Abstract
Hypertension, hypercholesterolemia, diabetes and obesity are among a growing list of conditions that have been designated as major risk factors for cardiovascular disease (CVD). While CVD risk factors are well known to enhance the development of atherosclerotic lesions in large arteries, there is also evidence that the structure and function of microscopic blood vessels can be profoundly altered by these conditions. The diverse responses of the microvasculature to CVD risk factors include oxidative stress, enhanced leukocyte- and platelet-endothelial cell adhesion, impaired endothelial barrier function, altered capillary proliferation, enhanced thrombosis, and vasomotor dysfunction. Emerging evidence indicates that a low-grade systemic inflammatory response that results from risk factor-induced cell activation and cell-cell interactions may underlie the phenotypic changes induced by risk factor exposure. A consequence of the altered microvascular phenotype and systemic inflammatory response is an enhanced vulnerability of tissues to the deleterious effects of secondary oxidative and inflammatory stresses, such as ischemia and reperfusion. Future efforts to develop therapies that prevent the harmful effects of risk factor-induced inflammation should focus on the microcirculation.
Keywords: Hypertension, obesity, diabetes, hypercholesterolemia, oxidative stress, immune cells
Introduction
The morbidity and mortality of many human diseases are linked to either acute or chronic inflammation. While inflammation often reflects a localized response to bacterial infection, trauma or neoplasia, a generalized or systemic inflammatory response can result when inflammatory mediators produced by an infected or injured tissue gain access to the blood stream, and subsequently activate the innate and adaptive immune systems. Whether localized or systemic, the inflammatory response appears to be a defensive measure to eliminate the inciting agent, whose precise identity and mechanisms of action are often unknown.
There is mounting evidence that the established risk factors for cardiovascular disease, i.e., hypertension, hypercholesterolemia, diabetes, obesity, and cigarette smoke, elicit a systemic inflammatory response that underlies much of the vascular pathology that is associated with these conditions. Although these risk factors induce very similar inflammatory and functional changes in blood vessels, it remains unclear whether the shared risk factor-induced vascular phenotype reflects common mechanisms through which the immune system is activated and whether the resultant inflammatory response reflects a defensive effort by the immune system to rid of inciting agents that are directly linked to the risk factor. [30, 34, 91]
The deleterious effects of CV risk factors are manifested in both macroscopic and microscopic blood vessels, and the endothelium lining the walls of these vessels appear to be the major cellular target for the risk factor-induced pathology. Risk factor-induced, endothelium-dependent vascular dysfunction is observed early in the microcirculation, and is evident in all vascular beds. However, the microcirculation may not only serve as a mere target for the deleterious effects of risk factors. Inflammatory events linked to risk factor-induced endothelial cell activation in the microcirculation, with its very large surface area, have been implicated in the initiation and/or progression of large vessel disease. As both a target and source of the inflammatory response induced by CV risk factors, a focus on the microcirculation for the development of therapies that preserve the salutary, while preventing the harmful, effects of risk factor-induced inflammation is well justified. [30, 34, 86, 88, 91]
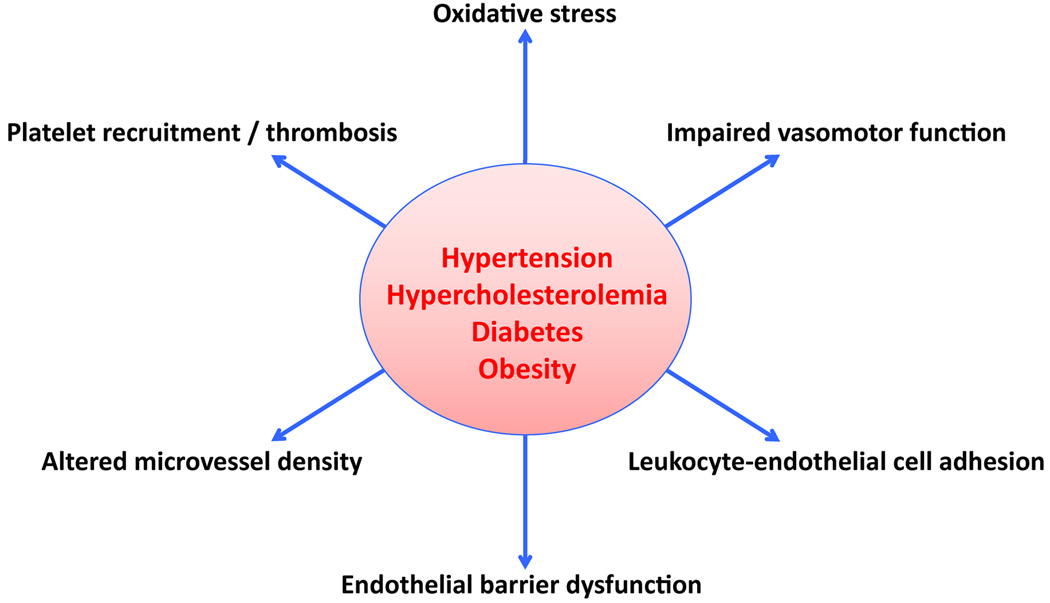
The overall objectives of this review are to summarize the responses of the microvasculature to CV risk factors (Figure 1) that are consistent with the induction of an inflammatory phenotype and to address the potential mechanisms that underlie these responses. Endothelium-dependent responses that are typically associated with inflammation, such as enhanced production of reactive oxygen species (ROS), impaired vasomotor function, leukocyte- and platelet-endothelial cell adhesion and endothelial barrier dysfunction, are addressed. In view of recent evidence linking both angiogenesis and thrombosis to inflammation, a brief discussion of these microvascular responses to CV risk factors is also included. The pathophysiological consequences of microvascular inflammation is also considered within the context of whether risk factors render tissues more vulnerable to damage induced by ischemia/reperfusion.
Figure 1.
Microvascular responses to cardiovascular risk factors.
Oxidative stress
An imbalance between the production and detoxification of ROS in vascular endothelial cells can result in the oxidative modification of cell components, impair cell function and/or can enhance cell death via apoptosis or necrosis. The oxidative activation of enzymes (e.g. phospholipase A2) and transcription factors (e.g. nuclear factor kB, NFkB) that accompanies excess ROS production can also result in an enhanced biosynthesis of lipids (e.g., platelet activating factor, leukotrienes) and proteins (adhesion molecules, cytokines) that promote inflammation. Superoxide, by virtue of its ability to inactivate nitric oxide (an anti-inflammatory molecule), is another link between oxidative stress and the induction of a pro-inflammatory phenotype in the vasculature.
There is evidence that vascular ROS production is enhanced by all of the major CV risk factors. This oxidative stress in the vessel wall is often accompanied by an increased production of superoxide anion by circulating immune cells, and there is evidence for a causal link between these two sources (circulating cells & vessel wall) of ROS. Different enzymatic sources have been implicated in the enhanced ROS production, including NADPH oxidase, xanthine oxidase, mitochondrial enzymes, and uncoupled nitric oxide synthase. A lower production of NO, which can scavenge the superoxide anion, has also been proposed as a mechanism of the enhanced ROS fluxes. [84, 88]
Many animal models of hypertension (HTN), including spontaneously hypertensive rats, chronic angiotensin II infusion and salt-retention (e.g. DOCA-salt) models, are associated with enhanced ROS production. While the elevated microvascular pressure associated with hypertension is largely confined to the arterial segment of the vascular tree, the increased oxidative stress induced by this condition is evident in both arterioles and postcapillary venules. The pathophysiological relevance of the increased ROS fluxes is evidenced by the anti-hypertensive actions of genetic SOD overexpression or SOD mimetic treatment in these models. It remains unclear whether the pro-hypertensive effects of the superoxide anion relate to its ability to inactivate NO or to indirectly promote the production of endogenous vasoconstrictors, such as endothelin. NADPH oxidase has received the most attention as a potential source of ROS in HTN, followed by xanthine oxidase. Both endothelial cell- and leukocyte-associated NADPH oxidase have been implicated in HTN-induced superoxide production, and there is evidence linking both cellular sources of the enzyme to activation of the angiotensin II type 1 receptor (AT1r) and to cytokines (TNF-alpha) derived from circulating immune cells. [16, 33, 88, 96]
Hypercholesterolemia (HCh) is also accompanied by increased ROS production in both arterioles and venules. This response can be completely reversed by dietary correction of the HCh. Endothelial cell denudation significantly reduces HCh-induced superoxide formation, suggesting that these cells account for most of the production. The vessel wall oxidative stress is not observed in either immunodeficient mice or in mice that are genetically deficient in interferon-γ. Since adoptive transfer of wild type T-lymphocytes into IFN-γ−/− mice restores the oxidative stress induced by HCh, it is proposed that T-cell derived IFN-γ mediates this response. Both NADPH oxidase (endothelial cell and leukocyte) and xanthine oxidase (endothelial cell) are proposed sources of the accelerated superoxide production rates. Oxidatively modified lipoproteins (e.g., oxidized LDL), which are elevated in blood during HCh, have been implicated in different HCh-induced responses, including inflammation and eNOS uncoupling. [16, 82, 85]
