Abstract
Overall, Latinas are more likely to be diagnosed with a more advanced stage of breast cancer, and are 20% more likely to die of breast cancer than non-Hispanic white women. It is estimated that from 2003–2006, $82.0 billion in direct medical care expenditures, in addition to 100,000 lives annually, could be saved by eliminating health disparities experienced by Latinos and increasing the use of up to five preventive services in the U.S. An additional 3,700 lives could be saved if 90% of women ≥40 years were recently screened for breast cancer. We examined risk for breast cancer in a case-control population-based sample of Mexican-origin women in Harris County, TX (n=714), where rates of breast cancer mortality for Latina women have doubled since 1990. Half of breast cancer cases (n=119) were diagnosed before the age of 50. In a multivariable model, women with a family history of breast cancer (OR=4.3), born in Mexico and having high levels of language acculturation (OR=2.5), and without health insurance (OR=1.6) were found to have the highest risk of breast cancer. Because Mexican-origin women were found to be of high-risk for early onset pre-menopausal breast cancer, we recommend policies targeting screening, education and treatment to prevent increased disparities in mortality. The inclusion of community members and policymakers as partners in these endeavors would further safeguard against an increase in cancer health disparities, and aid in formulating a policy agenda congruent with scientifically-based, community-driven policy efforts addressing breast cancer screening, education and treatment in this vulnerable population.
BACKGROUND
Breast cancer is the leading site of new cancer cases and the leading cause of death for Latinas in the United States,1 and breast cancer health disparities between Latinas and non-Latino populations are vast. Overall, Latinas are more likely to be diagnosed with a more advanced stage of breast cancer,1 and are 20% more likely to die of breast cancer than non-Hispanic white women.2 Moreover, by 2050, the Latino population is expected to double relative to current population estimates.3
While some reports suggest the overall cancer burden among Latino populations is lower than non-Hispanic whites, these reports are based on women who have access to treatment. Recent estimates show that Latinos have the highest rates of being uninsured and the lowest rates of employer-paid insurance,4 suggesting significant barriers to health care access, an increased need for “safety net” health centers, less access to cancer screening services, and consequently delayed cancer treatment and shorter survivorship.5 It is estimated that between 2003–2006, eliminating health disparities experienced by Latinos would have saved $82.0 billion in direct medical care expenditures.6
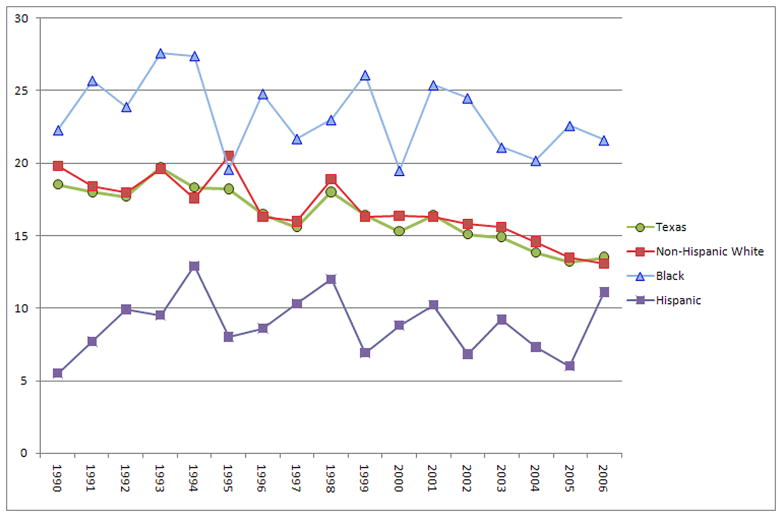
Furthermore, wide population variations may be masked as suggested by the finding that the rate of decline in cancer incidence in Latino populations is slower than that for non-Hispanic whites.7 From 1990–2006, in Harris County (Houston), Texas, while the gaps in breast cancer mortality narrowed between Latinos and non-Hispanic whites (from 14.3 to 2.0 per 100,000)8 and blacks (from 16.8 to 12.1 per 100,000)9 respectively, it was largely driven by the decreased mortality rates of non-Hispanic whites, and by the detrimental increased rate of Latino breast cancer mortality. In 2006, Latinos overall had a lower breast cancer mortality rate (11.1)10 compared to non-Hispanic whites (13.1)8 and blacks (21.6).9 However, since 1990 breast cancer mortality rates for Latinos have more than doubled,10 compared to a slight decrease for blacks (3.1%)9 and a significant decrease for non-Hispanic whites (33.8%)8 (see Figure 1). It has been estimated that an increase in utilization of five preventive services in the United States would annually save greater than 100,000 lives, and an additional 3,700 lives if 90% of women ≥40 years were recently screened for breast cancer.11 Among Latino populations, these findings would be further enhanced through a greater understanding of population-specific health determinants.
