Introduction
The recruitment, selection, and ovulation of follicles, termed folliculogenesis, result from a complex set of signals that are exchanged between the follicle and its environment. These interactions include circulating hormones, extracellular matrix (ECM) components, and mechanical signaling. The ovarian environment is highly dynamic, which has been commonly characterized by the cyclic changes in endocrine factors. Disruption of this dynamic interplay between the follicle and its environment, which can result from environmental toxins, disease, or disease therapies, underlies many causes of infertility. Although the significance of endocrine factors has been widely recognized, numerous other aspects of the ovarian environment are increasingly being recognized for their role in regulating folliculogenesis. Identifying the environmental mechanisms that regulate follicle development is essential for creating novel strategies to preserve fertility.
The field of biomaterials and regenerative medicine has been developing the tools to create tunable microenvironments, which can be employed to investigate the basic biology of tissue development and also to develop therapeutic strategies for tissue loss or organ failure. Biomaterials have been widely used for in vitro cell culture to provide support for cell growth and attachment within a three-dimensional architecture in the absence of the endogenous tissue. Many properties of tissue can be mimicked with biomaterials such as the mechanical strength, or the presentation or sequestration of biological signals. The potential of biomaterials to address significant clinical problems is exemplified by the biomaterial-based culture of urothelial and smooth muscle cells, which was employed to create a functional synthetic bladder [1]. The successful translation of this system from an animal model to human clinical use was reported in 2006 [2]. The success of this engineered bladder has motivated its methodology to be used in other areas of regenerative medicine. Using a similar method, an artificial vagina was recently developed for a rabbit-model that was integrated into the host tissue 6 months after vaginal replacement [3]. The need for fertility preservation for females facing cancer therapies provides an opportunity for biomaterials use in the field of reproductive biology [4-6]. Patients can elect to undergo hormonal stimulation prior to cancer treatment, but the feasibility of this option is dependent upon many variables, such as the patient’s age, the urgency of the cancer treatment, and the availability of a sperm donor. In the future, patients may be able to opt to bank a portion of an ovary for use with ovarian tissue transplantation or in vitro culture to preserve their fertility. The culture option, the focus of this chapter, requires a culture system for in vitro folliculogenesis that produces oocytes that are viable for in vitro maturation (IVM) and subsequent in vitro fertilization (IVF). It has been demonstrated that the biomaterial alginate, in the form of a hydrogel, can provide a permissive environment for the folliculogenesis of a two-layer secondary follicle, and the cultured oocyte can be fertilized to obtain a live birth in mice [7]. The recent successful growth of human two-layer secondary follicles into antral follicles in vitro [8] provides theoretical and practical basis to support the translation of this system to a clinical application. Recapitulating the native ovarian environment within an in vitro culture system is generally viewed as necessary to obtain the highest quality oocytes. Light micrographs typical of three stages of murine folliculogenesis in alginate culture are shown in Fig. 2.1. Further development and characterization of in vitro follicle culture is needed for translating this system from mice to the clinic.
Fig. 2.1.
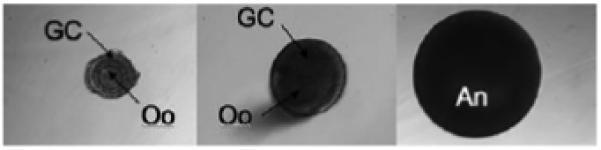
Stages of murine folliculogenesis during in vitro follicle culture. All follicles shown are encapsulated within alginate. A two-layered secondary follicle (left) is the most immature follicle that can be cultured to obtain a live birth in vitro. A multilayer secondary follicle (center) and antral follicle (right) are the subsequent stages of folliculogenesis. Both two-layered and multilayered secondary follicles are referred to as pre-antral. The oocyte (Oo), granulosa cells (GC), and antrum(An) are labeled. Note that the oocyte is obscured in the antral follicle by the multiple layers of granulosa cells.
The potential of biomaterials in reproductive biology is not limited to clinical applications and can be employed to investigate the mechanisms of follicle development. The goal of this chapter is to describe follicular interactions with their environment and the rational design of biomaterials to mimic and investigate those interactions. Biomaterial-based culture systems can be an enabling tool to investigate the spatio-temporal changes that occur within the follicle and the surrounding tissue. Follicle–environment interactions in this chapter are categorized as (i) extraovarian interactions and (ii) interactions between a follicle and the ovarian tissue.
These interactions are summarized in Fig. 2.2. This chapter reviews the recent advances in the design of biomaterials for follicle culture, and we refer to other reports for a more thorough history [9].
Fig. 2.2.