The mechanisms that contribute to the oxidative stress in obesity appear to be site specific. Vascular wall NADPH oxidase, which is presumably activated by the elevated blood levels of cytokines (adipokines) released from adipose tissue, has been implicated in the peripheral vascular oxidative stress in obese animals. However, mitochondrial uncoupling (caused by the processing of excess fatty acids) has also received much attention as a cause of the oxidative stress experienced by growing adipose tissue. The pro-oxidative environment in adipocytes that results from mitochondrial uncoupling is amplified by the enhanced ROS production that is linked to endoplasmic reticulum (ER)stress. Enhanced ROS production in adipose tissue is reflected in the accumulation of malondialdehyde and conjugated dienes, common surrogate markers of oxidative stress. [31, 91]
Hyperglycemia is widely viewed as a major stimulus for the oxidative stress associated with diabetes. Enhanced cellular uptake of glucose in insulin-independent tissues ultimately enhances oxidant production and impairs antioxidant defenses. Hyperglycemia increases superoxide production via glucose oxidase as well as NADPH oxidase, which is linked to both the generation of advanced glycosylation end-products (AGE) and protein kinase C activation. Mitochondria are the dominant site of the enhanced superoxide fluxes associated with hyperglycemia. Adiponectin, a cytokine derived from adipocytes, inhibits the increased ROS production by endothelial cells exposed to hyperglycemia via a cyclic AMP/protein kinase A pathway. [16, 44, 60, 78]
Impaired vasomotor function
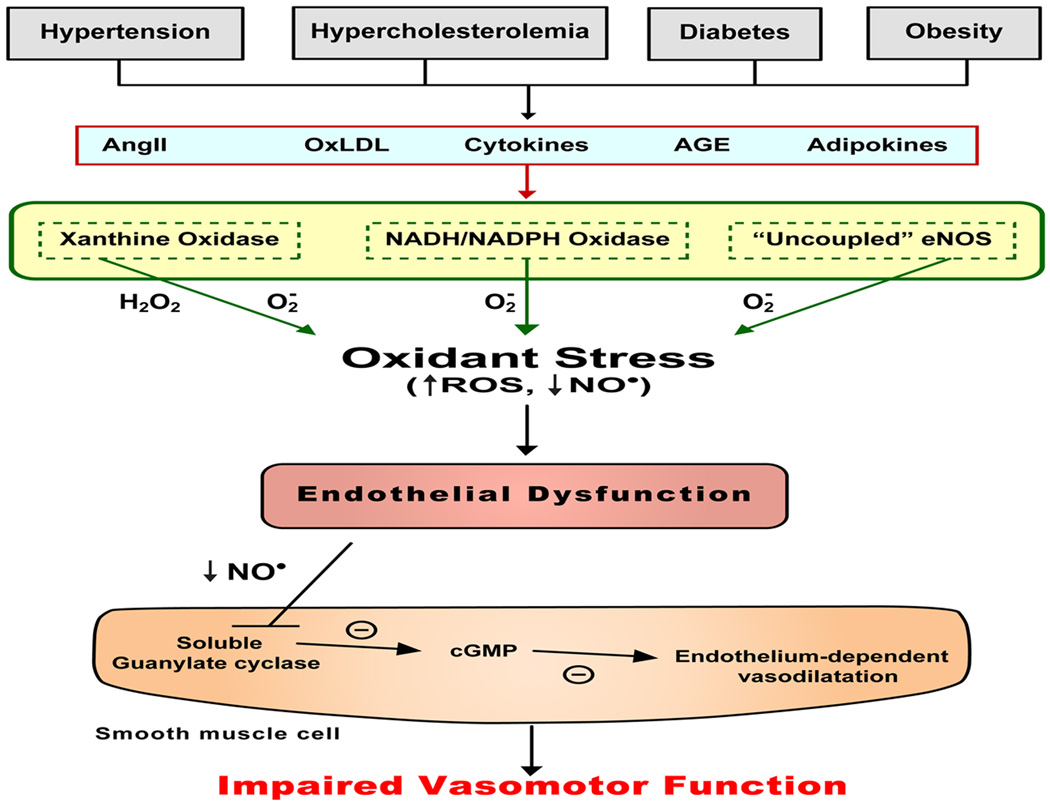
A well-characterized response of the vasculature to CV risk factors is impaired endothelium-dependent vasodilation (EDV) mediated by either pharmacological (e.g., acetylcholine) or physiological (increased flow) stimuli. This response is widely considered to be an early and sensitive indicator of the endothelial cell dysfunction in arterioles and arteries that accompanies a variety of pathological conditions. Impaired EDV has also gained acceptance as an early marker for the development of atherosclerosis and can be detected before structural changes to the vessel wall are evident with angiography or ultrasound. EDV has been linked to an altered bioavailability of endothelial cell derived nitric oxide, either resulting from diminished NO production and/or inactivation by superoxide (Figure 2). The systemic low-grade inflammatory state induced by CV risk factors appears to predispose arterial endothelial cells to both oxidative and nitrosative stresses that can lead to impaired EDV. [10, 17, 42, 92]
Figure 2.
Oxidant-dependent mechanism underlying the impaired vasomotor function induced by cardiovascular risk factors. AngII = angiotensin II, oxLDL = oxidized low density lipoprotein, AGE = advanced glycosylation end-products, H2O2 = hydrogen peroxide, O2- = superoxide, ROS = reactive oxygen species, NO = nitric oxide, cGMP = cyclic GMP.
Many of the cardiovascular complications associated with hypertension are assignable, at least in part, to endothelial cell activation and vasomotor dysfunction. While reduced NO bioactivity has been ascribed a key role in HTN-induced vasomotor dysfunction, the receptor-dependent and independent mechanisms that underlie the reduced NO levels have not been resolved and remain an area of intense investigation. Receptors for endothelin-1 and angiotensin II have been implicated in the impaired EDV of HTN, providing the basis for an improved tonic basal release of NO and lower vascular tone in hypertensive patients treated with an ACE inhibitor or calcium antagonist. Some of the benefit may result from the ability of these drugs to blunt the oxidative stress of HTN. There is also evidence implicating a role for immune cells in the impaired EDV in HTN. It has recently been proposed that AT1r-mediated T-lymphocyte activation leads to the release of TNF-α from T-cells, and the cytokine subsequently induces a pro-oxidative environment in endothelial cells, leading to impaired endothelium-dependent vasomotor function and hypertension. [33, 80]
Blood lipid levels also exert an influence on endothelium-dependent vasomotor tone, as evidenced by studies reporting impaired EDV within 2–4 hours after ingestion of a lipid meal and the ability of cholesterol-lowering statins to restore EDV in patients with coronary artery disease. Enhanced superoxide production in endothelial cells, mediated by either NADPH oxidase or xanthine oxidase, has been implicated in the HCh-induced impairment of EDV. A role for platelet-associated NADPH oxidase has also been proposed. Immune cell involvement in this response is indicated by the protection against HCh-induced arteriolar dysfunction observed in mice that either lack lymphocytes or genetically deficient in interferon-γ or either component of the CD40/CD40L dyad. [82, 83, 87, 94]
Oxidative stress, resulting from hyperglycemia and insulin resistance, has been implicated in the impaired vasomotor function associated with diabetes. The p47phox and gp91phox protein subunits of NADPH oxidase as well as the AT1r receptor are upregulated in diabetic vessels compared to non-diabetic controls. The pathophysiological relevance of these changes is evidenced by the ability of an AT1r antagonist or NADPH oxidase inhibitor to prevent the impaired NO-dependent vasodilation in diabetic animals. Other factors that have been implicated in the diabetes induced vasomotor dysfunction include cytokines, cyclooxygenase products, AGE, and endothelin. [4, 81]
Obese subjects and animal models of obesity are also characterized by an impairment of EDV. Since obesity is often accompanied by other conditions such as insulin resistance and/or hypertension, it is difficult to clearly distinguish the influence of excess fat deposition from its comorbidities. However, there is evidence that alterations in plasma adipokine levels, namely leptin and adiponectin, can reproduce the impaired EDV that accompanies obesity. [77, 81]
Leukocyte-endothelial cell adhesion (LECA)
Patients with essential hypertension exhibit enhanced LECA, reflected in an increased expression of adhesion molecules on leukocytes and a resultant increase in adhesivity to vascular endothelial cells, which is not observed following treatment with an AT1r antagonist. Animal studies have also revealed an ability of angiotensin II (AngII) to promote LECA in the microvasculature via an AT1-dependent mechanism. It has been proposed that engagement of the AT1r results in oxidant-mediated NFkB activation and the subsequent induction of redox-sensitive genes for certain endothelial CAMs (eg, VCAM-1), as well as the mobilization of preformed endothelial P-selectin (Figure 3). Ang II can also engage the AT1 receptors on leukocytes to promote β2-integrin upregulation. A role for blood pressure has also been implicated based on the observation that leukocyte adhesion, NFkB activation and endothelial CAM expression are significantly elevated (along with blood pressure) proximal to the site of aortic constriction in rats, with no changes noted distal to the constriction site, and despite a 10-fold increase circulating AngII concentration. A similar dependency of CAM expression and LECA on intravascular pressure has been demonstrated using in vitro models. However, there is evidence that the spontaneously hypertensive rat is protected against the leukocyte adhesion observed in other models, most likely resulting from a glucocorticoid-dependent, selectin mediated mechanism. [2, 30, 55, 69, 88,95]
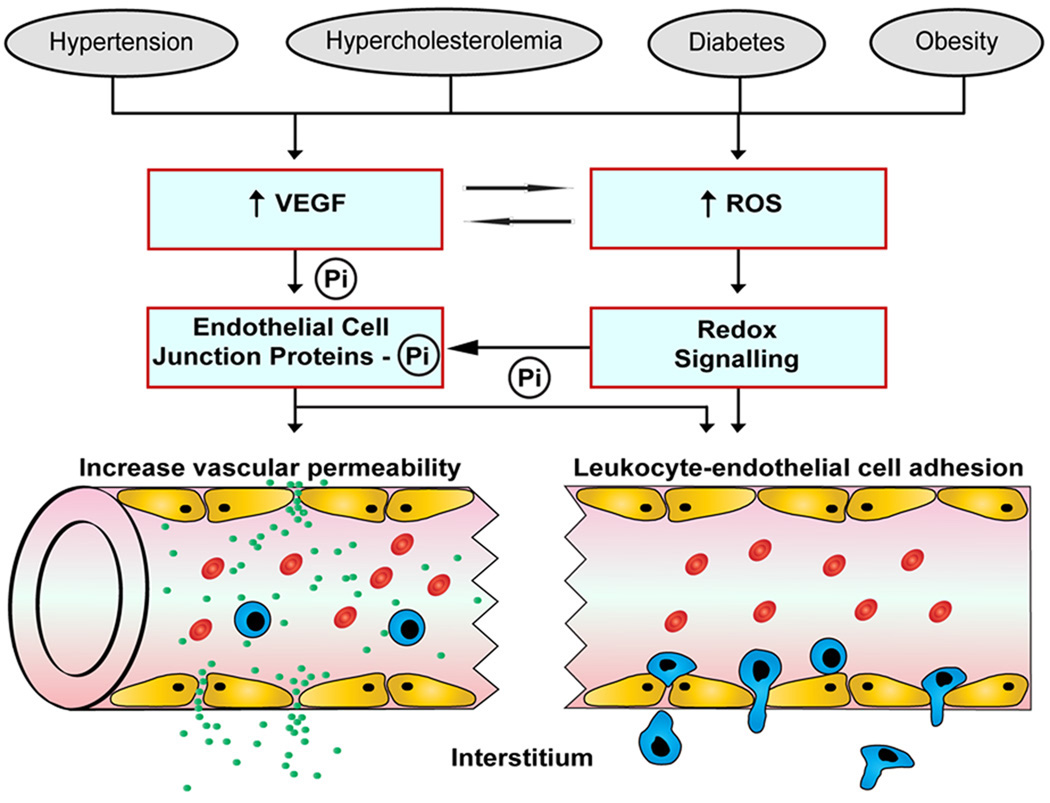
Figure 3.
