Abstract
We conducted a preliminary study of the 4 session Community-Friendly Health Recovery Program for HIV-infected drug users (CHRP+) which was adapted from a 12 session evidence-based risk reduction and antiretroviral adherence intervention. Improvements were found in the behavioral skills required to properly adhere to HIV medication regimens. Enhancements were found in all measured aspects of sex-risk reduction outcomes including HIV knowledge, motivation to reduce sex-risk behavior, behavioral skills related to engaging in reduced sexual risk, and reduced risk behavior. Improvements in drug use outcomes included enhancements in risk reduction skills as well as reduced heroin and cocaine use. Intervention effects also showed durability from Post-intervention to the Follow-up assessment point. Females responded particularly well in terms of improvements in risk reduction skills and risk behavior. This study suggests that an evidence-based behavioral intervention may be successfully adapted for use in community-based clinical settings where HIV-infected drug users can be more efficiently reached.
Keywords: HIV infection, HIV risk reduction, behavioral intervention, injection drug use, intervention adaptation, implementation
1.0 Introduction
Despite decades of HIV prevention campaigns targeting high risk populations and a rapidly expanding research literature on HIV-risk reduction, a significant percentage of HIV-infected injection drug users (IDUs) continue to engage in inter-related health risk behaviors (i.e., drug- and sex-related HIV risk and sub-optimal antiretroviral adherence)1,2. Thus, while remarkable advancements in highly active antiretroviral therapy (HAART) have led to longer and healthier lives for HIV-infected persons, there has been an increase in the rate of new HIV infections in the U.S.3
HIV prevention experts have suggested that broad dissemination of more effective interventions could result in a reduction in the incidence of HIV infection4 and a number of evidence-based interventions (EBIs) targeting addicted populations are now widely available (see www.effectiveinterventionveinterventions.org). Unfortunately, the ready availability of EBIs for treating drug dependence has not led to a correspondingly wide-scale implementation of these interventions in “real-world” settings5, a goal that can be impeded by a host of factors that typically differentiate clinical settings from the research settings in which interventions are usually developed and tested. Similarly, the length, duration, and complexity of HIV risk reduction EBIs targeting HIV-infected drug users have hindered their wide-scale implementation in clinical settings6.
Prior research has identified differences between clinical and research settings with respect to the quantity and quality of resources available to competently deliver, monitor, and evaluate what are often complex and lengthy interventions7,8. Such fundamental barriers can disrupt the deployment of an intervention at multiple organizational levels ranging from the intervention participants to organizational stakeholders9,10. Therefore, in the present study, we attempted to optimize an EBI – the Holistic Health Recovery Program for HIV-infected drug users (HHRP+1) - by conducting formative research with members of the target population of HIV-infected IDUs as well as organizational stakeholders, in addition to reviewing published empirical outcome data11.
Consistent with the Assessment-Decision-Administration-Production-Topical experts-Integration-Training-Testing (ADAPT-ITT) model of intervention adaptation12, our formative research process was designed to specify: (1) what intervention content was most relevant based on the target participants’ HIV risk behavior profiles; (2) what intervention design characteristics - including modality, frequency, and duration - would be feasible to implement in real-world settings; and (3) what would be the optimal intervention placement and source of participant referral within the target organization. We therefore present outcome data from a preliminary study, in a community-based clinical setting, of the final modified intervention that resulted from the elicitation research, the Community-Friendly Health Recovery Program for HIV-infected drug users (CHRP+).
2.0 Methods
2.1 The CHRP+ intervention
Based on formative research, we concluded that the intervention should (1) be brief but based on an EBI targeting HIV-infected IDUs, (2) consist of no more than 4–6 weekly group sessions each lasting no more than one hour, (3) contain content that focuses explicitly on drug- and sex-related HIV risk reduction and antiretroviral adherence, and (4) be readily available to in- and out-of-treatment HIV-infected IDUs. We determined that the intervention would be an abbreviated version of the 12-session, 24-hour, evidence-based Holistic Health Recovery Program (HHRP+1).
The CHRP+ is a theory driven13–14, manual-guided, behavioral intervention that consists of four 45-minute weekly group meetings (Table 1; Active Health Care Participation; Reducing Drug-related Risk; Risk Reduction With Male and Female Condoms; Negotiating Risk Reduction with Partners) that are specifically designed to address sex- and drug-related transmission risk behavior and antiretroviral adherence among HIV-infected IDUs. The sessions were provided by two graduate-level trained facilitators who delivered the intervention content using cognitive remediation strategies, such as multi-modal presentation of the material, designed to accommodate the mild to moderate cognitive difficulties that are frequently present in HIV-infected IDUs15.
Table 1.
