Abstract
Optical absorption is closely associated with many physiological important parameters, such as the concentration and oxygen saturation of hemoglobin, and it can be used to quantify the concentrations of non-fluorescent molecules. We propose a method to use acoustic spectra of photoacoustic signals to quantify the absolute optical absorption. This method is self-calibrating and thus insensitive to variations in the optical fluence. Factors, such as system bandwidth and acoustic attenuation, can affect the quantification but can be canceled by dividing the acoustic spectra measured at two optical wavelengths. Using the optical-resolution photoacoustic microscopy, we quantified the absolute optical absorption of black ink samples with various concentrations. We also quantified both the concentration and oxygen saturation of hemoglobin in a live mouse in absolute units.
Keywords: Photoacoustic tomography, acoustic spectrum, optical absorption coefficient, hemoglobin concentration, blood oxygen saturation
Total, oxygenated, and deoxygenated hemoglobin concentrations ([HbT], [HbO2], and [HbR]) are fundamental pathophysiological parameters in biomedicine. Several techniques have been developed to quantify hemoglobin concentration or blood oxygen saturation (sO2) in vivo, including near-infrared spectroscopy (NIRS), blood oxygen level dependent contrast magnetic resonance imaging, electron paramagnetic resonance imaging, positron emission tomography, and single photon emission computed tomography. However, all of these modalities have disadvantages: for example, poor spatial resolution, relative quantification, and undesirable contrast agent injection [1].
Photoacoustic (PA) tomography (PAT) has already demonstrated its ability to monitor biological hemodynamic functions without using exogenous contrast agents [2]. In PA imaging, the sample is illuminated, usually by a pulsed laser, and following the absorption of optical energy, an initial pressure is generated via thermo-elastic expansion. The PA waves then propagate and are detected by ultrasonic sensors. The strength of the initial pressure is directly proportional to the absorbed optical energy in the tissue, and therefore multi-wavelength PA measurements can yield optical absorption spectral information. Since ultrasound scattering is much weaker than optical scattering in biological tissues, PAT is capable of high resolution imaging at depths beyond the optical transport mean free path. Moreover, because PA imaging inherently exploits optical absorption contrast, it usually has a higher sensitivity to optical absorption than other optical imaging technologies [2]. By measuring the optical absorption spectrum with PA imaging, we can potentially quantify sO2 in the same way NIRS does [3], except with high spatial resolution and sensitivity.
Nevertheless, challenges remain. PA images are spatial mappings of the absorbed optical energy A(r⃑) (Jm−3), which is the product of the optical absorption coefficient μa(r⃑) (m−1) and the fluence F(r⃑) (Jm−2). To obtain the intrinsic tissue property μa(r⃑), we must somehow compensate for the extrinsic quantity F(r⃑). Currently, fluence compensation can be done invasively [1, 4] or non-invasively [5, 6]. The invasive method puts an optical absorber with a known spectrum close to the region of interest, and then normalizes the measured PA signals from the objects with the amplitudes of the PA signals from the absorber. The non-invasive method involves solving the radiative transfer equation (RTE) and PA wave equations with iterative algorithms. All of the methods mentioned above are based on the linear relationship between the local fluence incident on the blood vessel and the peak amplitude of the PA signal. The temporal profile of the PA signal was also used to quantify the optical absorption coefficients [7–10]. This method is self-calibrating since it depends on the relative temporal profile rather than the absolute amplitude of the PA signal. However, directly fitting the temporal profile may be inaccurate, since the temporal profiles are usually distorted by the limited bandwidth of ultrasonic detectors and the acoustic attenuation.
