Abstract
Phenylketonuria (PKU) is a metabolic genetic disease characterized by deficient phenylalanine hydroxylase (PAH) enzymatic activity. Brain hypomyelination has been reported in untreated patients, but its mechanism remains unclear. We therefore investigated the influence of phenylalanine (Phe), phenylpyruvate (PP), and phenylacetate (PA) on oligodendrocytes. We fisrt showed in a mouse model of PKU that the number of oligodendrocytes is not different in corpus callosum sections from adult mutants or from control brains. Then, using enriched oligodendroglial cultures, we detected no cytotoxic effect of high concentrations of Phe, PP, or PA. Finally, we analyzed the impact of Phe, PP, and PA on the myelination process in myelinating cocultures using both an in vitro index of myelination, based on activation of the myelin basic protein (MBP) promoter, and the direct quantification of myelin sheaths by both optical measurement and a bioinformatics method. None of these parameters was affected by the increased levels of Phe or its derivatives. Taken together, our data demonstrate that high levels of Phe, such as in PKU, are unlikely to directly induce brain hypomyelination, suggesting involvement of alternative mechanisms in this myelination defect.
Introduction
Folling characterized phenylketonuria (PKU; OMIM #261600) in 1934, associating mental retardation with the presence of phenylpyruvic acid (PP) in the urine (Williams et al. 2008). PKU is characterized by null or low activity of phenylalanine hydroxylase (PAH), an enzyme expressed in the liver and to a lesser extend in the kidney and converts phenylalanine (Phe) into tyrosine (Tyr). Multiple mutations of the PAH gene can alter the enzymatic activity, and the majority of these mutations impair protein folding, preventing its tetramerization (Pey et al. 2003). As a consequence of PAH deficiency, Phe accumulates and is metabolized by transamination into several compounds, mostly PP and phenylacetate (PA), which are undetectable in unaffected individuals (Scriver and Kaufman 2001).
In the absence of a strict diet (Ahring et al. 2009), PKU severely alters mental capability, and patient brains show reduced myelination in forebrain structures such as the corpus callosum, striatum, and subcortical white matter. A correlation between plasmatic Phe concentrations and white-matter lesions has been demonstrated (Dyer 2000), and hypomyelination and mental retardation are thought to be linked (Huttenlocher 2000; Rocha and Martel 2009). Hypomyelination was quantified in the PKU BTBR Pahenu2/J mouse model (Joseph and Dyer 2003), as only 50% of axons are myelinated in mutant animals (Shefer et al. 2000). The mechanisms involved in this altered myelination are still unknown. In most vertebrates, proliferation and maturation of oligodendrocytes occur mainly during early postnatal life (Baumann and Pham-Dinh 2001; Miller 2002) and are concomitant with the rise of Phe blood concentrations after birth. A cytotoxic effect of Phe, PP, or PA on oligodendrocytes was suggested (Kaufman 1989). It was also proposed that high Phe concentrations could convert oligodendrocytes from a myelinating to a nonmyelinating phenotype (Dyer et al. 1996) or decrease the rate of protein (Hughes and Johnson 1976) and cholesterol synthesis (Shefer et al. 2000), two major compounds of the myelin sheaths. Alternatively, indirect mechanisms were suggested. Phe crosses the blood–brain barrier through the L-type amino acid transporter 1 (LAT1), which is shared with other large neutral amino acids (LNAA). As the transporter has a high affinity for Phe (Pardridge 1998), elevated Phe concentrations can compete with the transport of other amino acids to the brain, resulting in decreased protein and neurotransmitter synthesis (Rocha and Martel 2009; van Spronsen et al. 2009).
As there is no consensus about these different hypotheses, we investigated in vivo and in vitro the influence of Phe and two of its metabolites on oligodendrocyte development and myelin-sheath formation.
Materials and methods
Animals
PKU model: BTBR Pahenu2/J mouse strain was obtained from Jackson Laboratory (Bar Harbor, MN, USA). The phenotype of homozygote animals (Pah−/−) has been previously described (Sarkissian et al. 2000a; Zagreda et al. 1999). Postnatal-day 1 (P1) Wistar rats (Charles River, France) were used for oligodendrocyte isolation. For myelinating cocultures, homozygous 1900-bp myelin basic protein (MBP) lacZ mice (Gow et al. 1992) were crossed with nontransgenic Oncis France Strain 1 (OF1) mice, (Charles River). The 1900-bp MBP lacZ transgene enables indirect quantification of the MBP promoter activity (Stankoff et al. 2002). All animal experimentation was approved by the local ethics committee (agreements: ULg : n° 864 ; Cr-Icm : 75–646).
Genotyping
Genetic characterization of Pahenu2/J animals was performed on tail DNA. The enu2 mutation was detected by polymerase chain reaction (PCR) amplification of exon 7 of the Pah gene and enzymatic digestion with Bacillus stearothermophilus maltogenic amylase 1 (BsmA1) (New England Biolabs Inc., UK) (McDonald and Charlton 1997).
BrdU exposure
Eight-day-old BTBR Pahenu2/J mice (P8) were injected twice intraperitoneally with 100µg/g 5-bromo-2-deoxyuridine (BrdU) (Sigma Aldrich, Bornem, Belgium) at 12 h of interval and sacrificed 24 h after the first injection.
Histology
Animals were transcardially perfused with 9‰ sodium chloride (NaCl) then 4% paraformaldehyde (PFA) for tissue fixation. Brains were dissected and frozen at −80°c for conservation; 50-µm-wide brain coronal sections were made and conserved at −20°C.
