Abstract
Problem
Histopathological chorioamnionitis (HCA) is caused by microbial-driven infiltration of leukocytes to the maternal-fetal interface resulting in adverse neonatal outcomes in a subset of pregnancies. The role of placental villus macrophages (i.e. Hofbauer cells, HBCs) in the pathophysiology of HCA is unelucidated.
Method of study
The number of HBCs in human term placental villi in HCA and control groups was compared using immunohistochemistry. Levels of monocyte chemotactic protein (MCP-1) expression were measured in primary cultures of syncytioytrophoblasts (SCTs) and fibroblasts (FIBs) treated with bacterial compounds [lipopolysaccharide (LPS) and peptidoglycan] and pro-inflammatory cytokines (TNF-α and IL-1β) using ELISA and quantitative real-time PCR.
Results
Immunohistochemistry revealed a focal increase in HBCs in HCA. Treatment of FIBs with LPS, IL-1β, and TNF-α significantly increased MCP-1 mRNA and protein expression. Conversely, MCP-1 mRNA and protein levels were virtually undetectable in treated and untreated SCTs.
Conclusion
These results demonstrate cell-type-specific regulation of MCP-1 expression in human placenta. A model is presented in which bacterial products and inflammatory cytokines initiate a fibroblast-driven cytokine cascade resulting in recruitment of fetal monocytes to placenta which focally increases levels of HBCs in pregnancies complicated by HCA.
Keywords: Chorioamnionitis, fetal macrophages, Hofbauer cells, monocyte chemotactic protein-1, placenta
Introduction
Histopathological chorioamnionitis (HCA) is most often caused by ascending bacteria, mycoplasma, or fungi, resulting in a marked infiltration of polymorphonuclear leukocytes in the decidua (deciduitis), chorion (chorionitis) amniotic membranes (amnionitis), and umbilical cord (funisitis).1 HCA, when accompanied by funisitis, is a risk factor for neurological impairment in newborns and cerebral palsy.2,3 Intravillous lymphocyte, plasma cell, and macrophage infiltrates are not usually present in acute HCA, while a large increase in villous lymphocytes and fetal macrophages (i.e. Hofbauer cells, HBCs) can be evident in the so-called ‘villitis of unknown etiology’, a relatively rare entity of uncertain significance.4,5
The role of HBCs in placental immune function in general, and in HCA specifically, remains largely unexplored. HBCs normally appear on the 18th day of gestation and can be found until term.6 However, because of the compression of the villous stroma, by the fourth or fifth month of gestation their identification becomes more difficult and requires the use of immunohistochemistry with antibodies that recognize macrophage proteins (e.g. CD68).4,7 HBCs were shown to be of fetal origin using Y chromosome-specific probes in pregnancies with male fetus.4,5 The function that specific placental cell types play in the generation and/or recruitment of HBCs remains unknown. Major cell types in placenta include syncytiotrophoblast (SCT), the cell layer that lines the intervillous space and is in direct contact with maternal blood, and underlying stromal cells adjacent to fetal capillaries largely consisting of fibroblasts (FIBs) and HBCs.8 Based on the association between fetal-placental infection/inflammation and devastating neonatal and pediatric outcomes,2,3 the goal of the current study was to examine the expression of HBCs in HCA and to determine the factor(s) that may be responsible for modulating their levels in the placental villus.
In the current study, we unexpectedly observed that HCA was associated with focally increased numbers of HBCs in the placental villous stroma. Monocyte-chemotactic protein-1 (MCP-1) is a major monocyte/macrophage chemoattractant that is produced in cells following binding of microbial compounds to Toll-like receptors (TLRs).9,10 To establish a potential etiology of villous macrophage influx in HCA, we determined whether MCP-1 levels were regulated in primary cultures of SCTs and FIBs following treatment with the Gram-negative bacterial compound lipopolysaccharide (LPS), or the Gram-positive bacterial component peptidoglycan (PG). Inflammatory processes at the maternal-fetal interface in HCA are suggested to be regulated by tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β),11 and these compounds are known to be major inflammatory cytokines synthesized by macrophages including HBCs.12,13 In addition, TNF-α has been demonstrated to be an important regulator of trophoblast damage and apoptosis.14,15 Therefore, we also examined the influence of IL-1β and TNF-α on MCP-1 expression in SCTs and FIBs. Our results are presented in the context of a model linking cell-type specific MCP-1 expression to infiltration of fetal monocytes and generation of HBCs in HCA.
Materials and methods
Tissue Procurement and Histological Analysis
Placentas were fixed in 10% buffered formalin. Cord, membrane rolls and full-thickness placental samples were paraffin-embedded and stained with hematoxylin–eosin for routine histological examination. HCA was determined by the presence of polymorphonuclear leukocytes in the Wharton jelly (funisitis), in the walls of umbilical cord vessels, in the chorionic plate stroma and/or vessels (chorionitis and/or vasculitis), or in the amniotic membranes (amniositis). Deciduitis was defined as the presence of numerous PMNs (20 PMNs or more in a 20×· high-power field) in the decidua basalis or decidua parietalis. A single examiner (PT) reviewed all slides. Histological diagnosis of chorioamnionitis was performed in 60 cases. Uncomplicated pregnancy and normal histological examination identified 39 control placentas matched for gestational age. Approval for this study was granted by the Human Institutional Investigation Committees (HIC) of the University of Siena and Yale University School of Medicine. Informed consent was obtained from all women.
