRs for both continuous and categorical BMI, and found that the RR per kg/m2 increase obtained by conversion was similar with the RR for continuous BMI shown in the paper (11).
We did not include four studies (27–30) on biochemical recurrence that did not present relative risk estimates or confidence intervals. Among these four studies, two studies in Canada showed that BMI was predictive of reduced biochemical disease free survival among patients treated with radiation therapy (27) or radical prostatectomy (30). Another two consecutive studies by Merrick et al showed null association between BMI and biochemical recurrence free survival in patients treated with brachytherapy, which were consistent with studies included in our meta-analysis (Table 3). We also excluded two studies that presented only univariate relative risk estimates since the association of BMI and prostate cancer outcome are potentially confounded by confounding factors such as age. Among these two, Motamedinia et al found no difference in the obese and nonobese patients’ actual observed biochemical failure rate, whereas Amling et al showed that obesity alone predicted biochemical recurrence with RR 1.20 (95% CI 1.02–1.42) for obese vs non-obese patients. The association was not significant in the multivariate model after adjusting for pathologic variables but the study unfortunately did not present the data. They also found that increased BMI was associated worse pathologic outcomes, i.e. BMI was an independent predictor of higher Gleason grade cancer, thus suggested that the association between obesity and poor biochemical recurrence could be mediated by pathologic factors. If true, our pooled RR would be a conservative estimate of the association between BMI and biochemical recurrence as majority of the studies included in our meta-analysis had adjusted for pathologic variables.
In conclusion, this meta-analysis provides the first quantitative assessment of the evidence accumulated up to date from 26 studies of a pooled population of 1,302,246 from different countries, various study designs, and majority were published within the past 5 years. It showed a consistent 15–21% increased risk of fatal prostate cancer or biochemical recurrence, and an estimated 12–20% of prostate cancer deaths could be attributable to overweight and obesity. Further investigations are needed to evaluate the role of BMI measured at different stages of life, before, at, or after prostate cancer diagnosis, the impact of weight control on prostate cancer-specific and all-cause mortality. Studies of biomarkers and genetic markers related to adiposity and energy metabolism will provide biological plausibility for a causal role and can guide the development of effective and targeted cancer prevention and therapeutic strategies. Randomized weight control interventions in clinical setting or community-based program could provide a more definitive answer.
Acknowledgments
We thank Dr. Yi Ning for programming support for the analyses in this paper and Daad Abraham for technical support in preparation of the meta-analysis.
Grant Support: The study was supported by grant # 019894 and NIH R01 CA42182-19.
References
- 1.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 2.MacInnis RJ, English DR. Body size and composition and prostate cancer risk: systematic review and meta-regression analysis. Cancer Causes Control. 2006;17:989–1003. doi: 10.1007/s10552-006-0049-z. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez C, Freedland SJ, Deka A, Jacobs EJ, McCullough ML, Patel AV, et al. Body mass index, weight change, and risk of prostate cancer in the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol Biomarkers Prev. 2007;16:63–9. doi: 10.1158/1055-9965.EPI-06-0754. [DOI] [PubMed] [Google Scholar]
