Abstract
Purpose
We recently reported that curcumin attenuates radiation (IR) induced survival signaling and proliferation in human neuroblastoma (NB) cells. Also, in endothelial system, we demonstrated that NFκB regulates IR-induced telomerase activity (TA). Accordingly, we investigated the effect of curcumin in inhibiting IR-induced NFκB dependent hTERT transcription, TA and cell survival in NB cells.
Methods and Materials
SK-N-MC or SH-SY5Y cells exposed to IR, treated with curcumin (10nM–100nM) with or without IR were harvested after 1h through 24h. NFκB dependent regulation was investigated either by luciferase reporter assays using pNFκB-, pGL3-354-, pGL3-347-, pUSE-IκBα-Luc, p50/p65 or RelA siRNA transfected cells. NFκB activity was analyzed using EMSA and hTERT expression using QPCR. TA was determined using TRAP assay and, cell survival using MTT and clonogenic assay.
Results
Curcumin profoundly inhibited IR-induced NFκB. Consequently, curcumin significantly inhibited IR-induced TA and hTERT mRNA at all time points investigated. Furthermore, IR-induced TA is regulated at the transcriptional level by triggering TERT promoter activation. Moreover, NFκB becomes functionally activated after IR and mediates TA upregulation by binding to the κB-binding region in the promoter region of the TERT gene. Consistently, elimination of NFκB-recognition site on telomerase promoter or inhibition of NFκB by IκBα mutant compromises IR-induced telomerase promoter activation. Significantly, curcumin inhibited IR-induced TERT transcription. Consequently, Curcumin inhibited hTERT mRNA and TA in NFκB overexpressed cells. Furthermore, curcumin enhanced the IR-induced inhibition of cell survival.
Conclusions
These results strongly suggest that curcumin inhibits IR-induced TA in an NFκB dependent manner in human NB cells.
Keywords: Telomerase activity, NFκB, Radiosensitization, Neuroblastoma, Curcumin
INTRODUCTION
The enzyme telomerase maintains the telomeric ends of chromosomes and is intimately involved in the proliferative potential of both normal and malignant cells (1). Telomerase is a ribonucleoprotein complex composed of: (i) telomerase RNA component (2); (ii) telomerase-associated proteins (3); and (iii) the most important catalytic subunit, telomerase reverse transcriptase (TERT) (4). Using hTR as a template, hTERT adds tandem repeats of DNA sequence TTAGGG to chromosome termini, elongating the telomeres. Loss of telomerase in differentiating cells and up-regulation of telomerase in cancer cells has been associated with the synthesis of hTERT mRNA (1, 4). Studies have demonstrated that expression of hTERT mRNA is essential for activation of telomerase in cancer cells (5). In fact, hTERT expression parallels TA in cancer cells suggesting a transcriptional mechanism of telomerase regulation (4). To that end, activation of a telomere maintenance mechanism seems to be indispensable for the immortalization of human cells (6). Given the prevalence of telomerase in tumors and its absence in normal cells, TA has been widely studied as a biomarker for the diagnosis and prognosis of various adult and childhood neoplasms including NB (7).
NB is one of the most frequent extra cranial solid tumors in children that accounts for 8–10% of all childhood cancers (8) and 15% of childhood cancer fatalities (9). NB exhibit a remarkable heterogeneity with respect to clinical behavior, ranging from spontaneous regression or differentiation with favorable outcome to a rapid progression with poor outcome, despite multimodal therapy. Recently, TA was shown to be a prognostic indicator of unusual predictive strength in NB (7). Evidently, studies have demonstrated that TA may discriminate between prognostically different subsets of NB (7, 10) and emerged as an independent predictor of clinical outcome with greater prognostic impact than even clinical stage (11).
Ionizing radiation (IR) delivered to NB sites has several well-recognized applications. Event-free survival was significantly improved (76%) with radiotherapy, compared to chemotherapy alone (46%) (12). Traditionally, IR is delivered in 2Gy fractions (5 days/week) to total doses of 50 to 75Gy in ~5–7 weeks. In this context, studies have shown that IR can induce TA (13). To that end, regulation of hTERT appears to be influenced by different transcription factors (TF) in various cellular contexts (5). The promoter region of TERT contains the recognition sequence for several TFs including AP1, AP2, AP4, NFκB (14), SP1, Myc/Max and CRE. More recently, we demonstrated that IR induces NFκB in human NB cells (15, 16) and NFκB mediates the IR-induced TA (17). Therefore inhibition of IR-induced NFκB may inhibit TA and associated clonal expansion in NB cells.
Curcumin is known to suppress NFκB (15) and down-regulate the expression of NFκB–regulated genes involved in survival, proliferation, angiogenesis, invasion, and metastasis. This phytochemical has been shown to modulate various mechanisms linked with radioresistance, such as quenching ROS, down-regulating COX-2, MRP, Bcl-2, and survivin expression, inhibiting PI3K/AKT activation, suppressing growth factor signaling pathways, and inhibiting STAT3 activation (18–20). Moreover, in clinical trials, cancer patients have not shown adverse effects with doses from 2000 to 8000 mg/day (21). In addition, it has been demonstrated that curcumin injected peripherally crossed the blood-brain barrier (22). Furthermore, studies have demonstrated that curcumin inhibits TA and induced apoptosis in human cancer cell lines (23). We recently demonstrated that curcumin inhibits NFΚB mediated radioprotection by reverting IR modulated apoptosis related genes in human NB cells (15). Accordingly, in this study we investigated whether curcumin can inhibit IR-induced NFΚB dependent TA and thereby confers radiosensitivity in NB cells.
MATERIALS AND METHODS
Cell Culture; Curcumin treatment and Irradiation experiments
SK-N-MC cells were cultured as reported in our earlier studies (15,16). SH-SY5Y cells were maintained as monolayer cultures in DMEM/F-12 50/50 supplemented with NEAA, MEM vitamins and 10% FBS. Curcumin (Sigma-Aldrich, St.Louis, MO) stock (500mM in DMSO) solution was further diluted in plain media to a ‘working’ concentration of 50µM. For curcumin treatment, cells were treated with 10, 20, 50,100nM curcumin and allowed to incubate for 24h. To determine the effect of curcumin on IR-induced modulations, cells treated with curcumin (3h) were then exposed to IR (2Gy) using Gamma Cell 40 Exactor (Nordion International Inc, Ontario, Canada) at a dose rate of 0.81 Gy/min. Mock irradiated cells were treated identical except that cells were not subjected to IR. Irradiated cells were incubated for additional 1 through 72h. All experiments were repeated at least three times in each group.
Plasmid preparation, DNA Transfection and Luciferase reporter assay
The plasmid constructs, pGL3-354-Luc, pGL3-347-Luc, pUSE-IκB(s32A/s36A)-Luc, pTAL-Luc, pNFκB-Luc and pCRE-Luc were amplified, purified and transfected as described earlier (17). Furthermore, NFκB overexpression studies were performed as described in our earlier studies (24). Cell lysates were assayed for luciferase activity as per the manufacturer’s protocol (Biovision Research Products, Mountain View, CA).
