Abstract
Background
Data on the association between the MTHFR 677C>T and ACE D/I polymorphisms and migraine including aura status are conflicting.
Objective
To perform a systematic review and meta-analysis on this topic.
Methods
We searched for studies published until March 2009 using electronic databases (MEDLINE, EMBASE, Science Citation Index) and reference lists of studies and reviews on the topic. Assessment for eligibility of studies and extraction of data was performed by two independent investigators. For each study we calculated the odds ratios (OR) and 95% confidence intervals (CI) assuming additive, dominant, and recessive genetic models. We then calculated pooled ORs and 95% CIs.
Results
Thirteen studies investigated the association between the MTHFR 677C>T polymorphism and migraine. The TT genotype was associated with an increased risk for any migraine, which only appeared for migraine with aura (pooled OR=1.48, 95% CI 1.02–2.13), but not migraine without aura. Nine studies investigated the association of the ACE D/I polymorphism with migraine. The II genotype was associated with a reduced risk for migraine with (pooled OR=0.71, 95% CI 0.55–0.93) and without aura (pooled OR=0.84, 95% CI 0.70–0.99). Results for both variants were driven by studies in non-Caucasian populations. Results among Caucasians did not suggest an association. Extractable data did not allow investigation of gene-gene-interactions.
Conslusions
The MTHFR 677TT genotype is associated with an increased risk for migraine with aura, while the ACE II genotype is protective against both migraine with and without aura. Results for both variants appeared only among non-Caucasian populations. There was no association among Caucasians.
Keywords: Migraine, MTHFR 677C>T polymorphism, ACE D/I polymorphism, meta-analysis
Introduction
Migraine is a common, chronic disorder characterized by recurrent headache attacks and combinations of gastrointestinal and autonomic nervous system symptoms.1 It affects 10–20% of the population; women 3–4-times more often than men. Up to one third of migraine patients experience an aura prior to or during the migraine headache.
Current concepts view migraine as an inherited brain disorder, but vascular mechanisms are implicated.2 For example, oxidative stress may lead to endothelial dysfunction,3 which may cause pathological vascular reactivity among migraineurs4 and also explain in part the association between migraine and cardiovascular disease.5 In addition, gene variants in the methylenetetrahydrofolate reductase gene (MTHFR 677C>T polymorphism, rs1801133) and in the angiotensin-converting enzyme gene (ACE D/I polymorphism, rs1799752) appear to play important roles in the vascular oxidative stress response.3 Consequently, an association between the MTHFR 677C>T and ACE D/I polymorphisms with migraine has been suggested.3
The MTHFR 677TT genotype impairs enzyme activity and carriers have increased homocysteine levels. A previous meta-analysis summarizing studies until December 2006 found an increased risk for migraine with aura among carriers of the TT genotype.6 However, a re-analysis is warranted because of methodological issues and the publication of five new studies since 2007,7–11 increasing the number of migraineurs with genetic information by a factor of 3 and the number of controls by a factor of 6 to a total of 8,000 migraineurs and 24,578 controls.
ACE is ubiquitously expressed and carriers of the ACE II genotype have plasma levels half that of DD subjects, with ID subjects having intermediate levels. Results on the association between the ACE D/I polymorphism and migraine are controversial.11–19 Furthermore, an interaction between certain genotypes of the MTHFR 677C>T and ACE D/I polymorphisms have been suggested.11, 13
We sought to summarize the current evidence on the association between the MTHFR 677C>T and ACE D/I polymorphisms and migraine including migraine with aura (MA) and migraine without aura (MO) by systematically reviewing the literature and performing a meta-analysis.
Methods
Selection of studies
We followed the guidelines for systematic reviews of genetic association studies.20 Two investigators (M.S., P.M.R.) independently searched MEDLINE, EMBASE, and Science Citation Index from inception to March 2009 combining text words and MESH terms, were appropriate, for methylenetetrahydrofolate reductase and angiotensin converting enzyme (“mthfr” or “methylenetetrahydrofolate reductase” or “ace” or “angiotensin converting enzyme” or “peptidyl-dipeptidase A”) with terms for genetic variations (“gene” or “polymorphism” or “genetic variation”) and terms for headache and migraine (“headache” or “headache disorders” or “migraine” or “migraine disorders”). The search terms were combined with the “explode” feature where applicable. We considered full articles published in English, German, French, and Spanish. In addition, we manually searched the reference list of all primary articles and review articles.
A priori, we defined the following criteria for inclusion:
Studies must have a cross-sectional, case-control or cohort design.
Authors must investigate patients with migraine and healthy control subjects.
Authors must provide information on genotype frequencies of the MTHFR 677C>T and/or ACE D/I polymorphism or sufficient data to calculate these.
In studies with overlapping cases and/or controls the largest study with extractable data was included.
In a first step, two investigators (M.S., T.K.) by consensus identified all studies not meeting any of the pre-specified criteria by screening the title and abstracts. These studies were excluded. In a second step, the same investigators evaluated the remaining studies in their entirety. Studies were excluded if they did not meet all criteria.
Data extraction
Two investigators (M.S., P.M.R.) independently extracted data from the published studies and entered them in a customized database. Disagreements were resolved by consensus. The extracted data included authors and title of study, year of publication, country of origin, ethnicity of population investigated, setting (clinic vs. population), study design, genotyping method, migraine status (any migraine, MA, MO), age range and gender of study individuals, study size, allele and genotype frequencies, and information on additional genetic variants as well as gene-gene and gene-environment interactions, if investigated. If not given, genotype frequencies were calculated where possible. If allele or genotype frequencies given did not match with the number of patients reported, the respective subgroup was excluded. We did not contact the authors to collect further information.
Statistical analysis
We first used logistic regression to calculate odds ratios (ORs) and 95% confidence intervals (CIs) for the association between the MTHFR 677C>T and the ACE D/I polymorphisms and migraine assuming additive, dominant, and recessive genetic models, based on the extracted or calculated genotype frequencies. The additive model assumes that the risk for migraine among carriers of the heterozygous genotype is half way between carriers of the homozygous genotypes. While the dominant model assumes that carriers of the heterozygous and homozygous mutant genotypes have the same risk of developing migraine compared with carriers of the homozygous wild-type genotype, a recessive model assumes that carrying the homozygous mutant genotype is necessary to alter the risk for migraine compared with carriers of the heterozygous and homozygous wild-type genotype. We also determined Hardy Weinberg-Equilibrium (HWE) for each study. We investigated any migraine, MA, and MO.
We then pooled results from all studies and subsequently stratified analyses by ethnicity to account for potential confounding.
