Abstract
Background
Exercise stimulates the vascular response in pathological conditions, including ischemia; however, the molecular mechanisms by which exercise improves the impaired hypoxia-induced factor (HIF)-1α–mediated response to hypoxia associated with aging are poorly understood. Here, we report that swimming training (ST) modulates the vascular response to ischemia in aged (24-month-old) mice.
Methods and Results
Aged wild-type mice (MMP-2+/+) that maintained ST (swimming 1 h/d) from day 1 after surgery were randomly assigned to 4 groups that were treated with either vehicle, LY294002, or deferoxamine for 14 days. Mice that were maintained in a sedentary condition served as controls. ST increased blood flow, capillary density, and levels of p-Akt, HIF-1α, vascular endothelial growth factor, Fit-1, and matrix metalloproteinase-2 (MMP-2) in MMP-2+/+ mice. ST also increased the numbers of circulating endothelial progenitor cells and their function associated with activation of HIF-1α. All of these effects were diminished by LY294002, an inhibitor of phosphatidylinositol 3-kinase; enhanced by deferoxamine, an HIF-1α stabilizer; and impaired by knockout of MMP-2. Finally, bone marrow transplantation confirmed that ST enhanced endothelial progenitor cell homing to ischemic sites in aged mice.
Conclusions
ST can improve neovascularization in response to hypoxia via a phosphatidylinositol 3-kinase–dependent mechanism that is mediated by the HIF-1α/vascular endothelial growth factor/MMP-2 pathway in advanced age.
Keywords: exercise, angiogenesis, physiological, phosphatidylinositol 3-kinase, hypoxia-inducible factor 1, α subunit, aging, neovascularization, physiological
Aging is associated with a decreased ability to form new blood vessels in response to ischemia, which results in higher rates of cardiovascular complications and diminished capacity for tissue regeneration.1 There is therefore considerable interest in understanding the mechanisms of angiogenesis in advanced age. Accumulating evidence suggests that the process of new blood vessel formation is associated with extracellular matrix remodeling, mainly involving the matrix metalloproteinase (MMP) family.2,3 In particular, aging reduces MMP-2 expression in vitro and in vivo.4,5 Genetic and pharmacological intervention studies have demonstrated in several animal models that MMP-2 plays an important role in angiogenesis and vasculogenesis.5,6 Recently, a few studies have shown that knee-extension exercise activates MMP expression in human skeletal muscle.7 On the basis of these findings and past reports that exercise increases coronary vascularization by promoting vascular growth and remodeling in response to stress,8 we hypothesize that the activation of MMP-2 might represent a crucial mediator by which exercise triggers protective vascular action.
Administration of bone marrow (BM)–derived or peripheral blood–derived endothelial progenitor cells (EPCs) has improved postischemic neovascularization in various experimental and clinical trials9,10; however, several recent randomized clinical trials of stem and progenitor cell treatment for ischemic diseases have been disappointing in subjects of advanced age.11 Impaired angiogenesis in advanced age might be due to an intrinsic decline in the regenerative capacity of vascular progenitors or a decline in a proregenerative niche.12 On the other hand, physical training increases circulating EPCs in patients with ischemic syndromes.13 Further work is necessary to determine whether exercise improves EPC mobilization and function in individuals of advanced age, as well as to determine the mechanisms underlying these processes.
Exercise promotes ischemic angiogenesis by increasing vascular endothelial growth factor (VEGF) in plasma or ischemic tissue in humans and animals14,15; however, angiogenic growth factors and related transcriptional factor hypoxia-induced factor-1α (HIF-1α) activity decreased in aged cell lines and animals.16,17 In the present study, we investigate the effects of swimming training (ST) on angiogenic mechanisms and HIF-1α function in a mouse model of limb ischemia at advanced age. We evaluated whether ST was able (1) to enhance HIF-1α transcriptional activity through activation of the insulin-like growth factor (IGF)-1–mediated phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway and attenuation of the prolyl hydroxylases (PHDs) degradation system, (2) to stimulate reactivation of VEGF and MMP-2 expression in ischemic tissue and BM-derived EPCs, (3) to improve EPC mobilization and homing to the vasculature, and (4) to enhance neovascularization in response to hypoxia.
Methods
An expanded Methods section is available in the online-only Data Supplement.
Mouse Model of Revascularization Without or With Exercise
Studies of wild-type (WT; MMP-2+/+; Chubu Kagaku Shizai Co., Ltd. Nagoya, Japan) and MMP-2 knockout (KO, MMP−/−, gifted by S. Itohara RIKEN Brain Science, Institute, Wako, Saitama, Japan)5 mice in a C57/BL6 background were approved by the Animal Studies Committee of Nagoya University. Male young (6 months) and aged (18 and 24 months) mice of both genotypes were subjected to unilateral hindlimb ischemic surgery and ST programs.
Statistical Analysis
Data are expressed as mean±standard error of the mean (SEM). Student t tests (for comparison between 2 groups) or 1-way ANOVA (for comparison of 3 or more groups) followed by Tukey post hoc tests were used for statistical analyses. The nonparametric Kruskal-Wallis test (Tukey-type multiple comparison) was used ANOVA for the gene expression data. Blood flow data were subjected to 2-way repeated-measures ANOVA and Bonferroni post hoc tests. The comparative incidence of limb amputation was evaluated by the χ2 test. SPSS software version 17.0 (SPSS Inc, Chicago, Ill) was used. A value of P<0.05 was considered statistically significant.