Outline of the CHRP+ HIV risk reduction and antiretroviral adherence intervention
| Group Topic | Information, Motivation, & Skills Taught |
|---|---|
| Active Health Care Participation | Understanding HIV And Your Immune System, Strategies for Improving Health, Developing a Partnership With Health Care Providers, and Enhancing Antiretroviral Adherence Skills |
| Reducing Drug-related Risk | Identifying Drug-Related HIV-Risks, Learning About Proper Needle Cleaning, and Managing Drug Cravings |
| Risk Reduction With Male and Female Condoms | Identifying Sex-Related HIV Risks, and Learning About Latex Products and Their Correct Use |
| Negotiating Risk Reduction With Partners | Negotiating Use of Latex, and Communicating About Sex- and Drug-Related HIV-Risk |
2.2 Participants
Participants (n = 39) were recruited in New Haven, CT from community-based, non-research oriented programs and health services centers (e.g., homeless shelters, addiction treatment centers, medical clinics). The intervention sessions were provided at an inner-city satellite clinic of the Yale University AIDS Program in New Haven, CT. Potential study participants were screened initially by phone, and if potentially eligible, invited to complete a brief, but detailed questionnaire in person. Invitation to participate required meeting the following criteria: 1) HIV-infected; 2) meeting DSM-IV criteria for opioid dependence; 3) confirmation of current prescription of antiretroviral medications; and 4) self-reported HIV risk behavior within the past month. The study protocol was approved by the Investigational Review Board (IRB) at the University of Connecticut and the Human Investigation Committee at Yale University. All participants signed an informed consent form prior to their participation. Participants were reimbursed for the time required to complete assessments.
2.3 Assessments
Participants were assessed at three standard time points (Pre-intervention, Post-intervention, and Follow-up). After providing informed consent, participants completed a Pre-intervention assessment. Immediately after completing the four weekly intervention groups, subjects underwent a Post-intervention assessment. A Follow-up assessment was then conducted 12 weeks after the intervention was completed to assess the durability of effects. Guided by the Information-Motivation- Behavioral Skills model of health behavior change13–14 on which the CHRP+ is based, assessments covered antiretroviral adherence and HIV risk reduction information, motivation, behavioral skills, and behavior. The assessments were delivered using audio computer-assisted structured interviews (ACASI) based upon an HIV risk assessment approach used in previous trials conducted within community-based settings16.
Table 2 displays a detailed description of the constructs measured including: Information (knowledge) about antiretroviral adherence (calculated as percent correct), Motivation to adhere to antiretroviral medication-taking, and Behavioral skills self-efficacy about antiretroviral adherence. Examples of antiretroviral-related items by construct are as follows: (1) Information, “What could happen if you do not take all of your medication as prescribed?”; (2) Motivation, “How strong is your intention to take all of your medications as directed by your health care provider in the next month”; and (3) Behavioral skills, “How confident are you that you would remember to take your medications with you to your friend’s house”.
Table 2.
Measures included in the analysis
| Measures | # of Items | Range of items | αa |
|---|---|---|---|
| Antiretroviral adherence | |||
| -Knowledge | 2 | 0% – 100% | |
| -Motivation | 14 | 0 – 3 | .73 |
| -Behavioral skills self-efficacy | 14 | 0 – 9 | .95 |
| Drug-related HIV reduction | |||
| -Knowledge | 5 | 0% – 100% | |
| -Personal motivation | 3 | 0/1 | N/A |
| -Social motivation | 3 | 0 – 4 | N/A |
| -Perceived difficulty | 3 | 0 – 4 | N/A |
| -Behavioral skills self-efficacy | 6 | 0 – 10 | .86 |
| -Reported safe drug use (frequency) | 3 | 0 – 4 | .85 |
| -Reported drug use (frequency -heroin) | 2 | days | .97 |
| -Reported drug use (frequency -cocaine) | 2 | days | .92 |
| -Reported drug use (quantity -heroin) | 2 | dime bags | .85 |
| -Reported drug use (quantity -cocaine) | 2 | dime bags | .72 |
| Sex-related HIV reduction | |||
| -Knowledge | 5 | 0% – 100% | |
| -Personal motivation | 2 | 0/1 | N/A |
| -Social motivation | 1 | 0 – 4 | N/A |
| -Behavioral skills self-efficacy | 9 | 0 – 10 | .94 |
| -Reported risky sex (use of condom) | 1 | 0 – 5 | N/A |
| -Reported risky sex (# regular partners) | 1 | 0 – 2 | N/A |
| -Reported risky sex (# of sexual partners) | 1 | persons | N/A |
Note. N/A indicates a single item was used or that a composite construct was not included due to the measurement of different facets of the construct. Multivariate analysis was conducted on items measuring different facets of the construct.
Cronbach’s alphas were reported according to the data at the Pre-intervention assessment point.
The assessment also focused on drug- and sex-related HIV-risk reduction knowledge (Information), personal and social motivation to reduce HIV risk behavior (Motivation), and self-efficacy about reducing HIV risk behavior (Behavioral Skills), as well as reported HIV risk behavior. Examples include “Anyone having sex with only one other person cannot get the AIDS virus” for knowledge about safer sex and “A person cannot get the AIDS virus from works/rigs bought on the street in a sealed wrapper” for knowledge about safer injection drug use. For motivation to engage in safe sex, items measured participants’ personal motivation to use condoms and to abstain from sex (yes or no), and their perception of significant others’ beliefs about the importance of using condoms (social motivation). Behavioral skills self-efficacy about engaging in safer sex was also recorded as well as reported frequency of sex-risk behavior (Table 2).
