Summary
Inhibitory antibodies to factor VIII (fVIII inhibitors) are the most significant complication in the management of hemophilia A. The immunogenicity of fVIII may be driven in part by structural determinants within the fVIII molecule itself. Regions of non-identity between human and porcine fVIII possibly could drive differential immune responses. The goal of this study was to compare the overall antibody response and levels of antibodies to the individual fVIII domains in naïve hemophilia A mice immunized with human or porcine fVIII. Hemophilia A mice were immunized with human or porcine fVIII using a protocol that mimics human clinical use. Inhibitor and total anti-fVIII antibody titers were measured and the domain-specificity of antibodies from 1759 anti-fVIII hybridomas was determined. The overall immunogenicity of human and porcine fVIII was similar but significant differences in domain recognition were discovered. Anti-A2 and anti-C2 antibodies constituted the majority of inhibitors in both the human and porcine fVIII groups, similar to inhibitors that develop in humans. The proportions of anti-A2 or anti-C2 antibodies were not significantly different between the two groups. However, the specific inhibitory activity of anti-A2 antibodies was higher in the human fVIII group. Additionally, proportion of anti-C1 antibodies was significantly higher in the human fVIII group. In contrast, anti-A3 antibodies were more common in the porcine fVIII group. The differential immune response to human and porcine fVIII suggests that it may be possible to reduce the immunogenicity of fVIII by mutagenesis of the A2, A3 and C1 domains.
Keywords: Factor VIII, hemophilia therapy, coagulation inhibitors
Introduction
Inhibitory antibodies (inhibitors) to factor VIII (fVIII) develop in approximately 30 percent of patients with moderate or severe hemophilia A (1;2),(3;4). Inhibitor development is considered the most significant complication in the management of hemophilia A. FVIII inhibitors also occur as autoantibodies in nonhemophiliacs, producing a condition called acquired hemophilia A, which is the most common autoimmune bleeding disorder involving the coagulation system. Human fVIII inhibitors consist of a polyclonal IgG population in which IgG4 is disproportionately high relative to the normal IgG subclass distribution. Despite the different immunological settings in which they arise, most inhibitors in either hereditary or acquired hemophilia A bind the A2 and/or C2 regions within the A1-A2-B-ap-A3-C1-C2 domain sequence of fVIII (5). However, human inhibitors targeting the A1, ap, A3 and C1 domains also have been observed (5–11), suggesting a broad spectrum immune response that has not been well characterized.
Hemophilia A mice produced by targeted disruption of the fVIII gene (12) have been used as a model system to study the immunogenicity of human fVIII (13). After serial intravenous injections of human fVIII using a dose per body mass similar to that used in humans, hemophilia A mice develop high titer inhibitors (13–16). The inhibitor response in hemophilia A mice includes antibodies that recognize the immunodominant Arg484-Ile508 A2 epitope that is recognized by human hemophilia A patients (16), although the extent to which other B cell epitopes in the murine model and human hemophilia are similar is unknown. On the whole, hemophilia A mice appear to provide a reasonable model system to study the immunogenicity of fVIII and potentially provide a model system to dissect the polyclonal immune response using B cell hybridoma technology.
Porcine fVIII has been used to treat fVIII inhibitor patients whose antibodies cross-react poorly with porcine fVIII. A commercial plasma-derived porcine fVIII concentrate, Hyate:C, was used for approximately 20 years until it was withdrawn because of concerns over the presence of viruses in the porcine blood supply (17;18). Additionally, a recombinant B – domain deleted form of porcine fVIII has been evaluated in a phase II clinical trial (19). Hyate:C and recombinant B domain-deleted porcine fVIII produce inhibitory anti-porcine fVIII antibodies in hemophilia A mice that have been pre-sensitized to human fVIII (20). However, the immunogenicity of porcine fVIII has not been studied in naïve hemophilia A mice. The comparison of the immunogenicity of human and porcine fVIII potentially is important because there may be species-specific determinants of immunogenicity that are intrinsic to fVIII structure. In this study, hemophilia A mice received recombinant human or porcine B domain – deleted intravenously using body mass – adjusted doses similar to those used in humans. A domain-specific ELISA was used to assign domain specificity to anti-fVIII hybridoma antibodies (21), resulting in the characterization of the relative immunodominance of fVIII domains and domain/inhibitor associations. Significant differences in the immunogenicity of human and porcine fVIII were observed, which may have therapeutic implications in the management of hemophilia A.
Materials and Methods
Materials
Citrated human hemophilia A plasma and normal pooled human plasma (FACT) were purchased from George King Biomedical, Inc. (Overland Park, KA). Polyoxyethylene sorbitan monooleate (Tween 80) was purchased from Pierce (Rockford, IL). Goat anti-mouse IgG-alkaline phosphatase conjugate and Alkaline Phosphatase Substrate Kit containing and p-nitrophenylphosphate were purchased from Bio-Rad Laboratories (Hercules, CA). Goat anti-mouse isotype and subclass specific antibodies were purchased from Southern Biotech (Birmingham, AL). SP Fast Flow-Sepharose was purchased from GE Healthcare Life Sciences (Piscataway, NJ). Recombinant full-length human fVIII and B-domain deleted (BDD) porcine fVIII were gifts from Baxter Biosciences (Duarte, CA) and Ipsen Pharmaceuticals (Milford, MA), respectively.