Results
Aging Reduces HIF-1α–Induced Growth Factors and Impairs Neovascularization in Response to Hypoxia
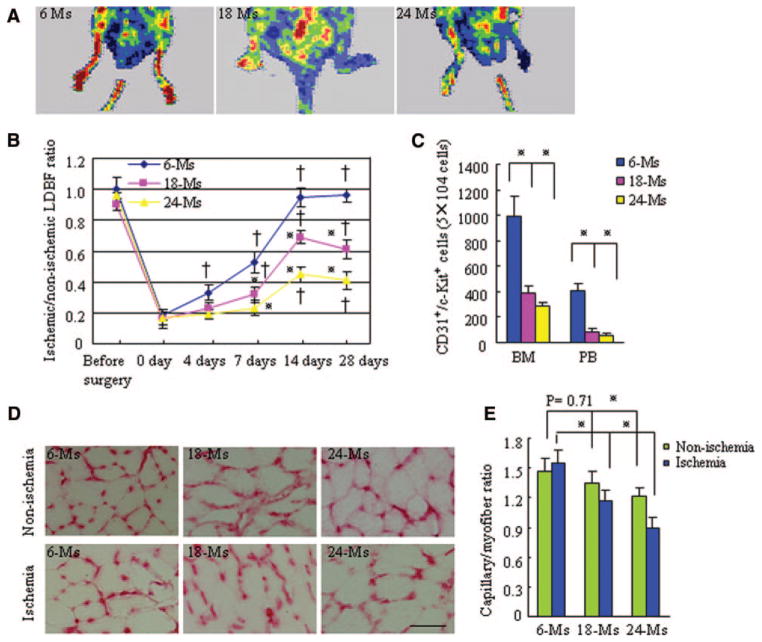
Serial laser Doppler blood flow measurements showed that aged WT mice (18 to 24 months old) had lower ratios of ischemic-to-nonischemic blood flow (Figure 1A and 1B) than young (6-month-old) WT mice. The ratio decreased further from 18 to 24 months of age. The numbers of CD31+/c-Kit+ progenitor cells in both BM and peripheral blood also decreased markedly in an age-dependent manner (Figure 1C), which suggests a vasculogenesis-specific impairment with age. The capillary density of nonischemic and ischemic muscle also correlated with age (Figure 1D and 1E).
Figure 1.
Aging reduces vessel density and blood flow in ischemic tissues of MMP-2+/+ mice. A, A low perfusion signal (dark blue) was observed in the ischemic hindlimbs of aged MMP-2+/+ mice (18 and 24 months old) with laser Doppler perfusion imaging, whereas a high signal (red) was detected in young (6-month-old) MMP-2+/+ mice. B, The ratio of ischemic-to-normal laser Doppler blood flow (LDBF) in aged MMP-2+/+ mice (n=10 per group; †P<0.05 vs each group at day 0, *P<0.05 vs the corresponding 6-month-old mice at days 7 to 28 after ischemia; 2-way repeated-measures ANOVA and Bonferroni post hoc tests). C, Quantitative analysis of the numbers of EPCs in BM and peripheral blood (PB) of WT mice (n=10 per group; *P<0.05, Tukey post hoc test). D, Immunohis-tostaining showed the capillaries in the thigh adductor muscle at postoperative day 28. Scale bar=100 αm. E, Quantitative analysis of capillary density in 3 groups of mice (n=8 per group; *P<0.05, paired Student t test). Ms indicates months.
ST Restores Ischemic Neovascularization in Mice of Advanced Age (24 Months)
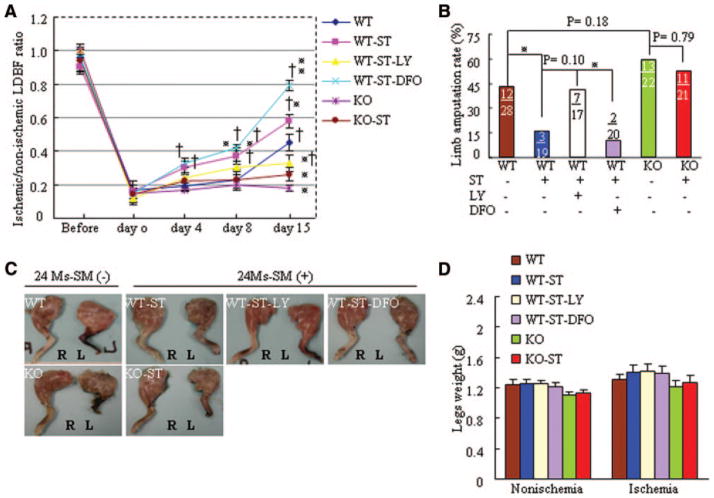
On day 15 after the induction of ischemia, aged WT mice exposed to ST (WT-ST mice) had markedly higher blood perfusion than WT mice (Figure 2A), which suggests that ST stimulated neovascularization in response to hypoxia. This was further supported by data from longer-term ST (29 days; online-only Data Supplement Figure I). ST also increased capillary density (Figure 3A). HIF-1α, which is regulated by the PI3K signaling pathway, is less stable and active in aged animals during ischemia.18,19 We hypothesized that ST protects against HIF-1α destabilization by activating the PI3K signaling pathway; the increased stability of HIF-1α would then increase ischemic neovascularization. We tested this hypothesis by treating aged WT mice with LY2940029 (LY), an inhibitor of PI3K, or deferoxamine (DFO). ST-mediated improvement of blood perfusion and capillary density were diminished by use of LY in WT-ST mice; these beneficial effects of ST treatment were enhanced by DFO (Figures 2A, 3A, and 3B).
Figure 2.
ST restores ischemic revascularization in angiogenesis-defective 24-month-old mice by postoperative day 15. A, The ratio of ischemic-to-normal laser Doppler blood flow (LDBF) in aged MMP-2+/+ mice (n=8 per group; †P<0.05 vs corresponding day 0, *P<0.05 vs corresponding MMP-2+/+ mice during ischemia; 2-way repeated-measures ANOVA and Bonferroni post hoc tests). B, Quantitative analysis of foot amputation in 6 groups (*P<0.05, χ2 test). Upper number indicates number of amputations; lower number, number of animals. C, Photographs of typical hindlimbs of the 6 groups of mice. R indicates right (nonischemic); L, left (ischemic). D, Weights of ischemic and nonischemic legs of mice (paired Student t test). Ms indicates months.
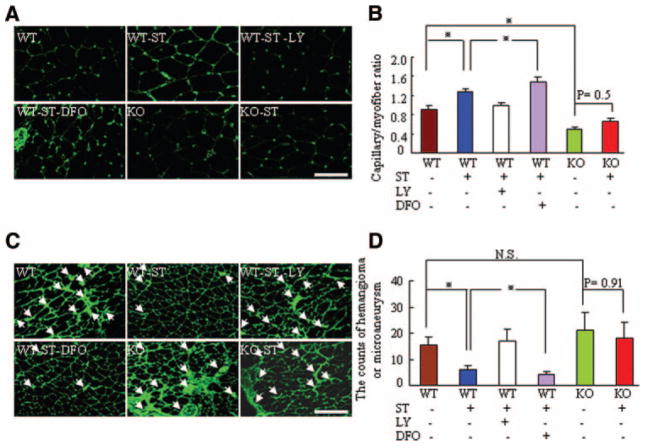
Figure 3.
