Abstract
Purpose
Recent advances in nanotechnology have resulted in the manufacture of a plethora of nanoparticles with different sizes, shapes, core physicochemical properties and surface modifications that are being investigated for potential medical applications, particularly for the treatment of cancer. This review focuses on the therapeutic use of customized gold nanoparticles, magnetic nanoparticles and carbon nanotubes that efficiently generate heat upon electromagnetic (light and magnetic fields) stimulation after direct injection into tumors or preferential accumulation in tumors following systemic administration. This review will also focus on the evolving strategies to improve the therapeutic index of prostate cancer treatment using nanoparticle-mediated hyperthermia.
Conclusions
Nanoparticle-mediated thermal therapy is a new and minimally invasive tool in the armamentarium for the treatment of cancers. Unique challenges posed by this form of hyperthermia include the non-target biodistribution of nanoparticles in the reticuloendothelial system when administered systemically, the inability to visualize or quantify the global concentration and spatial distribution of these particles within tumors, the lack of standardized thermal modeling and dosimetry algorithms, and the concerns regarding their biocompatibility. Nevertheless, novel particle compositions, geometries, activation strategies, targeting techniques, payload delivery strategies, and radiation dose enhancement concepts are unique attributes of this form of hyperthermia that warrant further exploration. Capitalizing on these opportunities and overcoming these challenges offers the possibility of seamless and logical translation of this nanoparticle-mediated hyperthermia paradigm from the bench to the bedside.
Keywords: Nanoparticles, magnetic, optical, activatable, hyperthermia, prostate cancer
Prostate cancer
Prostate cancer accounted for 25% of estimated newly diagnosed cases of non-skin cancer in men in the United States in 2009. It was predicted that 192,280 new cases of prostate cancer would be diagnosed in the US in 2009, with one in six men developing the disease during their lifetime. Nearly 90% of men with prostate cancer have clinically localized disease. The aggressiveness of prostate cancer is largely determined by the prostate specific antigen levels, clinical extent and volume of disease, and Gleason histologic score (a measure of glandular differentiation). By predicting the likelihood of adjacent organ invasion, nodal metastasis, distant metastasis, recurrence following treatment, and likelihood of progression without treatment, these factors help stratify tumors into low-, intermediate- and high-risk groups, and aid the choice of treatment options. For low-risk clinically localized disease, a potentially curable stage of disease, common treatments include watchful waiting, radical prostatectomy, external beam radiation therapy (RT) and interstitial RT (brachytherapy), freezing the prostate (cryotherapy), and androgen deprivation therapy (ADT). The choice of treatment is often governed by the patient’s life expectancy, the likelihood of cancer progression without treatment, efficacy of treatment, convenience of treatment, treatment costs, and adverse effects (treatment-related urinary, bowel, and sexual dysfunction). For locally advanced cancers, the treatment options are based on the extent of disease but typically involve combinations of the treatments mentioned above with ADT usually being one of them. Less frequently, patients present with metastatic disease and treatment begins with ADT and palliative interventions but may proceed to involve chemotherapy when tumors become resistant to ADT. Locally recurrent prostate cancer is often treated with ADT, salvage radical prostatectomy, salvage brachytherapy, cryotherapy or thermal therapy. The focus of this article is on one form thermal therapy – hyperthermia.
Hyperthermia for prostate cancer
Hyperthermia generally refers to temperatures between 40°C and 45°C whereas temperatures >45°C are considered thermoablative. Mild temperature hyperthermia mediates its antitumor effects via subtle influences on the tumor microenvironment (Horsman and Overgaard 1997), induction of apoptosis (Fuller et al. 1994; Harmon et al. 1991), activation of immunological processes (Servadio and Leib 1991; Stawarz et al. 1993; Zhang et al. 2008), and induction of gene and protein synthesis (Horsman and Overgaard 1997; Kampinga and Dikomey 2001; Roti Roti 2004). While these effects do not independently cause tumor cell cytotoxicity, they lead to greater effectiveness of other conventional treatment modalities such as RT, chemotherapy, and immunotherapy (van der Zee 2002) (Hildebrandt et al. 2002). In its role as an adjunct to RT, hyperthermia serves as a dose-modifying agent that increases the therapeutic ratio of RT (i.e., enhanced effectiveness of a given dose of RT without additional toxicity). Hyperthermia can be achieved a number of ways including local hyperthermia by external or internal energy sources, regional hyperthermia by irrigation of body cavities or perfusion of organs or limbs, and whole body hyperthermia. Regardless of the mechanism of heating, clinical trials of hyperthermia as stand-alone therapy or in combination with RT have demonstrated promising outcomes in the treatment of many cancers including prostate cancer (Anscher et al. 1997; Baronzio et al. 2009; Jones et al. 2005; Overgaard et al. 1995; Satoh et al. 1988; Sherar et al. 2004; Sherar et al. 2003; Shimm et al. 1988; Sneed et al. 1998; van der Zee et al. 2000; Vernon et al. 1996). Although an entirely non-invasive treatment approach is preferred, minimally invasive techniques such as intraluminal or intracavitary treatments are particularly appealing due to the flexibility of positioning endorectal applicators close to the posterior aspect of the prostate or transurethral catheters in the center of the prostate. This strategy has been employed successfully in the treatment of locally advanced prostate cancer using modalities such as ultrasound, radiofrequency and microwaves with appropriate applicators positioned either externally, intraluminally or interstitially to generate heat. Multiple studies report gratifying outcomes with the use of endorectal microwave or ultrasound applicator mediated hyperthermia in conjunction with conventional RT (thermoradiotherapy) for the treatment of locally advanced prostate cancer (Algan et al. 2000; Fosmire et al. 1993; Hurwitz et al. 2002; Hurwitz et al. 2005; Hurwitz et al. 2001; Mendecki et al. 1980; Servadio and Leib 1991; Yerushalmi et al. 1982). Similarly, radiofrequency induced hyperthermia has been used in the treatment of prostate tumors through intracavitary applicators (Bhowmick et al. 2001; Dawkins et al. 1997; Sofras et al. 1996; Zargar Shoshtari et al. 2006). The main challenge with the intracavitary or intraluminal delivery of hyperthermia is the generation of adequate heat in the prostate without excessive temperature in critical adjacent structures such as the neurovascular bundle, urethra, bladder and rectum (Gillett et al. 2004; Marberger 2007). Alternatively, external regional hyperthermia can be used in combination with RT for the treatment of prostate cancer (Anscher et al. 1997). The lack of significant temperature conformality at the interface between the prostate and the rectum remains a major challenge with this technique. Interstitial hyperthermia offers the possibility of generating uniform temperatures within the prostate without any significant temperature rise in surrounding normal structures, but is an invasive procedure (Emami et al. 1996; Lancaster et al. 1999; Prionas et al. 1994; Sherar et al. 2004; Sherar et al. 2003).
Clinical hyperthermia experience has led to the recognition that high minimum temperatures achieved in most parts of the target volume correlate better with clinical outcome than maximum temperatures attained in small parts of the target volume (Dewhirst et al. 1984). A standardized nomenclature has been proposed and validated for the representation of variable time-temperature data as an Arrhenius isoeffect relationship where the total thermal dose is expressed as the cumulative equivalent minutes at 43°C achieved or exceeded in 90% of the prostate (CEM 43°C T90) (Jones et al. 2005; Sapareto and Dewey 1984; Thrall et al. 2005). The targeted clinical thermal dose for hyperthermia when combined with RT is a CEM 43°C T90 of 5–10 minutes (Hurwitz et al. 2005; Jones et al. 2005; Oleson et al. 1993; Tilly et al. 2005). Despite the increasingly convincing evidence for clinical hyperthermic radiosensitization and the evolving consensus in reporting these data, it is underutilized in routine clinical practice due to: (a) the invasive means of achieving and maintaining hyperthermia, (b) the lack of good thermal dosimetry, and (c) the inability to achieve localized hyperthermic temperatures (Moros et al. 2007). Hence, a relatively non-invasive approach with externally regulatable and quantifiable prostate-specific hyperthermia could provide renewed enthusiasm for this treatment paradigm. The above mentioned hyperthermia strategies solely rely on the ability of the cancer tissues to convert the imparted electromagnetic energy into heat. In contrast to methods relying on modifying the energy source to generate heat, there is evolving interest in methods to preferentially enhance the heat generating capacity of the cancer tissues by introducing exogenous materials in to them. Along these lines, ferromagnetic seeds have been used in conjunction with a magnetic field to induce hyperthermia in prostate cancer (Brezovich and Meredith 1989; Meredith et al. 1989; Partington et al. 1989). Ferromagnetic seeds or thermoseeds are needle-shaped devices that are interstitially placed into the tumor, similar to brachytherapy implants, and the heating is accomplished by an externally applied magnetic field. The uniqueness of thermoseed hyperthermia are (i) the lack of requirement for external power connections and (ii) the automatic regulation of temperature of the implanted thermoseeds depending on the compositional characteristics of the implants (Meredith et al. 1989; Partington et al. 1989). Being an interstitial modality, thermoseed mediated hyperthermia has limitations similar to other interstitial hyperthermia techniques. Further, due to the heating equipment size and the requirement to limit electromagnetic radiation to meet federal (FCC) regulations, thermoseed implant hyperthermia treatments are performed in special electromagnetic shielded rooms located in dedicated hyperthermia suites. Alternatively, nanoscale materials, particularly metal nanoparticles that are activatable by externally applied electromagnetic fields, can be used to induce cancer-specific hyperthermia.
Nanoparticles
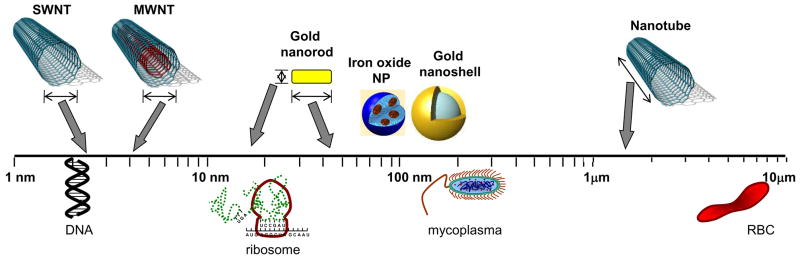
In the broadest sense, nanotechnology involves utilizing the unique properties and behaviors of materials made at the nanoscale, a scale that ranges from 1 to 100 nm. At the simplest level, what drives these unique behaviors and properties is the significantly larger surface area per unit volume of nanoscale materials than the same material in the bulk scale – the greater surface area affords greater opportunities for interactions with adjacent materials. Capitalizing on these observations, recent advances in nanotechnology have resulted in the manufacture of a plethora of nanoparticles with different sizes, shapes, core physicochemical properties and surface modifications that are being investigated for potential medical applications. From a biological perspective, the size of such particles tends to be similar to that of a DNA doublestrand (2 nm thick), a ribosome (20 nm), or the smallest bacteria (200 nm Mycoplasma) and considerably smaller than the typical eukaryotic cell (7 micron diameter of a small red blood cell). Therefore, systemically administered nanoparticles readily extravasate out of blood vessels and can interact with biomolecules at the cellular and molecular level. The most common nanoparticles studied for biomedical applications are liposomes and uni- or multi-lamellar vesicles (organic biolipid layers encapsulating imaging and therapeutic payloads), dendrimers (repeatedly branched polymers), quantum dots (metallic core-shell nanoparticles that are intensely fluorescent at specific wavelengths), gold nanoparticles (ranging in shape from spheres and shells to rods and cages), paramagnetic nanoparticles (iron oxide laden particles), and carbon nanotubes. In the arena of cancer research, there has been an explosion of knowledge and research regarding oncologic uses of such nanoparticles. In addition to several diagnostic applications of nanoparticles using optical, magnetic resonance, positron emission tomography, computed tomography and x-ray techniques, the therapeutic application of nanoparticles via tumor heating is emerging as a novel form of “nanothermal therapy” of tumors (Sharma and Chen 2009). Although several potential hyperthermic particles such as silver, lanthanum and zinc nanoparticles are available (Naruse et al. 1986), the thermal activation properties of gold nanoparticles, magnetic nanoparticles and carbon nanotubes have been extensively characterized preclinically and they are furthest along in potential translation to clinical biomedical applications. In addition, these particles serve as platforms for development of additional novel nanoparticle ensembles comprised of alloys, dopants and hybrid particles. Hence, this review will focus on the potential application of gold nanoparticles, magnetic nanoparticles and carbon nanotubes (see Figure 1) in the thermal therapy of prostate cancer.