As for safer drug use, items related to motivation were measured in terms of participants’ personal motivation to abstain from drug use or engage in safer drug use (yes or no), and their perceptions of significant others’ beliefs about the importance of safer drug use and abstinence from drug use (social motivation). Behavioral Skills self-efficacy measures included confidence about engaging in safer drug use and perceived difficulty engaging in safer drug use. Reported frequency of drug use (i.e., heroin and cocaine) and reported quantities of drug use (i.e., heroin and cocaine) were also measured. Reliabilities for these above constructs were acceptable, ranging from 0.68 to 0.97 (Table 2).
2.4 Analytical Plan
Because gender differences have been reported on HIV risk behavior outcomes17,18, we included participant gender in our data analytical model. The analytical strategy was a 2 (Pre-intervention/Post-intervention) × 2 (Gender) mixed effects model. Due to the limited number of participants, univariate analyses of variance with repeated measures were also adopted. The analysis examined antiretroviral adherence related constructs (i.e., Information, Motivation, and Behavioral Skills) and other dependent variables organized by HIV risk domain: Sex-related HIV risk reduction (HIV Knowledge, Social Motivation, Behavioral Skills, and Reported Behavior) and Drug-related HIV risk reduction (HIV Knowledge, Social Motivation, Behavioral Skills, and Reported Behavior). When there were sufficient cases (n > 10), we also conducted a 3 (Pre-intervention/Post-intervention/Follow-up) × 2 (Gender) mixed effects model to assess the durability of outcomes. For dichotomous items (only personal motivation), chi-square tests were conducted to examine changes at Post-Intervention and Follow-up. An Intervention effect was defined as improvement in a measured outcome from the Pre-intervention to the Post-intervention assessment point (p < 0.05).
3.0 Results
The 39 enrolled participants were 18% Caucasian, 64% African American, and 15% Latino. Most (79%) earned an income of less than $10,000 per year. On average, participants reported having been diagnosed with HIV since 1989 and having contracted HIV through both drug- and sex-related risk behaviors (45.9% drug-related, 45.9% sex-related, and 8.2% uncertain). All enrolled participants were currently taking antiretroviral medication.
Of the 39 enrolled participants, 10 dropped out immediately after completing only a Pre-intervention assessment, and were not included in the outcome analyses. There were no differences at the Pre-intervention assessment, however, between the 10 drop-outs and the remaining 29 enrolled participants in terms of demographic characteristics or outcome variables aside from reported frequency of heroin use and self-efficacy about sex-risk reduction (Table 3). The remaining 29 participants completed a Pre-intervention assessment and at least one group meeting; 21 (72%) of these participants completed a Post-intervention assessment, while 17 (59%) completed a 3-month Follow-up assessment.
Table 3.
Baseline characteristics of participants (drop-outs vs. completers)
| Variable | Completers (n = 29) | Drop-outs (n = 10) | p-value |
|---|---|---|---|
| Gender (M:F) | 18:11 | 4:6 | .28 |
| Mean age (years) | 45.8 | 43.9 | .43 |
| Employment status | .81 | ||
| ■ Disability | 15 (52%) | 6 (60%) | |
| ■ Unemployed | 9 (31%) | 4 (40%) | |
| HIV status (median year diagnosed) | 1993 | 1993 | .87 |
| Mode of contracting HIV | 12 drug-related, 12 sex-related | 5 drug-related, 5 sex-related | .55 |
| Antiretroviral adherence related | |||
| - Knowledge (0 – 100%) | 76.9% | 75.0% | .86 |
| - Motivation (0 – 3) | 2.10 | 1.93 | .37 |
| - Behavioral skills self-efficacy (0 – 9) | 5.21 | 4.64 | .50 |
| Drug-related | |||
| - Knowledge (0 – 100%) | 81.6% | 86.0% | .48 |
| - Personal motivation (0 – 1) | .86 | .93 | .41 |
| - Social motivation (0 – 4) | 3.05 | 3.10 | .68 |
| - Behavioral skills self-efficacy (0 – 10) | 7.79 | 6.55 | .22 |
| - Perceived difficulty of drug risk reduction (0 – 4) | 1.48 | 1.20 | .55 |
| - Heroin use (# days of past 7 days) | 1.62 | 4.60 | .003** |
| - Heroin use (# days of past 30 days) | 7.46 | 18.50 | .012** |
| - Cocaine use (# days of past 7 days) | 2.23 | 4.00 | .072 |
| - Cocaine use (# days of past 30 days) | 8.08 | 13.90 | .16 |
| Sex-related | |||
| - Knowledge (0 – 100%) | 86.4% | 90.0% | .59 |
| - Personal motivation (0 – 1) | .31 | .30 | .79 |
| - Social motivation (0 – 4) | 2.81 | 2.80 | .99 |
| - Behavioral skills self-efficacy (0 – 10) | 6.62 | 4.46 | .022** |
| - Condom use | 2.28 | 2.22 | .91 |
| - Number of regular sexual partners (past 30 days) | 1.15 | 1.20 | .83 |
| - Number of sexual partners (past 30 days) | 3.77 | 3.90 | .97 |
Note.
