Abstract
Hepatocyte growth factor (HGF) is a potent antifibrotic protein that inhibits kidney fibrosis through several mechanisms. To study its role in podocyte homeostasis, injury, and repair in vivo, we generated conditional knockout mice in which the HGF receptor, c-met, was specifically deleted in podocytes using the Cre–LoxP system. Mice with podocyte-specific ablation of c-met (podo-met−/−) developed normally. No albuminuria or overt pathologic lesions were detected up to 6 months of age, suggesting that HGF signaling is dispensable for podocyte maturation, survival, and function under normal physiologic conditions. However, after adriamycin treatment, podo-met−/− mice developed more severe podocyte injury and albuminuria than their control littermates. Ablation of c-met also resulted in more profound suppression of Wilms tumor 1 (WT1) and nephrin expression, and podocyte apoptosis after injury. When HGF was expressed ectopically in vivo, it ameliorated adriamycin-induced albuminuria, preserved WT1 and nephrin expression, and inhibited podocyte apoptosis. However, exogenous HGF failed to significantly reduce albuminuria in podo-met−/− mice, suggesting that podocyte-specific c-met activation by HGF confers renal protection. In vitro, HGF was able to preserve WT1 and nephrin expression in cultured podocytes after adriamycin treatment. HGF also protected podocytes from apoptosis induced by a lethal dose of adriamycin primarily through a phosphoinositide 3-kinase (PI3K)/Akt-dependent pathway. Collectively, these results indicate that HGF/c-met signaling has an important role in protecting podocytes from injury, thereby reducing proteinuria.
Keywords: adriamycin nephropathy, c-met, HGF, podocyte, proteinuria
Hepatocyte growth factor (HGF) is a pleiotropic protein that has an important role in the regulation of organ development, tissue homeostasis, injury repair, and regeneration.1,2 Extensive studies have demonstrated that HGF is also a potent antifibrotic factor that prevents the onset and progression of a wide variety of chronic kidney diseases, including diabetic nephropathy, obstructive nephropathy, remnant kidney after 5/6 renal ablation, and chronic allograft nephropathy.3–7 Interestingly, administration of HGF protein or its gene seems particularly effective in ameliorating proteinuric kidney diseases,8,9 in which podocyte dysfunction and/or depletion are thought to have a causative role.10–12 These observations suggest a possible linkage of HGF signaling to podocyte function under physiological and pathological conditions. However, the function of HGF in podocyte maturation, survival, and differentiation in vivo in normal physiologic setting remains poorly understood. Furthermore, very little is known about the role of HGF in regulating podocyte dysfunction and survival in vivo after a variety of insults.
Podocytes are specialized, terminally differentiated visceral epithelial cells that reside on the glomerular basement membrane outside the glomerular capillaries.13 These cells possess unique, sophisticated foot processes, and slit diaphragm, a membrane-like structure that is one of the principal components of the glomerular filtration barrier.14 Not surprisingly, disruption of podocyte integrity will lead to development of proteinuria and glomerulosclerosis. Depending on the severity and duration of injury, podocytes respond to various injurious stimuli in different ways, including dedifferentiation, detachment, and apoptosis.15,16 Emerging evidence indicates that podocytes also undergo epithelial-to-mesenchymal transition after injury by losing their unique features and acquiring mesenchymal markers.17,18 In view of the ability of HGF in regulating epithelial-to-mesenchymal transition and cell survival,5,19 it is plausible to speculate that HGF could have a role in preserving podocyte structure and function after injury, thereby preventing the development of proteinuria.
The biological activities of HGF are mediated by a single receptor, c-met, which belongs to a family of the transmembrane tyrosine kinase receptors that transmit growth factor signals across the plasma membrane by activating multiple downstream signaling events.1,20 Although HGF expression is limited to nonepithelial cells in the kidney, such as fibroblasts, mesangial cells, and endothelial cells, c-met protein is ubiquitously expressed in almost all types of cells.21 Podocytes in normal glomeruli are shown to express c-met protein,21 suggesting that these cells are capable of responding to HGF stimulation.
To elucidate the role of HGF/c-met signaling in podocyte homeostasis and injury/repair in vivo, here we have generated conditional knockout mice in which c-met is specifically ablated in podocytes by using Cre–LoxP recombination system. We show that mice with podocyte-specific deletion of c-met are healthy but develop more severe albuminuria, podocyte lesions, and podocyte loss after injury. In addition, ectopic expression of exogenous HGF ameliorates podocyte dysfunction and apoptosis and reduces proteinuria. Our findings suggest that HGF/c-met signaling is critical for preventing podocyte injury and proteinuria in pathological conditions.
RESULTS
Mice with podocyte-specific ablation of c-met are healthy
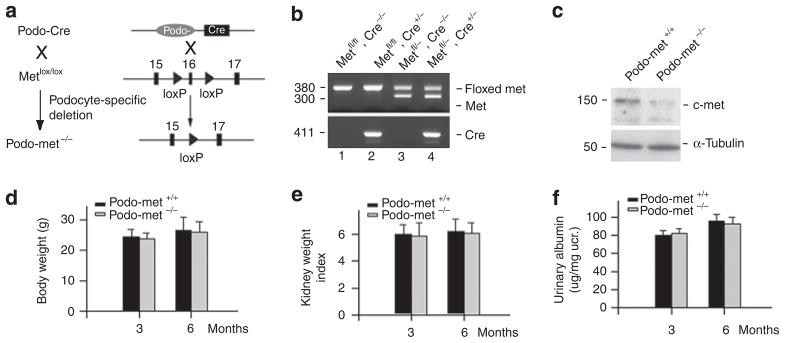
To explore the role of HGF/c-met signaling in podocyte homeostasis in vivo, we generated conditional knockout mice in which c-met gene was specifically disrupted in glomerular podocytes by using the Cre–LoxP system (Figure 1a). Figure 1b shows different genotypes of the mice by PCR analysis of genomic DNA. Mice with podocyte-specific ablation of c-met was designated as podo-met−/− (genotype: c-metfl/fl, Cre +/−; Figure 1b, lane 2), whereas c-met-floxed mice were used as podo-met+/+ controls (genotype: c-metfl/fl, Cre−/−; Figure 1b, lane 1). Western blot analysis demonstrated that c-met protein level was reduced in the isolated glomeruli from podo-met−/− mice, compared with those from control podo-met +/+ littermates (Figure 1c). Notably, the faint c-met protein band seen in the western blot analysis of podo-met−/− mice (Figure 1c) was probably derived from the nonpodocyte glomerular cells, as both mesangial and endothelial cells express c-met protein. There was no difference in body weight, kidney/body weight ratio, and urine albumin level between podo-met−/− mice and their control littermates at 3 and 6 months after birth, respectively (Figure 1d–f). Podocyte numbers and density as shown by Wilms tumor 1 (WT1) staining were similar in podo-met−/− mice and their control littermates. Furthermore, there was no alteration in major slit-diaphragm protein, nephrin, expression and distribution in the glomeruli of podo-met−/− mice, in comparison with the control littermates. Electron microscopy showed an intact ultrastructure of the glomerular filtration barrier in podo-met−/− mice (see below). Together, these results demonstrate that HGF/c-met signaling seems dispensable for podocyte maturation, survival, and function under normal physiologic conditions in vivo.
Figure 1. Mice with podocyte-specific ablation of c-met are healthy.
(a) Experimental design shows the strategy of the crossbreeding of mice to generate podocyte-specific deletion of c-met receptor. (b) Genotyping of the mice by PCR analysis of genomic DNA. Lane 1, genotype: c-metfl/fl, Cre−/−, designated as podo-met +/+ control; Lane 2, genotype: c-metfl/fl, Cre +/−, designated as podo-met−/−, podocyte-specific ablation of c-met; Lane 3, genotype: c-metfl/−, Cre−/−; Lane 4, genotype: c-metfl/−, Cre +/−. (c) Western blot analysis of glomerular c-met protein in podo-c-met−/− mice (c-metfl/fl, Cre +/−) and control podo-met +/+ littermates (c-metfl/fl, Cre−/−). Glomerular lysates were prepared from the mice and immunoblotted with antibodies against c-met and α-tubulin, respectively. (d–f) Mice with podocyte-specific ablation of c-met are phenotypically normal. There was little difference in body weight (d), kidney/body weight index (e), and urinary albumin excretion (f) between podo-c-met−/− mice and their control littermates at different time points (n = 4).