ST promotes ischemic revascularization and reduces pathological vascularity in 24-month-old mice by postoperative day 15. A, Immunohistostaining showing the capillary density of ischemic muscle. Scale bar=100 μm. B, Quantitative analysis of capillary density in the 6 experimental groups. (n=6 per group; *P<0.05, Tukey post hoc test). C, Immunofluorescence of the ischemic leg vasculature. Scale bar=100 μm. D, Quantitative analysis of the numbers of aberrant vessels, such as hemangiomas or microaneurysms, in the 6 experimental groups (n=6 per group; *P<0.05, Tukey post hoc test).
Aged MMP-2−/− mice exhibited a marked impairment of blood perfusion and capillary density after hindlimb ischemia compared with MMP-2+/+ mice; these changes were not improved by ST treatment (Figures 2A, 3B, and 3B). This finding supports the notion that ST stimulates an angiogenic response via MMP-2 activation in ischemic tissue of aged animals. Furthermore, spontaneous amputation occurred in 42.9% of the ischemic limbs of WT mice and in 15.7% of ischemic limbs of WT-ST mice. The beneficial effect of ST was diminished in WT-ST-LY mice (41.2% of limbs) and was slightly enhanced in WT-ST-DFO mice (10% of limbs; Figure 2B). The rate of spontaneous amputation in KO mice (59.1%) was slightly higher than in WT mice and was not affected by ST (52.3%). At day 15 after surgery, there were no significant differences between the weights of nonischemic and ischemic legs among the 6 experimental groups of mice (Figures 2C and 2D). Additionally, DFO alone increased blood perfusion and capillary density in aged mice without ST, whereas LT alone did not have these effects (online-only Data Supplement Figure II).
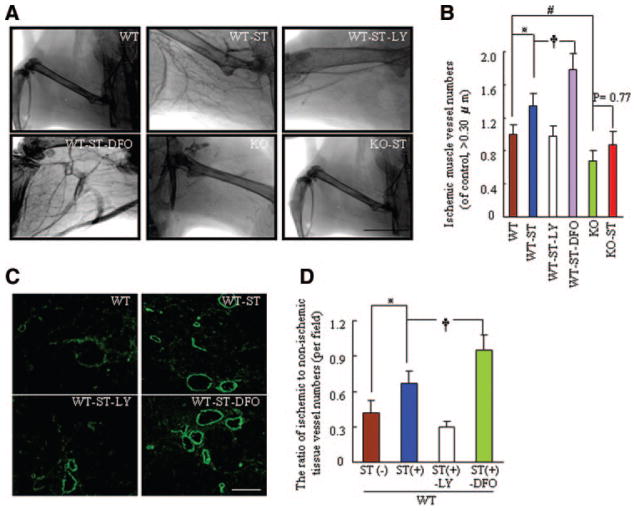
ST Prevents the Formation of Pathological Vessels and Restores Collateral Artery Formation in Response to Hypoxia in Mice at 24 Months
Abnormal corkscrewlike vessels with irregular lumens were visible in aged mice (Figure 3C). Some vessels were dilated and formed microaneurysms and hemangiomas. In contrast, fewer pathological vessels were observed in WT-ST and WT-ST-DFO mice than in WT mice; this effect was diminished in WT-ST-LY mice (Figure 3D). On postoperative day 15, the microangiography and angiographic score revealed that the ischemic hindlimbs of 24-month-old WT-ST mice had well-developed collateral arteries, and the numbers of collateral vessels (vessel diameter >0.30 μm, of non-ST control) was improved significantly by ST, resulting in an angiographic score 135% of that in WT-ST mice; this effect was attenuated to a score of 98% in ST-LY mice and enhanced to a score 178% of ST-DFO mice (Figure 4A and 4B). Similarly, fluorescent staining with α-smooth muscle actin showed that the ischemic-to-nonischemic ratio in mature neovessels was higher in WT-ST mice than in WT mice; this change was attenuated by LY and enhanced by DFO (Figures 4C and 4D). These findings suggest that ST might promote collateral vessel formation and maturation and prevent pathological vessel formation via PI3K-dependent HIF-1α activation and stabilization, which influences ischemic tissue blood perfusion. However, ST had no significant effect on the formation of collateral and pathological vessels in aged MMP-2−/− mice.
Figure 4.
ST stimulates collateral artery formation in response to hypoxia in 24-month-old MMP-2+/+ mice by postoperative day 15. A, Representative microangiography showing collateral artery formation of ischemic tissues of the 6 experimental groups. B, Quantitative analysis of microangiography showed the numbers of neovessels (>0.30 μm per field) in ischemic muscles of the 6 groups (n=4 per group; *P<0.05, †P<0.05, #P<0.05, Tukey post hoc tests). C, Fluorescence staining of ischemic tissue of the 4 indicated MMP-2+/+ experimental groups with mouse anti-human α-smooth muscle actin. D, Quantitative analysis of the ratio of neovessels in ischemic to nonischemic tissue in the 4 groups (n=6 per group; *P<0.05, †P<0.05, Tukey post hoc tests).
