Abstract
Background
Treatment of distal rectal cancer remains clinically challenging and includes proctectomy and coloanal anastomosis (CAA) or abdominoperineal resection (APR). The purpose of this study is to evaluate operative and pathologic factors associated with long-term survival and local recurrence outcomes in patients treated for distal rectal cancer.
Methods
A retrospective consecutive cohort study of 304 patients treated for distal rectal cancer with radical resection from 1993 to 2003 was performed. Patients were grouped by procedure (CAA or APR). Demographic, pathologic, recurrence, and survival data were analyzed utilizing chi-square analysis for comparison of proportions. Survival analysis was performed using Kaplan–Meier method and log-rank test for univariate and Cox regression for multivariate comparison.
Results
The median tumor distance from the anal verge was 2 cm [interquartile range (IQR) 0.5–4 cm]. Margins were negative in all but four patients (one distal, 0.3%; three radial, 1%). The 5-year overall survival rate was 82% (88.6% stage pI, 80.5% stage pII, 67.9% stage pIII). Older age, advanced pathologic stage, presence of lymphovascular or perineural invasion, earlier treatment period, and APR surgery type were associated with worse survival on multivariate analysis. The 5-year local recurrence rate was 5.3% after CAA and 7.9% after APR (p = 0.33).
Conclusions
Low rates of local recurrence and good overall survival can be achieved after treatment of distal rectal cancer with stage-appropriate chemoradiation and proctectomy with CAA or APR. Sphincter preservation can be achieved even with distal margins less than 2 cm.
An estimated 40,870 new cases of rectal cancer will be diagnosed within the USA in 2009, making this one of the most common cancers among men and women.1 Surgical treatment of distal rectal cancer includes sphincter preservation with proctectomy and coloanal anastomosis (CAA) or abdominoperineal resection (APR). The goals of surgical therapy are both control of the cancer and preservation of function. In addition to surgery, combined chemoradiation therapy (CRT) is recommended for patients with stage II or stage III disease to improve local control and overall survival.2,3 Despite these improvements, treatment of distal rectal cancer remains clinically challenging in terms of both the suitability of performing sphincter-preserving surgery and the historically poorer local control and survival outcomes associated with APR compared with sphincter preservation.4,5
Traditionally, the ability to perform sphincter preservation was related to the distance between the tumor and the anal sphincters.6 This was largely based on the finding that distal intramural extension of tumor occurred in 12% of cases and therefore a 5-cm distal margin was deemed necessary for a curative resection.6 More recently, studies have shown that distal intramural spread beyond 1 cm is uncommon and only occurs in 4–10% of cases.7–9 Currently, a distal resection margin of at least 2 cm is considered the standard for rectal cancer, but more recent literature has proposed that a 1-cm margin may be adequate for tumors without adverse histologic features.10 More importantly, the ability to obtain negative radial margins by total mesorectal excision (TME) and wide division of the levator ani in cases of APR may afford the best chance to decrease local recurrence, distant metastases, and death.10–14 This finding may be important in explaining the historical higher risk of local failure in patients who undergo APR than in patients who are able to undergo a sphincter-preserving operation.15
The purpose of this retrospective cohort study is to evaluate operative and pathologic factors associated with long-term survival and local recurrence in patients treated for primary distal rectal cancer with CAA or APR. Understanding these clinicopathologic factors could better aid in preoperative evaluation and patient counseling.
METHODS
Patient Identification and Management
All consecutive patients who had undergone resection for primary distal rectal cancer between January 1993 and January 2003 were identified from The University of Texas M. D. Anderson Cancer Center tumor registry, and their records were reviewed. Distal cancers were defined as those requiring coloanal reconstruction or abdominoperineal resection for oncologic control. This study was approved by the institutional review board.
Three-hundred four patients were identified and categorized into two groups: those who underwent CAA with sphincter preservation and those who required APR. Preoperative tumor staging was obtained by cross-sectional imaging [computed tomography (CT) or magnetic resonance imaging (MRI)] and endorectal ultrasound unless cross-sectional staging already established the presence of mesorectal nodal involvement (stage III). All patients had disease confined to the pelvis at the time of resection. Neoadjuvant chemoradiation therapy was administered to all patients with stage II or III rectal cancers after multi-disciplinary review as previously described and to selected stage I patients with tumors abutting the dentate line and in whom sphincter preservation was being considered.16 The dose of radiotherapy during the study period was 45–52.5 Gy, and concurrent chemotherapy consisted of infusional 5-fluorouracil (5-FU) during the earlier period but transitioned to oral capecitabine during the latter part of the study. Proctectomy was performed via anterior or combined anterior and transanal or transperineal approach. In all cases, sharp total mesorectal excision was performed to the level of the pelvic floor and, in the case of APR, the levator ani were divided widely. To maximize the ability to preserve sphincter function in the CAA group, the technique of intersphincteric resection was employed, if needed.17,18 Handsewn or double-stapled coloanal anastomosis was performed. Postoperatively, adjuvant chemotherapy was offered to all patients with stage II or III cancers and primarily consisted of 5-FU and leucovorin.