Figure 1.
Complex findings for Latino health outcomes contradictory to their socioeconomic profile may be explained by the dynamic nature of the Latino population, continuously influenced by social forces, shifting disease patterns, and demography.12, 13 However, the ways cancer screening behaviors are shaped by social determinants, such as access to screening and health care services, socioeconomic position and race/ethnicity, have been largely overlooked in cancer prevention research; thus, our understanding of health disparities in Latino populations has been hindered. The goal of the current study was to examine the association between social and cultural determinants and risk of breast cancer among women enrolled in an on-going cohort study.
METHODS
Self-identified adult women of Mexican-origin included in this study were drawn from an ongoing population-based cohort created by the Department of Epidemiology at The University of Texas M. D. Anderson Cancer Center, referred to as the Mexican American Cohort Study (MACS). Participant recruitment began in July 2001 using random-digit dialing, intercepts, door-to-door block walking, and networking, in neighborhoods in which at least 75% of the population is of Mexican origin according to the 2000 U.S. Census. A detailed description of the recruitment methodology and data collection protocol has been published elsewhere.14 Briefly, after obtaining written informed consent, trained bilingual interviewers conducted personal interviews using a structured questionnaire in either Spanish or English based on the participants’ preference. The interviews lasted approximately 45 minutes each and elicited information about personal and family health history, behavioral risks, socio-demographic characteristics, residential history, and exposure data. Participants received a $25 gift card for their participation. More than 19,000 individuals have been recruited into the study with the majority being women. The Institutional Review Board at M. D. Anderson Cancer Center approved all aspects of the study.
Sample and Matching Criteria
A total of 122 women in the MACS reported they had been diagnosed with breast cancer by a physician at baseline or follow-up. However, because we wanted to have complete data on all variables of interest, three women with missing health insurance data were excluded from the analysis. The remaining 119 cases were categorized into the following age groups: 30–39 years; 40–49 years; 50–59 years; and 60 years or more. Five controls per breast cancer case were randomly drawn from the MACS and frequency matched by age group. This resulted in the inclusion of 714 women (119 cases and 595 controls); each woman included in the study was drawn from a different household.
Variables
The outcome variable in this study is physician diagnosed breast cancer. Both diagnosis of breast cancer and age at diagnosis were self-reported. Family history of breast cancer also was self-reported by the participant.
Sociodemographic characteristics included age, marital status, parity status and educational attainment. Age at enrollment was calculated in years based on participants’ reported birth date, and used to match cases with controls. Marital status was categorized as married/living as married or not married (never married, divorced, separated, or widowed). Married women served as the reference category. Parity status was dichotomized as at least one live birth or none. Socioeconomic status was assessed based on educational attainment rather than household income or a combination of the two because roughly 50% of the participants did not report household income. Educational attainment was classified into three categories: less than high school, completed high school/General Educational Development equivalency, or more than high school.
Health insurance status was self-reported by the participant or participant’s spouse and categorized as yes or none.
Language acculturation was assessed using four items from the Bidimensional Acculturation Scale for Hispanics (BAS), a validated acculturation instrument designed for use with Hispanics15 (α=0.88). Scores ranged from 1 to 4, with higher scores reflecting fluency in English and higher levels of acculturation.
Country of birth was either USA or Mexico. We created a four level cultural determinant variable that combined level of language acculturation with country of birth. For this variable, language acculturation scores of 2.75 or more were coded as high language acculturation; those below 2.75 were coded as low. For the multivariable analysis we created four dummy variables with participants born in Mexico reporting low language acculturation serving as the reference category.
We examined four behavioral health risks (ever smoker, ever drinker, currently obese, and sedentary lifestyle) that are associated with breast cancer. Ever smokers were defined as having smoked at least 100 cigarettes in their lifetime. Ever drinkers were classified as having drunk at least one standard unit of alcohol a month for at least one year. We used body mass index (BMI), weight in kilograms divided by height in meters squared, to define obesity. Participants with a BMI of 30 or greater16 were classified as obese. Sedentary lifestyle was defined as less than 150 minutes of moderate-intensity activity per week or less than 75 minutes of vigorous activity per week, which represents the minimum physical activity level recommended for adults.17
Statistical analysis
Pearson’s χ2 test of association and Student’s t-test were used to examine distributional differences between the cases and controls for categorical and continuous variables, respectively. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using unconditional logistic regression analyses. Variables were maintained in the multivariable models based on two criteria: 1) if they were significant based on the results of Wald statistics at a Type I error rate of 0.05 and 2) if their inclusion resulted in a non-significant (defined as p > 0.1) Hosmer and Lemeshow goodness-of-fit statistic for the overall model.18 All analyses were completed using SAS version 9.2 software (SAS Institute Inc, Cary, NC, USA).