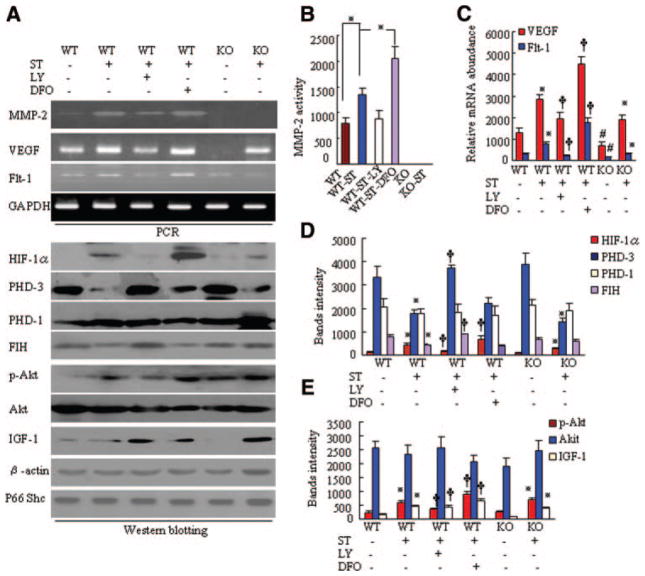
ST Enhances the Hypoxia Response Through Stimulation of the PI3K Signaling Pathway and Prevention of HIF-1α Destabilization in 24-Month-Old Mice
Western blot analysis revealed that the levels of p-Akt and HIF-1α were higher in WT-ST mice than in WT mice; these effects were diminished by LY (Figures 5A and 5E). We then evaluated whether ST prevents age-mediated HIF-1α destabilization. ST reduced the levels of PHD-3 and factor inhibiting HIF-1 (FIH) proteins from those in aged non-ST-WT mice (Figures 5A and 5D). Levels of VEGF and Flt-1 mRNAs, as well as of stromal cell–derived factor-1, were higher in WT-ST mice than in WT mice; these effects were diminished by LY and enhanced by DFO (Figure 5A and 5C; online-only Data Supplement Figure III). Similarly, the level of MMP-2 mRNA was also higher in WT-ST mice than in WT mice; this effect was diminished by LY and enhanced by DFO (Figure 5A and 5B). In addition, ST enhanced the IGF-1 protein level in ischemic muscle of aged mice of both genotypes (Figure 5A and 5E). VEGF has been shown to regulate MMP-2 expression in several cell lines.5 Taken together, these findings suggest that ST likely provides these beneficial effects on ischemic revascularization in aged mice through VEGF/Flt-1–mediated MMP-2 activation induced by stimulation of IGF-1/PI3K/Akt-dependent HIF-1α synthesis and prevention of HIF-1α destabilization. On the other hand, the level of VEGF mRNA was lower in the ischemic muscle of aged MMP-2−/− mice than in aged MMP-2+/+ mice. Immunostaining demonstrated that macrophage infiltration was impaired in the ischemic tissues of aged MMP-2−/− mice (online-only Data Supplement Figure IV). A previous study demonstrated that the reduction of VEGF in the plasma and ischemic tissues of young MMP-2−/− mice results from decreased inflammatory cell infiltration.5 On the basis of these findings, we can conclude that MMP-2−/−–mediated impairment of macrophage infiltration may also contribute to the decrease in the level of VEGF in aged mice.
Figure 5.
Expression of targeted genes in the 6 groups of 24-month-old mice on postoperative day 8. A, Representative polymerase chain reaction (PCR) and immunoblots show the levels of the targeted gene or protein in the ischemic muscles of mice in the 6 experimental groups. B, Quantitative analysis of gelatin zymography for MMP-2 gelatinolytic activity in the 6 groups (n=4 per group; *P<0.05). C, Quantitative analysis of PCR bands for levels of VEGF and Flt-1 mRNAs in the 6 groups (n=4 per group; *P<0.05, †P<0.05). D and E, Quantitative analysis of Western blots for levels of HIF-1α, p-Akt, IGF-1, PHD-3, FIH, total Akt, and PHD-1 proteins in the 6 groups (n=4 per group). Expression levels of targeted genes were normalized with a PCR or Western blot with primer or antibodies, respectively, to glyceraldehyde 3-phosphate dehydrogenase (GAPDH), β-actin, or Shc. Kruskal-Wallis tests and Tukey post hoc tests were each used for statistical analyses. *P<0.05 vs corresponding MMP-2+/+ or MMP-2−/−, †P<0.05 vs MMP-2+/+-ST, #P<0.05 vs MMP-2+/+.
To test this hypothesis, we examined the ability of ST to activate the PI3K/HIF-1α/VEGF signaling pathway in aged MMP-2−/− mice. The increase in levels of p-Akt, HIF-1α, VEGF, and Flt-1 in aged MMP-2−/−-ST mice (Figure 5A, 5C, 5D, and 5E) supports the notion that activation of the PI3K/HIF-1α/VEGF/MMP-2–dependent angiogenic pathway might represent a critical molecular mechanism by which ST triggers angiogenesis. The absence of the last step in this pathway (MMP-2) prevents the functional effects of activating this pathway. Otherwise, it appears that the ability of this pathway to be activated in KO mice actually does not support a role for the pathway. There were no significant differences in the levels of PHD-1 and total Akt proteins among the 6 experimental groups.
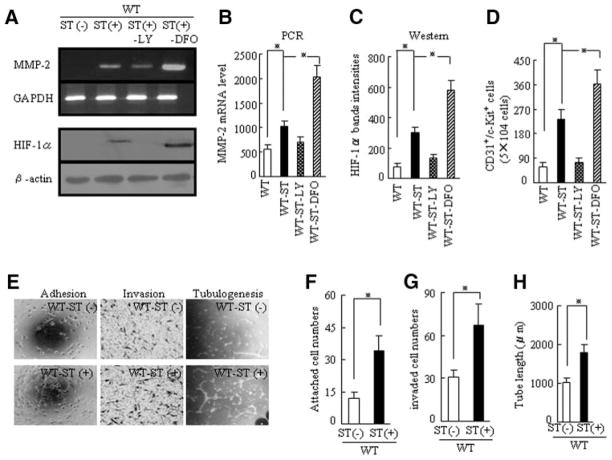
ST Stimulates EPC Mobilization and Improves the Intrinsic Function of EPCs in 24-Month-Old Mice
At day 7 after ST, the numbers of CD31+/c-Kit+ EPC-like cells were higher in WT-ST mice than in aged WT mice as measured by flow cytometry; this effect was diminished by LY and enhanced by DFO (Figure 6D). The levels of HIF-1α protein and MMP-2 mRNA in the EPCs from WT-ST mice were higher than in those from WT mice; these changes were diminished by LY and enhanced by DFO (Figure 6A through 6C). This finding led us to hypothesize that ST-mediated activation of HIF-1α and MMP-2 may facilitate the mobilization of EPC-like cells from the BM into the circulation to support revascularization in older mice. The numbers of adherent and invading EPCs were higher in WT-ST mice than in WT mice (Figure 6E and 6F). Strikingly, the tubulogenic response was also better in EPCs derived from WT-ST mice than in those derived from WT mice (Figure 6E and 6H). These findings suggest that an improvement of EPC cellular function may contribute, in part or entirely, to the restored revascularization seen with ST in advanced age.
Figure 6.
