Abstract
The endogenous cannabinoid system has been noted for its therapeutic potential, as well as the psychoactivity of cannabinoids such as Δ9-tetrahydrocannabinol (THC). However, less is known about the psychoactivity of anandamide (AEA), an endocannabinoid ligand. Thus, the goals of this study were to establish AEA as a discriminative stimulus in transgenic mice lacking fatty acid amide hydrolase (i.e., FAAH −/− mice unable to rapidly metabolize AEA), evaluate whether THC or oleamide, a fatty acid amide, produced AEA-like responding, and assess for CB1 mediation of AEA’s discriminative stimulus. Mice readily discriminated between 6 mg/kg AEA and vehicle in a two-lever drug discrimination task. AEA dose-dependently generalized to itself. THC elicited full AEA-like responding, whereas oleamide failed to substitute. The CB1 antagonist rimonabant attenuated AEA- and THC-induced AEA-appropriate responding, demonstrating CB1 mediation of AEA’s discriminative stimulus. These findings suggest that, in the absence of FAAH, AEA produces intoxication comparable to THC, and consequently to marijuana.
Keywords: anandamide, Δ9-tetrahydrocannabinol, fatty acid amide hydrolase, marijuana, drug discrimination, knockout mice
1. Introduction
The endocannabinoid system is comprised of two primary G-protein coupled receptors, cannabinoid 1 (CB1) and cannabinoid 2 (CB2), and two major endogenous ligands, anandamide (AEA) and 2-arachidonoylglycerol (2-AG), which activate these receptors. The endocannabinoid system appears to play a regulatory role in many physiological processes, including appetite (Wiley et al. 2005), pain (Lichtman et al. 2004), and cognition (Varvel et al. 2007). Although the breadth of processes with endocannabinoid involvement make this system a particularly attractive therapeutic target, as does the on-demand synthesis of endocannabinoids (Howlett et al., 2002; Di Marzo et al., 2005), the potential for endogenous cannabinoids to share marijuana-like psychoactive effects of exogenous cannabinoids such as Δ9-tetrahydrocannabinol (THC) [the principle psychoactive substituent of marijuana (Gaoni and Mechoulam, 1964)] represents a possible limitation to this endeavor.
Marijuana intoxication in humans can be modeled in animals using cannabinoid discrimination (Balster and Prescott, 1992). In this model, an animal is trained to make one response (e.g., lever press) under the presence of a drug such as THC (“training drug”) and to make a different response (e.g., pressing a different lever) after vehicle administration for food reward. Once the animal can reliably discriminate the interoceptive stimuli/discriminative stimulus produced by the training drug, they can demonstrate (i.e., by lever choice) whether other drugs elicit discriminative stimulus effects similar to or different from the training drug. THC has typically served as the drug of reference in cannabinoid discriminations and thus has been established in numerous species including rats (Wiley et al., 1995b; Burkey and Nation, 1997; Jarbe et al., 1998; Solinas et al., 2007; Vann et al., 2007), rhesus monkeys (Wiley et al., 1995a), pigeons (Henriksson et al., 1975), gerbils (Jarbe et al., 1975), and mice (McMahon et al., 2008; Vann et al., 2009; Walentiny et al., 2010). This well-established model possesses a high degree of pharmacological specificity (Balster and Prescott, 1992), and CB1 mediation of THC’s discriminative stimulus has been demonstrated (Wiley et al., 1995a).
Although the intoxicating effects of exogenous cannabinoids such as THC have been well-characterized in the discrimination model, previous attempts to train AEA have been unsuccessful (Wiley, 1999), most likely due to rapid catabolism by its primary metabolic enzyme, fatty acid amide hydrolase (FAAH) (Cravatt et al., 1996). FAAH −/− mice lacking this enzyme have been developed and possess higher brain levels of AEA (Cravatt et al., 2001) and display a phenotypic reduction in pain (Lichtman et al., 2004). In response to AEA administration, FAAH −/− mice exhibit more robust analgesia, catalepsy, and hypothermia (Wise et al., 2007) and more pronounced disruptions in working memory (Varvel et al., 2006) compared with wild-type mice. Although these findings have revealed much regarding the pharmacological properties of AEA, its discriminative stimulus effects have yet to be evaluated. We propose that FAAH −/− mice provide a unique opportunity to directly investigate the discriminative stimulus properties of AEA.
2. Methods and materials
2.1 Subjects
Male FAAH −/− mice (N=6) were individually housed and kept in a temperature-controlled (20–22°C) environment with a 14:10-h light:dark cycle. Mice were food restricted to 85–90% of free-feeding weight and received water ad libitum. All animals used in this study were cared for in accordance with the guidelines of the Institutional Animal Care and Use Committee of Virginia Commonwealth University and the ‘Guide for the Care and Use of Laboratory Animals’ (Institute of Laboratory Animal Resources, National Academy Press, 1996).
