Abstract
Background
Activated platelets have previously-unrecognized mechanisms of post-transcriptional gene expression that may influence hemostasis and inflammation. A novel pathway involves splicing of pre-mRNAs in resting platelets to mature, translatable mRNAs in response to cellular activation.
Objectives
We asked if bacterial products and host agonists present in the septic milieu induce tissue factor pre-mRNA splicing in platelets from healthy subjects. In parallel, we asked if spliced tissue factor mRNA is present in platelets from septic patients in a proof-of-principle analysis.
Patients/Methods
Tissue factor pre-mRNA and mRNA expression patterns were characterized in platelets from septic patients and in platelets isolated from healthy subjects activated with bacteria, toxins, and inflammatory agonists. Procoagulant activity was also measured.
Results and Conclusions
Live bacteria, staphylococcal α-toxin, and lipopolysaccharide induced tissue factor pre-mRNA splicing in platelets isolated from healthy subjects. Toxin-stimulated platelets accelerated plasma clotting, a response that was blocked by a previously-characterized splicing inhibitor and by an anti-tissue factor antibody. Platelets from septic patients expressed spliced tissue factor mRNA, whereas it was absent from unselected and age-matched control subjects. Tissue factor-dependent procoagulant activity was elevated in platelets from a subset of septic patients. Thus, bacterial and host factors induce splicing of tissue factor pre-mRNA, expression of tissue factor mRNA, and tissue factor-dependent clotting activity in human platelets. Tissue factor mRNA is present in platelets from some septic patients, indicating that it may be a marker of altered platelet phenotype and function in sepsis and that splicing pathways are induced in this syndrome.
Keywords: Gene Expression, Inflammation, Platelets, Post-Transcriptional Pathways, Sepsis, Thrombosis
INTRODUCTION
Platelet number and function are altered in sepsis, a complex systemic syndrome of injurious host inflammatory and thrombotic responses to infection [1–10]. Platelet activation – a general term for a daedal group of functional activities that traditionally includes adherence, aggregation, and rapid secretion of preformed thrombotic and inflammatory mediators [11, 12] – may be a ubiquitous effector mechanism in the complex pathophysiology of septic syndromes (reviewed in [1, 3, 4, 8, 10]). This is consistent with evolving concepts of platelets as inflammatory cells, in addition to their activities as cellular effectors of hemostasis [12, 13].
In addition to intensely-studied adhesive and secretory events that occur rapidly and transiently after cellular activation, platelets have newly-recognized functional responses that may be important in sepsis and other inflammatory and thrombotic disorders [13]. These changes in function and phenotype are induced by “outside-in” signals delivered via surface receptors and involve intracellular biochemical cascades, and thus also represent activation responses. Post-transcriptional gene expression triggered by cellular activation is among these novel activities [13]. For example, stimulation of platelets with physiological agonists induces translation of endogenous repressed messenger RNAs (mRNAs), leading to rapid and sustained synthesis of protein products with activities relevant to thrombosis and inflammation (“signal-dependent translation”) [13, 14]. Remarkably, the platelet transcriptome also contains pre-messenger RNAs (pre-mRNAs)1, and activation of platelets leads to splicing of constitutively-expressed pre-mRNAs into mature transcripts that are translated into functional proteins [15–18]. One of these pre-mRNAs encodes tissue factor (TF) [18], which initiates coagulation by inducing a proteolytic cascade that culminates in generation of thrombin and conversion of fibrinogen to fibrin [19]. TF activity is induced in experimental and clinical sepsis, and is a central mechanism in pathologic clotting in this disorder [20–24]. Procoagulant activity mediated by TF, together with cellular activation of platelets, results in deposition of platelet-fibrin thrombi in microvessels. This may then lead to inadequate tissue perfusion, vascular injury, and – potentially – end organ failure and/or multiple organ dysfunction in sepsis [1, 4, 9, 10, 20, 23]. Contributions of endogenous platelet TF activity to these events are unexplored, and it is unknown if expression of TF pre-mRNA and mRNA transcripts is altered in sepsis.
In the current study, we examined TF pre-mRNA and mRNA expression and TF procoagulant activity in platelets isolated from healthy subjects before and after activation with bacteria, bacterial toxins, thrombin, or platelet activating factor (PAF). These agonists were examined because they are present and/or are generated in the internal milieu of human sepsis (reviewed in [10]). Because platelets may be ubiquitously-activated in human subjects with sepsis [1], we also performed an initial characterization of TF transcripts in platelets isolated from septic patients as an in vivo proof-of-principle analysis, with the hypothesis that mature TF mRNA is expressed in platelets from subjects with this syndrome. We found that spliced TF mRNA is present in circulating platelets from septic patients, that platelet-dependent procoagulant activity is enhanced in samples from septic subjects, and that bacterial toxins and host inflammatory factors may contribute to these phenotypic and functional alterations.
METHODS
Platelet Isolation and Activation with Toxins, Bacteria, and Mediators
Detailed methods are described in supporting online text. CD45 leukocyte-depleted human platelets were isolated from healthy volunteers, patients with sepsis, or control patients and suspended in medium (1×109/ml) as previously described [14, 15, 18]. The protocols for this study were reviewed and approved by the University of Utah School of Medicine Institutional Review Board. Platelets from healthy subjects were left quiescent or activated with α-toxin (10ng/ml; List Biological Laboratories Inc.) or lipopolysaccharide (LPS, strain 0111:B4; 100 ng/ml; Sigma) [25, 26]. In some studies, platelets were pre-incubated (30 min) with vehicle alone or with Tg003 (Calbiochem), a previously-reported inhibitor of the platelet splicing pathway [18]. In a second group of experiments, platelets were preincubated (15 min) with CLI-095 (also called TAK-242) [27] or with vehicle (DMSO) prior to treatment with LPS or α-toxin. In additional studies platelets were activated with PAF (C-16 PAF, Biomol) or thrombin (Sigma, St. Louis, MO) as described in “Results”.
Bacterial Incubation
Platelets were incubated alone or in suspension with Staphylococcus aureus or Escherichia coli at a bacterium to platelet ratio of 1:10. Bacteria were isolated from blood cultures from septic patients.
Pre-mRNA and mRNA Detection
mRNA was detected using polymerase chain reactions (PCR), as previously described [15, 18]. Primers that targeted TF exonic sequences four (5'-CTCGGACAGCCAACAATTCAG-3') and five (5'-CGGGCTGTCTGTACTCTTCC-3'), thus spanning intron four, were used to identify unspliced and spliced TF transcripts using previously established conditions and criteria for detection [18]. For all samples, PCR conditions were set to 35 cycles to determine if the mature transcript (i.e., 297 bp) was present or absent at this threshold. To rule out contribution by contaminating leukocytes, we also assessed TF and integrin subunit αIIb mRNA expression levels (Supporting Figs 1–3) and monocyte chemotactic protein 1 (MCP-1) mRNA expression (Supporting Fig 4).
TF-dependent Procoagulant Activity and Clotting
Platelets or monocytes from healthy controls were stimulated with LPS, α-toxin, or thrombin in the presence of Tg003 or its vehicle. Preparation of platelet membranes and microparticles and assays of procoagulant activity were accomplished as described [18].
Septic Patients
TF pre-mRNA and mRNA expression patterns were examined in platelets from forty six patients meeting consensus criteria for sepsis [28] after approval of the study by the University of Utah School of Medicine Institutional Review Board and informed consent prior to study enrollment. Blood samples for analysis of platelet TF patterns were collected within 72 hours of admission to the Medical Intensive Care Unit (MICU). In a subset of 16 patients, platelets were collected within the first 72 hours and at later time points in serial fashion. TF pre-mRNA and mRNA expression patterns were examined in unstimulated platelets from healthy, medication-free volunteers (age 18–50) in parallel with each assay of platelets from septic subjects. In addition, we also examined platelets from non-hospitalized, healthy volunteers age-matched to the septic patients.