Data Collection
We recorded data on demographics, pre- and postoperative disease stage, grade, lymphovascular invasion (LVI), perineural invasion (PNI), radiotherapy, distal margin length, radial margin status, multivisceral resection, local recurrence, and overall survival. Cases in which complete response was seen on pathologic evaluation were classified as LVI and PNI negative unless otherwise identified on pretreatment biopsy. Distal margin length was measured and recorded by the pathologist prior to tissue fixation. The anastomotic rings from the circular stapler, in cases of double-stapled coloanal anastomosis, were not included in the assessment of margin length as they did not represent a circumferential distal margin of resection. Radial margin status was recorded as positive if a tumor was present on microscopic examination of the inked margin. Time to local recurrence was defined as time in months from date of operation to first radiographic or endoscopic documentation of disease. Total follow-up time was defined as time in months from date of operation to last clinic visit or correspondence with the institutional tumor registry.
Statistical Analysis
We performed summary comparisons between the two groups for age, gender, pre- and postoperative tumor-node-metastasis (TNM) stage, grade, lymphovascular or perineural invasion, CRT, multivisceral resection, and year of surgery (1998 and earlier versus after 1998) using univariate chi-square analysis. We chose 1998 as the cut-point year, when agents other than 5-FU became available for systemic therapy of colorectal cancer. Analyses for recurrence and survival were performed using Kaplan–Meier method and log-rank test for univariate comparisons. A fully saturated multivariate Cox proportional-hazards model incorporating all candidate variables based on univariate or clinical significance was constructed. Model assumptions were evaluated by examination of residual plots. p-Values <0.05 were considered statistically significant. Statistical analyses were performed by using Stata 10.1 MP software (StataCorp, College Station, TX).
RESULTS
Patient Population and Tumor Characteristics
A total of 304 patients who were treated for distal rectal cancer during the study period were identified. Of these 304 patients, 176 underwent CAA and 128 underwent APR. Median follow-up for all patients was 95 months [94 and 98 months for CAA and APR, respectively; interquartile range (IQR) 70–132 months]. Patient demographics are shown in Table 1. Median age was the same in the CAA and APR groups (57 years, IQR 48–66 years). The majority of patients were men (57% and 58% for CAA and APR, respectively). Median distance of the tumor from the anal verge was 2 cm (IQR 0.5–4 cm). The majority of patients in both groups had stage II or III disease on pre-operative evaluation, while postoperative evaluation reflected a shift towards downstaging due to neoadjuvant CRT. Rates of LVI and PNI did not differ between the two groups. Over 88% (n = 155 CAA, 115 APR) of patients underwent neoadjuvant CRT. In the CAA group, the median distal margin of resection was 1.8 cm (IQR 0.6–2.6 cm). Radial margins were negative in all but one (0.7%) patient after CAA and in all but two patients (1.8%) after APR. More patients in the CAA group (67%, n = 118) than in the APR group (59%, n = 75) completed postoperative chemotherapy (p < 0.01), and more patients in the APR group (29%, n = 37) than in the CAA group (4%, n = 7) required multivisceral resection (p < 0.01).
TABLE 1.