2.2 Apparatus
Eight standard sound and light attenuated mice operant conditioning chambers (MED Associates, St. Albans, VT) were used for behavioral training and testing. Each operant conditioning chamber (18 × 18 × 18 cm) was equipped with a house light, two levers (left and right), and a recessed dipper receptacle centered between the levers. A dipper arm delivered sweetened milk in a 0.05-ml cup, which was available for 5 s. Fan motors provided ventilation and masking noise for each chamber. House lights were illuminated during training and testing sessions. A computer with Logic ‘1’ interface (MED Associates) and MED-PC software (MED Associates) was used to control schedule contingencies and to record data.
2.3 Drugs
N-arachidonoyl ethanolamide (anandamide, AEA; Organix Inc., Woburn, MD), Δ9-tetrahydrocannabinol (THC; National Institute on Drug Abuse; NIDA, Rockville, MD), oleamide (ODA; Sigma-Alrich; St. Louis, MO) and rimonabant (NIDA) were dissolved in a vehicle solution of 7.8% Tween-80 and 92.2% saline. AEA, THC and ODA were administered intraperitoneally (i.p.) 30 min prior to start of the session at a volume of 0.01 mL/g body weight. For antagonism tests, rimonabant was administered i.p. 10 min prior to administration of either THC or AEA at a volume of 0.01 mL/g body weight.
2.4 Procedures
2.4.1. Lever-Press Training
Each mouse was placed in a standard operant chamber and trained to lever press according to a fixed ratio (FR) 1 schedule of reinforcement. During these sessions, both levers were extended, and an active stimulus light was located above the reinforced lever. Milk reinforcement was delivered after every press on the active lever while responses on the opposite lever had no consequence. The FR value was gradually increased to the final FR10 schedule of reinforcement in which 10 consecutive responses were required for delivery of milk reinforcement. After mice were trained on one lever, lever press training at the second lever proceeded identically to that at the first lever. When responding on the second lever under a FR10 schedule was acquired, discrimination training began.
2.4.2 Discrimination Training
Mice were trained to press one lever following i.p. injection of 6 mg/kg AEA and to press the opposite lever after injection with vehicle, each according to a FR10 schedule of milk reinforcement. Mice were injected and returned to their home cages until the start of the experimental session. During the experimental session, completion of 10 consecutive responses on the injection-appropriate lever resulted in delivery of sweetened milk. Each response on the incorrect lever reset the response requirement on the correct lever. The position of the drug lever was varied among the group of mice. The daily injections for each mouse were administered in a double alternation sequence of AEA and vehicle. Training occurred during 15-min sessions conducted 5 days a week (Monday-Friday) until the mice met three criteria during 7 of 8 consecutive sessions: (a) first completed FR10 on the correct lever, (b) percentage of correct-lever responding greater than 80% for the entire session, and (c) response rate greater than 0.17 responses/sec. When these three criteria were met, acquisition of the discrimination was established and substitution testing began.
2.4.3 Substitution tests
Following successful acquisition of AEA’s discriminative stimulus, substitution tests were conducted on Tuesdays and Fridays during 15-min test sessions. Training continued on Mondays, Wednesdays, and Thursdays. During test sessions, responses on either lever delivered reinforcement according to an FR10 schedule. Mice must have completed the first FR10 on the correct lever and ≥ 80% of the total responding must have occurred on the correct lever on the preceding day in order to be tested. In addition, mice must have met these same criteria during previous training sessions with the alternate training compound (AEA or vehicle). Prior to substitution tests a generalization curve for AEA was generated with all mice. Then, substitution tests were conducted with THC and oleamide. For antagonism tests, 1 mg/kg rimonabant was administered i.p. 10 min prior to AEA or THC administration. Control tests with vehicle and AEA were re-determined prior to conducting substitution tests. Each data point was collected in all subjects.
2.5 Data Analysis
Number of responses on the drug lever and total number of responses were recorded. Full substitution was defined as ≥ 80% AEA-lever responding. ED50 values were calculated for percentage of responses on the drug lever using least squares linear regression analysis followed by calculation of 95% confidence limits. Mice that failed to earn at least one reinforcer during a session had their percent drug lever responding (%DLR) data excluded for the corresponding dose; however, their response rate data were included in the analysis. Response rate suppression data were analyzed by repeated measures analysis of variance (ANOVA) using GBSTAT statistical software (GB-STAT software; Dynamic Microsystems, Silver Spring, MD). Significant ANOVAs were further analyzed by Fisher’s LSD post hoc tests (α = 0.05) to specify differences between means.