RESULTS
We observed significant differences between cases and controls based on several of the socio-demographic and risk factors examined (see Table 1). Results from the univariate unconditional logistic regression analyses (see Table 2), indicated that nativity and language acculturation, marital status, family history of breast cancer, having health insurance, and educational attainment were associated with the risk for breast cancer. These five variables were included in the final multivariable model (see Table 2). Results from the multivariable model indicated that women born in Mexico reporting high levels of language acculturation had a 2.5 times (95% CI: 1.19 – 5.17) increased risk for breast cancer compared to women born in Mexico reporting low levels of language acculturation. Compared to married women, single women were at 1.7 (95% CI: 1.10 – 2.67) times increased risk for breast cancer. The uninsured, compared to the insured were at 1.6 (95% CI: 1.01 – 2.38) times increased risk. The strongest risk factor we observed was for family history of breast cancer: women with a family history were at 4.3 (95% CI: 2.14–8.50) times increased risk for breast cancer compared to women without a family history.
Table 1.
Comparison of socio-demographic characteristics and risk factors between self-reported breast cancer cases and controls (N=714)
| Characteristic or Risk Factor | Controls (N=595) |
Cases (N=119) |
p-value |
|---|---|---|---|
| N (%) | N (%) | ||
| Age at enrollment | 1.00 | ||
| 30–39 years | 75 (12.6) | 15 (12.6) | |
| 40–49 years | 140 (23.5) | 28 (23.5) | |
| 50–59 years | 170 (28.6) | 34 (28.6) | |
| 60 years or older | 210 (35.3) | 42 (35.3) | |
| Mean (SD) | 54.3 (12.9) | 54.9 (12.1) | 0.652 |
| Age at diagnosis, Mean (SD) | -- | 50.5 (12.1) | |
| Range | |||
| Marital status | 0.003 | ||
| Married/living as married | 431 (72.4) | 70 (58.8) | |
| Not married | 164 (27.6) | 49 (41.2) | |
| Educational attainment | 0.032 | ||
| Less than high school | 416 (69.9) | 72 (60.5) | |
| High school graduate | 69 (11.6) | 24 (20.2) | |
| More than high school | 110 (18.5) | 23 (19.3) | |
| Health insurance status | 0.002 | ||
| None | 346 (58.2) | 51 (42.9) | |
| Yes | 249 (41.8) | 68 (57.1) | |
| Nativity & acculturation level | < 0.001 | ||
| Mexico & low acculturation | 388 (65.2) | 60 (50.4) | |
| Mexico & high acculturation | 30 (5.0) | 15 (12.6) | |
| US & low acculturation | 44 (7.4) | 5 (4.2) | |
| US & high acculturation | 133 (22.4) | 39 (32.8) | |
| Family history of breast cancer | < 0.001 | ||
| No | 574 (96.5) | 101 (84.9) | |
| Yes | 21 (3.5) | 18 (15.1) | |
| Parity status | 0.609 | ||
| No live births | 23 (3.9) | 6 (5.0) | |
| At least one live birth | 571 (96.1) | 113 (95.0) | |
| Smoking status | 0.498 | ||
| Never | 462 (77.7) | 89 (74.8) | |
| Ever | 133 (22.3) | 30 (25.2) | |
| Alcohol status | 0.081 | ||
| Never | 473 (79.5) | 86 (72.3) | |
| Ever | 122 (20.5) | 33 (27.7) | |
| Sedentary lifestyle | 0.791 | ||
| No | 78 (13.1) | 16 (13.5) | |
| Yes | 517 (86.9) | 103 (86.5) | |
| BMI | 0.615 | ||
| Obese | 310 (52.1) | 65 (54.6) | |
| Not | 285 (47.9) | 54 (45.4) |
Table 2.