Significant difference between groups (p < .05)
Of the 21 participants who completed a Post-intervention assessment, and were thus included in the outcome analyses, 19 (90%) completed all four of the intervention group sessions while 2 participants completed three of the four sessions. Overall, participants could be characterized as long-term drug users, with a mean duration of heroin use and cocaine use of 16 and 19 years, respectively. Though all participants were currently enrolled in drug treatment, they reported using both heroin and cocaine an average of 7 of the past 30 days. On average, participants reported their most recent unprotected sexual activity as within the past two months, having four sexual partners in the past month, and having one regular sexual partner in the past month.
3.1 Antiretroviral adherence
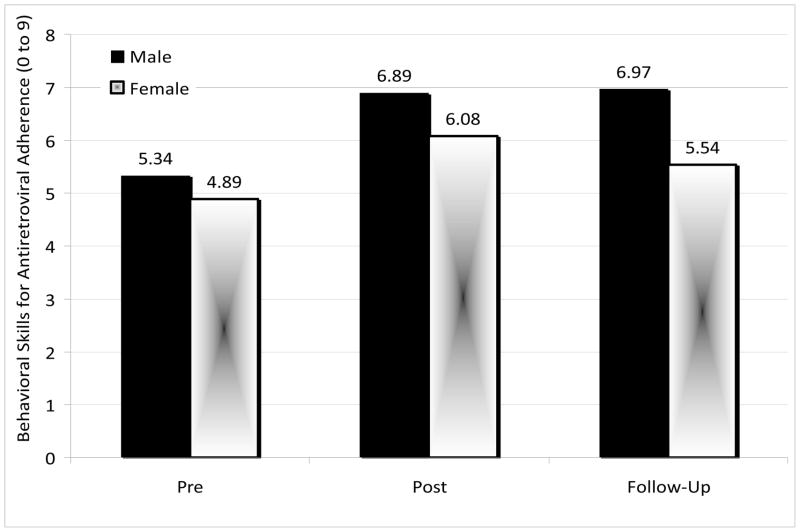
A significant Intervention effect was demonstrated, F (1, 19) = 10.71, p = .004), with regard to antiretroviral adherence self-efficacy (Behavioral Skills) from Pre-intervention (mean = 5.22, SD = 2.45) to Post-intervention (mean = 6.64, SD = 2.06). There were no Gender effects or Gender × Intervention interaction effects, indicating that both males and females benefitted equally well. This effect remained significant at Follow-up, F (2, 30) = 4.60, p = .02. There was no significant difference between Post-intervention and Follow-up scores, indicating no significant decay of effects (Figure 1).
Figure 1.
Mean behavioral skills for antiretroviral adherence by gender (n=17)
Note. A significant intervention effect was demonstrated from Pre-intervention to Post-intervention (p = 0.004), and this effect remained significant at Follow-up (p = 0.02). No other comparisons were significant (p > 0.33).
3.2 Sex-related HIV risk reduction
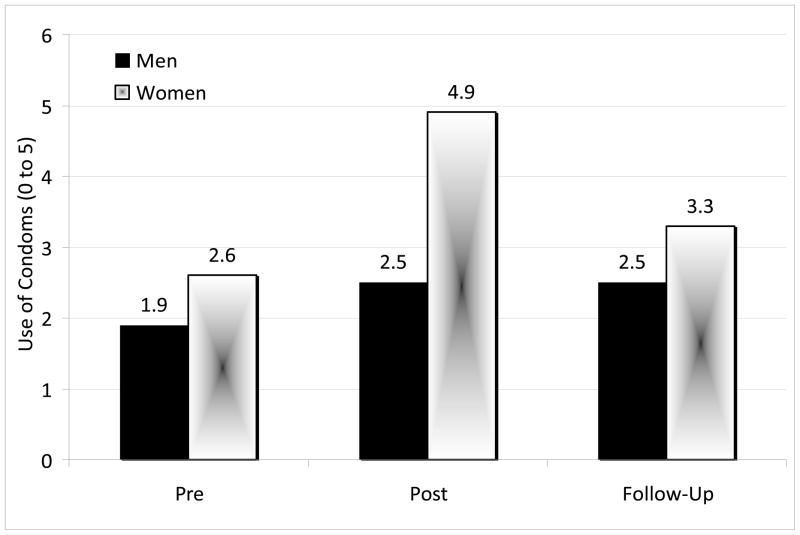
Univariate analyses of variance with repeated measures (on Information, Motivation, Behavioral Skills, and Reported Behaviors) were conducted to test for Intervention and Gender effects. A marginally significant improvement was demonstrated from Pre-intervention to Post-intervention in participants’ knowledge (Information) about safer sex, F (1, 18) = 4.25, p = .054. Overall, participants’ knowledge improved from 83% (SD = 20.8%) accuracy at Pre-intervention to 93% (SD = 13.4%) at Post-intervention. There were no additional effects pertaining to sex-related HIV knowledge. In terms of Motivation to use condoms or other protection, a marginally significant Intervention effect on personal motivation emerged at Post-Intervention. A chi-square test with Yates’ correction, χ2 (1, n = 21) = 3.49, p = .06, demonstrated that 67% of participants reported wanting to engage in safer sex at Pre-Intervention whereas 81% of participants reported wanting to engage in safer sex at Post-intervention. Improvement in motivation to engage in safer sex continued over time: 82.4% of participants reported wanting to engage in safer sex at Follow-up. With regard to Behavioral Skills, a significant Intervention effect was demonstrated when examining scores at Pre-intervention (mean = 6.41, SD = 2.55) vs. Post-intervention (mean = 7.74, SD = 2.01), F (1, 19) = 6.69, p = .018, and this upward trend continued through the Follow-up assessment (mean = 8.05, SD = 1.69) though there was no significant difference in this outcome at Post-intervention vs. Follow-up (p = 1.00). Finally, in terms of reported sexual-risk reduction Behavior, a significant Intervention effect was found for reported condom use when comparing scores at Pre-intervention vs. Post-intervention, F (1, 18) = 17.36, p < .001. In addition, a Gender effect and a Gender × Intervention effect showed that females reported increasing their use of condoms more than males, F (1, 18) = 5.69, p = .028, and females improved relatively more than males in this regard, F (1, 18) = 8.54, p = .009 (Figure 2). This pattern of effects remained significant at Follow-up, F (2, 30) = 4.31, p = .023, indicating no evidence of decay from Post-intervention to Follow-up (p = .21).