Immunolabeling
For immunohistology on brain sections, BrdU staining was performed after a two-normal hydrochloric acid (2N HCl) pretreatment of sections. For oligodendrocyte transcription factor 2 (Olig-2) and CC1 double staining, brain sections were incubated in antigen retrieval solution (Dako Belgium nv/sa, Heverlee, Belgium) for 10 min at 90°C. Nonspecific labeling was prevented by incubating tissue sections in 0.1 M phosphate-buffered saline (PBS)–0.3% triton X 100–0.25% gelatin for 2 h at room temperature. The sections were first incubated overnight at 4°C with primary antibodies directed against BrdU (ImmunoSource, Halle-Zoersel, Belgium; 1:500), Olig-2 (Millipore, Brussels, Belgium; 1:250) or CC1 (Calbiochem, Nottingham, UK; 1:250) diluted in 0.1 M PBS–0.3% triton X 100–0.25% gelatin, then incubated for 1 h at room temperature with appropriate secondary antibody diluted 1:500 in the same buffer (Invitrogen, Merelbeke, Belgium; Goat antirat Texas Red; Goat antirabbit Alexa 488; goat antimouse Texas Red). Diamidino-2-phenylindole (DAPI) nuclear labeling was performed before mounting the sections (Invitrogen, Merelbeke, Belgium). Rat oligodendrocytes or myelinating cocultures were grown on 14-mm glass coverslip. Specimens were fixed with 4% PFA for 10 min then incubated at room temperature for 30 min with Dulbecco's modified Eagle medium (DMEM) (Invitrogen, Merelbeke, Belgium) and 20% goat serum (Millipore). Samples were incubated overnight at 4°C in 0.1 M PBS–0.2% triton with primary antibodies: Olig-2 (Millipore; 1:200) and SMI31, SMI32 (Covance, Princeton, NJ, USA; 1:500) or oligodendrocyte marker 4 (O4) (Sommer and Schachner 1981) (1:4) for isolated oligodendrocytes; SMI31, SMI32, and proteolipid protein (PLP) (AA3 clone (Yamamura et al. 1991); 1:5) for myelinating cocultures. Labeling was revealed by incubation of the specimens during 30 min at room temperature with appropriate secondary antibodies diluted in 0.1 M PBS–0.2% triton (Invitrogen: goat anti-rabbit Alexa 488, goat anti-rat Alexa 488, goat anti-mouse Alexa 595). Nuclei were labeled with Hoetsch (Sigma Aldrich), and coverslips were mounted in Fluoromount G (SouthernBiotech, Birmingham, AL, USA) and stored at 4°C.
Oligodendrocyte proliferation
After exposure to BrdU of heterozygous and double-mutant BTBR P8, brain sections were immunolabeled against BrdU and Olig-2, and Z-stacks (step size 1µm) of the subventricular zone (SVZ) were analyzed at 40× magnification using confocal microscopy (MRC1024; Bio-Rad, Nazareth Eke, Belgium). Results were expressed as the mean percentage of double positive cells versus Olig-2+/BrdU-cells. Four animals were studied for each genotype, and two sections per animal were analyzed.
In vivo oligodendrocyte density
Oligodendrocyte density was quantified in the corpus callosum of 2-month-old wild-type or double-mutant BTBR Pahenu2/J mice using Olig-2 and CC1 labeling. On each section, three Z-stacks at 40× magnification were performed in the corpus callosum (one central and two median; step size 1.3 µm, total depth 9.1 µm; Fluoview FV1000; Olympus, Hamburg, Germany). Double-positive cells were counted and divided by the volume represented by the stack in µm3. Results are expressed as the mean density per genotype. Four animals per genotype were studied, and six stacks per animal were analyzed.
Rat oligodendrocyte isolation
Brain cortices from 12 postnatal-day-1 rat pups were dissected as described (Barbin et al. 2004; McCarthy and de Vellis 1980). After 10 days of culture, the oligodendrocytes and most of the microglia were separated from the astrocytes by shaking the flasks overnight at 260 rpm. The microglia was then eliminated after incubation for 20 min on uncoated plates. Cells from the supernatants were seeded on poly-L-lysine-coated coverslips in 24-well plates at the density of 50,000 cells per coverslip in Bottenstein and Sato medium (Bottenstein et al. 1979) to quantify oligodendrocyte enrichment. Cells were immunolabeled with anti Olig-2 and SMI31/32 antibodies and a DAPI nuclear staining. The oligodendrocyte proportion was assessed by counting Olig-2-positive/SMI-negative cells.
Reagents
Phe and PP were purchased from Sigma and PA from SERATEC (Courville-sur-Eure, France) and were used at either pathological or suprapathological concentrations (1.6/5 mM, 2.2/22 µM, and 7.4/74 µM, respectively).
Viability assay
Rat oligodendrocytes were seeded in 24-well plates coated with poly-L-lysine at 100,000 cells per well with Bottenstein and Sato medium. After 6 days of exposure to Phe, PP, or PA, methyl thiazolyldiphenyl-tetrazolium bromide (MTT) (Sigma Aldrich) was added to media at 0.5 mg/ml for two h, then cells were washed with PBS and lysed with 150-µl dimethyl sulfoxide per well. Then, 100 µl of the supernatant was transferred to a 96-well plate and absorbance was read at 570 nm. In living cells, MTT is converted into water-insoluble formazan by mitochondrial dehydrogenase. The viability index is provided by measuring the amount of formazan through absorbance determination.
Myelinating cocultures
Myelinating cultures from embryonic mouse brains were performed as previously described (Stankoff et al. 2002). In treated cultures, 5 mM of Phe, 22 µM of PP, or 74 µM of PA was added throughout the culture period. Myelination was quantified at day 25 in vitro (Charles et al. 2000; Demerens et al. 1999; Stankoff et al. 2002). Three independent sets of experiments were generated.