Mechanisms implicated in the diminished endothelial barrier function and enhanced leukocyte-endothelial cell adhesion associated with cardiovascular risk factors. Elevated levels of vascular endothelial growth factor (VEGF) and reactive oxygen species (ROS) result in the phosphorylation and subsequently disassembly of endothelial junctional proteins, which leads to open interendothelial junctions and an enhanced permeation of solutes and transendothelial migration of leukocytes. Transcription-dependent and –independent redox signaling events also promote leukocyte recruitment by increasing the expression of endothelial cell adhesion molecules. Pi, phosphate.
The increased LECA that accompanies diabetes has been linked to both hyperglycemia and oxidative stress. Acute increases in ambient glucose comparable to those seen in diabetic patients elicits an increased rolling, firm adhesion and emigration of leukocytes in postcapillary venules, and an increased expression of endothelial P-selectin. These responses are markedly blunted by treatment with either insulin, superoxide dismutase, or a protein kinase C inhibitor. Serum from diabetic patients enhances the adhesion of leukocytes to cultured endothelial cells and this response can be mimicked by exposure of the endothelial cells to advanced glycosylation end product-albumin. In this regard, it is noteworthy that RAGE (receptor for AGE) has been proposed to function as an endothelial adhesion receptor that binds to the leukocyte β2-integrin to promote leukocyte–endothelial cell adhesion. [7, 8, 14, 54, 77]
Hypercholesterolemia is associated with LECA in both large and microscopic blood vessels. A robust adhesion response is noted in postcapillary venules within 2 weeks after placing animals on a cholesterol-enriched diet. The rolling, adherent and emigrating leukocytes detected at this stage of HCh are largely neutrophils. NADPH oxidase-derived superoxide has been implicated in the HCh-induced leukocyte recruitment, along with T-lymphocyte-derived interferon-γ(IFN-γ) and the CD40/CD40L dyad. Studies in bone marrow chimeras created from IFN-γ- CD40- or CD40L-deficient mice have revealed a role for both circulating cells and vascular wall/extravascular cells as potential sources of the IFN-γ and CD40/CD40L that mediates the HCh-induced microvascular responses. Little attention has been devoted to identifying the specific cell populations involved. It has been proposed that T-cell derived cytokines may contribute to the activation of NADPH oxidase, with the resultant oxidative stress eliciting an upregulation of adhesion molecules that mediate the HCh-induced LECA. In the absence of AT1r on leukocytes, HCh does not elicit the usual LECA response, suggesting that AT1r activation is a critical component of the oxidant- and immune cell-dependent mechanism underlying HCh-induced vascular inflammation. [64, 77, 82, 83, 88]
While some adipokines have been shown to promote the expression of endothelial CAMs and LECA (e.g., leptin), others (e.g., adiponectin) are known to exert an inhibitory effect on these responses. The absence of LECA in the microcirculation of obese mice under basal conditions suggests either that the pro- and anti-adhesive adipokines are in balance or that the systemic plasma levels achieved by these mediators do not cause overt inflammation in tissues distant from their source (adipose tissue). The latter possibility is supported by evidence of an increased sensitivity (priming) of endothelial cells and leukocytes in obese animals to inflammatory stimuli. However, within the microvasculature of adipose tissue, a robust inflammatory response is noted under basal conditions, as reflected by an increased expression of the endothelial cell adhesion molecules ICAM-1 and E- and P-select in, with an accompanying recruitment of rolling and firmly adherent leukocytes, and the formation of platelet-leukocyte aggregates. The reduced LECA may be linked to adipnectin deficiency since the adipokine is a potent inhibitor of LECA and its production/release is diminished during adipogenesis. [56, 57, 77, 91]
Endothelial barrier dysfunction
The endothelial barrier plays an important role in the partitioning of fluid and protein between the plasma and interstitial compartments. Impaired barrier function alters extracellular fluid volume homeostasis by allowing the egress of plasma into the interstitial compartment. Interstitial edema, a consequence of endothelial barrier dysfunction, is a characteristic feature of most acute and chronic inflammatory conditions. In view of this link between inflammation and endothelial barrier function, it is not surprising that the CV risk factors are typically associated a state of hyperpermeability.
Patients with essential hypertension and animal models of HTN exhibit a state of vascular hyperpermeability that parallels other indices of endothelial cell dysfunction. This endothelial barrier dysfunction can be life-threatening, as evidenced by the blood brain barrier disruption, cerebral edema, and ischemia associated with hypertensive encephalopathy. While the barrier dysfunction associated with HTN may reflect a pressure-induced injury response, there is evidence linking the permeability response to angiotensin II receptor activation, oxidative stress, and inflammation. Angiotensin II can increase vascular permeability either via the release of reactive oxygen species, activation of redox-sensitive transcription factors (NFkB, AP-1, HIF-1) and the subsequent production/secretion of VEGF and prostaglandins. A role for the renin-angiotensin system is supported by reports describing the beneficial effects of treatment with angiotensin-converting enzyme (ACE) inhibitors or AT1 receptor blockers on the proteinuria elicited in patients with HTN. Studies of the coronary microcirculation suggest that nitric oxide may mediate the enhanced protein extravasation induced by angiotensin-II. [15, 76, 90, 93, 99]
Hypercholesterolemia is also associated with impaired endothelial barrier function. Oxidative stress, VEGF, and endothelin-1 have been implicated in the HCh-induced permeability response. Statin treatment has been shown to normalize the exaggerated transvascular albumin leakage observed in patients with hypercholesterolemic atherosclerosis, and this protection appears to be unrelated to changes in blood lipids. It remains unclear whether the barrier protection afforded by statin treatment relates to the ability of the drug to promote nitric oxide production from eNOS or to its anti-inflammatory properties. [9, 18, 66, 101]
Animal studies have revealed that coronary microvascular permeability is increased in early obesity, i.e., prior to the development of insulin resistance. Adipokines such as leptin and adiponectin are known to alter endothelial barrier function, with leptin increasing and adiponectin decreasing vascular permeability. Adiponectin appears to protect against barrier dysfunction induced by Ang II or TNF-α by modulating microtubule and cytoskeleton stability via a cAMP/ PKA signaling cascade. Enhanced exposure of endothelial cells to leptin and less exposure to adiponectin may account for the increased vascular permeability observed in fat tissue during adipogenesis. [12, 27, 56, 100]
Increased endothelial permeability is a common alteration observed both in animal models of diabetes and diabetic patients. The major complications related to the hyperpermeability of diabetes are retinopathy and nephropathy. The increased permeability has been linked to hyperglycemia and the oxidative stress elicited by the elevated extracellular glucose. There is also evidence implicating leukocyte adhesion, inflammatory mediators and the renin-angiotensin system in the hyperpermeability of diabetes. The transcription factor peroxisome proliferator-activated receptor γ (PPARγ), protein kinase C, and VEGF are also believed to play a role in diabetes-induced permeability responses. [1, 26, 43, 65, 79]
Microvessel density
While the structure and density of microvessels are relatively fixed under normal conditions, the cardiovascular risk factors appear to induce significant remodeling of the microvasculature with accompanying changes in microvessel density. Hypertension is associated with a degenerative, reverse angiogenesis (rarefaction) that can result in the loss of up to 50% of microvessels. The HTN-induced rarefaction may result from an impaired angiogenic response to vascular growth factors such as VEGF. Since angiotensin II (acting through the AT1r and VEGF) promotes vascular proliferation at subpressor concentrations, the reduced microvessel density associated with hypertensive states characterized by low AngII levels (e.g., low renin HTN or prolonged high salt intake) may reflect the loss of this pro-angiogenic signal. Studies in spontaneously hypertensive rats have revealed a role for apoptosis in the structural rarefaction occurring in arterioles, capillaries and venules. This apoptosis apoptosis appears to result from a glucocorticoid driven mechanism.