Figure 1.
A schematic representation of the typical nanoparticles utilized in thermal therapies.
Gold nanoparticle induced hyperthermia
A number of features of gold nanoparticles have rendered them particularly attractive to biomedical researchers and account for their popularity in preclinical research leading up to potential clinical translation. The most striking feature is the familiarity of the medical community with gold as a clinically useful therapeutic agent for various ailments such as melancholy, fainting, fevers, syphilis and arthritis (Higby 1982; Parish 1999). The most prominent use of gold has been for the treatment of rheumatic arthritis. Treatment of rheumatoid arthritis with a cumulative dose of a little less than 2gm/yr for 10 years without any appreciable toxicity speaks to the good overall tolerance of gold in humans (Abrams and Murrer 1993). Due to its physical inertness, gold is unlikely to interact chemically with biomolecules in humans. In addition to its apparent clinical safety and tolerability, gold can be used to synthesize nanoparticles with very precise sizes, shapes and surface chemistries at the nanoscale using simple techniques and relatively inexpensive reagents (Daniel and Astruc 2004). The most unique property that lends itself to hyperthermia applications is the photothermal activation of gold nanoparticles. However, there are concerns regarding the biocompatibility of these gold nanoparticles for clinical applications. For instance, a recent study noted activation of the immune complement system by gold (Hulander et al. 2009). While this raises concerns about its clinical safety, the study also reports that when coated on Bactriguard commercial surfaces the gold nanoparticles are less effective in activating the immune complement system, a feature attributed to the altered nanostructure and chemistry of gold nanoparticles and nanogalvanic effects. Furthermore, similar biocompatible coatings such as polyethylene glycol or dextran provide “stealth” characteristics to these particles for evasion of capture by and accumulation within the reticuloendothelial system, further enhancing their biocompatibility.
It is known that, at the nanoscale, bulk metals exhibit optical resonances of their surface plasmons. In colloidal form, these metals typically absorb and scatter light strongly at a characteristic wavelength (plasmon resonance) in the visible region of the spectrum. Working with wavelengths in the near infrared (NIR) region of the spectrum is clinically meaningful because light penetrates deep within tissue (up to several centimeters) at these wavelengths. Indeed, certain geometries (spheres, rods and shells) of metal nanoparticles have optical plasmon resonances that can be tuned to the NIR region (Oldenburg et al. 1999). While gold nanospheres and nanorods are made of solid gold, nanoshells consist of a dielectric core (e.g. silica) surrounded by a thin gold shell. Nanospheres exhibit resonances around 540 nm without much tunability of this peak whereas nanoshells and nanorods have peak resonances that can be tuned throughout the NIR spectrum (Jain et al. 2006; Oldenburg et al. 1998). Nanoshells are tuned via their core-to-shell ratio while nanorods are tunable through their aspect ratio (i.e. ratio of the length to diameter). For instance, gold nanoshells comprised of an aminated colloidal silica (120 nm diameter) core with a 14-nm-thick shell of gold colloid adsorbed onto it as sequential nucleating sites result in an absorption peak between 780 and 800nm. Furthermore, due to their metal structure, gold nanoparticles are extremely efficient photothermal coupling devices. Their large absorption cross sections convert light to heat, and their high thermal conductivity couples this heat to the surrounding tissue. Lastly, the handling of gold nanoparticles as devices rather than drugs could reduce time and expense incurred in translating their use from the bench to the bedside.
For clinically pertinent oncologic applications, interstitial injection of these particles within tumors is a readily available option but a more exciting and possibly elegant option is to deliver these nanoparticles systemically and have them accumulate within tumors either passively or via active targeting of tumor-specific molecules. Passive yet selective sequestration of nanoparticles within tumors capitalizes on a phenomenon termed enhanced permeability and retention (EPR) effect (Baluk et al. 2003; McDonald and Choyke 2003) where macromolecules and nanoparticles passively extravasate from leaky tumor vasculature containing wide interendothelial junctions, incomplete or absent basement membranes, dysfunctional lymphatics, and numerous transendothelial channels (Jain 1999). In contrast, active targeting is facilitated by functionalizing the gold nanoparticle with biomolecules including peptides, antibodies, and oligonucleotides that are specific to the target of interest (Han et al. 2000; Khan et al. 2008; Loo et al. 2005; Lowery et al. 2006).
The seminal report on the use of systemically administered nontargeted NIR-activatable gold nanoshells for thermal ablation of tumors in an animal model (Hirsch et al. 2003) built on the initial characterization of laser dosimetry studies and pharmacokinetic and biodistribution analyses. A subsequent efficacy study in BALB/c mice inoculated subcutaneously with CT26/wt murine colorectal cancer cells demonstrated that 100 μL of 2.4 × 1011 nanoshells/mL administered intravenously resulted in tumor accumulation at 6 hours – NIR laser treatment at 4 W/cm2 for 3 minutes resulted in 90% survival of nanoshell-treated and irradiated mice and 0% survival of nanoshell-treated and unirradiated controls and 0% survival of saline-treated and irradiated controls (O’Neal et al. 2004). Subsequently, several studies have been reported on the use of gold nanoparticles such as gold nanorods and nanocages for the thermal therapy of cancer (Dickerson et al. 2008; Hu et al. 2006; Huang et al. 2006; Huang et al. 2008a; Huff et al. 2007; Skrabalak et al. 2007; von Maltzahn et al. 2009; Wu et al. 2009). More recently, the feasibility of using gold nanoshells and optical fiber-based NIR illumination for interstitial thermoablation of intracranial tumors was demonstrated (Schwartz et al. 2009). While these reports involve passive targeting of gold nanoparticles to achieve the photothermal ablation, several other studies document the feasibility and efficacy of tumor-specific active targeting of gold nanoparticles for photothermal therapy of tumors (Bernardi et al. 2008; Cheng et al. 2009; Huang et al. 2008b; Kawano et al. 2009; Kumar et al. 2008; Liu et al. 2009; Ma et al. 2009; Sokolov et al. 2003). Gold nanoshells have also been used to induce mild temperature hyperthermia in murine tumor models to enhance the therapeutic efficacy of RT. Initial experiments defined the laser parameters for mild temperature hyperthermia and demonstrated that non-invasive magnetic resonance thermal imaging accurately predicted temperature measurements obtained using thermocouples inserted into the tumors (see Figure 2). When mild temperature hyperthermia (41°C for 20 minutes) was followed immediately by a single 10 Gy dose of radiation (125 kV X-rays), there was an approximately 2-fold increase in tumor growth delay (time taken for tumors to double in volume) when compared to the animals treated with radiation alone (Diagaradjane et al. 2008b). As expected, hyperthermia led to an immediate increase in perfusion of the center of tumors (documented on dynamic contrast enhanced magnetic resonance imaging). Unexpectedly, however, there were large areas of necrosis in the combined treatment group at a later time point. This necrotic pattern of tumor cytotoxicity was attributed to a decrease in microvessel density, possibly due to focal vascular disruption mediated by perivascularly concentrated gold nanoshells that generate intense focal temperature elevations. Presumably, gold nanoshells that are too large to diffuse freely into tumor interstitium but large enough to leak out of tumor vasculature remain sequestered in the perivascular region. Conceivably, as an extension of this concept, gold nanorods that are smaller than nanoshells will penetrate deeper into tumors and provide more global temperature elevations within tumors, particularly if they are conjugated to tumor-specific biomarkers. In spite of these extensive reports on the use of gold nanoparticle-mediated ablation and hyperthermic radiosensitization, very few reports document its utility in the treatment of prostate cancer. In vitro studies of gold nanoshell-mediated photothermal ablation of PC-3 and C4-2 prostate cancer cells demonstrated a complete loss of cell viability while maintaining intact cellular morphology (Stern et al. 2007). This observation correlates well with the intact cellular morphology of clinical biopsies obtained after radiofrequency ablation (Margulis et al. 2004). A subsequent in vivo study on a murine subcutaneous prostate cancer model compared the therapeutic efficacy of two doses of gold nanoshells (7μL/gm and 8.5μL/gm of body weight) and demonstrated enhanced therapeutic efficacy (93% tumor necrosis and regression with an average temperature rise to 65.4°C) with the high concentration of gold nanoshells (Stern et al. 2008). Targeted thermal therapy of PC-3 prostate cancer cell lines using prostate-specific EphrinA1-conjugated gold nanoshells demonstrated localized thermal damage to cells that were bound to the conjugates (Gobin et al. 2008). Prostate cancer cell specific uptake and toxicity studies of different nanoparticles (gold nanoshells and gold nanorods) have also demonstrated size dependent uptake and negligible toxicity (Malugin and Ghandehari 2009). It is reasonable to assume that findings from other tumors can be reproduced in prostate cancers to provide justification for advancing gold nanoparticle-mediated hyperthermia in clinical scenarios.
Figure 2.
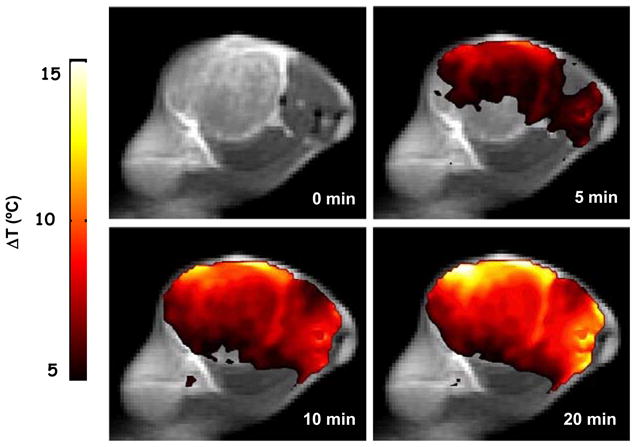
Spatial temperature map of magnetic resonance thermal imaging indicating temperature rise (°C) above the baseline following near infrared illumination of gold nanoshells-laden tumors (Reproduced with permission from.