β-galactosidase activity
Myelinating cocultures from embryonic mice carrying the 1900-bp MBP lacZ transgene were stopped at day 25. Cells were lysed and, after centrifugation, the supernatants were frozen at −80°C until use. β-galactosidase activity was measured with the Galacto-light system kit (Applied Biosystems, Foster City, CA, USA) on a microplate luminometer with a microinjection system (Victor2 1420; Perkin Elmer, Zaventem, Belgium). Results are expressed as counts per second per microgram of proteins. Global results are stated as ratio of the mean score of each condition divided by the score of the control samples from the same experiment. Three different sets of myelinating cocultures were analyzed, with a minimum of five samples per condition to generate the final results.
Quantification of in vitro myelinogenesis
The number of myelin sheaths was analyzed optically on a fluorescence microscope (Eclipse 90i; Nikon, Brussels, Belgium) at 40× magnification by counting the number of PLP-positive internodes per coverslip. Three different sets of myelinating cocultures were analyzed, with four to six samples per condition. Results are expressed as ratio between the mean number of sheaths for treated and control samples. In parallel, the whole surface of the immunolabeled samples was captured by an automated fluorescence microscope (Cell^R; Olympus, Hamburg, Germany), then myelin labeling was computed using a novel phase-based sheath extraction algorithm (Wu et al. unpublished data). The extraction algorithm included two steps: seed-line generation and sheath tracing. Phase symmetry information was used to generate reliable seed lines for sheaths. The orientation map was computed based on local gradient information and smoothed using low-pass filter. The sheath-tracing algorithm was orientation-guided so that a complete sheath map was produced by extending the seed lines in the original gray-scale image.
Statistical analysis
The statistical Student’s t test was used to analyze all data from treated versus control samples. A p value of 0.05 was chosen as a threshold for significance. All reported values are expressed as means ± standard error of the mean (SEM). The correlation between optical and bioinformatics methods was estimated using Pearson’s linear correlation coefficient.
Results
Oligodendrocyte development in PKU mutants
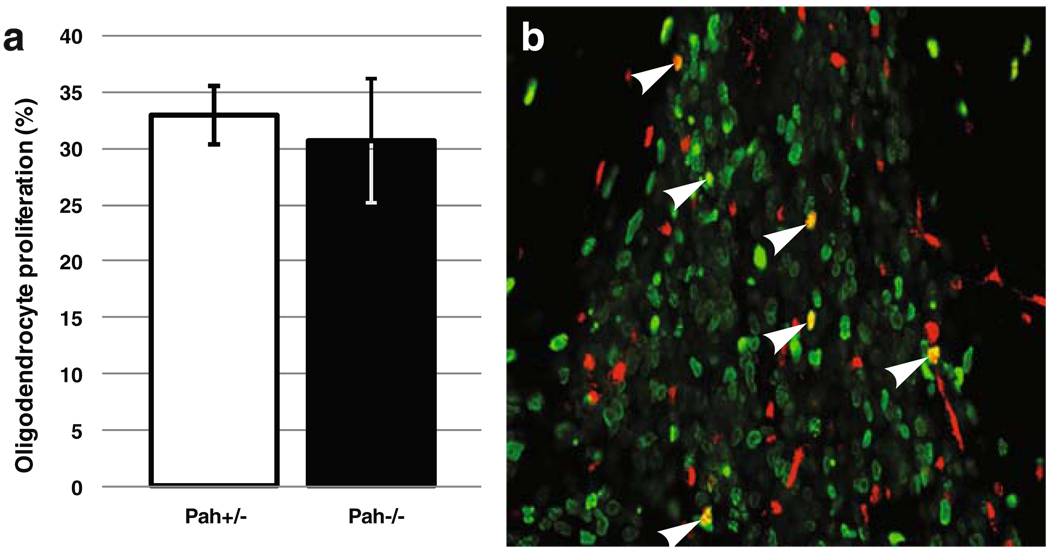
The BTBR Pahenu2/J double mutant animals show a typical PKU phenotype, including a 21-fold increase of the Phe plasmatic concentration (Smith and Kang 2000), whereas heterozygous individuals harbor almost normal plasmatic and brain Phe concentrations (Pascucci et al. 2002) and develop normally (Cabib et al. 2003). Moreover, we confirmed that, as previously reported (Shefer et al. 2000), homozygous mutants showed an hypomyelination of their corpus callosum (data not shown). As PKU treatment has to be initiated during the first weeks of life, a period when oligodendrocyte progenitor cells (OPC) proliferate actively, we investigated postnatal OPC proliferation after a 24 h of BRDU exposure of heterozygous and homozygous BTBR Pahenu2/J (−/−) 8-day-old mice pups. Proliferating oligodendrocytes were identified in the SVZ through double staining with antibodies against Olig-2 and BRDU. The percentage of cycling oligodendrocytes is very similar between control (+/−) and PKU (−/−) subjects [32.9% (+/−2.6) and 30.7% (+/−5.5), respectively], showing that hypomyelination is not due to an OPC proliferation defect (Fig. 1).