Electrophoretic Mobility Shift Assay
Nuclear protein extraction, EMSA and supershift assays were performed as described earlier (15, 25).
Immunoblotting
Total protein extraction and immunoblotting was performed as described earlier (26) using rabbit anti-p65, anti-p50 (Biolegend, San Diego, CA), anti-IκBα or mouse anti-α-tubulin (Santa Cruz Biotechnology Inc., Santa Cruz, CA) polyclonal antibodies.
Telomerase Assay
Telomerase repeat amplification protocol (TRAP) was performed as described earlier (17). Telomerase extended products were amplified using QPCR and the relative quantity was assessed after background (heat-inactivated) subtraction and calculating the ratio of telomeric extended products to that of 36bp internal control. Values were then normalized to mock-IR control and expressed as fold change. Group-wise comparisons were made using ANOVA with Tukey’s post-hoc correction.
Cell survival by MTT and clonogenic assay
Cell survival was analyzed using MTT and clonogenic assays as described in our previous studies (20).
QPCR
IR-induced NFκB dependent regulation of hTERT mRNA expression was analyzed by QPCR using hTERT primers (5’-TGACACCTCACCTCACCCAC-3’; 5’-CAG TGTCTTCCGCAAGTTCAC-3’). We used β-actin (5’-ATGACCCAGATCATGTTTGA-3’; 5’-TACGACCAGAGGCATACA G-3’) as a positive control and a negative control without template RNA was also included. QPCR was performed as described earlier (20).
RESULTS
Curcumin inhibits IR-induced NFκB-DNA binding activity
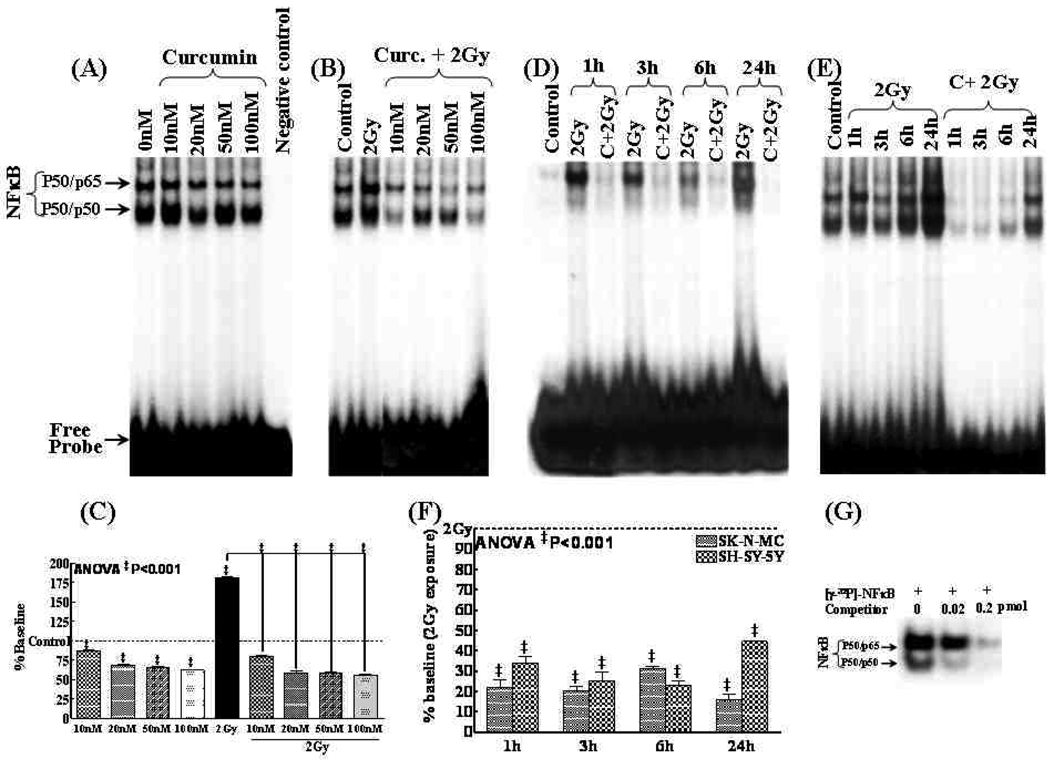
Previously we established a decreasing NFκB activity with increasing concentration of curcumin in SK-N-MC cells (15). Consistently, in this study we observed a dose dependent decrease (P<0.001) in NFκB activity in SH-SY5Y cells exposed to Curcumin (Fig. 1A) with a maximal inhibition after 100nM (Fig. 1C). Furthermore, we have shown a dose dependent suppression of IR-induced NFκB with Curcumin in SK-N-MC cells (15). Herein, while SH-SY5Y cells exposed to IR showed a significant NFκB induction (Fig 1B), we observed a complete suppression of IR-induced NFκB with increasing concentrations of curcumin (Fig. 1C). Finally, we investigated the kinetics of the curcumin influenced inhibition of IR-induced NFκB in SK-N-MC and SH-SY5Y. IR significantly induced NFκB at all time points investigated in these cells (Fig. 1D&E) and this induced NFκB activity is persistently suppressed (P<0.001) with curcumin (Fig. 1F). Competitively reduced NFκB (to 47% and 36.4%) with 0.02 and 0.2pmol of homologous unlabeled NF-κB specific-double stranded oligonucleotide (Fig. 1G) confirm the specificity of the shifted band observed.
Figure 1.
NFκB DNA-binding activity in SH-SY5Y cells (A) treated with curcumin (10, 20, 50, 100nM); (B) treated with curcumin 3h prior to 2Gy exposure and harvested after 24h. (C) Densitometry showing reduction in NFκB activity by curcumin in a dose dependent manner compared to either Mock-IR or 2Gy exposure. NFκB DNA-binding activity in (D) SK-N-MC and (E) SH-5YSYcells exposed to 2Gy with or without curcumin (100nM for 3h) and, harvested after 1–24h. (F) Densitometry revealing curcumin associated significant inhibition of IR-induced NFκB activity in NB cells. ANOVA with tukey’s post-hoc correction was used to compare between groups (G) Specificity of NFκB-DNA binding activity. Nuclear protein obtained from SK-N-MC cells exposed to IR and harvested after 3h was incubated in the absence (lane 1) or presence (lanes 2 and 3) of increased concentrations of homologous unlabelled competitor for five minutes and then probed with [γ-32P]-ATP labeled NFκB specific oligonucleotide.