ST stimulates MMP-2 and HIF-1α expression in BM-derived EPCs and improves EPC function in the WT, WT-ST, WT-ST-LY, and WT-ST-DFO groups on postoperative day 8. A, Representative polymerase chain reaction (PCR) and Western blots showing expression of MMP-2 mRNA and HIF-1α protein in the 4 indicated groups. B–D, Quantitative analysis of levels of MMP-2 mRNA and HIF-1α protein and the numbers of CD31+/c-Kit+ cells in the 4 groups (*P<0.05, Kruskal-Wallis and Tukey post hoc tests). E, Cellular function assays showing the beneficial effects of ST on the indicated cellular events in EPCs. F–H, Quantitative analysis of the capability for adhesion, invasion, and tubulogenesis for EPCs from the 4 groups (*P<0.05, Student t test).
ST Improves Ischemic Revascularization in Aged MMP-2−/− Mice That Received MMP-2+/+ BM–Derived EPCs
We observed a high blood flow signal on laser Doppler perfusion imaging in both aged MMP-2+/+-ST and MMP-2−/−-ST mice that received EPC-like c-Kit+ cells derived from aged MMP-2+/+ BM (Figure 7A). Capillary densities were also higher in the recipient mice than in the corresponding controls (Figure 7B). Cell therapy with aged MMP-2−/− BM abolished these ST-induced angiogenic actions in aged mice (online-only Data Supplement Figure V). To examine whether BM-derived EPC-like c-Kit+ cells home to the ischemic vasculature, we transplanted BM from aged green fluorescent protein (GFP)–positive (GFP+)-MMP-2+/+ mice into MMP-2−/− mice of advanced age. Blood perfusion and numbers of c-Kit+ cells were higher in MMP-2−/−-ST mice than in non-ST MMP-2+/+ mice (Figure 7C through 7E), which further suggests that a defect in MMP-2 signaling for EPC mobilization contributes in part to the decreased ischemic revascularization seen in aging without ST. Additionally, transfection GFP gene transfection had no effect on MMP-2 mRNA and activity (online-only Data Supplement Figure VI).
Figure 7.
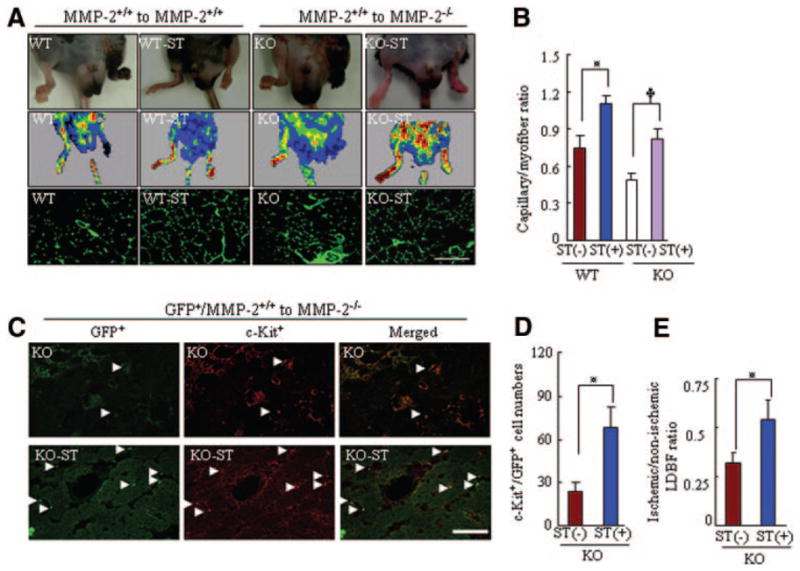
ST improves the effects of cell therapy on ischemic revascularization in both genotypes mice at age 24 months. A, Pictures of legs, laser Doppler imaging, and immunostaining showing ST-mediated protection against spontaneous amputation of ischemic feet and improvement of blood perfusion and capillary density in MMP-2+/+ and MMP-2−/− mice that received BM cells from 18-month-old MMP-2+/+ mice. B, ST also reversed the impairment of revascularization in MMP-2+/+ or MMP-2−/− mice that received cell therapy (n=5 per group; *P<0.05, 2-way ANOVA). C, Fluorescence staining with an anti–c-Kit monoclonal antibody (red) showing EPC homing into the vasculature of ischemic tissues of MMP-2−/− mice that received GFP+ MMP-2+/+ BM without or with ST. D and E, Quantitative analysis of the numbers of c-Kit+ EPCs and the ratio of ischemic-nonischemic laser Doppler blood flow in KO and KO-ST groups (n=5 per group; *P<0.05, Student t test).
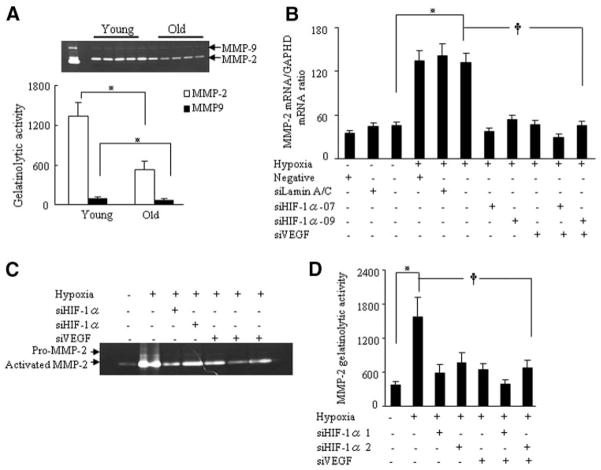
HIF-1α Regulates VEGF-Mediated MMP Expression in Response to Hypoxia in Human Umbilical Vein Endothelial Cells
Old human umbilical vein endothelial cells (HUVECs; 15 to 17 population doublings) had much less MMP-2 activity than young HUVECs (5 to 7 population doublings; Figure 8A), which suggests that MMP-2 transcription becomes impaired with age in vitro. To understand the mechanism by which HIF-1α stimulates MMP-2 expression via VEGF signaling in vivo, we used small interfering (si) RNAs targeting HIF-1α and VEGF in young HUVECs exposed to hypoxia. Quantitative real-time polymerase chain reaction analysis demonstrated that hypoxia stimulated MMP-2 mRNA expression; this effect was attenuated by siHIF-1α-07, siHIF-1α-09, and siVEGF, as well as their combinations (siHIF-1α-07 plus siVEGF or siHIF-1α-09 plus siVEGF; Figure 8B). In addition, hypoxia stimulated the gelatinolytic activity of MMP-2; this effect was also diminished by the siRNAs singly or in combination (Figure 8C and 8D). These findings suggest that HIF-1α can regulate VEGF-dependent MMP-2 expression and activity in vascular endothelial cells.