We weighted the log of the OR by the inverse of their variance to obtain pooled estimates. We ran random-effects models, which have fewer assumptions than fixed-effects models and thus provide more conservative estimates. We performed the DerSimonian and Laird Q test for heterogeneity and also calculated the I2 statistic for each analysis.21 This statistic describes the percentage of total variation across studies that is due to heterogeneity rather than chance (25%: low, 50%: medium, 75%: high heterogeneity). We used Galbraith plots to visually examine the impact of individual studies on the overall homogeneity test statistic and employed meta-regression to evaluate the impact of ethnicity (Caucasian vs. non-Caucasian) and age (adult vs. adolescent/mixed adult+adolescent) on the results. We evaluated potential publication bias visually by examining for possible skewness in funnel plots22 and statistically with the methods described by Begg and Mazumdar22 and Egger.23 This method uses a weighted regression approach to investigate the association between outcome effects (log relative risk) and its standard error in each study.
All analyses were performed using SAS version 9.1 (SAS Institute Inc, Cary, NC) and STATA 10.1 (Stata, College Station, Texas, USA).
Since we only utilized previously published data, we did not obtain approval of an ethics committee or written informed consent.
Results
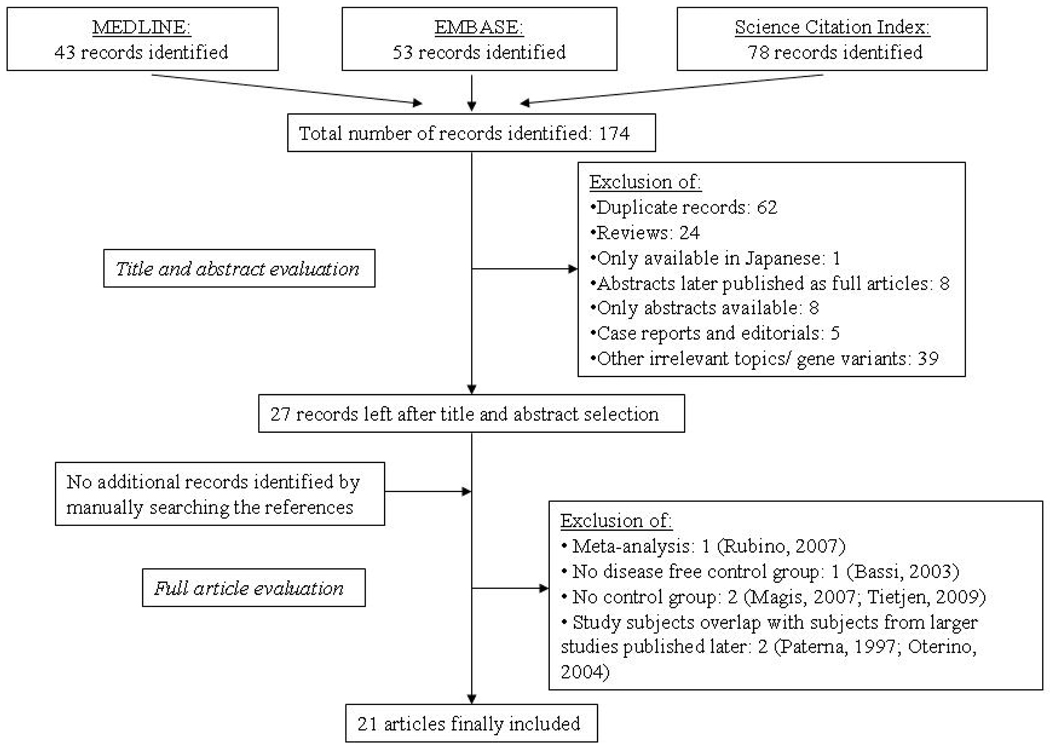
Figure 1 summarizes the process of identifying eligible studies. After title and abstract evaluation we were left with 27 studies.6–19, 24–36 We excluded six more studies after evaluating the remaining articles in their entirety and were left with 21 studies for this analysis.
Figure 1.
Process of study selection
Study characteristics
Table 1 summarizes the characteristics of the 21 studies included according to the MTHFR 677C>T and ACE D/I polymorphism. Twelve studies investigated the MTHFR 677C>T polymorphism,7–10, 25–29, 32, 34, 36 eight the ACE D/I polymorphism,12–19 and one study both variants.11
Table 1.
Characteristics of the 21 studies included according to MTHFR 677C>T and ACE D/I polymorphism
| a) MTHFR 677C>T polymorphism | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| MTHFR 677C>T polymorphism | |||||||||
| Total study size with genotypic information (women, %) |
|||||||||
| Author, year | Setting | Ethnicity | Study design |
Gender | controls | any migraine |
MA | MO | Comment |
| Kowa, 2000 (Japan)28 |
NS | Japanese | case-control | women+men | 261 (67) |
74 (78) |
22 (68) |
52 (83) |
----- |
| Kara, 2003 (Turkey)26 |
clinic | Turkish | case-control | women+men | 136 (88.2) |
93 (NS) |
23 (NS) |
70 (NS) |
Other gene variants investigated: MTHFR 1298A>C |
| Lea, 2004 (Australia)29 |
clinic | Caucasian | case-control | women+men | 269 (68) |
268 (68) |
168 (NS) |
100 (NS) |
----- |
| Oterino, 2005 (Spain)32 |
clinic | Caucasian | case-control | women+men | 237 (61.6) |
329 (77.5) |
138 (76.8) |
191 (78.0) |
Study base from Oterino, 2004,31 but additional cases and controls. Other gene variants investigated: TS 2R/3R; MS 919D>G; MTHFD1 653R>Q |
| Scher, 2006 (Netherlands)34 |
population | mostly Caucasian | cross-sectional | women+men | 1212 (44) |
413 (NS) |
187 (73) |
226 (76) |
----- |
| Todt, 2006 (Germany)36 |
clinic | Caucasian | case-control | NS | 625 (NS) |
----- | 656 (NS) |
----- | ----- |
| Kaunisto, 2006 (Finland)27 |
clinic+population | Caucasian | case-control | women+men | 900 (75.6; 50.4) |
----- | 898 (81.7; 79.2) |
----- |
Other gene variants investigated: 5 additional in MTHFR gene; 26 in ESR1 gene |
| Bottini, 2006 (Italy)25 |
clinic | Caucasian | case-control | women+men | 66 (57) |
45 (60) |
33 (63.6) |
12 (50) |
Other gene variants investigated: MTHFR 1298A>C; PRT 20210G>A; FVL 1698A>T |
| de Tommaso, 2007 (Italy)7 | clinic | Caucasian | case-control | women+men | 97 (74) |
105 (77) |
----- | ----- | ----- |
| Pezzini, 2007 (Italy)8 |
clinic | Caucasian | case-control | women+men | 105 (63.8) |
206 (NS) |
100 (68) |
106 (72.6) |
----- |
| Schürks, 2008 (US)9 |
population | Caucasian | cross-sectional | women | 20424 (100) |
4577 (100) |
1275 (100) |
1951 (100) |
----- |
| Ferro, 2008 (Portugal)10 |
clinic | Caucasian | case-control | women+men | 96 (NS) |
186 (82) |
78 (NS) |
108 (NS) |
Blood donor group chosen for control, because migraine was excluded. |
| Joshi, 2009 (India)11 |
clinic | North Indian | case-control | women+men, women, men | 150 (67) |
150 (67) |