Patient demographics and clinicopathologic characteristics (n = 304)
| APR (n = 128) | % | CAA (n = 176) | % | p-Value | |
|---|---|---|---|---|---|
| Age | 0.84 | ||||
| Median (years) | 57 | 57 | |||
| Gender (M:F) | 74:54 | 100:76 | 0.83 | ||
| Clinical stage | 0.52 | ||||
| I | 10 | 8 | 20 | 11 | |
| II | 68 | 53 | 74 | 42 | |
| III | 50 | 39 | 82 | 47 | |
| Pathologic stage | 0.23 | ||||
| 0 | 18 | 14 | 33 | 19 | |
| I | 41 | 32 | 54 | 31 | |
| II | 36 | 28 | 34 | 19 | |
| III | 33 | 26 | 55 | 31 | |
| Preoperative chemoradiation therapy | 115 | 90 | 155 | 88 | 0.63 |
| Postoperative chemotherapy | 75 | 59 | 118 | 67 | <0.01 |
| Lymphovascular invasion | 14 | 13 | 24 | 17 | 0.36 |
| Perineural invasion | 7 | 6 | 12 | 8 | 0.54 |
| Positive radial margin | 2 | 2 | 1 | 1 | 0.54 |
| Multivisceral resection | 37 | 29 | 7 | 4 | <0.01 |
| Year of surgery | |||||
| 1992–1998 | 66 | 52 | 71 | 40 | 0.05 |
| 1999–2003 | 62 | 48 | 105 | 60 | |
APR abdominoperineal resection, CAA coloanal anastomosis
Survival Outcomes
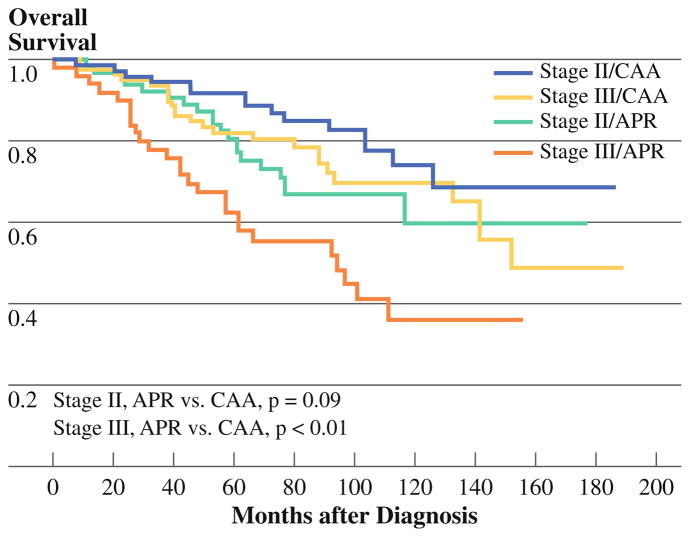
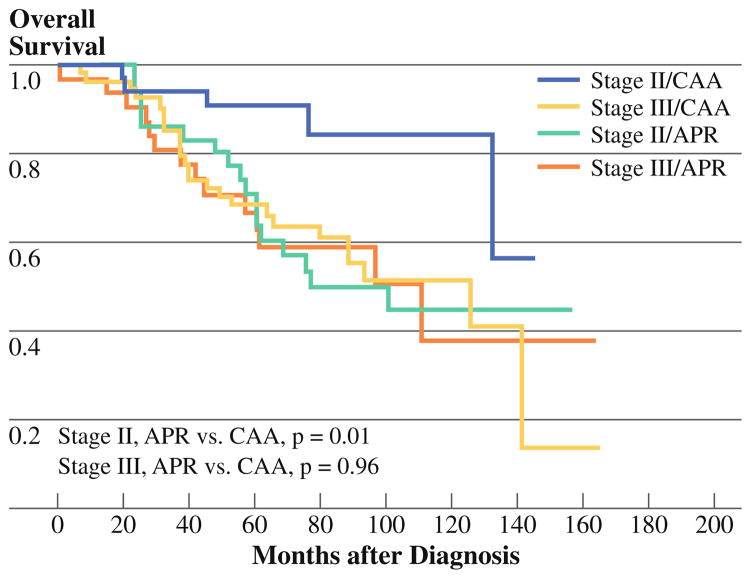
The 5-year overall survival rate was 82% [95% confidence interval (CI) 76.7–85.7%]: 90% for those with clinical stage I disease, 87% for clinical stage II disease, and 75% for clinical stage III disease. The 5-year overall survival for clinical stage II and III disease patients after CAA was 92% and 82%, respectively, and after APR was 81% and 63% (Fig. 1). After CAA, 5-year overall survival rates were 91% and 69% for patients with pathologic stage II and III disease, respectively. After APR, 5-year overall survival rates were 71% and 67% for pathologic stage II and III patients, respectively (Fig. 2). Patients who underwent APR with multivisceral resection appeared to have worse 5-year overall survival rate (66%, 95% CI 48.8–79.6%) than did those undergoing APR without multivisceral resection (78%, 95% CI 67.8–85.8%, p = 0.19) although not statistically significantly so. In contrast, 5-year overall survival rates for the CAA group with and without multivisceral resection were 85% (95% CI 33.4–97.8%) and 86% (95% CI 80.3–90.9%, p = 0.94), respectively.
FIG. 1.
Overall survival by clinical stage and surgery type
FIG. 2.