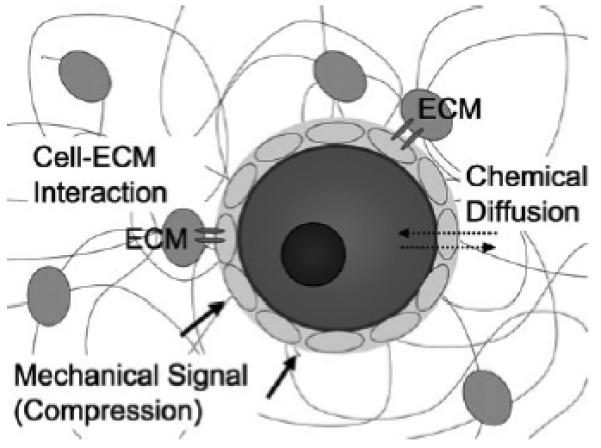
Interactions between the follicle and its environment in vitro. A representation of a follicle within a hydrogel matrix is shown. Extraovarian interactions are incorporated into an in vitro culture through diffusible factors. The physical properties of a hydrogel will impact how quickly diffusion occurs. Hydrogels mimic the role of ovarian tissue by presenting extracellular matrix (ECM) proteins, through which receptors on the plasma membrane can interact with the matrix. Mechanical signals are also presented by the hydrogel through a compressive force from the elastic rigidity of the matrix.
Extraovarian Interactions
The ovary receives numerous inputs from the systematic circulation, which we refer to here as extraovarian interactions. The systematic circulation is responsible for transporting biological signals, such as hormones, nutrients, waste, toxins, and oxygen. In mimicking the environment within the ovary, these factors are typically provided by addition to the cell culture media and are transported through the culture environment by diffusion. In particular, cells cultured within synthetic hydrogels recapitulate the 3D architecture observed in vivo; however, this matrix and the formation of the multi-cellular structure of the follicle can impose transport limitations with the potential to affect a range of cellular processes, which are discussed further below. Additionally, in vitro cultures can allow a molecule of interest to be investigated for its effects, which can identify the mechanisms of action that underlies in vivo biology. For instance, there has been an increasing concern on the effects of industrial chemicals, such as bisphenol A (BPA), on human fertility. The use of in vitro follicle culture as a bioassay [10, 11] provides a unique platform to investigate the mechanisms by which diffusible factors that can end up in the systemic circulation, such as hormones, nutrients, and environmental toxins, impact follicle growth and development. These systems have implications in the fertility of humans, livestock, and endangered species.
Introduction to Mass Transport in Hydrogels
Ahydrogel is a highly-water-swollen polymer network. Many hydrophilic polymers, such as fibrin, chitosan, and alginate will form hydrogels if they are cross-linked into a network. For instance, alginate is a naturally occurring polysaccharide that is composed of mannuronic acid and guluronic acid. When a divalent cation, such as calcium, is added to a solution of alginate, the anionic (carboxylic) group on two different residues of guluronic acid will form an electrostatic crosslink with a single calcium ion. The cross-linking between polymers will create a network, which creates the solid-like structure of the gel. This section provides a brief overview of hydrogels: what they are, how they form, and the influence their physical properties have on diffusible factors during cell culture.
Unlike an in vivo setting where there is an extensive vascular system to transport nutrients and waste efficiently, all transport in vitro must rely on diffusion. Direct interaction between a follicle and the surrounding biomaterial (such as ECM–integrin interactions) will be considered in later sections. Transport through the hydrogel network is determined largely by the hydrogel architecture and its chemistry. The architecture of the hydrogel’s network, as well as its chemical composition, is critical for providing a permissive environment for cell culture because it will determine the rate at which nutrient and waste move between the culture media and the encapsulated cells. The structure of the network can depend upon many conditions, such as the type(s) of polymers present, the concentration of the polymer, the molecular weight of the polymer, and the cross-linking conditions.
Two important properties of the hydrogel architecture that are influenced by polymer concentration and molecular weight are pore size and tortuosity. Any supplement provided in the culture media must diffuse into the hydrogel, as well as navigate from the surface of the hydrogel to the encapsulated cells. The time it takes for a solute to travel this route will be largely determined by the pore size and tortuosity. If the pore size is small, relative to the diffusing molecule, there may significant mass transport limitations. Consider bovine serum albumin (BSA), which has a Stokes radius of 3.6 nm [12], in 1.5% versus 3% alginate, which has a pore size of 17 and 15 nm, respectively [13]. In comparison to free diffusion in water, the diffusion rate of BSA in 1.5 and 3.0% alginate hydrogels is decreased by 27- and 48-fold, respectively [13]. Table 2.1 lists the diffusion coefficients for several solutes relevant to cell culture. Follicle-stimulating hormone (FSH) is another essential soluble factor for follicle culture [14]. The diffusion of FSH, a large protein hormone with a molecular weight of 3 × 104, has not been directly studied, but there is experimental evidence that the protein can be transported through the hydrogel; however, its rate of diffusion is hindered by alginate [15].
Table 2.1.