67 (76) |
83 (59) |
For MTHFR 677C>T the numbers of MA and MO cases do not match the numbers given earlier in paper. Other gene variants investigated: ACE D/I |
| Total number of subjects | 24,578 | 6,446 | 3,645 | 2,899 | |||||
| b) ACE D/I polymorphism | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| ACE D/I polymorphism | |||||||||
| Total study size with genotypic information (women, %) |
|||||||||
| Author, year | Setting | Ethnicity | Study design |
Gender | controls | any migraine |
MA | MO | Comment |
| Paterna, 2000 (Italy)16 | clinic | Caucasian | case-control | women+men | 201 (63.2) |
----- | ----- | 302 (66.2) |
New and larger sample of migraine patients, but same controls as in Paterna, 1997.33 Association of frequency and duration of migraine with ACE D/I also investigated. |
| Cakmak, 2003 (Turkey)17 | NS | Turkish | case-control | women+men, women, men | 231 (NS) |
200 (NS) |
----- | ----- | ----- |
| Lin, 2005 (Taiwan)14 | clinic | Han Chinese | case-control | women+men, women, men | 200 (70.5) |
240 (70.4) |
----- | ----- | ----- |
| Kowa, 2005 (Japan)15 | NS | Japanese | case-control | women+men | 248 (69.4) |
176 (NS) |
54 (68.5) |
122 (84.4) |
----- |
| Lea, 2005 (Australia)13 | NS | Caucasian | case-control | women+men | 244 (NS) |
250 (NS) |
151 (NS) |
99 (NS) |
Other gene variants investigated: MTHFR 677C>T; data are from Lea, 2004.29 |
| Kara, 2007 (Turkey)12 | NS | Turkish | case-control | women+men | 210 (85.7) |
180 (96.1) |
59 (96.7) |
109 (96.3) |
Other gene variants investigated: MMP-3 |
| Tronvik, 2008 (Norway)19 | clinic | Caucasian | case-control | women+men | 403 (57.8) |
347 (77.2) |
155 (NS) |
187 (NS) |
----- |
| Schürks, 2009 (US)18 | population | Caucasian | cross-sectional | women | 20423 (100) |
4577 (100) |
1275 (100) |
1951 (100) |
----- |
| Joshi, 2009 (India)11 | clinic | North Indian | case-control | women+men, women, men | 150 (67) |
150 (67) |
67 (76) |
83 (59) |
Other gene variants investigated: MTHFR 677C>T |
| Total number of subjects | 22,310 | 6,120 | 1,761 | 2,853 | |||||
NS: not specified.
Ten studies investigating the MTHFR 677C>T polymorphism were performed in Caucasians,7–10, 25, 27, 29, 32, 34, 36 one in Japanese,28 one in Turks,26 and one in a north Indians.11 Eleven studies presented results for any migraine,7–11, 25, 26, 28, 29, 32, 34 twelve for MA,8–11, 25–29, 32, 34, 36 and ten for MO.8–11, 25, 26, 28, 29, 32, 34 One study did not specify the genotyping method,36 all the others used standard methods.
Four studies investigating the ACE D/I polymorphism reported on Caucasian populations,13, 16, 18, 19 two on Turks,12, 17 one on Japanese,15 one on Han Chinese,14 and one on north Indians.11 Eight studies presented results for any migraine,11–15, 17–19 six for MA,11–13, 15, 18, 19 and seven for MO.11–13, 15, 16, 18, 19 All studies used standard genotyping methods.
The allele and genotype frequencies for the MTHFR 677C>T and the ACE D/I polymorphisms for migraineurs and controls in each of the studies included are summarized in Supplementary Table 1.
Supplementary Table 2 summarizes for each of the studies the p-value for the Hardy Weinberg-Equilibrium (HWE) in the controls as well as ORs (95% CI) for the association between the MTHFR 677C>T and the ACE D/I polymorphism and migraine assuming additive, dominant, and recessive genetic models.
Association between the MTHFR 677C>T polymorphism and migraine
The pooled effect estimates among all studies suggest that the T allele of the MTHFR 677C>T polymorphism is associated with an increased risk for any migraine (additive mode: pooled OR 1.15; 95% CI 1.00–1.31; p=0.05) (Table 2). The association was most pronounced for carriers of the TT genotype (recessive mode: pooled OR 1.39; 95% CI 1.02–1.90). However, there was moderate heterogeneity across all studies (recessive mode: I2=72%). Ethnicity was a significant source of heterogeneity as determined by meta-regression (recessive mode: p=0.04) and accounted for 34% of the variance across all studies. The positive association was driven by a Turkish26 (recessive mode: pooled OR 6.31; 95% CI 1.31–30.41) and an Asian28 study (recessive mode: pooled OR 2.40; 95% CI 1.19–4.84). However, among Caucasians the results were not significant (recessive mode: pooled OR 1.22; 95% CI 0.92–1.63). Formal investigation using Begg’s test indicated no publication bias, while Egger’s test did suggest some indication for publication bias across all studies (recessive model: p=0.008).
Table 2.