Overall survival by pathologic stage and surgery type
Table 2 shows that univariate predictors of overall survival were age, clinical and pathologic stage, LVI and PNI, need for multivisceral resection, and type of surgery (APR versus CAA). On multivariate analysis, advanced age, advanced pathologic stage, presence of lymphovascular and, perineural invasion, APR surgery type, and earlier year of surgery were associated with worse overall survival. In the multivariate Cox regression model, postoperative stage remained more predictive of outcome than did clinical stage and was therefore used in the final model. Patients treated during the study period after 1998 were noted to have improved overall survival, likely related to ongoing advances in systemic therapy. After adjusting for sex, clinical stage, CRT, LVI, PNI, and year of surgery, patients undergoing CAA had better overall survival than those after APR [hazard ratio (HR) 0.57, 95% CI 0.36–0.92, p <0.01]. Although the proportion of patients undergoing APR was slightly higher during the earlier years of study inclusion, there was no significant interaction between type of surgery performed and treatment period in the Cox model (p = 0.79).
TABLE 2.
Univariate and multivariate predictors of overall survival
| Variable | Univariate Cox regression |
Multivariate Cox regression |
||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Age (continuous) | 1.02 (1.00–1.04) | <0.01 | 1.03 (1.01–1.05) | <0.01 |
| Gender | ||||
| Male | 1.26 (0.84–1.91) | 0.25 | 1.25 (0.81–1.94) | 0.30 |
| Preoperative stage | ||||
| I | 1 | |||
| II | 1.24 (0.48–3.17) | 0.64 | ||
| III | 2.08 (0.83–5.24) | 0.11 | ||
| Postoperative stage | ||||
| 0–I | 1 | 1 | ||
| II | 2.05 (1.17–3.58) | 0.01 | 1.80 (1.01–3.21) | 0.04 |
| III | 3.35 (2.06–5.44) | <0.01 | 2.87 (1.68–4.90) | <0.01 |
| Preoperative chemoradiation therapy | 0.91 (0.47–1.76) | 0.79 | 1.31 (0.62–2.75) | 0.47 |
| Postoperative chemotherapy | 1.04 (0.67–1.63) | 0.836 | 0.99 (0.61–1.60) | 0.98 |
| Multivisceral resection | 1.85 (1.12–3.04) | 0.01 | 1.40 (0.77–2.54) | 0.25 |
| Lymphovascular invasion | 1.94 (1.05–3.58) | 0.03 | 2.02 (0.99–4.10) | 0.05 |
| Perineural invasion | 3.67 (1.91–7.06) | <0.01 | 2.29 (1.13–4.64) | 0.02 |
| Type of surgery | ||||
| APR | 1 | 1 | ||
| CAA | 0.56 (0.37–0.85) | <0.01 | 0.57 (0.36–0.92) | 0.02 |
| Year of surgery | ||||
| 1992–1998 | 1 | 1 | ||
| 1999–2003 | 0.68 (0.43–1.07) | 0.09 | 0.54 (0.33–0.90) | 0.02 |
HR hazard ratio, CI confidence interval, APR abdominoperineal resection, CAA coloanal anastomosis
With median follow-up of 81 months, we observed 21 local recurrences (7%). Eleven local recurrences occurred in the CAA group, with median time to recurrence of 18 months. Of those 11 patients, one had undergone multivisceral resection. Ten local recurrences occurred in the APR group, with median time to recurrence of 29 months. Of those ten patients, four had undergone multivisceral resection.
The overall local recurrence rate at 5 years was 5.3% for patients who had undergone CAA and 7.9% for patients who had undergone APR (p = 0.33). One patient had a positive distal margin, and only three patients had a positive radial margin. Complete quantitative distal margin information was available for 119 (68%) of the 176 CAA patients. Among these patients, distal margins were ≤1 cm in 37 patients (32%), 1.1–2 cm in 40 patients (33%), and >2 cm in 42 patients (35%). The distance of distal mucosal margin did not correlate with rates of local recurrence (p = 0.46 for ≤1 cm versus 1.1–2 cm; p = 0.26 for 1–2 cm versus >2 cm). Of the 11 CAA patients in whom cancer recurred locally, the distal margin was unspecified but reported negative in one patient, unknown in one patient, ≤1 cm in three patients, 1.1–2 cm in four patients, and >2 cm in two patients. None of the three patients with a positive radial margin had local recurrence.