Expression and purification of recombinant human, porcine and hybrid human/porcine fVIII
The cDNAs encoding BDD forms of human and porcine fVIII were prepared as described previously (22). The BDD single porcine domain (SPD) hybrid human/porcine fVIII constructs HP1, HP46 and HP20 and the single human domain (SHD) hybrid human/porcine fVIII constructs HP42, HP48, HP51, HP52, HP53, HP54 have been described previously (21;23;24). The SPD constructs HP58, HP71 and HP72 were prepared using previously published procedures (9). Expression of stably integrated fVIII cDNAs from baby hamster kidney–derived cells and purification of recombinant fVIII constructs was performed as described previously (22).
Immunization of hemophilia A mice with BDD human and porcine fVIII
Preparations of purified BDD human or porcine fVIII were stored in 0.4 M NaCl, 20 mM HEPES, 5 mM CaCl2, 0.01% Tween 80, pH 7.4 in small aliquots at −80 °C. Samples were diluted to 4 μg/mL in sterile normal saline immediately prior to injection. To compare plasma antibody responses to human and porcine fVIII, forty hemophilia A mice were randomized to two equal groups and were immunized as described previously (16). Mice received six tail vein injections of 10 μg/kg fVIII at 7 d intervals, followed by a final injection of 25 μg/kg fVIII two weeks after the sixth dose. Blood was collected by tail snip into 1/10 volume 3.8% trisodium citrate by terminal cardiac puncture three weeks after the final injection and plasma was prepared by centrifugation at 3000g for 15 min at 4° C. Separate groups of 7 and 8 mice were immunized with human or porcine fVIII, respectively for production of hybridomas.
Production of anti-fVIII B cell hybridomas
Three days after the last injection of fVIII, cell suspensions from individual spleens were fused with NS-1 myeloma cells and hybridomas were characterized as described previously (21). Approximately 300 anti-fVIII positive hybridomas were identified per spleen in the initial screen. A maximum of 192 positive hybridomas from each spleen was expanded, re-screened for anti-fVIII antibodies, and the resulting positives were subjected to domain mapping, Ig isotype/subclass determination, and fVIII inhibitor assays.
Purification of anti-fVIII MAbs
Anti-fVIII hybridomas were cloned by limiting dilution, expanded and secreted anti-fVIII MAbs were purified by SP-Sepharose chromatography as described previously (21). Antibodies were judged to be greater than 95% pure by SDS-PAGE analysis. IgG concentrations were estimated using an extinction coefficient at 280 nm of 1.4 (mg/mL)−1cm−1. Yields of purified IgG ranged from 0.4 to 4 mg per 50 mL of culture medium.
Bethesda assay for inhibitory anti-fVIII antibodies
FVIII inhibitor titers were measured by a modifications (20;21) of the Bethesda assay (25) in which human hemophilia A plasma was reconstituted with BDD human or BDD porcine fVIII to a final concentration of 0.8 – 1.2 units per mL. Residual fVIII activity was determined and compared to the control incubation, which was defined as 100% residual activity. One Bethesda unit (BU) per mL is defined as the dilution of inhibitor that produces 50% inhibition of fVIII activity using the published reference curve for values between 25% and 75% residual activity (25). The reported values represent the means of at least two separate 2 h, 37 °C incubations.
ELISA for anti-fVIII antibodies
Anti-fVIII antibodies were measured by direct ELISA using plates coated with human or porcine fVIII as described previously (26). Purified MAbs (2 μg/mL), dilutions of plasma, or undiluted hybridoma supernatants were incubated in wells for 1 h. After washing, bound antibodies were detected using alkaline phosphatase – conjugated goat anti-mouse IgG as the secondary antibody and p-nitrophenylphosphate as the chromogenic substrate. Plasma ELISA titers were determined by nonlinear least-squares fits of plots of A405 versus the reciprocal of the sample dilution to the four-parameter logistic equation. The ELISA titer was defined as the reciprocal of the plasma dilution that produced an A405 of 0.22 above the baseline on the fitted curve.
Anti-fVIII antibody isotype/subclass and domain-specificity
Anti-fVIII Ig isotype/subclass determinations were done by direct ELISA using goat anti-mouse isotype-specific and subclass-specific alkaline phosphatase conjugated antibodies as described previously (21). Domain-specific anti-human fVIII antibody mapping was carried out by direct ELISA on undiluted hybridoma supernatants in half-area 96-well plates using purified SHD hybrid human/porcine fVIII as described previously (21). Domain-specific anti-porcine fVIII antibody mapping was carried out similarly using purified SPD hybrid human/porcine fVIII.
Statistical analysis
Differences in the mean proportions of domain-specific hybridomas for groups of mice immunized with human or porcine fVIII were analyzed using a two-tailed Student’s t test. A P value of less than 0.05 was considered statistically significant.