Association between the MTHFR 677C>T polymorphism and migraine, heterogeneity, and publication bias (references are the same for additive, dominant, and recessive models and are only given for the additive models)
| Any migraine | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Genetic model |
Population | No of studies |
Relative Risk (95% CI)* |
Heterogeneity | Publication Bias | ||||
| Q | df | p-value | I2 in % | p-value Begg |
p-value Egger |
||||
| additive | All 7–11, 25, 26, 28, 29, 32, 34 | 11 | 1.15 (1.00–1.31) | 29.2 | 10 | 0.001 | 66 | 0.24 | 0.03 |
| Caucasian 7–10, 25, 29, 32, 34 | 8 | 1.06 (0.95–1.19) | 14.0 | 7 | 0.05 | 50 | 0.62 | 0.15 | |
| Turkish 26 | 1 | 1.80 (1.13–2.87) | ---- | ---- | ---- | ---- | ---- | ---- | |
| Asian 28 | 1 | 1.85 (1.24–2.77) | ---- | ---- | ---- | ---- | ---- | ---- | |
| Indian 11 | 1 | 1.03 (0.64–1.65) | ---- | ---- | ---- | ---- | ---- | ---- | |
| dominant | All 7–11, 25, 26, 28, 29, 32, 34 | 11 | 1.08 (0.96–1.22) | 14.1 | 10 | 0.17 | 29 | 0.24 | 0.09 |
| Caucasian 7–10, 25, 29, 32, 34 | 8 | 1.00 (0.94–1.06) | 5.0 | 7 | 0.66 | 0 | 1.0 | 0.48 | |
| Turkish 26 | 1 | 1.63 (0.95–2.79) | ---- | ---- | ---- | ---- | ---- | ---- | |
| Asian 28 | 1 | 2.06 (1.15–3.70) | ---- | ---- | ---- | ---- | ---- | ---- | |
| Indian 11 | 1 | 1.14 (0.69–1.87) | ---- | ---- | ---- | ---- | ---- | ---- | |
| recessive | All 7–10, 25, 26, 28, 29, 32, 34 | 10* | 1.39 (1.02–1.90) | 32.6 | 9 | <0.0001 | 72 | 0.13 | 0.008 |
| Caucasian 7–10, 25, 29, 32, 34 | 8 | 1.22 (0.92–1.63) | 20.8 | 7 | 0.004 | 66 | 0.32 | 0.08 | |
| Turkish 26 | 1 | 6.31 (1.31–30.41) | ---- | ---- | ---- | ---- | ---- | ---- | |
| Asian 28 | 1 | 2.40 (1.19–4.84) | ---- | ---- | ---- | ---- | ---- | ---- | |
| Indian | 0* | ---- | ---- | ---- | ---- | ---- | ---- | ---- | |
| Migraine with aura | |||||||||
| Genetic model |
Population | No of studies |
Relative Risk (95% CI)* |
Heterogeneity | Publication Bias | ||||
| Q | df | p-value | I2 in % | p-value Begg |
p-value Egger |
||||
| additive | All 8–11, 25–29, 32, 34, 36 | 12 | 1.13 (0.97–1.32) | 42.6 | 11 | <0.0001 | 76 | 0.41 | 0.05 |
| Caucasian 8–10, 25, 27, 29, 32, 34, 36 | 9 | 1.08 (0.93– 1.24) | 28.5 | 8 | <0.0001 | 72 | 0.68 | 0.13 | |
| Turkish 26 | 1 | 1.05 (0.46–2.39) | ---- | ---- | ---- | ---- | ---- | ---- | |
| Asian 28 | 1 | 3.80 (1.88–7.66) | ---- | ---- | ---- | ---- | ---- | ---- | |
| Indian 11 | 1 | 1.06 (0.59–1.90) | ---- | ---- | ---- | ---- | ---- | ---- | |
| dominant | All 8–11, 25–29, 32, 34, 36 | 12 | 1.03 (0.90–1.18) | 19.1 | 11 | 0.06 | 42 | 0.49 | 0.14 |
| Caucasian 8–10, 25, 27, 29, 32, 34, 36 | 9 | 1.01 (0.88–1.14) | 13.5 | 8 | 0.10 | 41 | 0.84 | 0.41 | |
| Turkish 26 | 1 | 0.94 (0.39–2.29) | ---- | ---- | ---- | ---- | ---- | ---- | |
| Asian 28 | 1 | 4.20 (1.21–14.54) | ---- | ---- | ---- | ---- | ---- | ---- | |
| Indian 11 | 1 | 1.17 (0.63–2.20) | ---- | ---- | ---- | ---- | ---- | ---- | |
| recessive | All 8–10, 25–29, 32, 34, 36 | 11* | 1.48 (1.02–2.13) | 51.3 | 10 | <0.0001 | 81 | 0.48 | 0.03 |
| Caucasian 8–10, 25, 27, 29, 32, 34, 36 | 9 | 1.28 (0.91–1.80) | 36.1 | 8 | <0.0001 | 78 | 0.53 | 0.08 | |
| Turkish 26 | 1 | 3.05 (0.27–35.03) | ---- | ---- | ---- | ---- | ---- | ---- | |
| Asian 28 | 1 | 6.54 (2.54–16.81) | ---- | ---- | ---- | ---- | ---- | ---- | |
| Indian | 0* | ---- | ---- | ---- | ---- | ---- | ---- | ---- | |
| Migraine without aura | |||||||||
| Genetic model |
Population | No of studies |
Relative Risk (95% CI)* |
Heterogeneity | Publication Bias | ||||
| Q | df | p-value | I2 in % | p-value Begg |
p-value Egger |
||||
| additive | All 8–11, 25, 26, 28, 29, 32, 34 | 10 | 1.02 (0.89–1.16) | 15.5 | 9 | 0.08 | 42 | 0.33 | 0.70 |
| Caucasian 8–10, 25, 29, 32, 34 | 7 | 1.00 (0.94–1.06) | 5.4 | 6 | 0.5 | 0 | 0.88 | 0.30 | |
| Turkish 26 | 1 | 2.16 (1.28–3.64) | ---- | ---- | ---- | ---- | ---- | ---- | |
| Asian 28 | 1 | 1.38 (0.86–2.19) | ---- | ---- | ---- | ---- | ---- | ---- | |
| Indian 11 | 1 | 1.01 (0.50–1.75) | ---- | ---- | ---- | ---- | ---- | ---- | |
| dominant | All 8–11, 25, 26, 28, 29, 32, 34 | 10 | 1.04 (0.92–1.17) | 10.5 | 9 | 0.31 | 14 | 0.33 | 0.77 |
| Caucasian 8–10, 25, 29, 32, 34 | 7 | 1.02 (0.94–1.11) | 3.9 | 6 | 0.69 | 0 | 0.65 | 0.20 | |
| Turkish 26 | 1 | 1.97 (1.09–3.59) | ---- | ---- | ---- | ---- | ---- | ---- | |
| Asian 28 | 1 | 1.63 (0.85–3.13) | ---- | ---- | ---- | ---- | ---- | ---- | |
| Indian 11 | 1 | 1.11 (0.62–2.00) | ---- | ---- | ---- | ---- | ---- | ---- | |
| recessive | All 8–10, 25, 26, 28, 29, 32, 34 | 9* | 0.94 (0.71–1.23) | 12.0 | 8 | 0.15 | 33 | 0.06 | 0.66 |
| Caucasian 8–10, 25, 29, 32, 34 | 7 | 0.94 (0.82–1.07) | 5.3 | 6 | 0.51 | 0 | 0.65 | 0.52 | |
| Turkish 26 | 1 | 7.44 (1.50–36.84) | ---- | ---- | ---- | ---- | ---- | ---- | |
| Asian 28 | 1 | 1.23 (0.48–3.17) | ---- | ---- | ---- | ---- | ---- | ---- | |
| Indian | 0* | ---- | ---- | ---- | ---- | ---- | ---- | ---- | |
one study is left out of the analysis 11 because there are no effect estimates (no homozygote TT).