Figure 8.
HIF-1α regulates VEGF-dependent MMP-2 expression in response to hypoxia in HUVECs. A, Representative gelatin zymography (upper panel) showing that VEGF induces MMP-2 and MMP-9 activities in the conditioned media of young and old HUVECs. Quantitative analysis of the zymography (lower panel) showed the gelatinolytic activities of MMP-2 and MMP-9 (*P<0.05, 3 independent experiments, Student t test). B, Quantitative analysis of MMP-2 mRNA expression in the lysates of young HUVECs treated with or without a nontargeting siRNA, siRNAlamin A/C (siLamin A/C), siHIF-1α-07, siHIF-1α-09, or siVEGF under hypoxic conditions (*P<0.05, †P<0.05, Kruskal-Wallis and Tukey post hoc tests; 6 independent experiments). C, Representative gelatin zymography showing the inhibitory effects of targeted siRNAs on MMP-2 activity. D, Quantitative analysis of MMP-2 activity in the conditioned media of HUVECs treated with or without the indicated siRNAs (*P<0.05, †P<0.05, Kruskal-Wallis and Tukey post hoc tests; 3 independent experiments).
siHIF-1α Impairs HUVEC Invasion and Tubulogenesis in Response to Hypoxia
Because HIF-1α supports EPC mobilization and the angiogenic response in vivo,17 we speculated that disruption of HIF-1α activity may influence cellular function. In hypoxic conditions (1% O2), siHIF-1α-07 attenuated the numbers of invading HUVECs in a cell invasion assay (online-only Data Supplement Figure VIIc and VIId). Similarly, siHIF-1α-07 inhibited the HUVEC tubulogenic response (online-only Data Supplement Figure VIIe). These findings provide evidence that hypoxia-induced HIF-1α may support revascularization by affecting EPC function.
Discussion
Here, we report the novel finding that ST stimulates the PI3K/Akt-dependent HIF-1α activation pathway and attenuates the PHD/FIH-mediated HIF-1α degradation pathway in ischemic tissues and within the cells of aged animals. The increase in HIF-1α transcriptional activity increased the expression of the angiogenic genes VEGF, Flt-1, and MMP-2 in ischemic tissue and BM-derived progenitor cells, thereby improving not only recruitment of EPCs but also their function. This resulted in a substantial number of circulating CD34+/c-Kit+ EPC-like cells and their homing to the vasculature, accelerating ischemic revascularization and blood flow recovery.
The ability of ST to increase HIF-1α levels is likely to contribute to the stimulation of revascularization under experimental conditions. The ability of HIF-1α to activate the expression of multiple angiogenic growth factor genes as a direct transcriptional activator has been demonstrated previously.20 Here, ST increased the protein levels of VEGF and Flt-1 as well as HIF-1α in ischemic muscles of aged WT mice. It has been reported previously that insulin or IGF-1 enhances HIF-1α activation via stimulation of the PI3K/Akt signaling pathway in several cancer lines.18,21 A recent study demonstrated that the IGF-1/IGF-1R signaling pathway may serve redundant roles in the cardiac response to ST.22 Here, ST increased IGF-1 protein levels in ischemic muscle, and the ability of ST to enhance beneficial vascular action was abrogated by LY in vivo. Thus, the increase in HIF-1α transcriptional activity by IGF-1–mediated activation of the PI3K/Akt signaling pathway could represent an ST-related mechanism that protects cardiovascular tissue from stress.
On the other hand, the present data show that HIF-1α stability is also required for ST-stimulated revascularization in ischemic tissue. The PHD family of proteins degrades HIF-1α,23 and ST inhibited increases in the levels of PHD-3 and FIH proteins in ischemic tissues. DFO is an HIF-1α stabilizer that prevents HIF-1α degradation by several PHD families,23,24 and DFO enhanced the ST-induced increase in ischemic muscular HIF-1α levels and angiogenic response to ischemia but slightly reduced the ST-related effects on PHD-3 and FIH. DFO has also been shown to stimulate angiogenic cytokines in aged animals.23 These findings provide evidence that the vasculoprotective action of ST is mediated, at least in part, through the increase in HIF-1α stabilization that occurs when PHD3 and FIH expression is reduced.
It has become clear that the function and the number of EPCs are modified by pathological conditions such as aging and cardiovascular disease and by therapeutic exercise intervention.5,13 In the present observations, the adhesiveness, invasiveness, and tubulogenesis responses of BM-derived EPCs to VEGF were higher in WT-ST mice than in WT mice; these effects were abrogated by LY and enhanced by DFO. Furthermore, siHIF-1α impaired HUVEC invasion and tubulogenic responses in vitro. Partial HIF-1α deficiency and impaired HIF-1α stabilization decreases EPC recruitment and revascularization in young and aged animals.17,23 Thus, the ability of ST to improve EPC function has a salutary effect on the vasculature under conditions of ischemic stress by enhancing HIF-1α activity pathways in EPCs, thereby promoting revascularization.
The present findings are consistent with the results of Chang et al,23 who demonstrated an impairment of BM-mediated vasculogenesis in the ischemic tissues of aged mice; however, unlike in the present study, they found no effects of age on EPC number and function in mice. This difference may be due to differences in the ischemic models or to differences in the cell type or specific cellular functions. Chang et al23 used the skin flap model for their evaluation. Furthermore, they studied only the migration of EPCs, defined as Flk-1+/CD11− cells, in healthy younger and older animals. In contrast, we used a more extensive hindlimb ischemic model, and we defined CD31+/c-Kit+ cells as EPCs and studied both their invasion and tubulogenic response. This may have provided a more robust determination of differences in EPC number and function.