Diffusion coefficients (D) of different solutes in alginate hydrogels. The molecular weight of the alginate polymers was 350 kD and the hydrogel was crosslinked for 5 min in a calcium
| Solute | Stoke’s radius (nm) [10] |
D, water (cm2/s) [10] |
D, 1.5% alginate (cm2/s) [18] |
D, 3.0% alginate (cm2/s) [18] |
|---|---|---|---|---|
| Oxygen | 0.15a | 2.7 × 10−5 | 1.4 × 10−5 | 1.2 × 10−5 |
| Glucose | 0.35 | 9.2 × 10−6 | 6.0 × 10−6 | 6.2 × 10−6 |
| BSA | 3.6 | 9.6 × 10−7 | 3.5 × 10−8 | 2.0 × 10−8 |
van der Waals radius
Even though oxygen is small relative to the pore size, its diffusion is still decreased in alginate (Table 2.1); thus, not all diffusion effects can be explained by pore size. The cause of the slower diffusion rate is likely due to the tortuosity of the hydrogel’s architecture [13]. The tortuosity of a material describes how tortuous or “windy” of a path the solute must take in the hydrogel. The concept of tortuosity is exploited in size-exclusion chromatography techniques where smaller molecules elute from a packed column after larger molecules because there are more pores that a small molecule can fit into; thus, smaller molecules have a more tortuous path through the column. Note that the decrease in the diffusion coefficient of oxygen is relatively small in alginate, approximately twofold, but this example illustrates that the diffusion of a solute through a hydrogel is more complex than the relative size between the diffusing solute and the pores of the hydrogel.
Cross-linking conditions, such as duration of cross-linking and the concentration of cross-linking agent, will play an important role in the formation of the hydrogel architecture, and thus the transport of solutes as well. For instance, the structure of fibrin-alginate interpenetrating networks (FA-IPN), which has been successfully used for follicle culture [16], depends upon thrombin concentration. Thrombin is a serine protease that actives factor XIII, a transglutaminase, which covalently cross links glutamine and lysine residues on fibrinogen fibers. If fibrin is cross-linked with a high concentration of thrombin (500 IU/mL), the resulting network has thin, dense fibers relative to a network cross-linked with 5 IU/mL thrombin [16]. A denser matrix would be more likely to impair the diffusion of solutes through the scaffold. Therefore, many factors contribute to overall transport properties of hydrogels that are used for follicle encapsulation. Understanding these properties is essential for developing the follicle culture system, as well as proper interpretation of experimental results.
Ovarian Tissue Interactions
The ovarian tissue, which is composed primarily of extracellular matrix (ECM) and stromal cells, directly interacts with a follicle through biological and mechanical signaling. The ECM is a complex composite of fibrous proteins and polysaccharides and is present in all tissues in the body. There are structural elements called “binding motifs” on the ECM components that are recognized by receptors on the plasma membrane of cells. Integrins are the primary family of proteins responsible for ECM recognition and binding, and are ubiquitous across species and cell lines. Replicating the role of the ECM with biomaterials provides researchers with a tool to create artificial tissue for clinical therapies and in vitro cell culture, and will be the focus of this section. Many cellular and biological processes that occur in vivo, such as migration, differentiation, and angiogenesis, are supported by biomaterials that mimic the biological activity of the ECM. The influence of mechanical signaling on cells by the surrounding tissue can be as significant as the presence of a biological signal, such as a growth factor. For example, if stem cells are cultured on a gel with a high, intermediate, and lowmodulus, theywill differentiate into bone,muscle and neural cells, respectively, which is representative of their native tissue [17]. In general, a guiding principle of tissue engineering is to create materials with mechanical properties similar to the native tissue.
A key aspect of a biomaterial is its bioactivity, which typically entails incorporating factors that will promote tissue growth while excluding factors that may be inhibitory. Two aspects of bioactivity that are incorporated into a biomaterial are the support of cell adhesion and the presentation of growth factors. Cell adhesion can be supported by hydrogels in at least three ways: (i) using natural polymers with intrinsic biological activity, (ii) chemically modifying a material not otherwise recognized by the encapsulated cells with biologically active factors, and (iii) create a mixture of natural and synthetic materials, such as an interpenetrating network. Polymers with intrinsic biological activity are generally components of the extracellular matrix found in native tissue, such as fibrin and collagen. Inert materials, such as alginate, chitosan, and PEG, are not recognized by mammalian cells, but can still enhance cell culture by providing a 3D environment for cell development. Chemical modification of inert materials, such as the covalent attachment of integrin binding sequences or growth factors, allows for precise control of interactions between the biomaterial and the cultured cells. In regards to growth factors, these materials are being modified to support binding and/or release of growth factors, which can stimulate responses by cells entrapped within the gel.
Designer Environments for Follicle Culture
Creating an artificial environment for follicle development that is representative of the native tissue presents a unique engineering challenge because the ECM of the ovarian tissue exhibits spatio-temporal dynamics with respect to stage of the folliculogenesis, particularly in the basal lamina surrounding the granulosa cells [18-20]. Non-degradable follicle environments, such as encapsulation within alginate, have been shown to provide a permissive environment for follicle development and fertilizable oocytes in both 3D [7] and 2D environments [21]. Results with alginate have led to the development of a degradable cell-responsive matrix for follicle culture that is based on a fibrin-alginate interpenetrating network, which greatly enhanced oocyte quality relative to the alginate culture system [16]. Synthetic environments with tunable properties provides researchers with a tool to isolate the mechanisms underlying follicle–tissue interactions to shed light on the basic biology of follicle development as well as provide insight into how to improve existing in vitro culture conditions.
