Abstract
Purpose
Sacral insufficiency (SI) fractures can occur as a late side effect of pelvic radiation therapy. Our goal was to determine the incidence, risk factors, and clinical course of SI fractures in patients treated with preoperative chemoradiation for rectal cancer.
Materials and Methods
Between 1989 and 2004, 562 patients with non-metastatic rectal adenocarcinoma were treated with preoperative chemoradiation followed by mesorectal excision. The median radiotherapy dose was 45 Gy. The hospital records and radiology reports of these patients were reviewed to identify those with pelvic fractures. Radiology images of patients with pelvic fractures were then reviewed to identify those with SI fractures.
Results
Among the 562 patients, 15 had SI fractures. The 3-year actuarial rate of SI fractures was 3.1%. The median time to SI fractures was 17 months (range, 2–34 months). The risk of SI fractures was significantly higher in women compared to men (5.8% vs. 1.6%, p = 0.014), and in whites compared with non-whites (4% vs. 0%, p = 0.037). On multivariate analysis, gender independently predicted for the risk of SI fractures (hazard ratio, 3.25; p = 0.031). Documentation about the presence or absence of pain was available for 13 patients; of these 7 (54%) had symptoms requiring pain medications. The median duration of pain was 22 months. No patient required hospitalization or invasive intervention for pain control.
Conclusions
SI fractures were uncommon in patients treated with preoperative chemoradiation for rectal cancer. The risk of SI fractures was significantly higher in women. Most cases of SI fractures can be managed conservatively with pain medications.
Keywords: Insufficiency fracture, Pelvic fracture, Rectal cancer, Radiation therapy, Toxicity
INTRODUCTION
Randomized trials have shown that adjuvant chemoradiation increases local control in patients with Stage II and III rectal cancer (1-4). More recently, a randomized trial showed that preoperative chemoradiation reduces local recurrence and toxicity compared with postoperative chemoradiation (5). Based on these trials, preoperative and postoperative chemoradiation are being commonly used for rectal cancer. However, pelvic radiotherapy for rectal cancer can result in a number of acute and late toxicities. The widespread use of radiotherapy for rectal cancer accentuates the importance of understanding these toxicities.
Sacral insufficiency fractures are uncommon late complications of pelvic radiotherapy that can cause significant morbidity (6-14). Although many studies have investigated insufficiency fractures after radiotherapy for gynecologic malignancies, the incidence and clinical course of insufficiency fractures in rectal cancer patients have not been well characterized. Hence, the goal of this study was to evaluate the incidence, risk factors, and clinical course of sacral insufficiency fractures among 562 patients treated with preoperative chemoradiation and mesorectal excision for rectal cancer.
MATERIALS AND METHODS
Patient selection
Between November 1989 and July 2004, 562 patients with newly diagnosed rectal adenocarcinoma (located ≤12 cm from the anal verge) and no evidence of distant metastasis received preoperative radiotherapy and concurrent chemotherapy followed by mesorectal excision at the University of Texas M. D. Anderson Cancer Center. The hospital, radiation oncology, and diagnostic radiology records of these patients were reviewed for this study. The University of Texas M. D. Anderson Institutional Review Board approved this study.
Patient characteristics and treatment
The patient, tumor, and treatment characteristics of these 562 patients have been reported previously (15). The clinical tumor classification, based on endoscopic ultrasound and/or computed tomography (CT) scan, was T2 in 31 patients (6%), T3 in 474 patients (84%), T4 in 49 patients (9%), and unknown in 8 patients (1%) (Table 1) (16). The clinical lymph node classification was N0 in 248 patients (44%), N1 in 301 patients (54%), N2 in 4 patients (1%), and unknown in 9 patients (2%).
Table 1.