Statistical Analyses
Each experimental result reflects at least 3–5 experiments. For all analyses, continuous variables were assessed for normality and if distributions were normal, parametric t-tests were used. If distributions were not normal, Wilcoxon Rank Sum tests were used. Categorical variables were compared using the Fisher’s Exact test. Significance was predetermined at p<0.05. Categorical variables were compared using the Fisher’s Exact test and t tests for comparison of continuous variables. The three study groups (unselected healthy volunteers, age-matched healthy volunteers, and septic patients) were compared on the dichotomous outcome of TF splicing using Logistic regression analyses to determine odds ratios (OR) controlled for the confounder of age.
RESULTS
Bacterial Pathogens and Host Factors Induce TF Pre-mRNA Splicing, Expression of TF mRNA, and Generation of TF-dependent Procoagulant Activity by Human Platelets
We first characterized the pattern of unspliced and spliced TF transcripts in platelets from healthy subjects using primers that flank intron 4. This strategy was chosen because intron 4 is the shortest intron in the TF pre-mRNA (Fig 1A), and is thus most tractable to amplification of the pre-mRNA (904bp) and mRNA (297bp) sequences. In previous studies, we validated this approach and also detected TF transcripts using primers that span other introns [18]. In the current studies the unspliced pre-mRNA was uniformly present, and the mature transcript absent, in unstimulated platelets from healthy volunteers (Fig 1B; also see Figs 2A, 3B, 4, and Supporting Figs 1–3). This is consistent with previous results in which we verified the presence of the pre-mRNA by amplification and sequencing of the entire transcript and by in situ detection of the pre-mRNA in quiescent platelets, and also found that the spliced mRNA is not present under basal conditions [18]. We also previously found that the TF pre-mRNA is spliced to the mature, translatable mRNA in platelets from normal subjects when platelets adherent to fibrinogen are activated with nanomolar concentrations of thrombin [18], which is generated in sepsis and may be a key mediator of platelet activation in septic patients [10, 20, 22, 23]. In the current study we confirmed by quantitative RT-PCR (qRT-PCR) that activated platelets splice TF pre-mRNA into mature mRNA (Supporting Figs 1–3). In addition, supporting figs 1–4 conclusively demonstrate that TF pre-mRNA splicing events detected in our platelet preparations were not due to the presence of contaminating leukocytes.
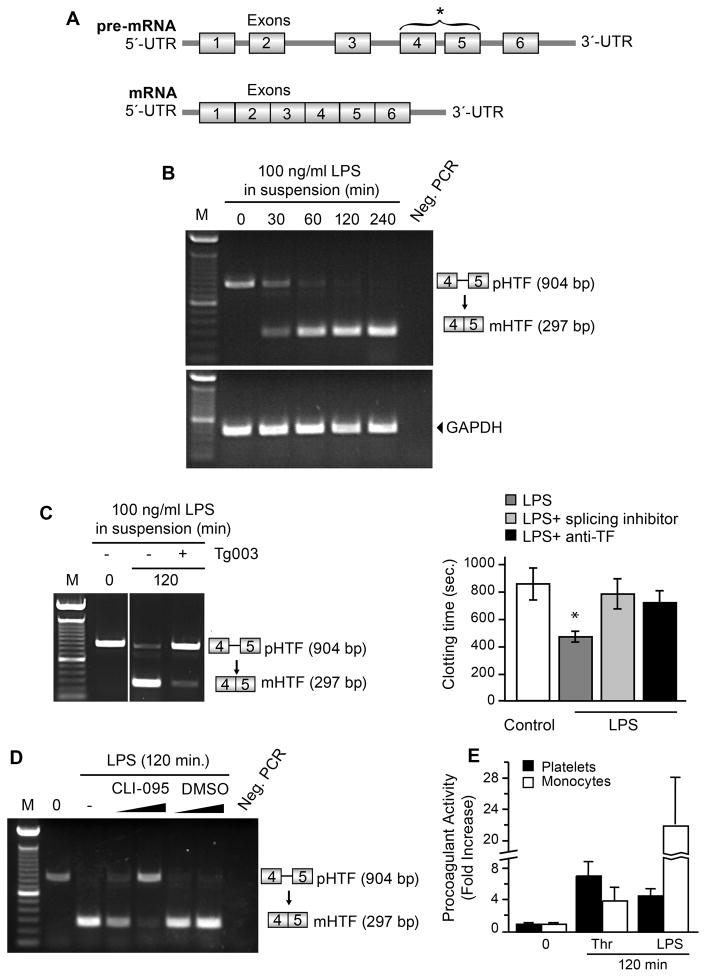
Figure 1. LPS induces splicing of TF pre-mRNA by human platelets.
(A) The intron-exon structure of the unspliced TF pre-mRNA and the mature, spliced TF mRNA are shown in diagrammatic form. Primers for exons 4 and 5 that span the intervening intron (bracket and asterisk) were constructed and used to examine platelets for unspliced and spliced TF mRNA transcripts as previously described [18]. (B) Platelets isolated from healthy controls were incubated in medium alone or with LPS for 30–240 minutes. The box-stick diagrams to the right identify human pre-mRNA for TF (pHTF) and mature mRNA for TF (mHTF) based on PCR analysis of the exon 4–5 region outlined in (A) and Methods. Size markers (M) are shown on the left and a lane without PCR primers (Neg. PCR) is shown on the right. The transcript expression patterns shown in this figure are representative of three independent experiments. (C) Platelets from healthy controls were assayed at time zero (second lane) or were activated with 100 ng/ml of LPS for 120 minutes in the presence or absence of the splicing inhibitor Tg003. Left Panel: TF mRNA expression patterns were examined as in (B). Right panel: Membranes from platelets preincubated with Tg003 or control buffer and then activated with LPS were added to human plasma and clotting times were measured in the presence or absence of a neutralizing antibody against TF. The bars represent the mean±SEM for four independent experiments. The asterisk (*) indicates a significant difference (p<0.05) between activated platelets and quiescent or inhibitor-treated platelets. (D) Platelets isolated from healthy subjects were analyzed at baseline (lane 2) or after stimulation with LPS (100ng/ml, 120 min) following preincubation (15 min) in buffer alone (lane 3), with CLI-095 dissolved in DMSO (lanes 4, 5), or with the same final concentrations of DMSO (lanes 6, 7). TF pre-mRNA and mRNA were analyzed as in B and C. CLI-095, an inhibitor of signaling via TLR4 [27], partially blocked TF splicing at the low concentration (2 μm; lane 4) and almost completely blocked it at a higher concentration (3 μm; lane 5). This figure is representative of three experiments. (E) Isolated platelets (2.1+0.2 ×109) or monocytes (4.0+0.03 ×106) were incubated with LPS (100 ng/ml) or with thrombin (0.1 U/ml) for 120 minutes. The number of platelets and monocytes for each study were based on the number of each cell type present in 10 ml of the volunteer donor’s blood, which was determined by differential platelet and monocyte counts assessed on the day of the experiment. TF-dependent procoagulant activity associated with isolated cell membranes was measured in 3 independent experiments. The bars indicate mean±SEM of the experiments and the data are displayed as fold increase over baseline TF activity in unstimulated membranes from platelets and monocytes.