3. Results
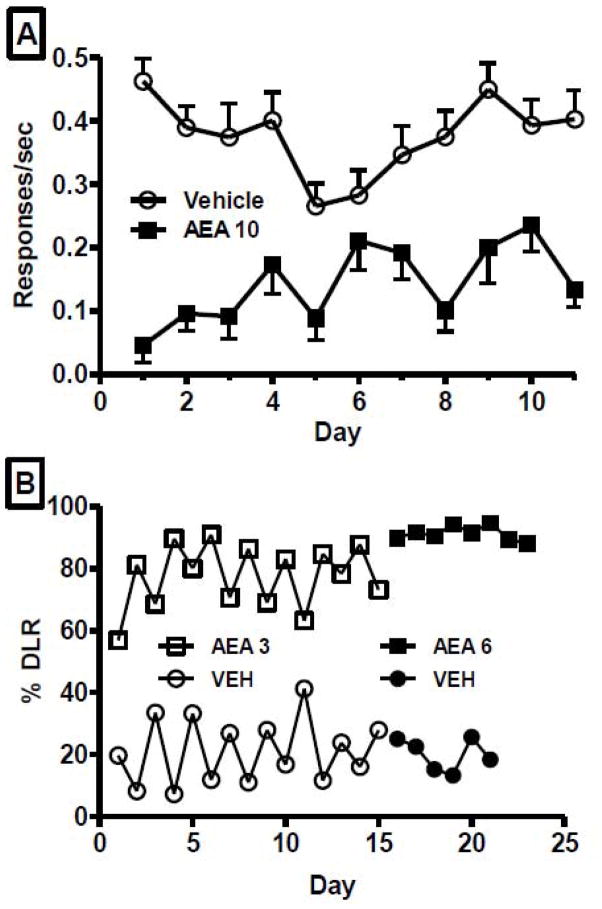
Initial attempts to train 10 mg/kg AEA as a discriminative stimulus in FAAH −/− mice were unsuccessful, as tolerance to the rate suppressing effects failed to develop (Fig. 1A). Response rates following AEA were significantly decreased relative to vehicle across all administrations, with an additional pronounced decrease in response rate on the first day of AEA on the double alternation schedule of drug administration. While an increase in rate was often seen on the second consecutive day of AEA administration, this tolerance effect was not maintained during subsequent intervening vehicle sessions. Further, rates under AEA never achieved the level occurring after vehicle injection. Efforts to fade the training dose to 3 mg/kg AEA were aborted due to failure by any animal to reliably discriminate after 30 sessions (representative data shown in Fig. 1B). Subsequently, the training dose was changed to 6 mg/kg AEA.
Fig. 1.
Effects of AEA and vehicle on response rates (resp/sec) during initial attempts to train FAAH −/− mice to discriminate 10 mg/kg AEA from vehicle. Values shown are for the first 22 sessions following administration of either 10 mg/kg AEA or vehicle. Values represent the mean (±S.E.M.) of 6 mice.
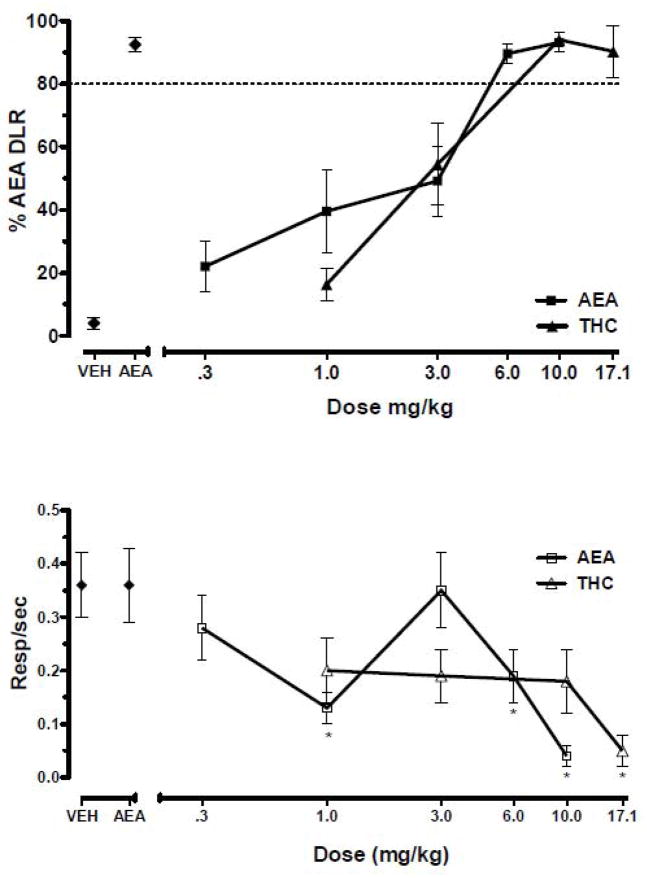
All FAAH −/− mice successfully learned to discriminate 6 mg/kg AEA from vehicle by meeting discrimination criteria on 7 out of 8 consecutive sessions. AEA fully and dose dependently substituted for itself with an ED50 = 1.48 mg/kg (95% CL: 0.98 – 2.22; Fig. 2, top panel). THC produced a similar substitution pattern for AEA with an ED50 = 2.85 (95% CL: 2.12 – 3.83; Fig. 2, top panel); however, THC’s ED50 was not significantly different than that of AEA’s. A repeated measures ANOVA indicated significant differences for response rates as a function of dose for AEA [F (6,42)=8.2, P < 0.05; Fig. 2, bottom panel], and THC [F (5,35)=3.4, P < 0.05; Fig. 2, bottom panel]. Post hoc analysis revealed significant decreases in response rates by 1 mg/kg, 6 mg/kg, and 10 mg/kg AEA (P < 0.05) and 17.1 mg/kg THC (P < 0.05) as compared with vehicle; however, 3 mg/kg AEA produced significantly higher response rates than 1 and 6 mg/kg doses (P < 0.05).