Patient, tumor, and treatment characteristics
| Characteristic | No. of patients (%) |
|---|---|
| Age, median (range) | 57.9 y (20.7–87.8 y) |
| Gender | |
| Male | 354 (63%) |
| Female | 208 (37%) |
| Race | |
| White | 438 (78%) |
| Others | 124 (22%) |
| Clinical T classification | |
| T2 | 31 (6%) |
| T3 | 474 (84%) |
| T4 | 49 (9%) |
| Unknown | 8 (1%) |
| Clinical N classification | |
| N0 | 248 (44%) |
| N1–2 | 305 (54%) |
| Unknown | 9 (2%) |
| Radiotherapy dose, median (range) | 45 Gy (19.8–58.6 Gy) |
| Concurrent chemotherapy | |
| Protracted infusional 5-fluorouracil | 430 (77%) |
| Capecitabine | 114 (20%) |
| Uracil/Tegafur | 9 (2%) |
| Other | 9 (2%) |
| Surgery | |
| Low anterior resection | 234 (42%) |
| Proctectomy with coloanal anastomosis | 140 (25%) |
| Abdominoperineal resection | 161 (29%) |
| Pelvic exenteration | 22 (4%) |
| Others | 5 (1%) |
All patients received preoperative radiation therapy with concurrent chemotherapy, followed by mesorectal excision. Of the 562 patients, 548 (98%) were treated with a three-field technique (one posterior field and two lateral fields) and an open tabletop (belly board) device for bowel exclusion. Radiation therapy was delivered in 1.8-Gy fractions, Monday through Friday, typically over 5 or 6 weeks, using 15–18 MV photons and customized blocking. The median dose of radiotherapy was 45 Gy (range, 19.8–58.6 Gy). A radiation dose of at least 45 Gy was administered to 555 (99%) patients, of whom 363 (65%) received a dose of exactly 45 Gy and 192 (34%) received a dose of 45 Gy, as well as a sequential or concurrent boost (5.4–7.5 Gy in most cases). Concurrent chemotherapy was given with continuous infusional 5-fluorouracil to 430 patients (77%) at a median dose of 300 mg/m2 (range, 250–300 mg/m2) administered Monday through Friday. Concurrent chemotherapy was given with capecitabine to 114 patients (20%) at a median dose of 1650 mg/m2 per day (range, 1,050–2,000 mg/m2/day) typically Monday through Friday. Concurrent chemotherapy was given with uracil and Tegafur to 9 patients (2%) and with other fluoropyrimidine-based regimens to 9 patients (2%). The surgical procedures included low anterior resection in 234 (42%) patients, proctectomy with coloanal anastomosis in 140 (25%) patients, abdominoperineal resection in 161 (29%) patients, pelvic exenteration in 22 (4%) patients, and other procedures in 5 (1%) patients.
Patients were scheduled for follow-up visits every 3 to 4 months for the first 2 years, every 6 months for up to 5 years, and annually thereafter with a medical oncologist, surgical oncologist, or radiation oncologist. For most patients, follow-up evaluations included abdomen and pelvic CT scans at least every 6 months for the first 2 years and every year thereafter. Patients underwent a median of seven follow-up pelvic CT scans, and 74% of patients had at least four follow-up pelvic CT scans. The median follow-up time was 49 months (range, 0–188 months).
Identification of insufficiency fractures
Radiology reports and clinical follow-up notes were carefully reviewed for all 562 patients to identify those with possible fractures in any pelvic site. For patients with suspected pelvic fracture, the CT scans were reviewed by an attending radiologist (P.R.B.) to identify the type of fracture and the earliest date at which imaging studies showed evidence of a fracture. The hospital records of patients with identified sacral insufficiency fractures were then further reviewed, with particular attention to history, reported symptoms, pain medications, invasive procedures, and other pertinent medical information.
Statistical analysis
The cumulative incidence of sacral insufficiency fracture was estimated by Kaplan-Meier methods (17). Log–rank tests were performed to identify significant univariate predictors of sacral insufficiency fracture risk. The following potential predictors were evaluated: age, race, gender, and radiation dose. Cox proportional hazards regression analysis was then used to identify significant multivariate predictors of sacral insufficiency fracture risk. A backward selection process was used, and all variables that were significant on the univariate analysis were entered into the multivariate model. A p value of <0.05 was considered significant.