Further analyses indicated that the positive association between the MTHFR 677TT genotype and migraine appears only among migraineurs with aura (recessive mode: pooled OR 1.48; 95% CI 1.02–2.13), but not among migraineurs without aura. However, heterogeneity among studies was high (I2=81%) and ethnicity was a significant source (p-value from meta-regression=0.02). The overall result was driven by a study among Asians (recessive mode: OR=6.54; 95% CI 2.54–16.81).28 The result for Caucasians were not statistically significant (recessive mode: pooled OR=1.28; 95% CI 0.91–1.80). For MO only one Turkish study26 suggested a positive association (recessive mode: OR 7.44; 95% CI 1.50–36.84). Results for the overall analysis (recessive mode: pooled OR=0.94; 95% CI 0.71–1.23) and Caucasians (recessive mode: pooled OR=0.94; 0.82–1.07) did not suggest an association.
Association between the ACE D/I polymorphism and migraine
The pooled effect estimates from all studies suggest a trend for a protective association of the I allele with any migraine, which was most pronounced for the II genotype (recessive mode: pooled OR 0.83; 95% CI 0.69–1.01) (Table 3). There was moderate heterogeneity across all studies (recessive mode: I2=58%). Begg’s test and Egger’s test did not suggest publication bias.
Table 3.
Association between the ACE D/I polymorphism and migraine, heterogeneity, and publication bias (references are the same for additive, dominant, and recessive models and are only given for the additive models)
| Any migraine | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Genetic model |
Population | No of studies |
Relative Risk (95% CI) |
Heterogeneity | Publication Bias | ||||
| Q | df | p-value |
I2 in % |
p-value Begg |
p-value Egger |
||||
| additive | All 11–15, 17–19 | 8 | 0.93 (0.86–1.02) | 10.0 | 7 | 0.19 | 30 | 0.05 | 0.08 |
| Caucasian 13, 18, 19 | 3 | 0.99 (0.95–1.03) | 1.0 | 2 | 0.6 | 0 | 0.12 | 0.25 | |
| Turkish 12, 17 | 2 | 0.88 (0.66–1.17) | 2.1 | 1 | 0.15 | 52 | 0.32 | ---- | |
| Asian 14, 15 | 2 | 0.88 (0.60–1.30) | 4.0 | 1 | 0.05 | 75 | 0.32 | ---- | |
| Indian 11 | 1 | 0.82 (0.57–1.17) | ---- | ---- | ---- | ---- | ---- | ---- | |
| dominant | All 11–15, 17–19 | 8 | 1.00 (0.94–1.07) | 4.7 | 7 | 0.70 | 0 | 0.01 | 0.12 |
| Caucasian 13, 18, 19 | 3 | 1.01 (0.95–1.09) | 0.001 | 2 | 1 | 0 | 0.60 | 0.33 | |
| Turkish 12, 17 | 2 | 0.96 (0.73–1.28) | 0.02 | 1 | 0.88 | 0 | 0.32 | ---- | |
| Asian 14, 15 | 2 | 0.80 (0.49–1.32) | 1.8 | 1 | 0.18 | 45 | 0.32 | ---- | |
| Indian 11 | 1 | 0.64 (0.30–1.38) | ---- | ---- | ---- | ---- | ---- | ---- | |
| recessive | All 11–15, 17–19 | 8 | 0.83 (0.69–1.01) | 16.7 | 7 | 0.02 | 58 | 0.05 | 0.12 |
| Caucasian 13, 18, 19 | 3 | 0.88 (0.72–1.08) | 3.6 | 2 | 0.17 | 44 | 0.12 | 0.27 | |
| Turkish 12, 17 | 2 | 0.62 (0.20–1.88) | 7.4 | 1 | 0.006 | 87 | 0.32 | ---- | |
| Asian 14, 15 | 2 | 0.88 (0.53–1.48) | 3.5 | 1 | 0.06 | 71 | 0.32 | ---- | |
| Indian 11 | 1 | 0.84 (0.53–1.35) | ---- | ---- | ---- | ---- | ---- | ---- | |
| Migraine with aura | |||||||||
| Genetic model |
Population | No of studies |
Relative Risk (95% CI) |
Heterogeneity | Publication Bias | ||||
| Q | df | p-value |
I2 in % |
p-value Begg |
p-value Egger |
||||
| additive | All 11–13, 15, 18, 19 | 6 | 0.82 (0.69–0.98) | 11.8 | 5 | 0.04 | 58 | 0.02 | 0.02 |
| Caucasian 13, 18, 19 | 3 | 0.97 (0.90–1.04) | 1.01 | 2 | 0.60 | 0 | 0.12 | 0.23 | |
| Turkish 12 | 1 | 0.75 (0.50–1.15) | ---- | ---- | ---- | ---- | ---- | ---- | |
| Asian 15 | 1 | 0.55 (0.36–0.84) | ---- | ---- | ---- | ---- | ---- | ---- | |
| Indian 11 | 1 | 0.63 (0.40–1.00) | ---- | ---- | ---- | ---- | ---- | ---- | |
| dominant | All 11–13, 15, 18, 19 | 6 | 0.86 (0.67–1.11) | 9.6 | 5 | 0.09 | 48 | 0.04 | 0.1 |
| Caucasian 13, 18, 19 | 3 | 1.02 (0.91–1.14) | 0.07 | 2 | 0.97 | 0 | 0.60 | 0.91 | |
| Turkish 12 | 1 | 0.85 (0.48–1.54) | ---- | ---- | ---- | ---- | ---- | ---- | |
| Asian 15 | 1 | 0.41 (0.20–0.84) | ---- | ---- | ---- | ---- | ---- | ---- | |
| Indian 11 | 1 | 0.44 (0.19–1.06) | ---- | ---- | ---- | ---- | ---- | ---- | |
| recessive | All 11–13, 15, 18, 19 | 6 | 0.71 (0.55–0.93) | 9.2 | 5 | 0.10 | 45 | 0.04 | 0.001 |
| Caucasian 13, 18, 19 | 3 | 0.85 (0.68–1.06) | 2.8 | 2 | 0.25 | 28 | 0.12 | 0.17 | |
| Turkish 12 | 1 | 0.41 (0.15–1.08) | ---- | ---- | ---- | ---- | ---- | ---- | |
| Asian 15 | 1 | 0.49 (0.26–0.95) | ---- | ---- | ---- | ---- | ---- | ---- | |
| Indian 11 | 1 | 0.64 (0.34–1.18) | ---- | ---- | ---- | ---- | ---- | ---- | |
| Migraine without aura | |||||||||
| Genetic model |
Population | No of studies |
Relative Risk (95% CI) |
Heterogeneity | Publication Bias | ||||
| Q | df | p-value |
I2 in % |
p-value Begg |
p-value Egger |
||||
| additive | All 11–13, 15, 16, 18, 19 | 7 | 0.92 (0.86–1.00) | 6.5 | 6 | 0.37 | 8 | 0.88 | 0.17 |
| Caucasian 13, 16, 18, 19 | 4 | 0.92 (0.82–1.03) | 4.3 | 3 | 0.23 | 31 | 0.17 | 0.44 | |
| Turkish 12 | 1 | 0.77 (0.55–1.09) | ---- | ---- | ---- | ---- | ---- | ---- | |
| Asian 15 | 1 | 0.82 (0.60–1.12) | ---- | ---- | ---- | ---- | ---- | ---- | |
| Indian 11 | 1 | 1.01 (0.66–1.57) | ---- | ---- | ---- | ---- | ---- | ---- | |
| dominant | All 11–13, 15, 16, 18, 19 | 7 | 0.95 (0.87–1.04) | 5.6 | 6 | 0.47 | 0 | 0.45 | 0.46 |
| Caucasian 13, 16, 18, 19 | 4 | 0.91 (0.74–1.11) | 5.2 | 3 | 0.16 | 42 | 1.0 | 0.65 | |
| Turkish 12 | 1 | 0.96 (0.60–1.55) | ---- | ---- | ---- | ---- | ---- | ---- | |
| Asian 15 | 1 | 0.77 (0.42–1.44) | ---- | ---- | ---- | ---- | ---- | ---- | |
| Indian 11 | 1 | 0.94 (0.36–2.50) | ---- | ---- | ---- | ---- | ---- | ---- | |
| recessive | All 11–13, 15, 16, 18, 19 | 7 | 0.84 (0.70–0.99) | 8.0 | 6 | 0.24 | 25 | 0.01 | 0.13 |