Magnetic nanoparticle induced hyperthermia
Thermotherapy using magnetic nanoparticles involves the coupling of an external magnetic field to tumor laden magnetic particles to generate high-energy photons through a magnetic field induced locally in the vicinity of the nanoparticle. These high-energy photons result in the observed magnetic hyperthermia effect by the Neel’s relaxation process (Hildebrandt et al. 2002). Although the concept of magnetic hyperthermia was introduced 50 years ago, its clinical potential has recently been recognized based on the achievable selective and relatively homogenous temperature distribution in deep-seated tumors when compared to conventional hyperthermia modalities (Moroz et al. 2002). Initial studies on the physical evaluation of ferromagnetic particles and magnetic fluids suggested that intratumoral power absorption from a moderate concentration of 5 mg ferrite per gram tumor (i.e. 0.5% w/w) and clinically acceptable magnetic fields is comparable to radiofrequency heating with local applicators and superior to regional radiofrequency heating (by comparison with clinical specific absorption rate measurements from regional and local hyperthermia treatments), which is attributed to the much larger number and surface area of magnetic particles (Jordan et al. 1993). Subsequent in vivo studies were performed in murine models of mammary carcinoma with intratumoral injection of 1.5×10−2mg ferrite/mm3 followed 20–30 minutes later by intratumoral hyperthermia (steady-state temperatures of 47°C for 30 minutes) generated by whole-body alternating magnetic fields of 6–12.5kA/m at 520 kHz. In spite of the inhomogeneity in the intratumoral distribution of magnetic nanoparticles and local tumor regrowth, widespread tumor necrosis was observed after hyperthermia treatment and tumor growth was slightly delayed in comparison with untreated controls (Jordan et al. 1997). The first in vivo preliminary evaluation in a rat model of prostate cancer demonstrated successful intraprostatic nanoparticle infiltration, excellent tolerability and stable steady-state thermoablative intratumoral temperatures (50°C using a field strength of 15 kA/m) induced by an alternating magnetic field (Johannsen et al. 2004). Further investigation of an orthotopic Dunning R3327 rat prostate cancer model revealed an intra-prostatic temperature of 70°C with a maximum field strength of 18 kA/m resulting in significant growth inhibition of 44–51% over control animals (Johannsen et al. 2005b). Subsequent studies of magnetic nanoparticle-mediated hyperthermia in combination with RT (20 Gy) in a rat prostate cancer model demonstrated a therapeutic efficacy equivalent to a single radiation of 60 Gy (Johannsen et al. 2006). Similar results were observed with complete tumor regression 30 days after hyperthermic treatment of DMBA-induced rat mammary cancers using magnetic nanoparticles (Motoyama et al. 2008). In an animal model of metastatic prostate cancer to bone, application of an alternating magnetic field to magnetic nanoparticles conjugated to cationic liposomes demonstrated potent suppression of tumor proliferation in the bone microenvironment (Kawai et al. 2008). The promising preclinical activity of magnetic nanoparticle-mediated hyperthermia has now been advanced to early clinical experiences discussed later.
Carbon nanotube induced hyperthermia
Carbon nanotubes (CNT) are another class of nanomaterials that holds great potential for various biomedical applications including extrinsically activated hyperthermia. CNTs are nested, cylindrical grapheme structures with diameters ranging from a few to hundreds of nanometers and lengths up to a few micrometers (Zheng et al. 2004). The extraordinary photon-to-thermal energy conversion efficiency of CNTs with high absorption cross-section in the NIR region of the electromagnetic spectrum stimulated several investigations to exploit their potential for anticancer therapy (Kam et al. 2005; O’Connell et al. 2002). Several in vitro studies have demonstrated the use of targeted and non-targeted CNTs (single walled) for photothermal ablation of cancer cells (Biris et al. 2009; Chakravarty et al. 2008; Kam et al. 2005; Mahmood et al. 2009; Torti et al. 2007; Wang et al. 2009). While these studies have used NIR irradiation to generate hyperthermia, radiofrequency fields have also been shown to induce thermal toxicity in malignant cells. In this study, internalized single walled CNTs (SWCNTs) in human cancer cells were exposed to a 13.56 mHz radiofrequency field to induce noninvasive, selective, concentration dependent thermal destruction. Direct intratumoral injection of SWCNTs followed by radiofrequency treatment at 48 hrs demonstrated complete necrosis of tumors when compared to the controls (Gannon et al. 2007). In vivo obliteration of solid human epidermoid tumor xenografts in mice without harmful side effects or tumor recurrence for 6 months was demonstrated by combining intratumoral injections of SWCNTs (~ 120 mg/ml, 100 μL) with NIR irradiation (808 nm, 76 W/cm3) for 3 min. SWCNTs were completely excreted (in 2 months) via the biliary or urinary pathways (Moon et al. 2009). Recently, multi walled CNTs (MWCNTs) have been explored as a mediator for photothermal therapy of cancer because of their enhanced absorption cross-section when compared to SWCNTs (Kam et al. 2005). A long-term survival study following a single treatment of kidney tumors with MWCNTs (100 μg/mouse) and NIR radiation (1064 nm; 3W/cm2) for 30 seconds demonstrated tumor ablation with minimal local or systemic toxicity. The tumor ablation achieved with a relatively low laser power and very minimal exposure time suggests that the photothermal efficacy of MWCNTs is considerably greater than SWCNTs (Burke et al. 2009). More recently, treatment of prostate cancer xenografts in nude mice with MWCNTs demonstrated that DNA-encasement enhanced the heat emission from MWCNTs following NIR irradiation with a 3-fold lower concentration of MWCNTs than that required to impart a 10 °C temperature increase in bulk solution. A single intratumoral injection of MWCNTs (100 μL of a 500 μg/mL solution) followed by laser irradiation at 1064 nm, 2.5 W/cm2 resulted in complete eradication of PC3 xenograft tumors (Ghosh et al. 2009). Despite these promising results, toxicity concerns about carbon nanotubes have been attributed to factors such as surface chemistry, degree of aggregation, and chemical functionalization (Dumortier et al. 2006; Magrez et al. 2006; Sayes et al. 2006; Wick et al. 2007). In addition, the route of administration also contributes the likelihood of toxicity. For instance, exposing the mesothelial lining of the body cavity of mice to long multiwalled carbon nanotubes resulted in granuloma formation similar to that noted with asbestos exposure (Poland et al. 2008). Similarly, in a manner reminiscent of asbestos-associated pleural fibrosis and mesothelioma formation, multiwalled carbon nanotubes reach the subpleura in mice after a single inhalation exposure (Ryman-Rasmussen et al. 2009). Nonetheless, at low doses, ranging from 20 – 850 μg/kg body weight, carbon nanotubes are non-toxic in mice (Liu et al. 2007; Liu et al. 2008; Schipper et al. 2008; Singh et al. 2006). Even at high oral doses of 1000 mg/kg body weight, a more recent study has demonstrated that carbon nanotubes are non-toxic (Kolosnjaj-Tabi et al.). Clearly, the toxicity of carbon nanotubes needs to be carefully evaluated in parallel with continued exploration of the use of carbon nanotubes for biomedical applications.
Clinical experience
The first indication of clinical feasibility of nanoparticle-based hyperthermia treatment was provided by a German pilot study in 10 patients with biopsy-proven locally recurrent prostate cancer following prior RT. In the absence of an extant standard treatment for recurrent prostate cancer, patients were eligible as long as they were either not suitable for or refused salvage radical prostatectomy. In an approach similar to prostate brachytherapy, patients under general anesthesia underwent transrectal ultrasound/fluoroscopy-guided external template-assisted transperineal intraprostatic injection of a nanoparticle dispersion to achieve a three-dimensional distribution paralleling a preplan (Johannsen et al. 2005a). The magnetic nanoparticles had an average core size of 15 nm with an aminosilane-type shell and remained stable in the injected location for all six weeks of treatment. Computed tomography images allowed visualization of the spatial distribution of nearly 90% of all magnetic nanoparticles in suspension (112 mg/ml of ferrites in aqueous solution) whereas ultrasound and MRI were incapable of providing similar anatomic definition. A customized magnetic field applicator MFH 300F (MagForce Nanotechnologies) (Gneveckow et al. 2004) provided the 100 kHz alternating magnetic field at a variable field strength of beginning at 2.5 kA/m and escalated to 18 kA/m as tolerated by the unanesthetized patient. A median temperature of 40.1°C was attained in 90% of prostates and a median CEM 43°C T90 of 7.8 min was achieved over six 60-min weekly treatments. A 4–5 kA/m constant magnetic field strength was tolerated for the entire hour by all patients. With a median follow-up of 17.5 months, no systemic toxicity was noted (Johannsen et al. 2007a). Acute urinary retention occurred in 4 patients with pre-existing urethral strictures. Quality of life assessments detected acute (midway through and upon completion of 6-week course) decline in social and sexual functioning, and increased fatigue, pain, and urinary symptoms. Later (3–6 months from treatment), only deterioration in social functioning was recorded.
Challenges with nanoparticle-mediated hyperthermia
Biodistribution
Even a single injection of nanoparticles directly into the prostate, in an idealized hypothetical scenario, leads to a non-uniform spatial distribution of particles that is defined by the injection volume, the injection rate, the concentration of the particles and the resilience of the tissue (Salloum et al. 2008). The heterogeneity in intraprostatic biodistribution of nanoparticles is compounded by the need for multiple injections to encompass the entire prostate. It is well recognized that the spatial distribution of nanoparticles dominates the resulting spatial distribution of temperature within the prostate (Salloum et al. 2009). In the case of systemically delivered nanoparticles not only is there heterogeneous accumulation within tumors, there is also considerable variability in organ/tissue biodistribution and pharmacokinetics. For instance, the kinetics of accumulation within tumors is influenced by the hydrodynamic diameter (Choi et al. 2007), shape (Champion and Mitragotri 2009), surface charge (Akiyama et al. 2009), and the extent (Akiyama et al. 2009), length (Choi et al. 2009) and branching (Veronese 2001) of the polyethylene glycol surface coating often used to confer “stealth” properties for immune evasion from the reticuloendothelial system. Experimental evidence suggests that spherical nanoparticles with a hydrodynamic diameter of approximately 5.5 nm and a zwitterionic surface charge are cleared by the kidneys and not entrapped within the reticuloendothelial system (Choi et al. 2007) whereas larger nanoparticles (20 nm) are captured by the reticuloendothelial macrophages (Diagaradjane et al. 2008a). At the tissue-level, leaky vascular fenestrations and chaotic immature vascular architecture largely determine the geographic distribution of nanoparticles within tumors - untargeted nanoparticles, irrespective of their size, leak out of tumor vasculature and remain sequestered and spatially confined to the perivascular zone (Diagaradjane et al. 2008a) (Diagaradjane et al. 2008b). The heterogeneity in vascular architecture and the consequent heterogeneity in intratumoral distribution of nanoparticles results in non-uniform temperature profiles within tumors when these nanoparticles are activated. Some reports would suggest that this heterogeneity is advantageous when combined with radiation since focal vascular disruption may ensue following the juxtaposition of this form of hyperthermia with RT (Diagaradjane et al. 2008b). Therefore, in contrast to the vascular compartment of tumors serving as a heat sink with traditional forms of hyperthermia, in this instance the preferential heating of nanoparticles entrapped within perivascular spaces focuses thermal energy on endothelial cells, a prime target for radiosensitization strategies. Despite this potential advantage, the heterogeneous distribution of nanoparticles, the inhomogeneity of NIR density (greater intensity closer to the illumination source), and difficulty with modeling and dosimetry make nanoparticle-mediated hyperthermia challenging. To some extent, the heterogeneity in the nanoparticle distribution can be overcome by specifically targeting tumor interstitium-penetrating small nanoparticles to tumor-specific biomarkers for a relatively uniform and homogeneous distribution (Diagaradjane et al. 2008a).