RESULTS
Incidence and risk factors
Among the 562 patients, 15 developed sacral insufficiency fractures. The estimated 3-year actuarial rate of sacral insufficiency fractures was 3.1% (Fig. 1). The median time to sacral insufficiency fractures was 17 months (range, 2–34 months). Five patients had pubic fractures associated with sacral insufficiency fractures, an association that has been reported previously (18, 19). Only 1 patient developed avascular necrosis of the femur, and no patients developed a femoral neck fracture.
Fig. 1.
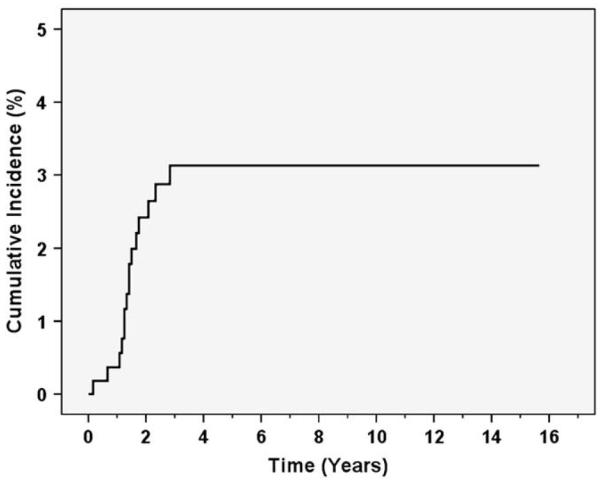
Kaplan-Meier estimates of cumulative incidence of sacral insufficiency fractures.
The risk of sacral insufficiency fractures was significantly higher in women compared with men (p = 0.014), and in whites compared with non-whites (p = 0.037), on univariate analysis (Table 2). The risk of sacral insufficiency fractures was not significantly associated with age (p = 0.418) or radiotherapy dose (p = 0.143), although the incidence of fracture was numerically higher in patients with age ≥60 years and in patients treated with radiation dose >45 Gy (Table 2). On multivariate analysis, gender was the only factor that independently predicted for the risk of sacral insufficiency fractures (p = 0.031), with a hazard ratio of 3.25 (95% confidence interval, 1.11–9.51). On multivariate analysis, race was not significantly associated with the risk of sacral insufficiency fractures (p = 0.959).
Table 2.
Univariate predictors of sacral insufficiency fractures
| Predictor | 3-y fracture risk (%) |
p |
|---|---|---|
| Gender | ||
| Female | 5.8 | 0.014* |
| Male | 1.6 | |
| Race | ||
| White | 4 | 0.037* |
| Others | 0 | |
| Age | ||
| <60 | 2.6 | 0.418 |
| ≥60 | 3.8 | |
| Radiotherapy dose | ||
| ≤45 Gy | 2.3 | 0.143 |
| >45 Gy | 4.6 |
p < 0.05.
Clinical course
Information regarding symptoms and management was available for 13 of the 15 patients with sacral insufficiency fractures. Of these 13 patients, 7 (54 %) required pain medications, including narcotic pain medications in 5 patients, 1 had pain not requiring medications, and 5 had no reported pain. Among patients that reported pain, the median duration of pain was 22 months. No patient required hospitalization, operative procedures, or other interventional procedures for pain control. Two patients underwent treatment with bisphosphonates.
DISCUSSION
We have here reported the first study to systematically characterize the incidence, risk factors, and clinical course of sacral insufficiency fractures in patients treated with chemoradiation for rectal cancer. The risk of developing sacral insufficiency fractures was relatively low, although the risk was higher in women compared with men and in whites compared with non-whites. Although pain symptoms could last for several months, patients could be managed conservatively with pain medications.
The widespread use of radiotherapy for rectal cancer makes it very important for clinicians to understand the presenting features and clinical course of sacral insufficiency fractures. Although sacral insufficiency fractures are relatively uncommon, these can cause considerable morbidity and need to be treated appropriately. The most common presenting symptom is pain that is typically severe and acute in onset, although more indolent onsets have also been described (20, 21). The pain can be localized to the low back, pelvis, or abdomen, and accompanied by hip, buttock, or thigh pain (22). Radicular symptoms can be present in a minority of patients. Blood tests are notable only for a mild elevation in serum alkaline phosphatase levels (23). Unfortunately, patients with sacral insufficiency fractures often face missed or delayed diagnosis. In patients with rectal cancer, sacral insufficiency fractures can mimic pelvic recurrences in their presentation, often prompting further evaluation which can be very stressful to patients.