Ablation of c-met aggravates albuminuria and exacerbates WT1 and nephrin loss after adriamycin injury
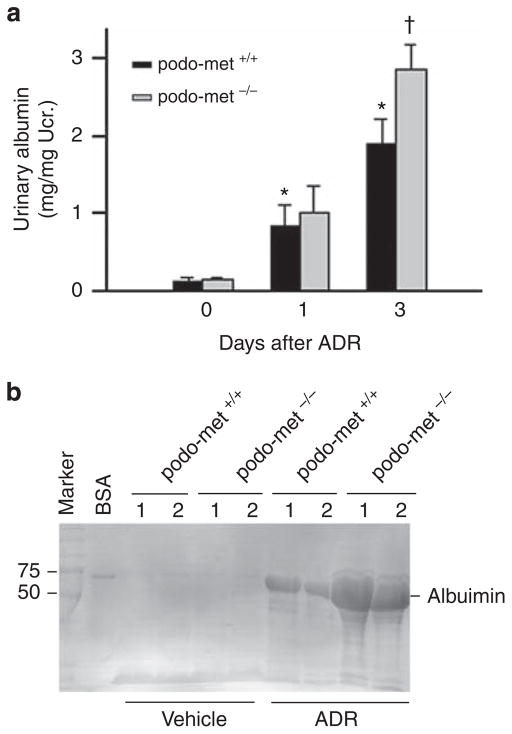
Next, we investigated the role of HGF/c-met signaling in podocyte injury under pathological conditions by challenging podo-met−/− mice with adriamycin (ADR), an agent that specifically damages glomerular podocytes.22 As shown in Figure 2, mice developed albuminuria at day 1 and 3, respectively, after intravenous injection of ADR at a dose of 25 mg/kg body weight. Under same conditions, podo-met−/− mice developed more severe albuminuria at day 3, compared with the control littermates. Sodium dodecyl sulfate–polya-crylamine gel electrophoresis analysis of urine samples after normalization to urinary creatinine revealed that albumin was the major constituent of urine proteins in mice after ADR injury (Figure 2b).
Figure 2. Mice with podocyte-specific ablation of c-met are more susceptible to the development of albuminuria induced by adriamycin (ADR).
(a) Urinary albumin levels in control and podo-c-met−/− mice after ADR injection (25 mg/kg body weight). *P<0.05 versus vehicle control (day 0); †P<0.05 versus control podo-met +/+ mice (n = 5–7). (b) Representative sodium dodecyl sulfate–polyacrylamine gel electrophoresis shows the urinary proteins after normalization to urinary creatinine (Ucr.) in podo-met +/+ and podo-met−/− mice as indicated. Urine proteins after separation were stained with Coomassie blue R-250. BSA, bovine serum albumin. Numbers (1, 2) indicate each individual animal in a given group.
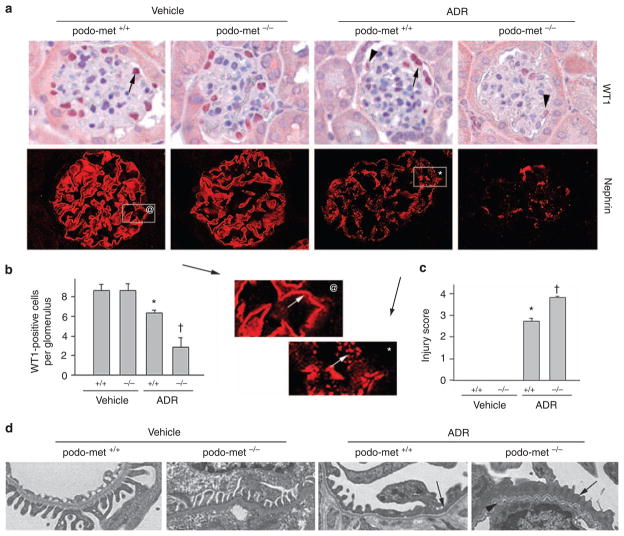
We further examined the expression of WT1, a nuclear zinc finger-containing transcription factor, that is a molecular signature of podocytes and has an essential role in establishing the podocyte-specific gene expression in adult kidney.23,24 As shown in Figure 3a and b, ADR caused a significant decrease in the number of the WT1-positive cells in the glomeruli of podo-met +/+ mice. Ablation of c-met, however, markedly exacerbated WT1 loss after ADR injury (Figure 3a and b). Notably, ablation of c-met not only reduced the number of WT1-positive cells after ADR injury, but also significantly decreased the level of WT1 in the positively stained podocytes (Figure 3a, arrowheads), suggesting the pivotal role of HGF/c-met signaling in preserving WT1 expression in vivo.
Figure 3. Ablation of c-met receptor aggravates podocyte injury induced by adriamycin (ADR).
(a) Immunohistochemical staining for Wilm’s tumor 1 (WT1) and nephrin in different groups as indicated. Arrows indicate WT1-positive cells in the glomeruli. Arrowheads denote weak staining for WT1 in the glomeruli after ADR injection (25 mg/kg body weight). Boxed areas were enlarged to show the distribution pattern of nephrin in control and ADR-treated glomeruli. A fine, linear nephrin staining pattern was observed in normal glomeruli (@), whereas a disorganized, granular staining was observed after ADR injection (*). (b) Quantitative determination of WT1-positive cells in the glomeruli in different groups as indicated. Data are expressed as WT1-positive cells per glomerular cross-section. (c) Semi-quantitative determination of podocyte injury score (as defined by nephrin loss and altered distribution) in different groups. *P<0.05 versus vehicle treatment (n = 4–11). †P<0.05 versus control podo-met +/+ mice after ADR (n = 11). (d) Electron microscopy (EM) shows the ultrastructure of glomerular filtration apparatus in different groups. Representative EM pictures demonstrate podocyte foot process effacement (arrows) and altered glomerular basement membrane (arrowhead) after ADR injection.
Immunofluorescent staining revealed that levels of nephrin were reduced and its distribution was changed from a linear to granular pattern in control podo-met+/+ mice after ADR injury (Figure 3a). As illustrated in the enlarged boxed area, nephrin staining in normal glomeruli exhibited a typical, fine line pattern in the podocytes (Figure 3a@, arrow). The ADR-caused injury not only reduced the overall abundance of nephrin protein, but also converted its linear staining into a disorganized, scattered, and granular pattern (Figure 3a*, arrow). These lesions in nephrin expression and distribution were more severe in podo-met−/− mice after ADR injury, compared with the control littermates (Figure 3a and c). We also examined the ultrastructure of podocyte foot processes and slit diaphragm by electron microscopy. As shown in Figure 3d, foot process effacement was evident and slit diaphragm disappeared in some area of the glomeruli in podo-met +/+ mice after ADR injection. More severe lesions in the foot processes and slit diaphragm, however, were observed in podo-met−/− mice under the same conditions (Figure 3d).