| Caucasian 13, 16, 18, 19 | 4 | 0.90 (0.81–1.00) | 1.9 | 3 | 0.60 | 0 | 0.04 | 0.26 | |
| Turkish 12 | 1 | 0.35 (0.16–0.77) | ---- | ---- | ---- | ---- | ---- | ---- | |
| Asian 15 | 1 | 0.77 (0.49–1.20) | ---- | ---- | ---- | ---- | ---- | ---- | |
| Indian 11 | 1 | 1.04 (0.60–1.80) | ---- | ---- | ---- | ---- | ---- | ---- | |
Further overall analyses indicated a protective association between the ACE II genotype and both MA (recessive mode: pooled OR 0.71; 95% CI 0.55–0.93) and MO (recessive mode: pooled OR 0.84; 95% CI 0.70–0.99). Heterogeneity among studies was low (MA: I2=45%; MO: I2=25%). Ethnicity appeared to be a significant source of heterogeneity in MA (recessive mode: p-value from meta-regression=0.02), but not in MO. The overall positive result in MA was driven by a study among Asians,15 in MO by a Turkish study.12 The results among Caucasians were not significant.
Sensitivity analyses
For some of our analyses, Galbraith plots identified individual studies as important sources of heterogeneity. We performed sensitivity analyses by excluding studies that fell outside the margin set by the z score ±2 standard deviations.
For all analyses on the association between the MTHFR 677C>T polymorphism and migraine the effect estimates were lower and none indicated a statistically significant association. For example, for the overall association of the MTHFR 677TT genotype with any migraine the pooled OR (95% CI) was 1.06 (0.81–1.38) after excluding four studies.7, 26, 28, 29 Likewise, for the overall association with MA the pooled OR (95% CI) was 1.08 (0.74–1.58) after excluding five studies.8, 9, 28, 29, 34 In addition, for the association with MA among Caucasians the pooled OR (95% CI) was 1.05 (0.71–1.56) after excluding four studies.8, 9, 29, 34 Results from sensitivity analysis for MO were virtually unchanged.
Sensitivity analyses for the association between the ACE D/I polymorphism and migraine did not change our results. For example, for the overall association of the ACE II genotype with any migraine the pooled OR (95% CI) was 0.91 (0.80–1.04) after excluding one study.12 Likewise, for the association with MO the pooled OR (95% CI) was 0.90 (0.81–0.99) after excluding one study.12 The Galbraith plot did not detect single studies as major sources of heterogeneity in MA.
Discussion
The results of this meta-analysis suggest that the MTHFR 677TT genotype is associated with an increased likelihood of having migraine, which only appears for MA. However, there is high heterogeneity among studies and sensitivity analysis did not support an association. While pooled analyses for studies among Caucasians did not show an association, single studies suggested an increased risk for carriers of the TT genotype for MA among Japanese28 and for MO among Turkish migraineurs.26 The ACE II genotype seems to be protective against both MA and MO, a result not changed in sensitivity analyses. While results among Caucasians did not suggest an association, single studies showed significant associations for MA (Asians15) and MO (Turkish12).
A meta-analysis6 summarizing studies on the association between the MTHFR 677C>T polymorphism and migraine until December 2006 found an increased risk among carriers of the TT genotype only for MA. However, five new studies have been published since 2007 dramatically increasing the number of migraineurs and controls with genetic information.7–11 In addition, there are methodological differences to our study. First, two studies with overlapping populations were included.31, 32 We have only considered the more recent and larger study for our analysis.32 Second, only overall results were presented, but not results stratified by ethnicity. However, our data indicate that ethnicity is an important source of heterogeneity across studies (recessive mode: p-value from meta-regression among MA=0.02). While our overall results for MA are similar to the previous meta-analysis (recessive mode: pooled OR=1.48; 95% CI=1.02–2.13), the results for Caucasians were not significant (recessive mode pooled OR=1.28; 95% CI=0.91–1.80). In contrast a single Japanese28 study indicated a >6-fold increased risk for MA. Finally, the previous meta-analysis has excluded a study among adolescents. However, there is no a priori reason to believe that the pathophysiology of migraine differs between adolescents and adults, and populations in other studies also included adolescents.11, 27
Studies investigating the association between the ACE D/I polymorphism and migraine are contradictory;11–19 reasons may include differences in ethnicity and limited sample sizes. The overall results suggested a trend for a protective association of the ACE II genotype with any migraine, which became statistically significant for MA and MO. The likely reason is that not all studies investigated any migraine and also categorized according to MA and MO. One study only investigated MO16 and two only any migraine.14, 17 Based on our overall results, the following pathophysiological association may be sketched: ACE II genotype is associated with reduced risk for MA and MO, because the ACE I allele results in lower ACE levels than the D allele. In addition, healthy controls have lower ACE levels compared with migraineurs with aura.37 However, our data do not support such an association for Caucasians. The association may be different in Turkish12 and Asian15 populations, yet, these results await confirmation in independent cohorts. Further, the ACE D/I polymorphism accounts for only about 50% of ACE activity variation, and elevated ACE activities may for example also be attributable to copy number variations of the ACE gene.