Unadjusted and Adjusted* odds ratios (OR) and 95% confidence intervals (CI) for risk of breast cancer among women of Mexican origin (N=714)
| Risk Characteristic | Unadjusted |
Adjusted* |
||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Nativity & acculturation level | ||||
| Mexico & low acculturation | 1.00 | 1.00 | ||
| Mexico & high acculturation | 3.23 | 1.64 – 6.36 | 2.48 | 1.19 – 5.17 |
| US & low acculturation | 0.74 | 0.28 – 1.93 | 0.56 | 0.21 – 1.52 |
| US & high acculturation | 1.90 | 1.21 – 2.97 | 1.15 | 0.67 – 1.96 |
| Marital status | ||||
| Married | 1.00 | 1.00 | ||
| Not married | 1.84 | 1.23 – 2.76 | 1.71 | 1.10 – 2.67 |
| Health insurance status | ||||
| Yes | 1.00 | 1.00 | ||
| None | 1.85 | 1.25 – 2.76 | 1.55 | 1.01 – 2.38 |
| Family history of breast cancer | ||||
| None | 1.00 | 1.00 | ||
| Yes | 4.87 | 2.51 – 9.46 | 4.27 | 2.14 – 8.50 |
| Educational attainment | ||||
| Less than high school | 1.00 | 1.00 | ||
| High school graduate | 2.01 | 1.19 – 3.41 | 1.52 | 0.84 – 2.75 |
| More than high school | 1.21 | 0.72 – 2.02 | 0.93 | 0.53 – 1.66 |
Adjusted for all other covariates in the table.
DISCUSSION
Among women self-reporting breast cancer, 85% reported a family history of breast cancer and the mean age of diagnosis was 50.5 (range 21–80 years); 49% of women with breast cancer were diagnosed before the age of 50. Overall, women reporting a family history of breast cancer and women born in Mexico with high levels of language acculturation had the highest risk of breast cancer in this population.
When compared to other studies, we found the unadjusted risk for breast cancer among our participants who report a family history of breast cancer (OR=4.87) fits within the range of previous findings, from OR=1.32 among Hispanic women diagnosed before the age of 5019 to OR=11.220 among high risk women in Mexico. Previous mean ages of diagnoses have ranged from 37 in a sample of Hispanic women at high-risk for breast cancer21 to a median age of 51 years in a study of Hispanic women in the Department of Defense health care system with known access to health care.22 While parity status (i.e. having children) has been previously shown to decrease risk of breast cancer in this population,23 we did not find parity status to be a significant factor in this sample, perhaps because the overwhelming majority of women reported at least one live birth.
These findings expand existing cancer studies to a population-based sample, which is important for a population that has the highest rates of being uninsured and the lowest rates of employer-paid insurance.4 Latinos experience higher rates of uninsured compared to other racial/ethnic groups, with Mexican-origin populations being 40%, Cubans 26% and Puerto Ricans 21% uninsured, respectively, compared to 16% of non-Latino whites.24 Mexican-American women have been shown to discover breast cancer through self-breast exams, but wait to seek medical care,25 possibly explained by high rates of uninsured. Among other minority women, access to health insurance has been shown to influence mammogram use even at the highest levels of education.26 Ever having a mammogram has been shown to have an inverse association with lack of health insurance.27, 28 Access to care and treatment, largely driven by access to health insurance, possibly explains the higher rates of mortality of Latinas due to breast cancer compared to non-Latino white women.29, 30
The findings presented here should be interpreted within the context of study limitations. First, the data are cross-sectional; causality cannot be determined. In addition, we do not know the extent of lifestyle changes over time, and mammography screening practices are not reported by participants in this study. Furthermore, the small sample size available to examine some of the risk factors (i.e. language, acculturation, nativity status, and religious/ethnic background) also limits our ability to interpret our findings. Also, because the Latino community is very heterogeneous, we do not know how well our findings will generalize to other Latinas. Finally, all the data in our study, including diagnosis of breast cancer and family history of breast cancer, were self-reported. However, studies examining the sensitivity and specificity of self-reported breast cancer diagnosis have found agreements ranging within 90th percentiles overall, even among populations with lower levels of education.31 Moreover, studies examining self-reported family history of cancer have shown 88–95% agreement using multiple methods to verify diagnoses, including cancer registries, death certificates and medical records,32–34 and find self-reported family histories to be effective in evaluating risk of breast cancer.35
Despite these limitations, a great amount of knowledge can be gained from this study, especially since our study is among the first to examine risk of breast cancer in this non-clinical, population-based sample. Following the recently revised U.S. Preventive Task Force recommendations,36 half of the women diagnosed with breast cancer and greater than one-third of women in this Mexican-origin population would not have been recommended for mammography screening. Given the dearth of clinically-based studies on underrepresented minority populations, the task force lacked crucial data from these high-risk populations when formulating the recommendations. Furthermore, the current change in recommendations for general breast cancer screening practices stresses the need for a focus on improving screening methodology for Mexican-origin women under the age of 50, and how risk is examined.