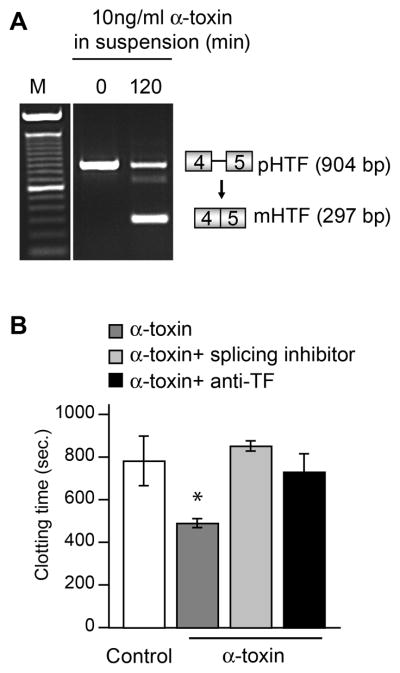
Figure 2. α-toxin induces splicing of TF pre-mRNA and expression of TF-dependent procoagulant activity by human platelets.
(A) TF mRNA expression patterns were assessed in platelets from healthy controls that were assayed at time zero (lane 2) or were activated with staphylococcal α-toxin for 120 minutes (lane 3) as described in Figure 1. This gel is representative of 3 independent experiments. (B) Platelets from healthy volunteers were incubated with control buffer or activated with α-toxin for 120 minutes in the presence or absence of Tg003. Plasma clotting was measured as described in Figure 1C. The bars represent the mean ± SEM for four independent experiments and the asterisk (*) indicates a significant difference (p<0.05) between activated platelets and quiescent or inhibitor treated platelets.
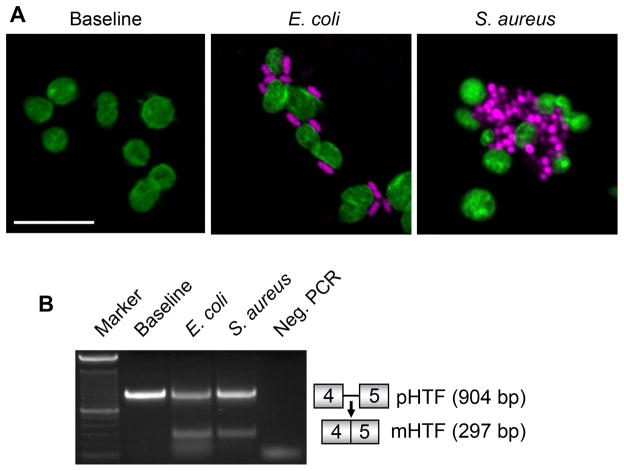
Figure 3. E. coli and S. aureus induce splicing of TF pre-mRNA in human platelets.
(A) Platelets isolated from healthy controls were incubated in medium alone (control) or in the presence of S. aureus or E. coli bacteria for 30 minutes. The platelets and bacteria were then fixed in suspension and subsequently stained with Alexa 488 phalloidin (for F-actin; green staining in platelets) and TOPRO-3 (for DNA; magenta in bacteria). This figure is representative of multiple independent studies. (B) TF pre-mRNA and mRNA expression patterns were assessed in platelets from healthy controls that were incubated with E. coli or S. aureus for 30 minutes. This gel is representative of 3 independent experiments.
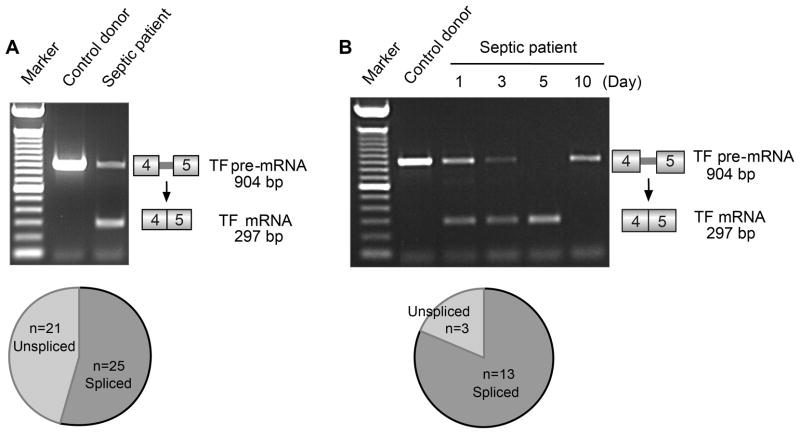
Figure 4. Platelets isolated from patients with sepsis express spliced TF mRNA.
(A) Patterns of TF pre-mRNA and mature TF mRNA expression were examined in platelets from healthy control donors and septic patients as outlined in Figure 1. No exogenous activating agonist was added. A representative experiment examining platelets isolated from a healthy control and a septic patient, studied in parallel, is shown. The pie chart below the gel illustrates the number of platelet preparations from septic patients (46 total), isolated within 72 hours of ICU admission, that expressed unspliced or spliced TF transcripts. Unspliced and spliced transcripts were present together in platelets from some septic subjects as shown, consistent with patterns detected in vitro (Figures 1–3). (B) A representative example of serial examination of platelets isolated from a septic patient at day 1, 3, 5, and 10 post-admission to the ICU. Platelets from control donors were assayed in parallel with each sample from the patient; one representative blot is shown in lane 2. The pie chart below the gel illustrates the total number of septic patients (13 of 16) whose platelets expressed spliced TF mRNA on at least one day during the septic episode and serial analysis.
To determine if microbial stimuli can also induce splicing activity and consequent processing of the TF pre-mRNA to the mRNA transcript, we examined bacterial toxins that can activate host effector cells. E. coli LPS, in concentrations of 10–1000ng/ml, did not directly increase P-selectin expression on the surfaces of platelets after a 60 min incubation (data not shown). Nevertheless, LPS induced splicing, yielding mature TF mRNA in isolated platelets within 30 minutes that increased over time (Fig 1B). Generation of mature TF mRNA was inhibited by Tg003 (Fig 1C), which interrupts activation-dependent splicing of TF pre-mRNA by blocking activity of Cdc2-like kinase 1, a key enzyme in the splicing pathway [18]. These results are similar to findings of Shaskin et al [29] who recently reported time-dependent splicing of IL-1β pre-mRNA in human platelets induced by LPS. In addition, we found that LPS-induced splicing of TF pre-mRNA was blocked by a small molecule inhibitor, CLI-095 (Figure 1D), that suppresses cellular responses triggered by TLR4 engagement by selectively interrupting signaling mediated by the intracellular domain of the toll-like receptor [27]. This is consistent with activation of the Cde2-like kinase splicing pathway [18] by a mechanism that involves molecular interactions at the cytoplasmic tail of TLR4.
In our previous studies, time-dependent splicing of the TF pre-mRNA transcript in thrombin-stimulated platelets was paralleled by enhanced procoagulant activity and accelerated TF-dependent clotting, indicating translation of the mature mRNA and synthesis of the functional protein product [18]. Similarly, we found that plasma clotting time was shortened in the presence of membranes isolated from LPS-stimulated platelets (Fig 1C, right panel). Tg003 reversed this response, confirming that accelerated clotting depends on pre-mRNA splicing. Furthermore, a neutralizing antibody against TF also reversed the shortened clotting times (Fig 1C, right panel).