Fig. 2.
Effects of AEA and THC on percentage of AEA-lever responding (% AEA DLR; upper panel) and response rates (resp/sec; lower panel) in FAAH −/− mice trained to discriminate 6 mg/kg AEA from vehicle. Points above VEH and AEA represent the results of control tests with vehicle and 6 mg/kg AEA conducted before each dose-effect determination. Values represent the mean (±S.E.M.) of 6 mice. Significant differences (P < 0.05) in response rate relative to vehicle controls are denoted by *.
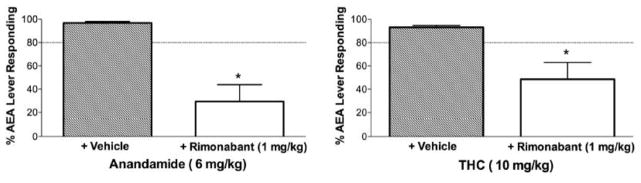
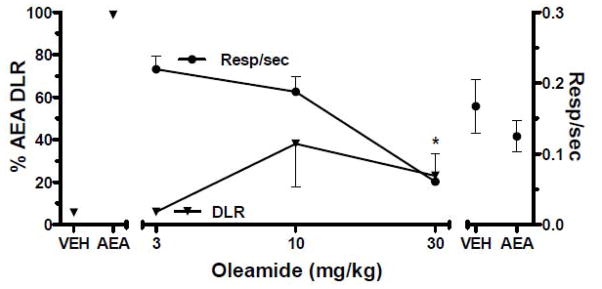
When co-administered with rimonabant, 6 mg/kg AEA significantly decreased responding on the AEA-appropriate lever relative to vehicle and AEA [F (1,7)=48.7, P < 0.05; Fig. 3]. Similarly, co-administration of 10 mg/kg THC with 1 mg/kg rimonabant significantly decreased AEA-appropriate responding as compared with vehicle and THC [F (1,7)=15.2, P < 0.05; Fig. 3]. ODA failed to substitute for AEA at all doses (Fig. 4). A repeated measures ANOVA indicated significant differences in response rates as a function of ODA dose [F (4,16)=3.6, P < 0.05; Fig. 4]. Post hoc analysis indicated a significant decrease in response rates by 30 mg/kg ODA as compared with vehicle (P < 0.05).
Fig. 3.
Effects of rimonabant blockade on AEA and THC discriminative stimulus effects in FAAH −/− mice. Points above + rimonabant and + vehicle represent antagonism tests with 1.0 mg/kg rimonabant or vehicle administered prior to an injection of 6.0 mg/kg AEA (top panel) or 10.0 mg/kg THC (bottom panel). Values represent the mean (±S.E.M.) of 6 mice. Significant differences (P < 0.05) in % AEA lever responding during antagonism tests relative to appropriate controls are denoted by *.
Fig. 4.
Effects of ODA on percentage of AEA-lever responding (triangles) and response rates (circles) in FAAH −/− mice trained to discriminate 6.0 mg/kg AEA from vehicle. Points above VEH and AEA represent the results of control tests with vehicle and 6.0 mg/kg AEA, respectively, conducted before each dose-effect determination. Values represent the mean (±S.E.M.) of 6 mice. Significant differences (P < 0.05) in response rate relative to vehicle controls are denoted by *.
4. Discussion
Initial efforts to compare the discriminative stimulus effects of THC and AEA generally found that AEA failed to occasion THC-like responding (Burkey and Nation 1997; Wiley et al. 1997; Wiley et al. 1998). Further, while AEA evoked a transient cannabimimetic profile as assessed through the tetrad (hypolocomotion, hypothermia, antinociception, catalepsy; Smith et al. 1994), AEA failed to influence other behaviors typically altered by cannabinoids, including anxiety-like behavior, memory and food intake (Crawley et al. 1993). Further, significant AEA binding in rat brain homogenate was attained only in the presence of the non-specific serine protease inhibitor phenylmethylsulfonyl fluoride (PMSF; Childers et al. 1994). These results, along with others, strongly suggested that AEA was rapidly metabolized, hence the general lack of cannabinoid activity in vivo. Thus, there was a clear imperative to better understand AEA’s degradative pathway and develop tools to facilitate AEA-based research.
Initially, the metabolically stable AEA analog, methanandamide (Abadji et al. 1994), was used extensively to make inferences regarding the pharmacological properties of the parent compound. Next, Cravatt et al. (1996) demonstrated that FAAH was the enzyme primarily responsible for AEA’s rapid metabolism. Subsequently, the FAAH −/− mouse was generated by isolating the FAAH gene from the 129SvJ genome, then inserting a phosphoglycerate kinase promotor to replace the first FAAH exon (Cravatt et al. 2001). Two of the resulting embryonic stem cell clones were chosen to produce chimeric mice on a C57BL/6 background. Subsequent mating between the 129SvJ and C57BL/6 FAAH +/− mice resulted in animals that lacked FAAH in central and peripheral tissue. These developments, along with others (e.g., pharmacologically selective FAAH inhibitors), have allowed for sophisticated analyses of AEA’s pharmacological properties.