In this letter, we propose to use the acoustic spectra of the PA signals to quantify optical absorption. This method is also self-calibrating since it deals only with the relative change in various acoustic frequencies. The acoustic spectrum S(ω) of the received PA signal depends on three factors: 1) the ‘real’ object spectrum O(ω) measured with unit fluence, which is related to the target object’s shape, size, optical properties, and fluence incident directions; 2) the system dependent response H(ω), which is the Fourier transform of the PA signal from an ideal point absorber measured with this system without acoustic attenuation in the tissue; 3) the acoustic attenuation effect a(ω), which is related to the acoustic properties of the tissue that lies between the target objects and the detector. Based on the system linearity assumption, we have S(ω)=F(λ1)O(ω)H(ω)a(ω). The last two terms remain unchanged when samples are measured with the same system under the same condition, and therefore are usually cancellable. An obvious example is where light at various optical wavelengths is used to excite one blood vessel. Therefore, by simply dividing the PA acoustic spectra measured at two optical wavelengths, we can eliminate the system dependent effects and the acoustic attenuation effect. As such, the absolute value of μa can be quantified.
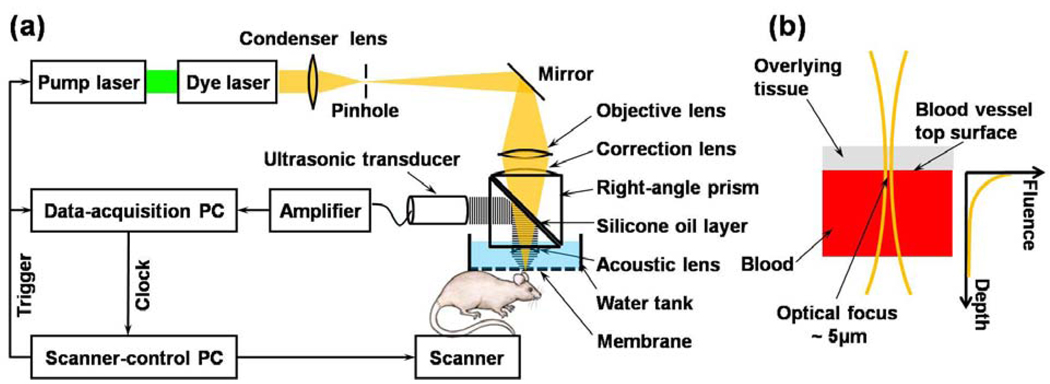
As the first feasibility study, we validated this idea using one form of PA imaging, optical resolution photoacoustic microscopy (OR-PAM) [11]. In OR-PAM, the lateral resolution of the system (5 µm) relied on optical focusing. The laser beam from the dye laser is focused by an objective lens, which is confocally configured with a focused ultrasonic transducer (50 MHz, 80% bandwidth) [Fig. 1(a)]. PA A-scan signals are acquired through time-resolved ultrasonic detections, and three-dimensional images were formed by raster scanning the ultrasonic transducer along the transverse plane. The surface of big blood vessels with >30 µm diameter can be roughly treated as a flat surface [Fig. 1(b)]. If we use F0 to denote the incident fluence on the surface of the blood vessel, the fluence inside the blood vessel obeys Beer’s law. O(t) = Γμa exp(−μact) (1), where c is the speed of sound in the biological tissue, and Γ is the Grüneisen coefficient. Here, the reduced scattering coefficient is much less than the absorption coefficient, because the anisotropy factor is so close to 1 in blood in the optical spectral region we used (around 585 nm); therefore, it is neglected. The 3D Gaussian beam profile (Rayleigh range~30 µm) should be considered if the assumption of laser beam collimation is invalid. If the PA signals of the blood vessel are measured at two optical wavelengths, the ratio of the spectra of the PA signals can be written as
| (2) |
where O(ω) is the Fourier transformation of Eq. (1). By fitting this ratio we can derive the absolute values of μa1, μa2, and F(λ1)/F(λ2).
Fig. 1. Schematic of the OR-PAM system and experimental setup.
(a) The schematic of the OR-PAM system. (b) The optical focus is much smaller than the targeted blood vessel, whose top surface within the optical focal diameter can therefore be approximated as a plane.