Ablation of c-met promotes podocyte apoptosis after injury
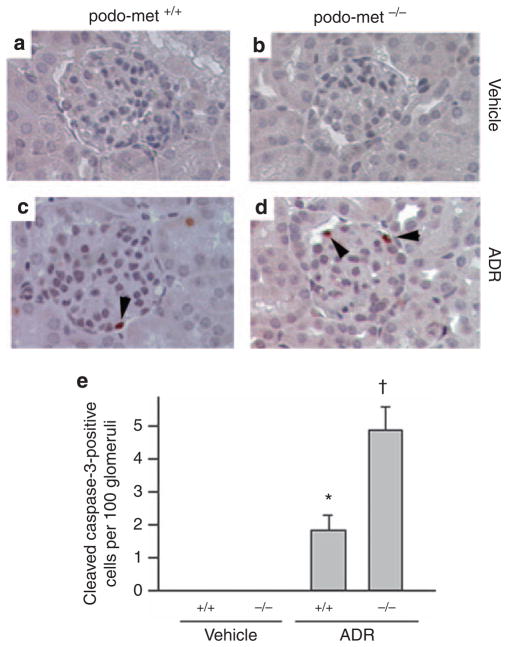
In view of a decrease in the WT1-positive cells in the glomeruli after ADR injury, we next examined whether podocytes undergo apoptosis. Immunohistochemical staining for the cleaved caspase-3 revealed that apoptosis was extremely rare in mice at day 3 after injection of ADR (Figure 4), a time point when significant albuminuria developed (Figure 2). Quantitative determination of apoptotic cells showed about two apoptotic cells among every 100 glomerular cross-sections (Figure 4e). Judged from their localization in the glomeruli, these apoptotic cells appeared to be podocytes (Figure 4c). Interestingly, ablation of c-met in a podocyte-specific manner increased the numbers of apoptotic cells in the glomeruli after ADR injection (Figure 4d and e), suggesting that HGF/c-met signaling has an important role in podocyte survival after injury.
Figure 4. Glomerular cells are more susceptible to apoptosis in mice with podocyte-specific ablation of c-met.
Immunohistochemical staining reveals the cleaved caspase-3-positive cells in glomeruli. (a–d) Representative micrographs show the cleaved caspase-3-positive glomerular cells in control or podo-met−/− mice after injection with vehicle (a and b) or adriamycin (ADR) (c and d) (25 mg/kg body weight). (e) Quantitative determination of the cleaved caspase-3-positive glomerular cells in different groups as indicated. Data are presented as cleaved caspase-3-positive cells per 100 glomerular cross-sections. *P<0.05 versus vehicle controls, †P<0.05 versus control podo-met +/+ mice after ADR injection (n = 4–8).
Expression of exogenous HGF in mice ameliorates podocyte injury and albuminuria
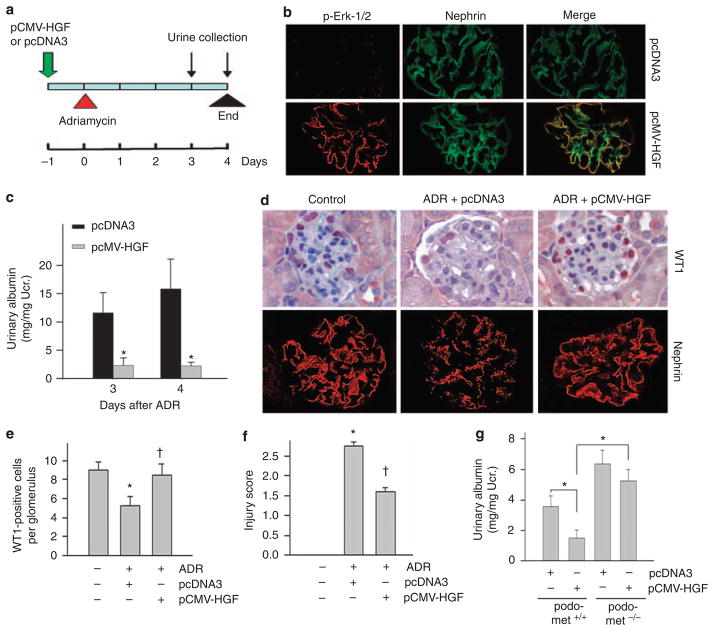
To confirm the protective role of HGF/c-met signaling in preserving podocyte function, we sought to evaluate the effect of exogenous HGF on proteinuric nephropathy in mice. To this end, BALB/c mice were injected intravenously with HGF expression plasmid (pCMV-HGF) or empty vector (pcDNA3) by using a hydrodynamic-based gene delivery approach. Notably, this method produced substantial exogenous HGF in kidney glomeruli and in circulation, as previously reported.8,25,26 To ascertain that glomerular podocytes respond to exogenous HGF, we examined the activation of extracellular signal-regulated kinase-1/2 (Erk-1/2), a major downstream mediator of HGF signaling. As shown in Figure 5, HGF plasmid injection induced a marked phosphorylation of Erk-1/2 in mouse glomeruli. Double immunostaining revealed that phosphospecific Erk-1/2 was largely colocalized with nephrin, suggesting a podocyte-specific response to exogenous HGF expression. We administrated ADR (10 mg/kg body weight) into BALB/c mice at 16 h after HGF plasmid injection (Figure 5a). Heavy albuminuria developed in mice at day 3 and day 4 after ADR injection (Figure 5c). Interestingly, delivery of exogenous HGF gene markedly ameliorated ADR-induced albuminuria in these mice (Figure 5c). Consistent with the albuminuria data, exogenous HGF expression also prevented a reduction of WT1 and nephrin expression induced by ADR. As shown in Figure 5d and e, ADR caused significant loss of WT1 expression in glomerular podocytes; and HGF expression almost completely preserved WT1 protein expression. Similarly, exogenous HGF also preserved nephrin expression and its distribution in podocytes after ADR injury (Figure 5d and f).
Figure 5. Expression of exogenous hepatocyte growth factor (HGF) protects against podocyte injury and albuminuria induced by adriamycin (ADR) in mice.
(a) Experimental design shows the timing of pCMV-HGF plasmid and ADR injections in BALB/c mice. (b) Gene therapy with HGF expression plasmid in vivo induced extracellular signal-regulated kinase-1/2 (Erk-1/2) phosphorylation in glomerular podocytes. BALB/c mice were injected with either empty vector pcDNA3 or pCMV-HGF. Kidney sections were immunostained with specific antibodies against phosphorylated Erk-1/2 and nephrin, respectively. (c) Gene therapy with HGF expression plasmid markedly reduced albuminuria induced by ADR (10 mg/kg body weight) in BALB/c mice. (d–f) Exogenous HGF expression ameliorated podocyte injury (as defined by Wilm’s tumor 1 (WT1) and nephrin loss) in mice. Representative micrographs of WT1 and nephrin staining (d) and quantitative determination of WT1-positive cell number (e) and injury score in different groups (f) are given. *P<0.05 versus vehicle controls; †P<0.05 versus pcDNA3 controls (n = 4–11). (g) Exogenous HGF did not significantly ameliorate albuminuria in podo-met−/− mice after ADR injury. Podo-met−/− mice and their control podo-met +/+ littermates were injected with ADR (25 mg/kg body weight). Urinary albumin was determined at 3 days after ADR injection. *P<0.05 (n = 4–5).
To assess whether the beneficial effect of exogenous HGF is directly related to c-met activation in podocytes, we examined the reno-protective role of exogenous HGF in podo-met−/− mice after ADR injury. As shown in Figure 5g, although ectopic expression of exogenous HGF reduced albuminuria in podo-met+/+ mice after ADR injection, it did not significantly affect albuminuria in podo-met−/− mice in which c-met was specifically ablated in podocytes, suggesting a direct and predominant role of podocyte-specific c-met signaling in conferring HGF renoprotection after ADR injury.
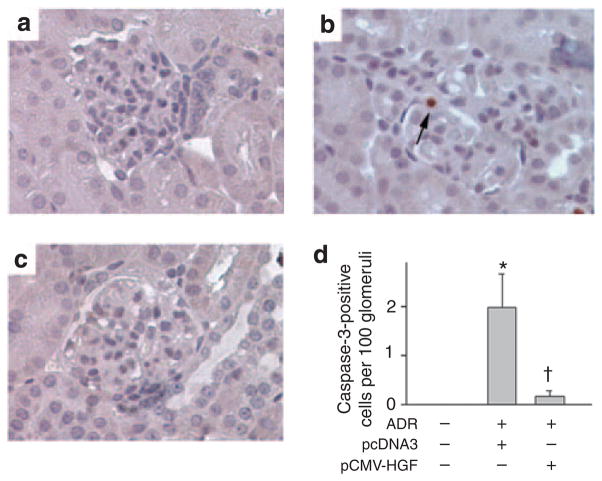
Figure 6 shows the effect of exogenous HGF on podocyte apoptosis induced by ADR. Glomerular cell apoptosis was also detectable, albeit extremely rare, in BALB/c mice after ADR injection. The incidence of apoptosis was about two cells per 100 glomerular cross-sections (Figure 6d). Delivery of exogenous HGF gene almost completely prevented glomerular cells from apoptosis induced by ADR in vivo (Figure 6c and d).