- 4.Wright ME, Chang SC, Schatzkin A, Albanes D, Kipnis V, Mouw T, et al. Prospective study of adiposity and weight change in relation to prostate cancer incidence and mortality. Cancer. 2007;109:675–84. doi: 10.1002/cncr.22443. [DOI] [PubMed] [Google Scholar]
- 5.Gong Z, Neuhouser ML, Goodman PJ, Albanes D, Chi C, Hsing AW, et al. Obesity, diabetes, and risk of prostate cancer: results from the prostate cancer prevention trial. Cancer Epidemiol Biomarkers Prev. 2006;15:1977–83. doi: 10.1158/1055-9965.EPI-06-0477. [DOI] [PubMed] [Google Scholar]
- 6.Andersson SO, Wolk A, Bergstrom R, Adami HO, Engholm G, Englund A, et al. Body size and prostate cancer: a 20-year follow-up study among 135006 Swedish construction workers. J Natl Cancer Inst. 1997;89:385–9. doi: 10.1093/jnci/89.5.385. [DOI] [PubMed] [Google Scholar]
- 7.Giovannucci E, Liu Y, Platz EA, Stampfer MJ, Willett WC. Risk factors for prostate cancer incidence and progression in the health professionals follow-up study. Int J Cancer. 2007;121:1571–8. doi: 10.1002/ijc.22788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodriguez C, Patel AV, Calle EE, Jacobs EJ, Chao A, Thun MJ. Body mass index, height, and prostate cancer mortality in two large cohorts of adult men in the United States. Cancer Epidemiol Biomarkers Prev. 2001;10:345–53. [PubMed] [Google Scholar]
- 9.Efstathiou JA, Bae K, Shipley WU, Hanks GE, Pilepich MV, Sandler HM, et al. Obesity and mortality in men with locally advanced prostate cancer: analysis of RTOG 85–31. Cancer. 2007;110:2691–9. doi: 10.1002/cncr.23093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gong Z, Agalliu I, Lin DW, Stanford JL, Kristal AR. Obesity is associated with increased risks of prostate cancer metastasis and death after initial cancer diagnosis in middle-aged men. Cancer. 2007;109:1192–202. doi: 10.1002/cncr.22534. [DOI] [PubMed] [Google Scholar]
- 11.Ma J, Li H, Giovannucci E, Mucci L, Qiu W, Nguyen PL, et al. Prediagnostic body-mass index, plasma C-peptide concentration, and prostate cancer-specific mortality in men with prostate cancer: a long-term survival analysis. Lancet Oncol. 2008;9:1039–47. doi: 10.1016/S1470-2045(08)70235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siddiqui SA, Inman BA, Sengupta S, Slezak JM, Bergstralh EJ, Leibovich BC, et al. Obesity and survival after radical prostatectomy: A 10-year prospective cohort study. Cancer. 2006;107:521–9. doi: 10.1002/cncr.22030. [DOI] [PubMed] [Google Scholar]
- 13.van Roermund JG, Hinnen KA, Battermann JJ, Witjes JA, Bosch JL, Kiemeney LA, et al. Body mass index is not a prognostic marker for prostate-specific antigen failure and survival in Dutch men treated with brachytherapy. BJU Int. 2009 doi: 10.1111/j.1464-410X.2009.08687.x. [DOI] [PubMed] [Google Scholar]
- 14.Efstathiou JA, Chen MH, Renshaw AA, Loffredo MJ, D’Amico AV. Influence of body mass index on prostate-specific antigen failure after androgen suppression and radiation therapy for localized prostate cancer. Cancer. 2007;109:1493–8. doi: 10.1002/cncr.22564. [DOI] [PubMed] [Google Scholar]
- 15.Hisasue S, Yanase M, Shindo T, Iwaki H, Fukuta F, Nishida S, et al. Influence of body mass index and total testosterone level on biochemical recurrence following radical prostatectomy. Jpn J Clin Oncol. 2008;38:129–33. doi: 10.1093/jjco/hym162. [DOI] [PubMed] [Google Scholar]
- 16.Jayachandran J, Banez LL, Aronson WJ, Terris MK, Presti JC, Jr, Amling CL, et al. Obesity as a predictor of adverse outcome across black and white race: results from the Shared Equal Access Regional Cancer Hospital (SEARCH) Database. Cancer. 2009;115:5263–71. doi: 10.1002/cncr.24571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 18.Halabi S, Ou SS, Vogelzang NJ, Small EJ. Inverse correlation between body mass index and clinical outcomes in men with advanced castration-recurrent prostate cancer. Cancer. 2007;110:1478–84. doi: 10.1002/cncr.22932. [DOI] [PubMed] [Google Scholar]