Some limitations need to be considered. First, migraine is biologically heterogeneous. Despite established diagnostic criteria for MA and MO,38 the clinical spectrum among patients is wide, which may be a source of misclassification in all studies. Also, in some studies the distinction between MA and MO relied on a single question.9, 18 Second, methods of migraine diagnosis differed between studies. Most studies used ICHD-1/ICHD-2 criteria, others employed self-administered questionnaires,9, 18 or combined methods.34 Two did not explicitly mention the criteria used.16, 36 While clinical diagnosis constitutes the gold standard, questionnaire based population-based studies have proven successful in reaching a valid migraine diagnosis.5, 39, 40 Third, we acknowledged ethnicity as an important source of heterogeneity in the association between both polymorphisms and migraine. Nevertheless, regarding the MTHFR 677C>T polymorphism, residual heterogeneity among Caucasians especially for MA was still high (I2=78%). This agrees with results from the previous meta-analysis (I2=73%).6 Our results did not indicate that age was a significant source of heterogeneity for both gene variants (data not shown). Regarding gender, there is no a priori reason to believe that migraine pathophysiology differs between women and men. Further, while there were too few studies on the MTHFR 677C>T polymorphism to assess an impact of gender on heterogeneity, our data on the ACE D/I polymorphism do not support this (data not shown). Hence, the effect estimates carry further unidentified sources of heterogeneity. In addition, the single results among Japanese28 and Turks26 suggesting a strong association of the MTHFR 677TT genotype with MA and MO await replication. Fourth, we only used extractable data from the papers. However, all papers provided information to determine genotype frequencies. Only one study of limited sample size investigating the MTHFR 677C>T7 and two studies investigating the ACE D/I polymorphism14, 17 did not present genotypic information stratified by aura status. Finally, two studies investigated both the MTHFR 677C>T and ACE D/I polymorphism in the same cohort.11, 13 However, since we did not have information on the genotypic distribution for both variants combined, we were not able to investigate for potential gene-gene interaction.
Additional research is warranted to further delineate the association between the two genetic variants and migraine, in particular among non-Caucasian populations. Future studies need to be adequately powered, should use standardized migraine classification including aura status, and should also present results stratified by gender and migraine aura status. Further, gene-gene interactions should be investigated, even if individual gene variants do not suggest an association with migraine. Finally, other than migraine status, age at onset or markers of migraine severity including attack frequency and aura frequency may be better outcomes.
Supplementary Material
Acknowledgments
Funding and Support
There was no specific funding to conduct this study.
Abbreviations
- MTHFR
Methylenetetrahydrofolate reductase
- ACE
Angiotensin-converting enzyme
- MA
Migraine with aura
- MO
Migraine without aura
- MESH
Medical Subject Headings
- OR
Odds ratio
- CI
Cconfidence interval
- HWE
Hardy Weinberg-Equilibrium
Footnotes
Full Disclosures for the last 5 years
Dr. Schürks has received an investigator-initiated research grant from the Deutsche Forschungsgemeinschaft and honoraria from L.E.K. Consulting for telephone surveys.
P. Rist has no disclosures.
Dr. Kurth has received investigator-initiated research funding from McNeil Consumer & Specialty Pharmaceuticals, Merck, the National Institutes of Health, and Wyeth Consumer Healthcare; he is a consultant to i3 Drug Safety and to World Health Information Science Consultants, LLC; and he received honoraria from Genzyme, Merck, and Pfizer for educational lectures.
References
- 1.Haut SR, Bigal ME, Lipton RB. Chronic disorders with episodic manifestations: focus on epilepsy and migraine. Lancet Neurol. 2006;5:148–157. doi: 10.1016/S1474-4422(06)70348-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pietrobon D, Striessnig J. Neurobiology of migraine. Nat Rev Neurosci. 2003;4:386–398. doi: 10.1038/nrn1102. [DOI] [PubMed] [Google Scholar]
- 3.Tietjen EG. Migraine and ischaemic heart disease and stroke: potential mechanisms and treatment implications. Cephalalgia. 2007;27:981–987. doi: 10.1111/j.1468-2982.2007.01407.x. [DOI] [PubMed] [Google Scholar]
- 4.Vanmolkot FH, Van Bortel LM, de Hoon JN. Altered arterial function in migraine of recent onset. Neurology. 2007;68:1563–1570. doi: 10.1212/01.wnl.0000260964.28393.ed. [DOI] [PubMed] [Google Scholar]
- 5.Kurth T, Gaziano JM, Cook NR, Logroscino G, Diener HC, Buring JE. Migraine and risk of cardiovascular disease in women. JAMA. 2006;296:283–291. doi: 10.1001/jama.296.3.283. [DOI] [PubMed] [Google Scholar]
- 6.Rubino E, Ferrero M, Rainero I, Binello E, Vaula G, Pinessi L. Association of the C677T polymorphism in the MTHFR gene with migraine: a meta-analysis. Cephalalgia. 2009;29:818–825. doi: 10.1111/j.1468-2982.2007.01400.x. [DOI] [PubMed] [Google Scholar]
- 7.de Tommaso M, Difruscolo O, Sardaro M, et al. Influence of MTHFR genotype on contingent negative variation and MRI abnormalities in migraine. Headache. 2007;47:253–265. doi: 10.1111/j.1526-4610.2006.00690.x. [DOI] [PubMed] [Google Scholar]
- 8.Pezzini A, Grassi M, Del Zotto E, et al. Migraine mediates the influence of C677T MTHFR genotypes on ischemic stroke risk with a stroke-subtype effect. Stroke. 2007;38:3145–3151. doi: 10.1161/STROKEAHA.107.491506. [DOI] [PubMed] [Google Scholar]
- 9.Schürks M, Zee RY, Buring JE, Kurth T. Interrelationships among the MTHFR 677C>T polymorphism, migraine, and cardiovascular disease. Neurology. 2008;71:505–513. doi: 10.1212/01.wnl.0000316198.34558.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferro A, Castro MJ, Lemos C, et al. The C677T polymorphism in MTHFR is not associated with migraine in Portugal. Dis Markers. 2008;25:107–113. doi: 10.1155/2008/178679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joshi G, Pradhan S, Mittal B. Role of the ACE ID and MTHFR C677T polymorphisms in genetic susceptibility of migraine in a north Indian population. J Neurol Sci. 2009;277:133–137. doi: 10.1016/j.jns.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Kara I, Ozkok E, Aydin M, et al. Combined effects of ACE and MMP-3 polymorphisms on migraine development. Cephalalgia. 2007;27:235–243. doi: 10.1111/j.1468-2982.2006.01269.x. [DOI] [PubMed] [Google Scholar]