Figure 6. Hepatocyte growth factor (HGF) prevents glomerular cells from apoptosis induced by adriamycin (ADR).
(a–c) Representative micrographs show the cleaved caspase-3-positive glomerular cells in vehicle controls (a), BALB/c mice injected with ADR and pcDNA3 plasmid (b), and BALB/c mice injected with ADR and pCMV-HGF plasmid (c). Arrow indicates cleaved caspase-3-positive cells. (d) Quantitative determination of the cleaved caspase-3-positive glomerular cells in different groups as indicated. Data are presented as cleaved caspase-3-positive cells per 100 glomerular cross-sections. *P<0.05 versus vehicle controls, †P<0.05 versus pcDNA3 controls (n = 4–11).
HGF preserves WT1 and nephrin expression in podocytes after injury in vitro
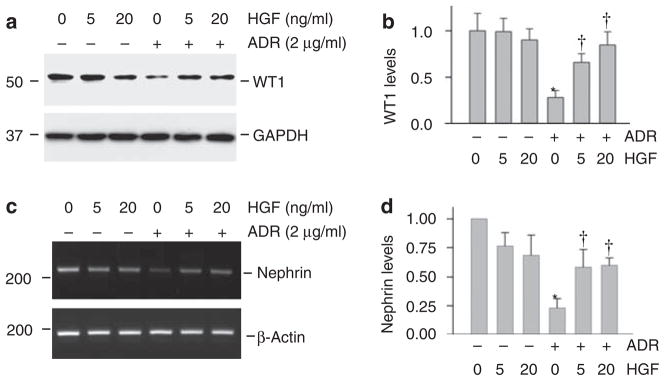
To further investigate HGF protection of podocytes from injury, we used cultured mouse podocytes as an in vitro system. As shown in Figure 7a and b, incubation of mouse podocytes with a sub-lethal dose of ADR (2 μg/ml) for 16 h markedly downregulated WT1 protein expression. Although HGF alone had little effect on WT1 expression, simultaneous incubation with it largely preserved WT1 protein expression after ADR treatment in a dose-dependent manner (Figure 7a and b).
Figure 7. Hepatocyte growth factor (HGF) preserves Wilm’s tumor 1 (WT1) and nephrin expression in cultured mouse podocytes in vitro.
Mouse podocytes were treated with ADR (2 μg/ml) in the absence or presence of different doses of HGF for 24 h. (a and b) HGF largely prevented adriamycin (ADR)-mediated WT1 suppression in mouse podocytes. Representative western blotting results (a) and quantitative data of WT1 protein levels after normalizing with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (b) are presented. Relative WT1 abundances (with value in control group = 1.0) are shown. *P<0.05 versus controls; †P<0.05 versus ADR alone (n = 3). (c and d) HGF preserved nephrin mRNA expression in podocytes after ADR treatment. Representative reverse transcriptase–PCR (RT–PCR) results (c) and quantitative data of nephrin mRNA levels after normalizing with β-actin (d) are presented. Relative nephrin mRNA abundances (with value in control group = 1.0) are shown. *P<0.05 versus controls; †P<0.05 versus ADR alone (n = 3).
We then examined the expression of nephrin, a major slit diaphragm-associated protein, which is a direct transcriptional target of WT1.27,28 In cultured podocytes, nephrin mRNA expression was suppressed after ADR treatment (Figure 7c and d). HGF alone seemed to have the tendency to slightly downregulate nephrin mRNA expression. Simultaneous incubation with HGF, however, clearly preserved nephrin mRNA expression in ADR-treated podocytes (Figure 7c and d).
HGF prevents podocyte apoptosis after injury in vitro
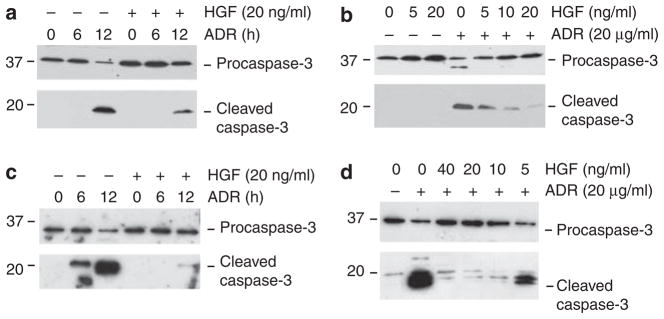
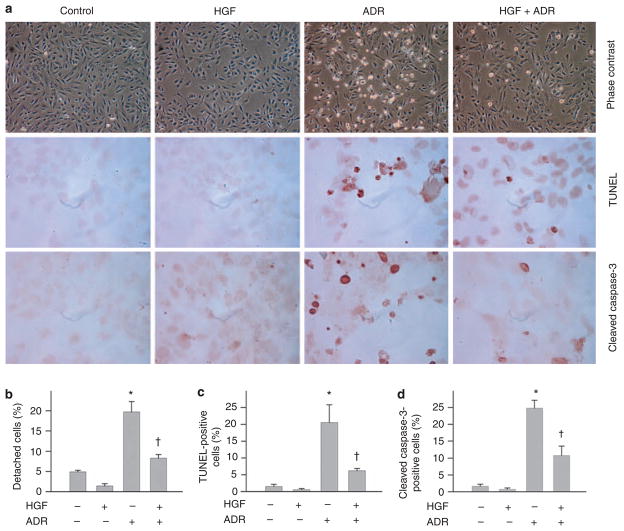
We also examined the ability of HGF in protecting podocytes from apoptosis after injury in vitro. When mouse podocytes were incubated with a lethal dose of ADR (20 μg/ml), these cells underwent apoptosis in a time-dependent manner, characterized by caspase-3 cleavage and activation (Figure 8a). Treatment of the cells with HGF largely prevented caspase-3 cleavage induced by ADR. The antiapoptotic activity of HGF was also dose dependent (Figure 8b). Similar pro-survival action of HGF was found in cultured human podocytes as well (Figure 8c and d). Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling and immunostaining for cleaved caspase-3 also confirmed the cytoprotective ability of HGF in podocytes after a lethal dose of ADR (Figure 9).
Figure 8. Hepatocyte growth factor (HGF) abolishes adriamycin (ADR)-induced caspase-3 cleavage in cultured podocytes.
Mouse or human podocytes were treated with a lethal dose of ADR (20 μg/ml) in the absence or presence of different concentrations of HGF for various periods of time as indicated. (a and b) Western blot results reveal that HGF prevented the ADR-induced caspase-3 cleavage in mouse podocytes in a time- and dose-dependent manner. (c and d) HGF also prevented the ADR-induced caspase-3 cleavage in human podocytes in a time- and dose-dependent manner. Human podocytes were treated with fixed amounts of ADR (20 μg/ml) and HGF (20 ng/ml) for various periods of time as indicated (c), or different doses of HGF for 12 h (d). Podocyte lysates were immunoblotted with specific antibodies against pro-caspase-3 or cleaved caspase-3, respectively.
Figure 9. Hepatocyte growth factor (HGF) prevents podocyte apoptosis induced by adriamycin (ADR) in vitro.
Human podocytes were treated with a lethal dose of ADR (20 μg/ml) in the absence or presence of HGF (20 ng/ml) for 12 h. (a) Representative micrographs of phase-contrast, terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) staining and immunostaining for cleaved caspase-3. (b) Quantitative data of the detached cells after various treatments as indicated. (c) Quantitative data showing the TUNEL-positive cells after various treatments as indicated. (d) Quantitative data showing the cleaved caspase-3-positive cells after various treatments as indicated. *P<0.05 versus controls; †P<0.05 versus ADR alone (n = 4).