Figure 2.
Mean reported condom use by gender (n=17)
Note. A significant Intervention effect was demonstrated from Pre-intervention to Post-intervention (p < 0.001); a Gender effect (p = 0.028) and a Gender × Intervention effect were also demonstrated (p = 0.009). The Intervention effect remained significant from Pre-intervention to Follow-up (p = 0.023), as did the Gender effect (p = 0.044).
3.3 Drug-related HIV risk reduction
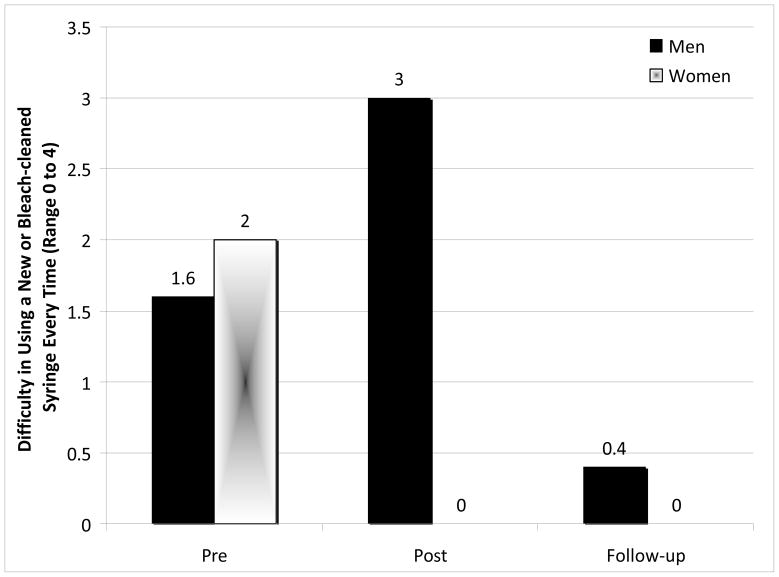
Univariate analyses of variance with repeated measures (on Information, Motivation, Behavioral Skills, and Reported Behaviors) were conducted to test for Intervention and Gender effects. No significant Intervention effects were demonstrated with regard to changes in drug-related HIV Knowledge (p =.16) nor Social Motivation (p >.71). An Intervention × Gender effect was demonstrated for drug-risk reduction Behavioral Skills, F (1, 10) = 7.05, p = .024. Females demonstrated a significantly more pronounced reduction in their perceived difficulty of incorporating drug-risk reduction skills (i.e., using new or bleach-cleaned needles) from Pre- to Post- intervention compared with males (Figure 3). A chi-square analysis with Yates’ correction showed that more participants reported wanting to engage in safer drug use at Post-intervention vs. Pre-intervention (95.2% vs. 81.0%, respectively), χ2 (1, n = 21) = 3.65, p = .055. This trend in motivation to engage in safer drug use continued over time: 94.1% of participants reported wanting to engage in safer drug use at Follow-up.
Figure 3.
Mean perceived difficulty using new or bleach-cleaned needles by gender (n=17)
Note. An Intervention × Gender effect was demonstrated for perceived difficulty using new or bleach-cleaned needles (p = 0.024). None of the effects remained significant at follow-up (p > 0.13).