Curcumin inhibits IR-induced functional activation of NFκB
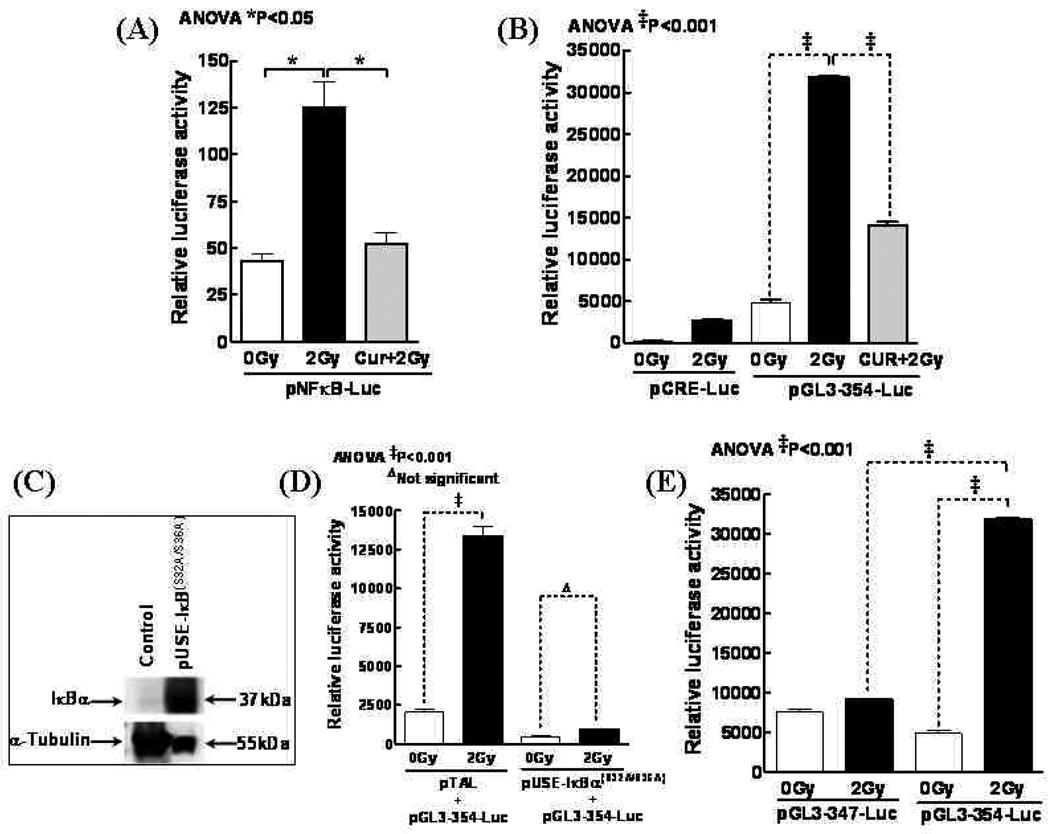
Furthermore, to determine whether IR initiates transcriptional activation of NFκB, and curcumin reverts this induced response, SH-SY5Y cells were transfected with a pNFκB-Luc plasmid construct, exposed to 2Gy with or without Curcumin, and analyzed for luciferase activity after 24h. Compared to mock-IR, 2Gy induced 2.9-fold increase in luciferase activity, indicating that IR could specifically initiate transcriptional activation of NFκB (Fig. 2A). Furthermore, curcumin significantly reduced IR-induced luciferase activity almost to the basal levels (1.2 fold) demonstrating its potential in attenuating IR-induced NFκB transcription.
Figure 2.
(A) Luciferase assay showing curcumin inhibits IR functionally activated NFκB. SH-SY5Y cells were transfected with pNFκB-Luc and then exposed to 2 Gy with or without Curcumin (100nM). (B) SH-SY5Y cells transfected with pCRE-Luc or pGL3-354 were mock irradiated, exposed to 2Gy or treated with Curcumin and exposed to 2Gy. The transfected cells were further incubated for 24h, and then lysed for the luciferase assay. (C) The ectopic expression of IκBα(S32A/S36A). SH-SY5Y cells were transiently transfected with the indicated plasmid and incubated for 24h. (D) A luciferase reporter assay showing the inhibitory effect of ectopically expressed IκBα(S32A/S36A) on IR-induced transcription of the telomerase gene. Cells co-transfected with either pGL3-354 and a non-specific construct pTAL, or pGL3-354 and pUSEI-IκBα(S32A/S36A), were mock irradiated or exposed to 2Gy. (E) Deleting NFκB binding site on the telomerase promoter attenuates 2Gy induced transcriptional activation. SH-SY5Y cells transfected with mutant (pGL3-347) or wild-type (pGL3-354) construct and incubated for 16h were mock irradiated or exposed to 2Gy. Cells were then harvested at 24h post-irradiation. Data shown represent mean and standard deviation (SD) of three independent experiments. Significant decrease in luciferase activity (P<0.001) in the absence of an intact NFκB binding site.
Curcumin inhibits IR-induced hTERT mRNA
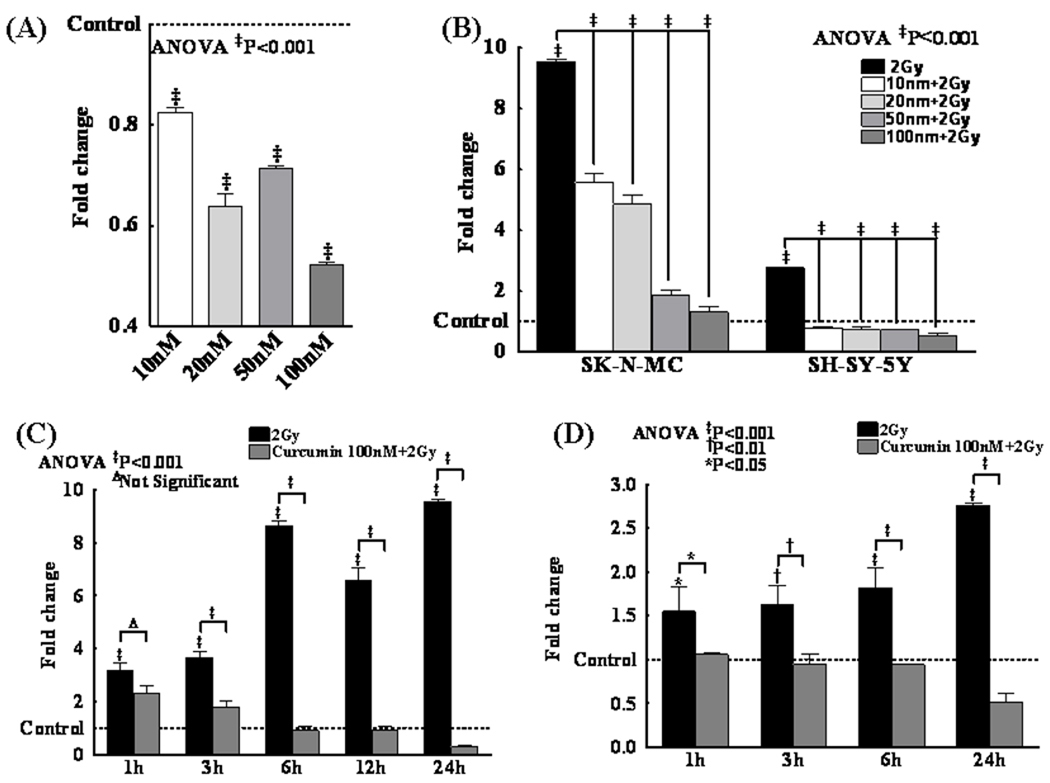
Curcumin significantly (P<0.001) inhibited hTERT mRNA levels (Fig. 3A) in NB cells. Conversely, IR significantly (P<0.001) induced hTERT mRNA in these cells (Fig. 3B) with a robust induction in SK-N-MC cells. However, curcumin profoundly (P<0.001) induced a dose dependent inhibition of IR-induced hTERT mRNA (Fig. 3B). Analyzing hTERT mRNA kinetics in this setting, IR-associated (P<0.001) persistent induction of hTERT mRNA in both SK-N-MC and SH-SY5Y cells was significantly inhibited in the presence of Curcumin (Fig. 3C&D).