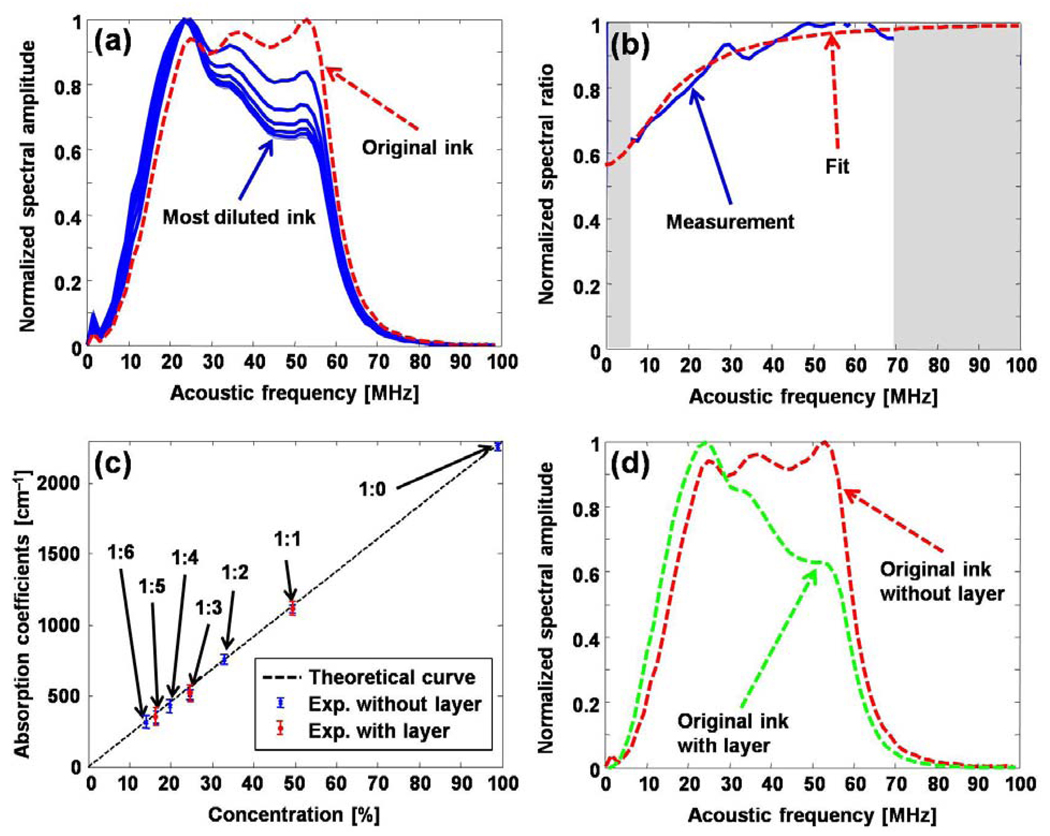
In a phantom study, the original black ink was diluted with water in six ratios ranging from 1:1 to 1:6. The original and diluted ink samples were sequentially placed in a container, sealed with plastic membrane, and then the container was placed in a water tank. PA A-line signals were acquired from these samples, and the acoustic spectra of the PA signals are shown in Fig. 2(a). Compared with the spectrum of the PA signal from the original ink sample, the spectra of the PA signals from the diluted ink samples are “shifted” to lower frequencies. Light penetrated deeper in lower concentration ink samples, and the corresponding PA signals decay more slowly in the time domain. Therefore, the spectra contain more low-frequency components. By dividing the measured spectra of any two ink samples frequency-by-frequency [Fig. 2(b)], we can find the absorption coefficients of both samples by fitting the resultant ratio curve with Eq. (2). Because parts of the spectra (grey bands) are unreliable due to the limited-band detection, they are not used for the fitting. By pairing the spectrum from the 1:1 diluted ink sample with the spectra from other six samples, we quantified the absorption coefficients of all seven samples [Fig. 2(c)]. To demonstrate that the recovered absorption coefficients are independent of acoustic attenuation and optical fluence, we covered three of the ink samples with an identical layer of optical phantom (~1.5 mm 2% Agar, 0.1% intralipid, 1% black ink). The spectra of the PA signals from the ink sample with and without this layer differ [Fig. 2(d)] because of the acoustic attenuation. Since the acoustic properties of the layer added between the samples and the detector are the same for the three ink samples, the acoustic attenuation can be cancelled by taking the ratio of the acoustic spectra of PA signals from any two covered ink samples. The quantification results are shown in Fig. 2(c).
Fig. 2. Quantifying the optical absorption coefficients of ink.