Imaging studies can help differentiate insufficiency fractures from recurrences, thus obviating the need for biopsies and other interventions. Plain radiographs of the pelvis can show sclerotic bands, cortical disruption, and fracture lines in the sacral ala. However, subtle findings are not usually identifiable or diagnostic on plain films. Bone scintigraphy can reveal the classic butterfly shaped or “H” pattern, which is produced when there are fractures of both sacral alae and a horizontal component involving the sacral body, although other patterns can also be seen (24). Characteristic findings on CT scans include fracture lines or sclerotic lines within the sacral ala or areas of sclerosis within the sacral ala parallel to the sacroiliac joints (Fig. 2). Frequently, there is disruption of the anterior cortex of the sacral ala with associated displacement of the fracture fragment. In the setting of an insufficiency fracture, the remainder of the bony trabeculae is intact, rather than destroyed by a space-occupying lesion, as in the case of a malignancy. On magnetic resonance imaging, presence of a fracture line on T1-weighted images and high signal intensity parallel to the sacroiliac joints on T2-weighted images are virtually diagnostic of insufficiency fractures. On diffusion-weighted magnetic resonance, an insufficiency fracture appears low in signal because of the bony sclerosis around fracture lines, whereas a pathologic fracture can show high signal intensity (25). In contrast, positron emission tomography with fluorodeoxyglucose is nonspecific and can show increased activity in tumors as well as insufficiency fractures.
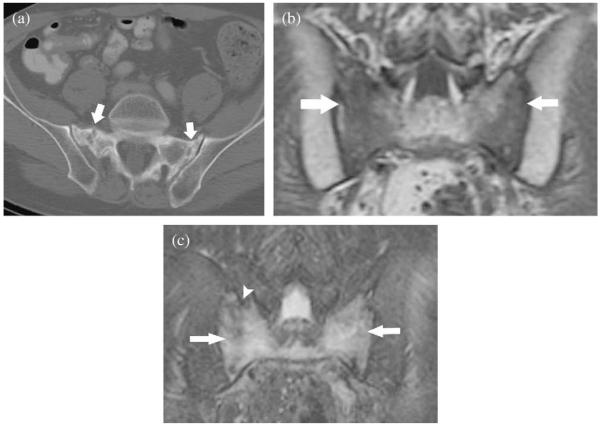
Fig. 2.
Characteristic radiological findings for sacral insufficiency fractures. (a) Axial computed tomography scan of the pelvis shows bilateral sacral fractures (white arrows). (b) Coronal T1-weighted magnetic resonance (MR) sequence shows low signal in the sacrum consistent with marrow edema (white arrows). (c) Coronal T2-weighted fat-saturated magnetic resonance sequence shows high signal intensity in the sacrum consistent with marrow edema (white arrows) and low signal intensity fracture line (white arrow head).
The incidence of sacral insufficiency fractures among the general population remains unknown (26). Weber et al. reported that the incidence of sacral insufficiency fractures was 1.8% among women older than 55 admitted to the rheumatology department at a hospital in Switzerland (27). The baseline incidence of sacral insufficiency fractures among the general population is likely to be much lower than that in the selected population studied by Weber et al. Although the patients in our study had a certain baseline risk of sacral insufficiency fractures even without radiotherapy, we believe that the insufficiency fractures could be predominantly attributed to radiotherapy, especially because this study included both genders and had relatively young patients.