To date, inferences regarding AEA’s psychoactive effects primarily have relied on pharmacological tools. Tests with pharmacological inhibitors of FAAH (URB597 or PMSF) have revealed that AEA fully substitutes for THC in rats (Solinas et al. 2007) and mice (Vann et al., 2009), respectively. Generally, methanandamide substitutes for THC’s discriminative stimulus in rats (Burkey and Nation, 1997; Jarbe et al., 2000) and non-human primates (McMahon, 2009) and THC substitutes for methanandamide’s discriminative stimulus in rats (Jarbe et al., 2001; Wiley et al., 2004), signifying cross-substitution between THC and methanandamide. Similarly, cross-substitution has been observed between THC and the methanandamide analog O-1812 in rats (Wiley et al. 2004). In studies of this nature, demonstration of cross-substitution is a key indicator that drugs exert comparable intoxicating effects that are mediated through similar or identical mechanisms. Such determinations have provided critical support in classifying drugs, both existing and newly developed. In contrast, Jarbe and colleagues (1998; 2000), demonstrated that methanandamide substitutes for a low training dose of THC (1.8 mg/kg), but only partially substitutes for a high THC training dose (5.6 mg/kg), establishing that training dose is a critical determinant of substitution patterns in cannabinoid discrimination. In another study, methanandamide failed to substitute for THC (McMahon et al., 2008), suggesting methanandamide was acting via non-CB1 mechanisms. FAAH −/− mice readily acquired the 6 mg/kg AEA discriminative stimulus, but failed to acquire a lower (3 mg/kg) or higher training dose of AEA (10 mg/kg). Albeit speculative, had the higher training dose of AEA served as a discriminative stimulus, THC may not have substituted due to increased efficacy of AEA at non-CB1 receptors such as TRPV1 (see Ross 2003). Inferences between previous findings with methanandamide and results of the present study should be made with several considerations in mind, including species differences and potential potency differences between methanandamide and AEA. For instance, methanandamide was reported to be equipotent in disrupting working memory performance in FAAH −/− and wild-type mice, but at higher doses than observed with AEA in FAAH −/− mice (Varvel et al., 2006).
Prior to this study, FAAH −/− mice have only been trained to discriminate THC vs. vehicle. In that study, we demonstrated that inhibition of 2-AG metabolism (i.e., indirect elevation of 2-AG) produces THC-like intoxicating effects (Long et al., 2009). By utilizing FAAH −/− mice as subjects, this study represents the first successful training of AEA as a discriminative stimulus. Here we demonstrated that, through genetic inhibition of AEA metabolism, AEA and THC share discriminative stimulus effects. Thus, if 2-AG occasions THC-like responding and THC occasions AEA-like responding in FAAH −/− mice, it is reasonable to suggest shared discriminative stimuli between endogenous and exogenous cannabinoids. Further, given that THC’s discriminative stimulus models the intoxicating properties of marijuana (Balster and Prescott 1992), endogenous cannabinoids appear to produce comparable intoxication. It also has been well established that THC’s discriminative stimulus effects are mediated by central CB1 receptors (see Wiley, 1999 for review). Challenge tests with rimonabant decreased AEA-appropriate responding for both AEA and THC in the present study. Similarly, we have shown previously that rimonabant reversed THC-like responding following indirect elevation of 2-AG in FAAH −/− mice (Long et al. 2009). Thus, combinations of direct (e.g., methanandamide) and indirect (i.e., endocannabinoid metabolic inhibitors) pharmacological tools, complemented by genetic models (e.g., FAAH −/− mice) have provided strong behavioral evidence that AEA and 2-AG produce CB1-mediated psychoactive effects similar to THC and marijuana.
Besides AEA, other centrally active fatty acid amides exist, including N-palmitoyl ethanolamine (PEA), N-oleoyl ethanolamine (OEA) and oleamide, all rapidly inactivated by FAAH. Oleamide has been noted for its ability to induce sleep following systemic administration (Basile et al., 1999). To our knowledge, oleamide has not been tested in any other published drug discrimination studies, thus its psychoactivity is unknown. Some data indicate oleamide is a full CB1 agonist (Leggett et al., 2004), while other data indicate it has negligible affinity (Lichtman et al., 2002) or none at all (Mechoulam et al., 1997). While AEA is a structurally similar fatty acid amide, its discriminative stimulus effects failed to generalize to oleamide, even at behaviorally active doses (i.e., those that suppress operant responding). While AEA and THC both produced full substitution for AEA at doses that significantly reduced operant responding, this was not observed during AEA control tests or with 10 mg/kg THC, which fully substituted for AEA. Thus drug-induced operant rate suppression is neither necessary nor sufficient to produce AEA-like discriminative stimulus effects. Considering the established molecular and pharmacological specificity of the drug discrimination paradigm (Balster and Prescott, 1992; Colpaert, 1999), the present data provide strong behavioral evidence that oleamide is not a psychoactive agonist at the CB1 receptor. Thus, it remains unclear whether other fatty acid amides would produce intoxicating effects similar to AEA. Given the therapeutic implications of several fatty acid amides, such as the anti-inflammatory properties of N-palmitoyl ethanolamine (Wise et al., 2008), this remains an important consideration and should be evaluated in future tests.