DISCUSSION
This study was performed to evaluate factors associated with long-term survival and local recurrence outcomes in a cohort of patients with distal rectal cancer undergoing CAA or APR at a referral center. We have demonstrated, after long-term follow-up, that surgical treatment of distal rectal cancers with stage-appropriate CRT and TME is associated with low rates of positive radial margins and local recurrence. While all but one of the patients within our retrospective cohort had a negative distal margin for resection, we did not observe am association between distance of tumor from the distal margin and local failure risk. Furthermore, local recurrence rates were not different among patients undergoing CAA or APR after adjusting for covariate influences. Overall survival was worse among older patients, patients with advanced stage, patients with tumors demonstrating LVI or PNI, patients treated during the earlier time period, and patients undergoing APR.
Surgical resection of localized rectal cancer with curative intent involves complete removal of the primary tumor and its lymphatic drainage by sharp mesorectal excision with or without sphincter preservation. The mesorectal dissection should be carried sharply to the pelvic floor. For low-lying cancers at the pelvic floor and adjacent to the anal sphincters, the historically higher risk for positive circumferential resection margins and local failure following APR versus anterior resection may in part be due to inadequate resection at the level of the pelvic floor with TME, resulting in increased risk for a positive radial margin.15,19–22 The TME plane brings the dissection margin very close to the lateral margin of the tumor where the need for wide resection is greatest.23 We routinely perform a wide cylindrical resection of the levator ani in the region of the tumor during APR.24,25 This technique avoids “coning in” along the distal mesorectum and allows for a wide radial margin to be achieved at the level of the tumor, resulting in a lower risk for local failure.15,26 Indeed we observed no significant difference in local recurrence rates between CAA and APR.
Although on univariate comparison survival was poorer among patients undergoing APR compared with those undergoing CAA, more patients required multivisceral resection and failed to receive adjuvant chemotherapy in the APR cohort. Thus this group had patient and tumor factors not fully characterized by American Joint Committee on Cancer (AJCC) stage assignment alone. After adjusting for covariate effects, there was worse overall survival among patients undergoing APR, likely reflecting patient, biologic, and treatment-related factors. These results are consistent with a recently published report showing poorer outcomes in patients undergoing APR for low rectal tumors.27
In the absence of distant metastasis, resection margin status may be the most important factor in determining long-term survival and local recurrence in distal rectal cancer. The proximal margin is determined by the level of vascular ligation of the superior rectal artery and, historically, it was thought that a 5-cm distal margin was required for curative resection.6,28,29 More recently a ≤ 2 cm distal margin has been considered for oncologic control; however, the true minimum distance required for oncologic control in the setting of preoperative CRT and TME remains undefined.8–10,12,30–34 In this study the rate of local failure after stage-appropriate CRT was low (6.3%), and among CAA patients, of whom all but one underwent margin-negative resection, an association with distal margin length was not observed.
Although traditionally the distal margin was the focus of attention in rectal cancer, recently the radial or circumferential resection margin (CRM) has been shown to be the most important determinant of local control. Positive CRM has been associated with an up to 86% local failure rate.35 A number of studies have demonstrated improvements in local recurrence, risk for distant metastases, and death with a negative CRM (≥1–2 mm).10–14,36–38 Only three patients in the present study had a positive CRM, none of whom recurred locally.
Surgery within the correct mesorectal dissection plane will result in a negative CRM except in cases in which an advanced tumor extends beyond the mesorectal envelope. The low overall local recurrence rate of 5% in this study’s large, long-term follow-up cohort may be in part be attributable to a combination of CRT with a standardized surgical technique of sharp mesorectal excision with wide lateral clearance and preservation of the integrity of the CRM. Thorough preoperative evaluation is emphasized so that appropriate en bloc resection of adjacent viscera, in the case of locally advanced tumors, can be planned in advance of surgery. This approach also allows for the recruitment of multidisciplinary reconstructive services when needed, but most importantly, it maximizes the surgeon’s ability to plan for and achieve a margin-negative resection.
Perineural invasion was another factor in our study associated with poorer overall survival on univariate and multivariate analysis, being present among 12 patients (8%) in the CAA group and 7 (6%) patients in the APR group. Presence of PNI has previously been associated with increased risk for distant metastases and local recurrence.23–25,39,40 Among our 13 patients with local recurrence, 5 (38%) had evidence of PNI versus 14 (4.8%) without recurrence.
We also evaluated the influence of clinical and pathologic stage on survival outcomes within our multivariate models and observed that pathologic stage was more predictive of outcome than was clinical stage. In our group of patients, most of whom had undergone neoadjuvant chemoradiotherapy, this finding suggests that pathologic stage is a marker of tumor biology and is consistent with previous reports.41,42 Although treatment strategy is based on complete clinical staging, prognosis may be related to pathologic stage.