(a) Acoustic spectra of PA signals from original and diluted black ink samples. (b) Ratio of the acoustic spectral amplitudes of PA signals from two samples and fitting with the theoretical formula. (c) Fitting result with 7 phantoms (without the covered layer) and 3 samples (with the covered layer). (d) The acoustic spectra measured from the original black ink with and without the covered layer.
In another phantom study, we quantified the optical absorption of oxygenated bovine blood at three optical wavelengths (580 nm, 585 nm, and 590 nm). We adjusted the power of the incident laser pulses so that the peaks of the PA signal amplitudes are the same, although not required. From the point-by-point acoustic spectral ratios [Fig. 3(a)], the optical absorption coefficients of the sample were quantified to be 266±5 cm−1, 169±4 cm−1 and 80±4 cm−1 at the three optical wavelengths. The incident fluence ratios F13 = F(λ1)/F(λ3) and F23 = F(λ2)/F(λ3) were quantified to be 0.30±0.01 and 0.47±0.01, which agree with the experimentally measured laser power.
Fig. 3. Quantifying the optical absorption coefficients of ex vivo bovine blood and in vivo blood vessels in a nude mouse ear.
(a) Ratios of the acoustic spectral amplitudes of PA signals from oxygenated bovine blood measured ex vivo at various optical wavelengths and fittings with the theoretical formula. (b) Structural image acquired at 570 nm (inset), and ratios of the acoustic spectral amplitudes of PA signals measured in vivo with two optical wavelengths (570 nm and 561 nm) from arteries and veins.
In an in vivo experiment, we imaged a 1-mm-by-1-mm region in a nude mouse ear with two optical wavelengths (561 nm and 570 nm). The PA maximum amplitude projection (MAP) image was acquired with an optical wavelength of 570 nm. Each point in the MAP image records the maximum value of the PA A-scan. Two vessels marked with V1 and V2 in Fig. 3 (b) were selected for a quantitative study. The A-scans acquired within these two vessels were properly aligned by their maximum values and then averaged. Since the PA method is sensitive to boundaries of blood vessels [12], we usually can see both the top and bottom boundaries of the blood vessel in each A-scan. The bottom boundaries, where the light beam is no longer collimated, were removed from the data. The absorption coefficients and the incident fluence ratios were quantified from the acoustic spectral ratios [Fig. 3 (b)]. The [HbT], [HbO2], and [HbR], together with the sO2 values were calculated based on the quantified optical absorption coefficients at the two optical wavelengths (Table 1). According to the sO2 values, V1 and V2 were identified to be an arteriole-venule pair. If we ignore the wavelength-dependent fluence, the quantified sO2 values become inaccurate by approximately 8% and 11% for the artery and the vein, respectively. Moreover, because H(ω) and a(ω) are roughly same for both vessels, the optical absorption coefficients of both vessels can be quantified with a single optical wavelength (561 nm) measurement. The μa values at 561 nm for both blood vessels were quantified from the acoustic spectral ratio [Fig. 3 (b)]. The fitted μa agree with the values in Table 1.
Table 1.
The absorption coefficients, the fluence ratio, the hemoglobin concentrations, and the sO2 quantified from two optical wavelength measurements.
| μa(λ1) (cm−1) |
μa(λ2) (cm−1) |
F(λ2)/F(λ1) | [HbT] (g/L) |
[HbO2] (g/L) |
[HbR] (g/L) |
sO2 | sO2a | |
|---|---|---|---|---|---|---|---|---|
| V1 (Artery) | 143±3 | 188±4 | 0.96±0.01 | 110.6±8.1 | 106.2±4.3 | 4.4±3.8 | 0.96±0.04 | 0.88 |
| V2 (Vein) | 159±4 | 186±5 | 0.96±0.01 | 110.2±9.2 | 77.1±4.9 | 33.1±4.3 | 0.70±0.07 | 0.62 |
With F(λ2)/F(λ1) = 1
In summary, we demonstrated the feasibility of using the acoustic spectra to quantify the optical absorption coefficients in vivo with OR-PAM. This method is self-calibrating and thus is insensitive to absolute optical fluence. By taking advantage of the cancellation effect, the acoustic attenuation and system limited bandwidth can be corrected with multi-wavelength measurements. Moreover, this method can quantify the absolute value of μa, which can be used to quantify hemoglobin concentrations in absolute units.