We compared TF-dependent procoagulant activity expressed by stimulated platelets to that of monocytes, a major cellular source of TF in sepsis and other prothrombotic conditions [19, 22, 23]. For these studies, the ratio of procoagulant activity in platelets versus monocytes was determined using cell numbers that reflected differential platelet and monocyte numbers in the volunteer donor’s blood on the day of the experiment. At 120 min of stimulation the procoagulant activity of thrombin-stimulated platelets was equal or greater to that of monocytes (Fig 1E). In contrast, when LPS was the agonist, the procoagulant response of monocytes was much greater than that of platelets (Fig 1E). This finding suggests that local generation of TF-dependent procoagulant activity depends on the agonist and cell type in sepsis, and that the contributions by platelets may be important depending on the stimulus and time of activation of target cells. Furthermore, platelet generation of TF-dependent procoagulant activity may be additive to that of other key cells [19, 23].
Staphylococcal infections are common initiators of sepsis and septic complications, including thrombosis and microvascular fibrin deposition [10]. A factor with potentially lethal activities that is released by S. aureus [25], α-toxin, induced TF pre-mRNA splicing in platelets (Fig 2A). Higher concentrations of α-toxin did not further increase TF pre-mRNA splicing in platelets (data not shown). We also found that membranes from platelets stimulated with α-toxin shortened plasma clotting time (Fig 2B). Accelerated clotting time was blocked when the platelets were pretreated with the splicing inhibitor Tg003 or a neutralizing antibody against TF (Fig 2B). In an initial experiment CLI-095, which blocked LPS-inhibited splicing (Figure 1D), did not inhibit splicing triggered by α-toxin (not shown).
In additional experiments, we found that E. coli or S. aureus adhere to human platelets and induce TF pre-mRNA splicing within 30 minutes, a response that increased over time (Fig 3A, 3B, and data not shown). We also found that incubation of whole blood from a control subject with S. aureus induced coagulation and that spliced TF mRNA was present in platelets extracted from the clot, whereas only unspliced TF pre-mRNA was present in the uncoagulated blood sample in the absence of bacteria (Supporting Fig 5).
Bacteria and bacterial products induce generation of endogenous host mediators in clinical and experimental sepsis [10]. In addition to thrombin, PAF is synthesized in the septic milieu and activates platelets and leukocytes in sepsis [10]. As with thrombin [18], PAF triggered splicing of TF pre-mRNA in a time- and concentration-dependent fashion (Supporting Fig 6).
Platelets Isolated from Septic Patients Express Spliced TF mRNA
Platelet activation is a common and perhaps ubiquitous feature of sepsis [1, 3, 4, 8–10], and bacterial factors and host mediators induce splicing of TF pre-mRNA by platelets from control subjects (Figs 1–3; Supporting Figs 5, 6). Therefore, we examined platelets from septic patients for spliced TF mRNA as an initial proof-of-principle analysis of in vivo relevance of our in vitro findings. We prospectively enrolled 46 patients (Table 1) who met consensus criteria for sepsis, the majority of whom (>85%) had the complicated syndromes of severe sepsis or septic shock [28, 30]. There were broad ranges of platelet and leukocyte counts and APACHE II scores (a severity index based on multiple variables in critical illness), as might be expected for a relatively small study population that included the full clinical spectrum of sepsis (Table 2).
Table 1.
Patient Characteristics (n=46)*
| Mean age (yrs) | 57±17 |
| Median age (yrs) | 57 |
| Male | 54% |
| APACHE II Score | 25.6±9.2 |
| Platelets count (K/ul) | 232±136 |
| WBC (K/ul) | 17.8±10.3 |
| Bacteremia | 52% |
| MICU LOS (days) | 6.7±9.7 |
| In-hospital mortality | 13% |
Values indicate mean ± standard deviation.
Table 2.
Clinical Features of Patients in Which Spliced, Mature TF mRNA Was or Was Not Detected in Circulating Platelets*
| Spliced TF Present in Platelets | Only Unspliced TF Present in Platelets | Odds Ratio (95% CI) | Odds Ratio (95% CI) Adjusted for Age | |
|---|---|---|---|---|
| Number of patients | 25 (54%) | 21 (46%) | -- | -- |
| Age ≥65 | 10 (40%) | 4 (19%) | 2.77 (0.63, 14.74) | -- |
| Female† | 12 (48%) | 9 (43%) | 1.23 (0.33, 4.64) | 1.40 (0.37, 5.66) |
| APACHE II >20* | 21 (84%) | 11 (52%) | 4.60 (1.03, 25.02) | 4.06 (0.87, 22.69) |
| Bacteremia§ | 15 (60%) | 8 (37%) | 2.45 (0.63, 10.11) | 2.45 (0.63, 10.11) |
| D-dimer>8.2 [units]‡ | 6/19 (32%) | 3/10 (30%) | 1.34 (0.65, 2.91) | 1.32 (0.64, 2.83) |
| MICU LOS (days)* | 8.3 | 4.8 | 1.05 (0.97, 1.17) | 1.06 (0.98, 1.18) |
| Survival to Hospital | 20 (80%) | 20 (95%) | 0.21 (0.004, 2.08) | 0.23 (0.004, 2.40) |
| Discharge |
Platelets were isolated from patients with sepsis (identified by consensus criteria) within 72 hours of admission to the MICU. Spliced TF mRNA was detected in platelets from one or more blood samples from 25 patients, and only the unspliced TF transcript was present in platelets from 21 patients (columns 1 and 2). [*mean (95% CI);
number (%);
D-dimer levels were only available for 29 patients upon MICU admission and study enrollment;
Culture data on 2 patients was unavailable.]
We found that platelets in samples from 54% of septic patients expressed mature, spliced TF mRNA in blood collected within 72 hours of admission to the ICU or in one or more later samples (see below) (Fig 4A, Table 2). We observed a similar frequency of TF pre-mRNA splicing when mRNA expression patterns were assessed by qRT-PCR (data not shown). In contrast, platelets isolated from 65 healthy young volunteers (mean ± SD age = 34 ± 9 years, range 18–50 years) examined in parallel side-by-side assays exclusively expressed unspliced TF pre-mRNA in the absence of an activating agonist such as thrombin or PAF (Fig 4 and data not shown). This result is consistent with previous studies [18], and with analysis of the transcripts in platelets from healthy volunteers in in vitro experiments in this study (Figs 1–3, Supporting Figs 1–3, and data not shown). In addition, unactivated platelets isolated from a group of 10 randomly selected, older healthy controls (mean ± SD age = 58.1±11 years, range 40–74 years) age-matched to the septic subjects (Table 1) exclusively expressed unspliced TF mRNA (data not shown).
In a subset of patients (n=16) we were able to collect serial blood samples to determine if the species of TF transcripts in platelets changed during the course of the septic episode. Fig 4B illustrates an example, in which platelets from a patient with aspiration pneumonia and sepsis due to Enterococcus faecalis were examined in serial fashion. Both unspliced pre-mRNA and mature, spliced mRNA were detected on days 1 and 3 but only spliced mRNA was detected on day 5. At day 10, when sepsis had resolved and this patient had improved clinically, the patient’s platelets only expressed unspliced TF pre-mRNA – a pattern equivalent to that of unactivated platelets from control subjects (Fig 4B). Serial analysis also revealed that platelets from some patients did not initially express spliced TF mRNA at the time of ICU admission but mature TF mRNA was subsequently detected at later times during the course of sepsis (data not shown). Of 16 patients in whom serial sampling was performed, 13 expressed spliced TF mRNA in at least one analysis (Fig 4B, pie chart).