To quantitate the number of CD68+ cells in placental specimens, 5-μm thick sections, obtained from paraffin-embedded samples, were mounted on electrostatically charged slides, and dried overnight at 37°C. Sections were de-waxed, rehydrated, and washed in Tris–buffered saline [TBS: 20 mM Tris–HCl, 150 mM NaCl (pH 7.6)]. Antigen retrieval was carried out by incubating sections in sodium citrate buffer (10 mM, pH 6.0) in a microwave oven at 750 W for 5 min. Endogenous peroxidases were blocked by rinsing the slides with 3% hydrogen peroxide solution. Slides were incubated for 1 hr at room temperature with a primary antibody against CD68 (clone KP1; Dakopatts, Glostrup, Denmark) diluted 1:100 in TBS. The reaction was developed by successive incubations with anti-mouse immunoglobulins labeled with biotin, avidin–biotin peroxidase complex (Vector Laboratories, Burlingame, CA, USA) and 3,3′-diaminobenzidine tetrahydrochloride (1 mg/mL; Sigma, St Louis, MO, USA) in TBS containing 0.3% H2O2. Harris hematoxylin was used for nuclear counterstaining. A positive reaction was characterized by the presence of granular brown staining in the cytoplasm of tissue macrophages. Morphometric evaluation was performed by counting the number of CD68+ cells inside a reference area of 100 villi. As in both HCA and control cases CD68+ cells were not homogenously distributed among the villi, only areas with positive cells were selected for CD68 evaluation. Specifically, CD68 positivity was assessed in tissue areas containing one or more macrophages in terminal villi (final grape-like projections of the villous tree) and five or more in intermediate villi (long, slender peripheral ramifications of the villous tree characterized by the absence of vessels). Thus, this methodology compared focal changes in CD68+ cells in HCA and control placentas.
Cell Culture
Cytotrophoblasts (CTs) and FIBs were isolated from the same placenta essentially as we have previously described.16 CTs were isolated from approximately 50 g of human villous tissue at term following trypsin digestion and centrifugation on Percoll gradients. We have previously used this method17,18 which is modified from those described by Kliman et al.19 and Douglas and King.20 Briefly, fresh placental tissue was finely chopped, washed with saline, and treated with trypsin and DNAse. The digestate was filtered through cheesecloth and two wire-mesh sieves with 0.0038 and 0.0021 inch openings. The effluent was centrifuged at 500 × g for 5 min. The cell pellets were resuspended and were centrifuged on a continuous Percoll gradient. CTs sedimenting at a density of approximately 1.05 g/mL were washed and resuspended in basal medium supplemented with 10% heat-inactivated fetal calf serum (FCS; Hyclone Laboratories, Logan, UT, USA), 10 mm L-glutamine, 50 μg/mL penicillin, and 50 μg/mL streptomycin (Cellgro, Herndon, VA, USA), i.e. FCS medium. Under these conditions, we and others have found that CT purity was approximately 90%, with the major contaminant being FIBs (~5%) and immune cells (~5%).17,19,20 Unpurified CTs were then suspended in culture medium at 107 cells per ml and mouse anti-human CD45 (clone F10-89-4; GeneTex, Irvine, CA, USA) and CD9 (clone 209306; R&D Systems, Minneapolis, MN, USA) were added at a ratio of per 1 μg of antibody per 107 cells. Incubations were performed at 4°C for 15 min. Then the cells were centrifuged and washed once using FCS medium. The cells were then resuspended in FCS medium at 107 cells/mL medium and incubated for 15 min at 4°C with goat anti-Mouse IgG Dynal beads (Invitrogen, San Diego, CA, USA) at a ratio of 107 cells/10 μL beads. The beads were then removed by a 2-min exposure to a magnet. The cells were again incubated for 15 min with Dynal beads, and the beads were completely removed by exposure to a magnet twice for 5 min each. Immunopurified CTs were washed and used to generate SCTs (see below).
The FCS medium was added to magnetic particles attached to CD9 and CD45 positive cells, and the mixture was plated in a T-75 culture flask. On reaching 80% confluency after approximately 2 weeks, the cells in the flask were sub-cultured. On first passage, the cells were trypsinized using 0.05% trypsin with EDTA solution (Invitrogen). That portion of the cells with beads still attached (~10%) were then completely removed by exposure to a magnet twice for 5 min. Then 106 bead-free cells were subcultured in a T-75 culture flask. Fresh FCS medium was added every 2 days. Eighty percent confluency was reached after about 1 week. The cells were used for experiments from passage 3 to passage 10.
The SCTs were generated by culturing CTs for 72 hr in FCS medium. Under these conditions, CTs spontaneously differentiate into SCTs as previously described by Kliman et al.19
For PCR studies, SCTs and FIBs were plated in FCS medium in 6-well culture dishes or T-25 flasks. The cells were plated in 24-well dishes for ELISA studies. The cells were treated for the indicated time and concentrations of LPS (Escherichia coli 055:B5, purified by phenol extraction, Sigma), PG (from Staphylococcus aureus; InvivoGen, San Diego, CA, USA), IL-1β (recombinant human, R&D Systems) and TNF-α (recombinant human, R&D Systems), based on previous studies conducted by our group and others.11,16
Cultures were maintained at 37°C in FCS medium in a humidified atmosphere of 5% CO2/95% air.
ELISA
The level of MCP-1 in culture media was determined using immunoassay (DuoSet ELISA kit, R&D Systems). For experiments, media were added to 96-well dishes coated with the MCP-1 antibody and after washing, the cells were treated with secondary antibody as described by the manufacturer. Levels of MCP-1 were derived from optical densities using a microtiter-plate reader and the Soft Max software program (Molecular Devices, Sunnyvale, CA, USA) and were normalized to total cell protein using the Dc Protein Assay from Bio-Rad (Hercules, CA, USA).