Quantification and visualization
One of the challenges of using nanoparticles for cancer therapy is the inability to readily quantify and/or visualize these particles after they have accumulated within tumors. As noted above, magnetic nanoparticles are not visualized on MRI due to a signal void in the areas containing high concentrations of interstitially injected iron oxide nanoparticles (Johannsen et al. 2007b). Similarly, they are not readily visualized on transrectal ultrasound. CT imaging was able to detect large deposits of injected magnetic nanoparticles but this sensitivity would be much lower if the concentration of nanoparticles is considerably lower, as in the case of systemically administered nanoparticles. For such instances, dedicated techniques are needed to quantify the amount of nanoparticles globally present within tumors as well as to visualize geographic locations of these nanoparticles within tumors. In the case of gold nanoparticles, one technique that non-invasively estimates the quantity of gold (and therefore, the number of gold nanoparticles) within tumors in real time is diffuse optical spectroscopy (DOS). In DOS, light is delivered to and collected from tissue via an optical fiber probe and the specific reflectance spectra of gold nanoparticles are used to indirectly measure gold concentrations after accounting for the contribution of oxy-hemoglobin and deoxy-hemoglobin. In one such study, DOS measurements accurately quantified the concentration of gold nanoshells in tissue phantoms within 10% of the known concentration as well as in vivo where gold content measurements were validated by neutron activation analysis, the standard method of measuring gold nanoshell concentrations in tissues that are excised, dehydrated and irradiated within a nuclear reactor (Zaman et al. 2007). While DOS provides a global estimate of gold nanoparticle concentration within tumors, it does not provide spatial information on the distribution of these nanoparticles within tumors. One technique that offers this option is narrowband imaging which capitalizes on the strong NIR absorption of gold nanoshells to distinguish between blood and nanoshells in the tumor by imaging in narrow wavelength bands in the visible and NIR, respectively (Puvanakrishnan et al. 2009). By clearly discriminating between blood and gold nanoshells, this technique allows imaging of the heterogeneous spatial distribution of nanoshells within tumors. This geographic distribution imaging can be verified ex vivo by two-photon luminescence imaging. Alternative strategies include radiolabeling the nanoparticle for visualization by positron emission tomography or single photon emission computed tomography, fluorescent dye labeling for optical tomography of enhanced fluorescence (Bardhan et al. 2009), optical coherence tomography (Gobin et al. 2007), or NIR diffuse optical tomography (Wu et al. 2005).
Modeling and dosimetry
Visualizing and quantifying gold nanoparticles accurately in tumors is a prelude to modeling and mapping the temperature elevations within tumors and generating dosimetry outputs similar to RT. For instance, continued development and clinical translation of gold nanoshell-mediated thermal therapy requires computational tools for estimating heat distribution within the tumor and surrounding tissues. One model for estimating heat from NIR laser activation of gold nanoshells uses a modified bioheat equation with a finite element model based on alterations in the optical properties of the medium when laden with nanoshells to predict temperature rise and magnetic resonance thermal imaging to validate it (Elliott et al. 2008). However, this model requires prior knowledge of the altered optical properties of the medium with nanoshells, which might not be readily obtained during routine in-vivo experiments or in future clinical applications. An alternative technique is to use the light transport theory with a diffusion approximation to model the temperature rise within nanoshell-free media due to NIR laser power dissipation and combine this with modeling of plasmonic heat generated by NIR irradiated individual gold nanoshells due to photothermal effect to estimate the global elevation of temperature within nanoshell-laden media (Cheong et al. 2009). This model accounts for the response of tissue to laser illumination as well as the optical and thermal effects due to embedded individual gold nanoshells on the temperature rise in tissues. Similarly, in the clinical study described earlier, real-time thermal measurements in four catheters (2 in each lobe of the prostate) were fitted to thermal dosimetry calculations using the bio-heat transfer equation solved on a finite element basis using iron-mass (derived from CT density), specific absorption rate (SAR) of magnetic nanoparticles, magnetic field strength, and an estimated perfusion (Johannsen et al. 2007b). In the earliest incarnation of individualized noninvasive three-dimensional thermal modeling based on spatial distributions of intraprostatic magnetic nanoparticles, the model overestimated temperatures near the urethra and bladder base, and underestimated temperatures at the prostatic apex and along skin folds. On the flip side, the heterogeneity of nanoparticle distribution (a function of injection flow rate, concentration and firmness of a previously radiated prostate) (Salloum et al. 2008) and consequent non-uniform intraprostatic temperatures also results in an overestimation of actual temperature by intraluminal measurements. Nonetheless, the reasonable agreement between noninvasive temperature calculations and invasive thermometry within the prostate suggests that thermal modeling could provide a global assessment of temperature across a target volume that complements focal thermal monitoring. Admittedly, unlike radiation dosimetry that is based on physical parameters (radiation quality, radiation quantity, tissue density and tissue geometry), thermal dosimetry also needs to account for tissue physiology (heat dissipation and transfer being modulated by vascularity, degree of necrosis/fibrosis, tissue conductivity/perfusion, and the influence of heat itself on these parameters) (Hurwitz 2007). Continued refinement of thermal dosimetry algorithms incorporating normal tissue avoidance constraints could guide the selection of injection coordinates for highly conformal thermotherapy (Salloum et al. 2009). If predictive models do not turn out to be sufficiently accurate, magnetic resonance thermometry currently provides accurate and real-time spatiotemporal resolution of thermal dose without requiring accurate heat-transfer models and precise knowledge of local particle concentrations. Magnetic resonance thermometry capitalizes on the correlation between a shift in proton resonance frequency and elevation of temperature within tissue to facilitate mapping of temperature distributions (Wust et al. 2006). Typically, after obtaining a reference (baseline) scan and a measurement scan, phase subtraction allows computation of a proton resonance frequency shift. Since the proton resonance frequency of adipose tissue does not shift as a function of temperature, nulling these regions (fat correction) can be utilized for calibration and accounting for baseline drift. This novel non-invasive thermal imaging remains one of the most significant technical advances in clinical applications of thermal therapy that allows real-time temperature monitoring, confirmation of adequate thermal dose coverage and adaptive course-correction during treatment.
Biocompatibility
A legitimate concern with all classes of nanoparticles is their toxicity, which is, in turn, determined by the dose, core composition, surface chemistry, and location and duration of confinement to the body. Whereas the use of gold and iron oxide in medicine for many decades offers some degree of familiarity with the safety profile of these bulk metals, the unique properties and biodistribution of nanoscale formulations of these metals calls for systematic evaluation and characterization of their biocompatibility. The National Institutes of Health also recognizes the need for toxicity testing of nanoparticles to parallel pre-clinical efficacy assessment - the Nanotechnology Characterization Laboratory, working in concert with the National Institute of Standards and Technology (NIST) and the U.S. Food and Drug Administration (FDA), facilitates such testing and regulatory review. Gold nanoshells have been extensively tested preclinically and are now in clinical trials as investigational new devices.
Unique opportunities with nanoparticle-mediated hyperthermia
Active targeting
As noted above, a unique feature of systemic administration of nanoparticles for hyperthermia is the ability to potentially target the nanoparticle preferentially to tumor cells, thereby increasing tumor-specificity and reducing collateral damage to surrounding critical structures. This has been demonstrated in vitro using ephrinA 1 (Gobin et al. 2008). Similarly, circulating nanoparticles (Gao et al. 2004) could be guided onto prostate-specific membrane antigen, an integraltransmembrane glycoprotein expressed on the surface of prostate carcinoma at all stages of the cancer (Rajasekaran et al. 2005) but highly restricted in extraprostatic tissues and normal endothelial cells (Silver et al. 1997). Alternatively, rather than focusing delivery on prostate cancer cells, the nanoparticles can be delivered to prostate cancer neovasculature by targeting molecules such as integrin αvβ3, a cell adhesion molecule that is significantly up-regulated onendothelia during angiogenesis and on fast-growing solid tumor cells, but not on quiescent endothelium and normal tissues (Hood and Cheresh 2002). Nevertheless, although targeted therapy is highly appealing as a means of treating prostate cancer without excessive collateral damage to surrounding normal tissue, a major limitation of such treatments for prostate cancer, which is typically a multifocal disease, is the lack of contemporary diagnostic imaging modalities to visualize localized foci of involvement.
Targeted payload delivery
Another unique feature of nanoparticle-mediated hyperthermia is the possibility of delivering an entirely different payload to the tumor when the nanoparticle concentrates within it. This is in contrast to using hyperthermia to trigger release of payloads contained with separate thermosensitive nanoparticles – this form of hyperthermia-triggered payload release would potentially be applicable to any form of hyperthermia. Instead, this feature refers to hybrid nanoparticles that serve as activatable thermal sources as well as therapeutic payload carriers. As a first step in achieving this goal, it has been shown that pretreatment with intravenously administered TNF-α-coated gold nanoparticles enhances thermally induced tumor growth delay in a mouse model of breast cancer (Visaria et al. 2006). Future applications could entail externally-triggered thermally-mediated release of payloads within the confines of the tumor alone - the payloads could include targeted therapeutic peptides/proteins, toxins, oligonucleotides and small interfering RNA.
Radiation dose enhancement
One additional benefit of combining RT with metal nanoparticle-mediated hyperthermia is the possibility of radiation dose-enhancement due greater photoelectric interactions in the presence of higher atomic number (Z) gold preferentially within tumors. Irradiation of tumor-bearing mice after injecting gold nanoparticles was shown to induce remarkable tumor regression and long-term survival without any significant toxicity when compared to mice irradiated without gold nanoparticles (Hainfeld et al. 2004). This dramatic outcome was attributed to significant increase in the photoelectron fluence within gold nanoparticle-laden tumors and their vasculature during x-ray irradiation (Hainfeld et al. 2008). A subsequent Monte Carlo computational study confirmed that the macroscopic (or average) tumor dose enhancement in the original animal study was dependent on gold concentration within the tumor and the photon beam quality, ranging from several hundred percent for diagnostic x-rays to a few percent for typical megavoltage photon beams (Cho 2005; Roeske et al. 2007). Since low energy gamma rays interact with gold nanoparticles within the tumor predominantly via photoelectric effect, additional computational studies suggested that macroscopic dose enhancement might be more pronounced in prostate brachytherapy applications (Cho et al. 2009).
Conclusions
Nanoparticle-mediated hyperthermia promises to be a new minimally invasive tool in the armamentarium for the treatment of prostate cancer. A greater understanding and increasing research related to novel particle compositions, geometries, activation strategies, targeting techniques, payload delivery strategies and radiation dose enhancement concepts are likely to energize this field in the coming years. Accurate modeling and dosimetry are likely to facilitate seamless and logical transition from the bench to the bedside.
Acknowledgments
We wish to thank David Aten from the medical graphics and photography department at MD Anderson Cancer Center for assistance with preparing figures.