Results
Comparative immunogenicity of BDD human and porcine fVIII in hemophilia A mice
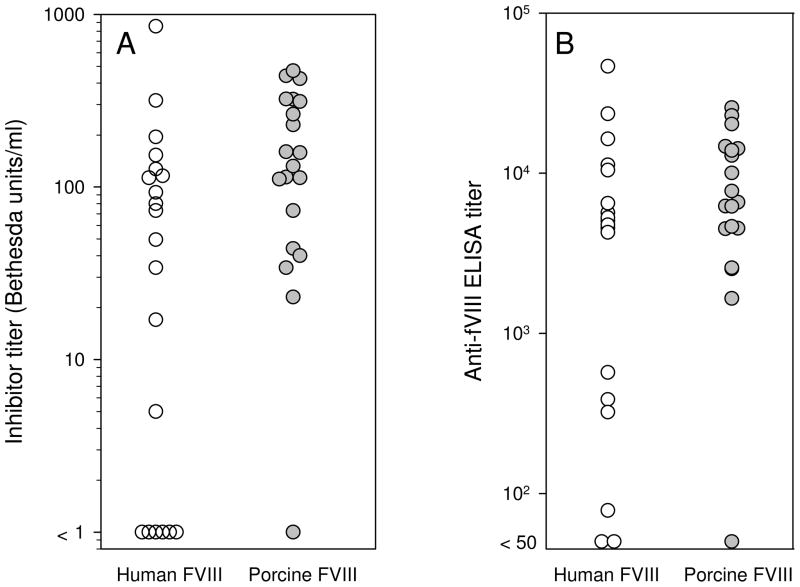
Hemophilia A mice were immunized with human or porcine fVIII (25 per group) using a dose per body mass and dosing frequency similar to that used in humans. The antibody response was measured by Bethesda assay and by ELISA using the isologous antigen (i.e., human or porcine fVIII depending on the immunogen). Porcine fVIII produced a strong immune response, with inhibitor titers and ELISA titers in most mice exceeding 100 Bethesda units per ml and 103, respectively (Fig. 1). This response was similar to the response to human fVIII (Fig. 1), which previously has been shown to produce high-titer antibodies in this model (13;14;16;21).
Figure 1. Anti-fVIII antibody response in hemophilia A mice immunized with BDD human fVIII or BDD porcine fVIII.
E16 hemophilia A mice were randomized to receive six weekly tail vein injections of 10 μg/kg BDD human fVIII (n = 20) or BDD porcine fVIII (n = 20), followed two weeks later by a final injection of 25 μg/kg. Plasma was obtained 14 d after the final injection and analyzed by Bethesda assay (A) and ELISA (B) against the isologous antigen (see “Materials and Methods”).
Domain-specific ELISA for anti-human fVIII and anti-porcine fVIII antibodies
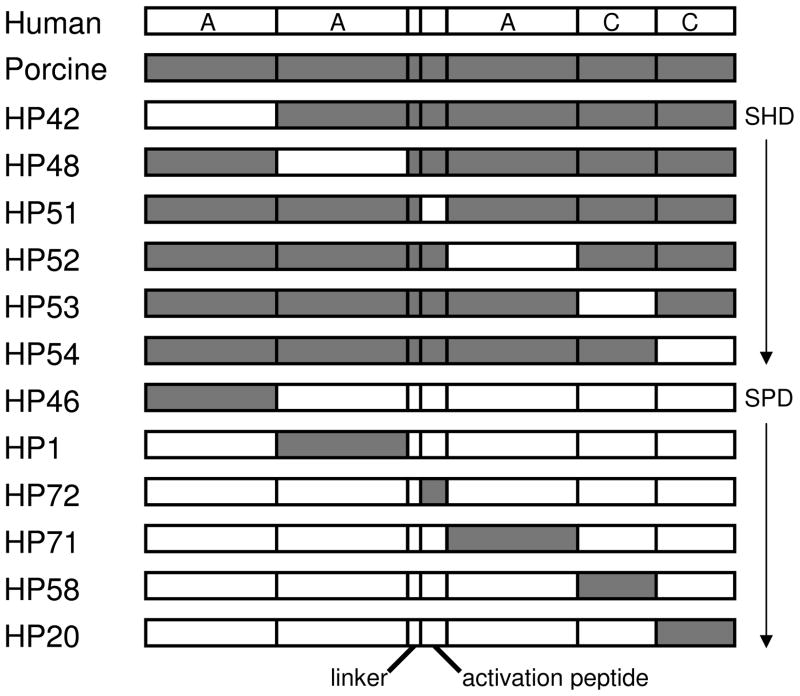
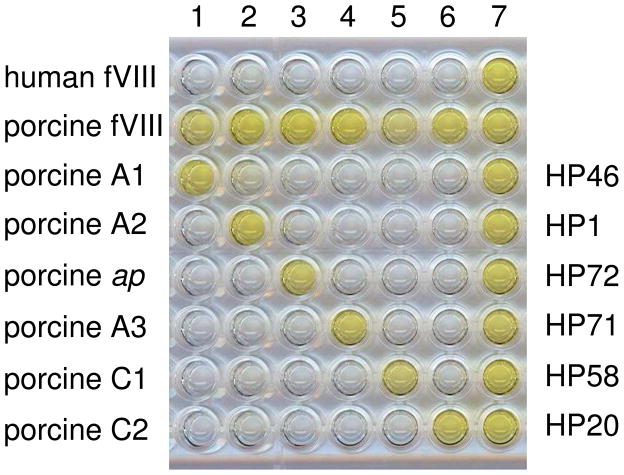
Active, recombinant BDD hybrid human/porcine fVIII molecules containing single human or porcine A1, A2, ap, A3, C1 or C2 domains (Fig. 2) were expressed in BHK-M cells and purified. The SHD or SPD hybrid fVIII molecules were coated onto microtiter plates to create a domain-specific ELISA. Results using SPD hybrids and anti-porcine fVIII MAbs are shown in Fig. 3 to illustrate the method. Clear domain-specific signals are present except for the rightmost column, which contains a MAb developed against porcine fVIII that cross-reacts with human fVIII, and thus cannot be interpreted.