PCR
RNA was extracted from cultures of SCTs and FIBs using Tri Reagent from Invitrogen, and 3 μg was converted to cDNA using AMV reverse transcriptase (Invitrogen) in a 20 μL reaction volume. One microliter of cDNA was used for quantitative real-time PCR (qRTPCR) using a LightCycler (Roche, Indianapolis, IN, USA) and a LightCycler FastStart DNA MasterPlus SYBR Green I kit. The following primers were synthesized and gel-purified at the Yale DNA Synthesis Laboratory, Critical Technologies. MCP-1: forward, 5′-GCTCAGCCAGATGCAA-3′ reverse, 5′-GTCCAGGTGGTCCATG-3′; 18S: forward: 5′-GATATGCTCATGTGGTGTTG-3′, reverse: 5′-AATCTTCTTCAG TCGCTCCA-3′. The reaction conditions employed were: 1 cycle of 10 min at 95°C, 40 cycles of 10 s at 95°C, 5s at 57°C, and 10s at 72°C. Data were collected and analyzed using Light Cycler software v3.5. For each qRTPCR endpoint, a quantitative standard curve ranging from 0.5 to 250 ng of cDNA was generated by monitoring increasing fluorescence of PCR products during amplification as we have previously described.21 Following establishment of standard curves, MCP-1 expression was quantitated and normalized to that of 18S RNA. Melting curve analysis was conducted to verify the specificity of amplified products and to ensure the absence of primer-dimers. Endpoint PCR was used to confirm the identity of the amplified products; 207 bp for MCP-1, and 236 bp for 18S RNA. No signal was observed for negative control reactions carried out in the absence of either reverse transcriptase or template. To examine MCP-1 and 18S RNA expression in treated and control SCTs and FIBs, 1 μL of cDNA was used for conventional PCR in a 25 μL reaction system. In this case, the reaction conditions used were:1 cycle of 5 min at 95°C, 35 cycles of 30 s at 95°C, 30 s at 57°C, and 30 s at 72°C, and 1 cycle of 10 min at 72°C. Ten microliters of PCR product was loaded onto a 2% agarose gel containing ethidium bromide.
Statistical Analysis
Results from immunohistochemistry were expressed as medians with inter-quartile range [25th and 75th percentiles] for non-normal distributions. The Mann–Whitney U-test was employed to compare the non-parametric continuous data using the MedCalc ver. 8.1.1 statistical package from MedCalc Software (Mariakerke, Belgium). Real-time PCR and ELISA results from in vitro studies were found to be normally distributed and were analyzed using ANOVA followed by pairwise comparison by Student–Newman–Keuls method using SigmaStat software from Jandel Scientific (San Rafael, CA, USA). A P value of <0.05 was considered to be statistically significant for all comparisons.
Results
Levels of HBCs in Placentas from Pregnancies with and without HCA
Immunohistochemistry with anti-CD68 antibody was initially carried out to identify macrophages (i.e. HBCs) in the placental villous stroma from pregnancies at term with and without evidence of HCA. We observed that CD68 staining, as indicated by the presence of the brown peroxidatic product, revealed increased numbers of macrophages in villi from pregnancies complicated by HCA compared with control (Fig. 1). Cumulative analysis of CD68 levels expressed as CD68+ cells/100 villi in HCA+ (n = 60) and control (n = 39) pregnancies was carried out and found to follow a non-Gaussian distribution. Neither the gestational age at delivery nor the percentage of specimens derived following labor were different in HCA versus the control group (Table I). We noted that the number of CD68+ cells/100 villi was significantly higher in HCA+ placentas when compared with control specimens [HCA+: median 150 (interquartile range: 128–180; extreme values range: 45–310 versus control: median 62 (interquartile range: 57–75; extreme values range: 10–140) (Mann–Whitney U-test)]. These results indicate that HCA was associated with focally increased numbers of macrophages in placental villi.
Fig. 1.
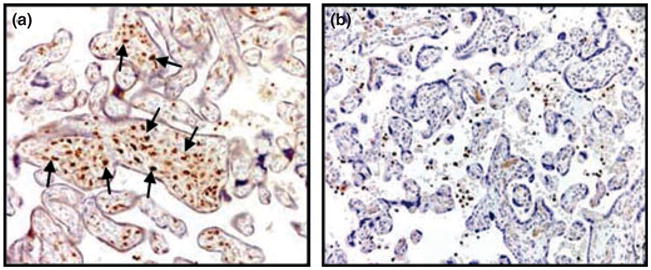
Immunohistochemistry reveals focally increased numbers of macrophages in chorionic villi from pregnancies complicated by histological chorioamnionitis (HCA). Formalin-fixed paraffin embedded term placental specimens from a pregnancy with HCA (a) and an uncomplicated pregnancy (b). Arrows refer to CD68+ cells in the placental villous stroma. One representative micrograph out of 60 HCA+ and 39 control placentas is shown (100×· magnification).
Table I.
Comparison of CD68+ Cells in Pregnancies With and Without Histological Chorioamnionitis (HCA)
| Variable | HCA+ | Control | Statistical comparison |
|---|---|---|---|
| N | 60 | 39 | |
| Gestational age (weeks) | 39 (39–40) | 39 (36–40) | NS |
| Deliveries with labor/total | 46/60 | 30/39 | NS |
| CD68+ cells/100 Villi | 150 | 62 | P = 0.0324* |
Immunohistochemical detection of CD68+ cells was carried out for the indicated number of specimens and analysis of results was performed as described in ‘Methods’.
HCA+ versus Control by Mann–Whitney U-test.