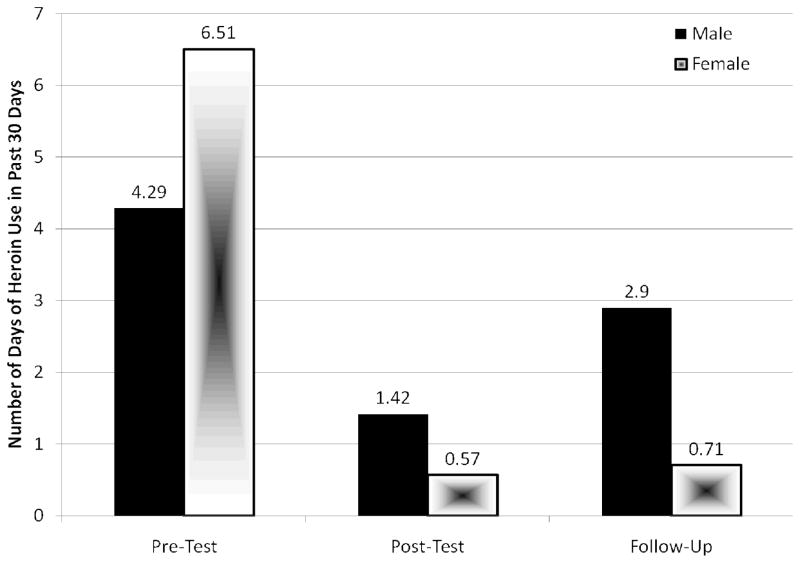
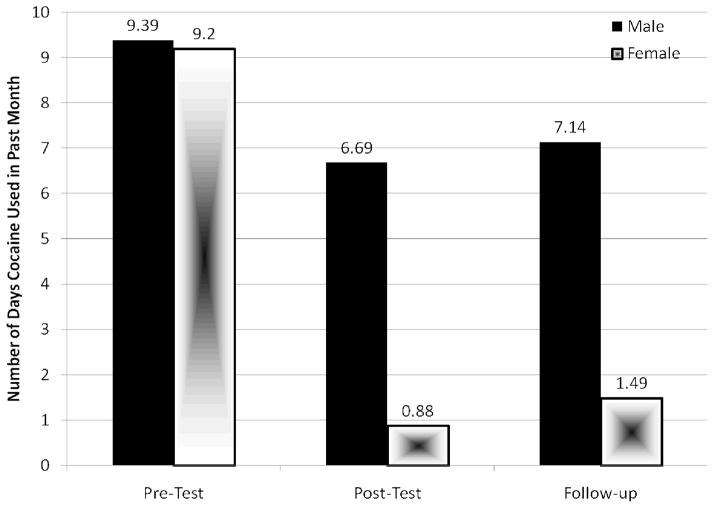
A significant Intervention effect was demonstrated with regard to reported drug use Behavior following the intervention. Specifically, participants reported significantly reducing the frequency of heroin use from Pre-intervention (mean = 7.02 days in the past month, SD = 10.2) to Post-intervention (mean = 0.87 days in the past month, SD = 2.1), F (1, 19) = 8.19, p = .010, and this effect was marginally significant at Follow-up (mean = 2.00 days in the past month, SD = 4.13), F (2, 30) = 4.02, p = .06 (Figure 4). Participants also reported significant reductions in cocaine use from Pre-intervention (mean = 7.96 days in the past month, SD = 8.56) to Post-intervention (mean = 3.90 days in the past 30 month, SD = 5.59), F (1, 19) = 4.55, p = .046, to Follow-up (mean = 4.81 days in the past month), F (2, 30) = 4.48, p = .02 (Figure 5).
Figure 4.
Mean frequency of heroin use in the past 30 days by gender (n = 17)
Note. A significant intervention effect was found from Pre-intervention to Post-intervention (p = 0.01) and this effect remained marginal at Follow-up (p = 0.06).
Figure 5.
Mean frequency of cocaine use in the past 30 days by gender (n = 17)
Note. A significant intervention effect was found from Pre-intervention to Post-intervention (p = 0.046) and this effect remained at Follow-up (p = 0.020).
4.0 Discussion
We conducted a preliminary study of the brief Community-Friendly Health Recovery Program for HIV-infected persons (CHRP+), adapted from a longer evidence-based risk reduction and antiretroviral adherence intervention targeting HIV-infected IDUs. This study provides evidence of feasibility and preliminary evidence of efficacy of the brief CHRP+ intervention. Enhancements were demonstrated in antiretroviral adherence skills as well as key HIV risk reduction outcomes. The encouraging effect sizes produced by CHRP+ were similar to previous EBIs targeting this priority population11, yet it is premature to make direct comparisons until prospective, randomized controlled trials are conducted.
Significant enhancements in the antiretroviral adherence-related outcomes were observed with regard to behavioral skills required to take HIV medications according to regimen. Improvements were found in all measured aspects of sex-risk reduction. Thus, participants demonstrated significant gains in sex-risk reduction knowledge, motivation to reduce sex-risk behavior, behavioral skills related to engaging in reduced sexual risk, and reduced risk behavior. The injection drug use outcomes included enhancements in behavioral skills for drug risk reduction as well as reduced frequency of heroin and cocaine use.
Overall, the CHRP+ intervention had a particularly robust influence on participants’ behavioral skills, with improvements emerging across sex- and drug-risk reduction as well as antiretroviral adherence domains. The intervention effects also remained durable from Post-intervention to the 3-month Follow-up point. Due to the small sample size, the design only allowed for a detection of relatively large effect sizes although we observed improvement across several different types of HIV risk reduction outcomes. These findings are particularly encouraging with regard to sex-risk reduction outcomes as prior behavioral interventions have typically produced relatively smaller effect sizes that have decayed precipitously following the intervention19.
