Abstract
Aged rat brain is more sensitive to reperfusion injury induced by cardiac arrest and resuscitation. The mitochondrial respiratory chain, the major source of free radicals during reperfusion, is likely to be the target of lipid peroxidation. Previous work has shown a higher mortality and lower hippocampal neuronal survival in older rats. 4-hydroxy-2-nonenal (HNE), a major product of lipid peroxidation, was found to be elevated in cortex and brainstem after resuscitation. In this study we investigated the acute changes of mitochondrial function in aging rat brain following cardiac arrest and resuscitation; the effect of an antioxidant, alpha-phenyl-tert-butyl-nitrone (PBN) was also tested. Fischer 344 rats, 6 and 24-month old, were subjected to cardiac arrest (7-10 minutes) and allowed to recover 1 hour after resuscitation. Mitochondria of cortex and brainstem were isolated and assayed for respiratory function. Compared to their respective non-arrested control group, 1h untreated groups (both 6 month and 24 month) had similar state 3 (ADP-stimulated) but higher state 4 (resting state) respiratory rates. The respiratory control ratio (state 3/state 4) of cortex in the 1h untreated group was 26% lower than the non-arrested control group; similar results were found in brainstem. The decreased mitochondrial respiratory function was improved by PBN treatment. HNE–modified mitochondrial proteins were elevated 1h after resuscitation, with an evident change in the aged. Treatment with PBN reduced the elevated HNE production in mitochondria of cortex. The data suggest (i) there is increased sensitivity to lipid peroxidation with aging, (ii) mitochondrial respiratory function related to coupled oxidation decreases following cardiac arrest and resuscitation, and (iii) treatment with antioxidant, such as PBN, reduces the oxidative damage following cardiac arrest and resuscitation.
1. INTRODUCTION
Transient global brain ischemia induced by cardiac arrest and resuscitation results in reperfusion injury associated with oxidative stress1. We have previously shown that aged rats are more sensitive to reperfusion injury induced by cardiac arrest and resuscitation. Higher mortality and lower hippocampal neuronal survival were found in the older rats2. 4-hydroxy-2-nonenal (HNE), a major product of lipid peroxidation, was found to be elevated in cortex and brainstem after resuscitation3. An antioxidant, alpha-phenyl-tert-butyl-nitrone (PBN)4, improved the outcome following cardiac arrest and resuscitation2. The cellular mechanism that contributes to the age-related changes in brain aerobic capacity remains to be discerned. Mitochondria are both a major source and target of free radicals during insult of oxidative stress. Modification of mitochondrial proteins by lipid peroxidation products HNE, have been described to decrease oxidative capacity5. Oxidative damage is therefore likely to play a role in the decline of mitochondrial function6. With aging, increased susceptibility to oxidative stress is known to lead to declined mitochondrial function, especially following an ischemic-reperfusion insult5. In this study we investigated the effects of aging on mitochondrial function in brain following cardiac arrest and resuscitation. Mitochondrial oxidative capacity was assessed by measuring respiratory rates (state 3/state 4) from freshly isolated mitochondria. Western blot analysis was used to detect HNE-modified mitochondrial proteins. We hypothesized that an antioxidant treatment with PBN in the aged rats would result in improved mitochondrial function following cardiac arrest and resuscitation by attenuation of lipid peroxidation.
2. METHODS AND MATERIALS
2.1. Animal Preparation
Male Fischer 344 rats (6- and 24-month-old) were purchased and allowed to acclimate in the animal facility at Case Western Reserve University for one week before being utilized. Surgical procedures for each experiment were as follows7: anesthesia was induced by isoflurane (2.5% isoflurane, 70% N2O in O2) and maintained with 1-2% isoflurane, 70% N2O in O2 through a nasal cone. Cannulae were placed in: (i) Ventral tail artery using polyethylene tubing (PE-50, 0.023“ i.d., 0.038” o.d.) for the purpose of monitoring of systemic arterial blood pressure and to obtain samples for blood gas, plasma glucose and lactate determinations (ii) External jugular vein into the right atrium using a Silastic catheter (0.025“ i.d., 0.047” o.d.) for administration of drug. After surgery, the rats were allowed to recover for at least 1 hour while restrained in plastic cages. Throughout the experiment, the body temperature was maintained at 37°C by an infrared heat lamp (250W, 45 cm above the body) regulated by feedback from a rectal probe.