Modification of Synthetic Biomaterials for Follicle Culture
Integrin binding motifs and growth factors are frequently attached to synthetic biomaterials to provide bioactivity. A commonly used integrin-binding motif is the peptide sequence RGD (arginine–glycine–aspartic acid). The RGD motif is found on most ECM proteins, including laminin, collagen, and fibronectin. The presence of an RGD peptide on an otherwise inert hydrogel will support a variety of cell processes such as attachment, migration [22], proliferation, and differentiation [23]. Growth factors can similarly be presented from the matrix. Growth factors are not covalently attached to the ECM in vivo; however, the ECM does sequester growth factors through relatively weak interactions. Growth factors have been directly conjugated to the matrix, though a key consideration is that the chemistry for attachment must not affect the activity of the protein. Alternatively, hydrogels have been modified with motifs, such as heparin [24], that support the reversible binding of growth factors. The response of the cell to an immobilized growth factor may differ from the response to a soluble growth factor. For example, an immobilized growth factor may be more potent than a soluble growth factor, meaning that a lower concentration of an immobilized growth factor will have the same influence as a greater concentration of soluble growth factor.
The immobilization of growth factors and integrin binding motifs are enabling tools to precisely control the environment of the cell that would not otherwise be feasible. In a landmark study, it was demonstrated that endothelial cell shape controlled apoptosis, and cells that were able to spread out over a larger surface area had a significantly lower rate of apoptosis [25]. In addition to controlling the size of the domain, gradients of biological factors can be imprinted on a material, which has been employed to investigate the chemotactic response of cells. Chemotaxis is the biased migration of a cell from a low to a high concentration of a chemical agent, as opposed to a random walk if there is no biasing force. A gradient will be present anywhere that there is a chemical source (a cell secreting a biological factor) and a sink (the rest of the tissue). A well-characterized chemotactic response in reproductive biology is the directed movement of sperm toward the oocyte for fertilization. These chemotactic factors have been shown to accumulate in the follicular fluid of the follicle [26]. Although not currently used for follicle culture, gradients have been extensively studied for their use in other disciplines in regenerative medicine, particularly for nerve regeneration and the chemotactic response of axon growth cones.
The application of synthetic matrices to support follicle development has been a recent advance in the field. Inclusion of an RGD peptide on a hydrogel can influence murine [27] and ovine [28] granulosa cells cultured in a 2D environment. Murine granulosa cells cultured on RGD-modified alginate have increased survival and proliferation, as well as a different morphology, in comparison to alginate alone. Hormone secretion was also influenced by the attachment of an RGD sequence and was dependent on the density of the RGD peptide [27]. The success of RGD peptides influencing granulosa cell function motivated their application to follicle culture. Alginate modified with an RGD-binding motif significantly increased the growth of two-layer secondary follicles in comparison to alginate alone, and meiotic competency rates were improved as well [29]. Steroid release was also significantly different in the presence or absence of RGD peptides. The presence of RGD led to an increase in progesterone secretion and a decrease in estradiol and inhibin A secretion [29]. These results suggest that integrin interaction with the environment can enhance the development of follicles in vitro.
Incorporation of Natural Biomaterials for Follicle Culture
Polymers that are isolated from the ECM of tissue, such as collagen and fibrin, are intrinsically bioactive. There are several sub-types of collagen, which gives tissues, such as connective tissue and muscle tissue, its characteristic elastic strength. Fibrin is the ECM protein responsible for blood clotting and is formed via enzymatic crosslinking. Both fibrin and collagen have been used extensively in the field of biomaterials for creating artificial tissues. As mentioned previously, these materials can be formed into a single-component hydrogel, or they can be blended with another polymer to create an interpenetrating network (IPN).
Collagen was one of the first biomaterials used for 3D in vitro follicle culture [30]. In this study, which used hydrogels composed only of collagen, follicles survived in vitro for 2 weeks and multilayered follicles were formed, but no follicles proceeded to the antral stage [30]. More recently, buffalo pre-antral follicles encapsulated in collagen have been shown to develop an antrum [31]. Follicles have also been encapsulated in fibrin. However, enzymes secreted by the encapsulated follicle rapidly degraded the fibrin, and the 3D integrity of the follicle architecture was lost when it fell to the bottom of the culture plate [16]. Thus, fibrin alone cannot support 3D in vitro culture of follicles. Blends of an ECM component, either laminin, or fibrinogen, or collagen I, or collagen IV and alginate were used to study follicle-ECM interactions [29]. Interestingly, the influence of the ECM component on follicle development depended on the stage of the follicle upon encapsulation. For instance, relative to follicle growth in pure alginate, collagen IV enhanced the survival of two-layer secondary follicles, but diminished the survival of multilayer follicles [29]. Optimization of dynamic, synthetic materials has the potential to enhance follicle culture, and to understand how the follicle interacts with the ovarian tissue in vivo.
More recently, a fibrin-alginate interpenetrating network (FA-IPN) was developed for in vitro growth of follicles in order to combine the bioactive properties of fibrin, while maintaining the 3D structure of the follicle [16]. An IPN is a blend of at least two polymers where at least one polymer is cross-linked in the presence of another [32]. This results in an entangled network that gives the IPN its name. IPN’s can be advantageous because desirable properties, such as bioactivity and degradability, of more than one material can be utilized in a single system. In the case of the FA-IPN, alginate maintains the structure of the follicle because it is not degradable, and the fibrin provides bioactivity.