Figure 2. Hybrid human/porcine fVIII molecules.
BDD fVIII molecules containing human (white) or porcine (gray) regions as single human domains or single porcine domains are shown. The linker region represents a short peptide containing a PACE/furin recognition sequence. FVIII domain boundaries are defined using human fVIII amino acid sequence numbering as follows: residues 1-372 (A1), 373-740 (A2), 1649-1689 (light chain activation peptide, ap), 1690-2019 (A3), 2020-2172 (C1) and 2173-2332 (C2) (36).
Figure 3. Domain-specificity of anti-porcine fVIII MAbs.
Purified domain-specific anti-porcine fVIII MAbs were used as primary antibodies in a ELISA using plates coated with human fVIII, porcine fVIII, or SPD hybrid fVIII molecules. MAbs 4-3, 4-39, 4-23, 4-5, 4-1 or 4-63, which bind to the A1, A2, ap, A3, C1 or C2 domains, respectively, were added to wells in columns 1 through 6, respectively. Anti-porcine fVIII MAb 4-17, which cross-reacts with human fVIII, was added to wells in column 7. MAb binding was detected using goat anti-mouse IgG-alkaline phosphatase conjugate as secondary antibody and p-nitrophenylphosphate as substrate (see “Materials and Methods”).
Domain specificity of anti-fVIII B cell hybridomas
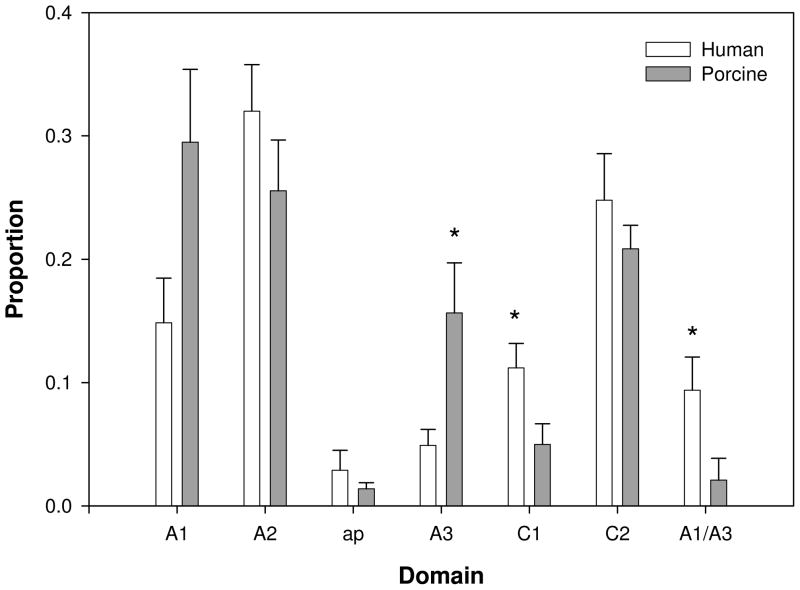
B cell hybridomas were produced from spleens obtained from 7 mice immunized with human fVIII and 8 mice immunized with porcine fVIII. The robust immune responses to human and porcine fVIII resulted in the production of an average of nearly 300 hybridomas per spleen. An average of 126 and 110 anti-human fVIII and anti-porcine fVIII hybridomas, respectively, were subjected to domain mapping. Domain specificity was assigned to approximately 70% of the hybridomas. The remaining hybridomas were not evaluable largely due to cross-reactivity. Figure 4 shows the distribution of antibodies to the different domains of human and porcine fVIII.
Figure 4. Domain-specificity of anti-fVIII antibodies.
Supernatants from B cell hybridomas obtained from hemophilia A mice immunized with human fVIII (open bars, N = 7) or porcine fVIII (gray bars, N = 8) were analyzed by domain-specific anti-fVIII ELISA (see “Materials and Methods”). Shown are the relative proportions of assignable domain specificities and the standard errors of the mean proportions. Asterisks represent statistically significant differences between groups.
Anti-C1 antibodies were significantly more frequent in the human fVIII group (p = 0.03). In contrast, anti-A3 antibodies were significantly more frequent in the porcine fVIII group (p = 0.03). Additionally, there was trend toward significantly more frequent anti-porcine fVIII A1 antibodies (p = 0.06). Anti-porcine fVIII antibodies with dual specificity for the A1 and A3 domains were identified, similar to results reported previously for hemophilia A mice immunized with human fVIII (21). However, anti-porcine A1/A3 antibodies were significantly less frequent than anti-human A1/A3 antibodies (p = 0.04). There was no significant difference between the human fVIII and porcine fVIII groups in the proportion of anti-A2 or anti-C2 antibodies. Anti-ap antibodies were not significantly different between the groups, although the statistical power was low due to the small number of positive samples.