Septic patients who were ≥65 years, had an APACHE II score >20, or had documented bacteremia were more likely to express mature, spliced TF mRNA in one or more samples (single or serial analysis) (Table 2). The odds of mature, spliced TF mRNA being present in platelets isolated from septic patients within 72 hours of ICU admission also increased with age ≥65 and APACHE II score >20. In addition, patients with sepsis whose platelets expressed mature TF mRNA at one or more time points were more likely to die prior to hospital discharge, regardless of age. Nevertheless, the confidence intervals for these trends and subgroups were wide. This may be due in part to a relatively small sample size and inclusion of patients with a spectrum of septic syndromes.
Platelets Isolated from Septic Patients have Enhanced TF-Dependent Procoagulant Activity
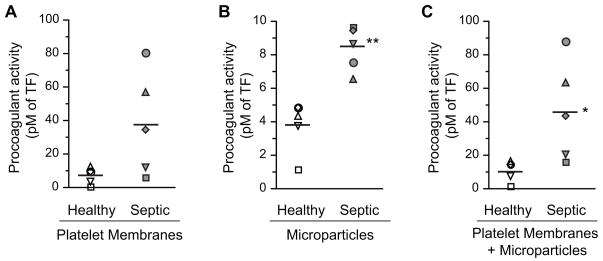
We were unable to collect enough blood from patients to retrieve sufficient numbers of platelets for simultaneous analysis TF pre-mRNA splicing and procoagulant activity. Therefore, we measured TF-dependent procoagulant activity expressed by platelets from 5 additional septic patients and 5 healthy volunteers. We found that TF activity was increased in membrane preparations from platelets isolated from patients with sepsis compared to platelets from healthy subjects assayed in a side-by-side, parallel fashion in order to control for individual and day-today variability (Fig 5). The mean TF activity of combined platelet membrane and microparticle fractions from platelets isolated from septic patients (45.9±29.6 pM) was significantly higher than that in preparations from control subjects (11.1±6.3 pM; p=0.03). TF activity was also higher in preparations from septic patients versus control subjects when platelet membrane and microparticle fractions were examined separately, although the difference achieved statistical significance in the microparticle analysis only (Fig 5). Enhanced procoagulant activity associated with platelets from septic patients compared to controls (Fig 5) is consistent with expression of mature, spliced TF mRNA in platelets from septic subjects (Fig 4, Table 2), although other mechanisms could also account for this observation [19, 23].
Figure 5. Platelets from patients with sepsis have increased TF-dependent procoagulant activity compared to activity associated with platelets from control subjects.
TF-dependent procoagulant activity was measured using membranes and microparticles isolated from platelets in samples from healthy control subjects (n=5) and patients with sepsis (n=5). No exogenous activating agonist was added. Samples from control subjects and septic patients were assayed in side-by-side fashion on the same day. The paired samples are indicated by matching symbols in each panel. The single asterisk (*) indicates a statistical significance of p=0.03. The double asterisks (**) denotes a statistical significance of p<0.01.
DISCUSSION
Our findings demonstrate that platelets from some patients with sepsis express mature, spliced TF mRNA, in contrast to platelets from control subjects. While inflammatory conditions such as sepsis can alter transcript expression and other characteristics of circulating platelets by inducing changes in megakaryocyte function [31, 32], our parallel in vitro studies suggest a mechanism that may operate independently, or in concert, involving systemic activation-dependent splicing of TF pre-mRNA and translation of the spliced, mature transcript by human platelets. Splicing in the circulation may occur when platelets encounter microbial toxins, live bacteria, or endogenous host factors that are generated in the septic milieu. The studies also suggest that sepsis-driven splicing may contribute to enhanced TF-dependent procoagulant activity of platelets sequestered in the microvasculature of affected patients, potentially contributing to dysregulated hemostasis and inflammation. These are central features of the pathobiology of septic syndromes [4, 10, 20].
We previously found that the transcriptome of quiescent platelets isolated from healthy volunteers includes pre-mRNAs, and that activation of platelets with thrombin induces spliceosomal activity and processing of unspliced transcripts to mature, translatable mRNAs [15, 18]. This represents a novel and unexpected activity of human platelets [13, 15, 33]. In addition to TF [18] the unspliced transcripts that are constitutively present in resting human platelets include IL-1β [15], which encodes a pleiotropic cytokine important in sepsis and other inflammatory disorders [10, 20]. Stimulation of platelets with thrombin while the cells are adherent to fibrinogen, conditions that model events that occur in micro-thrombi in inflamed vessels in sepsis [4, 10, 20], induces splicing of TF pre-mRNA, expression of TF mRNA, and translation of the mRNA into TF protein [18]. Interruption of pre-mRNA splicing or translation of the mature mRNA prevents increased TF protein expression in activated platelets [18]. Inhibition of TF pre-mRNA splicing also prevents TF-dependent procoagulant activity from accumulating in activated platelet membranes and microparticles [18]. It is possible that “de-encryption” [34] may be required for full TF activity in these membranous domains. In addition to our original observations, other investigators have reported that human platelets synthesize TF protein [35–37] and confirmed that activating signals induce splicing of pre-mRNAs, including the TF precursor transcript, in human platelets [29, 36, 37].
In this study we demonstrate that bacterial factors that induce functional alterations in target cells in vitro and in vivo [10, 25, 26] are agonists for splicing of TF pre-mRNA, accumulation of mature TF mRNA, and generation of TF-dependent procoagulant activity by platelets from normal subjects. We found that LPS induces time-dependent TF pre-mRNA splicing and splicing-dependent procoagulant activity (Fig 1). Induction of splicing and post-transcriptional TF expression in platelets stimulated by LPS identifies a new mechanism by which LPS can trigger procoagulant events in sepsis or in localized infections caused by gram negative bacteria [23, 26]. This may occur if LPS is released into the blood systemically or locally or, potentially, by direct encounters between platelets and gram negative pathogens (Fig 3).
LPS-induced splicing of pre-mRNA transcripts is consistent with multiple observations demonstrating that human and mouse platelets express members of the toll-like receptor family, including TLR4 [10, 29] (reviewed in [38]). This is one molecular mechanism by which platelets act as “sentinels” for microbes and recognize, respond to, and participate in clearance of bacteria and other pathogens [4, 10, 12, 38]. In vivo studies indicate that human and murine platelet TLR4 is functional, and that it mediates LPS-induced thrombocytopenia, cytokine production, and other cellular responses [29, 38–42]. Nevertheless, mechanisms by which engagement of TLR4 induces alterations in function and phenotype of platelets (reviewed in [38]), are incompletely defined. In some reports LPS did not induce aggregation, secretion, or surface translocation of P-selectin (CD62P) [41, 43] whereas in others these responses were detected depending on incubation time and other variables [29, 39, 42]. While TLR4 signaling in platelets may be “unconventional” [41], recent observations indicate that LPS-induced activation of murine platelets requires myD88, a key intracellular component of TLR4-mediated signaling cascades, and that myD88 is present in human platelets [42]. Engagement of TLR4 by LPS triggers dimerization and association of myD88 with the intracellular TIR (toll/IL-1 receptor) domain of TLR4, followed by additional signal transduction events [10, 44]. We found that a small molecule inhibitor that selectively blocks TLR4 signaling and that may target the TIR domain [27] interrupts splicing of TF in LPS-stimulated platelets (Figure 1D). This result suggests that interaction of the TIR domain with myD88 and/or other adaptor proteins is a component of the molecular mechanism leading to LPS-induced activation of the Cdc2-like kinase1 pathway and spliceosomal function in human platelets (Figure 1) [15, 18, 29]. This possibility remains to be further explored, and additional “downstream” signaling intermediates that link the cytoplasmic domain of TLR4 to the splicing pathway remain to be defined.