MCP-1 is a major monocyte/macrophage chemoattractant that is produced in cells following TLR ligation.9,10 It is often difficult to measure using immunohistochemistry as it is actively secreted from cells.22 Therefore, it is not surprising that in the current study the use of three different antibodies to MCP-1 failed to reveal consistent evidence of MCP-1 staining in control and HCA+ placentas. Therefore, to obtain information concerning the cellular source of placental MCP-1, its expression was studied in primary cultures of SCTs and FIBs (i.e. major placental cell types) isolated from human term placentas.
Regulation of MCP-1 mRNA Levels in SCTs and FIBs by LPS, PG, IL-1, and TNF-α
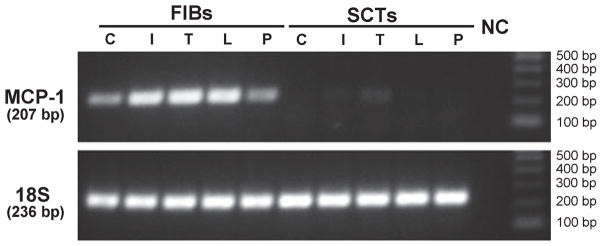
As HCA is associated with bacterial infection and inflammation at the maternal-fetal interface,1 conventional PCR was used to examine MCP-1 mRNA expression in cultures of SCTs and FIBs treated for 24 hr with and without 1 μg/mL PG (a component of Gram-positive bacteria and a TLR-2 ligand), 1 μg/mL LPS (a component of Gram-negative bacteria and a TLR-4 ligand), as well as IL-1β (1 ng/mL) and TNF-α (10 ng/mL), major pro-inflammatory cytokines found in placenta across gestation.23,24 Following extraction of RNA and conversion to cDNA, expression of MCP-1 and 18S RNA was examined using PCR (Fig. 2). MCP-1 was detected in untreated FIBs, and this baseline level of expression was increased in the presence of LPS, IL-1β, and TNF-α. PG treatment did not affect MCP-1 expression in FIBs which is consistent with our previous observation of TLR-2 absence in this cell type.16 Conversely, no MCP-1 expression was noted in control or treated SCTs. Levels of 18S RNA were similar in FIBs and SCTs confirming the finding that MCP-1 mRNA is expressed and regulated only in FIBs. To specifically quantitate the effects of these treatments, FIBs were maintained for 24 hr as described above and levels of MCP-1 mRNA were examined using qRTPCR and normalized to levels of 18S RNA (Fig. 3). We noted that treatment with IL-1β and TNF-α significantly induced MCP-1 mRNA levels 19- and 27-fold, respectively. LPS treatment enhanced MCP-1 expression sevenfold, although this effect did not reach statistical significance.
Fig. 2.
Expression of MCP-1 in FIBs and SCTs using conventional PCR. Cultures of FIBs and SCTs were maintained for 24 hr in the absence (C, control) or presence of 1 ng/ml IL-1β (I), 10 ng/mL TNF-α (T), 1 μg/mL LPS (L), or 1 μg/mL PG (P). Cellular RNA was then isolated and converted to cDNA and conventional PCR was then carried out to examine expression of MCP-1 (Upper Panel) and 18S RNA (Lower Panel). The size of the amplified products for MCP-1 and 18S are shown at the left of the gel, and the molecular weight of the DNA standards is shown at the right. NC, negative control. A representative experiment of three identically conducted ones is shown.
Fig. 3.
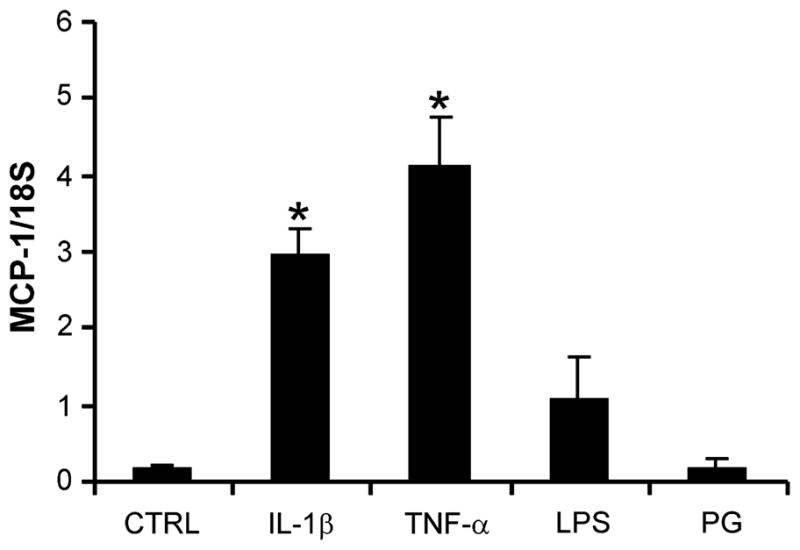
Analysis of LPS, PG, IL-1β, and TNF-α effect on MCP-1 expression in FIBs using quantitative real-time PCR (qRTPCR). FIBs were maintained for 24 hr without (Ctrl) and with 1 μg/mL PG, 1 μg/mL LPS, 1 ng/mL IL-1β or 10 ng/mL TNF-α. Following RNA extraction and cDNA synthesis, levels of MCP-1 mRNA normalized to that of 18S RNA were then determined using qRTPCR. Results are expressed as a mean MCP-1/18S ratio + SEM from seven independent experiments. *P < 0.001 versus Control by ANOVA
Regulation of MCP-1 Protein Levels in SCTs and FIBs by LPS, PG, IL-1, and TNF-α
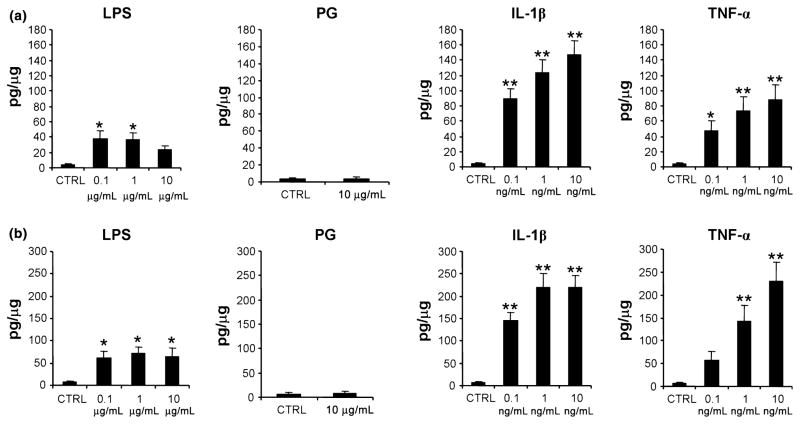