Ig isotype/subclass determinations were made on the anti-human fVIII and anti-porcine fVIII hybridomas. IgG1 and IgG2a antibodies dominated the immune response, each representing 40 – 45 % of the total antibodies in both the human and porcine fVIII groups (data not shown), consistent with previous studies in the murine hemophilia A model (15;27–29). This pattern is characteristic of a combined TH1 and TH2 response ((30), review). There was no significant association between domain specificity and Ig subclass in mice immunized with human or porcine fVIII, indicating that helper T cell responses are not strongly polarized with respect to domain specificity. As is typical of murine (and human) immune responses, κ light chain antibodies were dominant, averaging 98% and 95% of antibodies in the human and porcine fVIII groups, respectively.
Inhibitory activity of domain-specific anti-fVIII antibodies
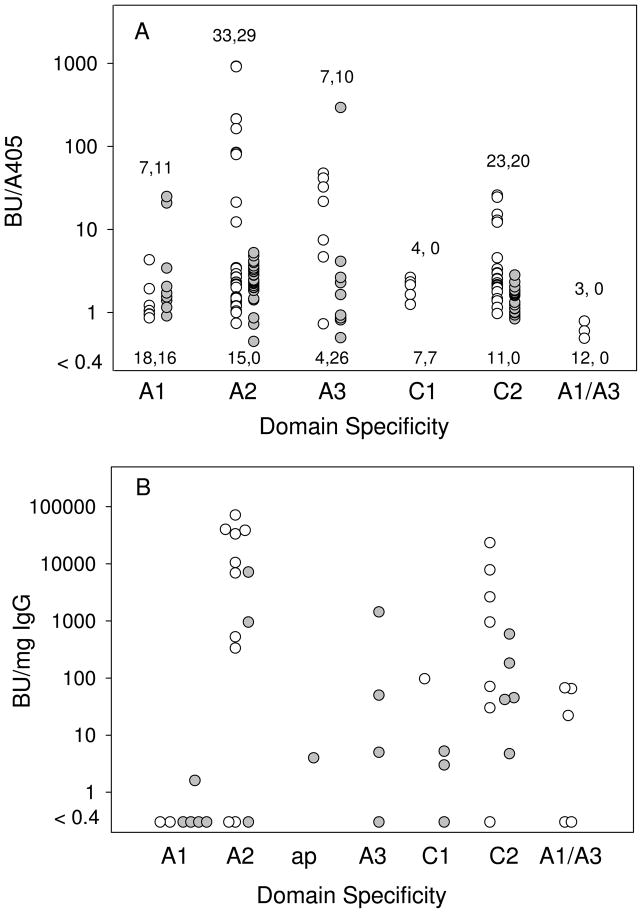
Hybridoma supernatants were analyzed for inhibitory activity using the Bethesda assay. The relationship between specific activity, BU/ELISA A405, which provides a measure of inhibitory activity relative to the amount of bound antibody, and domain specificity, is shown in Fig. 5A. Inhibitory activity was detected in 77/144 (53%) and 70/119 (59%) of anti-human fVIII and anti-porcine fVIII antibodies, respectively. Most of the anti-A2 and anti-C2 antibodies were inhibitory. However, there were several anti-human A2 antibodies with no detectable inhibitory activity, suggesting the presence of at least one additional human fVIII A2 epitope in addition to previously described inhibitory Arg484-Ile508 epitope (21;31). Higher anti-A2 specific activities were seen in the human fVIII group than the porcine fVIII group. Inhibitory anti-A1 and anti-A3 antibodies were identified in both groups. Inhibitory anti-C1 antibodies were identified only in the human fVIII group. Most, but not all, of the anti-A1/A3 antibodies had no detectable inhibitory activity. Overall, the inhibitor response in both groups was dominated by anti-A2 and anti-C2 antibodies, which accounted for 56/77 (73%) and 49/70 (70%) of the detectable inhibitory antibodies in the human and porcine fVIII groups, respectively.
Figure 5. Inhibitory activity of anti-fVIII antibodies.
A. Supernatants from anti-human fVIII hybridomas (open circles) and anti-porcine fVIII hybridomas (gray circles) were analyzed for total anti-fVIII antibodies by ELISA and for inhibitory anti-fVIII antibodies by Bethesda assay (see “Materials and Methods”). The ordinate axis represents the ratio of the inhibitory activity (BU/ml) and ELISA reading (A405/ml). Numbers below the data points represent the numbers of hybridomas with no detectable inhibitory activity (BU/A405 < 0.4). Numbers above the data points represent the numbers of hybridomas with detectable inhibitory activity. B. Specific inhibitory activities of purified anti-human (open circles) and anti-porcine (gray circles) fVIII MAbs were determined using the Bethesda assay and the concentration of purified antibody.
The analysis of inhibitory activity was extended by purifying 25 anti-human fVIII MAbs and 21 anti-porcine fVIII MAbs (Fig. 5B). The higher proportion of MAbs with detectable inhibitory activity compared to the hybridoma supernatants was due to the higher available concentrations of purified MAbs, which increases assay sensitivity. Inhibitory activity was detected in 72% and 67% of anti-human and anti-porcine MAbs, respectively. Consistent with the hybridoma supernatant results, most of the inhibitory MAbs had anti-A2 or anti-C2 specificities. Inhibitory antibodies with anti-A1 or anti-A2 specificity were less frequent in the MAb sample than in the hybridoma supernatant sample, possibly due to the small sample number in the former group.