Many previous studies have investigated pelvic fractures after radiotherapy. Using the Surveillance, Epidemiology, and End Results/Medicare database, Baxter et al. reported that elderly women (age ≥65 years) who underwent radiotherapy had a higher risk of pelvic fracture than women who did not undergo radiotherapy (28). The cumulative 5-year fracture rates were 14%, 8.2%, and 11.2%, respectively, in women with anal, cervical, and rectal cancer who underwent radiotherapy, whereas the rates were 7.5%, 5.9%, and 8.7%, respectively, in women with anal, cervical, and rectal cancer who did not undergo radiotherapy. In the study by Baxter et al., hip fractures constituted 90% of the pelvic fractures (28). In contrast, none of the patients in our study was found to have a femoral neck fracture. The risk of developing a femoral neck fracture is likely associated with the use of groin irradiation, which was not administered to any of the patients in this study (29). Moreover, the median age was nearly 58 years, and women represented only 37% of patients in this study; these demographic characteristics may also explain the lack of femoral fractures in our study. The study by Baxter et al. did not specifically discuss insufficiency fractures; the Surveillance, Epidemiology, and End Results/Medicare database may not have had sufficiently detailed information to identify insufficiency fractures.
A number of previous studies have investigated pelvic insufficiency fractures after radiotherapy for cervical and other gynecologic malignancies, whereas some case reports have described insufficiency fractures after radiotherapy for rectal cancer (6-14).Oh et al. and Ogino et al. have reported that older age, lower body weight, and higher radiotherapy dose are associated with a higher risk of pelvic insufficiency fractures (6, 8). In our study, older patients and patients treated with higher radiotherapy dose had numerically higher rates of insufficiency fractures, but the differences were not statistically significant. Three studies from Japan and Korea have reported that the cumulative incidence of insufficiency fractures in patients treated with radiotherapy for gynecologic cancers was 11.4%, 17%, and 19.7% (6, 8, 13). In contrast, the 3-year actuarial incidence of sacral insufficiency fractures was only 3.1% among all patients and 5.8% among women in the present study. Differences in patient characteristics, such as age, body weight, and ethnicity, and differences in treatment parameters, such as radiotherapy dose and technique, could have contributed to the difference in incidence of insufficiency fractures. However, the clinical course of sacral insufficiency fractures in our study is similar to that reported in patients with gynecologic cancers(6-8, 13, 14).
As with all retrospective studies, there are some limitations in our findings. Complete follow-up information was not available for all patients. Imaging studies were reviewed only in patients with known pelvic fractures and not on all 562 patients in the study, although radiology reports and medical records were reviewed in detail for all patients. The study was restricted to medical records at M. D. Anderson Cancer Center, and could have potentially left out sacral insufficiency fractures identified at other institutions. However, the majority of patients had prolonged follow-up at M.D. Anderson; hence, it is unlikely that this study significantly underestimated the risk of sacral insufficiency fractures. We did not have adequate information to evaluate the impact of certain potential predictors, such as body weight. Finally, because this is a retrospective study, it is difficult to draw firm conclusions regarding the clinical course of these patients.
Many opportunities exist for further studies on sacral insufficiency fractures. For example, investigations could be conducted on whether bone mineral density tests can predict which patients are most likely to develop insufficiency fractures. Studies could be conducted on whether medications such as bisphosphonates could reduce the risk of sacral insufficiency fractures among high-risk groups, such as white women. However, the low rate of insufficiency fractures makes it difficult to design and conduct such studies. A case report has suggested that pentoxifylline can cause clinical improvement of sacral insufficiency fracture (30). A new therapeutic intervention, sacroplasty, has been developed recently to treat sacral insufficiency fracture (31, 32). Such interventions need to be studied further to determine their indications and efficacy.
In conclusion, sacral insufficiency fractures are an uncommon but important late side effect in patients treated with pelvic radiotherapy for rectal cancer. Women and whites have a higher risk of developing sacral insufficiency fractures. Distinguishing sacral insufficiency fractures from pelvic recurrences may help reduce unnecessary biopsies and other interventions. Most patients with sacral insufficiency fractures can be managed conservatively with pain medications; however, new therapeutic interventions are now under investigation.
Acknowledgments
Presented in part at the International Society of Gastrointestinal Oncology Annual Meeting, September 27–29, 2007, Philadelphia, PA.
Footnotes
Conflicts of interest: none.