To evaluate the release of MCP-1 protein, the cells were maintained for 24 or 48 hr in medium without (Control) and with PG, LPS (0.1–10 μg/mL), IL-1β and TNF-α (0.1–10 ng/mL) and levels of MCP-1 in culture media were determined using ELISA following normalization to cell protein. In five independent experiments, we observed that levels of MCP-1 expression in SCTs ranged from undetectable (<0.02 pg/μg protein) to 0.08 pg/μg protein under all conditions tested (not shown). hCG levels in SCTs ranged from 40 to 80 mU/μg protein (not shown). This is consistent with our earlier results17 and demonstrates that low or undetectable levels of MCP-1 in SCTs do not reflect compromised function in this cell type. Conversely, in FIBs we noted that a 24 hr (Fig. 4a) and 48 hr (Fig. 4b) treatment with LPS, IL-1β, and TNF-α induced significant increases in MCP-1 levels of up to 9-, 37-, and 33-fold, respectively compared with control levels which varied from 2 to 5 pg/μg protein. Taken together, these results indicate that MCP-1 is expressed in FIBs, but not in SCTs, and is regulated in FIBs by bacterial products and inflammatory cytokines.
Fig. 4.
Effect of LPS, PG, IL-1β and TNF-α treatments on MCP-1 protein levels in conditioned media of FIBs. Cultures of FIBs were maintained for 24 hr (a) or 48 hr (b) in the medium without (Ctrl) and with the indicated concentration of LPS, PG, IL-1β, and TNF-α. Levels of MCP-1 in culture media were then determined using ELISA following normalization to cell protein. Results are expressed as a mean + SEM from six to nine independent experiments. *P < 0.05 versus Control, **P < 0.001 versus Control by ANOVA
Discussion
Cerebral palsy and related neurological disorders occur in approximately 2 to 3/1000 live births, and are a major cause of neonatal mortality, morbidity and long-term disability.25,26 In some affected individuals, pregnancies proceed unremarkably until term at which time non-specific signs of fetal distress occur.27 In other pregnancies, cerebral palsy is associated with preterm delivery, low birth weight, and HCA when accompanied by fetal inflammatory response syndrome [FIRS, a multisystemic dysfunction with the attendant septic chemokine response and the anatomic hallmarks of funisitis, or umbilical cord inflammation].1,27,28 Acute HCA and chronic villitis involve microbial-driven pathways.1,27,28 HCA is most often caused by ascending genital tract bacteria, mycoplasma or fungi, which result in a marked infiltration of neutrophils in maternal decidua and fetal membranes, with or without a neutrophil response in the placenta and fetus.1 Gram-negative microbes, including Escherichia coli and Ureaplasma urealyticum as well as the Gram-positive microbes Group B streptococcus and Staphylococcus aureus have been implicated in HCA with funistis.3,29 Villitis of unknown etiology (VUE) is a destructive inflammatory lesion of the chorionic villi characterized by infiltration of chronic inflammatory cells, mainly maternal T lymphocytes and CD68+ fetal macrophages i.e. HBCs.4 To date, the appearance, or role of HBCs in HCA remains largely unexplored.
In the current study, using immunohistochemistry we noted that HCA was accompanied by a two- to threefold increase in CD68+ cells in the placental villus stroma. This is consistent with the findings of another group demonstrating increased infiltration of CD68+ cells in the placental villous stroma and fetal membrane choriodecidua in pregnancies complicated by HCA delivered at term.30 They reported that HCA was also associated with increased expression of TNF-α converting enzyme (TACE) in these tissues, suggesting that release of TNF-α by TACE is part of an inflammatory cascade in HCA. Conversely, it was reported that CD68+ cells decrease in the placental villus in association with advancing gestational age and HCA.31 In this study, CD68+ cells in an entire villous area were systematically calculated using computerized imaging. As we noted that in both HCA and control cases, CD68+ cells were not homogenously distributed among the villi, we quantitated only those areas in which we observed one or more macrophages in terminal villi and five or more in intermediate villi. Although this is an arbitrary designation, our sample sizes of 99 placentas and 100 reference areas examined per placenta are sufficiently large to make meaningful observations. Taken together, this suggests that term placentas show focal increases in numbers of CD68+ cells in pregnancies with HCA even if the total numbers of villous CD68+ cells may decrease or not change.