Interactions with the Mechanical Environment
Engineering synthetic tissues for cell culture requires an understanding of the biological cues presented by the system, and the mechanical signals that are presented as well. The mechanism by which cells translate a mechanical signal to a biological one, a process known as mechanotransduction, is currently under investigation. Although not completely understood, mechanotransduction is exhibited by many cell types, and disruptions in the mechanical environment of tissues is associated with disease phenotypes, as is the case with arthrosclerosis, where hardening of the arteries is observed. Tissue rigidity is also thought to play a role in the progression of breast cancer [33, 34], which is often detected physically through palpation.
Polycystic ovarian syndrome (PCOS) is a common cause of infertility in young women. It has been suggested that a change in the mechanical environment of developing follicles contributes to the anovulation observed in patients with PCOS [35]. This hypothesis is supported by the observation that immature follicles cultured in a more rigid 3D environment are less able to proceed through folliculogenesis to the antral stage [35, 36]. In a proteomic comparison of polycystic ovaries (PCOs) and normal ovaries [37], alterations in protein expression were observed that could lead to accumulation of fibrin and collagen. Specifically, an increase in the level of fibrinogen precursors was present, which could potentially impair the fibrinolysis pathway in the PCOs. Additionally, both a collagen precursor and a chaperone protein (HSP47), the latter of which stabilizes pre-collagen and aids in the assembly of collagen fibers [38], had increased expression in the PCOs [37]. An increase in the deposition of ECM from these observed changes in protein expression could lead to hardening of the ovarian tissue. Most studies investigating mechanotransduction have used 2D culture conditions because the physical properties of the system can be independently manipulated more easily. However, if cultured in a 2D environment, granulosa cells will detach and migrate from the developing oocyte. Thus, an in vitro system for folliculogenesis presents a novel system in which to study basic biological questions governing interactions between cells and a 3D mechanical environment.
The mechanical properties of the environment can determine if the environment is permissive for follicle development [35, 36]. As a follicle develops in a 3D environment in vitro, its diameter increases, and the surrounding hydrogel will exert a compressive force on the follicle in response to the expansion. The compressive force is dependent upon the elastic strength of the hydrogel, as well as the change in size of the follicle. The volume of the hydrogel that is displaced by the developing follicle increases as r3, where r is the radius of the follicle, but the surface area that is acted on by the compressive force increases only as r2. During murine folliculogenesis, the approximate changes in dimensions are an 11-fold increase in surface area and 37-fold increase in volume, starting from a two-layered secondary size of!120 μm and ending at an antral size of!400 μm. To date, researchers have been successful in creating systems that are permissive for follicles at this stage of development. In primates, the change in volume relative to surface area during folliculogenesis is much more significant than in murine follicles, which may present a challenge in translating a murine to a human follicle culture system that could be used clinically. Specifically, a human follicle, if cultured from a two-layered secondary follicle (!120 μm) to a large antral follicle (!20 mm), would have a 28,000-fold increase in surface area and a 4.7 million fold increase in volume. Therefore, the stress profile in a human follicle culture may be significantly different than a murine follicle culture, and this may contribute to the reason why materials optimized for murine follicle culture remain sub-optimal in human follicle culture. Creating a permissive in vitro system for human follicle growth that has clinical applications for fertility preservation must be able to reproducibly yield large antral follicles, so that IVM and IVF could be successfully administered at a reasonable success rate.
Characterizing the physical properties of biomaterials is essential to investigating the influence of the mechanical environment on cells created by an in vitro culture system, as well as to determine the mechanics of healthy and diseased tissue. Materials are characterized using techniques from rheology, which is the study of flow phenomenon, and a rheometer is the instrument commonly used to determine the mechanical properties of a material. Most biomaterials, both synthetic and natural, are polymers, and thus exhibit properties of both a liquid and a solid – a phenomenon known as viscoelasticity due to the viscous nature of liquids and the elastic nature of solids. The underlying cause of the viscoelasticity of polymeric materials is their chain length. As the chains of polymers become entangled, their movement becomes increasingly restricted. A liquid material that is entangled at the molecular level cannot flow as freely as a non-polymeric liquid, which results in an elastic response, as opposed to a viscous response. Therefore, parameters such as the polymer concentration, the molecular weight, and the degree of polymer branching will increase chain entanglements, and thus the mechanical strength of the material. Although viscoelastic properties are beneficial for tissue engineering, they are difficult to characterize rigorously because the viscosity and the modulus (the measure of elasticity) are dependent upon the time scale of the experiment. For instance, the elastic and viscous response of a material can be separated into the storage (i.e., a solid stores and remembers its original shape) and loss (i.e., a liquid losses and forgets its original shape) modulus, respectively, in small amplitude oscillatory shear (SAOS) experiments, which is a common technique used in rheometry to study viscoelasticity. However, the measured moduli are dependent upon the frequency of the oscillations, which contrasts with a material such as glycerol, which has a viscosity independent of frequency. A schematic of a rheometer for SAOS is shown in Fig. 2.3.