Figure 3.
(A) hTERT mRNA expression in SK-N-MC and SH-5YSY cells following treatment with different concentrations of curcumin (10, 20, 50, 100nM) alone or (B) in combination with IR (2Gy). Compared to control or 2Gy irradiated groups, curcumin significantly reduced the hTERT mRNA levels in a dose dependent manner. Sustained inhibition (1h through 24h) of IR-induced hTERT mRNA by curcumin (100nM) in (C) SK-N-MC and (D) SH-SY5Y cells.
Curcumin inhibits IR-induced TA
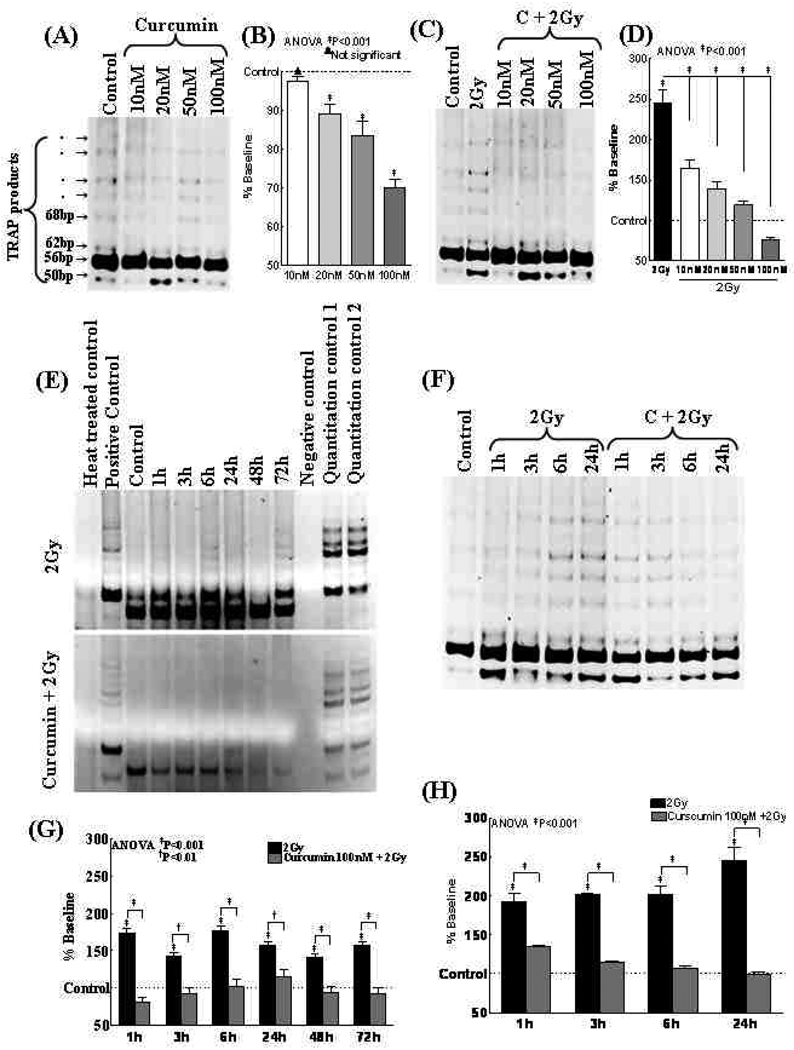
As a stand-alone compound Curcumin significantly (P<0.001) decreased TA with increasing concentrations (Fig. 4A&B). However, IR markedly (P<0.001) induced TA in NB cells (Fig. 4C). Conversely, we observed a significant (P<0.001) and dose-dependent inhibition of IR-induced TA levels with curcumin (Fig. 4D). Furthermore, IR persistently and significantly (P<0.001) induced TA in SK-N-MC (Fig. 4E) and SH-SY5Y (Fig. 4G) cells. Conversely, curcumin attenuated the IR-induced TA to the basal levels or even to much lesser degree in both cell lines (Fig. 4F&H).
Figure 4.
(A) Representative gel showing TA in SH-SY5Y cells treated with 10, 20, 50, 100nM Curcumin. (B) Densitometry analysis showing curcumin-induced dose dependent inhibition of TA in SH-SY5Y cells. (C) Representative gel showing TA in SH-SY5Y cells exposed to either mock-IR, IR (2Gy) or treated with Curcumin (10, 20, 50 or 100nM) and exposed to IR. (D) Densitometric analysis showing a dose dependent inhibition of IR-induced TA by Curcumin. (E & F) Representative gel pictures showing TA in (E) SK-N-MC and (F) SH-SY5Y cells exposed to either mock-IR, IR (2Gy) or treated with Curcumin (100nM) and exposed to IR. TA was assessed after 1, 3, 6, 24, 48 and 72h post-IR. (G & H) Densitometric analysis showing IR persistently activates TA in NB cells. Curcumin treatment persistently and significantly inhibited IR-induced TA.
Curcumin inhibits IR-induced hTERT transcription
SH-SY5Y cells transfected with pGL3-354-Luc plasmid, which contains TERT promoter with recognition sequences for several transcription factors including NFκB, were exposed to 0 Gy or 2 Gy. Luciferase activity revealed a significant (P<0.001) hTERT transcription increase in cells exposed to 2Gy (Fig. 2B). Parallel experiment with pCRE-Luc showed no such induction (Fig. 2B), suggesting that the observed hTERT induction may not reflect a general increase in transcription following IR. More importantly, curcumin profoundly (P<0.001) reverted IR-induced hTERT transcription in these cells (Fig. 2B).