Moreover, the combination of nativity and acculturation provides novel findings that furthers the discussion on the “healthy migrant” hypothesis.37, 38 Among our study participants, there was no overlap between this composite cultural determinant and the two highest risk factors (family history and health insurance status). In other words, none of the highly acculturated women born in Mexico reported a family history of breast cancer; nor were there significant differences in the number of women with health insurance or not. We included linguistic acculturation because previous studies have noted that low levels of English fluency are a barrier to accessing healthcare. Therefore, while it is possible that the Mexican-born women in our study were unaware of their family history for breast cancer, our findings underscore the possibility that, and consistent with results from a recent study based on the National Health Interview Survey,39 the highly acculturated women born in Mexico were more likely to obtain a mammogram and hence more likely to obtain an accurate diagnosis, than their less acculturated peers. Or perhaps, because language is only one component of the acculturation process,39, 40 it is other aspects of acculturation, such as lifestyle changes, that impact health habits over time that are important. Changes in health habits not only influence an individual’s risk for developing breast cancer, they also impact an individual’s risk of detecting breast cancer.
The higher prevalence of more aggressive breast cancers among young Latinas is established.41 Though Latinos are the fastest-growing population in the U.S., they have been severely under-represented in research; combined with the overall young demographic profile of the Latino population, our findings are particularly alarming and posit a number of policy implications. First, among this population, behaviors such as smoking, physical activity and obesity are not observed significant risk factors. However, lack of health insurance was associated with a 50% higher risk of breast cancer. This further supports the need to shift away from individual-level intervention efforts to ones promoting access through employment opportunities that provide health insurance coverage, or state or national efforts that provide such coverage. Furthermore, our study suggests an impending increase in breast cancer disparities disproportionately affecting Mexican-origin women if physicians implement the currently discussed change in recommendations for mammography screening. Further information regarding the impact of early mammography among this population, its implications for interventions addressing costs related to medical expenses of individuals without health insurance, and developing new educational materials targeting Mexican-origin populations is warranted.
One potential explanation for our findings may be related to the higher prevalence of BRCA mutations in Mexican women with European (Spanish, and particularly of Ashkenazi Jewish descent) ancestry;21 future studies may consider examining the migratory patterns from Europe and the region of Mexico women migrate from, and their possible role in higher rates of breast cancer in this population. Furthermore, in this population, individual-level behavioral risk factors were not found to be significant. Future studies should consider examining change in behaviors and social environments over time to further understand the underlying mechanisms for these increased risks in breast cancer among Mexican-origin women. Existing mixed findings regarding the role of neighborhoods in the prevention, detection, and survivorship of breast cancer for this population suggest further consideration of multilevel studies of breast cancer, and policy efforts targeting neighborhood empowerment zones in areas of highest risk for breast cancer. The inclusion of community members and policymakers as partners in these endeavors would further safeguard against an increase in cancer health disparities,42 and aid in formulating a policy agenda congruent with scientifically-based, community-driven policy efforts addressing breast cancer screening, education and treatment in this vulnerable population.
Acknowledgments
This research was supported in part by the Kellogg Health Scholars Program, [P0117943 from the W.K. Kellogg Foundation to the Center for Advancing Health to PYM]; by the National Cancer Institute [CA126988 to AVW]; funds collected pursuant to the Comprehensive Tobacco Settlement of 1998 and appropriated by the 76th legislature to The University of Texas M. D. Anderson Cancer Center; by the Caroline W. Law Fund for Cancer Prevention; by the a grant from the Duncan Family Institute for Cancer Prevention and Risk Assessment; and by the National Center on Minority Health and Health Disparities [5P60MD000503 to LAJ].
Contributor Information
Patricia Y. Miranda, Email: pymiranda@mdanderson.org, University of Texas M. D. Anderson Cancer Center, Department of Health Disparities Research, PO BOX 301402 – Unit 639, Houston, TX 77230-1402.
Anna V. Wilkinson, Email: awilkins@mdanderson.org, University of Texas M. D. Anderson Cancer Center, Department of Epidemiology, 1155 Pressler - Unit Number: 1340, Houston, TX 77030.
Carol J. Etzel, Email: cetzel@mdanderson.org, University of Texas M. D. Anderson Cancer Center, Department of Epidemiology, 1155 Pressler - Unit Number: 1340, Houston, TX 77030.
Renke Zhou, Email: rzhou@mdanderson.org, University of Texas M. D. Anderson Cancer, Center Department of Epidemiology, 1155 Pressler - Unit Number: 1340, Houston, TX 77030.
Lovell A. Jones, Email: lajones@mdanderson.org, University of Texas M. D. Anderson Cancer Center, Department of Health Disparities Research, PO BOX 301402 – Unit 639, Houston, TX 77230-1402.