Fig. 2.3.
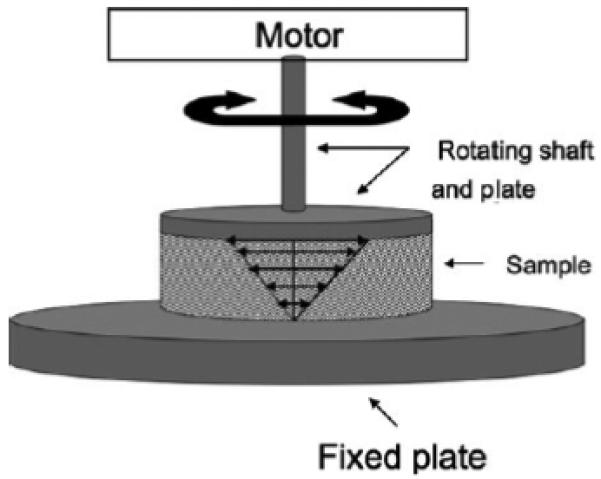
Rheometer schematic for small amplitude oscillatory shear. A motor oscillates a rotating shaft and plate at varying frequencies at a fixed strain amplitude. An idealized velocity profile within the sample is shown. In response to the applied strain, the material will exert a torque on the rheometer. If the sample is viscoelastic, the torque will be dependent on the applied frequency, as will the phase angle between the torque and the strain. The torque and phase angle are the only measurements needed to calculate the storage and loss modulus of the sample
The initial study to indicate an impact of environmental rigidity on follicle growth in vitro used varying concentrations of alginate to control the hydrogel rigidity [36]. Increasing the concentration of the polymer in solution is a well-known method of increasing the modulus of a hydrogel. A 0.25% alginate hydrogel, which creates relatively soft beads, was more permissive for follicle growth than the other concentrations tested (0.5, 1, and 1.5%). The 0.25% hydrogel improved growth, increased steroidogenesis of estradiol and androstenedione, and had a higher yield of meiotically competent oocytes. Varying the solids content of a hydrogel is a simple method to modulate the mechanics; however, as previously discussed, the solids content can also impact the transport of solutes due to a changing pore size.
A subsequent study attempted to isolate the impact of the mechanical properties from the transport effects. Chemical modification is an alternative means to control the mechanical properties of alginate hydrogels [39]. By using an irradiation source or an oxidizing agent, the individual polymer chains can be broken to reduce the average molecular weight, which will decrease the rigidity of the crosslinked hydrogel at a fixed solids concentration. Hydrogels with a decreased elastic modulus, created either through irradiation or through oxidation of the alginate, led to improved follicle growth. Furthermore, follicles encapsulated in materials with a lower solids content had higher rates of antrum formation than follicles encapsulated in materials with a higher solids content, but a similar gel stiffness. Alginate hydrogels formed with a lower solids content have a larger pore size, suggesting that transport of diffusible factors is significant in follicle culture even at low concentrations of alginate. Interestingly, the antrum has been hypothesized as a means for the follicle to overcome transport limitations as the diameter of follicle rapidly expands during the latter stages of folliculogenesis [40, 41].
The mechanism underlying mechanical signaling on folliculogenesis was investigated through gene expression profiles, which were compared between and mechanically permissive (soft) and non-permissive (rigid) environments [42]. Gene expression profiles associated with steroidogenic pathways (Star, Cyp11a1, Hsd3b1, Cyp17a1, Cyp19a1 and Lhcgr), oxidative stress (Hif1a), and water transport (Aqp7 and Aqp8) differed significantly between the two mechanical conditions. The follicle-stimulating hormone (FSH) receptor gene (Fshr) was the only gene reported that did not differ significantly between the two conditions at any time point [42]. These results indicate that mechanical environment impacts numerous biochemical pathways that influence follicle growth. In light of this, genomic techniques, such as gene microarrays, may give significant insight into the complex interactions between a follicle and its environment.
Conclusion
The application of biomaterials to reproductive biology provides a means to advance scientific understanding of reproduction and holds promise for translation to clinical use. Currently, an alginate encapsulation system can be used to obtain a live birth in mice, but further optimization is required to achieve this result in humans. Interaction between a material and a follicle during in vitro culture are complex and result from changes in transport properties, physical support of a 3D architecture, and biological and mechanical signaling. Characterizing follicular interactions with its environment draws from principles in engineering, biology, and medicine; exemplifying the need for an interdisciplinary approach to improve existing methods for in vitro folliculogenesis. By creating systems with tunable properties, scientists and engineers can advance reproductive technologies and provide scientific insight to the field of reproduction.
Acknowledgments
This research was supported by the Oncofertility Consortium NIH 8UL1DE019587, 5RL1HD058296.