2.2. Induction of Total Cerebral Ischemia in Rat
Reversible total cerebral ischemia was achieved using a cardiac arrest and resuscitation model7. Cardiac arrest was induced in the conscious rat by rapid sequential intra-atrial injection of d-tubocurare (0.3mg) and ice-cold KCl solution (0.5 M; 0.12 ml/100g of body weight). Resuscitation was initiated 5 minutes after arrest following orotreacheal intubation with a 14-gauge catheter attached to a rodent ventilator (100% O2, tidal volume: 10cc/kg, respiratory rate: 80 breaths/min). Simultaneous chest compressions and the infusion of normal saline (0.5 ml/min) were given until a spontaneous heartbeat returned. Epinephrine (4-10g) was given intravenously to establish a mean blood pressure greater than 80% of the pre-arrest value, at which point the animal was considered to be resuscitated (7-10 min ischemia). Ventilation was then adjusted to ~30% O2 and 70% N2O, depending on the normal range of blood gas, until spontaneous respiration was regained. For the PBN-treated rats, PBN (100 mg/kg) was infused intravenously immediately after resuscitation for 60 minutes. The untreated rats were given normal saline for the same period of time. Non-arrested rats went through the same surgical procedures except cardiac arrest. Non-arrested controls and resuscitated rats (1 h post-resuscitation) were decapitated and brains were removed for further process.
2.3. Isolation of Brain Mitochondria
Brain mitochondria were isolated using a method previously described with slight modifications8. In brief, tissue of cortex (whole layer, bilateral, 0.7-1.0g) and brainstem (~ 0.3g) were dissected (see Diagram below) and rinsed in ice-cold isolation buffer (200 mM Mannitol, 70 mM Sucrose, and 5.0 mM MOPS, pH 7.4). The tissue was blotted dry, freed of visible blood vessels, then weighed and minced thoroughly. The tissue was suspended in isolation buffer (10 ml/g tissue) containing defatted bovine albumin (BSA, 0.2%) and EDTA (0.2 mM) and then treated with the protease Subtilisin A (5mg/g), for 30 second with light shaking. The suspension was then homogenized with a Teflon pestle (4 strokes). The homogenate was then centrifuged at 4 °C. The resulting mitochondrial pellet was washed (x2) with isolation buffer, centrifuged and resuspended to a final protein concentration of approximately 25 mg/ml and 10 mg/ml, cortex and brainstem, respectively.
Diagram.
Dissection of cortex and brainstem (gray-shaded area) of a rat brain.
2.4. Measurement of Mitochondrial Respiratory Rates
Oxidative rates were assessed by measuring oxygen consumption using a polarographic system consisting of a Clark-type electrode in the presence of the substrates glutamate plus malate8. The NADH-linked oxidative rates (state 3: ADP-stimulated; state 4: resting state, ADP-limited) were then calculated (natom oxygen/min/mg protein). The respiratory control ratio (RCR) was determined (state 3 / state 4). ADP-to-oxygen (ADP/O) ratios (nmol ADP per nanoatom O) were calculated as previously described.
2.5. Detection of HNE-Modified Mitochondrial Protein
Western blot analysis was used to detect HNE-modified mitochondrial protein. Samples of mitochondria were prepared by addition of isolation buffer containing 1 mM dithiothreitol and 1 mM phenylmethylsulfonyl. Samples (100 μg of protein) were electrophoresed on 10% SDS-polyacrilamide gels. The proteins on the gels were transferred to nitrocellulose membranes then incubated with 5% skim milk blocking buffer for 1 hour (room temperature). HNE modified proteins were detected by incubating the membranes with a 1:500 dilution of polyclonal anti-HNE antibody (Calbiochem) overnight (4 °C) followed by incubation with horseradish peroxidase-conjungated anti-rabbit IgG (1:5000) for 1 hour (Jackson ImmunoResearch). The primary antibody immunoreactive protein bands were visualized using enhanced chemiluminescence detection system (ECL kit, Amersham).
2.6. Statistical Methods
All values were represented as mean ± S.D. Statistical analyses were performed using SPSS v13.0 for Windows. Group comparisons are made by one-way analysis of variance (ANOVA) using t-test. Significance was considered at the level of p < 0.05.
3. RESULTS
3.1. Mitochondrial Respiratory Function
To determine the effect of aging on the overall mitochondrial oxidative capacity following cardiac arrest and resuscitation, we measured polarographically, oxygen consumption in the presence of the substrates of glutamate plus malate. As seen in Table 1, there were no significant differences in state 3 oxidative rates between 6-month and 24 -month-old rat brain mitochondria (cortex and brainstem) under all conditions. However, in brainstem, the state 3 oxidative rates in the 24-month-old rat brains were decreased in both conditions (22% and 17%, non-arrested and 1 h recovery groups, respectively), compared to the 6-month-old. The state 4 rates in cortex and brainstem were significantly higher at 1 h recovery compared to their respective non-arrested controls (6 and 24- month-old). In both the cortex and brainstem, there appeared to be no aging effect on the state 4 respiratory rates (non-arrested and 1 h recovery), acutely. In both age groups, the respiratory control ratios in cortex and brainstem were significantly lower at 1 h recovery compared to non-arrested controls. Since the state 3 oxidative rates were similar, the lower respiratory control ratios were as a result of higher state 4 rates. There were no differences in ADP/O in any conditions or between age groups (Table 1).