5. Conclusions
The present study demonstrated that AEA can serve as a discriminative stimulus in FAAH −/− mice and highlighted similarities between the intoxicating properties of AEA and those elicited by THC. The findings of the present study strongly suggest that AEA produces CB1-mediated cannabis-like intoxication and should be factored heavily into the development of endocannabinoid-based therapeutics, as they might possess abuse liability similar to marijuana. Future challenges for this line of research include further evaluating the mechanism(s) of AEA’s discriminative stimulus (e.g., role of TRPV1), and assessing the intoxicating properties of other endocannabinoids such as 2-AG.
Acknowledgments
Research supported by NIH grants DA-026449, DA-003672 and DA-09789.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abadji V, Lin S, Taha G, Griffin G, Stevenson LA, Pertwee RG, Makriyannis A. (R)-methanandamide: a chiral novel anandamide possessing higher potency and metabolic stability. J Med Chem. 1994;37:1889–1893. doi: 10.1021/jm00038a020. [DOI] [PubMed] [Google Scholar]
- Balster RL, Prescott WR. Delta 9-tetrahydrocannabinol discrimination in rats as a model for cannabis intoxication. Neurosci Biobehav Rev. 1992;16:55–62. doi: 10.1016/s0149-7634(05)80051-x. [DOI] [PubMed] [Google Scholar]
- Basile AS, Hanus L, Mendelson WB. Characterization of the hypnotic properties of oleamide. Neuroreport. 1999;10:947–951. doi: 10.1097/00001756-199904060-00010. [DOI] [PubMed] [Google Scholar]
- Burkey RT, Nation JR. (R)-methanandamide, but not anandamide, substitutes for delta 9-THC in a drug-discrimination procedure. Exp Clin Psychopharmacol. 1997;5:195–202. doi: 10.1037//1064-1297.5.3.195. [DOI] [PubMed] [Google Scholar]
- Childers SR, Sexton T, Roy MB. Effects of anandamide on cannabinoid receptors in rat brain membranes. Biochem Pharmacol. 1994;47:711–715. doi: 10.1016/0006-2952(94)90134-1. [DOI] [PubMed] [Google Scholar]
- Colpaert FC. Drug discrimination in neurobiology. Pharmacol Biochem Behav. 1999;64:337–345. doi: 10.1016/s0091-3057(99)00047-7. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, Lichtman AH. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci U S A. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Corwin RL, Robinson JK, Felder CC, Devane WA, Axelrod J. Anandamide, an endogenous ligand of the cannabinoid receptor, induces hypomotility and hypothermia in vivo in rodents. Pharmacol Biochem Behav. 1993;46:967–972. doi: 10.1016/0091-3057(93)90230-q. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, De Petrocellis L, Bisogno T. The biosynthesis, fate and pharmacological properties of endocannabinoids. Handb Exp Pharmacol. 2005:147–185. doi: 10.1007/3-540-26573-2_5. [DOI] [PubMed] [Google Scholar]
- Gaoni Y, Mechoulam R. Isolation, structure and partial synthesis of an active constituent of hashish. J Am Chem Soc. 1964;86:1646–1647. [Google Scholar]
- Henriksson BG, Johansson JO, Jarbe TU. Delta 9-tetrahydrocannabinol produced discrimination in pigeons. Pharmacol Biochem Behav. 1975;3:771–774. doi: 10.1016/0091-3057(75)90105-7. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- Jarbe TU, Johansson JO, Henriksson BG. Delta9-tetrahydrocannabinol and pentobarbital as discriminative cues in the Mongolian Gerbil (Meriones unguiculatus) Pharmacol Biochem Behav. 1975;3:403–410. doi: 10.1016/0091-3057(75)90048-9. [DOI] [PubMed] [Google Scholar]
- Jarbe TU, Lamb RJ, Lin S, Makriyannis A. Delta9-THC training dose as a determinant for (R)-methanandamide generalization in rats: a systematic replication. Behav Pharmacol. 2000;11:81–86. doi: 10.1097/00008877-200002000-00009. [DOI] [PubMed] [Google Scholar]
- Jarbe TU, Lamb RJ, Lin S, Makriyannis A. (R)-methanandamide and Delta 9-THC as discriminative stimuli in rats: tests with the cannabinoid antagonist SR-141716 and the endogenous ligand anandamide. Psychopharmacology (Berl) 2001;156:369–380. doi: 10.1007/s002130100730. [DOI] [PubMed] [Google Scholar]
- Jarbe TU, Lamb RJ, Makriyannis A, Lin S, Goutopoulos A. Delta9-THC training dose as a determinant for (R)-methanandamide generalization in rats. Psychopharmacology (Berl) 1998;140:519–522. doi: 10.1007/s002130050797. [DOI] [PubMed] [Google Scholar]