Hypercholesterolemia is also associated with an impaired angiogenic response to VEGF and other vascular growth factors, when compared with normocholesterolemic controls. Superoxide-mediated inactivation of nitric oxide and an increased expression of the antiangiogenic protein, endostatin have also been implicated in the impaired angiogenic responses associated with HCh. [6, 32, 74]
An imbalance between pro-angiogenic and anti-angiogenic factors is also a key component of the microvasculopathy associated with diabetes. In early disease development (non-proliferative phase), hyperglycemia, oxidative stress, inflammation appear to contribute to the loss of pericytes and endothelial cells, basement membrane thickening and capillary closure in the retina. The resultant hypoxia and other biochemical changes lead to the upregulation of different growth factors and an unregulated angiogenic response. Studies in experimental models of diabetes have revealed that pharmacological blockade of the renin-angiotensin system, with either ACE inhibitors or angiotensin receptor antagonists) reduces retinal angiogenesis, and is accompanied by down-regulation of VEGF and its receptor.[6, 28, 97]
Adipokines produced by growing fat tissue (e.g., leptin) have been shown to exhibit potent pro-angiogenic properties. While these substances do not appear to exert an endocrine effect on distant organs, the local paracrine actions on the microvasculature appear to be critical for adipogenesis. This contention is supported by the close spatial and temporal relationships noted between angiogenesis and adipogenesis, and is consistent with studies showing that inhibition of angiogenesis reduces adipose tissue mass and ameliorates obesity. A role for VEGF is implicated by studies demonstrating that anti-VEGF inhibits both angiogenesis and adipogenesis. The dependency of adipogenesis on angiogenesis raised the possibility of targeting vascular growth for the treatment of morbid obesity. [12, 35, 51, 56]
Platelet recruitment & thrombosis
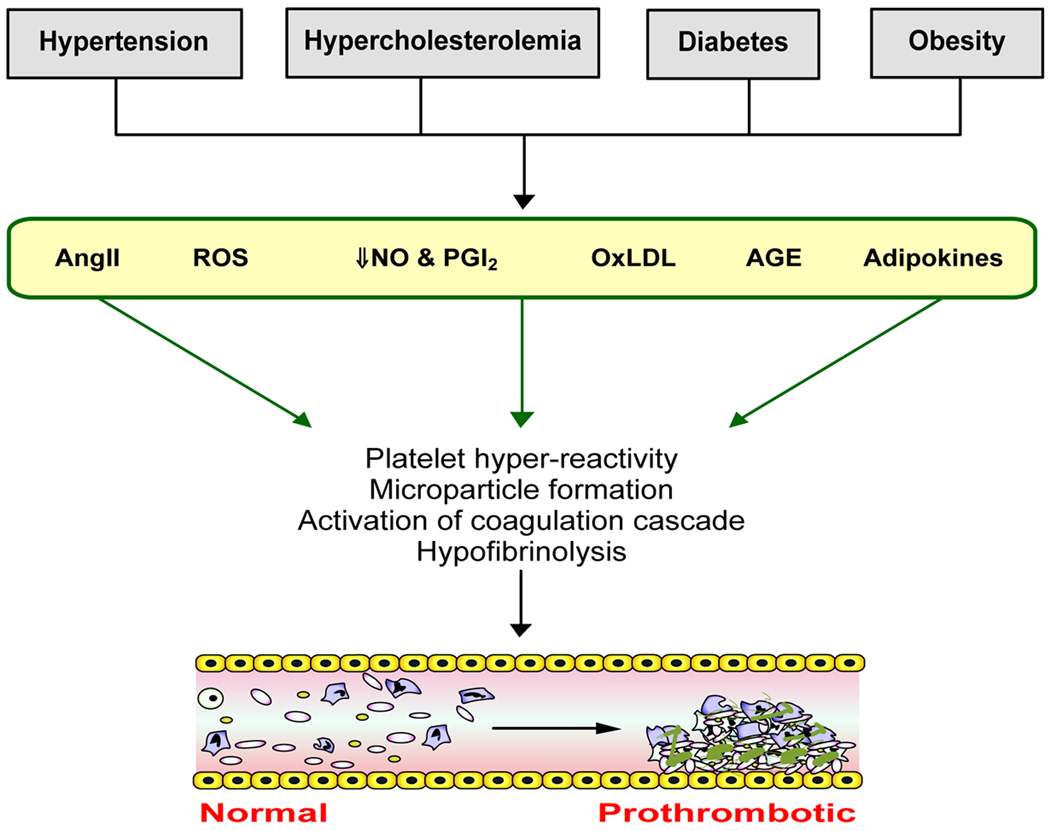
Alterations in platelet function and coagulation often accompany the CV risk factors, which translates clinically into an increased risk for thrombosis. While the underlying cause(s) of the risk factor-induced changes in platelet function and circulating levels of soluble components of the coagulation pathway are poorly understood, some of the factors that have been implicated in the risk factor-induced vascular dysfunction, such as oxidative stress and reduced NO bioavailability, are also viewed as potential initiators/mediators of the altered coagulant/thrombotic state (Figure 4). Inflammation, which is known to shift hemostatic mechanisms in favor of thrombosis, may also play a role in initiating the altered platelet and coagulation factor responses. [22]
Figure 4.
Cardiovascular risk factors induce a prothrombotic phenotype in the vasculature. AngII = angiotensin II, oxLDL = oxidized low density lipoprotein, AGE = advanced glycosylation end-products, ROS = reactive oxygen species, NO = nitric oxide, PGI2 = prostacyclin
Hypertensive patients exhibit abnormalities in clotting factors, platelet activation/reactivity, fibrinolysis and an increased risk for thrombosis. The platelet activation in HTN has been attributed to increased shear forces, activation of the RAS, and an altered endothelial cell production of platelet stimulants (endothelin) and inhibitors (prostacyclin, NO). Alterations in platelet intracellular Ca++, pHi and NO are associated with the increased tendency for aggregation, both under basal conditions’ and following exposure to agonists. The abnormalities in platelet morphology and functional activity in HTN may also be linked with defects of megakaryocytopoiesis and the production of immature, hyper-responsive platelets. The prothrombotic state and accompanying abnormalities in endothelial and platelet function, coagulation and fibrinolysis that are associated with HTN can be recapitulated by angiotensin II administration. A role for the renin-angiotensin system is supported by some clinical studies that demonstrate a reduction in prothrombotic events in HTN patients treated with an ACE inhibitor or angiotensin receptor blocker. [11, 21, 52, 62, 67]
Platelet hyper-reactivity, hypofibrinolysis and enhanced thrombus development are evident in obese subjects and in obesity-associated animal models. While these responses may result from the comorbidity of diabetes (increased insulin resistance) and/hypertension, there are several lines of evidence that implicate adipokines. Obese subjects with increased plasma leptin levels have a higher risk for thrombosis. Leptin administration promotes agonist-induced activation and aggregation of human and murine platelets and promotes thrombosis in vivo. When leptin binds with its receptor on platelets, it activates an intracellular signaling cascade that involves JAK2, insulin receptor substrate, phosphatidiylinositol 3 kinases (PIK3), and protein kinases B/Akt, which elicits the release of intracellular calcium stores, and the activation of phospholipase C and proteinkinase C. Platelets from leptin-deficient ob/ob mice exhibit a defect in platelet aggregation. Both ob/ob mice and wild type mice treated with a leptin-neutralizing antibody exhibit unstable thrombi and a depressed arterial thrombotic response. Adiponectin-knockout mice, on the other hand, exhibit accelerated thrombus formation, suggesting an antithrombotic effect of adiponectin. Overall, these findings suggest that the increased leptin and reduced adiponectin levels associated with adipogenesis may account for the prothrombotic state associated with obesity. [3, 23, 29, 45]
The hyper-responsiveness of platelets and enhanced thrombosis associated with hypercholesterolemia appears to be linked to the increased levels and chemical modification of low density lipoprotein (LDL) that results in this condition. Both native (nLDL) and oxidatively modified LDL (oxLDL) enhance platelet responsiveness to agonists via apoE receptor 2' (nLDL)- and lysophosphatidic acid (oxLDL)-mediated signaling pathways. HCh also induces the formation of platelet-leukocyte aggregates and platelet microparticles, which can activate the coagulation cascade through the induction of tissue factor. Platelet adhesion in postcapillary venules is another consequence of HCh that occurs via a mechanism that is dependent on platelet-associated P-selectin and NADPH oxidase. Nonetheless, it has been demonstrated that HCh enhances thromboembolism in arterioles (but not venules) and this response can be completely reversed by L-arginine supplementation. The latter findings suggest that HCh (possibly through oxLDL) promotes thrombosis by reducing NO bioavailability, which can be overcome by stimulating endogenous NO production with excess L-arginine.[25, 46, 47, 87]
The hypercoagulable, prothrombotic state in diabetes is associated with elevated plasma concentrations of different clotting factors (fVII, fVIII, fXII), and markers of platelet activation (soluble P-selectin, platelet microparticles), and there is evidence of a down-regulated fibrinolytic system. Basal and agonist-induced platelet reactivity is enhanced, along with the production of thromboxane, and the expression of platelet surface adhesion molecules and receptors. These changes are associated with abnormal platelet calcium homeostasis, enhanced production of thromboxane and thrombin and disturbances in platelet calcium homeostasis and the nitric oxide-cyclic GMP pathway. The ability of nitric oxide to inhibit platelet activation is impaired. The platelet hyper-reactivity of diabetes appears to be independent of changes in blood glucose and insulin levels. Advanced glycosylation end-products have been implicated in the activation of tissue factor via an oxidant-sensitive mechanism. While a pro-coagulant, prothrombotic phenotype is evident in streptozotocin-induced diabetes, mice with a genetic predisposition to diabetes (db/db) or with diet-induced type 2 diabetes do not demonstrate the hypercoagulability, platelet hyper-reactivity, increased production of microparticles or elevated P-selectin expression observed in humans with diabetes. [13, 20, 36–38, 53, 72, 75, 98]