Table 1.
Respiratory properties of rat brain mitochondria. Values are mean ± standard deviation.
| Age (mos) |
Condition | n | State 3 (nO/mg/min) |
State 4 (nO/mg/min) |
RCR | ADP/O | |
|---|---|---|---|---|---|---|---|
| Cortex | 6 | Non-arrested | 5 | 338.4 ± 34.5 | 29.0 ± 3.0 | 11.7 ± 1.0 | 2.1 ± 0.3 |
| 6 | 1 h recovery | 3 | 313.1 ± 16.8 | 34.9 ± 2* | 9.0 ± 0.1* | 2.4 ± 0.3 | |
| 24 | Non-arrested | 4 | 317.0 ± 34.4 | 27.5 ± 2.7 | 11.5 ± 0.8 | 2.2 ± 0.3 | |
| 24 | 1 h recovery | 3 | 287.8 ± 51.2 | 34.2 ± 1.3* | 8.5 ± 1.0* | 2.2 ± 0.1 | |
| 24 | PBN-treated | 3 | 302.5 ± 71.9 | 28.3 ± 8.8 | 10.9 ± 1.2 | 2.5 ± 0.4 | |
|
| |||||||
| Brainstem | 6 | Non-arrested | 5 | 319.7 ± 40.0 | 27.4 ± 3.9 | 11.9 ± 2.2 | 2.3 ± 0.3 |
| 6 | 1 h recovery | 3 | 297.7 ± 31.7 | 35.2 ± 1.2* | 8.5 ± 0.8* | 2.3 ± 0.4 | |
| 24 | Non-arrested | 4 | 248.3 ± 39.0 | 25.5 ± 2.7 | 9.7 ± 1.1 | 2.2 ± 0.1 | |
| 24 | 1 h recovery | 3 | 246.8 ± 33.8 | 35.1 ± 1.3* | 7.0 ± 0.8*§ | 2.3 ± 0.1 | |
| 24 | PBN-treated | 3 | 278.5 ± 66.4 | 28.5 ± 4.9 | 9.7 ± 0.7 | 2.3 ± 0.2 | |
RCR, respiratory control ratio; ADP/O, ADP-to-oxygen ratio.
indicates significant difference (t-test, p<0.05) from the pre-arrest value in the same age group.
indicates significant difference (t-test, p<0.05) between the untreated and PBN-treated groups.
Figure 1 shows the RCR in the 24-month group (cortex and brainstem), non-arrested, 1 h recovery-untreated and 1 h recovery-PBN treated. The data show a decrease of 26% in cortex and 28% in brainstem, compared to the values of non-arrested controls. PBN treatment resulted in similar RCR values to the non-arrested baseline.
Figure 1.
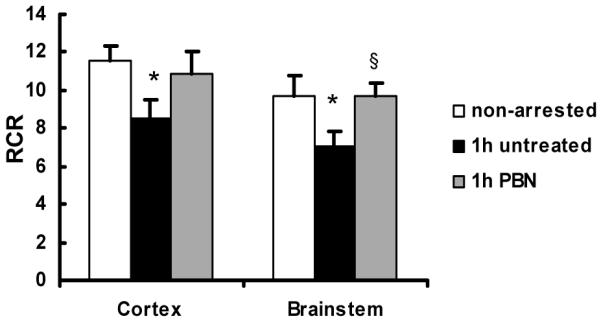
Respiratory control ratio (RCR) in mitochondria isolated from cortex and brainstem of 24-month-old rats, non-arrested control (n = 4), untreated (n = 3) and alpha-phenyl-tert-butyl-nitrone (PBN)-treated rats (n = 3). Values are mean ± S.D., * indicates significant difference (t-test, p<0.05) from the values of non-arrested controls. § indicates significant difference between (t-test, p<0.05) the untreated and PBN-treated group.
3.2. HNE Detection in Isolated Mitochondria of Brain
HNE-modified proteins were detected by Western blot analysis in isolated mitochondria from of cortex in 6 and 24-month-old rats. HNE adduct formation was observed within the molecular weight range of 65 to 110 kDa, with intense bands at 1h recovery in both age groups compared to the non-arrested controls (Figure 2). The increase of HNE production was more evident in the aged group. The elevated levels of HNE adducts were reduced with PBN treatment in the 24-month group.
Figure 2.