We then determined which of the major placental cell types may play a role in macrophage recruitment to the placental villus. SCT, the outer layer of the placental chorionic villus in direct contact with maternal blood and underlying FIBs, adjacent to HBCs in the stromal core, were likely candidates. MCP-1 was chosen as a molecule for study as it is a major monocyte/macrophage chemoattractant expressed in placenta and other gestational tissues.9,32,33 Unfortunately, immunohistochemistry using three different antibodies did not consistently detect MCP-1 in placental tissue, most likely due to its rapid secretion.22 Therefore, to establish a potential etiology of villous macrophage influx in HCA, we determined whether MCP-1 levels in cultures of SCTs and FIBs were altered following treatment with the LPS and PG, components of Gram-negative and Gram-positive bacteria, respectively. As inflammatory processes at the maternal-fetal interface in HCA are suggested to be regulated by TNF-α and IL-1β,11 and these compounds are known to be major inflammatory cytokines synthesized by macrophages including HBCs,12,13 the effect of these cytokines on MCP-1 levels in placental cultures was also studied. Conventional PCR and qRTPCR revealed that FIBs expressed MCP-1 mRNA under basal conditions, and treatment with LPS, IL-β, and TNF-α markedly enhanced MCP-1 expression in these cultures. As expected, PG that binds to the TLR-2 receptor9 had no effect on MCP-1 expression in FIBs supporting our previous observation of the absence of TLR-2 in this cell type.16 Conversely, PCR indicated that SCTs do not express MCP-1 under basal conditions nor was its expression consistently detectable or enhanced in the presence of the TLR ligands or cytokines. Similarly, immunoassays revealed that FIBs, but not SCTs, released MCP-1 protein to the culture medium, and the presence of LPS, IL-1β, and TNF-α stimulated MCP-1 protein levels in FIBs in a time-and dose-dependent manner. Taken together, these results demonstrate that FIBs synthesize and release MCP-1, which may promote migration of fetal monocytes to the placental villus. In addition, based on the juxtaposition of FIBs to HBCs and fetal capillaries, it is more likely that compounds released by FIBs will affect fetal monocyte recruitment and HBC function compared with the SCT which is separated from stromal elements of the villus by a basement membrane.8
Based on our results, a model is presented linking villus MCP-1 expression to enhanced recruitment of fetal monocytes to the placenta in pregnancies with HCA (Fig. 5). We postulate that during HCA, LPS or other microbial compounds stimulate MCP-1 expression in FIBs, and not SCTs, leading to increased focal recruitment of fetal monocytes which differentiate and give rise to elevated HBCs at that site in the placental villus. Production of IL-1β and TNF-α by HBCs is then suggested to further increase MCP-1 production in FIBs resulting in enhanced MCP-1 levels in the villous stroma and additional fetal monocyte recruitment. Microbial compounds may directly enhance IL-1β and TNF-α levels in HBCs themselves, further stimulating MCP-1 production and HBC recruitment to the villous stroma. Increased expression of TLR-4 in HBCs was noted in HCA34 supporting a direct role of infection in the regulation of HBC function in HCA. A recent study using mixed stromal cell cultures from first and second trimester placentas suggest a role of HBCs in maturation of the placental mesenchyme.35 The source of HBCs in the placental villus has been proposed to change across gestation.8 As HBCs appear in the placental villus at day 18 of gestation, prior to the appearance of a fetal circulation, it is suggested that HBCs are derived from villous mesenchymal stem cells early in pregnancy, whereas later in pregnancy they are suggested to largely arise following differentiation of recruited fetal monocytes.4,5,8 It is also important to consider the proliferative properties of stem cells, fetal monocytes, or HBCs, when assessing factors which may regulate their levels in the placental villus. Thus, in this study we expect that, in addition to recruitment of fetal monocytes, proliferation of HBC precursors may also contribute to focal elevations of HBCs in pregnancies with HCA.
Fig. 5.
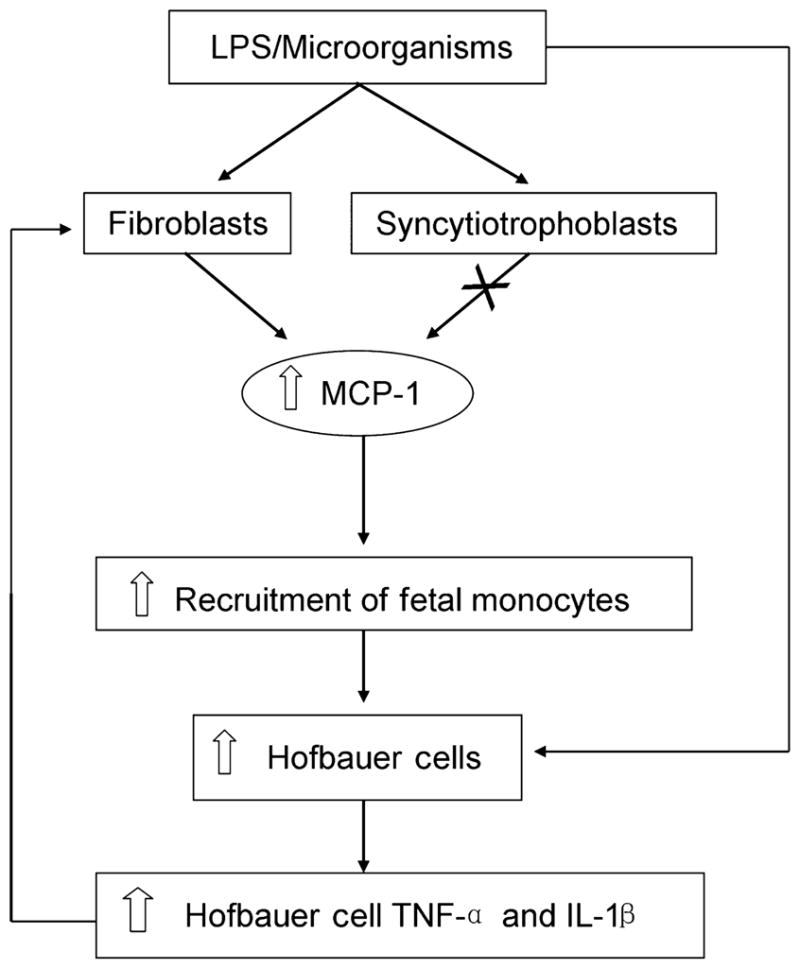
Model: the role of placental stromal FIBs and MCP-1 in promoting focally increased levels of HBCs in placentas from pregnancies with HCA.