REFERENCES
- 1.Prolongation of the disease-free interval in surgically treated rectal carcinoma. Gastrointestinal Tumor Study Group. N Engl J Med. 1985;312:1465–1472. doi: 10.1056/NEJM198506063122301. [DOI] [PubMed] [Google Scholar]
- 2.Douglass HO, Jr., Moertel CG, Mayer RJ, et al. Survival after post-operative combination treatment of rectal cancer. N Engl J Med. 1986;315:1294–1295. doi: 10.1056/NEJM198611133152014. [DOI] [PubMed] [Google Scholar]
- 3.Krook JE, Moertel CG, Gunderson LL, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med. 1991;324:709–715. doi: 10.1056/NEJM199103143241101. [DOI] [PubMed] [Google Scholar]
- 4.Wolmark N, Wieand HS, Hyams DM, et al. Randomized trial of postoperative adjuvant chemotherapy with or without radiotherapy for carcinoma of the rectum: National Surgical Adjuvant Breast and Bowel Project Protocol R-02. J Natl Cancer Inst. 2000;92:388–396. doi: 10.1093/jnci/92.5.388. [DOI] [PubMed] [Google Scholar]
- 5.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 6.Ogino I, Okamoto N, Ono Y, et al. Pelvic insufficiency fractures in postmenopausal woman with advanced cervical cancer treated by radiotherapy. Radiother Oncol. 2003;68:61–67. doi: 10.1016/s0167-8140(03)00128-2. [DOI] [PubMed] [Google Scholar]
- 7.Huh SJ, Kim B, Kang MK, et al. Pelvic insufficiency fracture after pelvic irradiation in uterine cervix cancer. Gynecol Oncol. 2002;86:264–268. doi: 10.1006/gyno.2002.6756. [DOI] [PubMed] [Google Scholar]
- 8.Oh D, Huh SJ, Nam H, et al. Pelvic insufficiency fracture after pelvic radiotherapy for cervical cancer: Analysis of risk factors. Int J Radiat Oncol Biol Phys. 2008;70:1183–1188. doi: 10.1016/j.ijrobp.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 9.Inoue Y, Miki C, Ojima E, et al. Pelvic insufficiency fractures after preoperative radiotherapy for rectal carcinoma. Int J Clin Oncol. 2003;8:336–339. doi: 10.1007/s10147-003-0340-x. [DOI] [PubMed] [Google Scholar]
- 10.Parikh VA, Edlund JW. Sacral insufficiency fractures—rare complication of pelvic radiation for rectal carcinoma: Report of a case. Dis Colon Rectum. 1998;41:254–257. doi: 10.1007/BF02238256. [DOI] [PubMed] [Google Scholar]
- 11.Peh WC, Khong PL, Sham JS, et al. Sacral and pubic insufficiency fractures after irradiation of gynaecological malignancies. Clin Oncol (R Coll Radiol) 1995;7:117–122. doi: 10.1016/s0936-6555(05)80814-3. [DOI] [PubMed] [Google Scholar]
- 12.Abe H, Nakamura M, Takahashi S, et al. Radiation-induced insufficiency fractures of the pelvis: Evaluation with 99mTc-methylene diphosphonate scintigraphy. AJR Am J Roentgenol. 1992;158:599–602. doi: 10.2214/ajr.158.3.1739002. [DOI] [PubMed] [Google Scholar]
- 13.Ikushima H, Osaki K, Furutani S, et al. Pelvic bone complications following radiation therapy of gynecologic malignancies: Clinical evaluation of radiation-induced pelvic insufficiency fractures. Gynecol Oncol. 2006;103:1100–1104. doi: 10.1016/j.ygyno.2006.06.038. [DOI] [PubMed] [Google Scholar]
- 14.Moreno A, Clemente J, Crespo C, et al. Pelvic insufficiency fractures in patients with pelvic irradiation. Int J Radiat Oncol Biol Phys. 1999;44:61–66. doi: 10.1016/s0360-3016(98)00534-3. [DOI] [PubMed] [Google Scholar]
- 15.Das P, Skibber JM, Rodriguez-Bigas MA, et al. Predictors of tumor response and downstaging in patients who receive preoperative chemoradiation for rectal cancer. Cancer. 2007;109:1750–1755. doi: 10.1002/cncr.22625. [DOI] [PubMed] [Google Scholar]