Injury responses to ischemia-reperfusion
There is growing evidence that all of the cardiovascular risk factors render tissues more vulnerable to the deleterious effects of ischemia and reperfusion (I/R). In postischemic brain and heart, this influence of the risk factors is manifested as larger infarcts and a more intense inflammatory response. The risk factors also appear to suppress the cardio- and cerebro-protective responses conferred by ischemic preconditioning. Although the mechanisms that underlie the injury promoting actions of risk factors remain poorly understood, the exaggerated injury responses may be related to the ability of I/R and risk factors to independently induce a pro-oxidative, pro-inflammatory and pro-thrombogenic phenotype in the microvasculature. Evidence indicates, for example, that the oxidative stress experienced by microvessels is much higher following I/R in either diabetic or hypercholesterolemic animals, compared to control (no risk factor) animals. In both diabetes and HCh, the aggravated brain injury and exaggerated oxidative stress appears to be linked to a greater induction in NADPH oxidase. The risk factor-enhanced oxidative stress following I/R would be expected to yield larger increases in oxidant-sensitive transcription factors (e.g., NFkB), with corresponding enhancements of endothelial adhesion molecule expression, blood cell-endothelial cell interactions, barrier dysfunction, and a further impairment of endothelium-dependent vasodilation. The pro-oxidative environment produced by the combination of I/R and risk factors is worsened as a result of the impaired production of NO by eNOS that accompanies either condition. [5, 24, 41, 50, 58, 73, 89]
Hypercholesterolemia, diabetes and obesity have been shown to greatly intensify the leukocyte- and platelet-endothelial cell adhesion induced in venules by I/R. However, the oxidant-dependent inflammatory mediators that underlie this exaggerated recruitment response differs among risk factors. The lipid mediators, leukotriene B4 and platelet activating factor have been implicated in the diabetes-enhanced leukocyte recruitment following I/R, while mast cell degranulation products appear to mediate the HCh-enhanced response. In obese mice, the exaggerated recruitment of both adherent leukocytes and platelets has been linked to MCP-1 and IL-6. With all risk factors, the increased blood cell recruitment is associated with an exaggerated vascular permeability response to I/R. In the presence of HCh (but not diabetes), the enhanced barrier dysfunction appears to be directly linked to leukocyte recruitment, in as much as interfering with leukocyte adhesion also prevents the enhanced permeability response. Although there is a limited amount of work that addresses the influence of hypertension on the LECA and vascular permeability responses to I/R, the available data indicates that LECA responds normally to I/R, while vascular permeability is enhanced in spontaneously hypertensive rats (SHR). Furthermore, the exaggerated permeability response to HTN + I/R is abolished by blocking LECA. [41, 48, 49, 61, 73, 89]
Angiotensin II receptor (AT1r) activation has been implicated in the inflammatory and injury responses to I/R, both in the presence or absence of a CV risk factor. Studies on the cerebral and intestinal microcirculation have revealed a role for AT1r in I/R-induced leukocyte and platelet adhesion. A link between I/R-induced AT1r activation is indicated by the comparable effectiveness of AT1r antagonists and an NADPH oxidase inhibitor (diphenyliodonium) in reducing I/R-induced blood cell-endothelial cell adhesion and oxidant production after treatment with an AT1r antagonist. Since bone marrow chimeras produced by the transplantation of marrow from AT1r−/− mice into wild type recipients show similar protection against the I/R-induced blood cell recruitment as mice treated with an AT1r antagonist, it appears likely that blood cell-associated AT1r activation accounts for the observed microvascular responses. AT1r antagonists are also effective in blunting the exaggerated I/R-induced inflammatory and tissue injury responses observed in experimental diabetes, hypertension, and obesity. For example, transgenic mice carrying human renin and human angiotensinogen genes exhibit enhanced superoxide anion production in brain tissue and arteries, and worse neurological deficits following focal ischemic stroke (compared to normal mice), and the exaggerated responses to I/R are blocked by an AT1r antagonist. [19, 39, 40, 59, 63, 64, 68]
Conclusions & Future Directions
While the mechanisms that underlie the microvascular and inflammatory responses to different cardiovascular risk factors have not been precisely defined, the available evidence reveals the involvement of several common events and factors in the initiation and/or progression of these responses. Endothelial cell activation, with the accompanying oxidative stress and diminished bioavailability of nitric oxide, appears to be an early and critical component of the microvascular responses to all of the risk factors. Activated leukocytes and platelets also contribute to the risk factor-induced microvascular alterations. Whether the cell activation responses to risk factors are the result of changes in circulating soluble factors and/or cell receptor sensitivity/density induced by the risk factors remains unclear. In this regard, cytokines and angiotensin II type-1 receptors are likely candidates. Another unresolved issue is the extent to which the endothelial cell and blood cell (leukocytes, platelets) activation responses are interdependent, i.e., is endothelial cell activation a prerequisite for blood activation or vice versa. Irrespective of if/how these events are linked, it is clear that the low-grade systemic inflammatory response that results from risk factor-induced cell activation and cell-cell interactions renders tissues much more vulnerable to the deleterious effects of secondary stresses, such as ischemia and reperfusion. Consequently, the risk factors are a “double-edged sword” that enhances both the likelihood that tissues experience an ischemic episode and then yield more tissue damage in response to a given ischemic insult.
Although much progress has been made in characterizing and defining mechanisms underlying the microvascular responses to single risk factors, much less attention has been devoted to determining if/how combinations of risk factors influence the microvasculature. Since several risk factors are known to individually induce similar phenotypic changes (pro-oxidative, pro-inflammatory, pro-thrombogenic) in the microvasculature and appear to use some common signaling pathways, it may be expected that combined risk factors would not yield either additive nor synergistic responses. However, the limited available evidence in the literature suggests otherwise. For example, in pigs with renovascular hypertension (HTN) and diet-induced hypercholesterolemia (HCh), the two risk factors exert a synergistic deleterious effect on endothelium-dependent dilation and oxidative stress in coronary arteries, compared to either risk factor alone, and the hypertension appears to amplify the HCh-induced endothelial barrier dysfunction in coronary venules. Whether other risk factor combinations also yield such synergistic deleterious effects on the microvasculature and how this synergism might occur are worthy topics for future investigation. [70,71]
Acknowledgments
Supported by a grant from the National Heart Lung & Blood Institute (HL26441).