References
- Abrams MJ, Murrer BA. Metal compounds in therapy and diagnosis. Science. 1993;261:725–30. doi: 10.1126/science.8102010. [DOI] [PubMed] [Google Scholar]
- Akiyama Y, Mori T, Katayama Y, Niidome T. The effects of PEG grafting level and injection dose on gold nanorod biodistribution in the tumor-bearing mice. J Control Release. 2009;139:81–4. doi: 10.1016/j.jconrel.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Algan O, Fosmire H, Hynynen K, Dalkin B, Cui H, Drach G, Stea B, Cassady JR. External beam radiotherapy and hyperthermia in the treatment of patients with locally advanced prostate carcinoma. Cancer. 2000;89:399–403. [PubMed] [Google Scholar]
- Anscher MS, Samulski TV, Dodge R, Prosnitz LR, Dewhirst MW. Combined external beam irradiation and external regional hyperthermia for locally advanced adenocarcinoma of the prostate. Int J Radiat Oncol Biol Phys. 1997;37:1059–65. doi: 10.1016/s0360-3016(97)00109-0. [DOI] [PubMed] [Google Scholar]
- Baluk P, Morikawa S, Haskell A, Mancuso M, McDonald DM. Abnormalities of basement membrane on blood vessels and endothelial sprouts in tumors. Am J Pathol. 2003;163:1801–15. doi: 10.1016/S0002-9440(10)63540-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardhan R, Grady NK, Cole JR, Joshi A, Halas NJ. Fluorescence enhancement by Au nanostructures: nanoshells and nanorods. ACS Nano. 2009;3:744–52. doi: 10.1021/nn900001q. [DOI] [PubMed] [Google Scholar]
- Baronzio G, Gramaglia A, Fiorentini G. Review. Current role and future perspectives of hyperthermia for prostate cancer treatment. In Vivo. 2009;23:143–6. [PubMed] [Google Scholar]
- Bernardi RJ, Lowery AR, Thompson PA, Blaney SM, West JL. Immunonanoshells for targeted photothermal ablation in medulloblastoma and glioma: an in vitro evaluation using human cell lines. J Neurooncol. 2008;86:165–72. doi: 10.1007/s11060-007-9467-3. [DOI] [PubMed] [Google Scholar]
- Bhowmick S, Swanlund DJ, Coad JE, Lulloff L, Hoey MF, Bischof JC. Evaluation of thermal therapy in a prostate cancer model using a wet electrode radiofrequency probe. J Endourol. 2001;15:629–40. doi: 10.1089/089277901750426436. [DOI] [PubMed] [Google Scholar]
- Biris AS, Boldor D, Palmer J, Monroe WT, Mahmood M, Dervishi E, Xu Y, Li Z, Galanzha EI, Zharov VP. Nanophotothermolysis of multiple scattered cancer cells with carbon nanotubes guided by time-resolved infrared thermal imaging. J Biomed Opt. 2009;14:021007. doi: 10.1117/1.3119135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezovich IA, Meredith RF. Practical aspects of ferromagnetic thermoseed hyperthermia. Radiol Clin North Am. 1989;27:589–602. [PubMed] [Google Scholar]
- Burke A, Ding X, Singh R, Kraft RA, Levi-Polyachenko N, Rylander MN, Szot C, Buchanan C, Whitney J, Fisher J, Hatcher HC, D’Agostino R, Jr, Kock ND, Ajayan PM, Carroll DL, Akman S, Torti FM, Torti SV. Long-term survival following a single treatment of kidney tumors with multiwalled carbon nanotubes and near-infrared radiation. Proc Natl Acad Sci U S A. 2009;106:12897–902. doi: 10.1073/pnas.0905195106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarty P, Marches R, Zimmerman NS, Swafford AD, Bajaj P, Musselman IH, Pantano P, Draper RK, Vitetta ES. Thermal ablation of tumor cells with antibody-functionalized single-walled carbon nanotubes. Proc Natl Acad Sci U S A. 2008;105:8697–702. doi: 10.1073/pnas.0803557105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champion JA, Mitragotri S. Shape induced inhibition of phagocytosis of polymer particles. Pharm Res. 2009;26:244–9. doi: 10.1007/s11095-008-9626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng FY, Chen CT, Yeh CS. Comparative efficiencies of photothermal destruction of malignant cells using antibody-coated silica@Au nanoshells, hollow Au/Ag nanospheres and Au nanorods. Nanotechnology. 2009;20:425104. doi: 10.1088/0957-4484/20/42/425104. [DOI] [PubMed] [Google Scholar]
- Cheong SK, Krishnan S, Cho SH. Modeling of plasmonic heating from individual gold nanoshells for near-infrared laser-induced thermal therapy. Med Phys. 2009;36:4664–71. doi: 10.1118/1.3215536. [DOI] [PubMed] [Google Scholar]
- Cho SH. Estimation of tumour dose enhancement due to gold nanoparticles during typical radiation treatments: a preliminary Monte Carlo study. Phys Med Biol. 2005;50:N163–73. doi: 10.1088/0031-9155/50/15/N01. [DOI] [PubMed] [Google Scholar]
- Cho SH, Jones BL, Krishnan S. The dosimetric feasibility of gold nanoparticle-aided radiation therapy (GNRT) via brachytherapy using low-energy gamma-/x-ray sources. Phys Med Biol. 2009;54:4889–905. doi: 10.1088/0031-9155/54/16/004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HS, Ipe BI, Misra P, Lee JH, Bawendi MG, Frangioni JV. Tissue- and organ-selective biodistribution of NIR fluorescent quantum dots. Nano Lett. 2009;9:2354–9. doi: 10.1021/nl900872r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, Bawendi MG, Frangioni JV. Renal clearance of quantum dots. Nat Biotechnol. 2007;25:1165–70. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel MC, Astruc D. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev. 2004;104:293–346. doi: 10.1021/cr030698+. [DOI] [PubMed] [Google Scholar]
- Dawkins GP, Harrison NW, Ansell W. Radiofrequency heat-treatment to the prostate for bladder outlet obstruction associated with benign prostatic hyperplasia: a 4-year outcome study. Br J Urol. 1997;79:910–4. doi: 10.1046/j.1464-410x.1997.00184.x. [DOI] [PubMed] [Google Scholar]
- Dewhirst MW, Sim DA, Sapareto S, Connor WG. Importance of minimum tumor temperature in determining early and long-term responses of spontaneous canine and feline tumors to heat and radiation. Cancer Res. 1984;44:43–50. [PubMed] [Google Scholar]
- Diagaradjane P, Orenstein-Cardona JM, Colon-Casasnovas NE, Deorukhkar A, Shentu S, Kuno N, Schwartz DL, Gelovani JG, Krishnan S. Imaging epidermal growth factor receptor expression in vivo: pharmacokinetic and biodistribution characterization of a bioconjugated quantum dot nanoprobe. Clin Cancer Res. 2008a;14:731–41. doi: 10.1158/1078-0432.CCR-07-1958. [DOI] [PubMed] [Google Scholar]
- Diagaradjane P, Shetty A, Wang JC, Elliott AM, Schwartz J, Shentu S, Park HC, Deorukhkar A, Stafford RJ, Cho SH, Tunnell JW, Hazle JD, Krishnan S. Modulation of in vivo tumor radiation response via gold nanoshell-mediated vascular-focused hyperthermia: characterizing an integrated antihypoxic and localized vascular disrupting targeting strategy. Nano Lett. 2008b;8:1492–500. doi: 10.1021/nl080496z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson EB, Dreaden EC, Huang X, El-Sayed IH, Chu H, Pushpanketh S, McDonald JF, El-Sayed MA. Gold nanorod assisted near-infrared plasmonic photothermal therapy (PPTT) of squamous cell carcinoma in mice. Cancer Lett. 2008;269:57–66. doi: 10.1016/j.canlet.2008.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumortier H, Lacotte S, Pastorin G, Marega R, Wu W, Bonifazi D, Briand JP, Prato M, Muller S, Bianco A. Functionalized carbon nanotubes are non-cytotoxic and preserve the functionality of primary immune cells. Nano Lett. 2006;6:1522–8. doi: 10.1021/nl061160x. [DOI] [PubMed] [Google Scholar]
- Elliott A, Schwartz J, Wang J, Shetty A, Hazle J, Stafford JR. Analytical solution to heat equation with magnetic resonance experimental verification for nanoshell enhanced thermal therapy. Lasers Surg Med. 2008;40:660–5. doi: 10.1002/lsm.20682. [DOI] [PubMed] [Google Scholar]
- Emami B, Scott C, Perez CA, Asbell S, Swift P, Grigsby P, Montesano A, Rubin P, Curran W, Delrowe J, Arastu H, Fu K, Moros E. Phase III study of interstitial thermoradiotherapy compared with interstitial radiotherapy alone in the treatment of recurrent or persistent human tumors. A prospectively controlled randomized study by the Radiation Therapy Group. Int J Radiat Oncol Biol Phys. 1996;34:1097–104. doi: 10.1016/0360-3016(95)02137-x. [DOI] [PubMed] [Google Scholar]
- Fosmire H, Hynynen K, Drach GW, Stea B, Swift P, Cassady JR. Feasibility and toxicity of transrectal ultrasound hyperthermia in the treatment of locally advanced adenocarcinoma of the prostate. Int J Radiat Oncol Biol Phys. 1993;26:253–9. doi: 10.1016/0360-3016(93)90205-a. [DOI] [PubMed] [Google Scholar]
- Fuller KJ, Issels RD, Slosman DO, Guillet JG, Soussi T, Polla BS. Cancer and the heat shock response. Eur J Cancer. 1994;30A:1884–91. doi: 10.1016/0959-8049(94)00362-9. [DOI] [PubMed] [Google Scholar]
- Gannon CJ, Cherukuri P, Yakobson BI, Cognet L, Kanzius JS, Kittrell C, Weisman RB, Pasquali M, Schmidt HK, Smalley RE, Curley SA. Carbon nanotube-enhanced thermal destruction of cancer cells in a noninvasive radiofrequency field. Cancer. 2007;110:2654–65. doi: 10.1002/cncr.23155. [DOI] [PubMed] [Google Scholar]
- Gao X, Cui Y, Levenson RM, Chung LW, Nie S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat Biotechnol. 2004;22:969–76. doi: 10.1038/nbt994. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Dutta S, Gomes E, Carroll D, D’Agostino R, Jr, Olson J, Guthold M, Gmeiner WH. Increased heating efficiency and selective thermal ablation of malignant tissue with DNA-encased multiwalled carbon nanotubes. ACS Nano. 2009;3:2667–73. doi: 10.1021/nn900368b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillett MD, Gettman MT, Zincke H, Blute ML. Tissue ablation technologies for localized prostate cancer. Mayo Clin Proc. 2004;79:1547–55. doi: 10.4065/79.12.1547. [DOI] [PubMed] [Google Scholar]