Discussion
Although intravenous injections of human and porcine fVIII produce similar inhibitor titers and total anti-fVIII antibodies in naïve hemophilia mice (Fig. 1), they differ significantly with respect to the immunogenicity at the domain level. The higher immune response to the human C1 domain (Fig. 4) suggests that substitution of the porcine C1 into human fVIII might produce a less immunogenic fVIII molecule. Additionally, although the overall immune response to the human and porcine fVIII A2 domains is similar (Fig. 4), further epitope mapping at the subdomain level may reveal differences that can be exploited therapeutically. This is suggested by the observation that anti-human A2 antibodies have greater inhibitory activity than anti-porcine A2 antibodies (Fig. 5A), which indicates that there are two or more A2 epitopes that are detected by the domain-specific ELISA. Consistent with this, we recently identified a human A2 inhibitor epitope that is distinct from the well-known A2 epitope that is bounded by residues Arg484-Ile508 (21).
The C2 domain, along with the A2 domain, represents the most immunogenic domains in human fVIII in inhibitor patients. The strong immunogenicity of the porcine C2 domain in hemophilia A mice (Figure 4) is disappointing because it indicates that simple porcine C2 domain substitution will not reduce the immunogenicity of fVIII. However, in this study we have produced a large number of anti-C2 antibodies that may reveal significant differences between human and porcine fVIII using high-resolution mapping methods. We recently used 56 anti-human C2 MAbs in a competition ELISA and site-directed C2 mutants to develop a comprehensive map of the human C2 epitope surface recognized by hemophilia A mice (32). In addition to previously described antibodies that block the phospholipid binding site of fVIII (33;34), “non-classical” antibodies were identified that inhibit the activation of fVIII by thrombin and factor Xa. These antibodies also are present in the plasmas of most human inhibitor patients (35). Similar mapping of anti-porcine C2 antibodies may reveal differences in epitope specificity that can be exploited therapeutically.
In the immunization protocol used in this and other studies (13;14;16), which mimics dose and dosing frequency of fVIII in humans, most mice develop high titer inhibitors, whereas the incidence of inhibitors in previously untreated hemophilia A patients is only ~30%. Additionally, most fVIII inhibitor patients do not develop high-titer inhibitors (greater than 10 BU/mL) (1). The fVIII inhibitor response in the murine hemophilia A model is dose-dependent in a therapeutically relevant range (20). Thus, although the reason for the robust immune response in hemophilia A mice is not known, it may simply indicate that doses used are supra-normal and expose the murine immune system to relatively higher concentrations of fVIII. The immune response in hemophilia A mice represents a strong signal that is useful for testing differences between treatment groups. A less immunogenic fVIII molecule might significantly reduce the inhibitor titer but not the incidence of inhibitors in the murine hemophilia A model, yet reduce the incidence of inhibitor development in previously untreated human hemophilia A patients.
What is known on this topic
Inhibitory antibodies to factor VIII are the most significant complication in the management of hemophilia A.
Porcine factor VIII has been used to treat inhibitor patients whose antibodies cross-react poorly with porcine factor VIII.
Although the epitope specificity of anti-human factor VIII antibodies has been extensively studied, anti-porcine factor VIII antibodies have not been characterized.
What this paper adds
A comprehensive analysis of the overall antibody response and levels of antibodies to the individual fVIII domains in naïve hemophilia A mice immunized with human or porcine fVIII was obtained.
Although the overall immunogenicity of human and porcine fVIII was similar, significant differences in domain recognition were discovered.
Acknowledgments
Supported by a grants from the National Institutes of Health (HL082609 and HL040921) (P.L.).
Footnotes
J.F.H. designed and performed research, analyzed data, and co-wrote the paper; E.T.P., R.T.B, and T.J.L. designed and performed research; W.R.C. designed research, analyzed data, and co-wrote the paper; P.L. designed research, analyzed data, and co-wrote the paper.
P.L. holds patents and has declared a financial interest in a company, Octagen, whose product was studied in the present work. W.R.C is employed by a company, Green Mountain Antibodies, whose potential product was studied in the present work.