We also found that α-toxin from S. aureus, a hydrophilic molecule that is one of the most frequent toxins of bacterial origin encountered by humans [25], induces splicing of TF pre-mRNA by human platelets. Staphylococcal α-toxin activates cells by aggregating to form pores in the plasma membrane rather than via surface receptors [25]. Post-transcriptional processing of TF pre-mRNA by platelets in response to α-toxin, and subsequent generation of procoagulant activity by platelets activated by α-toxin, establish a new pathogenetic mechanism that is potentially relevant to sepsis driven by staphylococcal pathogens. We also found that intact S. aureus microorganisms induce pre-mRNA splicing by platelets, indicating that direct encounters with the pathogen in the blood or at vascular surfaces may activate this response.
Together, our experiments demonstrate that both bacteria and bacterial toxins – the primary agents of sepsis – can directly induce expression of mature TF mRNA by human platelets. This may then result in translation of the mRNA and synthesis of TF protein, contributing to cascades of procoagulant activity, fibrin deposition, and proinflammatory signaling [10, 22, 23, 45]. In addition, host-derived mediators that are also present in the septic milieu [10, 20–24], such as PAF (Supporting Fig 6) or thrombin ([18]; Fig 2), also trigger splicing of TF pre-mRNA and accumulation of the mature mRNA transcript by target platelets. Therefore, under the pathologic conditions of sepsis splicing of TF pre-mRNA in platelets may be inappropriately induced by a variety of agonists and, indeed, “misplaced” as suggested by others [33].
To explore the possibility that spliced TF mRNA is expressed by human platelets in vivo, we performed an initial study in platelets from septic patients. The rationale was two-fold. First, platelet activation is a common feature of septic syndromes [1, 4, 9, 10], suggesting that – if activation-dependent splicing occurs in circulating platelets in vivo – sepsis might be an index condition in which to detect it. Second, murine models of sepsis were not appropriate for this question because mouse platelets do not express TF pre-mRNA [46]. While other pre-mRNAs and a splicing pathway may be conserved in murine platelets, differences in hemostatic and inflammatory mechanisms clearly exist [47, 48]. Thus, for an initial in vivo analysis of spliced TF transcripts in platelets, studies of samples from human subjects with clinical sepsis were necessary. We found that mature TF mRNA was expressed in platelets isolated from 60% of septic patients with documented bacteremia (Table 2), consistent with activation-dependent splicing triggered by bacteria and/or bacterial products in vitro (Figs 1–3). This is a previously-unrecognized phenotypic feature of platelets from patients with sepsis. We also found that spliced TF mRNA is present in platelets from some septic patients without documented bacteremia (Table 2), consistent with activation of platelets by host factors such as thrombin or PAF alone or in concert with circulating microbial toxins [10]. In contrast, we found that platelets from control subjects, including a subset of healthy volunteers age-matched to septic patients in this study, expressed only unspliced TF pre-mRNA. These results are similar to our original findings that platelets from healthy volunteers exclusively express unspliced TF pre-mRNA [18]. Similarly, others reported that only unspliced TF pre-mRNA is present in control, unactivated human platelets and that, upon cellular activation, the mature TF mRNA transcript is generated and TF protein is synthesized as a result of splicing [36, 37]. In preliminary studies that are ongoing, we also found that platelets from subjects at risk for deep vein thrombosis after knee or hip surgery expressed only unspliced TF pre-mRNA (Rondina MT et al. unpublished observations). Thus, while we predict that platelets from patients with other inflammatory or prothrombotic conditions besides sepsis may also express spliced TF mRNA, our findings in this study indicate that specific stimuli induce expression of spliced TF mRNA by platelets in septic syndromes. Increased TF-dependent procoagulant activity of platelets from a subgroup of septic patients (Fig 5), albeit a small sampling, is consistent with synthesis of TF protein from spliced TF mRNA. We cannot, however, exclude other variables such as in vivo binding of procoagulant TF-bearing microparticles to circulating platelets [23] from this study alone.
Our findings also raise a number of other important issues including effects of comorbidities [37, 49], the possibility that constitutive endogenous tissue factor mRNA and/or protein [35, 50] are present in platelets from some septic patients, and the effects of variables such as drugs and antithrombotics, specific pathogens, levels of endogenous host factors that can induce splicing, and changes in megakaryocyte function in response to inflammatory stimuli. Whether more severe sepsis induces greater amounts of mature TF mRNA and its corresponding protein is also not known. Additional quantitative studies of TF transcript profiles in platelets from patients with sepsis, post-surgical deep vein thrombosis, and acute lung injury have been initiated to begin to address these issues (Rondina MT, Schwertz H, Weyrich AS, et al, unpublished observations). Why mature TF mRNA and evidence for splicing was not detected in platelets from all subjects with sepsis – if platelet activation is ubiquitous in this condition [1, 4, 9, 10] – is not yet clear. One explanation may be the presence of endogenous splicing inhibitors [15] that modulate the process in the cells of some subjects with sepsis. Another may be that pre-mRNA splicing is far more prevalent in platelets sequestered in the local milieu of peripheral vascular beds [10] than in those we retrieved from the systemic circulation.
Although largely unexplored, recent reports indicate that mRNA expression patterns differ in circulating platelets from healthy subjects and those with disease [32, 51–55]. Thus, it is possible that expression of mature TF mRNA, alone or in combination with alterations in expression of other transcripts that undergo post-transcriptional processing in activated platelets [13, 15–18], may be a signature of sepsis and other inflammatory and prothrombotic disorders in some patients.
Supplementary Material
Acknowledgments
Sources of Support: Work in this report was supported by grants from the N.I.H. (HL066277, HL091754, HL092746, HL044525, HL048872, HL090870, and K231440921); public health service grants ULI-RRO25764 and CO6-RR11234 from the National Center for Research Resources; a Deutsche Forschlungsgemenschaft (DFG) grant (SCHW 1167/1-2); and Western States Affiliate American Heart Association awards 0625098Y and 09BGIA2250381.
The contributions of Diana Lim in preparation of the figures; Jenny Pierce, Sharla Watts, and Sharren Brewer in preparation of the manuscript; Maria Hamilton and Neal Tolley in sample analysis; Kristina Schwertz in patient data acquisition; and Molly Leecaster in statistical analyses and support were invaluable. The authors appreciate the help of Cathy Petti, M.D., in studies of bacterial activation of platelets and analysis of culture data from patient records.
Abbreviations
- pre-mRNA
precursor mRNA
- mRNA
messenger RNA
- TF
tissue factor pre-mRNA or mRNA transcript
- TF
tissue factor protein or procoagulant activity
- ALI
Acute Lung Injury
- ICU
Intensive Care Unit
- LPS
lipopolysaccharide
- MSSA
Methicillin-sensitive S. aureus
- PAF
platelet-activating factor
- APACHE II
Acute Physiology and Chronic Health Evaluation II
- WBC
White Blood Cells
- MICU
Medical Intensive Care Unit
- LOS
Length of Stay
ADDENDUM
Author contributions: M.T.R. – Corresponding author; patient identification, sample collection and analysis, overall data analysis, manuscript drafting. H.S. – Performed central in vitro experiments, overall data analysis, manuscript drafting. E.S.H. – Patient identification, sample collection and analysis, overall data analysis. B.F.K. – Performed key experiments. R.A.C. – Performed key experiments. N.M. – Interpretation and analysis of key experiments, overall data analysis. C.K.G. – Patient identification, sample collection, overall data analysis. A. S. W. – Co-directed all aspects of the study including in vitro experiments, analysis of clinical samples; manuscript preparation. G. A. Z. – Corresponding author; co-directed all aspects of the study; manuscript drafting.