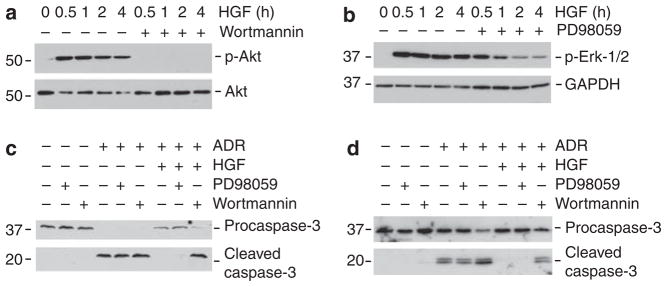
We next investigated the potential signal pathway leading to HGF protection of podocytes. As shown in Figure 10a and b, HGF markedly and rapidly activated protein kinase B/Akt and mitogen-activated protein kinase Erk-1/2 phosphorylation and activation. Incubation with wortmannin, a specific inhibitor of Akt upstream phosphoinositide 3-kinase (PI3K), or PD98058, a specific inhibitor of Erk-1/2 upstream Mek1, blocked Akt and Erk-1/2 phosphorylation, respectively (Figure 10a and b). As shown in Figure 10c and d, blockade of Akt activation by wortmannin, but not Erk-1/2 activation by PD98059, abolished the ability of HGF to prevent podocyte apoptosis induced by ADR, suggesting that HGF promotes podocyte survival through a PI3K/Akt-dependent pathway.
Figure 10. Hepatocyte growth factor (HGF) inhibits adriamycin (ADR)-induced caspase-3 cleavage through the phosphoinositide 3-kinase (PI3K)/Akt signal pathway.
(a and b) Western blot analysis shows that HGF induced Akt (a) and Erk-1/2 (b) phosphorylation and activation in human podocytes. Cell lysates were immunoblotted with specific antibodies against phosphospecific Akt and total Akt (a), or phospho-specific Erk1/2 and glyceraldehyde 3 phosphate dehydrogenase (GAPDH) (b). PI3K inhibitor (wortmannin, 10 nM) or Mek1 inhibitor (PD98059, 10 μM) effectively blocked the HGF-induced Akt and Erk-1/2 activation, respectively. (c and d) Blockade of Akt activation by wortmannin, but not Erk-1/2 activation by PD98059, abolished the antiapoptotic effect of HGF in both mouse (c) and human podocytes (d). Cell lysates were immunoblotted with specific antibodies against pro-caspase-3 or cleaved caspase-3, respectively.
DISCUSSION
Podocyte dysfunction and depletion are increasingly recognized as causative events in the pathogenesis of proteinuria and glomerulosclerosis in many common forms of proteinuric kidney diseases, such as diabetic nephropathy and focal and segmental glomerulosclerosis.10,16,29 In this context, identification of crucial factors that regulate podocyte survival, differentiation, function, and injury/repair will be of vital importance in understanding the mechanism underlying the onset of proteinuria, and in exploring new therapeutic strategies. In this study, we demonstrate that HGF/c-met signaling has a critical role in preventing podocyte injury under pathologic conditions. The ablation of HGF signaling in podocytes by conditional knockout of its c-met receptor renders the mice more susceptible to ADR-induced podocyte injury, resulting in more severe albuminuria. Conversely, ectopic expression of exogenous HGF gene in mice protects podocytes and ameliorates albuminuria in ADR nephropathy. Our findings clearly establish that HGF/c-met signaling is cytoprotective in preventing podocyte dysfunction and apoptosis in diseased kidney.
Despite its expression in podocytes, knockout of c-met receptor in mice does not result in any overt abnormality in glomerular structure and function. This suggests that HGF signaling is dispensable for podocyte maturation, survival, and function under physiological conditions. Given the functional redundancy of many peptide growth factors in vivo,30 it is not completely surprising that mice with podocyte-specific deletion of c-met receptor have normal phenotype, with no effect on body and kidney growth, and on kidney function. This finding is compatible with previous observation in liver, in which knockout of c-met receptor specifically in hepatocytes does not cause any detrimental effect under physiological conditions.31 It is worthwhile to point out that the Cre recombinase driven under podocin promoter begins to express in podocytes during late capillary loop stage of glomerular development in the newborn mice.32 Therefore, it remains to be determined whether HGF/c-met signaling has a role in mediating the initial differentiation of podocytes during early kidney development.
This study provides a clear in vivo evidence for a critical role of HGF/c-met signaling in preventing podocyte dysfunction and apoptosis after injury. Both loss- and gain-of-function strategies indicate that HGF specifically acts on podocytes to protect against dedifferentiation and apoptosis triggered by injurious stimuli. Although recent studies point to alterations in glomerular endothelium at 6 days after ADR injection,33 the cause–effect relationship of these endothelial lesions with proteinuria remains uncertain in this model. Podocyte dysfunction occurs early in ADR nephropathy,34 in which albuminuria is eminent in 1–3 days (Figure 2). Indeed, exogenous HGF only ameliorates albuminuria in podo-met +/+ mice, but not in podo-met−/− mice (Figure 5g), supporting a direct and predominant role of podocyte-specific c-met activation in conferring HGF renoprotection against albuminuria in this model. Adriamycin is well known to specifically damage podocytes in the glomeruli and to trigger kidney lesions characterized by proteinuria and glomerulosclerosis,22 though its pathogenic mechanism in vivo remains poorly understood. We show here that ADR induces WT1 and nephrin loss in vivo, suggesting that podocyte dedifferentiation could be attributable to the development of albuminuria. Given that podocyte apoptosis is an extremely rare event (Figure 4), it seems unlikely that podocyte depletion is the initial cause that results in the onset of albuminuria in this model. In this context, downregulation of WT1 gene and suppression of nephrin expression seem to be two major pathogenic alterations in glomerular podocytes in vivo after ADR administration that undoubtedly leads to podocyte dysfunction. Indeed, low dose of ADR (2 μg/ml) also suppresses both WT1 and nephrin expression in cultured mouse podocytes in vitro (Figure 7). The observation that HGF preserves WT1 and nephrin in podocytes both in vivo and in vitro suggests that this signaling is able to specifically target the key pathogenic events in ADR nephropathy. Notably, in addition to a reduced mRNA expression, nephrin loss could also result from a shedding of its protein from the cell surface due to protease-mediated cleavage and cytoskeleton redistribution.35 It remains to be determined whether HGF also protects podocytes from nephrin shedding after injury.
WT1 is a key transcription factor that has an essential role in early kidney development. In adult kidney, WT1 expression is restricted to mature podocytes, and is thought to be crucial in trans-activating multiple podocyte-specific genes, including nephrin and podocalyxin. Along this line, downregulation of WT1 expression by ADR could be an important pathway leading to suppression of podocyte-specific genes, thereby causing podocyte dysfunction and the onset of proteinuria. This idea is substantiated by the observation that nephrin is downregulated in podocytes by ADR. It should be pointed out that HGF alone seems to have the tendency to inhibit nephrin expression in cultured podocytes (Figure 7), consistent with a previous report.36 However, this slight inhibition of nephrin expression by HGF treatment alone in cultured podocytes in vitro is unlikely relevant to physiological conditions in vivo, as previous studies show that sustained expression of exogenous HGF for a long period of time (8 weeks) in mice does not cause any impairment of glomerular filtration and kidney function.37 Importantly, concomitant incubation with HGF and ADR, a situation that closely resembles HGF administration in the pathological setting, is able to upregulate and preserve nephrin expression.