- Jarbe TU, Li C, Vadivel SK, Makriyannis A. Discriminative stimulus functions of methanandamide and delta(9)-THC in rats: tests with aminoalkylindoles (WIN55,212-2 and AM678) and ethanol. Psychopharmacology (Berl) 2010;208:87–98. doi: 10.1007/s00213-009-1708-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggett JD, Aspley S, Beckett SR, D’Antona AM, Kendall DA. Oleamide is a selective endogenous agonist of rat and human CB1 cannabinoid receptors. Br J Pharmacol. 2004;141:253–262. doi: 10.1038/sj.bjp.0705607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman AH, Hawkins EG, Griffin G, Cravatt BF. Pharmacological activity of fatty acid amides is regulated, but not mediated, by fatty acid amide hydrolase in vivo. J Pharmacol Exp Ther. 2002;302:73–79. doi: 10.1124/jpet.302.1.73. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Shelton CC, Advani T, Cravatt BF. Mice lacking fatty acid amide hydrolase exhibit a cannabinoid receptor-mediated phenotypic hypoalgesia. Pain. 2004;109:319–327. doi: 10.1016/j.pain.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Long JZ, Nomura DK, Vann RE, Walentiny DM, Booker L, Jin X, Burston JJ, Sim-Selley LJ, Lichtman AH, Wiley JL, Cravatt BF. Dual blockade of FAAH and MAGL identifies behavioral processes regulated by endocannabinoid crosstalk in vivo. Proc Natl Acad Sci U S A. 2009;106:20270–20275. doi: 10.1073/pnas.0909411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon LR. Apparent affinity estimates of rimonabant in combination with anandamide and chemical analogs of anandamide in rhesus monkeys discriminating Delta9-tetrahydrocannabinol. Psychopharmacology (Berl) 2009;203:219–228. doi: 10.1007/s00213-008-1230-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon LR, Ginsburg BC, Lamb RJ. Cannabinoid agonists differentially substitute for the discriminative stimulus effects of Delta(9)-tetrahydrocannabinol in C57BL/6J mice. Psychopharmacology (Berl) 2008;198:487–495. doi: 10.1007/s00213-007-0900-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R, Fride E, Hanus L, Sheskin T, Bisogno T, Di Marzo V, Bayewitch M, Vogel Z. Anandamide may mediate sleep induction. Nature. 1997;389:25–26. doi: 10.1038/37891. [DOI] [PubMed] [Google Scholar]
- Moreira FA, Kaiser N, Monory K, Lutz B. Reduced anxiety-like behaviour induced by genetic and pharmacological inhibition of the endocannabinoid-degrading enzyme fatty acid amide hydrolase (FAAH) is mediated by CB1 receptors. Neuropharmacology. 2008;54:141–150. doi: 10.1016/j.neuropharm.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Pertwee RG, Ross RA. Cannabinoid receptors and their ligands. Prostaglandins Leukot Essent Fatty Acids. 2002;66:101–121. doi: 10.1054/plef.2001.0341. [DOI] [PubMed] [Google Scholar]
- Pich EM, Epping-Jordan MP. Transgenic mice in drug dependence research. Ann Med. 1998;30:390–396. doi: 10.3109/07853899809029939. [DOI] [PubMed] [Google Scholar]
- Riedel G, Fadda P, McKillop-Smith S, Pertwee RG, Platt B, Robinson L. Synthetic and plant-derived cannabinoid receptor antagonists show hypophagic properties in fasted and non-fasted mice. Br J Pharmacol. 2009;156:1154–1166. doi: 10.1111/j.1476-5381.2008.00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RA. Anandamide and vanilloid TRPV1 receptors. Br J Pharmacol. 2003;140:790–801. doi: 10.1038/sj.bjp.0705467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrabek RQ, Galimova L, Ethans K, Perry D. Nabilone for the treatment of pain in fibromyalgia. J Pain. 2008;9:164–173. doi: 10.1016/j.jpain.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Smith PB, Compton DR, Welch SP, Razdan RK, Mechoulam R, Martin BR. The pharmacological activity of anandamide, a putative endogenous cannabinoid, in mice. J Pharmacol Exp Ther. 1994;270:219–227. [PubMed] [Google Scholar]
- Solinas M, Scherma M, Tanda G, Wertheim CE, Fratta W, Goldberg SR. Nicotinic facilitation of delta9-tetrahydrocannabinol discrimination involves endogenous anandamide. J Pharmacol Exp Ther. 2007;321:1127–1134. doi: 10.1124/jpet.106.116830. [DOI] [PubMed] [Google Scholar]
- Solinas M, Tanda G, Justinova Z, Wertheim CE, Yasar S, Piomelli D, Vadivel SK, Makriyannis A, Goldberg SR. The endogenous cannabinoid anandamide produces delta-9-tetrahydrocannabinol-like discriminative and neurochemical effects that are enhanced by inhibition of fatty acid amide hydrolase but not by inhibition of anandamide transport. J Pharmacol Exp Ther. 2007;321:370–380. doi: 10.1124/jpet.106.114124. [DOI] [PubMed] [Google Scholar]
- Vann RE, Cook CD, Martin BR, Wiley JL. Cannabimimetic properties of ajulemic acid. J Pharmacol Exp Ther. 2007;320:678–686. doi: 10.1124/jpet.106.111625. [DOI] [PubMed] [Google Scholar]