- 16.Americal Joint Committee on Cancer . Colon and rectum. In: Greene FL, Page DL, Fleming ID, editors. AJCC cancer staging handbook. 6th ed Springer-Verlag; New York: 2002. pp. 127–138. [Google Scholar]
- 17.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 18.De Smet AA, Neff JR. Pubic and sacral insufficiency fractures: Clinical course and radiologic findings. AJR Am J Roentgenol. 1985;145:601–606. doi: 10.2214/ajr.145.3.601. [DOI] [PubMed] [Google Scholar]
- 19.Aretxabala I, Fraiz E, Perez-Ruiz F, et al. Sacral insufficiency fractures. High association with pubic rami fractures. Clin Rheumatol. 2000;19:399–401. doi: 10.1007/pl00011178. [DOI] [PubMed] [Google Scholar]
- 20.Saraux A, Valls I, Guedes C, et al. Insufficiency fractures of the sacrum in elderly subjects. Rev Rhum Engl Ed. 1995;62:582–586. [PubMed] [Google Scholar]
- 21.Gotis-Graham I, McGuigan L, Diamond T, et al. Sacral insufficiency fractures in the elderly. J Bone Joint Surg Br. 1994;76:882–886. [PubMed] [Google Scholar]
- 22.Rawlings CE, 3rd, Wilkins RH, Martinez S, et al. Osteoporotic sacral fractures: A clinical study. Neurosurgery. 1988;22:72–76. doi: 10.1227/00006123-198801010-00011. [DOI] [PubMed] [Google Scholar]
- 23.Grasland A, Pouchot J, Mathieu A, et al. Sacral insufficiency fractures: An easily overlooked cause of back pain in elderly women. Arch Intern Med. 1996;156:668–674. doi: 10.1001/archinte.156.6.668. [DOI] [PubMed] [Google Scholar]
- 24.Peh WC, Khong PL, Yin Y, et al. Imaging of pelvic insufficiency fractures. Radiographics. 1996;16:335–348. doi: 10.1148/radiographics.16.2.8966291. [DOI] [PubMed] [Google Scholar]
- 25.Byun WM, Jang HW, Kim SW, et al. Diffusion-weighted magnetic resonance imaging of sacral insufficiency fractures: Comparison with metastases of the sacrum. Spine. 2007;32:E820–E824. doi: 10.1097/BRS.0b013e31815ce70c. [DOI] [PubMed] [Google Scholar]
- 26.Tsiridis E, Upadhyay N, Giannoudis PV. Sacral insufficiency fractures: Current concepts of management. Osteoporos Int. 2006;17:1716–1725. doi: 10.1007/s00198-006-0175-1. [DOI] [PubMed] [Google Scholar]
- 27.Weber M, Hasler P, Gerber H. Insufficiency fractures of the sacrum. Twenty cases and review of the literature. Spine. 1993;18:2507–2512. doi: 10.1097/00007632-199312000-00021. [DOI] [PubMed] [Google Scholar]
- 28.Baxter NN, Habermann EB, Tepper JE, et al. Risk of pelvic fractures in older women following pelvic irradiation. JAMA. 2005;294:2587–2593. doi: 10.1001/jama.294.20.2587. [DOI] [PubMed] [Google Scholar]
- 29.Grigsby PW, Roberts HL, Perez CA. Femoral neck fracture following groin irradiation. Int J Radiat Oncol Biol Phys. 1995;32:63–67. doi: 10.1016/0360-3016(95)00546-B. [DOI] [PubMed] [Google Scholar]
- 30.Bese NS, Ozguroglu M, Kamberoglu K, et al. Pentoxifylline in the treatment of radiation-related pelvic insufficiency fractures of bone. Radiat Med. 2003;21:223–227. [PubMed] [Google Scholar]
- 31.Betts A. Sacral vertebral augmentation: Confirmation of fluoroscopic landmarks by open dissection. Pain Physician. 2008;11:57–65. [PubMed] [Google Scholar]
- 32.Heron J, Connell DA, James SL. CT-guided sacroplasty for the treatment of sacral insufficiency fractures. Clin Radiol. 2007;62:1094–1100. doi: 10.1016/j.crad.2007.04.017. [DOI] [PubMed] [Google Scholar]