Footnotes
References
- 1.Oberpenning F, Meng J, Yoo JJ, Atala A. De novo reconstitution of a functional mammalian urinary bladder by tissue engineering. Nat Biotechnol. 1999;17:149–55. doi: 10.1038/6146. [DOI] [PubMed] [Google Scholar]
- 2.Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367:1241–6. doi: 10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- 3.De Philippo R, Bishop C, Filho L, Yoo J, Atala A. Tissue engineering a complete vaginal replacement from a small biopsy of autologous tissue. Transplantation. 2008;86:208–14. doi: 10.1097/TP.0b013e31817f1686. [DOI] [PubMed] [Google Scholar]
- 4.Jeruss J, Woodruff T. Preservation of Fertility in Patients with Cancer. N Engl J Med. 2009;360:902–11. doi: 10.1056/NEJMra0801454. 2 Designing Follicle–Environment Interactions with Biomaterials 23
- 5.Nieman C, Kazer R, Brannigan R, Zoloth L, Chase-Lansdale P, Kinahan K, Dilley K, Roberts D, Shea L, Woodruff T. Cancer survivors and infertility: a review of a new problem and novel answers. J Support Oncol. 2006;4:171–8. [PubMed] [Google Scholar]
- 6.West E, Zelinski M, Kondapalli L, Gracia C, Chang J, Coutifaris C, Critser J, Stouffer R, Shea L, Woodruff T. Preserving female fertility following cancer treatment: current options and future possibilities. Pediatr Blood Cancer. 2009;53:289–95. doi: 10.1002/pbc.21999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu M, Kreeger PK, Shea LD, Woodruff TK. Tissue-engineered follicles produce live, fertile offspring. Tissue Eng. 2006;12:2739–46. doi: 10.1089/ten.2006.12.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu M, Barrett S, West-Farrell E, Kondapalli L, Kiesewetter S, Shea L, Woodruff T. In vitro grown human ovarian follicles from cancer patients support oocyte growth. Hum Reprod. 2009;24:2531–40. doi: 10.1093/humrep/dep228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.West ER, Shea LD, Woodruff TK. Engineering the follicle microenvironment. Semin Reprod Med. 2007;25:287–99. doi: 10.1055/s-2007-980222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lenie S, Cortvrindt R, Eichenlaub-Ritter U, Smitz J. Continuous exposure to bisphenol A during in vitro follicular development induces meiotic abnormalities. Mutat Res. 2008;651:71–81. doi: 10.1016/j.mrgentox.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 11.Sun F, Betzendahl I, Shen Y, Cortvrindt R, Smitz J, Eichenlaub-Ritter U. Preantral follicle culture as a novel in vitro assay in reproductive toxicology testing in mammalian oocytes. Mutagenesis. 2004;19:13–25. doi: 10.1093/mutage/geg040. [DOI] [PubMed] [Google Scholar]
- 12.Frigon RP, Leypoldt JK, Uyeji S. Disparity between Stokes radii of dextrans and proteins as determined by retention volume in Gel Permeation Chromatography. Anal Chem. 1983;55:1349–54. doi: 10.1021/ac00259a037. [DOI] [PubMed] [Google Scholar]
- 13.Li RH, Altreuter DH, Gentile FT. Transport characterization of hydrogel matrices for cell encapsulation. Biotechnol Bioeng. 1996;50:365–73. doi: 10.1002/(SICI)1097-0290(19960520)50:4<365::AID-BIT3>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 14.Cortvrindt R, Smitz J, VanSteirteghem AC. Assessment of the need for follicle stimulating hormone in early preantral mouse follicle culture in vitro. Hum Reprod. 1997;12:759–68. doi: 10.1093/humrep/12.4.759. [DOI] [PubMed] [Google Scholar]
- 15.Heise M, Koepsel R, Russell AJ, McGee EA. Calcium alginate microencapsulation of ovarian follicles impacts FSH delivery and follicle morphology. Reprod Biol Endocrinol. 2005;3:1–8. doi: 10.1186/1477-7827-3-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shikanov A, Xu M, Woodruff T, Shea L. Interpenetrating fibrin–alginate matrices for in vitro ovarian follicle development. Biomaterials. 2009;30:5476–85. doi: 10.1016/j.biomaterials.2009.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 18.Berkholtz C, Lai B, Woodruff T, Shea L. Distribution of extracellular matrix proteins type I collagen, type IV collagen, fibronectin, and laminin in mouse folliculogenesis. Histochem Cell Biol. 2006;126:583–92. doi: 10.1007/s00418-006-0194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodgers RJ, Irving-Rodgers HF, Russell DL. Extracellular matrix of the developing ovarian follicle. Reproduction. 2003;126:415–24. doi: 10.1530/rep.0.1260415. [DOI] [PubMed] [Google Scholar]
- 20.Rodgers RJ, Irving-Rodgers HF, van Wezel IL. Extracellular matrix in ovarian follicles. Mol Cell Endocrinol. 2000;163:73–9. doi: 10.1016/s0303-7207(00)00219-7. [DOI] [PubMed] [Google Scholar]
- 21.Cortvrindt R, Smitz J, Van Steirteghem AC. In-vitro maturation, fertilization and embryo development of immature oocytes from early preantral follicles from prepuberal mice in a simplified culture system. Hum Reprod. 1996;11:2656–66. doi: 10.1093/oxfordjournals.humrep.a019188. [DOI] [PubMed] [Google Scholar]
- 22.Maheshwari G, Brown G, Lauffenburger DA, Wells A, Griffith LG. Cell adhesion and motility depend on nanoscale RGD clustering. J Cell Sci. 2000;113:1677–86. doi: 10.1242/jcs.113.10.1677. [DOI] [PubMed] [Google Scholar]
- 23.Rowley J, Mooney D. Alginate type and RGD density control myoblast phenotype. J Biomed Mater Res. 2002;60:217–23. doi: 10.1002/jbm.1287. [DOI] [PubMed] [Google Scholar]
- 24.Cushing MC, Liao JT, Jaeggli MP, Anseth KS. Material-based regulation of the myofibroblast phenotype. Biomaterials. 2007;28:3378–87. doi: 10.1016/j.biomaterials.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–8. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 26.Ralt D, Manor M, Cohen-Dayag A, Tur-Kaspa I. Chemotaxis and chemokinesis of human spermatozoa to follicular factors. Biol Reprod. 1994;50:774–85. doi: 10.1095/biolreprod50.4.774. 24 R.M. Smith et al.