Fig. 1.
a Oligodendrocyte proliferation rate in the subventricular zone (SVZ) from P8 BTBR Pahenu2/J heterozygous (Pah+/−) and double-mutant (Pah−/−) mice. Results are expressed in percentages of oligodendrocyte transcription factor 2 (Olig-2)-positive cells having incorporated 5-bromo-2-deoxyuridine (BrdU) after 24-h exposure versus the total number of Olig-2 cells present in the SVZ. b Immunohistochemistry of P8 BTBR Pahenu2/J brain slice along the SVZ at ×40 magnification. Red labelling is Olig-2 and green is BrdU. Arrows indicate double-positive cells
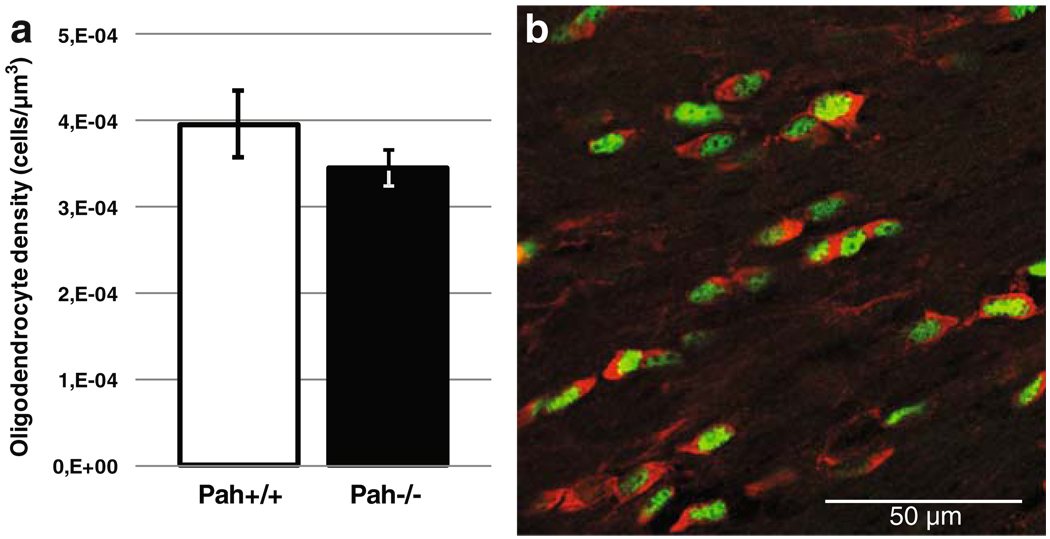
As the best characterized hypomyelinated site in the BTBR Pahenu2/J strain is the corpus callosum, we investigated oligodendrocyte density in this structure. Adult wild-type and mutant coronal brain slices were labeled with antibodies against Olig-2 and CC1 to stain the whole oligodendrocyte population and mature oligodendrocytes, respectively. As shown in Fig. 2, the density of mature Olig-2 and CC1-positive oligodendrocytes was not statistically different in wild-type versus mutant mice [4 × 10−4 (+/ − 3, 9 × 10−5) and 3, 5 × 10−4 (+/ −2, 2 × 10−5) cells / µm3 respectively]. Therefore, the reduced myelin sheath number found in the corpus callosum in mutant animals is not due to an oligodendrocyte migratory defect or an enhanced mortality.
Fig. 2.
a Oligodendroglial density in the corpus callosum of 2-month-old BTBR Pahenu2/J wild-type (Pah+/+) and double mutant (Pah−/−) mice. Data represent the density of mature oligodendrocytes, expressing both oligodendrocyte transcription factor 2 (Olig-2) and CC1. b Immunolabeling of a brain section across the corpus callosum from BTBR Pahenu2/J with anti-Olig-2 (green) and anti-CC1 (red) antibodies. Most cells are coexpressing the two markers and thus correspond to mature oligodendrocytes
Ex vivo oligodendrocyte exposure to Phe or its metabolites
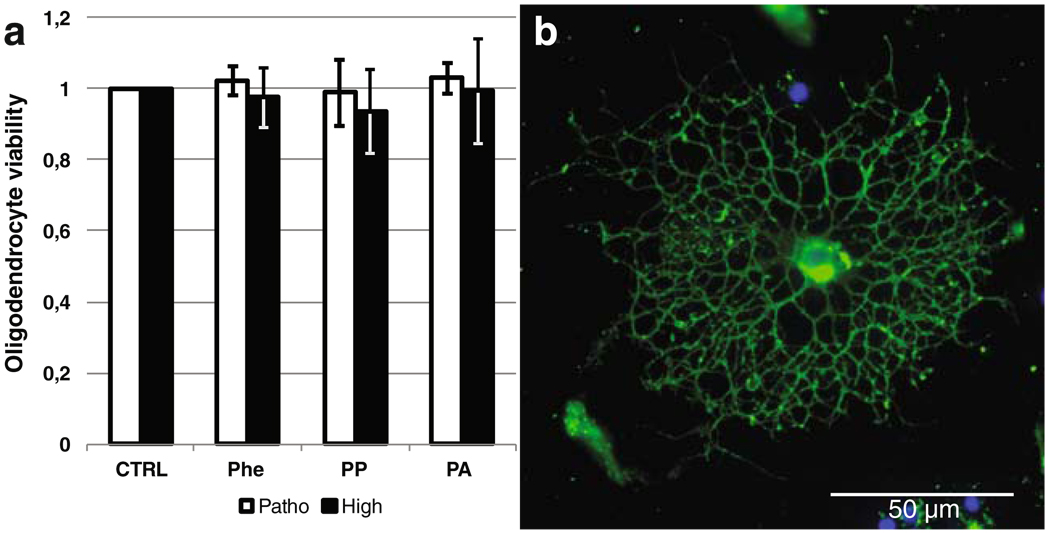
We then assessed the toxicity of Phe, PP, and PA ex vivo on enriched oligodendroglial cultures from P1 rat brains. In these cultures, the mean percentage of Olig-2-positive and SMI31/32-negative oligodendrocytes was 77% (±7.1%). This experiment showed no significant oligodendrocyte toxicity induced by the three molecules at either pathological (Sarkissian et al. 2000b) or suprapathological concentrations (Fig. 3).