- 13.Lea RA, Ovcaric M, Sundholm J, Solyom L, Macmillan J, Griffiths LR. Genetic variants of angiotensin converting enzyme and methylenetetrahydrofolate reductase may act in combination to increase migraine susceptibility. Brain Res Mol Brain Res. 2005;136:112–117. doi: 10.1016/j.molbrainres.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Lin JJ, Wang PJ, Chen CH, Yueh KC, Lin SZ, Harn HJ. Homozygous deletion genotype of angiotensin converting enzyme confers protection against migraine in man. Acta Neurol Taiwan. 2005;14:120–125. [PubMed] [Google Scholar]
- 15.Kowa H, Fusayasu E, Ijiri T, et al. Association of the insertion/deletion polymorphism of the angiotensin I-converting enzyme gene in patients of migraine with aura. Neurosci Lett. 2005;374:129–131. doi: 10.1016/j.neulet.2004.10.041. [DOI] [PubMed] [Google Scholar]
- 16.Paterna S, Di Pasquale P, D'Angelo A, et al. Angiotensin-converting enzyme gene deletion polymorphism determines an increase in frequency of migraine attacks in patients suffering from migraine without aura. Eur Neurol. 2000;43:133–136. doi: 10.1159/000008151. [DOI] [PubMed] [Google Scholar]
- 17.Cakmak EA, Cataloluk O, Yoldas T, et al. Migraine and angiotensin-converting enzyme association in Turkish patients. Pain Clinic. 2003;15:473–477. [Google Scholar]
- 18.Schürks M, Zee RYL, Buring JE, Kurth T. ACE D/I Polymorphism, Migraine, and Cardiovascular Disease in Women. Neurology. 2009;72:650–656. doi: 10.1212/01.wnl.0000342517.97178.f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tronvik E, Stovner LJ, Bovim G, et al. Angiotensin-converting enzyme gene insertion/deletion polymorphism in migraine patients. BMC Neurol. 2008;8:4. doi: 10.1186/1471-2377-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sagoo GS, Little J, Higgins JP. Systematic reviews of genetic association studies. PLoS Med. 2009;6:e28. doi: 10.1371/journal.pmed.1000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 23.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bassi B, Parodi E, Messina M, et al. Screening for genetic and acquired thrombophilia in a cohort of young migrainous patients. J Headache Pain. 2003;4:138–145. [Google Scholar]
- 25.Bottini F, Celle ME, Calevo MG, et al. Metabolic and genetic risk factors for migraine in children. Cephalalgia. 2006;26:731–737. doi: 10.1111/j.1468-2982.2006.01107.x. [DOI] [PubMed] [Google Scholar]
- 26.Kara I, Sazci A, Ergul E, Kaya G, Kilic G. Association of the C677T and A1298C polymorphisms in the 5,10 methylenetetrahydrofolate reductase gene in patients with migraine risk. Brain Res Mol Brain Res. 2003;111:84–90. doi: 10.1016/s0169-328x(02)00672-1. [DOI] [PubMed] [Google Scholar]
- 27.Kaunisto MA, Kallela M, Hamalainen E, et al. Testing of variants of the MTHFR and ESR1 genes in 1798 Finnish individuals fails to confirm the association with migraine with aura. Cephalalgia. 2006;26:1462–1472. doi: 10.1111/j.1468-2982.2006.01228.x. [DOI] [PubMed] [Google Scholar]
- 28.Kowa H, Yasui K, Takeshima T, Urakami K, Sakai F, Nakashima K. The homozygous C677T mutation in the methylenetetrahydrofolate reductase gene is a genetic risk factor for migraine. Am J Med Genet. 2000;96:762–764. doi: 10.1002/1096-8628(20001204)96:6<762::aid-ajmg12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 29.Lea RA, Ovcaric M, Sundholm J, MacMillan J, Griffiths LR. The methylenetetrahydrofolate reductase gene variant C677T influences susceptibility to migraine with aura. BMC Med. 2004;2:3. doi: 10.1186/1741-7015-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magis D, Allena M, Coppola G, Di Clemente L, Gerard P, Schoenen J. Search for correlations between genotypes and electrophysiological patterns in migraine: the MTHFR C677T polymorphism and visual evoked potentials. Cephalalgia. 2007;27:1142–1149. doi: 10.1111/j.1468-2982.2007.01412.x. [DOI] [PubMed] [Google Scholar]
- 31.Oterino A, Valle N, Bravo Y, et al. MTHFR T677 homozygosis influences the presence of aura in migraineurs. Cephalalgia. 2004;24:491–494. doi: 10.1111/j.1468-2982.2004.00692.x. [DOI] [PubMed] [Google Scholar]
- 32.Oterino A, Valle N, Pascual J, et al. Thymidylate synthase promoter tandem repeat and MTHFD1 R653Q polymorphisms modulate the risk for migraine conferred by the MTHFR T677 allele. Brain Res Mol Brain Res. 2005;139:163–168. doi: 10.1016/j.molbrainres.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 33.Paterna S, Di Pasquale P, Cottone C, et al. Migraine without aura and ACE-gene deletion polymorphism: is there a correlation? Preliminary findings. Cardiovasc Drugs Ther. 1997;11:603–604. doi: 10.1023/a:1007704324135. [DOI] [PubMed] [Google Scholar]
- 34.Scher AI, Terwindt GM, Verschuren WM, et al. Migraine and MTHFR C677T genotype in a population-based sample. Ann Neurol. 2006;59:372–375. doi: 10.1002/ana.20755. [DOI] [PubMed] [Google Scholar]
- 35.Tietjen GE, Herial NA, Utley C, White L, Yerga-Woolwine S, Joe B. Association of von Willebrand factor activity with ACE I/D and MTHFR C677T polymorphisms in migraine. Cephalalgia. 2009 doi: 10.1111/j.1468-2982.2008.01824.x. [DOI] [PubMed] [Google Scholar]
- 36.Todt U, Freudenberg J, Goebel I, et al. MTHFR C677T polymorphism and migraine with aura. Ann Neurol. 2006;60:621–622. doi: 10.1002/ana.20911. author reply 622–623. [DOI] [PubMed] [Google Scholar]
- 37.Fusayasu E, Kowa H, Takeshima T, Nakaso K, Nakashima K. Increased plasma substance P and CGRP levels, and high ACE activity in migraineurs during headache-free periods. Pain. 2007;128:209–214. doi: 10.1016/j.pain.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 38.The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24 Suppl 1:9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 39.Launer LJ, Terwindt GM, Ferrari MD. The prevalence and characteristics of migraine in a population-based cohort: the GEM study. Neurology. 1999;53:537–542. doi: 10.1212/wnl.53.3.537. [DOI] [PubMed] [Google Scholar]
- 40.Lipton RB, Stewart WF, Diamond S, Diamond ML, Reed M. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache. 2001;41:646–657. doi: 10.1046/j.1526-4610.2001.041007646.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.