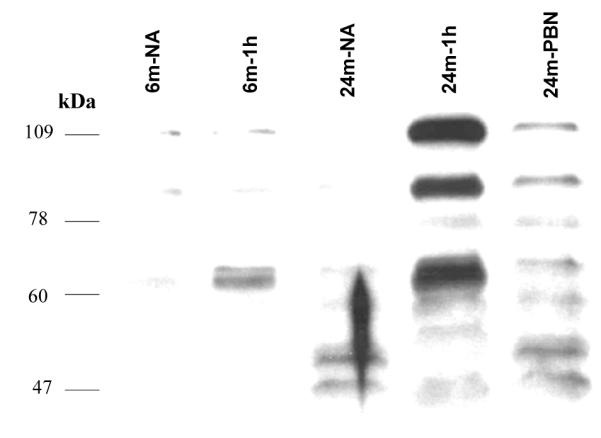
Western blot analyses showing HNE adducts formation in th cortex of 6 and 24-month-old rats. NA: non-arrested controls; 6m-NA: 6-month non-arrested rat; 6m-1h: 6-month untreated 1 h recovery rat; 24m-NA: 24-month non-arrested rat; 24m-1h: 24-month 1 untreated 1h recovery rat; 24m-PBN: alpha-phenyl-tert-butyl-nitrone (PBN)-treated 24-month-old rat at 1 h recovery.
4. DISCUSSION
This study focused on the early recovery phase from cardiac arrest. Our data showed that at 1 h recovery following cardiac arrest and resuscitation in both young and old rat brain RCR are decreased. This was evident by increased state 4 rates and not decreased state 3 rates. These data indicate that ischemic reperfusion injury acutely affects mitochondrial oxidative function through uncoupling. Furthermore, the HNE–modified mitochondrial proteins were elevated 1 h after resuscitation and were more apparent in the aged rats compared to the younger rats, suggesting that mitochondria in aged brain are more susceptible to damage as a result of lipid peroxidation. The data also show that with PBN treatment, there was improved mitochondrial respiratory function and reduced HNE modified mitochondrial proteins, possibly through free radical scavenging properties of PBN. The importance of this work is to provide information which may aid in potential therapeutic strategies aimed at early phase treatment in brain towards oxidative stress induced damage following cardiac arrest and resuscitation.
5. ACKNOWLEDGEMENTS
This work was supported by NIH grants NS 46074 and GM 066309. We would like to especially thank Constantinos Tsipis for his assistance in preparation of the manuscript.
6. REFERENCES
- 1.Lehotsky J, Murin R, Strapkova A, Urikova A, Tatarkova Z, Kaplan P. Time course of ischemia/reperfusion-induced oxidative modification of neural proteins in rat forebrain. Gen. Physiol Biophys. 2004;23(4):401–415. [PubMed] [Google Scholar]
- 2.Xu K, Sun X, Puchowicz MA, LaManna JC. Increased sensitivity to transient global ischemia in aging rat brain. Adv. Exp. Med. Biol. 2007;599:199–206. doi: 10.1007/978-0-387-71764-7_26. [DOI] [PubMed] [Google Scholar]
- 3.LaManna JC, Neubauer NL, Chavez JC. In: Matuation Phenomenon in Cerebral Ischemia IV. Bazen NG, Ito U, Maecheselli VL, Kuroiwa T, Klatzo I, editors. Springer-Verlag; Berlin Heidelberg: 2001. pp. 223–227. [Google Scholar]
- 4.Phillis JW, Clough-Helfman C. Protection from cerebral ischemic injury in gerbils with the spin trap agent N-tert-butyl-alpha-phenylnitrone (PBN) Neurosci. Lett. 1990;116(3):315–319. doi: 10.1016/0304-3940(90)90093-o. [DOI] [PubMed] [Google Scholar]
- 5.Lucas DT, Szweda LI. Cardiac reperfusion injury: aging, lipid peroxidation, and mitochondrial dysfunction. Proc. Natl. Acad. Sci. U. S. A. 1998;95(2):510–514. doi: 10.1073/pnas.95.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson MF, Sims NR. Mitochondrial respiratory function and cell death in focal cerebral ischemia. J. Neurochem. 1999;73(3):1189–1199. doi: 10.1046/j.1471-4159.1999.0731189.x. [DOI] [PubMed] [Google Scholar]
- 7.Xu K, Puchowicz MA, Lust WD, LaManna JC. Adenosine treatment delays postischemic hippocampal CA1 loss after cardiac arrest and resuscitation in rats. Brain Res. 2006;1071(1):208–217. doi: 10.1016/j.brainres.2005.11.060. [DOI] [PubMed] [Google Scholar]
- 8.Kerner J, Turkaly PJ, Minkler PE, Hoppel CL. Aging skeletal muscle mitochondria in the rat: decreased uncoupling protein-3 content. Am. J. Physiol Endocrinol. Metab. 2001;281(5):E1054–E1062. doi: 10.1152/ajpendo.2001.281.5.E1054. [DOI] [PubMed] [Google Scholar]