Footnotes
This article has supporting online data, which is accessible from this issue’s table of content online.
References
- 1.Aird WC. The hematologic system as a marker of organ dysfunction in sepsis. Mayo Clin Proc. 2003;78:869–81. doi: 10.4065/78.7.869. [DOI] [PubMed] [Google Scholar]
- 2.Cohen J, Guyatt G, Bernard GR, Calandra T, Cook D, Elbourne D, Marshall J, Nunn A, Opal S. New strategies for clinical trials in patients with sepsis and septic shock. Crit Care Med. 2001;29:880–6. doi: 10.1097/00003246-200104000-00039. [DOI] [PubMed] [Google Scholar]
- 3.Levi M. Platelets at a crossroad of pathogenic pathways in sepsis. J Thromb Haemost. 2004;2:2094–5. doi: 10.1111/j.1538-7836.2004.01004.x. [DOI] [PubMed] [Google Scholar]
- 4.Schwertz H, Weyrich AS, Zimmerman GA. Cellular interactions of platelets, leukocytes, and endothelium in systemic inflammatory responses and sepsis. In: Castro-Faria-Neto HC, David CM, editors. Sepsis: From Bench to Bedside. Rio de Janeiro: Revinter Press; 2007. pp. 107–20. [Google Scholar]
- 5.Stephan F, Hollande J, Richard O, Cheffi A, Maier-Redelsperger M, Flahault A. Thrombocytopenia in a surgical ICU. Chest. 1999;115:1363–70. doi: 10.1378/chest.115.5.1363. [DOI] [PubMed] [Google Scholar]
- 6.Strauss R, Wehler M, Mehler K, Kreutzer D, Koebnick C, Hahn EG. Thrombocytopenia in patients in the medical intensive care unit: bleeding prevalence, transfusion requirements, and outcome. Crit Care Med. 2002;30:1765–71. doi: 10.1097/00003246-200208000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Vanderschueren S, De Weerdt A, Malbrain M, Vankersschaever D, Frans E, Wilmer A, Bobbaers H. Thrombocytopenia and prognosis in intensive care. Crit Care Med. 2000;28:1871–6. doi: 10.1097/00003246-200006000-00031. [DOI] [PubMed] [Google Scholar]
- 8.Vincent JL, Yagushi A, Pradier O. Platelet function in sepsis. Crit Care Med. 2002;30:S313–7. doi: 10.1097/00003246-200205001-00022. [DOI] [PubMed] [Google Scholar]
- 9.Warkentin TE, Aird WC, Rand JH. Platelet-endothelial interactions: sepsis, HIT, and antiphospholipid syndrome. Hematology Am Soc Hematol Educ Program. 2003:497–519. doi: 10.1182/asheducation-2003.1.497. [DOI] [PubMed] [Google Scholar]
- 10.Harris EH, Rondina MT, Schwertz H, Weyrich AS, Zimmerman GA. Pathogenesis of sepsis and sepsis-induced acute lung injury. In: Choi A, editor. Acute Respiratory Distress Syndrome. 2. 2010. pp. 369–419. [Google Scholar]
- 11.Freedman JE. Molecular regulation of platelet-dependent thrombosis. Circulation. 2005;112:2725–34. doi: 10.1161/CIRCULATIONAHA.104.494468. [DOI] [PubMed] [Google Scholar]
- 12.Weyrich AS, Zimmerman GA. Platelets: signaling cells in the immune continuum. Trends Immunol. 2004;25:489–95. doi: 10.1016/j.it.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Zimmerman GA, Weyrich AS. Signal-dependent protein synthesis by activated platelets: new pathways to altered phenotype and function. Arterioscler Thromb Vasc Biol. 2008;28:s17–24. doi: 10.1161/ATVBAHA.107.160218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weyrich AS, Denis MM, Schwertz H, Tolley ND, Foulks J, Spencer E, Kraiss LW, Albertine KH, McIntyre TM, Zimmerman GA. mTOR-dependent synthesis of Bcl-3 controls the retraction of fibrin clots by activated human platelets. Blood. 2007;109:1975–83. doi: 10.1182/blood-2006-08-042192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denis MM, Tolley ND, Bunting M, Schwertz H, Jiang H, Lindemann S, Yost CC, Rubner FJ, Albertine KH, Swoboda KJ, Fratto CM, Tolley E, Kraiss LW, McIntyre TM, Zimmerman GA, Weyrich AS. Escaping the nuclear confines: signal-dependent pre-mRNA splicing in anucleate platelets. Cell. 2005;122:379–91. doi: 10.1016/j.cell.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindemann S, McIntyre TM, Prescott SM, Zimmerman GA, Weyrich AS. Platelet signal-dependent protein synthesis. In: Quinn M, Fitzgerald D, editors. Platelet Function: Assessment, Diagnosis, and Treatment. Totowa: The Humana Press, Inc; 2005. pp. 151–76. [Google Scholar]
- 17.Lindemann S, Tolley ND, Dixon DA, McIntyre TM, Prescott SM, Zimmerman GA, Weyrich AS. Activated platelets mediate inflammatory signaling by regulated interleukin 1beta synthesis. J Cell Biol. 2001;154:485–90. doi: 10.1083/jcb.200105058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwertz H, Tolley ND, Foulks JM, Denis MM, Risenmay BW, Buerke M, Tilley RE, Rondina MT, Harris EM, Kraiss LW, Mackman N, Zimmerman GA, Weyrich AS. Signal-dependent splicing of tissue factor pre-mRNA modulates the thrombogenicity of human platelets. J Exp Med. 2006;203:2433–40. doi: 10.1084/jem.20061302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mackman N, Tilley RE, Key NS. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arterioscler Thromb Vasc Biol. 2007;27:1687–93. doi: 10.1161/ATVBAHA.107.141911. [DOI] [PubMed] [Google Scholar]
- 20.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–91. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 21.Gando S, Nanzaki S, Sasaki S, Kemmotsu O. Significant correlations between tissue factor and thrombin markers in trauma and septic patients with disseminated intravascular coagulation. Thromb Haemost. 1998;79:1111–5. [PubMed] [Google Scholar]
- 22.Opal SM, Esmon CT. Bench-to-bedside review: functional relationships between coagulation and the innate immune response and their respective roles in the pathogenesis of sepsis. Crit Care. 2003;7:23–38. doi: 10.1186/cc1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pawlinski R, Mackman N. Tissue factor, coagulation proteases, and protease-activated receptors in endotoxemia and sepsis. Crit Care Med. 2004;32:S293–7. doi: 10.1097/01.ccm.0000128445.95144.b8. [DOI] [PubMed] [Google Scholar]
- 24.Zeerleder S, Schroeder V, Hack CE, Kohler HP, Wuillemin WA. TAFI and PAI-1 levels in human sepsis. Thromb Res. 2006;118:205–12. doi: 10.1016/j.thromres.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 25.Bhakdi S, Tranum-Jensen J. Alpha-toxin of Staphylococcus aureus. Microbiol Rev. 1991;55:733–51. doi: 10.1128/mr.55.4.733-751.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Munford RS. Severe sepsis and septic shock: the role of gram-negative bacteremia. Annu Rev Pathol. 2006;1:467–96. doi: 10.1146/annurev.pathol.1.110304.100200. [DOI] [PubMed] [Google Scholar]