- Vann RE, Gamage TF, Warner JA, Marshall EM, Taylor NL, Martin BR, Wiley JL. Divergent effects of cannabidiol on the discriminative stimulus and place conditioning effects of Delta(9)-tetrahydrocannabinol. Drug Alcohol Depend. 2008;94:191–198. doi: 10.1016/j.drugalcdep.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann RE, Warner JA, Bushell K, Huffman JW, Martin BR, Wiley JL. Discriminative stimulus properties of delta9-tetrahydrocannabinol (THC) in C57Bl/6J mice. Eur J Pharmacol. 2009;615:102–107. doi: 10.1016/j.ejphar.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varvel SA, Cravatt BF, Engram AE, Lichtman AH. Fatty acid amide hydrolase (−/−) mice exhibit an increased sensitivity to the disruptive effects of anandamide or oleamide in a working memory water maze task. J Pharmacol Exp Ther. 2006;317:251–257. doi: 10.1124/jpet.105.095059. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Wise LE, Niyuhire F, Cravatt BF, Lichtman AH. Inhibition of fatty-acid amide hydrolase accelerates acquisition and extinction rates in a spatial memory task. Neuropsychopharmacology. 2007;32:1032–1041. doi: 10.1038/sj.npp.1301224. [DOI] [PubMed] [Google Scholar]
- Walentiny DM, Vann RE, Warner JA, King LS, Seltzman HH, Navarro HA, Twine CE, Jr, Thomas BF, Gilliam AF, Gilmour BP, Carroll FI, Wiley JL. Kappa opioid mediation of cannabinoid effects of the potent hallucinogen, salvinorin A, in rodents. Psychopharmacology (Berl) 2010;210:275–284. doi: 10.1007/s00213-010-1827-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL. Cannabis: discrimination of “internal bliss”? Pharmacol Biochem Behav. 1999;64:257–260. doi: 10.1016/s0091-3057(99)00059-3. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Balster RL, Martin BR. Discriminative stimulus effects of anandamide in rats. European Journal of Pharmacology. 1995;276:49–54. doi: 10.1016/0014-2999(95)00010-i. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Barrett RL, Lowe J, Balster RL, Martin BR. Discriminative stimulus effects of CP 55,940 and structurally dissimilar cannabinoids in rats. Neuropharmacology. 1995;34:669–676. doi: 10.1016/0028-3908(95)00027-4. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Burston JJ, Leggett DC, Alekseeva OO, Razdan RK, Mahadevan A, Martin BR. CB1 cannabinoid receptor-mediated modulation of food intake in mice. Br J Pharmacol. 2005;145:293–300. doi: 10.1038/sj.bjp.0706157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Golden KM, Ryan WJ, Balster RL, Razdan RK, Martin BR. Evaluation of cannabimimetic discriminative stimulus effects of anandamide and methylated fluoroanandamide in rhesus monkeys. Pharmacol Biochem Behav. 1997;58:1139–1143. doi: 10.1016/s0091-3057(97)00327-4. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Huffman JW, Balster RL, Martin BR. Pharmacological specificity of the discriminative stimulus effects of delta 9-tetrahydrocannabinol in rhesus monkeys. Drug Alcohol Depend. 1995;40:81–86. doi: 10.1016/0376-8716(95)01193-5. [DOI] [PubMed] [Google Scholar]
- Wiley JL, LaVecchia KL, Karp NE, Kulasegram S, Mahadevan A, Razdan RK, Martin BR. A comparison of the discriminative stimulus effects of delta(9)-tetrahydrocannabinol and O-1812, a potent and metabolically stable anandamide analog, in rats. Exp Clin Psychopharmacol. 2004;12:173–179. doi: 10.1037/1064-1297.12.3.173. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Lowe JA, Balster RL, Martin BR. Antagonism of the discriminative stimulus effects of delta 9-tetrahydrocannabinol in rats and rhesus monkeys. J Pharmacol Exp Ther. 1995;275:1–6. [PubMed] [Google Scholar]
- Wiley JL, Ryan WJ, Razdan RK, Martin BR. Evaluation of cannabimimetic effects of structural analogs of anandamide in rats. Eur J Pharmacol. 1998;355:113–118. doi: 10.1016/s0014-2999(98)00502-0. [DOI] [PubMed] [Google Scholar]
- Wise LE, Cannavacciulo R, Cravatt BF, Martin BF, Lichtman AH. Evaluation of fatty acid amides in the carrageenan-induced paw edema model. Neuropharmacology. 2008;54:181–188. doi: 10.1016/j.neuropharm.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise LE, Shelton CC, Cravatt BF, Martin BR, Lichtman AH. Assessment of anandamide’s pharmacological effects in mice deficient of both fatty acid amide hydrolase and cannabinoid CB1 receptors. Eur J Pharmacol. 2007;557:44–48. doi: 10.1016/j.ejphar.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Wise LE, Thorpe AJ, Lichtman AH. Hippocampal CB(1) receptors mediate the memory impairing effects of Delta(9)-tetrahydrocannabinol. Neuropsychopharmacology. 2009;34:2072–2080. doi: 10.1038/npp.2009.31. [DOI] [PMC free article] [PubMed] [Google Scholar]