Fig. 3.
a Viability test using methylthiazolyldiphenyl-tetrazolium bromide (MTT) after a 6 days exposure to phenylalanine (Phe), phenylpyruvate (PP), and phenylacetate (PA) at pathologically relevant (Patho) and suprapathological (High) concentrations of an enriched population of oligodendrocytes isolated from postnatal-day 1 (P1) rat brains. Pathological/high concentrations are 1.6/5 mM, 2.2/22 µM, 7.4/74 µM for Phe, PP, and PA, respectively. b Anti-oligodendrocyte marker 4 (anti-O4) immunolabeling of rat preoligodendrocyte population 48 h after purification
In vitro myelin sheath formation
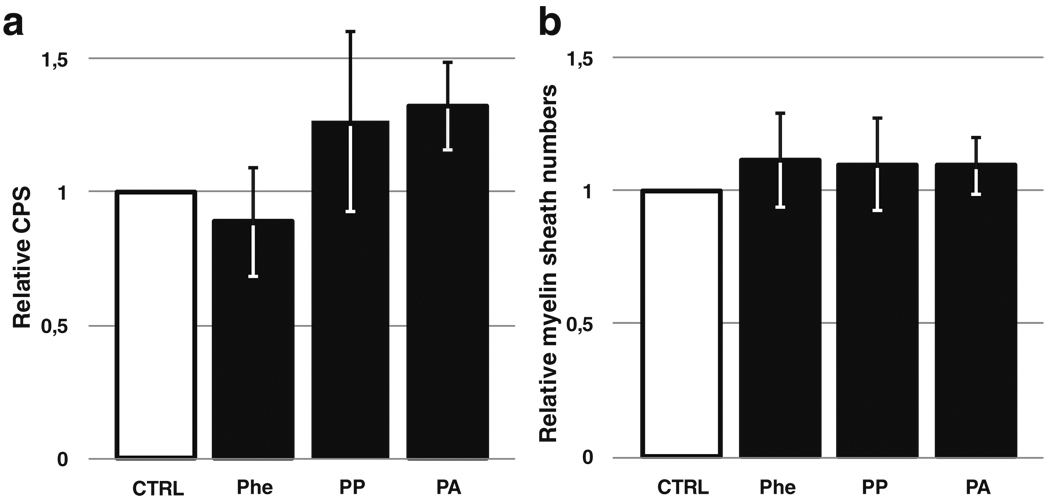
After isolation of primary cells from 1900-bp MBP lacZ transgenic mice, a myelinating coculture model allowed us to investigate in vitro the oligodendrocyte capacity to differentiate and synthesize myelin sheaths around axons. The β-galactosidase assays, reflecting MBP promoter activity, showed no statistically significant difference between control cocultures and cultures incubated with Phe, PP, or PA, with the exception of a minor increase in β-galactosidase activity after treatment with PA (Fig. 4a).
Fig. 4.
a β-Galactosidase activity assay from murine myelinating cocultures. The degree of maturation of oligodendrocytes in the cultures was assessed by quantifying the activation of the myelin basic protein (MBP) promoter through luminometric β-galactosidase activity assay. Results are expressed as relative counts per second (CPS) compared with control condition. b Optical counting of proteolipid protein (PLP)-positive internodes (i.e., myelin sheaths) in murine myelinating cocultures. Results are expressed as ratios of the count from cultures exposed to phenylalanine (Phe) (5 mM), phenylpyruvate (PP) (22 µM), or phenylacetate (PA) (74 µM) to the score from the corresponding control samples. P>0.05 versus control group
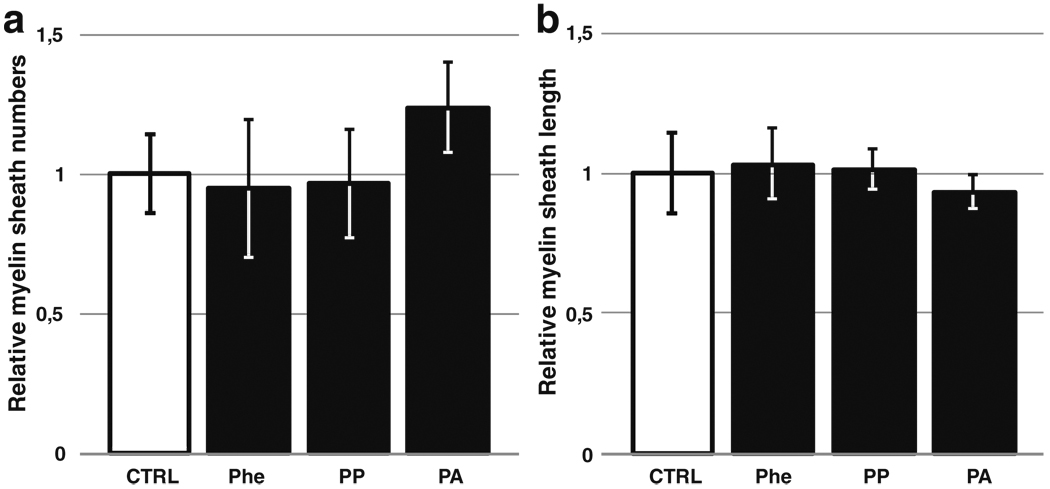
In parallel, myelin sheath formation was analyzed after immunolabeling for PLP and SMI31/32 to reveal the myelin and axons, respectively. Optical counting of myelinated internodes on the whole surface of the samples revealed no difference between control and Phe-, PP-, or PA-treated conditions (Fig. 4b). To improve quantification, whole-sample surfaces were captured, and structures corresponding to the myelin labeling were computed using novel phase-based sheaths extraction algorithm (Wu et al. unpublished data). This analysis accurately recognized the sheaths and counted them, as well as measured the length of each structure. Results showed a very good correlation coefficient (0.9) with optical counting regarding myelin-sheath numbers and showed no variation of sheath length and number between control and treated cultures, excepting a slight increase in the number of myelin sheaths produced after treatment with PA (Fig. 5).
Fig. 5.