IR induced NFκB regulates TA
To examine the effect of ecotopic expression of IκBα on the hTERT transcription, SH-SY5Y cells transfected with pUSE-IκB(S32A/S36A) were first confirmed with immunoblotting for increased expression of IκB(S32A/S36A) (Fig. 2C). EMSA analysis showed complete inhibition of IR-induced NFκB activity (data not shown). Furthermore, SH-SY5Y cells were co-transfected with pGL3-354 and pUSE-IκB(S32A/S36A) and exposed to 2Gy. Ectopic expression of IκBα(S32A/S36A) completely shutdown IR-induced hTERT transcription (Fig. 2D). In contrast, cells co-transfected with pGL3-354 and a non-specific pTAL-LUC did not show such suppression of telomerase promoter activation.
Purging NFκB recognition site on the telomerase promoter compromises transcriptional induction by IR
SH-SY5Y cells transfected with pGL3-347, a derivative of pGL3-354 lacking a functional NFκB recognition motif (19), or pGL3-354 were exposed to 2 Gy. Cells transfected with pGL3-354 plasmid showed a significant (P<0.001) increase in telomerase transcription after 2Gy (Fig. 2E). Deleting NFκB binding site (pGL3-347 plasmid) led to a significant reduction (p<0.001) in the ability of IR to induce telomerase transcription (Fig. 2E).
Blocking NFκB inhibits IR-induced hTERT mRNA, TA and cell survival
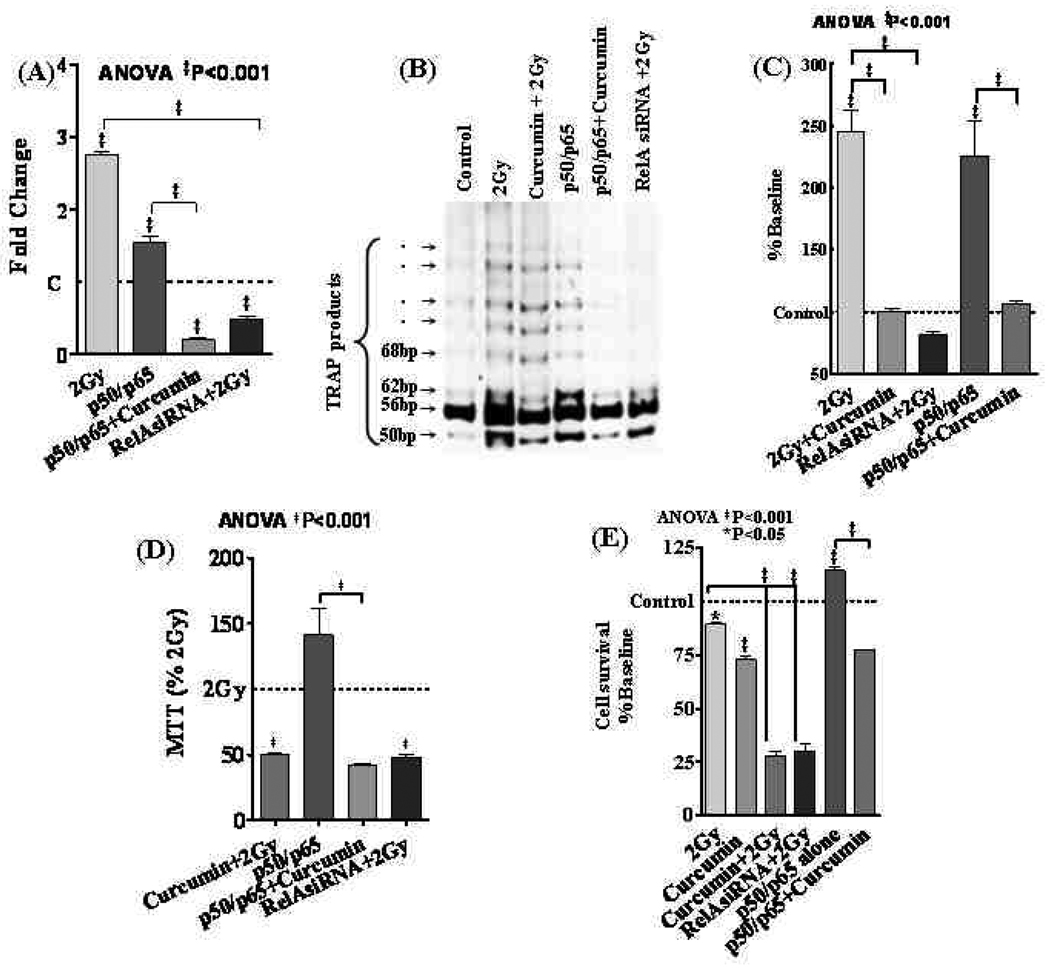
Forced inhibition of NFκB with RelA siRNA significantly inhibited IR-induced NFκB activity (Figs. 5A,C&D). Consistently, muting NFκB resulted in a significant (P<0.001) inhibition of IR-induced hTERT mRNA levels (Fig. 6A). More importantly, we observed that NFκB inhibition resulted in a profound (P<0.001) inhibition of IR-induced TA (Fig. 6B–C) and further reduced IR-inhibited cell survival (Fig. 6D&E).
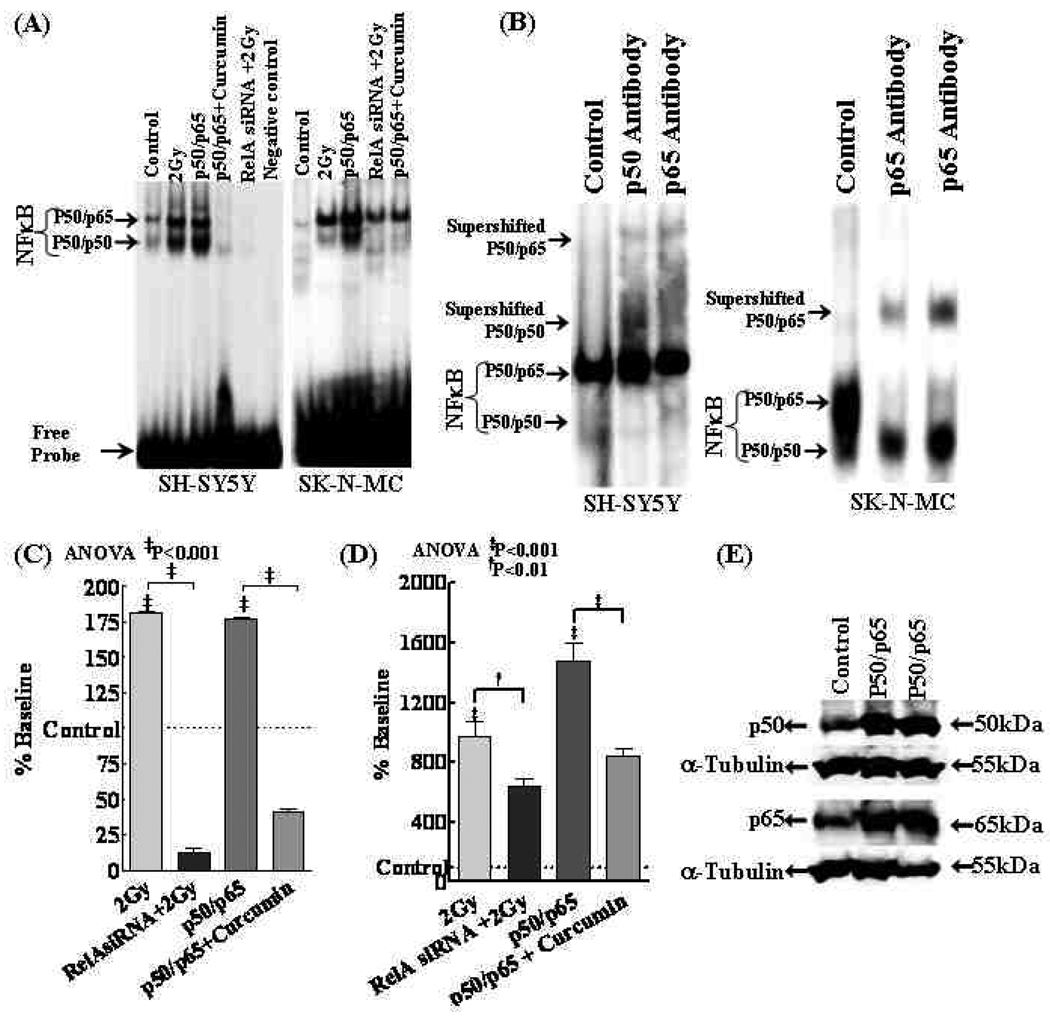
Figure 5.