This study has notable strengths as well as potential limitations. This retrospective cohort of 304 patients represents a large series of patients with long-term follow-up after treatment for distal rectal cancer, which allowed us to carry out robust examination of recurrence and survival outcomes. The long follow-up time minimizes the potential for detection and follow-up time bias for the identification of local recurrences that may have been delayed by use of adjuvant radiation. The relatively small number of surgeons allowed for standardization of technique and surgical quality control. However, as this is a retrospective study and pathologic reporting was not standardized, quantitative data regarding tumor distance from the distal margins were missing in 25% (n = 37) of the cases of CAA, although none of these patients were noted to have a recurrence. Furthermore, quantitative data regarding the radial margin were available only for 28.4% (n = 73) of patients in this retrospective cohort. Thus, if a criteria of >1 mm for a negative radial margin was applied to all cases, we may have identified a greater proportion of cases with a positive radial resection margin.
In summary, our study demonstrates that treatment of distal rectal cancer with stage-appropriate CRT, total mesorectal excision, and negative distal and radial margins with en bloc resection of involved viscera results in low rates of local recurrence after either CAA or APR. We have found that age, pathologic stage, lymphovascular invasion, perineural invasion, treatment period, and surgery type were associated with overall survival. By understanding the anatomy of the distal mesorectum and adhering to oncologic principles for surgery and combined modality therapy, sphincter preservation with low rates of local recurrence can be achieved in patients undergoing APR or CAA even with distal margins less than 1 cm.
Acknowledgments
Financial support from National Cancer Institute Core Grant CA16672 (MDACC), an American Society of Clinical Oncology Foundation Career Development Award (G.J.C.), and a National Cancer Institute K07-CA133187 research grant (G.J.C.). The authors would also like to thank Kristi Speights for her assistance with the preparation of this manuscript and Irma Medrano for her technical assistance.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009 doi: 10.3322/caac.200006. [DOI] [Google Scholar]
- 2.NIH Consensus Conference. Adjuvant therapy for patients with colon and rectal cancer. JAMA. 1990;264:1444–50. [PubMed] [Google Scholar]
- 3.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. New Engl J Med. 2004;351:1731–40. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 4.Kapiteijn E, Marijnen C, Negtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638–46. doi: 10.1056/NEJMoa010580. [DOI] [PubMed] [Google Scholar]
- 5.Law WL, Chu KW. Abdominoperineal resection is associated with poor oncologic outcome. Br J Surg. 2004;91:1493–9. doi: 10.1002/bjs.4723. [DOI] [PubMed] [Google Scholar]
- 6.Grinnell RS. Distal intramural spread of carcinoma of the rectum and rectosigmoid. Surg Gynecol Obstet. 1954;99:421–30. [PubMed] [Google Scholar]
- 7.Wolmark N, Fisher B. An analysis of survival and treatment failure following abdominoperineal and sphincter-saving resection in Dukes’ B and C rectal carcinoma. A report of the NSABP clinical trials. National Surgical Adjuvant Breast and Bowel Project. Ann Surg. 1986;204:480–9. doi: 10.1097/00000658-198610000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams NS, Dixon MF, Johnston D. Reappraisal of the 5 centimetre rule of distal excision for carcinoma of the rectum: a study of distal intramural spread and of patients’ survival. Br J Surg. 1983;70:150–4. doi: 10.1002/bjs.1800700305. [DOI] [PubMed] [Google Scholar]
- 9.Pollett WG, Nicholls RJ. The relationship between the extent of distal clearance and survival and local recurrence rates after curative anterior resection for carcinoma of the rectum. Ann Surg. 1983;198:159–63. doi: 10.1097/00000658-198308000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tjandra JJ, Kilkenny JW, Buie WD, et al. Practice parameters for the management of rectal cancer [revised] Dis Colon Rectum. 2005;48:411–23. doi: 10.1007/s10350-004-0937-9. [DOI] [PubMed] [Google Scholar]