References
- 1.Lusher JM, Arkin S, Abildgaard CF, Schwartz RS the Kogenate Previously Untreated Patient Study Group. Recombinant factor VIII for the treatment of previously untreated patients with hemophilia A. Safety, efficacy, and the development of inhibitors. N Engl J Med. 1993;328:453–459. doi: 10.1056/NEJM199302183280701. [DOI] [PubMed] [Google Scholar]
- 2.Bray GL, Gomperts ED, Courtier S, Gruppo R, Gordon EM, Manco-Johnson M, et al. A multicenter study of recombinant factor VIII (Recombinate): Safety, efficacy, and inhibitor risk in previously untreated patients with hemophilia A. Blood. 1994;83:2428–2435. [PubMed] [Google Scholar]
- 3.Lusher JM, Lee CA, Kessler CM, Bedrosian CL the Refacto Phase 3 Study Group. The safety and efficacy of B-domain deleted recombinant factor VIII concentrate in patients with severe haemophilia A. Haemophilia. 2003;9:38–49. doi: 10.1046/j.1365-2516.2003.00708.x. [DOI] [PubMed] [Google Scholar]
- 4.Kreuz W, Ettingshausen CE, Zyschka A, Oldenburg J, Saguer IM, Ehrenforth S, et al. Inhibitor development in previously untreated patients with hemophilia A: a prospective long-term follow-up comparing plasma-derived and recombinant products. Semin Thromb Hemost. 2002;28:285–290. doi: 10.1055/s-2002-32664. [DOI] [PubMed] [Google Scholar]
- 5.Prescott R, Nakai H, Saenko EL, Scharrer I, Nilsson IM, Humphries J, et al. The inhibitory antibody response is more complex in hemophilia A patients than in most nonhemophiliacs with fVIII autoantibodies. Blood. 1997;89:3663–3671. [PubMed] [Google Scholar]
- 6.Lubahn BC, Ware J, Stafford DW, Reisner HM. Identification of a F. VIII epitope recognized by a human hemophilic inhibitor. Blood. 1989;73:497–499. [PubMed] [Google Scholar]
- 7.Zhong D, Saenko EL, Shima M, Felch M, Scandella D. Some human inhibitor antibodies interfere with factor VIII binding to factor IX. Blood. 1998;92:136–142. [PubMed] [Google Scholar]
- 8.Jacquemin M, Benhida A, Peerlinck K, Desqueper B, Vander EL, Lavend’homme R, et al. A human antibody directed to the factor VIII C1 domain inhibits factor VIII cofactor activity and binding to von Willebrand factor. Blood. 2000;95:156–163. [PubMed] [Google Scholar]
- 9.Barrow RT, Healey JF, Gailani D, Scandella D, Lollar P. Reduction of the antigenicity of factor VIII toward complex inhibitory plasmas using multiply-substituted hybrid human/porcine factor VIII molecules. Blood. 2000;95:557–561. [PubMed] [Google Scholar]
- 10.Nogami K, Shima M, Giddings JC, Hosokawa K, Nagata M, Kamisue S, et al. Circulating factor VIII immune complexes in patients with type 2 acquired hemophilia A and protection from activated protein C-mediated proteolysis. Blood. 2001;97:669–677. doi: 10.1182/blood.v97.3.669. [DOI] [PubMed] [Google Scholar]
- 11.Kallas A, Pooga M, Benhida A, Jacquemin M, Saint-Remy JM. Epitope specificity of anti-FVIII antibodies during immune tolerance therapy with factor VIII preparation containing von Willebrand factor. Thromb Res. 2002;107:291–302. doi: 10.1016/s0049-3848(02)00352-3. [DOI] [PubMed] [Google Scholar]
- 12.Bi L, Lawler AM, Antonarakis SE, High KA, Gearhart JD, Kazazian HH., Jr Targeted disruption of the mouse factor VIII gene produces a model of haemophilia A. Nat Genet. 1995;10:119–121. doi: 10.1038/ng0595-119. [DOI] [PubMed] [Google Scholar]
- 13.Qian J, Borovak M, Bi L, Kazazian HH, Jr, Hoyer LW. Inhibitor antibody development and T cell response to human factor VIII in murine hemophilia A. Thromb Haemost. 1999;81:240–244. [PubMed] [Google Scholar]
- 14.Reipert BM, Ahmad RU, Turecek PL, Schwarz HP. Characterization of antibodies induced by human factor VIII in a murine knockout model of hemophilia A. Thromb Haemost. 2000;84:826–832. [PubMed] [Google Scholar]
- 15.Wu H, Reding M, Qian J, Okita DK, Parker E, Lollar P, et al. Mechanism of the immune response to human factor VIII in murine hemophilia A. Thromb Haemost. 2001;85:125–133. [PubMed] [Google Scholar]
- 16.Parker ET, Healey JF, Barrow RT, Craddock HN, Lollar P. Reduction of the inhibitory antibody response to human factor VIII in hemophilia A mice by mutagenesis of the A2 domain B cell epitope. Blood. 2004;104:704–710. doi: 10.1182/blood-2003-11-3891. [DOI] [PubMed] [Google Scholar]
- 17.Hay CR. Porcine factor VIII: past, present and future. Haematologica. 2000;85:21–24. [PubMed] [Google Scholar]
- 18.Soucie JM, Erdman DD, Evatt BL, Anderson LJ, Torok TJ, El-Jamil M, et al. Investigation of porcine parvovirus among persons with hemophilia receiving Hyate:C porcine factor VIII concentrate. Transfusion. 2000;40:708–711. doi: 10.1046/j.1537-2995.2000.40060708.x. [DOI] [PubMed] [Google Scholar]