Among the members of the MMP family, MMP-2 is sensitive to the hypoxia caused by ischemia.5,25 Recently, we have shown that exposure to VEGF or basic fibroblast growth factor causes the predominant accumulation of MMP-2 transcripts in vascular endothelial cells.5,6 In the data presented here, ST stimulated MMP-2 mRNA expression in ischemic muscle and EPCs of MMP-2+/+ mice; these changes were abolished by ST-LY and enhanced by ST-DFO. In HUVECs, aging impairs MMP-2 expression and activity, and both siHIF-1α and siVEGF reduced MMP-2 gene and protein expression. Because genetic deletion or selective pharmacological inhibition of MMP-2 inhibits angiogenesis in vitro and vivo,5,6 we propose that the hypoxia-induced MMP-2 reactivation by stimulation of the PI3K/HIF-1α/VEGF signaling pathway may be responsible for the vasculoprotective action of ST. This notion is further supported by the lack of effect of ST on angiogenesis in MMP-2−/− mice. More importantly, after transplantation with c-Kit+ EPC-like cells derived from aged MMP-2+/+ BM, ST restored blood perfusion and capillary formation in aged MMP-2−/− mice. MMP-2 deficiency impairs the mobilization of CD31+/c-Kit+ EPC-like cells in young and old animals.5 These data suggest that that ST-mediated improvement of vascularization may be attributable to the increase of EPC mobilization and to their functional incorporation into the vasculature in MMP-2−/−-ST mice.
The data presented here show that aging promotes the formation of pathological vessels with irregular lumens. Previous studies have reported that the reduction of PI3K/Akt expression or inhibition of HIF-1α activity can contribute to vessel maturation in gastric tumors.26 In the present study, ST reduced the formation of abnormal vessels and enhanced vessel maturation in ischemic muscle, and the ability of ST to favorably affect angiogenesis was abrogated in WT-ST-LY mice and was enhanced in WT-ST-DFO mice. Conversely, ST promoted collateral artery formation in the ischemic limbs of aged WT mice; this change was abolished by ST-LY and was enhanced by ST-DFO. We hypothesize that ST prevented the aberrant vascular structures and enhanced collateral artery formation by increasing HIF-1α activity, which resulted in improvement of blood perfusion recovery in ischemic limbs of aged WT mice. To the best of our knowledge, this is the first report to have clearly demonstrated that ST significantly enhances functional therapeutic neovascularization.
The present study confirmed the dysfunctional hypoxia response in aged ischemic tissue with subsequent impairment in downstream gene expression (VEGF, Flt-1, and MMP-2), offering a potential explanation for the disappointing outcome of clinical trials with cell therapies to date. Therapeutic interventions with exercise training in advanced age designed to restore the “young” hypoxic response can be recommended as a powerful strategy to prevent age-associated declines in vascular regeneration and function by recruiting and improving delivery of EPCs to the vasculature of ischemic tissues through PI3K signaling pathway–dependent HIF-1α/VEGF/MMP-2 activation. Future studies with patients of advanced age will be required to investigate whether healthy subjects and specific patient subsets with peripheral artery disease or coronary artery disease achieve the potential vascular benefit with exercise. This might also allow the therapeutic use of nonpharmacological interventions, such as voluntary exercising, to complement pharmacological or genetic interventions such as statins or vascular-related gene therapy.
Supplementary Material
Acknowledgments
We wish to acknowledge the technical assistance of T. Shibata, E. Asai, Y. Iwatsuki, and W. Adachi.
Sources of Funding
This work was supported in part by grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (Nos. 19590812 and 21590952 to Dr Cheng) and the Japan Heart Foundation (No. 26-007508 to Dr Cheng). This work was also supported in part by grants from the National Natural Science Foundation of China (No. 30960128 to Dr Cheng) and the Bio R&D program through the National Research Foundation of Korea, funded by the Ministry of Education, Science and Technology (No. 2010-0019913 to Dr Kim).
Footnotes
Disclosures
None.
References
- 1.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 2.van Hinsbergh VW, Engelse MA, Quax PH. Pericellular proteases in angiogenesis and vasculogenesis. Arterioscler Thromb Vasc Biol. 2006;26:716–728. doi: 10.1161/01.ATV.0000209518.58252.17. [DOI] [PubMed] [Google Scholar]
- 3.Werb Z. ECM and cell surface proteolysis: regulating cellular ecology. Cell. 1997;91:439–442. doi: 10.1016/s0092-8674(00)80429-8. [DOI] [PubMed] [Google Scholar]
- 4.Reed MJ, Corsa AC, Kudravi SA, McCormick RS, Arthur WT. A deficit in collagenase activity contributes to impaired migration of aged micro-vascular endothelial cells. J Cell Biochem. 2000;77:116–126. [PubMed] [Google Scholar]
- 5.Cheng XW, Kuzuya M, Nakamura K, Maeda K, Tsuzuki M, Kim W, Sasaki T, Liu Z, Inoue N, Kondo T, Jin H, Numaguchi Y, Okumura K, Yokota M, Iguchi A, Murohara T. Mechanisms underlying the impairment of ischemia-induced neovascularization in matrix metalloproteinase 2-deficient mice. Circ Res. 2007;100:904–913. doi: 10.1161/01.RES.0000260801.12916.b5. [DOI] [PubMed] [Google Scholar]
- 6.Silletti S, Kessler T, Goldberg J, Boger DL, Cheresh DA. Disruption of matrix metalloproteinase 2 binding to integrin alpha(v)beta3 by an organic molecule inhibits angiogenesis and tumor growth in vivo. Proc Natl Acad Sci U S A. 2001;98:119–124. doi: 10.1073/pnas.011343298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rullman E, Norrbom J, Stromberg A, Wagsater D, Rundqvist H, Haas T, Gustafsson T. Endurance exercise activates matrix metalloproteinases in human skeletal muscle. J Appl Physiol. 2009;106:804–812. doi: 10.1152/japplphysiol.90872.2008. [DOI] [PubMed] [Google Scholar]