- 11.Heald RJ, Husband EM, Ryall ED. The mesorectum in rectal cancer surgery: clue to pelvic recurrence? Br J Surg. 1982;69:613–6. doi: 10.1002/bjs.1800691019. [DOI] [PubMed] [Google Scholar]
- 12.Heald RJ, Ryall ED. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986;1:1479–82. doi: 10.1016/s0140-6736(86)91510-2. [DOI] [PubMed] [Google Scholar]
- 13.Wibe A, Rendedal PR, Svensson E. Prognostic significance of the circumferential resection margin following total mesorectal excision for rectal cancer. Br J Surg. 2002;89:327–34. doi: 10.1046/j.0007-1323.2001.02024.x. [DOI] [PubMed] [Google Scholar]
- 14.Sugihara K, Kobayashi H, Kato T, et al. Indication and benefit of pelvic sidewall dissection for rectal cancer. Dis Colon Rectum. 2006;49:1663–72. doi: 10.1007/s10350-006-0714-z. [DOI] [PubMed] [Google Scholar]
- 15.Nagtegaal ID, van de Velde CJH, Marijnen AM, et al. Low rectal cancer: a call for a change of approach in abdominoperineal resection. J Clin Oncol. 2005;23:9257–64. doi: 10.1200/JCO.2005.02.9231. [DOI] [PubMed] [Google Scholar]
- 16.Das P, Skibber JM, Rodriguez-Bigas MA, et al. Predictors of tumor response and downstaging in patients who receive preoperative chemoradiation for rectal cancer. Cancer. 2007;109:1750–5. doi: 10.1002/cncr.22625. [DOI] [PubMed] [Google Scholar]
- 17.Schiessel R, Karner-Hanusch J, Herbst F, et al. Intersphincteric resection for low rectal tumours. Br J Surg. 1994;81:1376–8. doi: 10.1002/bjs.1800810944. [DOI] [PubMed] [Google Scholar]
- 18.Rullier E, Laurent C, Bretagnol F, et al. Sphincter-saving resection for all rectal carcinomas: the end of the 2-cm distal rule. Ann Surg. 2005;241:465–9. doi: 10.1097/01.sla.0000154551.06768.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chuwa EW, Seow-Choen F. Outcomes for abdominoperineal resections are not worse than those of anterior resections. Dis Colon Rectum. 2006;49:41–9. doi: 10.1007/s10350-005-0227-1. [DOI] [PubMed] [Google Scholar]
- 20.Wibe A, Syse A, Anderson E, et al. Oncological outcomes after total mesorectal excision for cure for cancer of the lower rectum: anterior vs. abdominoperineal resection. Dis Colon Rectum. 2004;47:48–58. doi: 10.1007/s10350-003-0012-y. [DOI] [PubMed] [Google Scholar]
- 21.Law WL, Chu KW. Abdominoperineal resection is associated with poor oncological outcome. Br J Surg. 2004;91:1493–9. doi: 10.1002/bjs.4723. [DOI] [PubMed] [Google Scholar]
- 22.Heald RJ, Smedh RK, Kald A, et al. Abdominoperineal excision of the rectum—an endangered operation. Norman Nigro Lectureship. Dis Colon Rectum. 1997;40:747–51. doi: 10.1007/BF02055425. [DOI] [PubMed] [Google Scholar]
- 23.Stewart D, Yan Y, Mutch M, et al. Predictors of disease-free survival in rectal cancer patients undergoing curative proctectomy. Colorectal Dis. 2008;10:879–86. doi: 10.1111/j.1463-1318.2008.01508.x. [DOI] [PubMed] [Google Scholar]
- 24.Das P, Skibber JM, Rodriguez-Bigas MA, et al. Clinical and pathologic predictors of locoregional recurrence, distant metastasis, and overall survival in patients treated with chemoradiation and mesorectal excision for rectal cancer. Am J Clin Oncol. 2006;29:219–24. doi: 10.1097/01.coc.0000214930.78200.4a. [DOI] [PubMed] [Google Scholar]
- 25.Guillem JG, Chessin DB, Cohen AM, et al. Long-term oncologic outcome following preoperative combined modality therapy and total mesorectal excision of locally advanced rectal cancer. Ann Surg. 2005;241:829–38. doi: 10.1097/01.sla.0000161980.46459.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang GJ. Abdominoperineal resection for distal rectal cancer—for the future, look to the past. Am J Oncol Rev. 2006;5:2–4. [Google Scholar]
- 27.Weiser MR, Hak-Mien Q, Shia J, et al. Sphincter preservation in low rectal cancer is facilitated by preoperative chemoradiation and intersphincteric dissection. Ann Surg. 2009;249:236–42. doi: 10.1097/SLA.0b013e318195e17c. [DOI] [PubMed] [Google Scholar]