(A) NFκB-DNA binding activity in SH-SY5Y and SK-N-MC cells either exposed to 2Gy, transiently transfected with NFκB (p50/p65 subunits) with or without curcumin or transfected with RelA siRNA and exposed to IR. (B) Identification of NFκB subunits by supershift assay. The addition of antibodies directed against potential components of NFκB complex resulted in supershift when antibodies of p50 or p65 were used. (C&D) Densitometry analysis showing inhibition of IR-induced NFκB DNA binding activity in RelA siRNA transfected cells and, curcumin associated complete suppression of NFκB-DNA binding activity in p50/p65 overexpressed (C) SH-SY5Y and (D) SK-N-MC cells. (E) Representative immunoblot showing robust induction of p50 and p65 protein levels in NFκB (p50, p65 subunits) overexpressed cells. α-tubulin was used to validate equal loading of samples.
Figure 6.
(A) hTERT mRNA expression in SH-SY5Y cells. Transfection with RelAsiRNA significantly reduced IR-induced hTERT mRNA. Similarly, curcumin treatment significantly suppressed NFκB overexpression-induced hTERT mRNA. (B) TA in SH-SY5Y cells exposed either to 2Gy, treated with curcumin and exposed to 2Gy, transfected with p50/p65 with or without curcumin and transfected with RelAsiRNA and exposed to 2Gy. (C) Densitrometric analysis showing significant inhibition of either 2Gy or p50/p65 induced TA by RelA siRNA and Curcumin respectively. (D) MTT cell survival analysis showing curcumin-associated inhibition of IR-induced and/or NFκB overexpression-induced cell survival in SH-SY5Y cells. Muting NFκB with RelA siRNA significantly reduced IR-induced cell survival. (E) Clonogenic assay showing IR-induced clonal expansion in SH-SY5Y cells. Curcumin (100nM) treatment completely suppressed IR-induced clonal expansion in NB cells. Also, Curcumin significantly suppressed clonal expansion in NFκB overexpressed cells. Furthermore, muting NFκB with RelA siRNA showed a significant inhibition of IR-induced clonal expansion in SH-SY5Y cells.
Curcumin attenuates IR-induced hTERT mRNA, TA and cell survival by targeting IR-induced NFκB
Robust induction of NFκB activity observed in NFκB overexpressed cells serves as the positive controls for the study (Fig. 5A,C&D). Supershift analysis with p50 and p65 antibodies confirmed that the gel shifted bands are indeed NFκB (Fig. 5B). Furthermore, immunoblotting validated a profound increase in p50 and p65 in these cells (Fig. 5E). Conversely, curcumin significantly (P<0.001) inhibited overexpressed NFκB activity. Like-wise, while NFκB overexpression induced hTERT mRNA, curcumin markedly (P<0.001) reverted NFκB-induced transcription of hTERT (Fig. 6A). TRAP showed an induced TA in NFκB overexpressed cells (Fig. 6B) and this induced TA was completely inhibited with curcumin (Fig. 6C). Furthermore, NFκB overexpression induced cell survival (Fig. 6D) and clonal expansion (Fig. 6E) was markedly reduced with curcumin.
DISCUSSION
The fact that most tumors rely on telomerase for immortalization and genomic stabilization makes this enzyme an attractive target for cancer therapy. Telomerase is a ribonucleoprotein reverse transcriptase that is suppressed in normal somatic cells and is activated in cancer cells and immortalized cell lines (28). hTERT acts as a limiting factor for TA. Expression of hTERT mRNA closely coincides with the presence of TA in cancer (29). Inhibition of hTERT expression results in loss of telomere and limits the growth of cancer cells. To that end, studies have clearly demonstrated that IR profoundly induced hTERT expression and TA in a number of tumor types (30). Our group has recently demonstrated that IR induced TA in normal human aortic endothelial cells (17). More importantly, concordant studies have shown that selective inhibition of TA enhances radiosensitization (31, 32). Consistently, here we demonstrate that IR significantly induced hTERT expression and TA and, selective inhibition of IR-induced TA profoundly inhibited the cell survival and clonal expansion. To that note, we observed a maximum induction of hTERT mRNA and TA levels at 24h after IR exposure, consistent with ours (17) and other studies and serves as positive controls for the study.
In addition, we have clearly shown that NFκB mediates IR-induced hTERT expression, transcription and TA and, subsequent cell survival. Regulation of TA is known to occur at various levels, including transcription, mRNA splicing, maturation and modification of hTER and hTERT, transport and sub-cellular localization of each component, and assembly of active telomerase (33). Among the several components of telomerase, only the abundance of hTERT mRNA strongly correlated with the TA (4). Expression of hTERT in human cells is regulated primarily at the level of transcription (33). Results demonstrating the key role of NFκB in the transcriptional control of hTERT presented in the current study suggests that NFκB activates the hTERT promoter. Furthermore, we have demonstrated that IR-induced functionally activated NFκB mediates the upregulation of TA by binding to the κB-binding region in the promoter region of the TERT gene. Consistently, several studies have demonstrated that NFκB regulates the transcriptional activation of hTERT (14). However, this current study, the results clearly provide evidence that IR-induced NFκB is responsible for the induced hTERT transcription and subsequent TA in human NB cells. IR-induced telomerase activation might then possibly play a role in extending cellular lifespan. In support of this hypothesis, the association of NFκB with telomerase-mediated survival advantage has been shown in a number of earlier studies (34).