Patricia Thompson, Email: pthompson@azcc.arizona.edu, University of Arizona, Arizona Cancer Center, 1515 N. Campbell, Ave. Tucson, Arizona 85724.
Melissa L. Bondy, Email: mbondy@mdanderson.org, University of Texas M. D. Anderson Cancer Center, Department of Epidemiology, 1155 Pressler - Unit Number: 1340, Houston, TX 77030.
References
- 1.Cancer Facts & Figures for Hispanics/Latinos 2006–2008. American Cancer Society; 2006. [Google Scholar]
- 2.Jemal A, Clegg LX, Ward E, Ries LAG, Wu X, Jamison PM, et al. Annual report to the nation on the status of cancer, 1975–2001, with a special feature regarding survival. Cancer. 2004;101(1):3–27. doi: 10.1002/cncr.20288. Available from http://www.scopus.com/inward/record.url?eid=2-s2.0-3042628862&partnerID=40. [DOI] [PubMed]
- 3.Table 1a. Projected Population of the United States, by Race and Hispanic Origin: 2000 to 2050. U.S. Census Bureau; 2004. [Google Scholar]
- 4.James C, Thomas M, Lillie-Blanton M, Garfield R. Key Facts: Race, Ethnicity & Medical Care. 2007. [Google Scholar]
- 5.Cancer Facts & Figures 2008. 2008. [Google Scholar]
- 6.LaVeist TA, Gaskin DJ, Richard P. The economic burden of health inequalities. 2009 [Google Scholar]
- 7.SEER Cancer Statistics, Review, 1975–2003. National Cancer Institute; 2006. [Google Scholar]
- 8.Cancer mortality rates in Harris County, Texas: Breast, Non-Hispanic White, 1990–2006. Texas Cancer Registry; 2010. [Google Scholar]
- 9.Cancer mortality rates in Harris County, Texas: Breast, Black, 1990–2006. Texas Cancer Registry; 2010. [Google Scholar]
- 10.Cancer mortality rates in Harris County, Texas: Breast, Hispanic, 1990–2006. Texas Cancer Registry; 2010. [Google Scholar]
- 11.Preventive care: A national profile on use, disparities, and health benefits. National Commission on Prevention Priorities. 2007 [Google Scholar]
- 12.Zsembik BA, Fennell D. Ethnic variation in health and the determinants of health among Latinos. Social Science and Medicine. 2005;61(1):53–63. doi: 10.1016/j.socscimed.2004.11.040. Available from http://www.scopus.com/inward/record.url?eid=2-s2.0-17444376002&partnerID=40. [DOI] [PubMed]
- 13.Vega WA, Rodriguez MA, Gruskin E. Health disparities in the Latino population. Epidemiologic Reviews. 2009 doi: 10.1093/epirev/mxp008. Online Advance Access publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilkinson AV, Spitz MR, Strom SS, Prokhorov AV, Barcenas CH, Cao YM, et al. Effects of nativity, age at migration, and acculturation on smoking among adult Houston residents of Mexican descent. American Journal of Public Health. 2005;95(6):1043–49. doi: 10.2105/AJPH.2004.055319. Available from <Go to ISI>://000229381800031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marin G, Gamba RJ. A new measurement of acculturation for hispanics: The bidimensional acculturation scale for hispanics (BAS) Hispanic Journal of Behavioral Sciences. 1996;18(3):297–316. Available from http://www.scopus.com/inward/record.url?eid=2-s2.0-0030201461&partnerID=40.