- 8.Leosco D, Rengo G, Iaccarino G, Golino L, Marchese M, Fortunato F, Zincarelli C, Sanzari E, Ciccarelli M, Galasso G, Altobelli GG, Conti V, Matrone G, Cimini V, Ferrara N, Filippelli A, Koch WJ, Rengo F. Exercise promotes angiogenesis and improves beta-adrenergic receptor signalling in the post-ischaemic failing rat heart. Cardiovasc Res. 2008;78:385–394. doi: 10.1093/cvr/cvm109. [DOI] [PubMed] [Google Scholar]
- 9.Tateishi-Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, Masaki H, Amano K, Kishimoto Y, Yoshimoto K, Akashi H, Shimada K, Iwasaka T, Imaizumi T. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002;360:427–435. doi: 10.1016/S0140-6736(02)09670-8. [DOI] [PubMed] [Google Scholar]
- 10.Assmus B, Honold J, Schachinger V, Britten MB, Fischer-Rasokat U, Lehmann R, Teupe C, Pistorius K, Martin H, Abolmaali ND, Tonn T, Dimmeler S, Zeiher AM. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–1232. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 11.Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, Kalantzi M, Herbots L, Sinnaeve P, Dens J, Maertens J, Rademakers F, Dymarkowski S, Gheysens O, Van Cleemput J, Bormans G, Nuyts J, Belmans A, Mortelmans L, Boogaerts M, Van de Werf F. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006;367:113–121. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- 12.Heiss C, Keymel S, Niesler U, Ziemann J, Kelm M, Kalka C. Impaired progenitor cell activity in age-related endothelial dysfunction. J Am Coll Cardiol. 2005;45:1441–1448. doi: 10.1016/j.jacc.2004.12.074. [DOI] [PubMed] [Google Scholar]
- 13.Sandri M, Adams V, Gielen S, Linke A, Lenk K, Krankel N, Lenz D, Erbs S, Scheinert D, Mohr FW, Schuler G, Hambrecht R. Effects of exercise and ischemia on mobilization and functional activation of blood-derived progenitor cells in patients with ischemic syndromes: results of 3 randomized studies. Circulation. 2005;111:3391–3399. doi: 10.1161/CIRCULATIONAHA.104.527135. [DOI] [PubMed] [Google Scholar]
- 14.Jensen L, Bangsbo J, Hellsten Y. Effect of high intensity training on capillarization and presence of angiogenic factors in human skeletal muscle. J Physiol. 2004;557:571–582. doi: 10.1113/jphysiol.2003.057711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryan NA, Zwetsloot KA, Westerkamp LM, Hickner RC, Pofahl WE, Gavin TP. Lower skeletal muscle capillarization and VEGF expression in aged vs. young men. J Appl Physiol. 2006;100:178–185. doi: 10.1152/japplphysiol.00827.2005. [DOI] [PubMed] [Google Scholar]
- 16.Edelberg JM, Lee SH, Kaur M, Tang L, Feirt NM, McCabe S, Bramwell O, Wong SC, Hong MK. Platelet-derived growth factor-AB limits the extent of myocardial infarction in a rat model: feasibility of restoring impaired angiogenic capacity in the aging heart. Circulation. 2002;105:608–613. doi: 10.1161/hc0502.103672. [DOI] [PubMed] [Google Scholar]
- 17.Bosch-Marce M, Okuyama H, Wesley JB, Sarkar K, Kimura H, Liu YV, Zhang H, Strazza M, Rey S, Savino L, Zhou YF, McDonald KR, Na Y, Vandiver S, Rabi A, Shaked Y, Kerbel R, Lavallee T, Semenza GL. Effects of aging and hypoxia-inducible factor-1 activity on angiogenic cell mobilization and recovery of perfusion after limb ischemia. Circ Res. 2007;101:1310–1318. doi: 10.1161/CIRCRESAHA.107.153346. [DOI] [PubMed] [Google Scholar]
- 18.Fukuda R, Hirota K, Fan F, Jung YD, Ellis LM, Semenza GL. Insulin-like growth factor 1 induces hypoxia-inducible factor 1-mediated vascular endothelial growth factor expression, which is dependent on MAP kinase and phosphatidylinositol 3-kinase signaling in colon cancer cells. J Biol Chem. 2002;277:38205–38211. doi: 10.1074/jbc.M203781200. [DOI] [PubMed] [Google Scholar]
- 19.Liu L, Marti GP, Wei X, Zhang X, Zhang H, Liu YV, Nastai M, Semenza GL, Harmon JW. Age-dependent impairment of HIF-1alpha expression in diabetic mice: correction with electroporation-facilitated gene therapy increases wound healing, angiogenesis, and circulating angiogenic cells. J Cell Physiol. 2008;217:319–327. doi: 10.1002/jcp.21503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Semenza GL. HIF-1, O(2), and the 3 PHDs: how animal cells signal hypoxia to the nucleus. Cell. 2001;107:1–3. doi: 10.1016/s0092-8674(01)00518-9. [DOI] [PubMed] [Google Scholar]
- 21.Treins C, Giorgetti-Peraldi S, Murdaca J, Semenza GL, Van Obberghen E. Insulin stimulates hypoxia-inducible factor 1 through a phosphatidylinositol 3-kinase/target of rapamycin-dependent signaling pathway. J Biol Chem. 2002;277:27975–27981. doi: 10.1074/jbc.M204152200. [DOI] [PubMed] [Google Scholar]
- 22.Ikeda H, Shiojima I, Ozasa Y, Yoshida M, Holzenberger M, Kahn CR, Walsh K, Igarashi T, Abel ED, Komuro I. Interaction of myocardial insulin receptor and IGF receptor signaling in exercise-induced cardiac hypertrophy. J Mol Cell Cardiol. 2009;47:664–675. doi: 10.1016/j.yjmcc.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang EI, Loh SA, Ceradini DJ, Lin SE, Bastidas N, Aarabi S, Chan DA, Freedman ML, Giaccia AJ, Gurtner GC. Age decreases endothelial progenitor cell recruitment through decreases in hypoxia-inducible factor 1alpha stabilization during ischemia. Circulation. 2007;116:2818–2829. doi: 10.1161/CIRCULATIONAHA.107.715847. [DOI] [PubMed] [Google Scholar]
- 24.Appelhoff RJ, Tian YM, Raval RR, Turley H, Harris AL, Pugh CW, Ratcliffe PJ, Gleadle JM. Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J Biol Chem. 2004;279:38458–38465. doi: 10.1074/jbc.M406026200. [DOI] [PubMed] [Google Scholar]
- 25.Muhs BE, Plitas G, Delgado Y, Ianus I, Shaw JP, Adelman MA, Lam-parello P, Shamamian P, Gagne P. Temporal expression and activation of matrix metalloproteinases-2, −9, and membrane type 1-matrix metalloproteinase following acute hindlimb ischemia. J Surg Res. 2003;111:8–15. doi: 10.1016/s0022-4804(02)00034-3. [DOI] [PubMed] [Google Scholar]
- 26.Stoeltzing O, McCarty MF, Wey JS, Fan F, Liu W, Belcheva A, Bucana CD, Semenza GL, Ellis LM. Role of hypoxia-inducible factor 1alpha in gastric cancer cell growth, angiogenesis, and vessel maturation. J Natl Cancer Inst. 2004;96:946–956. doi: 10.1093/jnci/djh168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.