In conclusion, our results clearly demonstrate that FIBs, and not SCTs, are a cellular source of inducible MCP-1 expression in the human placenta. We postulate that a cascade in the placental villus involving FIBs, bacterial products, MCP-1, and inflammatory cytokines play an important role in enhancing focal recruitment of fetal monocytes to placenta leading to elevated levels of HBCs in pregnancies complicated by CA.
Acknowledgments
The authors would like to thank Luisa Coraluzzi and Erin Kustan for their procurement of placentas for in vitro studies. This work was supported in part by ARRA R01 grant HD33909-13 (SG) and P01 Grant HD054713-01 (GM) from the NIH.
References
- 1.Redline RW. Placental inflammation. Semin Neonatol. 2004;9:265–274. doi: 10.1016/j.siny.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Redline RW. Severe fetal placental vascular lesions in term infants with neurologic impairment. Am J Obstet Gynecol. 2005;192:452–457. doi: 10.1016/j.ajog.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 3.Redline RW. Inflammatory responses in the placenta and umbilical cord. Semin Fetal Neonatal Med. 2006;11:296–301. doi: 10.1016/j.siny.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 4.Kim JS, Romero R, Kim MR, Kim YM, Friel L, Espinoza J, Kim CJ. Involvement of Hofbauer cells and maternal T cells in villitis of unknown aetiology. Histopathology. 2008;52:457–464. doi: 10.1111/j.1365-2559.2008.02964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim MJ, Romero R, Kim CJ, Tarca AL, Chhauy S, LaJeunesse C, Lee DC, Draghici S, Gotsch F, Kusanovic JP, Hassan SS, Kim JS. Villitis of unknown etiology is associated with a distinct pattern of chemokine up-regulation in the feto-maternal and placental compartments: implications for conjoint maternal allograft rejection and maternal anti-fetal graft-versus-host disease. J Immunol. 2009;182:3919–3927. doi: 10.4049/jimmunol.0803834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castellucci M, Zaccheo D, Pescetto G. A three-dimensional study of the normal human placental villous core I. The Hofbauer cells. Cell Tissue Res. 1980;210:235–247. doi: 10.1007/BF00237612. [DOI] [PubMed] [Google Scholar]
- 7.Fox H. The incidence and significance of Hofbauer cells in the mature human placenta. J Pathol Bacteriol. 1967;93:710–717. doi: 10.1002/path.1700930239. [DOI] [PubMed] [Google Scholar]
- 8.Castellucci M, Kaufmann P. Basic structure of villous trees. In: Benirschke K, Kaufmann P, editors. Pathology of the Human Placenta. 4. New York: Springer-Verlag; 2000. pp. 50–115. [Google Scholar]
- 9.Abrahams VM, Mor G. Toll-like receptors and their role in the trophoblast. Placenta. 2005;26:540–547. doi: 10.1016/j.placenta.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 10.Schaefer TM, Desouza K, Fahey JV, Beagley KW, Wira CR. Toll-like receptor (TLR) expression and TLR-mediated cytokine/chemokine production by human uterine epithelial cells. Immunology. 2004;112:428–436. doi: 10.1111/j.1365-2567.2004.01898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lockwood CJ, Arcuri F, Toti P, Felice CD, Krikun G, Guller S, Buchwalder LF, Schatz F. Tumor necrosis factor-alpha and interleukin-1 beta regulate interleukin-8 expression in third trimester decidual cells: implications for the genesis of chorioamnionitis. Am J Pathol. 2006;169:1294–1302. doi: 10.2353/ajpath.2006.060185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berkowitz RS, Faris HM, Hill JA, Anderson DJ. Localization of leukocytes and cytokines in chorionic villi of normal placentas and complete hydatidiform moles. Gynecol Oncol. 1990;37:396–400. doi: 10.1016/0090-8258(90)90375-u. [DOI] [PubMed] [Google Scholar]
- 13.Lin WJ, Yeh WC. Implication of Toll-like receptor and tumor necrosis factor alpha signaling in septic shock. Shock. 2005;24:206–209. doi: 10.1097/01.shk.0000180074.69143.77. [DOI] [PubMed] [Google Scholar]
- 14.Huppertz B, Rote NS, Nelson DM, Reister F, Black S, Hunt JS. Apoptosis: molecular control of placental function – a workshop report. Placenta. 2001;22(Suppl A):S101–S103. doi: 10.1053/plac.2001.0645. [DOI] [PubMed] [Google Scholar]
- 15.Yui J, Garcia-Lloret M, Wegmann TG, Guilbert LJ. Cytotoxicity of tumour necrosis factor-alpha and gamma-interferon against primary human placental trophoblasts. Placenta. 1994;15:819–835. doi: 10.1016/s0143-4004(05)80184-5. [DOI] [PubMed] [Google Scholar]
- 16.Ma Y, Krikun G, Abrahams VM, Mor G, Guller S. Cell type-specific expression and function of toll-like receptors 2 and 4 in human placenta: implications in fetal infection. Placenta. 2007;28:1024–1031. doi: 10.1016/j.placenta.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guller S, LaCroix NC, Kirkun G, Wozniak R, Markiewicz L, Wang EY, Kaplan P, Lockwood CJ. Steroid regulation of oncofetal fibronectin expression in human cytotrophoblasts. J Steroid Biochem Mol Biol. 1993;46:1–10. doi: 10.1016/0960-0760(93)90202-8. [DOI] [PubMed] [Google Scholar]