References
- 1.Allen CL, Bayraktutan U. Antioxidants attenuate hyperglycaemia-mediated brain endothelial cell dysfunction and blood-brain barrier hyperpermeability. Diabetes Obesity Metab. 2009;11:480–490. doi: 10.1111/j.1463-1326.2008.00987.x. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez A, Sanz MJ. Reactive oxygen species mediate angiotensin II-induced leukocyte-endothelial cell interactions in vivo. J Leukoc Biol. 2001;70:199–206. [PubMed] [Google Scholar]
- 3.Anfossi G, Russo I, Trovati M. Platelet dysfunction in central obesity. Nutr Metab Cardiovasc Dis. 2009;19:440–449. doi: 10.1016/j.numecd.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Bagi Z, Feher A, Beleznai T. Preserved coronary arteriolar dilatation in patients with type 2 diabetes mellitus: Implications for reactive oxygen species. Pharmacological Reports. 2009;61:99–104. doi: 10.1016/s1734-1140(09)70011-8. [DOI] [PubMed] [Google Scholar]
- 5.Balakumar P, Singh H, Singh M, Anand-Srivastava MB. The impairment of preconditioning-mediated cardioprotection in pathological conditions. Pharmacol Res. 2009;60:18–23. doi: 10.1016/j.phrs.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Boodhwani M, Sellke FW. Therapeutic angiogenesis in diabetes and hypercholesterolemia: influence of oxidative stress. Antioxid Redox Signal. 2009 doi: 10.1089/ars.2009.2439. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Booth G, Stalker TJ, Lefer AM. Mechanisms of ameliorization of glucose-induced endothelial dysfunction following inhibition of protein kinase C in vivo. Diabetes. 2002;51:1556–1564. doi: 10.2337/diabetes.51.5.1556. [DOI] [PubMed] [Google Scholar]
- 8.Booth G, Stalker Tj, Lefer AM, Scalia R. Elevated ambient glucose induces acute inflammatory events in the microvasculature: effects of insulin. Am J Physiol Endocrinol Metab. 2001;280:E848–E856. doi: 10.1152/ajpendo.2001.280.6.E848. [DOI] [PubMed] [Google Scholar]
- 9.Bonetti PO, Best PJ, Rodriguez-Porcel M, Holmes DR, Jr, Lerman LO, Lerman A. Endothelin type A receptor antagonism restores myocardial perfusion response to adenosine in experimental hypercholesterolemia. Atherosclerosis. 2003;168:367–373. doi: 10.1016/s0021-9150(03)00141-2. [DOI] [PubMed] [Google Scholar]
- 10.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: The role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 11.Camilletti A, Moretti N, Giacchetti G, Faloia E, Martarelli D, Mantero F, Mazzanti L. Decreased nitric oxide levels and increased calcium content in platelets of hypertensive patients. Am J Hypertens. 2001;14:382–386. doi: 10.1016/s0895-7061(00)01297-8. [DOI] [PubMed] [Google Scholar]
- 12.Cao R, Brakenhielm E, Wahlestedt C, Thyberg J, Cao Y. Leptin induces vascular permeability and synergistically stimulates angiogenesis with FGF-2 and VEGF. Proc Natl Acad Sci U S A. 2001;98:6390–6395. doi: 10.1073/pnas.101564798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carr ME. Diabetes mellitus: a hypercoagulable state. J Diabetes Complications. 2001;15:44–54. doi: 10.1016/s1056-8727(00)00132-x. [DOI] [PubMed] [Google Scholar]
- 14.Chavakis T, Bierhaus A, Al-Fakhri N, Schneider D, Witte S, Linn T, Nagashima M, Morser J, Arnold B, Preissner KT, Nawroth PP. The pattern recognition receptor (RAGE) is a counter receptor for leukocyte integrins: a novel pathway for inflammatory cell recruitment. J Exp Med. 2003;198:1507–1515. doi: 10.1084/jem.20030800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chua CC, Hamdy RC, Chua BH. Upregulation of vascular endothelial growth factor by angiotensin II in rat heart endothelial cells. Biochim Biophys Acta. 1998;1401:187–194. doi: 10.1016/s0167-4889(97)00129-8. [DOI] [PubMed] [Google Scholar]
- 16.Crimi E, Ignarro LJ, Napoli C. Microcirculation and oxidative stress. Free Radic Res. 2007;41:1364–1375. doi: 10.1080/10715760701732830. [DOI] [PubMed] [Google Scholar]
- 17.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109:III-27–III-32. doi: 10.1161/01.CIR.0000131515.03336.f8. [DOI] [PubMed] [Google Scholar]
- 18.Dell'Omo G, Bandinelli S, Penno G, Pedrinelli R, Mariani M. Simvastatin, capillary permeability, and acetylcholine-mediated vasomotion in atherosclerotic, hypercholesterolemic men. Clin Pharmacol Ther. 2000;68:427–434. doi: 10.1067/mcp.2000.109787. [DOI] [PubMed] [Google Scholar]
- 19.duToit EF, Nabben M, Lochner A. A potential role for angiotensin II in obesity induced cardiac hypertrophy and ischaemic/reperfusion injury. Basic Res Cardiol. 2005;100:346–354. doi: 10.1007/s00395-005-0528-5. [DOI] [PubMed] [Google Scholar]
- 20.El Haouari M, Rosado JA. Platelet signaling abnormalities in patients with type 2 diabetes mellitus: a review. Blood Cells Mol Dis. 2008;41:119–123. doi: 10.1016/j.bcmd.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 21.El Haouari M, Rosado JA. Platelet function in hypertension. Blood Cells Mol Dis. 2009;42:38–43. doi: 10.1016/j.bcmd.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Esmon CT. Crosstalk between inflammation and thrombosis. Maturitas. 2008;61:122–131. doi: 10.1016/j.maturitas.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Faber DR, de Groot PG, Visseren FL. Role of adipose tissue in haemostasis, coagulation and fibrinolysis. Obes Rev. 2009 doi: 10.1111/j.1467-789X.2009.00593.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Ferdinandy P, Schulz R, Baxter GF. Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacol Rev. 2007;59(4):418–458. doi: 10.1124/pr.107.06002. [DOI] [PubMed] [Google Scholar]
- 25.Ferroni P, Basili S, Santilli F, Davì G. Low-density lipoprotein-lowering medication and platelet function. Pathophysiol Haemost Thromb. 2006;35:346–354. doi: 10.1159/000093226. [DOI] [PubMed] [Google Scholar]
- 26.Funatsu H, Noma H, Mimura T, Eguchi S, Hori S. Association of vitreous inflammatory factors with diabetic macular edema. Ophthalmology. 2009;116:73–79. doi: 10.1016/j.ophtha.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 27.Galili O, Versari D, Sattler KJ, Olson ML, Mannheim D, McConnell JP, Chade AR, Lerman LO, Lerman A. Early experimental obesity is associated with coronary endothelial dysfunction and oxidative stress. Am J Physiol Heart Circ Physiol. 2007;292:H904–H911. doi: 10.1152/ajpheart.00628.2006. [DOI] [PubMed] [Google Scholar]
- 28.Gariano RF, Gardner TW. Retinal angiogenesis in development and disease. Nature. 2005;438:960–966. doi: 10.1038/nature04482. [DOI] [PubMed] [Google Scholar]
- 29.Giandomenico G, Dellas C, Czekay RP, Koschnick S, Loskutoff DJ. The leptin receptor system of human platelets. J Thromb Haemost. 2005;3:1042–1049. doi: 10.1111/j.1538-7836.2005.01327.x. [DOI] [PubMed] [Google Scholar]
- 30.Granger DN, Vowinkel T, Petnehazy T. Modulation of the inflammatory response in cardiovascular disease. Hypertension. 2004;43:924–931. doi: 10.1161/01.HYP.0000123070.31763.55. [DOI] [PubMed] [Google Scholar]
- 31.Gregor MG, Hotamisligil GS. Adipocyte stress: the endoplasmic reticulum and metabolic disease. J. Lipid Res. 2007;48:1905–1914. doi: 10.1194/jlr.R700007-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Greene AS. Life and death in the microcirculation: a role for angiotensin II. Microcirculation. 1998;5:101–107. [PubMed] [Google Scholar]
- 33.Harrison DG, Gongora MC. Oxidative stress and hypertension. Med Clin North Am. 2009;93:621–635. doi: 10.1016/j.mcna.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 34.Harrison DG, Guzik TJ, Goronzy J, Weyand C. Is hypertension an immunologic disease? Curr Cardiol Rep. 2008;10:464–469. doi: 10.1007/s11886-008-0073-6. [DOI] [PubMed] [Google Scholar]
- 35.Hausman GJ, Richardson RL. Adipose tissue angiogenesis. J Anim Sci. 2004;82:925–934. doi: 10.2527/2004.823925x. [DOI] [PubMed] [Google Scholar]
- 36.Henry M, Davidson L, Cohen Z, McDonagh PF, Nolan PE, Ritter LS. Whole blood aggregation, coagulation, and markers of platelet activation in diet-induced diabetic C57BL/6J mice. Diabetes Res Clin Pract. 2009;84:11–18. doi: 10.1016/j.diabres.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 37.Henry ML, Davidson LB, Wilson JE, McKenna BK, Scott SA, McDonagh PF, Ritter LS. Whole blood aggregation and coagulation in db/db and ob/ob mouse models of type 2 diabetes. Blood Coagul Fibrinolysis. 2008;19:124–134. doi: 10.1097/MBC.0b013e3282f41e56. [DOI] [PubMed] [Google Scholar]