- 19.Mahlangu J, Andreeva TA, Macfarlane D, Reding MT, Walsh C, Ritchie B, et al. Phase II open-label study evaluating hemostatic activity, pharmacokinetics and safety of recombinant porcine factor VIII (OBI-1) in hemophilia A patients with alloantibody inhibitors directed against human factor VIII. Blood. 2007;110:783a. [Google Scholar]
- 20.Parker ET, Craddock HN, Barrow RT, Lollar P. Comparative immunogenicity of recombinant B domain-deleted porcine factor VIII and Hyate:C in hemophilia A mice pre-sensitized to human factor VIII. Journal of Thrombosis and Haemostasis. 2004;2:605–611. doi: 10.1111/j.1538-7836.2004.00685.x. [DOI] [PubMed] [Google Scholar]
- 21.Healey JF, Parker ET, Barrow RT, Langley TJ, Church WR, Lollar P. The humoral response to human factor VIII in hemophilia A mice. Journal of Thrombosis & Haemostasis. 2007;5:512–517. doi: 10.1111/j.1538-7836.2007.02373.x. [DOI] [PubMed] [Google Scholar]
- 22.Doering CB, Healey JF, Parker ET, Barrow RT, Lollar P. High-level expression of recombinant porcine coagulation factor VIII. J Biol Chem. 2002;277:38345–38349. doi: 10.1074/jbc.M206959200. [DOI] [PubMed] [Google Scholar]
- 23.Healey JF, Barrow RT, Tamim HM, Lubin IM, Shima M, Scandella D, et al. Residues Glu2181-Val2243 contain a major determinant of the inhibitory epitope in the C2 domain of human factor VIII. Blood. 1998;92:3701–3709. [PubMed] [Google Scholar]
- 24.Doering CB, Healey JF, Parker ET, Barrow RT, Lollar P. Identification of porcine coagulation factor VIII domains responsible for high level expression via enhanced secretion. J Biol Chem. 2004;279:6546–6552. doi: 10.1074/jbc.M312451200. [DOI] [PubMed] [Google Scholar]
- 25.Kasper CK, Aledort LM, Counts RB, Edson JR, Fratantoni J, Green D, et al. A more uniform measurement of factor VIII inhibitors. Thromb Diath Haemorrh. 1975;34:869–872. [PubMed] [Google Scholar]
- 26.Doering CB, Parker ET, Healey JF, Craddock HN, Barrow RT, Lollar P. Expression and characterization of recombinant murine factor VIII. Thromb Haemost. 2002;88:450–458. [PubMed] [Google Scholar]
- 27.Rossi G, Sarkar J, Scandella D. Long-term induction of immune tolerance after blockade of CD40-CD40L interaction in a mouse model of hemophilia A. Blood. 2001;97:2750–2757. doi: 10.1182/blood.v97.9.2750. [DOI] [PubMed] [Google Scholar]
- 28.Reipert BM, Sasgary M, Ahmad RU, Auer W, Turecek PL, Schwarz HP. Blockade of CD40/CD40 ligand interactions prevents induction of factor VIII inhibitors in hemophilic mice but does not induce lasting immune tolerance. Thromb Haemost. 2002;86:1345–1352. [PubMed] [Google Scholar]
- 29.Sasgary M, Ahmad RU, Schwarz HP, Turecek PL, Reipert BM. Single cell analysis of factor VIII-specific T cells in hemophilic mice after treatment with human factor VIII. Thromb Haemost. 2002;87:266–272. [PubMed] [Google Scholar]
- 30.Lollar P. Pathogenic antibodies to coagulation factors. I. Factor VIII and factor IX. Journal of Thrombosis & Haemostasis. 2004;2:1082–1095. doi: 10.1111/j.1538-7836.2004.00802.x. [DOI] [PubMed] [Google Scholar]
- 31.Healey JF, Lubin IM, Nakai H, Saenko EL, Hoyer LW, Scandella D, et al. Residues 484-508 contain a major determinant of the inhibitory epitope in the A2 domain of human factor VIII. J Biol Chem. 1995;270:14505–14509. doi: 10.1074/jbc.270.24.14505. [DOI] [PubMed] [Google Scholar]
- 32.Meeks SL, Healey JF, Parker ET, Barrow RT, Lollar P. Anti-human factor VIII C2 domain antibodies in hemophilia A mice recognize a functionally complex continuous spectrum of epitopes dominated by inhibitors of factor VIII activation. Blood. 2007;110:4234–4242. doi: 10.1182/blood-2007-06-096842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arai M, Scandella D, Hoyer LW. Molecular basis of factor-VIII inhibition by human antibodies - antibodies that bind to the factor-VIII light chain prevent the interaction of factor-VIII with phospholipid. J Clin Invest. 1989;83:1978–1984. doi: 10.1172/JCI114107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spiegel PC, Jr, Jacquemin M, Saint-Remy JM, Stoddard BL, Pratt KP. Structure of the factor VIII C2 domain-immunoglobulin G4κ Fab complex: identification of an inhibitory antibody epitope on the surface of factor VIII. Blood. 2001;98:13–19. doi: 10.1182/blood.v98.1.13. [DOI] [PubMed] [Google Scholar]
- 35.Meeks SL, Healey JF, Parker ET, Barrow RT, Lollar P. Non-classical anti-C2 domain antibodies are present in patients with factor VIII inhibitors. Blood. 2008;112:1151–1153. doi: 10.1182/blood-2008-01-132639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vehar GA, Keyt B, Eaton D, Rodriguez H, O’Brien DP, Rotblat F, et al. Structure of human factor VIII. Nature. 1984;312:337–342. doi: 10.1038/312337a0. [DOI] [PubMed] [Google Scholar]