Severe and/or sustained injury undoubtedly causes podocyte apoptosis that eventually leads to podocyte depletion and glomerulosclerosis in the kidney.16,38 Studies in animal models have established that podocyte numbers and density have a decisive role in the genesis of proteinuria and glomerulosclerosis.12,38 In that regard, the ability of HGF in promoting cell survival could also have a role in protecting podocyte after injury.39,40 Indeed, this study demonstrates that HGF is able to protect cultured podocytes against apoptosis induced by a lethal dose of ADR (20 μg/ml), a concentration that is 10-fold higher than that induces podocyte dedifferentiation, as manifested by WT1 and nephrin suppression. Even at that high concentration of ADR, HGF is able to protect podocytes from undergoing apoptotic cell death, as evidenced by caspas-3 activation and terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling staining. This cytoprotective effect of HGF against lethal dose of ADR is largely mediated by PI3K/Akt pathway, as blockade of Akt activation, but not of Erk-1/2, abolishes the ability of HGF to inhibit caspase-3 activation in podocytes. Notably, HGF seems to be more potent in protecting human podocytes from apoptosis induced by ADR, in comparison with their mouse counterparts (Figure 8). This probably reflects that human HGF preferentially works on human podocytes, despite a high degree of homology between human and mouse HGF proteins.41 Accordingly, as the HGF gene injected into mice is of human origin, the effects of exogenous HGF in vivo are probably underestimated in this study.
In summary, we have demonstrated that HGF/c-met signaling has an important role in protecting podocytes from injury. HGF not only prevents podocytes from dedifferentiation and dysfunction after sublethal injury, but also protects podocytes against apoptosis after more severe insult. Our studies suggest that protection of podocyte from injury and depletion by exogenous HGF could be an effective way to prevent proteinuria and glomerulosclerosis under pathological conditions.
MATERIALS AND METHODS
Generation of the podocyte-specific c-met knockout mice and genotyping
The c-met-floxed mice were created by homologous recombination using a c-met gene fragment with loxP sites flanking exon 16, as described previously,31,42 and kindly provided by Dr Snorri S. Thorgeirsson (National Cancer Institute, NIH, Bethesda, MD, USA). Transgenic mice expressing Cre recombinase under the control of a 2.5-kb fragment of the human podocin promoter (2.5P-Cre mice) are reported elsewhere.32 By mating c-met-floxed mice with podocin-Cre transgenic mice, mice that were heterozygous for the c-met-floxed allele were generated (genotype: c-metfl/+, Cre+/−). These mice were crossbred to inactivate both c-met alleles by Cre-mediated excision, thereby creating conditional knockout mice in which c-met gene was specifically disrupted in glomerular podocytes (genotype: c-metfl/fl, Cre+/−). The breeding protocol also generated heterozygous littermates (genotype: c-metfl/+, Cre+/−), and wild type and several groups with different genotypes (c-metfl/fl, Cre−/−; c-metfl/+, Cre−/−; c-met +/+, Cre+/−). Polymerase chain reaction was used for c-met loxP and Cre genotyping. All mice with ages between 1–3 months were used in the study except otherwise indicated. Littermates with genotypes (c-metfl/fl, Cre−/−) were used as podo-met +/+ controls throughout the experiments. Experimental protocols for animals were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh.
Animal models of podocyte injury and proteinuria
Mouse model for podocyte injury and proteinuria was established by intravenous injection of ADR.22 Different genetic backgrounds and strains of mice displayed significant divergences in response to ADR injury. All transgenic mice with C57BL/6J or mixed backgrounds were injected with a high dose of ADR protocol (25 mg/kg body weight),34 whereas BALB/c mice were sensitive to and only received a low dose of the drug (10 mg/kg body weight). Briefly, podo-met−/− mice and their control podo-met +/+ littermates were injected with ADR (doxorubicin hydrochloride; Sigma, St Louis, MO, USA) at the dosage of 25 mg/kg body weight in saline solution through tail vein. All male BALB/c mice weighing 20–22 g were obtained from Harlan Sprague–Dawley (Indianapolis, IN, USA), and injected through the tail vein with ADR at 10 mg/kg body weight. At different time points after ADR injection, mice were killed. Urine, kidney tissue and glomeruli were collected. For introducing exogenous HGF in vivo, mice were administrated with HGF expression plasmid (pCMV-HGF) at 1 mg/kg by a hydrodynamic-based gene transfer technique through rapid injection of a large volume of DNA solution through the tail vein, as described previously.8,25,37 At 16 h after plasmid injection, mice were injected with ADR through tail vein, and killed at day 4. Urine and kidney samples were collected. Mouse backgrounds and ADR protocol used in this study are listed in Table 1.
Table 1.
Mouse model and ADR protocol used in this study
| Mouse strain | ADR protocol (mg/kg body weight) | Albuminuriaa (mg/mg Ucr.) | Figures in this study |
|---|---|---|---|
| Podo-met+/+ and podo-met−/− (with mixed backgrounds) | 25 | 2–6 | 1–4, 5g |
| BALB/c | 10 | 12 | 5(a–f), 6 |
Abbreviation: ADR, adriamycin.
Denotes typical urinary albuminuria levels at 3 days after ADR injection.
Urinary albumin and creatinine assay
Urine albumin level was measured by using a mouse Albumin ELISA Quantification kit, according to the manufacturer’s protocol (Bethyl Laboratories, Montgomery, TX, USA). Urine creatinine was determined by a routine procedure as described previously.43 Urinary proteins were also analyzed by sodium dodecyl sulfate–polyacrylamine gel electrophoresis after normalization to urinary creatinine. After separation by sodium dodecyl sulfate–polyacrylamine gel electrophoresis, urine proteins were stained with Coomassie blue R-250.
Immunofluorescent staining and confocal microscopy
Kidney cryosections were fixed with 3.7% paraformalin for 15 min at room temperature. After blocking with 10% donkey serum for 30 min, slides were immunostained with primary antibodies against phosphospecific Erk-1/2 (Thr202/Tyr204; cat. no. 4370; Cell Signaling Technology, Danvers, MA, USA) and nephrin (Progen, Heidelberg, Germany), respectively. Slides were viewed under a Leica TCS-SL confocal microscope (Bannockburn, IL, USA).43 For determination of the podocyte injury, as defined by nephrin loss and altered distribution, a semiquantitative scoring method was used. Score 0 represents no lesion, whereas 1, 2, 3, and 4 represent the nephrin loss and altered distribution, involving <25%, 25–50%, 50–75%, and >75% of the glomerular tuft area, respectively. At least five randomly chosen glomeruli were evaluated for each mouse and an average composite score was calculated. Paraffin-embedded mouse kidney sections (3 μm thickness) were prepared by a routine procedure. Immunohistochemical staining for WT1 (sc-192; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and cleaved caspase-3 (cat. no. 9661; Cell Signaling Technology) was performed using a routine protocol as described previously.43,44
Glomerular isolation
Glomerular isolation was performed according to the method described elsewhere.43,45 Briefly, mice were anesthetized by intraperitoneal injection of pentobarbital (50 mg/kg) and perfused, through the abdominal artery, with 8 ×107 Dynabeads M-450 (Dynal Biotech ASA, Oslo, Norway) diluted in 5 ml of phosphate buffered saline. After perfusion, the kidneys were removed and cut into 1-mm3 pieces and digested in collagenase A (1 mg/ml) at 37 °C for 30 min with gentle shaking. The tissue was then pressed gently through a 100-μm cell strainer (BD Falcon, Bedford, MA, USA). Glomeruli-containing Dynabeads were then gathered by using a magnetic particle concentrator. The isolated glomeruli were washed three times with cold phosphate buffered saline and used for subsequent studies. The purity of the isolated glomeruli reached to more than 95% by this method.
Western blot analysis
The isolated glomeruli were pooled and lysed with radioimmunoprecipitation assay buffer containing 1% NP-40, 0.1% sodium dodecyl sulfate, 100 μg/ml phenylmethylsulfonyl fluoride, 1% protease inhibitor cocktail, and 1% phosphatase I and II inhibitor cocktail (Sigma) in phosphate buffered saline on ice. The supernatants were collected after centrifugation at 13,000 × g at 4 °C for 20 min. Cultured podocytes were lysed in sodium dodecyl sulfate sample buffer. Protein expression was detected by western blot analysis as described previously.43 The primary antibodies used were as follows: anti-WT1 (Santa Cruz Biotechnology); anti-c-met (cat. no. 3127), anti-cleaved caspase-3, anti-phosphospecific Akt (Ser473; cat no. 4060) and total Akt (cat. no. 9272), anti-phopshospecific Erk-1/2 (cat. no. 4370;Cell Signaling Technology); anti-glyceraldehyde-3-phosphate dehydrogenase (cat. no. 4300; Ambion), anti-procaspase-3 (sc-7148; Santa Cruz Biotechnology), and anti-α-tubulin (cat. no. T9026; Sigma).