Bioinformatics analysis: a Counting of myelin sheaths from the whole surface of murine myelinating coculture samples. b Evaluation of the mean length of myelin sheaths from the same samples. Results are expressed as ratios of the score from cultures exposed to phenylalanine (Phe) (5 mM), phenylpyruvate (PP) (22 µM), or phenylacetate (PA) (74 µM) to the score from the corresponding control samples. P>.05 versus control group
Discussion
Untreated PKU patients show hypomyelination in their CNS, and this defect participates in irreversible mental retardation. This defect in the myelination process could also explain why an early treatment is crucial, as myelination occurs in early postnatal life. Several hypotheses have been proposed to explain this hypomyelination, but none had been experimentally demonstrated. As one of these hypotheses suggests a direct toxic effect of Phe and its metabolites on oligodendrocytes, we evaluated, in vitro and in vivo, the effect of Phe, PP, and PA on oligodendrocyte development, survival, and function.
Our results found that, although PKU mice show hypomyelination in their corpus callosum, the number of oligodendrocytes present in these hypomyelinated areas is normal, confirming a previous study (Shefer et al. 2000). Moreover, we detected no direct cytoxicity on primary oligodendrocytes cultivated in the presence of very high Phe, PP, or PA concentrations. These data indicate that PKU-associated myelination defect is not due to direct toxicity on oligodendrocyte development or survival, although such a direct link had been suggested by several authors (Huttenlocher 2000; Kaufman 1989; Scriver and Kaufman 2001). Therefore, alternative mechanisms might be involved. It was previously reported that synthesis of cholesterol was inhibited by Phe and that oligodendrocytes switched to a nonmyelinating glial fibrillary acidic protein (GFAP)-positive phenotype when exposed to Phe in vitro (Dyer et al. 1996; Shefer et al. 2000). On the other hand, it was also shown that exposing mixed cortical cultures to enhanced amounts of Phe did not modify the ratio between glial and neuronal cells (Horster et al. 2006). In a different experimental setting, Silberberg previously investigated the effect of Phe, PP, and PA on cytotoxicity and myelination in the cerebellum (Silberberg 1967), but that study was limited to conventional bright-light microscopy. We therefore used an overall approach to further quantify in vitro the impact of Phe, PP, and PA on the myelination process. The myelinating coculture model allowed us to measure the final product, myelin sheaths, and thus evaluate the performance of the whole process, including oligodendrocyte differentiation, axonal interaction, synthesis, and sheath wrapping. Moreover, in cultures derived from the 1900-bp MBP lacZ transgenic animals, a good correlation was demonstrated between galactosidase expression and the number of terminally differentiated oligodendrocytes (Stankoff et al. 2002). In these conditions, we showed that Phe, PA, or PP had no impact on sheath synthesis, even at suprapathological concentrations. Our results are not directly comparable with previous in vitro studies, as this is, to our knowledge, the first time such an integrated technique has been used to study PKU physiopathology. Therefore, as high Phe, PP, or PA concentrations do not alter myelin synthesis by either oligodendrocytes nor myelin sheaths wrapping around axons, a direct Phe toxic effect on the myelination process in unlikely. An indirect relation between high Phe levels and hypomyelination should thus be considered.
Hyperphenylalaninemia generates a competition between Phe and the other LNAAs for the LAT1 transporter at the blood–brain barrier. The result is a lowered flux of LNAA to the brain (Smith and Kang 2000; Surtees and Blau 2000), and decreased cerebral protein synthesis rate in PKU patients was indeed reported (Hoeksma et al. 2009). Lowered protein synthesis might thus impair myelinogenesis and myelin maintenance (Surtees and Blau 2000). On the basis of this hypothesis, a supplementation with high amounts of LNAAs have been given to some PKU patients to modify competition for the transporter, and thus to reduce Phe brain concentrations and increase the transport of other LNAAs across the blood–brain barrier. Such treatment reduced Phe blood levels, probably as a consequence of competition for intestinal transporters, and showed some clinical benefits in patients unable to follow the diet (Matalon et al. 2007; Schindeler et al. 2007). The effects of this treatment on brain Phe and LNAA concentrations still need to be precisely evaluated (Rocha and Martel 2009). Alternatively, if such a hypothesis could be experimentally confirmed, the LAT1 transporter would be considered a putative target for novel therapeutic agents to increase the cerebral influx of LNAA.
In conclusion, this report could not demonstrate any direct Phe oligodendroglial toxicity in an in vitro assay recapitulating all major steps of myelin-sheath synthesis. Moreover, oligodendrocyte proliferation and survival are not affected in in vivo and ex vivo models. Therefore, novel in vivo models investigating the role of the LAT1 transporter and LNAAs should be developed to explore the indirect hypotheses for hypomyelination and identify new therapeutic targets.
Acknowledgments
The authors thank the Imaging Platform, the SPF Animal Facility, and the Neurosciences Department from GIGA-Research for their experimental help. We also thank Olympus Belgium N.V. for providing the Cell^R system.
List of abbreviations
- PKU
Phenylketonuria
- PAH
Phenylalanine hydroxylase
- Phe
Phenylalanine
- Tyr
Tyrosine
- PP
Phenylpyruvate
- PA
Phenylacetate
- LNAA
Large neutral amino acids
- OPC
Oligodendrocyte progenitor cells
- SVZ
Subventricular zone
Footnotes
Competing interest None declared.
Contributor Information
Renaud Schoemans, Human Genetics, GIGA-Research, University of Liège, Liège, Belgium.
Marie-Stéphane Aigrot, Cr-Icm, Inserm 711, UPMC, Paris, France.
Chaohong Wu, Michtom School of Computer Science, Volen Center for Complex Systems, Room 261, Brandeis University, Waltham, MA 02454, USA.