The gender effects that were observed across sex- and drug-risk domains indicate that females responded remarkably well with regard to risk reduction skills and reduced risk behavior. These findings are consistent with prior research demonstrating that HIV-infected drug-using women and at-risk women respond best to HIV interventions that focus on effective communication, partner negotiation skills, and developing healthier relationships relationships20, 21, all of which are addressed in multiple modalities throughout the CHRP+ intervention. These specific findings are noteworthy as they suggests that female drug users – who often face the challenge of negotiating safer practices with their partners22,23 – were successful at learning and applying the HIV risk reduction skills and behaviors that were emphasized.
An important objective of the present study was to contribute to the growing research literature devoted to adapting and implementing EBIs in community-based clinical settings settings9–10,12. Adaptation models have recently become available (e.g., ADAPT-ITT12) that may greatly assist with the formidable process of maximizing both the acceptability and efficacy of EBIs in clinical settings. In the present study, the adaptation process resulted in a significantly abbreviated intervention that produced favorable effect sizes across the primary HIV risk domains (e.g., from Pre- to Post-Intervention: increased condom use, Cohen f = 0.43 and decreased frequency of drug use, Cohen f = 0.31 for heroin use and Cohen f = 0.19 for cocaine use) and a higher than average retention rate among engaged participants (72% vs. 65%) compared with previous interventions targeting IDUs11. Optimizing EBIs in this manner may thus allow treatment providers to more efficiently reach priority populations in clinical settings where they are concentrated.
The limitations of this study should also be acknowledged. Principally, this preliminary efficacy trial was conducted without a control group. Thus, we cannot rule out the possibility that outcomes were influenced by factors extraneous to the intervention tested. Among our primary outcomes, however, were documented objective improvements (e.g., expected effect size for gains in participants’ HIV risk reduction knowledge11). Particularly in view of the sensitivity of the areas queried, these improvements would seem to be unaffected by demand characteristics, although there is always a chance that participants increased their risk reduction knowledge via non-intervention sources. Second, assessment of antiretroviral adherence was limited. Although improving outcomes relevant to antiretroviral adherence was among our intervention targets, we did not implement a stringent assessment of adherence such as the use of MEMS caps. We did, however, use established ACASI self-report measures that have been accepted as ways to measure changes in adherence outcomes16. Finally, due to the study design and sample size, it is premature to make direct comparisons to existing EBIs or to specify how particular IDU subpopulations (e.g., homeless persons) may respond to the CHRP+ intervention.
Study limitations notwithstanding, the reported outcomes are promising and indicate the benefits of systematically adapting and implementing less-cumbersome EBIs targeting priority populations. Such efforts may maximize the utility of EBIs and thereby more efficiently address the new HIV infections transmitted via priority populations. Indeed, the primary intervention effects (sex and drug risk reduction and antiretroviral adherence) produced by CHRP+ were very similar or stronger in terms of effect size when compared with previous EBI approaches targeting HIV-infected IDUs11. This study thus conveys hope that EBIs may be successfully adapted for optimal use in clinical settings where they are most needed.
Acknowledgments
The authors would like to thank the National Institute on Drug Abuse (NIDA) for ongoing research and career development support [K23 DA017015 (Copenhaver), K23 DA022143 (Bruce), and K24 017072 (Altice)].
References
- 1.Margolin A, Avants SK, Warburton LA, Hawkins KA, Shi J. A randomized clinical trial of a Manual-guided risk reduction intervention for HIV- positive injection drug users. Health Psychology. 2003;22 (2):223–228. [PubMed] [Google Scholar]
- 2.Avants SK, Margolin A, Usubiaga MH, Doebrick C. Targeting HIV-related outcomes with intravenous drug users maintained on methadone: A randomized clinical trial of a harm reduction group therapy. Journal of Substance Abuse Treatment. 2004;26:67–78. doi: 10.1016/S0740-5472(03)00159-4. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. HIV/AIDS in the United States. Atlanta: US Department of Health and Human Services; 2008. Specific populations. Retrieved May 20, 2009 from www.cdc.gov/hiv/resources/reports/hiv3rddecade/pdf/chapter4.pdf. [Google Scholar]
- 4.Copenhaver M, Fisher J. Experts outline ways to improve the decade-long U.S. trend of 40,000 new HIV infections per year. AIDS & Behavior. 2006 doi: 10.1007/s10461-005-9034-x. (published online 1/06) [DOI] [PubMed] [Google Scholar]