- 16.Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. 1998 [PubMed] [Google Scholar]
- 17.Cunningham GO, Michael YL. Concepts guiding the study of the impact of the built environment on physical activity for older adults: A review of the literature. American Journal of Health Promotion. 2004;18(6):435–43. doi: 10.4278/0890-1171-18.6.435. Available from <Go to ISI>://000222423800006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hosmer DW, Lemeshow S. Applied Logistic Regression. New York: Wiley; 1989. [Google Scholar]
- 19.Risendal B, Hines LM, Sweeney C, Slattery ML, Giuliano AR, Baumgartner KB, et al. Family history and age at onset of breast cancer in Hispanic and non-Hispanic white women. Cancer Causes and Control. 2008;19(10):1349–55. doi: 10.1007/s10552-008-9206-x. Available from http://www.scopus.com/inward/record.url?eid=2-s2.0-55849098886&partnerID=40. [DOI] [PMC free article] [PubMed]
- 20.Calderon-Garciduenas AL, Paras-Barrientos FU, Cardenas-Ibarra L, Gonzalez-Guerrero JF, Villarreal-Rios E, Staines-Boone T, et al. Risk factors of breast cancer in Mexican women. Salud Publica de Mexico. 2000;42(1):26–33. doi: 10.1590/s0036-36342000000100006. Available from http://www.scopus.com/inward/record.url?eid=2-s2.0-0342264370&partnerID=40. [DOI] [PubMed]
- 21.Weitzel JN, Lagos V, Blazer KR, Nelson R, Ricker C, Herzog J, et al. Prevalence of BRCA mutations and founder effect in high-risk Hispanic families. Cancer Epidemiology Biomarkers and Prevention. 2005;14(7):1666–71. doi: 10.1158/1055-9965.EPI-05-0072. Available from http://www.scopus.com/inward/record.url?eid=2-s2.0-22244470367&partnerID=40. [DOI] [PubMed]
- 22.Zaloznik AJ. Breast cancer stage at diagnosis: Caucasians versus Hispanics. Breast Cancer Research and Treatment. 1997;42(2):121–24. doi: 10.1023/a:1005760719089. Available from http://www.scopus.com/inward/record.url?eid=2-s2.0-0031032895&partnerID=40. [DOI] [PubMed]
- 23.Friedenreich CM, Cust AE. Physical activity and breast cancer risk: Impact of timing, type and dose of activity and population subgroup effects. British Journal of Sports Medicine. 2008;42(8):636–47. doi: 10.1136/bjsm.2006.029132. Available from http://www.scopus.com/inward/record.url?eid=2-s2.0-49949095417&partnerID=40. [DOI] [PubMed]
- 24.Rutledge MS, McLaughlin CG. Hispanics and health insurance coverage: The rising disparity. Medical Care. 2008;46(10):1086–92. doi: 10.1097/MLR.0b013e31818828e3. Available from http://www.scopus.com/inward/record.url?eid=2-s2.0-53449099417&partnerID=40. [DOI] [PubMed]
- 25.Voelker R. Breast cancer probed in Hispanic women. JAMA - Journal of the American Medical Association. 2009;301(13):1325–26. doi: 10.1001/jama.2009.402. Available from http://www.scopus.com/inward/record.url?eid=2-s2.0-63849317658&partnerID=40. [DOI] [PubMed]
- 26.Rosenberg L, Wise LA, Palmer JR, Horton NJ, Adams-Campbell LL. A multilevel study of socioeconomic predictors of regular mammography use among African-American women. Cancer Epidemiology Biomarkers and Prevention. 2005;14(11 I):2628–33. doi: 10.1158/1055-9965.EPI-05-0441. Available from http://www.scopus.com/inward/record.url?eid=2-s2.0-28644435277&partnerID=40. [DOI] [PubMed]
- 27.Tejeda S, Thompson B, Coronado GD, Martin DP, Heagerty PJ. Predisposing and enabling factors associated with mammography use among hispanic and non-hispanic white women living in a rural area. Journal of Rural Health. 2009;25(1):85–92. doi: 10.1111/j.1748-0361.2009.00203.x. Available from http://www.scopus.com/inward/record.url?eid=2-s2.0-58049174555&partnerID=40. [DOI] [PMC free article] [PubMed]
- 28.Tejeda S, Thompson B, Coronado GD, Martin DP. Barriers and facilitators related to mammography use among lower educated Mexican women in the USA. Social Science and Medicine. 2009;68(5):832–39. doi: 10.1016/j.socscimed.2008.12.023. Available from http://www.scopus.com/inward/record.url?eid=2-s2.0-60249099541&partnerID=40. [DOI] [PMC free article] [PubMed]
- 29.Freedman RA, He Y, Winer EP, Keating NL. Trends in racial and age disparities in definitive local therapy of early-stage breast cancer. Journal of Clinical Oncology. 2009;27(5):713–19. doi: 10.1200/JCO.2008.17.9234. Available from http://www.scopus.com/inward/record.url?eid=2-s2.0-59949093293&partnerID=40. [DOI] [PubMed]