Raphaël Marée, Bioinformatics platform, GIGA-Research, University of Liège, Liège, Belgium.
Pengyu Hong, Michtom School of Computer Science, Volen Center for Complex Systems, Room 261, Brandeis University, Waltham, MA 02454, USA.
Shibeshi Belachew, Neurosciences, GIGA-Research, University of Liège, Liège, Belgium.
Claire Josse, Human Genetics, GIGA-Research, University of Liège, Liège, Belgium.
Catherine Lubetzki, Cr-Icm, Inserm 711, UPMC, Paris, France.
Vincent Bours, Email: vbours@ulg.ac.be, Human Genetics, GIGA-Research, University of Liège, Liège, Belgium; Genetics Center, CHU Liège, Liège, Belgium; Department of Genetics, CHU Liège, Université de Liège B34, Avenue de l’hôpital 1, 4000 Liège, Belgique, Belgium.
References
- Ahring K, Belanger-Quintana A, Dokoupil K, et al. Dietary management practices in phenylketonuria across European centres. Clin Nutr. 2009;28(3):231–236. doi: 10.1016/j.clnu.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Barbin G, Aigrot MS, Charles P, et al. Axonal cell-adhesion molecule L1 in CNS myelination. Neuron Glia Biol. 2004;1(1):65–72. doi: 10.1017/S1740925X04000092. [DOI] [PubMed] [Google Scholar]
- Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev. 2001;81(2):871–927. doi: 10.1152/physrev.2001.81.2.871. [DOI] [PubMed] [Google Scholar]
- Bottenstein J, Hayashi I, Hutchings S, et al. The growth of cells in serum-free hormone-supplemented media. Methods Enzymol. 1979;58:94–109. doi: 10.1016/s0076-6879(79)58127-0. [DOI] [PubMed] [Google Scholar]
- Cabib S, Pascucci T, Ventura R, Romano V, Puglisi-Allegra S. The behavioral profile of severe mental retardation in a genetic mouse model of phenylketonuria. Behav Genet. 2003;33(3):301–310. doi: 10.1023/a:1023498508987. [DOI] [PubMed] [Google Scholar]
- Charles P, Hernandez MP, Stankoff B, et al. Negative regulation of central nervous system myelination by polysialylated-neural cell adhesion molecule. Proc Natl Acad Sci U S A. 2000;97(13):7585–7590. doi: 10.1073/pnas.100076197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerens C, Stankoff B, Zalc B, Lubetzki C. Eliprodil stimulates CNS myelination: new prospects for multiple sclerosis? Neurology. 1999;52(2):346–350. doi: 10.1212/wnl.52.2.346. [DOI] [PubMed] [Google Scholar]
- Dyer CA. Comments on the neuropathology of phenylketonuria. Eur J Pediatr. 2000;159(Suppl 2):S107–S108. doi: 10.1007/pl00014369. [DOI] [PubMed] [Google Scholar]
- Dyer CA, Kendler A, Philibotte T, Gardiner P, Cruz J, Levy HL. Evidence for central nervous system glial cell plasticity in phenylketonuria. J Neuropathol Exp Neurol. 1996;55(7):795–814. doi: 10.1097/00005072-199607000-00005. [DOI] [PubMed] [Google Scholar]
- Gow A, Friedrich VL, Jr, Lazzarini RA. Myelin basic protein gene contains separate enhancers for oligodendrocyte and Schwann cell expression. J Cell Biol. 1992;119(3):605–616. doi: 10.1083/jcb.119.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeksma M, Reijngoud DJ, Pruim J, de Valk HW, Paans AM, van Spronsen FJ. Phenylketonuria: high plasma phenylalanine decreases cerebral protein synthesis. Mol Genet Metab. 2009;96(4):177–182. doi: 10.1016/j.ymgme.2008.12.019. [DOI] [PubMed] [Google Scholar]
- Horster F, Schwab MA, Sauer SW, et al. Phenylalanine reduces synaptic density in mixed cortical cultures from mice. Pediatr Res. 2006;59(4 Pt 1):544–548. doi: 10.1203/01.pdr.0000203091.45988.8d. [DOI] [PubMed] [Google Scholar]
- Hughes JV, Johnson TC. The effects of phenylalanine on amino acid metabolism and protein synthesis in brain cells in vitro. J Neurochem. 1976;26(6):1105–1113. doi: 10.1111/j.1471-4159.1976.tb06993.x. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. The neuropathology of phenylketonuria: human and animal studies. Eur J Pediatr. 2000;159(Suppl 2):S102–S106. doi: 10.1007/pl00014371. [DOI] [PubMed] [Google Scholar]
- Joseph B, Dyer CA. Relationship between myelin production and dopamine synthesis in the PKU mouse brain. J Neurochem. 2003;86(3):615–626. doi: 10.1046/j.1471-4159.2003.01887.x. [DOI] [PubMed] [Google Scholar]
- Kaufman S. An evaluation of the possible neurotoxicity of metabolites of phenylalanine. J Pediatr. 1989;114(5):895–900. doi: 10.1016/s0022-3476(89)80161-1. [DOI] [PubMed] [Google Scholar]
- Matalon R, Michals-Matalon K, Bhatia G, et al. Double blind placebo control trial of large neutral amino acids in treatment of PKU: effect on blood phenylalanine. J Inherit Metab Dis. 2007;30(2):153–158. doi: 10.1007/s10545-007-0556-4. [DOI] [PubMed] [Google Scholar]
- McCarthy KD, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980;85(3):890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JD, Charlton CK. Characterization of mutations at the mouse phenylalanine hydroxylase locus. Genomics. 1997;39(3):402–405. doi: 10.1006/geno.1996.4508. [DOI] [PubMed] [Google Scholar]