- 5.Institute of Medicine. Bridging the Gap Between Practice and Research: Forging Partnerships With Community-Based Drug and Alcohol Treatment. Washington, DC: National Academy Press; 1998. [PubMed] [Google Scholar]
- 6.Safren SA, Wingood G, Altice FL. Strategies for primary HIV prevention that target behavioral change. Clinical Infectious Disease. 2007;15(45):300–307. doi: 10.1086/522554. [DOI] [PubMed] [Google Scholar]
- 7.Sholomskas DE, Syracuse-Siewert G, Rounsaville BJ, Ball SA, Nuro KF, Carroll KM. We don’t train in vain: a dissemination trial of three strategies of training clinicians in cognitive-behavioral therapy. Journal of Consulting and Clinical Psychology. 2005;3(1):06–15. doi: 10.1037/0022-006X.73.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morgenstern J, Morgan TJ, McCrady BS, Keller DS, Carroll KM. Manual-guided cognitive behavioral therapy training: A promising method for disseminating empirically supported substance abuse treatments to the practice community. Psychology of Addictive Behaviors. 2001;5:83–88. [PubMed] [Google Scholar]
- 9.Knudsen HK, Roman PM. Modeling the use of innovations in private treatment organizations: The role of absorptive capacity. Journal of Substance Abuse Treatment. 2004;16:353–361. doi: 10.1016/s0740-5472(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 10.Schoenwald SK, Hoagwood K. Effectiveness, transportability, and dissemination of interventions: What matters when? Psychiatric Services. 2001;52(9):1190–1197. doi: 10.1176/appi.ps.52.9.1190. [DOI] [PubMed] [Google Scholar]
- 11.Copenhaver MM, Johnson BT, Lee IC, Harman JJ, Carey MP the SHARP Research Team. Behavioral HIV risk reduction among people who inject drugs: Meta-analytic evidence of efficacy. Journal of Substance Abuse Treatment. 2006;31:163–171. doi: 10.1016/j.jsat.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wingood GM, DiClemente RJ. The ADAPT-ITT Model: A novel method of adapting evidence-based HIV interventions. Journal of Acquired Immune Deficiency Syndrome. 2008;47:S40–S46. doi: 10.1097/QAI.0b013e3181605df1. [DOI] [PubMed] [Google Scholar]
- 13.Fisher JD, Fisher WA. Changing AIDS-risk behavior. Psychological Bulletin. 1992;111:455–447. doi: 10.1037/0033-2909.111.3.455. [DOI] [PubMed] [Google Scholar]
- 14.Fisher JD, Fisher WA, Amico KR, Harman JJ. An information-motivation-behavioral skills model of adherence to antiretroviral therapy. Journal of Health Psychology. 2006;25:462–473. doi: 10.1037/0278-6133.25.4.462. [DOI] [PubMed] [Google Scholar]
- 15.Copenhaver M, Margolin A, Avants K, Warburton L. Intervening effectively with drug abusers infected with HIV: Taking into account the potential for cognitive impairment. Journal of Psychoactive Drugs. 2003;35(2):209–218. doi: 10.1080/02791072.2003.10400002. [DOI] [PubMed] [Google Scholar]
- 16.Fisher JD, Cornman DH, Osborn CY, Amico KR, Fisher WA, Friedland GA. Clinician–initiated HIV risk reduction intervention for HIV-positive persons: Formative research, acceptability, and fidelity of the OPTIONS project. Journal of Acquired Immune Deficiency Syndrome. 2004;37(2):S78–S87. doi: 10.1097/01.qai.0000140605.51640.5c. [DOI] [PubMed] [Google Scholar]
- 17.Station M, Leukefeld C, Logan T, Zimmerman R, Lynam D, Milich R, Martin C, McClanahan K, Clayton R. Gender differences in substance use and initiation of sexual activity. Population Research and Policy Review. 1999;18:89–100. [Google Scholar]
- 18.Stein JA, Nyamathi A. Gender differences in relationships among stress, coping, and health risk behaviors in impoverished, minority populations. Personality and Individual Differences. 1999;26:141–157. [Google Scholar]
- 19.Copenhaver MM, Lee IC, Margolin A. Successfully integrating an HIV risk reduction intervention into a community-based substance abuse treatment program. The American Journal of Drug and Alcohol Abuse. 2007;33(1):109–120. doi: 10.1080/00952990601087463. [DOI] [PubMed] [Google Scholar]
- 20.Wingood GM, DiClemente R, Mikhail I, Lang DL, McCree DH, Davies SL, Hardin JW, Hook EW, Saag M. A randomized controlled trial to reduce HIV transmission risk behaviors and sexually transmitted diseases among women living with HIV: The WiLLOW program. Journal of Acquired Immune Deficiency Syndrome. 2004;37(21):58–67. doi: 10.1097/01.qai.0000140603.57478.a9. [DOI] [PubMed] [Google Scholar]
- 21.Exner TM, Seal DW, Ehrhardt AA. A review of HIV interventions for at-risk women. AIDS & Behavior. 1997;1(2):93–124. [Google Scholar]
- 22.Tross S, Campbell AN, Cohen LR, Calsyn D, Pavlicova M, Miele GM, Hu MC, Haynes L, Nugent N, Gan W, Hatch-Maillette M, Mandler R, McLaughlin P, El-Bassel N, Crits-Christoph P, Nunes EV. Effectiveness of HIV/STD Sexual Risk Reduction Groups for Women in Substance Abuse Treatment Programs: Results of NIDA Clinical Trials Network Trial. Journal of acquired immune deficiency syndromes. 2008;48(5):581–589. doi: 10.1097/QAI.0b013e31817efb6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams SP, Gardos PS, Ortiz-Torres B, Tross S, Ehrhardt AA. Urban women’s negotiation strategies for safer sex with their male partners. Women & health. 2001;33(3–4):33–48. [PubMed] [Google Scholar]