- 19.Halabi S, Small EJ, Vogelzang NJ. Elevated body mass index predicts for longer overall survival duration in men with metastatic hormone-refractory prostate cancer. J Clin Oncol. 2005;23:2434–5. doi: 10.1200/JCO.2005.05.890. author reply 5. [DOI] [PubMed] [Google Scholar]
- 20.Mallah KN, DiBlasio CJ, Rhee AC, Scardino PT, Kattan MW. Body mass index is weakly associated with, and not a helpful predictor of, disease progression in men with clinically localized prostate carcinoma treated with radical prostatectomy. Cancer. 2005;103:2030–4. doi: 10.1002/cncr.20991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith MR, Bae K, Efstathiou JA, Hanks GE, Pilepich MV, Sandler HM, et al. Diabetes and mortality in men with locally advanced prostate cancer: RTOG 92-02. J Clin Oncol. 2008;26:4333–9. doi: 10.1200/JCO.2008.16.5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snowdon DA, Phillips RL, Choi W. Diet, obesity, and risk of fatal prostate cancer. Am J Epidemiol. 1984;120:244–50. doi: 10.1093/oxfordjournals.aje.a113886. [DOI] [PubMed] [Google Scholar]
- 23.Freedland SJ, Aronson WJ, Kane CJ, Presti JC, Jr, Amling CL, Elashoff D, et al. Impact of obesity on biochemical control after radical prostatectomy for clinically localized prostate cancer: a report by the Shared Equal Access Regional Cancer Hospital database study group. J Clin Oncol. 2004;22:446–53. doi: 10.1200/JCO.2004.04.181. [DOI] [PubMed] [Google Scholar]
- 24.Freedland SJ, Terris MK, Presti JC, Jr, Amling CL, Kane CJ, Trock B, et al. Obesity and biochemical outcome following radical prostatectomy for organ confined disease with negative surgical margins. J Urol. 2004;172:520–4. doi: 10.1097/01.ju.0000135302.58378.ae. [DOI] [PubMed] [Google Scholar]
- 25.Freedland SJ, Grubb KA, Yiu SK, Humphreys EB, Nielsen ME, Mangold LA, et al. Obesity and risk of biochemical progression following radical prostatectomy at a tertiary care referral center. J Urol. 2005;174:919–22. doi: 10.1097/01.ju.0000169459.78982.d7. [DOI] [PubMed] [Google Scholar]
- 26.Magheli A, Rais-Bahrami S, Trock BJ, Humphreys EB, Partin AW, Han M, et al. Impact of body mass index on biochemical recurrence rates after radical prostatectomy: an analysis utilizing propensity score matching. Urology. 2008;72:1246–51. doi: 10.1016/j.urology.2008.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palma D, Pickles T, Tyldesley S. Obesity as a predictor of biochemical recurrence and survival after radiation therapy for prostate cancer. BJU Int. 2007;100:315–9. doi: 10.1111/j.1464-410X.2007.06897.x. [DOI] [PubMed] [Google Scholar]
- 28.Merrick GS, Galbreath RW, Butler WM, Wallner KE, Allen ZA, Adamovich E. Obesity is not predictive of overall survival following permanent prostate brachytherapy. Am J Clin Oncol. 2007;30:588–96. doi: 10.1097/COC.0b013e318068b506. [DOI] [PubMed] [Google Scholar]
- 29.Merrick GS, Butler WM, Wallner KE, Galbreath RW, Allen Z, Lief JH, et al. Influence of body mass index on biochemical outcome after permanent prostate brachytherapy. Urology. 2005;65:95–100. doi: 10.1016/j.urology.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 30.Chun FK, Briganti A, Graefen M, Erbersdobler A, Walz J, Schlomm T, et al. Body mass index does not improve the ability to predict biochemical recurrence after radical prostatectomy. Eur J Cancer. 2007;43:375–82. doi: 10.1016/j.ejca.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 31.Amling CL, Riffenburgh RH, Sun L, Moul JW, Lance RS, Kusuda L, et al. Pathologic variables and recurrence rates as related to obesity and race in men with prostate cancer undergoing radical prostatectomy. J Clin Oncol. 2004;22:439–45. doi: 10.1200/JCO.2004.03.132. [DOI] [PubMed] [Google Scholar]