- Miller RH. Regulation of oligodendrocyte development in the vertebrate CNS. Prog Neurobiol. 2002;67(6):451–467. doi: 10.1016/s0301-0082(02)00058-8. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. Blood-brain barrier carrier-mediated transport and brain metabolism of amino acids. Neurochem Res. 1998;23(5):635–644. doi: 10.1023/a:1022482604276. [DOI] [PubMed] [Google Scholar]
- Pascucci T, Ventura R, Puglisi-Allegra S, Cabib S. Deficits in brain serotonin synthesis in a genetic mouse model of phenylketonuria. NeuroReport. 2002;13(18):2561–2564. doi: 10.1097/00001756-200212200-00036. [DOI] [PubMed] [Google Scholar]
- Pey AL, Desviat LR, Gamez A, Ugarte M, Perez B. Phenylketonuria: genotype-phenotype correlations based on expression analysis of structural and functional mutations in PAH. Hum Mutat. 2003;21(4):370–378. doi: 10.1002/humu.10198. [DOI] [PubMed] [Google Scholar]
- Rocha JC, Martel F. Large neutral amino acids supplementation in phenylketonuric patients. J Inherit Metab Dis. 2009;32(4):472–480. doi: 10.1007/s10545-009-1132-x. [DOI] [PubMed] [Google Scholar]
- Sarkissian CN, Boulais DM, McDonald JD, Scriver CR. A heteroallelic mutant mouse model: a new orthologue for human hyperphenylalaninemia. Mol Genet Metab. 2000a;69(3):188–194. doi: 10.1006/mgme.2000.2974. [DOI] [PubMed] [Google Scholar]
- Sarkissian CN, Scriver CR, Mamer OA. Measurement of phenyllactate, phenylacetate, and phenylpyruvate by negative ion chemical ionization-gas chromatography/mass spectrometry in brain of mouse genetic models of phenylketonuria and non-phenylketonuria hyperphenylalaninemia. Anal Biochem. 2000b;280(2):242–249. doi: 10.1006/abio.2000.4542. [DOI] [PubMed] [Google Scholar]
- Schindeler S, Ghosh-Jerath S, Thompson S, et al. The effects of large neutral amino acid supplements in PKU: an MRS and neuropsychological study. Mol Genet Metab. 2007;91(1):48–54. doi: 10.1016/j.ymgme.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Scriver CR, Kaufman S. Hyperphenylalaninemia: phenylalanine hydroxylase deficiency. In: Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Kinzler KW, Vogelstein B, editors. The metabolic and molecular bases of inherited disease. 8th edn. New York: McGraw-Hill; 2001. pp. 1667–1724. (associated eds) [Google Scholar]
- Shefer S, Tint GS, Jean-Guillaume D, et al. Is there a relationship between 3-hydroxy-3-methylglutaryl coenzyme a reductase activity and forebrain pathology in the PKU mouse? J Neurosci Res. 2000;61(5):549–563. doi: 10.1002/1097-4547(20000901)61:5<549::AID-JNR10>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Silberberg DH. Phenylketonuria metabolites in cerebellum culture morphology. Arch Neurol. 1967;17(5):524–529. doi: 10.1001/archneur.1967.00470290078010. [DOI] [PubMed] [Google Scholar]
- Smith CB, Kang J. Cerebral protein synthesis in a genetic mouse model of phenylketonuria. Proc Natl Acad Sci U S A. 2000;97(20):11014–11019. doi: 10.1073/pnas.97.20.11014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer I, Schachner M. Monoclonal antibodies (O1 to O4) to oligodendrocyte cell surfaces: an immunocytological study in the central nervous system. Dev Biol. 1981;83(2):311–327. doi: 10.1016/0012-1606(81)90477-2. [DOI] [PubMed] [Google Scholar]
- Stankoff B, Aigrot MS, Noel F, Wattilliaux A, Zalc B, Lubetzki C. Ciliary neurotrophic factor (CNTF) enhances myelin formation: a novel role for CNTF and CNTF-related molecules. J Neurosci. 2002;22(21):9221–9227. doi: 10.1523/JNEUROSCI.22-21-09221.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surtees R, Blau N. The neurochemistry of phenylketonuria. Eur J Pediatr. 2000;159(Suppl 2):S109–S113. doi: 10.1007/pl00014370. [DOI] [PubMed] [Google Scholar]
- van Spronsen FJ, Hoeksma M, Reijngoud DJ. Brain dysfunction in phenylketonuria: is phenylalanine toxicity the only possible cause? J Inherit Metab Dis. 2009;32(1):46–51. doi: 10.1007/s10545-008-0946-2. [DOI] [PubMed] [Google Scholar]
- Williams RA, Mamotte CD, Burnett JR. Phenylketonuria: an inborn error of phenylalanine metabolism. Clin Biochem Rev. 2008;29(1):31–41. [PMC free article] [PubMed] [Google Scholar]
- Wu C, Schulte J, Sepp KJ, Littleton JT, Hong P. Automatic robust neurite detection and morphological analysis of neuronal cell cultures in high-content screening. Neuroinformatics. 2010 Jun;8(2):83–100. doi: 10.1007/s12021-010-9067-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamura T, Konola JT, Wekerle H, Lees MB. Monoclonal antibodies against myelin proteolipid protein: identification and characterization of two major determinants. J Neurochem. 1991;57(5):1671–1680. doi: 10.1111/j.1471-4159.1991.tb06367.x. [DOI] [PubMed] [Google Scholar]
- Zagreda L, Goodman J, Druin DP, McDonald D, Diamond A. Cognitive deficits in a genetic mouse model of the most common biochemical cause of human mental retardation. J Neurosci. 1999;19(14):6175–6182. doi: 10.1523/JNEUROSCI.19-14-06175.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]