- 38.Ichikawa K, Yoshinari M, Iwase M, Wakisaka M, Doi Y, Iino K, Yamamoto M, Fujishima M. Advanced glycosylation end products induced tissue factor expression in human monocyte-like U937 cells and increased tissue factor expression in monocytes from diabetic patients. Atherosclerosis. 1998;136:281–287. doi: 10.1016/s0021-9150(97)00221-9. [DOI] [PubMed] [Google Scholar]
- 39.Inaba S, Iwai M, Tomono Y, Senba I, Furuno M, Kanno H, Okayama H, Mogi M, Higaki J, Horiuchi M. Exaggeration of focal cerebral ischemia in transgenic mice carrying human Renin and human angiotensinogen genes. Stroke. 2009;40:597–603. doi: 10.1161/STROKEAHA.108.519801. [DOI] [PubMed] [Google Scholar]
- 40.Ishikawa M, Sekizuka E, Yamaguchi N, Nakadate H, Terao S, Granger DN, Minamitani H. Angiotensin II type 1 receptor signaling contributes to platelet-leukocyte-endothelial cell interactions in the cerebral microvasculature. Am J Physiol Heart Circ Physiol. 2007;292:H2306–H2315. doi: 10.1152/ajpheart.00601.2006. [DOI] [PubMed] [Google Scholar]
- 41.Ishikawa M, Stokes KY, Zhang JH, Nanda A, Granger DN. Cerebral microvascular responses to hypercholesterolemia: roles of NADPH oxidase and P-selectin. Circ Res. 2004;94:239–244. doi: 10.1161/01.RES.0000111524.05779.60. [DOI] [PubMed] [Google Scholar]
- 42.John S, Schmieder RE. Impaired endothelial function in arterial hypertension and hypercholesterolemia: potential mechanisms and differences. Journal Hypertension. 2000;18:363–374. doi: 10.1097/00004872-200018040-00002. [DOI] [PubMed] [Google Scholar]
- 43.Kim JH, Kim JH, Yu YS, Cho CS, Kim KW. Blockade of angiotensin II attenuates VEGF-mediated blood-retinal barrier breakdown in diabetic retinopathy. J Cereb Blood Flow Metab. 2009;29:621–628. doi: 10.1038/jcbfm.2008.154. [DOI] [PubMed] [Google Scholar]
- 44.King GL, Locken MR. Hyperglycemia-induced oxidative stress in diabetic complications. Histo chem Cell Biol. 2004;122:333–338. doi: 10.1007/s00418-004-0678-9. [DOI] [PubMed] [Google Scholar]
- 45.Konstantinides S, Schäfer K, Koschnick S, Loskutoff DJ. Leptin-dependent platelet aggregation and arterial thrombosis suggests a mechanism for atherothrombotic disease in obesity. J Clin Invest. 2001;108:1533–1540. doi: 10.1172/JCI13143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Korporaal SJ, Akkerman JW. Platelet activation by low density lipoprotein and high density lipoprotein. Pathophysiol Haemost Thromb. 2006;35:270–280. doi: 10.1159/000093220. [DOI] [PubMed] [Google Scholar]
- 47.Korporaal SJ, Van Eck M, Adelmeijer J, Ijsseldijk M, Out R, Lisman T, Lenting PJ, Van Berkel TJ, Akkerman JW. Platelet activation by oxidized low density lipoprotein is mediated by CD36 and scavenger receptor-A. Arterioscler Thromb Vasc Biol. 2007;27:2476–2483. doi: 10.1161/ATVBAHA.107.150698. [DOI] [PubMed] [Google Scholar]
- 48.Kurose I, Wolf R, Cerwinka W, Granger DN. Microvascular responses to ischemia/reperfusion in normotensive and hypertensive rats. Hypertension. 1999;34:212–216. doi: 10.1161/01.hyp.34.2.212. [DOI] [PubMed] [Google Scholar]
- 49.Kurose I, Wolf RE, Grisham MB, Granger DN. Hypercholesterolemia enhances oxidant production in mesenteric venules exposed to ischemia/reperfusion. Arterioscler Thromb Vasc Biol. 1998;18:1583–1588. doi: 10.1161/01.atv.18.10.1583. [DOI] [PubMed] [Google Scholar]
- 50.Kusaka I, Kusaka G, Zhou C, Ishikawa M, Nanda A, Granger DN, Zhang JH, Tang J. Role of AT1 receptors and NAD(P)H oxidase in diabetes-aggravated ischemic brain injury. Am J Physiol Heart Circ Physiol. 2004;286:H2442–H2451. doi: 10.1152/ajpheart.01169.2003. [DOI] [PubMed] [Google Scholar]
- 51.Lijnen HR. Angiogenesis and obesity. Cardiovascular Res. 2008;78:286–293. doi: 10.1093/cvr/cvm007. [DOI] [PubMed] [Google Scholar]
- 52.Lip GY, Li-Saw-Hee FL. Does hypertension confer a hypercoagulable state? J Hypertens. 1998;16:913–916. doi: 10.1097/00004872-199816070-00003. [DOI] [PubMed] [Google Scholar]
- 53.McDonagh PF, Hokama JY, Gale SC, Logan JJ, Davis-Gorman G, Goldman S, et al. Chronic expression of platelet adhesion proteins is associated with severe heart disease in type 2 diabetic patients. Chronic platelet activation in diabetic heart patients. J Diabetes Complications. 2003;17:269–278. doi: 10.1016/s1056-8727(02)00246-5. [DOI] [PubMed] [Google Scholar]
- 54.Morigi M, Angioletti S, Imberti B, Donadelli R, Micheletti G, Figliuzzi M, Remuzzi A, Zoja C, Remuzzi G. Leukocyte-endothelial interaction is augmented by high glucose concentrations and hyperglycemia in a NF-kB-dependent fashion. J Clin Invest. 1998;101:1905–1915. doi: 10.1172/JCI656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nickenig G, Harrison DG. The AT1-type angiotensin receptor in oxidative stress and atherogenesis, part I: oxidative stress and atherogenesis. Circulation. 2002;105:393–396. doi: 10.1161/hc0302.102618. [DOI] [PubMed] [Google Scholar]
- 56.Nishimura S, Manabe I, Nagasaki M, Hosoya Y, Yamashita H, Fujita H, Ohsugi M, Tobe K, Kadowaki T, Nagai R, Sugiura S. Adipogenesis in obesity requires close interplay between differentiating adipocytes, stromal cells, and blood vessels. Diabetes. 2007;56:1517–1526. doi: 10.2337/db06-1749. [DOI] [PubMed] [Google Scholar]
- 57.Nishimura S, Manabe I, Nagasaki M, Seo K, Yamashita H, Hosoya Y, Ohsugi M, Tobe K, Kadowaki T, Nagai V, Sugiura S. In vivo imaging in mice reveals local cell dynamics and inflammation in obese adipose tissue. J. Clin. Invest. 2008;118:710–721. doi: 10.1172/JCI33328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Osmond JM, Mintz JD, Dalton B, Stepp DW. Obesity increases blood pressure, cerebral vascular remodeling, and severity of stroke in the Zucker rat. Hypertension. 2009;53:381–386. doi: 10.1161/HYPERTENSIONAHA.108.124149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ouedraogo R, Gong Y, Berzins B, Wu X, Mahadev K, Hough K, Chan L, Goldstein BJ, Scalia R. Adiponectin deficiency increases leukocyte-endothelium interactions via upregulation of endothelial cell adhesion molecules in vivo. J Clin Invest. 2007;117:1718–1726. doi: 10.1172/JCI29623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ouedraogo R, Wu X, Xu SQ, Fuchsel L, Motoshima H, Mahadev K, Hough K, Scalia R, Goldstein BJ. Adiponectin suppression of high-glucose-induced reactive oxygen species in vascular endothelial cells: evidence for involvement of a cAMP signaling. Diabetes. 2006;55:1840–1846. doi: 10.2337/db05-1174. [DOI] [PubMed] [Google Scholar]
- 61.Panés J, Kurose I, Rodriguez-Vaca D, Anderson DC, Miyasaka M, Tso P, Granger DN. Diabetes exacerbates inflammatory responses to ischemia-reperfusion. Circulation. 1996;93:161–167. doi: 10.1161/01.cir.93.1.161. [DOI] [PubMed] [Google Scholar]
- 62.Pathansali R, Smith NM, Bath PM. Prothromboticmegakaryocyte and platelet changes in hypertension are reversed following treatment: a pilot study. Platelets. 2001;12:144–149. doi: 10.1080/09537100120039000. [DOI] [PubMed] [Google Scholar]
- 63.Petnehazy T, Cooper D, Stokes KY, Russell J, Wood KC, Granger DN. Angiotensin II type 1 receptors and the intestinal microvascular dysfunction induced by ischemia and reperfusion. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1203–G1210. doi: 10.1152/ajpgi.00578.2005. [DOI] [PubMed] [Google Scholar]
- 64.Petnehazy T, Stokes KY, Wood KC, Russell J, Granger DN. Role of blood cell-associated AT1 receptors in the microvascular responses to hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2006;26:313–318. doi: 10.1161/01.ATV.0000193625.32499.71. [DOI] [PubMed] [Google Scholar]
- 65.Phipps JA, Clermont AC, Sinha S, Chilcote TJ, Bursell SE, Feener EP. Plasma kallikrein mediates angiotensin II type 1 receptor-stimulated retinal vascular permeability. Hypertension. 2009;53:175–181. doi: 10.1161/HYPERTENSIONAHA.108.117663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ray D, Mishra M, Ralph S, Read I, Davies R, Brenchley P. Association of the VEGF gene with proliferative diabetic retinopathy but not proteinuria in diabetes. Diabetes. 2004;53:861–864. doi: 10.2337/diabetes.53.3.861. [DOI] [PubMed] [Google Scholar]
- 67.Remková A, Remko M. The role of renin - angiotensin system in prothrombotic state in essential hypertension. Physiol Res. 2009 doi: 10.33549/physiolres.931525. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 68.Riaz AA, Wang Y, Schramm R, Sato T, Menger MD, Jeppsson B, Thorlacius H. Role of angiotensin II in ischemia/reperfusion-induced leukocyte-endothelium interactions in the colon. FASEB J. 2004;18:881–883. doi: 10.1096/fj.03-0502fje. [DOI] [PubMed] [Google Scholar]