- 32.Motamedinia P, Korets R, Spencer BA, Benson MC, McKiernan JM. Body mass index trends and role of obesity in predicting outcome after radical prostatectomy. Urology. 2008;72:1106–10. doi: 10.1016/j.urology.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 33.Freedland SJ, Sun L, Kane CJ, Presti JC, Jr, Terris MK, Amling CL, et al. Obesity and oncological outcome after radical prostatectomy: impact of prostate-specific antigen-based prostate cancer screening: results from the Shared Equal Access Regional Cancer Hospital and Duke Prostate Center databases. BJU Int. 2008;102:969–74. doi: 10.1111/j.1464-410X.2008.07934.x. [DOI] [PubMed] [Google Scholar]
- 34.Orisini NBR, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. Stata Journal. 2006;6:40–57. [Google Scholar]
- 35.Ning Y, Wang L, Giovannucci EL. A quantitative analysis of body mass index and colorectal cancer: findings from 56 observational studies. Obes Rev. 2009 doi: 10.1111/j.1467-789X.2009.00613.x. [DOI] [PubMed] [Google Scholar]
- 36.Freedland SJ, Isaacs WB, Mangold LA, Yiu SK, Grubb KA, Partin AW, et al. Stronger association between obesity and biochemical progression after radical prostatectomy among men treated in the last 10 years. Clin Cancer Res. 2005;11:2883–8. doi: 10.1158/1078-0432.CCR-04-2257. [DOI] [PubMed] [Google Scholar]
- 37.Spangler E, Zeigler-Johnson CM, Coomes M, Malkowicz SB, Wein A, Rebbeck TR. Association of obesity with tumor characteristics and treatment failure of prostate cancer in African-American and European American men. J Urol. 2007;178:1939–44. doi: 10.1016/j.juro.2007.07.021. discussion 45. [DOI] [PubMed] [Google Scholar]
- 38.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 39.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 40.Eichholzer M, Bernasconi F, Jordan P, Stahelin HB. Body mass index and the risk of male cancer mortality of various sites: 17-year follow-up of the Basel cohort study. Swiss Med Wkly. 2005;135:27–33. doi: 10.4414/smw.2005.10415. [DOI] [PubMed] [Google Scholar]
- 41.Davies BJ, Smaldone MC, Sadetsky N, Dall’era M, Carroll PR. The impact of obesity on overall and cancer specific survival in men with prostate cancer. J Urol. 2009;182:112–7. doi: 10.1016/j.juro.2009.02.118. discussion 7. [DOI] [PubMed] [Google Scholar]
- 42.Bassett WW, Cooperberg MR, Sadetsky N, Silva S, DuChane J, Pasta DJ, et al. Impact of obesity on prostate cancer recurrence after radical prostatectomy: data from CaPSURE. Urology. 2005;66:1060–5. doi: 10.1016/j.urology.2005.05.040. [DOI] [PubMed] [Google Scholar]
- 43.Strom SS, Wang X, Pettaway CA, Logothetis CJ, Yamamura Y, Do KA, et al. Obesity, weight gain, and risk of biochemical failure among prostate cancer patients following prostatectomy. Clin Cancer Res. 2005;11:6889–94. doi: 10.1158/1078-0432.CCR-04-1977. [DOI] [PubMed] [Google Scholar]
- 44.Strom SS, Kamat AM, Gruschkus SK, Gu Y, Wen S, Cheung MR, et al. Influence of obesity on biochemical and clinical failure after external-beam radiotherapy for localized prostate cancer. Cancer. 2006;107:631–9. doi: 10.1002/cncr.22025. [DOI] [PubMed] [Google Scholar]
- 45.Stroup SP, Cullen J, Auge BK, L’Esperance JO, Kang SK. Effect of obesity on prostate-specific antigen recurrence after radiation therapy for localized prostate cancer as measured by the 2006 Radiation Therapy Oncology Group-American Society for Therapeutic Radiation and Oncology (RTOG-ASTRO) Phoenix consensus definition. Cancer. 2007;110:1003–9. doi: 10.1002/cncr.22873. [DOI] [PubMed] [Google Scholar]
- 46.Efstathiou JA, Skowronski RY, Coen JJ, Grocela JA, Hirsch AE, Zietman AL. Body mass index and prostate-specific antigen failure following brachytherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;71:1302–8. doi: 10.1016/j.ijrobp.2007.11.073. [DOI] [PubMed] [Google Scholar]
- 47.King CR, Spiotto MT, Kapp DS. Obesity and risk of biochemical failure for patients receiving salvage radiotherapy after prostatectomy. Int J Radiat Oncol Biol Phys. 2009;73:1017–22. doi: 10.1016/j.ijrobp.2008.05.041. [DOI] [PubMed] [Google Scholar]