Acknowledgements
This work was sponsored in part by National Institutes of Health grants R01 EB000712, R01 EB008085, R01 CA113453901, U54 CA136398, and 5P60 DK02057933. L.W. has a financial interest in Microphotoacoustics, Inc. and Endra, Inc., which, however, did not support this work.
References
- 1.Zhang HF, Maslov K, Sivaramakrishnan M, Stoica G, Wang LHV. Imaging of hemoglobin oxygen saturation variations in single vessels in vivo using photoacoustic microscopy. Applied Physics Letters. 2007;90:053901. [Google Scholar]
- 2.Wang LV, Wu H. Biomedical Optics: Principles and Imaging. Hoboken, NJ: Wiley; 2007. [Google Scholar]
- 3.Chance B, Borer E, Evans A, Holtom G, Kent J, Maris M, Mccully K, Northrop J, Shinkwin M. Optical and Nuclear-Magnetic-Resonance Studies of Hypoxia in Human Tissue and Tumors. Annals of the New York Academy of Sciences. 1988;551:1–16. doi: 10.1111/j.1749-6632.1988.tb22316.x. [DOI] [PubMed] [Google Scholar]
- 4.Maslov K, Zhang HF, Wang LV. Effects of wavelength-dependent fluence attenuation on the noninvasive photoacoustic imaging of hemoglobin oxygen saturation in subcutaneous vasculature in vivo. Inverse Problems. 2007;23:S113–S122. [Google Scholar]
- 5.Laufer J, Delpy D, Elwell C, Beard P. Quantitative spatially resolved measurement of tissue chromophore concentrations using photoacoustic spectroscopy: application to the measurement of blood oxygenation and haemoglobin concentration. Physics in Medicine and Biology. 2007;52:141–168. doi: 10.1088/0031-9155/52/1/010. [DOI] [PubMed] [Google Scholar]
- 6.Yuan Z, Jiang HB. Quantitative photoacoustic tomography: Recovery of optical absorption coefficient maps of heterogeneous media. Applied Physics Letters. 2006;88:231101. [Google Scholar]
- 7.Esenaliev RO, Larina IV, Larin KV, Deyo DJ, Motamedi M, Prough DS. Optoacoustic technique for noninvasive monitoring of blood oxygenation: a feasibility study. Applied Optics. 2002;41:4722–4731. doi: 10.1364/ao.41.004722. [DOI] [PubMed] [Google Scholar]
- 8.Laufer J, Elwell C, Delpy D, Beard P. In vitro measurements of absolute blood oxygen saturation using pulsed near-infrared photoacoustic spectroscopy: accuracy and resolution. Physics in Medicine and Biology. 2005;50:4409–4428. doi: 10.1088/0031-9155/50/18/011. [DOI] [PubMed] [Google Scholar]
- 9.Petrov YY, Petrova IY, Patrikeev IA, Esenaliev RO, Prough DS. Multiwavelength optoacoustic system for noninvasive monitoring of cerebral venous oxygenation: a pilot clinical test in the internal jugular vein. Optics Letters. 2006;31:1827–1829. doi: 10.1364/ol.31.001827. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Wang RK. Photoacoustic recovery of an absolute optical absorption coefficient with an exact solution of a wave equation. Physics in Medicine and Biology. 2008;53:6167–6177. doi: 10.1088/0031-9155/53/21/018. [DOI] [PubMed] [Google Scholar]
- 11.Maslov K, Zhang HF, Hu S, Wang LV. Optical-resolution photoacoustic microscopy for in vivo imaging of single capillaries. Optics Letters. 2008;33:929–931. doi: 10.1364/ol.33.000929. [DOI] [PubMed] [Google Scholar]
- 12.Guo Z, Li V, Wang LV. On the speckle-free nature of photoacoustic tomography. Medical Physics. 2009;36:4084–4088. doi: 10.1118/1.3187231. [DOI] [PMC free article] [PubMed] [Google Scholar]