A number of different therapeutic strategies targeting telomerase are currently in various stages of development and testing. The major strategic paradigm for exploiting telomerase therapeutically is to capitalize on a tumor's dependence on telomerase enzymatic activity. To that note, the relative level of TA carries prognostic information that is particularly accentuated in NB (7) with an ability to discriminate between different subsets (7, 10). In this regard, telomerase inhibitory approaches including antisense oligonucleotides, dominant negative hTERT, nucleoside analogues, dietary polyphenols, G-quadruplex interactive agents, and small molecule inhibitors are being explored. However, one should consider the facts that (a) unlike other anti-cancer agents, telomerase inhibitors exhibit a delayed response proportional to telomere lengths of the tumor cells, (b) long-term administration of the agent may be required depending on the telomeric reserves in the tumor and (c) potential for adverse side effects in highly proliferative normal tissues that possess some level of TA. To that end, curcumin has been demonstrated to have antitumor effects by modulating many potential molecular targets (35). Because of its use as a food additive and its potential for cancer chemoprevention, curcumin has undergone extensive toxicological screening and preclinical investigation in rats, mice, dogs, and monkeys (36). Curcumin has been demonstrated to inhibit NFκB by directly interacting with IKK (35). Consistently, herein, we have shown that curcumin exerts a dose dependent inhibition of IR-induced NFκB activity. Concordantly, the results have clearly demonstrated that curcumin inhibits telomerase transcription and TA in human NB cells. Few previous studies have elucidated the efficacy of curcumin in inhibiting TA in tumor cells (23, 37). However, we present here that curcumin effectively attenuate the IR-induced hTERT transcription, TA and subsequent cell death. More importantly, NFκB overexpression studies clearly delineate the sequential regulation of IR-induced NFκB with curcumin that regulates IR-induced hTERT expression, TA, cell survival and clonal expansion.
Taken together, we demonstrate, for the first time in this study, the potential efficacy of curcumin and a sequential molecular link that is involved in inhibiting IR-induced NFκB dependent TA and clonal expansion in human NB cells. Firstly, exposing human NB cells to low-LET IR induces TA. Secondly, IR-induced TA appeared to be regulated at the transcriptional level by activation of the telomerase catalytic subunit promoter TERT. Next, IR-triggered activation of NFκB is critical for hTERT transcription and activation of telomerase. Purging NFκB recognition site on the telomerase promoter compromises transcriptional induction by IR. Curcumin treatment inhibits IR-induced NFκB transcription and activity. Inhibiting IR-induced NFκB effectively compromised the IR-induced TA and clonal expansion. Consequently, curcumin inhibited IR-induced hTERT transcription in these cells. These findings provide an important insight into the mechanisms involved in local failure of cancer control after radiotherapy. Furthermore, understanding the efficacy of curcumin in targeting and selective manipulation of Rel proteins in induced radioresistance and clonal expansion provide us the platform to the development of a potential “deliverable” to mitigate local failure of NB control after radiotherapy.
ACKNOWLEDGEMENTS
This work was supported by Department of Radiation Oncology at OUHSC research development funds, American Cancer Society (ACS- IRG-05-066-01) and Presbyterian Health Foundation to Natarajan Aravindan, NIH grant (R01 CA112175) from National Cancer Institute, National Institutes of Health, Department of Health and Human Services and Office of Science (BER), US Department of Energy DEFG02-03ER63449 Research grant to Mohan Natarajan.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Notification
Authors (Natarajan Aravindan, Jamunarani Veeraraghavan, Rakhesh Madhusoodhanan, Terence Herman, and Mohan Natarajan) have no conflict of interest. Sources of funding are declared in an Acknowledgements section
REFERENCES
- 1.Ahmed A, Tollefsbol TO. Telomerase, telomerase inhibition, and cancer. J Anti Aging Med. 2003;6:315–325. doi: 10.1089/109454503323028911. [DOI] [PubMed] [Google Scholar]
- 2.Feng J, Funk WD, Wang SS, et al. The RNA component of human telomerase. Science. 1995;269:1236–1241. doi: 10.1126/science.7544491. [DOI] [PubMed] [Google Scholar]
- 3.Harrington L, McPhail T, Mar V, et al. A mammalian telomerase-associated protein. Science. 1997;275:973–977. doi: 10.1126/science.275.5302.973. [DOI] [PubMed] [Google Scholar]
- 4.Meyerson M, Counter CM, Eaton EN, et al. hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell. 1997;90:785–795. doi: 10.1016/s0092-8674(00)80538-3. [DOI] [PubMed] [Google Scholar]
- 5.Takakura M, Kyo S, Kanaya T, et al. Cloning of human telomerase catalytic subunit (hTERT) gene promoter and identification of proximal core promoter sequences essential for transcriptional activation in immortalized and cancer cells. Cancer Res. 1999;59:551–557. [PubMed] [Google Scholar]
- 6.Kim NW, Piatyszek MA, Prowse KR, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 7.Poremba C, Willenbring H, Hero B, et al. Telomerase activity distinguishes between neuroblastomas with good and poor prognosis. Ann Oncol. 1999;10:715–721. doi: 10.1023/a:1008333500733. [DOI] [PubMed] [Google Scholar]
- 8.Bernstein ML, Leclerc JM, Bunin G, et al. A population-based study of neuroblastoma incidence, survival, and mortality in North America. J Clin Oncol. 1992;10:323–329. doi: 10.1200/JCO.1992.10.2.323. [DOI] [PubMed] [Google Scholar]
- 9.Kushner BH. Neuroblastoma: a disease requiring a multitude of imaging studies. J Nucl Med. 2004;45:1172–1188. [PubMed] [Google Scholar]
- 10.Streutker CJ, Thorner P, Fabricius N, et al. Telomerase activity as a prognostic factor in neuroblastomas. Pediatr Dev Pathol. 2001;4:62–67. doi: 10.1007/s100240010108. [DOI] [PubMed] [Google Scholar]