- 27.Kawamoto T, Ii M, Kitazaki T, Iizawa Y, Kimura H. TAK-242 selectively suppresses Toll-like receptor 4-signaling mediated by the intracellular domain. Eur J Pharmacol. 2008;584:40–8. doi: 10.1016/j.ejphar.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 28.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 29.Shashkin PN, Brown GT, Ghosh A, Marathe GK, McIntyre TM. Lipopolysaccharide is a direct agonist for platelet RNA splicing. J Immunol. 2008;181:3495–502. doi: 10.4049/jimmunol.181.5.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–6. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 31.Bozza FA, Shah AM, Weyrich AS, Zimmerman GA. Amicus or adversary: platelets in lung biology, acute injury, and inflammation. Am J Respir Cell Mol Biol. 2009;40:123–34. doi: 10.1165/rcmb.2008-0241TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freishtat RJ, Natale J, Benton AS, Cohen J, Sharron M, Wiles AA, Ngor WM, Mojgani B, Bradbury M, Degnan A, Sachdeva R, Debiase LM, Ghimbovschi S, Chow M, Bunag C, Kristosturyan E, Hoffman EP. Sepsis alters the megakaryocyte-platelet transcriptional axis resulting in granzyme B-mediated lymphotoxicity. Am J Respir Crit Care Med. 2009;179:467–73. doi: 10.1164/rccm.200807-1085OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meshorer E, Misteli T. Splicing misplaced. Cell. 2005;122:317–8. doi: 10.1016/j.cell.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 34.Bach RR. Tissue factor encryption. Arterioscler Thromb Vasc Biol. 2006;26:456–61. doi: 10.1161/01.ATV.0000202656.53964.04. [DOI] [PubMed] [Google Scholar]
- 35.Panes O, Matus V, Saez CG, Quiroga T, Pereira J, Mezzano D. Human platelets synthesize and express functional tissue factor. Blood. 2007;109:5242–50. doi: 10.1182/blood-2006-06-030619. [DOI] [PubMed] [Google Scholar]
- 36.Qian K, Xie F, Gibson AW, Edberg JC, Kimberly RP, Wu J. Functional expression of IgA receptor FcalphaRI on human platelets. J Leukoc Biol. 2008;84:1492–500. doi: 10.1189/jlb.0508327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gerrits AJ, Koekman CA, van Haeften TW, Akkerman JW. Platelet tissue factor synthesis in type 2 diabetic patients is resistant to inhibition by insulin. Diabetes. 2010;59:1487–95. doi: 10.2337/db09-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Semple JW, Freedman J. Platelets and innate immunity. Cell Mol Life Sci. 2010;67:499–511. doi: 10.1007/s00018-009-0205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cognasse F, Hamzeh-Cognasse H, Lafarge S, Delezay O, Pozzetto B, McNicol A, Garraud O. Toll-like receptor 4 ligand can differentially modulate the release of cytokines by human platelets. Br J Haematol. 2008;141:84–91. doi: 10.1111/j.1365-2141.2008.06999.x. [DOI] [PubMed] [Google Scholar]
- 40.Aslam R, Speck ER, Kim M, Crow AR, Bang KW, Nestel FP, Ni H, Lazarus AH, Freedman J, Semple JW. Platelet Toll-like receptor expression modulates lipopolysaccharide-induced thrombocytopenia and tumor necrosis factor-alpha production in vivo. Blood. 2006;107:637–41. doi: 10.1182/blood-2005-06-2202. [DOI] [PubMed] [Google Scholar]
- 41.Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair GD, Keys EM, Allen-Vercoe E, Devinney R, Doig CJ, Green FH, Kubes P. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–9. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 42.Zhang G, Han J, Welch EJ, Ye RD, Voyno-Yasenetskaya TA, Malik AB, Du X, Li Z. Lipopolysaccharide stimulates platelet secretion and potentiates platelet aggregation via TLR4/MyD88 and the cGMP-dependent protein kinase pathway. J Immunol. 2009;182:7997–8004. doi: 10.4049/jimmunol.0802884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ward JR, Bingle L, Judge HM, Brown SB, Storey RF, Whyte MK, Dower SK, Buttle DJ, Sabroe I. Agonists of toll-like receptor (TLR)2 and TLR4 are unable to modulate platelet activation by adenosine diphosphate and platelet activating factor. Thromb Haemost. 2005;94:831–8. [PubMed] [Google Scholar]
- 44.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 45.Mackman N. Role of tissue factor in hemostasis and thrombosis. Blood Cells Mol Dis. 2006;36:104–7. doi: 10.1016/j.bcmd.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 46.Pawlinski R, Wang JG, Owens AP, 3rd, Williams J, Antoniak S, Tencati M, Luther T, Rowley JW, Low EN, Weyrich AS, Mackman N. Hematopoietic and nonhematopoietic cell tissue factor activates the coagulation cascade in endotoxemic mice. Blood. 2010;116:806–14. doi: 10.1182/blood-2009-12-259267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–8. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 48.Tsakiris DA, Scudder L, Hodivala-Dilke K, Hynes RO, Coller BS. Hemostasis in the mouse (Mus musculus): a review. Thromb Haemost. 1999;81:177–88. [PubMed] [Google Scholar]
- 49.Brambilla M, Camera M, Colnago D, Marenzi G, De Metrio M, Giesen PL, Balduini A, Veglia F, Gertow K, Biglioli P, Tremoli E. Tissue factor in patients with acute coronary syndromes: expression in platelets, leukocytes, and platelet-leukocyte aggregates. Arterioscler Thromb Vasc Biol. 2008;28:947–53. doi: 10.1161/ATVBAHA.107.161471. [DOI] [PubMed] [Google Scholar]
- 50.Mezzano D, Matus V, Saez CG, Pereira J, Panes O. Tissue factor storage, synthesis and function in normal and activated human platelets. Thromb Res. 2008;122 (Suppl 1):S31–6. doi: 10.1016/S0049-3848(08)70016-1. [DOI] [PubMed] [Google Scholar]
- 51.Healy AM, Pickard MD, Pradhan AD, Wang Y, Chen Z, Croce K, Sakuma M, Shi C, Zago AC, Garasic J, Damokosh AI, Dowie TL, Poisson L, Lillie J, Libby P, Ridker PM, Simon DI. Platelet expression profiling and clinical validation of myeloid-related protein-14 as a novel determinant of cardiovascular events. Circulation. 2006;113:2278–84. doi: 10.1161/CIRCULATIONAHA.105.607333. [DOI] [PubMed] [Google Scholar]
- 52.Raghavachari N, Xu X, Harris A, Villagra J, Logun C, Barb J, Solomon MA, Suffredini AF, Danner RL, Kato G, Munson PJ, Morris SM, Jr, Gladwin MT. Amplified expression profiling of platelet transcriptome reveals changes in arginine metabolic pathways in patients with sickle cell disease. Circulation. 2007;115:1551–62. doi: 10.1161/CIRCULATIONAHA.106.658641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun L, Gorospe JR, Hoffman EP, Rao AK. Decreased platelet expression of myosin regulatory light chain polypeptide (MYL9) and other genes with platelet dysfunction and CBFA2/RUNX1 mutation: insights from platelet expression profiling. J Thromb Haemost. 2007;5:146–54. doi: 10.1111/j.1538-7836.2006.02271.x. [DOI] [PubMed] [Google Scholar]
- 54.Gnatenko DV, Zhu W, Xu X, Samuel ET, Monaghan M, Zarrabi MH, Kim C, Dhundale A, Bahou WF. Class prediction models of thrombocytosis using genetic biomarkers. Blood. 2010;115:7–14. doi: 10.1182/blood-2009-05-224477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nagalla S, Bray PF. Platelet RNA chips dip into thrombocytosis. Blood. 2010;115:2–3. doi: 10.1182/blood-2009-10-246405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.