- 48.van Roermund JG, Kok DE, Wildhagen MF, Kiemeney LA, Struik F, Sloot S, et al. Body mass index as a prognostic marker for biochemical recurrence in Dutch men treated with radical prostatectomy. BJU Int. 2009;104:321–5. doi: 10.1111/j.1464-410X.2009.08404.x. [DOI] [PubMed] [Google Scholar]
- 49.Robinson WR, Poole C, Godley PA. Systematic review of prostate cancer’s association with body size in childhood and young adulthood. Cancer Causes Control. 2008;19:793–803. doi: 10.1007/s10552-008-9142-9. [DOI] [PubMed] [Google Scholar]
- 50.Banez LL, Hamilton RJ, Partin AW, Vollmer RT, Sun L, Rodriguez C, et al. Obesity-related plasma hemodilution and PSA concentration among men with prostate cancer. JAMA. 2007;298:2275–80. doi: 10.1001/jama.298.19.2275. [DOI] [PubMed] [Google Scholar]
- 51.Freedland SJ, Platz EA, Presti JC, Jr, Aronson WJ, Amling CL, Kane CJ, et al. Obesity, serum prostate specific antigen and prostate size: implications for prostate cancer detection. J Urol. 2006;175:500–4. doi: 10.1016/S0022-5347(05)00162-X. discussion 4. [DOI] [PubMed] [Google Scholar]
- 52.Kranse R, Beemsterboer P, Rietbergen J, Habbema D, Hugosson J, Schroder FH. Predictors for biopsy outcome in the European Randomized Study of Screening for Prostate Cancer (Rotterdam region) Prostate. 1999;39:316–22. doi: 10.1002/(sici)1097-0045(19990601)39:4<316::aid-pros14>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 53.Millender LE, Aubin M, Pouliot J, Shinohara K, Roach M., 3rd Daily electronic portal imaging for morbidly obese men undergoing radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2004;59:6–10. doi: 10.1016/j.ijrobp.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 54.Li H, Stampfer MJ, Mucci L, Rifai N, Qiu W, Kurth T, et al. A 25-year prospective study of plasma adiponectin and leptin concentrations and prostate cancer risk and survival. Clin Chem. 56:34–43. doi: 10.1373/clinchem.2009.133272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Platz EA, Leitzmann MF, Rifai N, Kantoff PW, Chen YC, Stampfer MJ, et al. Sex steroid hormones and the androgen receptor gene CAG repeat and subsequent risk of prostate cancer in the prostate-specific antigen era. Cancer Epidemiol Biomarkers Prev. 2005;14:1262–9. doi: 10.1158/1055-9965.EPI-04-0371. [DOI] [PubMed] [Google Scholar]
- 56.Severi G, Morris HA, MacInnis RJ, English DR, Tilley W, Hopper JL, et al. Circulating steroid hormones and the risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:86–91. doi: 10.1158/1055-9965.EPI-05-0633. [DOI] [PubMed] [Google Scholar]
- 57.Chan JM, Stampfer MJ, Ma J, Gann P, Gaziano JM, Pollak M, et al. Insulin-like growth factor-I (IGF-I) and IGF binding protein-3 as predictors of advanced-stage prostate cancer. J Natl Cancer Inst. 2002;94:1099–106. doi: 10.1093/jnci/94.14.1099. [DOI] [PubMed] [Google Scholar]
- 58.Lee IM, Manson JE, Hennekens CH, Paffenbarger RS., Jr Body weight and mortality. A 27-year follow-up of middle-aged men. JAMA. 1993;270:2823–8. doi: 10.1001/jama.270.23.2823. [DOI] [PubMed] [Google Scholar]
- 59.Manson JE, Willett WC, Stampfer MJ, Colditz GA, Hunter DJ, Hankinson SE, et al. Body weight and mortality among women. N Engl J Med. 1995;333:677–85. doi: 10.1056/NEJM199509143331101. [DOI] [PubMed] [Google Scholar]
- 60.Huncharek M, Haddock S, Reid RBK. Smoking as a Risk Factor for Prostate Cancer: A Meta-Analysis of 24 Prospective Cohort Studies. Am J Public Health. 2009 doi: 10.2105/AJPH.2008.150508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zu K, Giovannucci E. Smoking and aggressive prostate cancer: a review of the epidemiologic evidence. Cancer Causes Control. 2009 doi: 10.1007/s10552-009-9387-y. [DOI] [PubMed] [Google Scholar]
- 62.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 63.Higano CS. Side effects of androgen deprivation therapy: monitoring and minimizing toxicity. Urology. 2003;61:32–8. doi: 10.1016/s0090-4295(02)02397-x. [DOI] [PubMed] [Google Scholar]