- 18.Lee MJ, Wang Z, Yee H, Ma Y, Swenson N, Yang L, Kadner SS, Baergen RN, Logan SK, Garabedian MJ, Guller S. Expression and regulation of glucocorticoid receptor in human placental villous fibroblasts. Endocrinology. 2005;146:4619–4626. doi: 10.1210/en.2005-0235. [DOI] [PubMed] [Google Scholar]
- 19.Kliman HJ, Nestler JE, Sermasi E, Sanger JM, Strauss JF., III Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology. 1986;118:1567–1582. doi: 10.1210/endo-118-4-1567. [DOI] [PubMed] [Google Scholar]
- 20.Douglas GC, King BF. Isolation of pure villous cytotrophoblast from term human placenta using immunomagnetic microspheres. J Immunol Methods. 1989;119:259–268. doi: 10.1016/0022-1759(89)90405-5. [DOI] [PubMed] [Google Scholar]
- 21.Guller S, Buhimschi CS, Ma YY, Huang ST, Yang L, Kuczynski E, Zambrano E, Lockwood CJ, Buhimschi IA. Placental expression of ceruloplasmin in pregnancies complicated by severe preeclampsia. Lab Invest. 2008;88:1057–1067. doi: 10.1038/labinvest.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serbina NV, Jia T, Hohl TM, Pamer EG. Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol. 2008;26:421–452. doi: 10.1146/annurev.immunol.26.021607.090326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen HL, Yang YP, Hu XL, Yelavarthi KK, Fishback JL, Hunt JS. Tumor necrosis factor alpha mRNA and protein are present in human placental and uterine cells at early and late stages of gestation. Am J Pathol. 1991;139:327–335. [PMC free article] [PubMed] [Google Scholar]
- 24.Hu XL, Yang Y, Hunt JS. Differential distribution of interleukin-1 alpha and interleukin-1 beta proteins in human placentas. J Reprod Immunol. 1992;22:257–268. doi: 10.1016/0165-0378(92)90047-8. [DOI] [PubMed] [Google Scholar]
- 25.Hagberg B, Hagberg G, Beckung E, Uvebrant P. Changing panorama of cerebral palsy in Sweden VIII. Prevalence and origin in the birth year period 1991–94. Acta Paediatr. 2001;90:271–277. [PubMed] [Google Scholar]
- 26.Greenwood C, Newman S, Impey L, Johnson A. Cerebral palsy and clinical negligence litigation: a cohort study. BJOG. 2003;110:6–11. [PubMed] [Google Scholar]
- 27.Redline RW, Heller D, Keating S, Kingdom J. Placental diagnostic criteria and clinical correlation – a workshop report. Placenta. 2005;26(Suppl A):S114–S117. doi: 10.1016/j.placenta.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 28.Romero R, Chaiworapongsa T, Espinoza J. Micronutrients and intrauterine infection, preterm birth and the fetal inflammatory response syndrome. J Nutr. 2003;133:1668S–1673S. doi: 10.1093/jn/133.5.1668S. [DOI] [PubMed] [Google Scholar]
- 29.Gibbs RS. Emerging infections in obstetric and gynecologic practice. Obstet Gynecol. 2006;108:480–481. doi: 10.1097/01.AOG.0000236127.48688.3c. [DOI] [PubMed] [Google Scholar]
- 30.Hung TH, Chen SF, Hsu JJ, Hsieh CC, Hsueh S, Hsieh TT. Tumour necrosis factor-alpha converting enzyme in human gestational tissues from pregnancies complicated by chorioamnionitis. Placenta. 2006;27:996–1006. doi: 10.1016/j.placenta.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Vinnars MT, Rindsjo E, Ghazi S, Sundberg A, Papadogiannakis N. The number of CD68+ (Hofbauer) cells is decreased in placentas with chorioamnionitis and with advancing gestational age. Pediatr Dev Pathol. 2009;13:300–304. doi: 10.2350/09-03-0632-OA.1. [DOI] [PubMed] [Google Scholar]
- 32.Abrahams VM, Bole-Aldo P, Kim YM, Straszewski-Chavez SL, Chaiworapongsa T, Romero R, Mor G. Divergent trophoblast responses to bacterial products mediated by TLRs. J Immunol. 2004;173:4286–4296. doi: 10.4049/jimmunol.173.7.4286. [DOI] [PubMed] [Google Scholar]
- 33.Esplin MS, Peltier MR, Hamblin S, Smith S, Fausett MB, Dildy GA, Branch DW, Silver RM, Adashi EY. Monocyte chemotactic protein-1 expression is increased in human gestational tissues during term and preterm labor. Placenta. 2005;26:661–671. doi: 10.1016/j.placenta.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 34.Kumazaki K, Nakayama M, Yanagihara I, Suehara N, Wada Y. Immunohistochemical distribution of Toll-like receptor 4 in term and preterm human placentas from normal and complicated pregnancy including chorioamnionitis. Hum Pathol. 2004;35:47–54. doi: 10.1016/j.humpath.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 35.Ingman K, Cookson VJ, Jones CJ, Aplin JD. Characterisation of Hofbauer cells in first and second trimester placenta: incidence, phenotype, survival in vitro and motility. Placenta. 2010;31:535–544. doi: 10.1016/j.placenta.2010.03.003. [DOI] [PubMed] [Google Scholar]