Electron microscopy
Electron microscopy of kidney samples was carried out by routine procedures as described previously.43 Briefly, mouse kidneys were perfusion fixed with 2.5% glutaraldehyde in phosphate buffered saline by left cardiac ventricular injection and post-fixed in aqueous 1%OsO4. Specimens were dehydrated through an ethanol series, infiltrated in a 1:1 mixture of propylene oxide/Polybed 812 epoxy resin (Polysciences, Warrington, PA, USA), and then embedded. Ultrathin sections were stained with 2% uranyl acetate, followed by 1% lead citrate. Sections were observed and photographed using a JEOL JEM 1210 transmission electron microscope (Peabody, MA, USA).
Cell culture and treatment
The conditionally immortalized mouse podocyte cell line was kindly provided by Dr Peter Mundel (Mount Sinai School of Medicine, New York, NY, USA) and described previously.46 Immortalized human podocytes were described previously.47 Cells were cultured at 33 °C in RPMI-1640 medium supplemented with 10% fetal bovine serum and recombinant interferon-γ (Invitrogen, Carlsbad, CA, USA). To induce differentiation, podocytes were grown under nonpermissive conditions at 37 °C in the absence of interferon-γ. After serum starvation for 16 h, cells were treated with ADR in the absence or presence of human recombinant HGF (R&D Systems, Minneapolis, MN, USA) for various periods of time as indicated. In some experiments, cells were pretreated with either various inhibitors at given concentrations or vehicle (0.1% dimethylsulfoxide) 0.5 h before incubation with HGF. Wortmannin (PI3K inhibitor) and PD98059 (Mek1 inhibitor) were purchased from Calbiochem (La Jolla, CA, USA).
RNA extraction and reverse transcriptase–PCR
For determination of nephrin mRNA expression, a semi-quantitative reverse transcriptase–PCR was used. Total RNA isolation and reverse transcriptase–PCR amplification of nephrin mRNA were carried out using the procedures described previously.17 Briefly, the first-strand complementary DNA synthesis was carried out using a Reverse Transcription System kit, according to the instructions of the manufacturer (Promega, Madison, WI, USA). Polymerase chain reaction amplification was performed using HotStar Taq Master Mix Kit (Qiagen, Valencia, CA, USA). The sequences of nephrin and β-actin primer pairs have been described previously.17 After quantifying bands using densitometry, the relative steady-state level of mRNA was calculated and compared after normalizing to β-actin.
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling staining
Apoptotic cell death was determined using terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling staining using Apoptosis Detection System (Promega), as previously reported.43
Statistical analyses
Statistical analysis of the data was performed using SigmaStat software (Jandel Scientific, San Rafael, CA, USA). Comparison between groups was made using one-way analysis of variance, followed by the Student–Newman–Keuls test. P<0.05 was considered statistically significant.
Acknowledgments
We thank Dr S Thorgeirsson of the National Institutes of Health (NIH) for kindly providing c-met-floxed mice. This work was supported by NIH Grants DK061408, DK064005, and DK071040. CD was supported by the American Heart Association beginning Grant-in-Aid (0865392D) and UPMC Health System Competitive Medical Research Fund.
Footnotes
DISCLOSURE
All the authors declared no competing interests.
References
- 1.Liu Y. Hepatocyte growth factor in kidney fibrosis: therapeutic potential and mechanisms of action. Am J Physiol Renal Physiol. 2004;287:F7–F16. doi: 10.1152/ajprenal.00451.2003. [DOI] [PubMed] [Google Scholar]
- 2.Mizuno S, Matsumoto K, Nakamura T. HGF as a renotrophic and anti-fibrotic regulator in chronic renal disease. Front Biosci. 2008;13:7072–7086. doi: 10.2741/3211. [DOI] [PubMed] [Google Scholar]
- 3.Mizuno S, Nakamura T. Suppressions of chronic glomerular injuries and TGF-beta1 production by HGF in attenuation of murine diabetic nephropathy. Am J Physiol Renal Physiol. 2004;286:F134–F143. doi: 10.1152/ajprenal.00199.2003. [DOI] [PubMed] [Google Scholar]
- 4.Dworkin LD, Gong R, Tolbert E, et al. Hepatocyte growth factor ameliorates progression of interstitial fibrosis in rats with established renal injury. Kidney Int. 2004;65:409–419. doi: 10.1111/j.1523-1755.2004.00417.x. [DOI] [PubMed] [Google Scholar]
- 5.Yang J, Liu Y. Blockage of tubular epithelial to myofibroblast transition by hepatocyte growth factor prevents renal interstitial fibrosis. J Am Soc Nephrol. 2002;13:96–107. doi: 10.1681/ASN.V13196. [DOI] [PubMed] [Google Scholar]
- 6.Herrero-Fresneda I, Torras J, Franquesa M, et al. HGF gene therapy attenuates renal allograft scarring by preventing the profibrotic inflammatory-induced mechanisms. Kidney Int. 2006;70:265–274. doi: 10.1038/sj.ki.5001510. [DOI] [PubMed] [Google Scholar]
- 7.Azuma H, Takahara S, Matsumoto K, et al. Hepatocyte growth factor prevents the development of chronic allograft nephropathy in rats. J Am Soc Nephrol. 2001;12:1280–1292. doi: 10.1681/ASN.V1261280. [DOI] [PubMed] [Google Scholar]
- 8.Dai C, Yang J, Bastacky S, et al. Intravenous administration of hepatocyte growth factor gene ameliorates diabetic nephropathy in mice. J Am Soc Nephrol. 2004;15:2637–2647. doi: 10.1097/01.ASN.0000139479.09658.EE. [DOI] [PubMed] [Google Scholar]
- 9.Cruzado JM, Lloberas N, Torras J, et al. Regression of advanced diabetic nephropathy by hepatocyte growth factor gene therapy in rats. Diabetes. 2004;53:1119–1127. doi: 10.2337/diabetes.53.4.1119. [DOI] [PubMed] [Google Scholar]
- 10.Reddy GR, Kotlyarevska K, Ransom RF, et al. The podocyte and diabetes mellitus: is the podocyte the key to the origins of diabetic nephropathy? Curr Opin Nephrol Hypertens. 2008;17:32–36. doi: 10.1097/MNH.0b013e3282f2904d. [DOI] [PubMed] [Google Scholar]
- 11.Wolf G, Ziyadeh FN. Cellular and molecular mechanisms of proteinuria in diabetic nephropathy. Nephron. 2007;106:26–31. doi: 10.1159/000101797. [DOI] [PubMed] [Google Scholar]
- 12.Susztak K, Raff AC, Schiffer M, et al. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55:225–233. [PubMed] [Google Scholar]
- 13.Pavenstadt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev. 2003;83:253–307. doi: 10.1152/physrev.00020.2002. [DOI] [PubMed] [Google Scholar]
- 14.Huber TB, Benzing T. The slit diaphragm: a signaling platform to regulate podocyte function. Curr Opin Nephrol Hypertens. 2005;14:211–216. doi: 10.1097/01.mnh.0000165885.85803.a8. [DOI] [PubMed] [Google Scholar]
- 15.Wiggins RC. The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int. 2007;71:1205–1214. doi: 10.1038/sj.ki.5002222. [DOI] [PubMed] [Google Scholar]
- 16.Shankland SJ. The podocyte’s response to injury: role in proteinuria and glomerulosclerosis. Kidney Int. 2006;69:2131–2147. doi: 10.1038/sj.ki.5000410. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Kang YS, Dai C, et al. Epithelial-to-mesenchymal transition is a potential pathway leading to podocyte dysfunction and proteinuria. Am J Pathol. 2008;172:299–308. doi: 10.2353/ajpath.2008.070057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamaguchi Y, Iwano M, Suzuki D, et al. Epithelial–mesenchymal transition as a potential explanation for podocyte depletion in diabetic nephropathy. Am J Kidney Dis. 2009;54:653–664. doi: 10.1053/j.ajkd.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J, Yang J, Liu Y. Role of Bcl-xL induction in HGF-mediated renal epithelial cell survival after oxidant stress. Int J Clin Exp Pathol. 2008;1:242–253. [PMC free article] [PubMed] [Google Scholar]