- 27.Kreeger PK, Woodruff TK, Shea LD. Murine granulosa cell morphology and function are regulated by a synthetic Arg-Gly-Asp matrix. Mol Cell Endocrinol. 2003;205:1–10. doi: 10.1016/s0303-7207(03)00209-0. [DOI] [PubMed] [Google Scholar]
- 28.Huet C, Pisselet C, Mandon-Pepin B, Monget P, Monniaux D. Extracellular matrix regulates ovine granulosa cell survival, proliferation and steroidogenesis: relationships between cell shape and function. J Endocrinol. 2001;169:347–60. doi: 10.1677/joe.0.1690347. [DOI] [PubMed] [Google Scholar]
- 29.Kreeger P, Deck J, Woodruff T, Shea L. The in vitro regulation of ovarian follicle development using alginate-extracellular matrix gels. Biomaterials. 2006;27:714–23. doi: 10.1016/j.biomaterials.2005.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Torrance C, Telfer E, Gosden RG. Quantitative study of the development of isolated mouse pre-antral follicles in collagen gel culture. Reproduction. 1989;87:367–74. doi: 10.1530/jrf.0.0870367. [DOI] [PubMed] [Google Scholar]
- 31.Sharma GT, Dubey PK, Meur SK. Survival and developmental competence of buffalo preantral follicles using three-dimensional collagen gel culture system. Anim Reprod Sci. 2009;114:115–24. doi: 10.1016/j.anireprosci.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 32.Sperling LH, Mishra V. The current status of interpenetrating polymer networks. Polym Adv Technol. 1996;7:197–208. [Google Scholar]
- 33.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–54. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 34.Peyton S, Ghajar C, Khatiwala C, Putnam A. The emergence of ECM mechanics and cytoskeletal tension as important regulators of cell function. Cell Biochem Biophys. 2007;47:300–20. doi: 10.1007/s12013-007-0004-y. [DOI] [PubMed] [Google Scholar]
- 35.West E, Xu M, Woodruff T, Shea L. Physical properties of alginate hydrogels and their effects on in vitro follicle development. Biomaterials. 2007;28:4439–48. doi: 10.1016/j.biomaterials.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu M, West E, Shea LD, Woodruff TK. Identification of a stage-specific permissive in vitro culture environment for follicle growth and oocyte development. Biol Reprod. 2006;75:916–23. doi: 10.1095/biolreprod.106.054833. [DOI] [PubMed] [Google Scholar]
- 37.Ma X, Fan L, Meng Y, Hou Z, Mao Y, Wang W, Ding W, Liu J. Proteomic analysis of human ovaries from normal and polycystic ovarian syndrome. Mol Hum Reprod. 2007;13:527–35. doi: 10.1093/molehr/gam036. [DOI] [PubMed] [Google Scholar]
- 38.Tasab M, Batten MR, Bulleid NJ. Hsp47: a molecular chaperone that interacts with and stabilizes correctly-folded procollagen. EMBO J. 2000;19:2204–11. doi: 10.1093/emboj/19.10.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boontheekul T, Kong H, Mooney D. Controlling alginate gel degradation utilizing partial oxidation and bimodal molecular weight distribution. Biomaterials. 2005;26:2455–65. doi: 10.1016/j.biomaterials.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 40.Gosden RG, Byattsmith JG. Oxygen concentration gradient across the ovarian follicular epithelium – model, predictions and implications. Hum Reprod. 1986;1:65–8. doi: 10.1093/oxfordjournals.humrep.a136362. [DOI] [PubMed] [Google Scholar]
- 41.Redding GP, Bronlund JE, Hart AL. Mathematical modelling of oxygen transport-limited follicle growth. Reproduction. 2007;133:1095–106. doi: 10.1530/REP-06-0171. [DOI] [PubMed] [Google Scholar]
- 42.West-Farrell E, Xu M, Gomberg M, Chow Y, Woodruff T, Shea L. The mouse follicle microenvironment regulates antrum formation and steroid production: alterations in gene expression profiles. Biol Reprod. 2008;80:432–9. doi: 10.1095/biolreprod.108.071142. [DOI] [PMC free article] [PubMed] [Google Scholar]