- Gneveckow U, Jordan A, Scholz R, Bruss V, Waldofner N, Ricke J, Feussner A, Hildebrandt B, Rau B, Wust P. Description and characterization of the novel hyperthermia- and thermoablation-system MFH 300F for clinical magnetic fluid hyperthermia. Med Phys. 2004;31:1444–51. doi: 10.1118/1.1748629. [DOI] [PubMed] [Google Scholar]
- Gobin AM, Lee MH, Halas NJ, James WD, Drezek RA, West JL. Near-infrared resonant nanoshells for combined optical imaging and photothermal cancer therapy. Nano Lett. 2007;7:1929–34. doi: 10.1021/nl070610y. [DOI] [PubMed] [Google Scholar]
- Gobin AM, Moon JJ, West JL. EphrinA I-targeted nanoshells for photothermal ablation of prostate cancer cells. Int J Nanomedicine. 2008;3:351–8. [PMC free article] [PubMed] [Google Scholar]
- Hainfeld JF, Dilmanian FA, Slatkin DN, Smilowitz HM. Radiotherapy enhancement with gold nanoparticles. J Pharm Pharmacol. 2008;60:977–85. doi: 10.1211/jpp.60.8.0005. [DOI] [PubMed] [Google Scholar]
- Hainfeld JF, Slatkin DN, Smilowitz HM. The use of gold nanoparticles to enhance radiotherapy in mice. Phys Med Biol. 2004;49:N309–15. doi: 10.1088/0031-9155/49/18/n03. [DOI] [PubMed] [Google Scholar]
- Han S, Lin J, Zhou F, Vellanoweth RL. Oligonucleotide-capped gold nanoparticles for improved atomic force microscopic imaging and enhanced selectivity in polynucleotide detection. Biochem Biophys Res Commun. 2000;279:265–9. doi: 10.1006/bbrc.2000.3943. [DOI] [PubMed] [Google Scholar]
- Harmon BV, Takano YS, Winterford CM, Gobe GC. The role of apoptosis in the response of cells and tumours to mild hyperthermia. Int J Radiat Biol. 1991;59:489–501. doi: 10.1080/09553009114550441. [DOI] [PubMed] [Google Scholar]
- Higby GJ. Gold in medicine: a review of its use in the West before 1900. Gold Bull. 1982;15:130–40. doi: 10.1007/BF03214618. [DOI] [PubMed] [Google Scholar]
- Hildebrandt B, Wust P, Ahlers O, Dieing A, Sreenivasa G, Kerner T, Felix R, Riess H. The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol. 2002;43:33–56. doi: 10.1016/s1040-8428(01)00179-2. [DOI] [PubMed] [Google Scholar]
- Hirsch LR, Stafford RJ, Bankson JA, Sershen SR, Rivera B, Price RE, Hazle JD, Halas NJ, West JL. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc Natl Acad Sci U S A. 2003;100:13549–54. doi: 10.1073/pnas.2232479100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2:91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- Horsman MR, Overgaard J. Can mild hyperthermia improve tumour oxygenation? Int J Hyperthermia. 1997;13:141–7. doi: 10.3109/02656739709012378. [DOI] [PubMed] [Google Scholar]
- Hu M, Chen J, Li ZY, Au L, Hartland GV, Li X, Marquez M, Xia Y. Gold nanostructures: engineering their plasmonic properties for biomedical applications. Chem Soc Rev. 2006;35:1084–94. doi: 10.1039/b517615h. [DOI] [PubMed] [Google Scholar]
- Huang X, El-Sayed IH, Qian W, El-Sayed MA. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J Am Chem Soc. 2006;128:2115–20. doi: 10.1021/ja057254a. [DOI] [PubMed] [Google Scholar]
- Huang X, Jain PK, El-Sayed IH, El-Sayed MA. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med Sci. 2008a;23:217–28. doi: 10.1007/s10103-007-0470-x. [DOI] [PubMed] [Google Scholar]
- Huang YF, Sefah K, Bamrungsap S, Chang HT, Tan W. Selective photothermal therapy for mixed cancer cells using aptamer-conjugated nanorods. Langmuir. 2008b;24:11860–5. doi: 10.1021/la801969c. [DOI] [PubMed] [Google Scholar]
- Huff TB, Tong L, Zhao Y, Hansen MN, Cheng JX, Wei A. Hyperthermic effects of gold nanorods on tumor cells. Nanomed. 2007;2:125–32. doi: 10.2217/17435889.2.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulander M, Hong J, Andersson M, Gerven F, Ohrlander M, Tengvall P, Elwing H. Blood Interactions with Noble Metals: Coagulation and Immune Complement Activation. Acs Applied Materials & Interfaces. 2009;1:1053–1062. doi: 10.1021/am900028e. [DOI] [PubMed] [Google Scholar]
- Hurwitz M. Editorial comment on: Thermotherapy of prostate cancer using magnetic nanoparticles: feasibility, imaging, and three-dimensional temperature distribution. Eur Urol. 2007;52:1661–2. [PubMed] [Google Scholar]
- Hurwitz MD, Kaplan ID, Hansen JL, Prokopios-Davos S, Topulos GP, Wishnow K, Manola J, Bornstein BA, Hynynen K. Association of rectal toxicity with thermal dose parameters in treatment of locally advanced prostate cancer with radiation and hyperthermia. Int J Radiat Oncol Biol Phys. 2002;53:913–8. doi: 10.1016/s0360-3016(02)02809-2. [DOI] [PubMed] [Google Scholar]
- Hurwitz MD, Kaplan ID, Hansen JL, Prokopios-Davos S, Topulos GP, Wishnow K, Manola J, Bornstein BA, Hynynen K. Hyperthermia combined with radiation in treatment of locally advanced prostate cancer is associated with a favourable toxicity profile. Int J Hyperthermia. 2005;21:649–56. doi: 10.1080/02656730500331967. [DOI] [PubMed] [Google Scholar]
- Hurwitz MD, Kaplan ID, Svensson GK, Hynynen K, Hansen MS. Feasibility and patient tolerance of a novel transrectal ultrasound hyperthermia system for treatment of prostate cancer. Int J Hyperthermia. 2001;17:31–7. doi: 10.1080/02656730150201570. [DOI] [PubMed] [Google Scholar]
- Jain PK, Lee KS, El-Sayed IH, El-Sayed MA. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: applications in biological imaging and biomedicine. J Phys Chem B. 2006;110:7238–48. doi: 10.1021/jp057170o. [DOI] [PubMed] [Google Scholar]
- Jain RK. Transport of molecules, particles, and cells in solid tumors. Annu Rev Biomed Eng. 1999;1:241–63. doi: 10.1146/annurev.bioeng.1.1.241. [DOI] [PubMed] [Google Scholar]
- Johannsen M, Gneveckow U, Eckelt L, Feussner A, Waldofner N, Scholz R, Deger S, Wust P, Loening SA, Jordan A. Clinical hyperthermia of prostate cancer using magnetic nanoparticles: presentation of a new interstitial technique. Int J Hyperthermia. 2005a;21:637–47. doi: 10.1080/02656730500158360. [DOI] [PubMed] [Google Scholar]
- Johannsen M, Gneveckow U, Taymoorian K, Thiesen B, Waldofner N, Scholz R, Jung K, Jordan A, Wust P, Loening SA. Morbidity and quality of life during thermotherapy using magnetic nanoparticles in locally recurrent prostate cancer: results of a prospective phase I trial. Int J Hyperthermia. 2007a;23:315–23. doi: 10.1080/02656730601175479. [DOI] [PubMed] [Google Scholar]
- Johannsen M, Gneveckow U, Thiesen B, Taymoorian K, Cho CH, Waldofner N, Scholz R, Jordan A, Loening SA, Wust P. Thermotherapy of prostate cancer using magnetic nanoparticles: feasibility, imaging, and three-dimensional temperature distribution. Eur Urol. 2007b;52:1653–61. doi: 10.1016/j.eururo.2006.11.023. [DOI] [PubMed] [Google Scholar]
- Johannsen M, Jordan A, Scholz R, Koch M, Lein M, Deger S, Roigas J, Jung K, Loening S. Evaluation of magnetic fluid hyperthermia in a standard rat model of prostate cancer. J Endourol. 2004;18:495–500. doi: 10.1089/0892779041271715. [DOI] [PubMed] [Google Scholar]
- Johannsen M, Thiesen B, Gneveckow U, Taymoorian K, Waldofner N, Scholz R, Deger S, Jung K, Loening SA, Jordan A. Thermotherapy using magnetic nanoparticles combined with external radiation in an orthotopic rat model of prostate cancer. Prostate. 2006;66:97–104. doi: 10.1002/pros.20324. [DOI] [PubMed] [Google Scholar]
- Johannsen M, Thiesen B, Jordan A, Taymoorian K, Gneveckow U, Waldofner N, Scholz R, Koch M, Lein M, Jung K, Loening SA. Magnetic fluid hyperthermia (MFH)reduces prostate cancer growth in the orthotopic Dunning R3327 rat model. Prostate. 2005b;64:283–92. doi: 10.1002/pros.20213. [DOI] [PubMed] [Google Scholar]
- Jones EL, Oleson JR, Prosnitz LR, Samulski TV, Vujaskovic Z, Yu D, Sanders LL, Dewhirst MW. Randomized trial of hyperthermia and radiation for superficial tumors. J Clin Oncol. 2005;23:3079–85. doi: 10.1200/JCO.2005.05.520. [DOI] [PubMed] [Google Scholar]
- Jordan A, Scholz R, Wust P, Fahling H, Krause J, Wlodarczyk W, Sander B, Vogl T, Felix R. Effects of magnetic fluid hyperthermia (MFH) on C3H mammary carcinoma in vivo. Int J Hyperthermia. 1997;13:587–605. doi: 10.3109/02656739709023559. [DOI] [PubMed] [Google Scholar]
- Jordan A, Wust P, Fahling H, John W, Hinz A, Felix R. Inductive heating of ferrimagnetic particles and magnetic fluids: physical evaluation of their potential for hyperthermia. Int J Hyperthermia. 1993;9:51–68. doi: 10.3109/02656739309061478. [DOI] [PubMed] [Google Scholar]
- Kam NW, O’Connell M, Wisdom JA, Dai H. Carbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destruction. Proc Natl Acad Sci U S A. 2005;102:11600–5. doi: 10.1073/pnas.0502680102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampinga HH, Dikomey E. Hyperthermic radiosensitization: mode of action and clinical relevance. Int J Radiat Biol. 2001;77:399–408. doi: 10.1080/09553000010024687. [DOI] [PubMed] [Google Scholar]
- Kawai N, Futakuchi M, Yoshida T, Ito A, Sato S, Naiki T, Honda H, Shirai T, Kohri K. Effect of heat therapy using magnetic nanoparticles conjugated with cationic liposomes on prostate tumor in bone. Prostate. 2008;68:784–92. doi: 10.1002/pros.20740. [DOI] [PubMed] [Google Scholar]
- Kawano T, Niidome Y, Mori T, Katayama Y, Niidome T. PNIPAM gel-coated gold nanorods for targeted delivery responding to a near-infrared laser. Bioconjug Chem. 2009;20:209–12. doi: 10.1021/bc800480k. [DOI] [PubMed] [Google Scholar]
- Khan MK, Minc LD, Nigavekar SS, Kariapper MS, Nair BM, Schipper M, Cook AC, Lesniak WG, Balogh LP. Fabrication of {198Au0} radioactive composite nanodevices and their use for nanobrachytherapy. Nanomedicine. 2008;4:57–69. doi: 10.1016/j.nano.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolosnjaj-Tabi J, Hartman KB, Boudjemaa S, Ananta JS, Morgant G, Szwarc H, Wilson LJ, Moussa F. In Vivo Behavior of Large Doses of Ultrashort and Full-Length Single-Walled Carbon Nanotubes after Oral and Intraperitoneal Administration to Swiss Mice. ACS Nano. doi: 10.1021/nn901573w. [DOI] [PubMed] [Google Scholar]