- 30.Ward E, Halpern M, Schrag N, Cokkinides V, DeSantis C, Bandi P, et al. Association of insurance with cancer care utilization and outcomes. CA Cancer Journal for Clinicians. 2008;58(1):9–31. doi: 10.3322/CA.2007.0011. Available from http://www.scopus.com/inward/record.url?eid=2-s2.0-38349138848&partnerID=40. [DOI] [PubMed]
- 31.Abraham L, Geller BM, Yankaskas BC, Bowles EJA, Karliner LS, Taplin SH, et al. Accuracy of self-reported breast cancer among women undergoing mammography. Breast Cancer Research and Treatment. 2009;118(3):583–92. doi: 10.1007/s10549-009-0375-4. Available from http://www.scopus.com/inward/record.url?eid=2-s2.0-70449589766&partnerID=40&md5=d8e674b5b7ea69e552e109c023c618bb. [DOI] [PMC free article] [PubMed]
- 32.Bondy ML, Strom SS, Colopy MW, Brown BW, Strong LC. Accuracy of family history of cancer obtained through interviews with relatives of patients with childhood sarcoma. Journal of Clinical Epidemiology. 1994;47(1):89–96. doi: 10.1016/0895-4356(94)90037-x. Available from http://www.scopus.com/inward/record.url?eid=2-s2.0-0028089242&partnerID=40&md5=a4e70eb86dc9f644dcd2d41b0e106e24. [DOI] [PubMed]
- 33.Douglas FS, O'Dair LC, Robinson M, Evans DGR, Lynch SA. The accuracy of diagnoses as reported in families with cancer: A retrospective study. Journal of Medical Genetics. 1999;36(4):309–12. Available from http://www.scopus.com/inward/record.url?eid=2-s2.0-0032902796&partnerID=40&md5=8546eb0af3cdc50d81cf8627f77b1ef1. [PMC free article] [PubMed]
- 34.Garbers V, Toniolo PG, Taioli E. Changes in self-reported family history of breast cancer with change in case-control status. European Journal of Epidemiology. 2001;17(6):517–20. doi: 10.1023/a:1014500204757. Available from http://www.scopus.com/inward/record.url?eid=2-s2.0-0035723999&partnerID=40&md5=d8203d6e2f27a9699b078d19c201418e. [DOI] [PubMed] [Google Scholar]
- 35.Murff HJ, Spigel DR, Syngal S. Does this patient have a family history of cancer? An evidence-based analysis of the accuracy of family cancer history. Journal of the American Medical Association. 2004;292(12):1480–89. doi: 10.1001/jama.292.12.1480. Available from http://www.scopus.com/inward/record.url?eid=2-s2.0-4544308586&partnerID=40&md5=cd3d451223c8d7f0e43637efe9fddc9e. [DOI] [PubMed]
- 36.Nelson HD, Tyne K, Naik A, Bougatsos C, Chan BK, Humphrey L. Screening for breast cancer: An update for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151:727–37. doi: 10.1059/0003-4819-151-10-200911170-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abraido-Lanza AF, Dohrenwend BP, Ng-Mak DS, Turner JB. The Latino mortality paradox: A test of the 'salmon bias' and healthy migrant hypotheses. American Journal of Public Health. 1999;89(10):1543–48. doi: 10.2105/ajph.89.10.1543. Available from http://www.scopus.com/inward/record.url?eid=2-s2.0-2942540654&partnerID=40. [DOI] [PMC free article] [PubMed]
- 38.Lu Y. Test of the 'healthy migrant hypothesis': A longitudinal analysis of health selectivity of internal migration in Indonesia. Social Science and Medicine. 2008;67(8):1331–39. doi: 10.1016/j.socscimed.2008.06.017. Available from http://www.scopus.com/inward/record.url?eid=2-s2.0-50349103143&partnerID=40. [DOI] [PubMed]
- 39.Abraido-Lanza AF, Chao MT, Florez KR. Do healthy behaviors decline with greater acculturation? Implications for the Latino mortality paradox. Social Science & Medicine. 2005;61(6):1243–55. doi: 10.1016/j.socscimed.2005.01.016. Available from <Go to ISI>://000230557200010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lara M, Gamboa C, Kahramanian MI, Morales LS, Bautista DEH. Acculturation and Latino health in the United States: A review of the literature and its sociopolitical context. Annual Review of Public Health. 2005;26:367–97. doi: 10.1146/annurev.publhealth.26.021304.144615. Available from <Go to ISI>://000228981500017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Biffl WL, Myers A, Franciose RJ, Gonzalez RJ, Darnell D. Is breast cancer in young Latinas a different disease? American Journal of Surgery. 2001;182(6):596–600. doi: 10.1016/s0002-9610(01)00789-9. Available from http://www.scopus.com/inward/record.url?eid=2-s2.0-0035707148&partnerID=40. [DOI] [PubMed]
- 42.King DW, Miranda PY, Gor B, Fuchs-Young R, Chilton J, Hajek R, et al. Addressing cancer health disparities using a global biopsychosocial approach. Cancer. 2010;116(2):264–9. doi: 10.1002/cncr.24765. Available from http://www3.interscience.wiley.com/cgi-bin/fulltext/122687699/PDFSTART. [DOI] [PMC free article] [PubMed]