- 69.Riou S, Mees B, Esposito B, Merval R, Vilar J, Stengel D, Ninio E, van Haperen R, de Crom R, Tedqui A, Lehoux S. High blood pressure promotes monocyte adhesion to the vascular wall. Circ Res. 2007;100:1226–1233. doi: 10.1161/01.RES.0000265231.59354.2c. [DOI] [PubMed] [Google Scholar]
- 70.Rodriguez-Porcel M, Lerman LO, Herrmann J, Sawamura T, Napoli C, Lerman A. Hypercholesterolemia and hypertension have synergistic deleterious effects on coronary endothelial function. Arterioscler Thromb Vasc Biol. 2003;23:885–891. doi: 10.1161/01.ATV.0000069209.26507.BF. [DOI] [PubMed] [Google Scholar]
- 71.Rodriguez-Porcel M, Lerman A, Herrmann J, Schwartz RS, Sawamura T, Condorelli M, Napoli C, Lerman LO. Hypertension exacerbates the effect of hypercholesterolemia on the myocardial microvasculature. Cardiovasc Res. 2003;58:213–221. doi: 10.1016/s0008-6363(03)00250-5. [DOI] [PubMed] [Google Scholar]
- 72.Rosenblum WI, El-Sabban F, Loria RM. Platelet aggregation in the cerebral and mesenteric microcirculation of mice with genetically determined diabetes. Diabetes. 1981;30:89–92. doi: 10.2337/diab.30.2.89. [DOI] [PubMed] [Google Scholar]
- 73.Salas A, Panés J, Elizalde JI, Granger DN, Piqué JM. Reperfusion-induced oxidative stress in diabetes: cellular and enzymatic sources. J Leukoc Biol. 1999;66:59–66. doi: 10.1002/jlb.66.1.59. [DOI] [PubMed] [Google Scholar]
- 74.Sane DC, Anton L, Brosnihan KB. Angiogenic growth factors and hypertension. Angiogenesis. 2004;7:193–201. doi: 10.1007/s10456-004-2699-3. [DOI] [PubMed] [Google Scholar]
- 75.Schafer A, Alp NJ, Cai S, Lygate CA, Neubauer S, Eigenthaler M, Bauersachs J, Channon KM. Reduced vascular NO bioavailability in diabetes increases platelet activation in vivo. Arterioscler Thromb Vasc Biol. 2004;24:1720–1726. doi: 10.1161/01.ATV.0000138072.76902.dd. [DOI] [PubMed] [Google Scholar]
- 76.Sigusch HH, Ou R, Katwa LC, Campbell SE, Ganjam VK, Reddy HK, Weber KT. Angiotensin-II-induced increase in transcoronary protein clearance: role of hypertension vs. nitric oxide or cyclo-oxygenase products. Cardiovasc Res. 2005;30:291–298. [PubMed] [Google Scholar]
- 77.Singer G, Granger DN. Inflammatory responses underlying the microvascular dysfunction associated with obesity and insulin resistance. Microcirculation. 2007;14:375–387. doi: 10.1080/10739680701283158. [DOI] [PubMed] [Google Scholar]
- 78.Son SM, Whalin MK, Harrison DG, Taylor WR, Griendling KK. Oxidative stress and diabetic vascular complications. Curr Diab Rep. 2004;4:247–252. doi: 10.1007/s11892-004-0075-8. [DOI] [PubMed] [Google Scholar]
- 79.Sotiropoulos KB, Clermont A, Yasuda Y, Rask-Madsen C, Mastumoto M, Takahashi J, Della Vecchia K, Kondo T, Aiello LP, King GL. Adipose-specific effect of rosiglitazone on vascular permeability and protein kinase C activation: novel mechanism for PPARgamma agonist's effects on edema and weight gain. FASEB J. 2006;20:1203–1205. doi: 10.1096/fj.05-4617fje. [DOI] [PubMed] [Google Scholar]
- 80.Stauffera BL, Westbya CM, DeSouza CA. Endothelin-1, aging and hypertension. Curr Opin Cardiol. 2008;23:350–355. doi: 10.1097/HCO.0b013e328302f3c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stepp DW. Impact of obesity and insulin resistance on vasomotor tone: nitric oxide beyond. Clin Exp Pharm Physiol. 2006;33:407–414. doi: 10.1111/j.1440-1681.2006.04381.x. [DOI] [PubMed] [Google Scholar]
- 82.Stokes KY. Microvascular responses to hypercholesterolemia: the interactions between innate and adaptive immune responses. Antioxid Redox Signal. 2006;8:1141–1151. doi: 10.1089/ars.2006.8.1141. [DOI] [PubMed] [Google Scholar]
- 83.Stokes KY, Calahan L, Hamric CM, Russell JM, Granger DN. CD40/CD40L contributes to hypercholesterolemia-induced microvascular inflammation. Am J Physiol Heart Circ Physiol. 2009;296:H689–H697. doi: 10.1152/ajpheart.00962.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stokes KY, Clanton EC, Russell JM, Ross CR, Granger DN. NAD(P)H oxidase-derived superoxide mediates hypercholesterolemia-induced leukocyte-endothelial cell adhesion. Circ Res. 2001;88:499–505. doi: 10.1161/01.res.88.5.499. [DOI] [PubMed] [Google Scholar]
- 85.Stokes KY, Cooper D, Tailor A, Granger DN. Hypercholesterolemia promotes inflammation and microvascular dysfunction: role of nitric oxide and superoxide. Free Radic Biol Med. 2002;33:1026–1036. doi: 10.1016/s0891-5849(02)01015-8. [DOI] [PubMed] [Google Scholar]
- 86.Stokes KY, Granger DN. The microcirculation: a motor for the systemic inflammatory response and large vessel disease induced by hypercholesterolaemia? J Physiol. 2007;562:647–653. doi: 10.1113/jphysiol.2004.079640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stokes KY, Russell JM, Jennings MH, Alexander JS, Granger DN. Platelet-associated NAD(P)H oxidase contributes to the thrombogenic phenotype induced by hypercholesterolemia. Free Radical Biology & Medicine. 2007;43:22–30. doi: 10.1016/j.freeradbiomed.2007.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Suematsu M, Suzuki H, Delano FA, Schmid-Schönbein GW. The inflammatory aspect of the microcirculation in hypertension: oxidative stress, leukocytes-endothelial interaction, apoptosis. Microcirculation. 2002;9:259–276. doi: 10.1038/sj.mn.7800141. [DOI] [PubMed] [Google Scholar]
- 89.Terao S, Yilmaz G, Stokes KY, Ishikawa M, Kawase T, Granger DN. Inflammatory and injury responses to ischemic stroke in obese mice. Stroke. 2008;39:943–950. doi: 10.1161/STROKEAHA.107.494542. [DOI] [PubMed] [Google Scholar]
- 90.Touyz RM. Molecular and cellular mechanisms in vascular injury in hypertension: role of angiotensin II. Curr Opin Nephrol Hypertens. 2005;14:125–131. doi: 10.1097/00041552-200503000-00007. [DOI] [PubMed] [Google Scholar]
- 91.Vachharajani V, Granger DN. Adipose tissue: a motor for the inflammation associated with obesity. IUBMB Life. 2009;61:424–430. doi: 10.1002/iub.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vanhoutte PM. Endothelial control of vasomotor function, from health to coronary disease. Circ J. 2003;67:572–575. doi: 10.1253/circj.67.572. [DOI] [PubMed] [Google Scholar]
- 93.Viazzi F, Leoncini G, Ratto E, Parodi A, Falqui V, Conti N, Tomolillo C, Ravera G, Deferrari G, Pontremoli R. Vascular permeability, blood pressure, and organ damage in primary hypertension. Hypertension Res. 2008;31:873–879. doi: 10.1291/hypres.31.873. [DOI] [PubMed] [Google Scholar]
- 94.Vogel RA, Corretti MC, Plotnic GD. Effect of a single high-fat meal on endothelial function in healty subjects. Am. J. Cardiol. 1997;79:350–354. doi: 10.1016/s0002-9149(96)00760-6. [DOI] [PubMed] [Google Scholar]
- 95.Wang H, Nawata J, Kakudo N, Sugimura K, Suzuki J, Sakuma M, Ikeda J, Shirato K. The upregulation of ICAM-1 and P-selectin requires high blood pressure but not circulating renin-angiotensin system in vivo. J Hypertension. 2004;22:1323–1332. doi: 10.1097/01.hjh.0000125437.28861.40. [DOI] [PubMed] [Google Scholar]
- 96.Wilcox CS, Pearlman A. Chemistry and antihypertensive effects of tempol and other nitroxides. Pharmacol Rev. 2008;60:418–469. doi: 10.1124/pr.108.000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wilkinson-Berka JL. Angiotensin and diabetic retinopathy. Int J Biochem Cell Biol. 2006;38:752–765. doi: 10.1016/j.biocel.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 98.Winocour PD, Perry DW, Kinlough-Rathbone RL. Hypersensitivity to ADP of platelets from diabetic rats associated with enhanced fibrinogen binding. Eur J Clin Invest. 1992;22:19–23. doi: 10.1111/j.1365-2362.1992.tb01930.x. [DOI] [PubMed] [Google Scholar]
- 99.Ying CJ, Xu JW, Ikeda K, Takahashi K, Nara Y, Yamori Y. Tea polyphenols regulate nicotinamide adenine dinucleotide phosphate oxidase subunit expression and ameliorate angiotensin II-induced hyperpermeability in endothelial cells. Hypertension Res. 2003;26:823–828. doi: 10.1291/hypres.26.823. [DOI] [PubMed] [Google Scholar]
- 100.Xu SQ, Mahadev K, Wu X, Fuchsel L, Donnelly S, Scalia RG, Goldstein BJ. Adiponectin protects against angiotensin II or tumor necrosis factor alpha-induced endothelial cell monolayer hyperpermeability: role of cAMP/PKA signaling. Arterioscler Thromb Vasc Biol. 2008;28:899–905. doi: 10.1161/ATVBAHA.108.163634. [DOI] [PubMed] [Google Scholar]
- 101.Zhu XY, Bentley MD, Chade AR, Ritman EL, Lerman A, Lerman LO. Early changes in coronary artery wall structure detected by microcomputed tomography in experimental hypercholesterolemia. Am J Physiol Heart Circ Physiol. 2007;293:H1997–H2003. doi: 10.1152/ajpheart.00362.2007. [DOI] [PubMed] [Google Scholar]