- Kumar S, Aaron J, Sokolov K. Directional conjugation of antibodies to nanoparticles for synthesis of multiplexed optical contrast agents with both delivery and targeting moieties. Nat Protoc. 2008;3:314–20. doi: 10.1038/nprot.2008.1. [DOI] [PubMed] [Google Scholar]
- Lancaster C, Toi A, Trachtenberg J. Interstitial microwave thermoablation for localized prostate cancer. Urology. 1999;53:828–31. doi: 10.1016/s0090-4295(98)00383-5. [DOI] [PubMed] [Google Scholar]
- Liu SY, Liang ZS, Gao F, Luo SF, Lu GQ. In vitro photothermal study of gold nanoshells functionalized with small targeting peptides to liver cancer cells. J Mater Sci Mater Med. 2009 doi: 10.1007/s10856-009-3895-x. [DOI] [PubMed] [Google Scholar]
- Liu Z, Cai W, He L, Nakayama N, Chen K, Sun X, Chen X, Dai H. In vivo biodistribution and highly efficient tumour targeting of carbon nanotubes in mice. Nat Nanotechnol. 2007;2:47–52. doi: 10.1038/nnano.2006.170. [DOI] [PubMed] [Google Scholar]
- Liu Z, Davis C, Cai W, He L, Chen X, Dai H. Circulation and long-term fate of functionalized, biocompatible single-walled carbon nanotubes in mice probed by Raman spectroscopy. Proc Natl Acad Sci U S A. 2008;105:1410–5. doi: 10.1073/pnas.0707654105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo C, Lowery A, Halas N, West J, Drezek R. Immunotargeted nanoshells for integrated cancer imaging and therapy. Nano Lett. 2005;5:709–11. doi: 10.1021/nl050127s. [DOI] [PubMed] [Google Scholar]
- Lowery AR, Gobin AM, Day ES, Halas NJ, West JL. Immunonanoshells for targeted photothermal ablation of tumor cells. Int J Nanomedicine. 2006;1:149–54. doi: 10.2147/nano.2006.1.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma LL, Feldman MD, Tam JM, Paranjape AS, Cheruku KK, Larson TA, Tam JO, Ingram DR, Paramita V, Villard JW, Jenkins JT, Wang T, Clarke GD, Asmis R, Sokolov K, Chandrasekar B, Milner TE, Johnston KP. Small multifunctional nanoclusters (nanoroses) for targeted cellular imaging and therapy. ACS Nano. 2009;3:2686–96. doi: 10.1021/nn900440e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrez A, Kasas S, Salicio V, Pasquier N, Seo JW, Celio M, Catsicas S, Schwaller B, Forro L. Cellular toxicity of carbon-based nanomaterials. Nano Lett. 2006;6:1121–5. doi: 10.1021/nl060162e. [DOI] [PubMed] [Google Scholar]
- Mahmood M, Karmakar A, Fejleh A, Mocan T, Iancu C, Mocan L, Iancu DT, Xu Y, Dervishi E, Li Z, Biris AR, Agarwal R, Ali N, Galanzha EI, Biris AS, Zharov VP. Synergistic enhancement of cancer therapy using a combination of carbon nanotubes and anti-tumor drug. Nanomed. 2009;4:883–93. doi: 10.2217/nnm.09.76. [DOI] [PubMed] [Google Scholar]
- Malugin A, Ghandehari H. Cellular uptake and toxicity of gold nanoparticles in prostate cancer cells: a comparative study of rods and spheres. J Appl Toxicol. 2009 doi: 10.1002/jat.1486. [DOI] [PubMed] [Google Scholar]
- Marberger M. Energy-based ablative therapy of prostate cancer: high-intensity focused ultrasound and cryoablation. Curr Opin Urol. 2007;17:194–9. doi: 10.1097/MOU.0b013e3280dd8a65. [DOI] [PubMed] [Google Scholar]
- Margulis V, Matsumoto ED, Lindberg G, Tunc L, Taylor G, Sagalowsky AI, Cadeddu JA. Acute histologic effects of temperature-based radiofrequency ablation on renal tumor pathologic interpretation. Urology. 2004;64:660–3. doi: 10.1016/j.urology.2004.05.023. [DOI] [PubMed] [Google Scholar]
- McDonald DM, Choyke PL. Imaging of angiogenesis: from microscope to clinic. Nat Med. 2003;9:713–25. doi: 10.1038/nm0603-713. [DOI] [PubMed] [Google Scholar]
- Mendecki J, Friedenthal E, Botstein C, Paglione R, Sterzer F. Microwave applicators for localized hyperthermia treatment of cancer of the prostate. Int J Radiat Oncol Biol Phys. 1980;6:1583–8. doi: 10.1016/0360-3016(80)90019-x. [DOI] [PubMed] [Google Scholar]
- Meredith RF, Brezovich IA, Weppelmann B, Henderson RA, Brawner WR, Jr, Kwapien RP, Bartolucci AA, Salter MM. Ferromagnetic thermoseeds: suitable for an afterloading interstitial implant. Int J Radiat Oncol Biol Phys. 1989;17:1341–6. doi: 10.1016/0360-3016(89)90547-6. [DOI] [PubMed] [Google Scholar]
- Moon HK, Lee SH, Choi HC. In vivo near-infrared mediated tumor destruction by photothermal effect of carbon nanotubes. ACS Nano. 2009;3:3707–13. doi: 10.1021/nn900904h. [DOI] [PubMed] [Google Scholar]
- Moros EG, Corry PM, Orton CG. Thermoradiotherapy is underutilized for the treatment of cancer. Med Phys. 2007;34:1–4. doi: 10.1118/1.2404790. [DOI] [PubMed] [Google Scholar]
- Moroz P, Jones SK, Gray BN. Magnetically mediated hyperthermia: current status and future directions. Int J Hyperthermia. 2002;18:267–84. doi: 10.1080/02656730110108785. [DOI] [PubMed] [Google Scholar]
- Motoyama J, Yamashita N, Morino T, Tanaka M, Kobayashi T, Honda H. Hyperthermic treatment of DMBA-induced rat mammary cancer using magnetic nanoparticles. Biomagn Res Technol. 2008;6:2. doi: 10.1186/1477-044X-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naruse S, Higuchi T, Horikawa Y, Tanaka C, Nakamura K, Hirakawa K. Radiofrequency hyperthermia with successive monitoring of its effects on tumors using NMR spectroscopy. Proc Natl Acad Sci U S A. 1986;83:8343–7. doi: 10.1073/pnas.83.21.8343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell MJ, Bachilo SM, Huffman CB, Moore VC, Strano MS, Haroz EH, Rialon KL, Boul PJ, Noon WH, Kittrell C, Ma J, Hauge RH, Weisman RB, Smalley RE. Band gap fluorescence from individual single-walled carbon nanotubes. Science. 2002;297:593–6. doi: 10.1126/science.1072631. [DOI] [PubMed] [Google Scholar]
- O’Neal DP, Hirsch LR, Halas NJ, Payne JD, West JL. Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer Lett. 2004;209:171–6. doi: 10.1016/j.canlet.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Oldenburg SJ, Averitt RD, Westcott SL, Halas NJ. Nanoengineering of optical resonances. Chem Phys Lett. 1998;288:243–247. [Google Scholar]
- Oldenburg SJ, Jackson JB, Westcott SL, Halas NJ. Infrared extinction properties of gold nanoshells. Appl Phys Lett. 1999;75:2897–2899. [Google Scholar]
- Oleson JR, Samulski TV, Leopold KA, Clegg ST, Dewhirst MW, Dodge RK, George SL. Sensitivity of hyperthermia trial outcomes to temperature and time: implications for thermal goals of treatment. Int J Radiat Oncol Biol Phys. 1993;25:289–97. doi: 10.1016/0360-3016(93)90351-u. [DOI] [PubMed] [Google Scholar]
- Overgaard J, Gonzalez Gonzalez D, Hulshof MC, Arcangeli G, Dahl O, Mella O, Bentzen SM. Randomised trial of hyperthermia as adjuvant to radiotherapy for recurrent or metastatic malignant melanoma. European Society for Hyperthermic Oncology. Lancet. 1995;345:540–3. doi: 10.1016/s0140-6736(95)90463-8. [DOI] [PubMed] [Google Scholar]
- Parish RV. Biologically-Active Gold(III) Complexes. Met Based Drugs. 1999;6:271–6. doi: 10.1155/MBD.1999.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partington BP, Steeves RA, Su SL, Paliwal BR, Dubielzig RR, Wilson JW, Brezovich IA. Temperature distributions, microangiographic and histopathologic correlations in normal tissue heated by ferromagnetic needles. Int J Hyperthermia. 1989;5:319–27. doi: 10.3109/02656738909140458. [DOI] [PubMed] [Google Scholar]
- Poland CA, Duffin R, Kinloch I, Maynard A, Wallace WA, Seaton A, Stone V, Brown S, Macnee W, Donaldson K. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Nanotechnol. 2008;3:423–8. doi: 10.1038/nnano.2008.111. [DOI] [PubMed] [Google Scholar]
- Prionas SD, Kapp DS, Goffinet DR, Ben-Yosef R, Fessenden P, Bagshaw MA. Thermometry of interstitial hyperthermia given as an adjuvant to brachytherapy for the treatment of carcinoma of the prostate. Int J Radiat Oncol Biol Phys. 1994;28:151–62. doi: 10.1016/0360-3016(94)90153-8. [DOI] [PubMed] [Google Scholar]
- Puvanakrishnan P, Park J, Diagaradjane P, Schwartz JA, Coleman CL, Gill-Sharp KL, Sang KL, Payne JD, Krishnan S, Tunnell JW. Near-infrared narrow-band imaging of gold/silica nanoshells in tumors. J Biomed Opt. 2009;14:024044. doi: 10.1117/1.3120494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekaran AK, Anilkumar G, Christiansen JJ. Is prostate-specific membrane antigen a multifunctional protein? Am J Physiol Cell Physiol. 2005;288:C975–81. doi: 10.1152/ajpcell.00506.2004. [DOI] [PubMed] [Google Scholar]
- Roeske JC, Nunez L, Hoggarth M, Labay E, Weichselbaum RR. Characterization of the theorectical radiation dose enhancement from nanoparticles. Technol Cancer Res Treat. 2007;6:395–401. doi: 10.1177/153303460700600504. [DOI] [PubMed] [Google Scholar]
- Roti Roti JL. Introduction: radiosensitization by hyperthermia. Int J Hyperthermia. 2004;20:109–14. doi: 10.1080/0265673032000173898. [DOI] [PubMed] [Google Scholar]
- Ryman-Rasmussen JP, Cesta MF, Brody AR, Shipley-Phillips JK, Everitt JI, Tewksbury EW, Moss OR, Wong BA, Dodd DE, Andersen ME, Bonner JC. Inhaled carbon nanotubes reach the subpleural tissue in mice. Nat Nanotechnol. 2009;4:747–51. doi: 10.1038/nnano.2009.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salloum M, Ma R, Zhu L. Enhancement in treatment planning for magnetic nanoparticle hyperthermia: optimization of the heat absorption pattern. Int J Hyperthermia. 2009;25:309–21. doi: 10.1080/02656730902803118. [DOI] [PubMed] [Google Scholar]
- Salloum M, Ma RH, Weeks D, Zhu L. Controlling nanoparticle delivery in magnetic nanoparticle hyperthermia for cancer treatment: experimental study in agarose gel. Int J Hyperthermia. 2008;24:337–45. doi: 10.1080/02656730801907937. [DOI] [PubMed] [Google Scholar]
- Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys. 1984;10:787–800. doi: 10.1016/0360-3016(84)90379-1. [DOI] [PubMed] [Google Scholar]
- Satoh T, Seilhan TM, Stauffer PR, Sneed PK, Fike JR. Interstitial helical coil microwave antenna for experimental brain hyperthermia. Neurosurgery. 1988;23:564–9. doi: 10.1227/00006123-198811000-00004. [DOI] [PubMed] [Google Scholar]
- Sayes CM, Liang F, Hudson JL, Mendez J, Guo W, Beach JM, Moore VC, Doyle CD, West JL, Billups WE, Ausman KD, Colvin VL. Functionalization density dependence of single-walled carbon nanotubes cytotoxicity in vitro. Toxicol Lett. 2006;161:135–42. doi: 10.1016/j.toxlet.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Schipper ML, Nakayama-Ratchford N, Davis CR, Kam NW, Chu P, Liu Z, Sun X, Dai H, Gambhir SS. A pilot toxicology study of single-walled carbon nanotubes in a small sample of mice. Nat Nanotechnol. 2008;3:216–21. doi: 10.1038/nnano.2008.68. [DOI] [PubMed] [Google Scholar]
- Schwartz JA, Shetty AM, Price RE, Stafford RJ, Wang JC, Uthamanthil RK, Pham K, McNichols RJ, Coleman CL, Payne JD. Feasibility study of particle-assisted laser ablation of brain tumors in orthotopic canine model. Cancer Res. 2009;69:1659–67. doi: 10.1158/0008-5472.CAN-08-2535. [DOI] [PubMed] [Google Scholar]
- Servadio C, Leib Z. Local hyperthermia for prostate cancer. Urology. 1991;38:307–9. doi: 10.1016/0090-4295(91)80140-3. [DOI] [PubMed] [Google Scholar]
- Sharma R, Chen CJ. Newer nanoparticles in hyperthermia treatment and thermometry. Journal of Nanoparticle Research. 2009;11:671–689. [Google Scholar]
- Sherar MD, Trachtenberg J, Davidson SR, Gertner MR. Interstitial microwave thermal therapy and its application to the treatment of recurrent prostate cancer. Int J Hyperthermia. 2004;20:757–68. doi: 10.1080/02656730410001734146. [DOI] [PubMed] [Google Scholar]
- Sherar MD, Trachtenberg J, Davidson SR, McCann C, Yue CK, Haider MA, Gertner MR. Interstitial microwave thermal therapy for prostate cancer. J Endourol. 2003;17:617–25. doi: 10.1089/089277903322518626. [DOI] [PubMed] [Google Scholar]
- Shimm DS, Hynynen KH, Anhalt DP, Roemer RB, Cassady JR. Scanned focussed ultrasound hyperthermia: initial clinical results. Int J Radiat Oncol Biol Phys. 1988;15:1203–8. doi: 10.1016/0360-3016(88)90205-2. [DOI] [PubMed] [Google Scholar]
- Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3:81–5. [PubMed] [Google Scholar]
- Singh R, Pantarotto D, Lacerda L, Pastorin G, Klumpp C, Prato M, Bianco A, Kostarelos K. Tissue biodistribution and blood clearance rates of intravenously administered carbon nanotube radiotracers. Proc Natl Acad Sci U S A. 2006;103:3357–62. doi: 10.1073/pnas.0509009103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrabalak SE, Au L, Lu X, Li X, Xia Y. Gold nanocages for cancer detection and treatment. Nanomed. 2007;2:657–68. doi: 10.2217/17435889.2.5.657. [DOI] [PubMed] [Google Scholar]
- Sneed PK, Stauffer PR, McDermott MW, Diederich CJ, Lamborn KR, Prados MD, Chang S, Weaver KA, Spry L, Malec MK, Lamb SA, Voss B, Davis RL, Wara WM, Larson DA, Phillips TL, Gutin PH. Survival benefit of hyperthermia in a prospective randomized trial of brachytherapy boost +/− hyperthermia for glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 1998;40:287–95. doi: 10.1016/s0360-3016(97)00731-1. [DOI] [PubMed] [Google Scholar]
- Sofras F, Sakkas G, Kontothanassis D, Lyssiotis F, Tamvakis N. Transurethral thermotherapy in the management of benign prostatic hyperplasia. Int Urol Nephrol. 1996;28:673–9. doi: 10.1007/BF02552163. [DOI] [PubMed] [Google Scholar]
- Sokolov K, Follen M, Aaron J, Pavlova I, Malpica A, Lotan R, Richards-Kortum R. Real-time vital optical imaging of precancer using anti-epidermal growth factor receptor antibodies conjugated to gold nanoparticles. Cancer Res. 2003;63:1999–2004. [PubMed] [Google Scholar]
- Stawarz B, Zielinski H, Szmigielski S, Rappaport E, Debicki P, Petrovich Z. Transrectal hyperthermia as palliative treatment for advanced adenocarcinoma of prostate and studies of cell-mediated immunity. Urology. 1993;41:548–53. doi: 10.1016/0090-4295(93)90102-g. [DOI] [PubMed] [Google Scholar]
- Stern JM, Stanfield J, Kabbani W, Hsieh JT, Cadeddu JA. Selective prostate cancer thermal ablation with laser activated gold nanoshells. J Urol. 2008;179:748–53. doi: 10.1016/j.juro.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Stern JM, Stanfield J, Lotan Y, Park S, Hsieh JT, Cadeddu JA. Efficacy of laser-activated gold nanoshells in ablating prostate cancer cells in vitro. J Endourol. 2007;21:939–43. doi: 10.1089/end.2007.0437. [DOI] [PubMed] [Google Scholar]
- Thrall DE, LaRue SM, Yu D, Samulski T, Sanders L, Case B, Rosner G, Azuma C, Poulson J, Pruitt AF, Stanley W, Hauck ML, Williams L, Hess P, Dewhirst MW. Thermal dose is related to duration of local control in canine sarcomas treated with thermoradiotherapy. Clin Cancer Res. 2005;11:5206–14. doi: 10.1158/1078-0432.CCR-05-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly W, Gellermann J, Graf R, Hildebrandt B, Weissbach L, Budach V, Felix R, Wust P. Regional hyperthermia in conjunction with definitive radiotherapy against recurrent or locally advanced prostate cancer T3 pN0 M0. Strahlenther Onkol. 2005;181:35–41. doi: 10.1007/s00066-005-1296-8. [DOI] [PubMed] [Google Scholar]
- Torti SV, Byrne F, Whelan O, Levi N, Ucer B, Schmid M, Torti FM, Akman S, Liu J, Ajayan PM, Nalamasu O, Carroll DL. Thermal ablation therapeutics based on CN(x) multi-walled nanotubes. Int J Nanomedicine. 2007;2:707–14. [PMC free article] [PubMed] [Google Scholar]
- van der Zee J. Heating the patient: a promising approach? Ann Oncol. 2002;13:1173–84. doi: 10.1093/annonc/mdf280. [DOI] [PubMed] [Google Scholar]
- van der Zee J, Gonzalez Gonzalez D, van Rhoon GC, van Dijk JD, van Putten WL, Hart AA. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: a prospective, randomised, multicentre trial. Dutch Deep Hyperthermia Group. Lancet. 2000;355:1119–25. doi: 10.1016/s0140-6736(00)02059-6. [DOI] [PubMed] [Google Scholar]
- Vernon CC, Hand JW, Field SB, Machin D, Whaley JB, van der Zee J, van Putten WL, van Rhoon GC, van Dijk JD, Gonzalez Gonzalez D, Liu FF, Goodman P, Sherar M. Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer: results from five randomized controlled trials. International Collaborative Hyperthermia Group. Int J Radiat Oncol Biol Phys. 1996;35:731–44. doi: 10.1016/0360-3016(96)00154-x. [DOI] [PubMed] [Google Scholar]
- Veronese FM. Peptide and protein PEGylation: a review of problems and solutions. Biomaterials. 2001;22:405–17. doi: 10.1016/s0142-9612(00)00193-9. [DOI] [PubMed] [Google Scholar]
- Visaria RK, Griffin RJ, Williams BW, Ebbini ES, Paciotti GF, Song CW, Bischof JC. Enhancement of tumor thermal therapy using gold nanoparticle-assisted tumor necrosis factor-alpha delivery. Mol Cancer Ther. 2006;5:1014–20. doi: 10.1158/1535-7163.MCT-05-0381. [DOI] [PubMed] [Google Scholar]
- von Maltzahn G, Park JH, Agrawal A, Bandaru NK, Das SK, Sailor MJ, Bhatia SN. Computationally guided photothermal tumor therapy using long-circulating gold nanorod antennas. Cancer Res. 2009;69:3892–900. doi: 10.1158/0008-5472.CAN-08-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CH, Huang YJ, Chang CW, Hsu WM, Peng CA. In vitro photothermal destruction of neuroblastoma cells using carbon nanotubes conjugated with GD2 monoclonal antibody. Nanotechnology. 2009;20:315101. doi: 10.1088/0957-4484/20/31/315101. [DOI] [PubMed] [Google Scholar]
- Wick P, Manser P, Limbach LK, Dettlaff-Weglikowska U, Krumeich F, Roth S, Stark WJ, Bruinink A. The degree and kind of agglomeration affect carbon nanotube cytotoxicity. Toxicol Lett. 2007;168:121–31. doi: 10.1016/j.toxlet.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Wu CF, Liang XP, Jiang HB. Metal nanoshells as a contrast agent in near-infrared diffuse optical tomography. Opt Commun. 2005;253:214–221. [Google Scholar]
- Wu X, Ming T, Wang X, Wang P, Wang J, Chen J. High-Photoluminescence-Yield Gold Nanocubes: For Cell Imaging and Photothermal Therapy. ACS Nano. 2009 doi: 10.1021/nn901064m. [DOI] [PubMed] [Google Scholar]
- Wust P, Cho CH, Hildebrandt B, Gellermann J. Thermal monitoring: invasive, minimal-invasive and non-invasive approaches. Int J Hyperthermia. 2006;22:255–62. doi: 10.1080/02656730600661149. [DOI] [PubMed] [Google Scholar]
- Yerushalmi A, Servadio C, Leib Z, Fishelovitz Y, Rokowsky E, Stein JA. Local hyperthermia for treatment of carcinoma of the prostate: a preliminary report. Prostate. 1982;3:623–30. doi: 10.1002/pros.2990030612. [DOI] [PubMed] [Google Scholar]
- Zaman RT, Diagaradjane P, Wang J, Swartz J, Gill-Sharp K, Rajaram N, Payne DJ, Krishnan S, Tunnell JW. In vivo detection of gold nanoshells in tumors using diffuse optical spectroscopy. IEEE J Sel Top Quant Elec. 2007;13:1715–20. doi: 10.1109/jstqe.2007.910804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zargar Shoshtari MA, Mirzazadeh M, Banai M, Jamshidi M, Mehravaran K. Radiofrequency-induced thermotherapy in benign prostatic hyperplasia. Urol J. 2006;3:44–8. [PubMed] [Google Scholar]
- Zhang HG, Mehta K, Cohen P, Guha C. Hyperthermia on immune regulation: a temperature’s story. Cancer Lett. 2008;271:191–204. doi: 10.1016/j.canlet.2008.05.026. [DOI] [PubMed] [Google Scholar]
- Zheng LX, O’Connell MJ, Doorn SK, Liao XZ, Zhao YH, Akhadov EA, Hoffbauer MA, Roop BJ, Jia QX, Dye RC, Peterson DE, Huang SM, Liu J, Zhu YT. Ultralong single-wall carbon nanotubes. Nat Mater. 2004;3:673–6. doi: 10.1038/nmat1216. [DOI] [PubMed] [Google Scholar]