- 11.Poremba C, Scheel C, Hero B, et al. Telomerase activity and telomerase subunits gene expression patterns in neuroblastoma: a molecular and immunohistochemical study establishing prognostic tools for fresh-frozen and paraffin-embedded tissues. J Clin Oncol. 2000;18:2582–2592. doi: 10.1200/JCO.2000.18.13.2582. [DOI] [PubMed] [Google Scholar]
- 12.Castleberry RP, Kun LE, Shuster JJ, et al. Radiotherapy improves the outlook for patients older than 1 year with Pediatric Oncology Group stage C neuroblastoma. J Clin Oncol. 1991;9:789–795. doi: 10.1200/JCO.1991.9.5.789. [DOI] [PubMed] [Google Scholar]
- 13.Finnon P, Silver AR, Bouffler SD. Upregulation of telomerase activity by X-irradiation in mouse leukaemia cells is independent of Tert, Terc, Tnks and Myc transcription. Carcinogenesis. 2000;21:573–578. doi: 10.1093/carcin/21.4.573. [DOI] [PubMed] [Google Scholar]
- 14.Yin L, Hubbard AK, Giardina C. NF-kappa B regulates transcription of the mouse telomerase catalytic subunit. J Biol Chem. 2000;275:36671–36675. doi: 10.1074/jbc.M007378200. [DOI] [PubMed] [Google Scholar]
- 15.Aravindan N, Madhusoodhanan R, Ahmad S, et al. Curcumin inhibits NFkappaB mediated radioprotection and modulate apoptosis related genes in human neuroblastoma cells. Cancer Biol Ther. 2008;7:569–576. doi: 10.4161/cbt.7.4.5534. [DOI] [PubMed] [Google Scholar]
- 16.Aravindan N, Madhusoodhanan R, Natarajan M, et al. Alteration of apoptotic signaling molecules as a function of time after radiation in human neuroblastoma cells. Mol Cell Biochem. 2008;310:167–179. doi: 10.1007/s11010-007-9678-0. [DOI] [PubMed] [Google Scholar]
- 17.Natarajan M, Mohan S, Konopinski R, et al. Induced telomerase activity in primary aortic endothelial cells by low-LET gamma-radiation is mediated through NF-kappaB activation. Br J Radiol. 2008;81:711–720. doi: 10.1259/bjr/57867919. [DOI] [PubMed] [Google Scholar]
- 18.Kunnumakkara AB, Diagaradjane P, Guha S, et al. Curcumin sensitizes human colorectal cancer xenografts in nude mice to gamma-radiation by targeting nuclear factor-kappaB-regulated gene products. Clin Cancer Res. 2008;14:2128–2136. doi: 10.1158/1078-0432.CCR-07-4722. [DOI] [PubMed] [Google Scholar]
- 19.Javvadi P, Segan AT, Tuttle SW, et al. The chemopreventive agent curcumin is a potent radiosensitizer of human cervical tumor cells via increased reactive oxygen species production and overactivation of the mitogen-activated protein kinase pathway. Mol Pharmacol. 2008;73:1491–1501. doi: 10.1124/mol.107.043554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Narang H, Krishna M. Inhibition of radiation induced nitration by curcumin and nicotinamide in mouse macrophages. Mol Cell Biochem. 2005;276:7–13. doi: 10.1007/s11010-005-2241-y. [DOI] [PubMed] [Google Scholar]
- 21.Cheng AL, Hsu CH, Lin JK, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–2900. [PubMed] [Google Scholar]
- 22.Yang F, Lim GP, Begum AN, et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005;280:5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- 23.Cui SX, Qu XJ, Xie YY, et al. Curcumin inhibits telomerase activity in human cancer cell lines. Int J Mol Med. 2006;18:227–231. [PubMed] [Google Scholar]
- 24.Aravindan N, Mohan S, Herman TS, et al. Nitric oxide-mediated inhibition of NFkappaB regulates hyperthermia-induced apoptosis. J Cell Biochem. 2009;106:999–1009. doi: 10.1002/jcb.22079. [DOI] [PubMed] [Google Scholar]
- 25.Madhusoodhanan R, Natarajan M, Veeraraghavan J, et al. NFkappaB signaling related molecular alterations in human neuroblastoma cells after fractionated irradiation. J Radiat Res (Tokyo) 2009;50:311–324. doi: 10.1269/jrr.08110. [DOI] [PubMed] [Google Scholar]
- 26.Natarajan M, Aravindan N, Meltz ML, et al. Post-translational modification of I-kappa B alpha activates NF-kappa B in human monocytes exposed to 56Fe ions. Radiat Environ Biophys. 2002;41:139–144. doi: 10.1007/s00411-002-0143-x. [DOI] [PubMed] [Google Scholar]
- 27.Brockman JA, Scherer DC, McKinsey TA, et al. Coupling of a signal response domain in I kappa B alpha to multiple pathways for NF-kappa B activation. Mol Cell Biol. 1995;15:2809–2818. doi: 10.1128/mcb.15.5.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsu YH, Lin JJ. Telomere and telomerase as targets for anti-cancer and regeneration therapies. Acta Pharmacol Sin. 2005;26:513–518. doi: 10.1111/j.1745-7254.2005.00098.x. [DOI] [PubMed] [Google Scholar]
- 29.Boldrini L, Faviana P, Gisfredi S, et al. Regulation of telomerase and its hTERT messenger in colorectal cancer. Oncol Rep. 2004;11:395–400. [PubMed] [Google Scholar]
- 30.Xing J, Zhu Y, Zhao H, et al. Differential induction in telomerase activity among bladder cancer patients and controls on gamma-radiation. Cancer Epidemiol Biomarkers Prev. 2007;16:606–609. doi: 10.1158/1055-9965.EPI-06-0615. [DOI] [PubMed] [Google Scholar]
- 31.Agarwal M, Pandita S, Hunt CR, et al. Inhibition of telomerase activity enhances hyperthermia-mediated radiosensitization. Cancer Res. 2008;68:3370–3378. doi: 10.1158/0008-5472.CAN-07-5831. [DOI] [PubMed] [Google Scholar]
- 32.Huang CH, Liao ZK, Zhou FX, et al. hTERT promoter enhances the radiosensitivity to gene-radiation therapy of human laryngeal carcinoma transplanted in nude mice. Zhonghua Zhong Liu Za Zhi. 2008;30:733–736. [PubMed] [Google Scholar]
- 33.Cong YS, Wright WE, Shay JW. Human telomerase and its regulation. Microbiol Mol Biol Rev. 2002;66:407–425. doi: 10.1128/MMBR.66.3.407-425.2002. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buser R, Montesano R, Garcia I, et al. Bovine microvascular endothelial cells immortalized with human telomerase. J Cell Biochem. 2006;98:267–286. doi: 10.1002/jcb.20715. [DOI] [PubMed] [Google Scholar]
- 35.Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol. 2006;71:1397–1421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 36.Chainani-Wu N. Safety and anti-inflammatory activity of curcumin: a component of tumeric (Curcuma longa) J Altern Complement Med. 2003;9:161–168. doi: 10.1089/107555303321223035. [DOI] [PubMed] [Google Scholar]
- 37.Mukherjee Nee Chakraborty S, Ghosh U, Bhattacharyya NP, et al. Curcumin-induced apoptosis in human leukemia cell HL-60 is associated with inhibition of telomerase activity. Mol Cell Biochem. 2007;297:31–39. doi: 10.1007/s11010-006-9319-z. [DOI] [PubMed] [Google Scholar]