- 28.Rouffet F, Hay JM, Vacher B, et al. Curative resection for left colonic carcinoma: hemicolectomy vs. segmental colectomy. A prospective, controlled, multicenter trial. French association for surgical research. Dis Colon Rectum. 1994;37:651–9. doi: 10.1007/BF02054407. [DOI] [PubMed] [Google Scholar]
- 29.Nelson H, Petrelli N, Carlin A, et al. Guidelines 2000 for colon and rectal cancer surgery. J Natl Cancer Inst. 2001;93:583–96. doi: 10.1093/jnci/93.8.583. [DOI] [PubMed] [Google Scholar]
- 30.Scott N, Jackson P, Al-Jaberi T, et al. Total mesorectal excision and local recurrence: a study of tumor spread in the mesorectum distal to rectal cancer. Br J Surg. 1995;82:1031–3. doi: 10.1002/bjs.1800820808. [DOI] [PubMed] [Google Scholar]
- 31.Andreola S, Leo E, Belli F, et al. Distal intramural spread in adenocarcinoma of the lower third of the rectum treated with total rectal resection and coloanal anastomosis. Dis Colon Rectum. 1997;40:25–9. doi: 10.1007/BF02055677. [DOI] [PubMed] [Google Scholar]
- 32.Kuvshinoff B, Maghfoor I, Miedema B, et al. Distal margin requirements after preoperative chemoradiotherapy for distal rectal carcinomas: are ≤1 cm distal margins sufficient? Ann Surg Oncol. 2001;8:163–9. doi: 10.1007/s10434-001-0163-9. [DOI] [PubMed] [Google Scholar]
- 33.Moore HG, Riedel E, Minsky BD, et al. Adequacy of 1-cm distal margin after restorative rectal cancer with sharp mesorectal excision and preoperative combined-modality therapy. Ann Surg Oncol. 2003;10:80–5. doi: 10.1245/aso.2003.04.010. [DOI] [PubMed] [Google Scholar]
- 34.Rutkowski A, Bujko K, Nowacki MP, et al. Distal bowel surgical margin shorter than 1 cm after preoperative radiation for rectal cancer: is it safe? Ann Surg Oncol. 2008;15:3124–31. doi: 10.1245/s10434-008-0125-6. [DOI] [PubMed] [Google Scholar]
- 35.Quirke P, Durdey P, Dixon MF, et al. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumour spread and surgical excision. Lancet. 1986;2:996–9. doi: 10.1016/s0140-6736(86)92612-7. [DOI] [PubMed] [Google Scholar]
- 36.Nagtegaal ID, Marijnen CA, Kranenbarg EK, et al. Circumferential margin involvement is still an important predictor of local recurrence in rectal carcinoma: not one millimeter but two millimeters is the limit. Am J Surg Pathol. 2002;26:350–7. doi: 10.1097/00000478-200203000-00009. [DOI] [PubMed] [Google Scholar]
- 37.Glynne-Jones R, Mawdsley S, Novell JR. The clinical significance of the circumferential resection margin following preoperative pelvic chemo-radiotherapy in rectal cancer: why we need a common language. Colorectal Dis. 2006;8:800–7. doi: 10.1111/j.1463-1318.2006.01139.x. [DOI] [PubMed] [Google Scholar]
- 38.Quirke P, Steele R, Monson J, et al. Effect of the plane of surgery achieved on local recurrence in patients with operable rectal cancer: a prospective study using data from the MRC CR07 and NCIC-CTG CO 16 randomised clinical trial. Lancet. 2009;373:821–8. doi: 10.1016/S0140-6736(09)60485-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fujita S, Nakanisi Y, Taniguchi H, et al. Cancer invasion to Auerbach’s plexus is an important prognostic factor in patients with pT3-pT4 colorectal cancer. Dis Colon Rectum. 2007;50:1860–6. doi: 10.1007/s10350-007-9072-8. [DOI] [PubMed] [Google Scholar]
- 40.Akasu T, Takawa M, Yamamoto S, et al. Intersphincteric resection for very low rectal adenocarcinoma: univariate and multivariate analyses of risk factors for recurrence. Ann Surg Oncol. 2008;15:2668–76. doi: 10.1245/s10434-008-0047-3. [DOI] [PubMed] [Google Scholar]
- 41.Chang GJ, Rodriguez-Bigas MA, Eng C, et al. Lymph node status after neoadjuvant radiotherapy for rectal cancer is a biologic predictor of outcome. Cancer. 2009 doi: 10.1002/cncr.24622. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 42.Quah HM, Chou JF, Gonen M, et al. Pathologic stage is most prognostic of disease-free survival in locally advanced rectal cancer patients after preoperative chemoradiation. Cancer. 2008;113:57–64. doi: 10.1002/cncr.23516. [DOI] [PubMed] [Google Scholar]