- 20.Maulik G, Shrikhande A, Kijima T, et al. Role of the hepatocyte growth factor receptor, c-Met, in oncogenesis and potential for therapeutic inhibition. Cytokine Growth Factor Rev. 2002;13:41–59. doi: 10.1016/s1359-6101(01)00029-6. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X, Li Y, Dai C, et al. Sp1 and Sp3 transcription factors synergistically regulate HGF receptor gene expression in kidney. Am J Physiol Renal Physiol. 2003;284:F82–F94. doi: 10.1152/ajprenal.00200.2002. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Wang YP, Tay YC, et al. Progressive adriamycin nephropathy in mice: sequence of histologic and immunohistochemical events. Kidney Int. 2000;58:1797–1804. doi: 10.1046/j.1523-1755.2000.00342.x. [DOI] [PubMed] [Google Scholar]
- 23.Morrison AA, Viney RL, Saleem MA, et al. New insights into the function of the Wilms tumor suppressor gene WT1 in podocytes. Am J Physiol Renal Physiol. 2008;295:F12–F17. doi: 10.1152/ajprenal.00597.2007. [DOI] [PubMed] [Google Scholar]
- 24.Guo JK, Menke AL, Gubler MC, et al. WT1 is a key regulator of podocyte function: reduced expression levels cause crescentic glomerulonephritis and mesangial sclerosis. Hum Mol Genet. 2002;11:651–659. doi: 10.1093/hmg/11.6.651. [DOI] [PubMed] [Google Scholar]
- 25.Dai C, Yang J, Liu Y. Single injection of naked plasmid encoding hepatocyte growth factor prevents cell death and ameliorates acute renal failure in mice. J Am Soc Nephrol. 2002;13:411–422. doi: 10.1681/ASN.V132411. [DOI] [PubMed] [Google Scholar]
- 26.Dai C, Liu Y. Hepatocyte growth factor antagonizes the profibrotic action of TGF-beta1 in mesangial cells by stabilizing Smad transcriptional corepressor TGIF. J Am Soc Nephrol. 2004;15:1402–1412. doi: 10.1097/01.asn.0000130568.53923.fd. [DOI] [PubMed] [Google Scholar]
- 27.Wagner N, Wagner KD, Xing Y, et al. The major podocyte protein nephrin is transcriptionally activated by the Wilms’ tumor suppressor WT1. J Am Soc Nephrol. 2004;15:3044–3051. doi: 10.1097/01.ASN.0000146687.99058.25. [DOI] [PubMed] [Google Scholar]
- 28.Guo G, Morrison DJ, Licht JD, et al. WT1 activates a glomerular-specific enhancer identified from the human nephrin gene. J Am Soc Nephrol. 2004;15:2851–2856. doi: 10.1097/01.ASN.0000143474.91362.C4. [DOI] [PubMed] [Google Scholar]
- 29.Wiggins JE, Goyal M, Sanden SK, et al. Podocyte hypertrophy, ‘adaptation,’ and ‘decompensation’ associated with glomerular enlargement and glomerulosclerosis in the aging rat: prevention by calorie restriction. J Am Soc Nephrol. 2005;16:2953–2966. doi: 10.1681/ASN.2005050488. [DOI] [PubMed] [Google Scholar]
- 30.Ishibe S, Karihaloo A, Ma H, et al. Met and the epidermal growth factor receptor act cooperatively to regulate final nephron number and maintain collecting duct morphology. Development. 2009;136:337–345. doi: 10.1242/dev.024463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huh CG, Factor VM, Sanchez A, et al. Hepatocyte growth factor/c-met signaling pathway is required for efficient liver regeneration and repair. Proc Natl Acad Sci USA. 2004;101:4477–4482. doi: 10.1073/pnas.0306068101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moeller MJ, Sanden SK, Soofi A, et al. Podocyte-specific expression of cre recombinase in transgenic mice. Genesis. 2003;35:39–42. doi: 10.1002/gene.10164. [DOI] [PubMed] [Google Scholar]
- 33.Jeansson M, Bjorck K, Tenstad O, et al. Adriamycin alters glomerular endothelium to induce proteinuria. J Am Soc Nephrol. 2009;20:114–122. doi: 10.1681/ASN.2007111205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dai C, Stolz DB, Kiss LP, et al. Wnt/β-catenin signaling promotes podocyte dysfunction and albuminuria. J Am Soc Nephrol. 2009;20:1997–2008. doi: 10.1681/ASN.2009010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doublier S, Ruotsalainen V, Salvidio G, et al. Nephrin redistribution on podocytes is a potential mechanism for proteinuria in patients with primary acquired nephrotic syndrome. Am J Pathol. 2001;158:1723–1731. doi: 10.1016/S0002-9440(10)64128-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takano Y, Yamauchi K, Hiramatsu N, et al. Recovery and maintenance of nephrin expression in cultured podocytes and identification of HGF as a repressor of nephrin. Am J Physiol Renal Physiol. 2007;292:F1573–F1582. doi: 10.1152/ajprenal.00423.2006. [DOI] [PubMed] [Google Scholar]
- 37.Yang J, Chen S, Huang L, et al. Sustained expression of naked plasmid DNA encoding hepatocyte growth factor in mice promotes liver and overall body growth. Hepatology. 2001;33:848–859. doi: 10.1053/jhep.2001.23438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wharram BL, Goyal M, Wiggins JE, et al. Podocyte depletion causes glomerulosclerosis: diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol. 2005;16:2941–2952. doi: 10.1681/ASN.2005010055. [DOI] [PubMed] [Google Scholar]
- 39.Fornoni A, Li H, Foschi A, et al. Hepatocyte growth factor, but not insulin-like growth factor I, protects podocytes against cyclosporin A-induced apoptosis. Am J Pathol. 2001;158:275–280. doi: 10.1016/S0002-9440(10)63966-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Y. Hepatocyte growth factor promotes renal epithelial cell survival by dual mechanisms. Am J Physiol. 1999;277:F624–F633. doi: 10.1152/ajprenal.1999.277.4.F624. [DOI] [PubMed] [Google Scholar]
- 41.Liu Y, Michalopoulos GK, Zarnegar R. Molecular cloning and characterization of cDNA encoding mouse hepatocyte growth factor. Biochim Biophys Acta. 1993;1216:299–303. doi: 10.1016/0167-4781(93)90159-b. [DOI] [PubMed] [Google Scholar]
- 42.Dai C, Huh CG, Thorgeirsson SS, et al. β-cell-specific ablation of the hepatocyte growth factor receptor results in reduced islet size, impaired insulin secretion, and glucose intolerance. Am J Pathol. 2005;167:429–436. doi: 10.1016/s0002-9440(10)62987-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dai C, Stolz DB, Bastacky SI, et al. Essential role of integrin-linked kinase in podocyte biology: bridging the integrin and slit diaphragm signaling. J Am Soc Nephrol. 2006;17:2164–2175. doi: 10.1681/ASN.2006010033. [DOI] [PubMed] [Google Scholar]
- 44.Giannopoulou M, Dai C, Tan X, et al. Hepatocyte growth factor exerts its anti-inflammatory action by disrupting nuclear factor-κB signaling. Am J Pathol. 2008;173:30–41. doi: 10.2353/ajpath.2008.070583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takemoto M, Asker N, Gerhardt H, et al. A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol. 2002;161:799–805. doi: 10.1016/S0002-9440(10)64239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mundel P, Reiser J, Zuniga Mejia Borja A, et al. Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res. 1997;236:248–258. doi: 10.1006/excr.1997.3739. [DOI] [PubMed] [Google Scholar]
- 47.Saleem MA, O’Hare MJ, Reiser J, et al. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol. 2002;13:630–638. doi: 10.1681/ASN.V133630. [DOI] [PubMed] [Google Scholar]