Abstract
Multimodality treatments that combine conventional cancer therapies with antigen-specific immunotherapy have emerged as promising approaches for the control of cancer. In the current study, we have explored the effect of doxorubicin on the antigen-specific immune responses generated in mice vaccinated with calreticulin (CRT)/E6 and/or Ii-PADRE DNA. We observed that pretreatment with doxorubicin suppressed the E6-specific CD8+ T-cell immune responses generated by CRT/E6 DNA vaccination in vaccinated mice. In contrast, pretreatment with doxorubicin enhanced the PADRE-specific CD4+ T-cell immune responses generated by Ii-PADRE DNA vaccination. Furthermore, coadministration of Ii-PADRE DNA could not only reverse the suppression, but also enhanced the E6-specific CD8+ T-cell responses in CRT/E6-vaccinated mice pretreated with doxorubicin. Finally, treatment with doxorubicin followed by CRT/E6 combined with Ii-PADRE DNA vaccination led to enhanced antitumor effects and prolonged survival in TC-1 tumor-bearing mice. The clinical implications of the current study are discussed.
Keywords: doxorubicin, DNA vaccine, calreticulin, E6, human papillomavirus
Introduction
Antigen-specific immunotherapy is an attractive approach for the treatment of cancers. DNA vaccines have emerged as a potentially promising strategy for cancer immunotherapy based on their excellent safety profile and stability (for review, see Donnelly et al.1 and Gurunathan et al.2). However, due to the poor immunogenicity of DNA vaccines, these vaccines have a low potency. The potency of DNA vaccines can be enhanced by direct delivery into the professional antigen-presenting cells (APCs), such as using intradermal administration of DNA through gene gun. Furthermore, the potency of DNA vaccines can be improved using innovative strategies that modify the properties of APCs to boost the antigen-specific immune responses (for review see Hung and Wu3 and Tsen et al.4).
DNA vaccine potency has been shown to be enhanced by using intracellular targeting strategies that enhance major histocompatibility complex (MHC) class I/II presentation of the target antigen in dendritic cells. Our previous studies have focused on the employment of calreticulin (CRT), a Ca2+-binding protein located in the endoplasmic reticulum (ER) (for review, see Gelebart et al.5) linked to a model tumor antigen, human papilloma virus type-16 (HPV-16) E6, for the development of a DNA vaccine, CRT/E6.6 Since E6 is constantly expressed in a majority of HPV-associated malignancies, E6 represents an ideal target for the development of therapeutic HPV vaccines. We have demonstrated that mice vaccinated with CRT/E6 DNA generated significant E6-specific CD8+ T-cell immune responses and could protect vaccinated mice from challenge with E6-expressing TC-1 tumors compared to vaccination with wild-type E6 DNA.6,7 Thus, DNA vaccines encoding CRT are capable of significantly enhancing the DNA vaccine potency.
Another strategy that has been employed to enhance DNA vaccine potency involves the generation of high numbers of antigen-specific CD4+ T cells in vaccinated mice. It is now clear that CD4+ T cells are important in the production of cytotoxic and memory T-cell populations (for review, see Castellino and Germain8). We previously generated a DNA construct encoding an invariant (Ii) chain in which the class II-associated Ii peptide region is replaced with a CD4+ T-helper epitope, PADRE (invariant Pan HLA-DR reactive epitope) to form Ii-PADRE. C57BL/6 mice vaccinated with DNA encoding Ii-PADRE showed significantly greater PADRE-specific CD4+ T-cell immune responses than mice vaccinated with DNA encoding the Ii chain alone. Furthermore, administration of DNA vaccines encoding HPV antigen with DNA-encoding Ii-PADRE led to significantly stronger HPV antigen-specific CD8+ T-cell immune responses and more potent protective and therapeutic antitumor effects against TC-1 tumors in mice.9
Although DNA vaccines have been shown to be effective in preclinical models against small tumors, such immunotherapeutic strategies alone may not be able to control rapidly growing, bulky tumors. This challenge may be overcome by the employment of multimodality treatment regimens that combine immunotherapy with chemotherapy in order to generate a much stronger antitumor effect (for reviews, see Emens and Jaffee10 and Emens11). Chemotherapeutic agents have been successfully used in combination with DNA vaccines to generate significant antitumor effects.12,13 Chemotherapeutic reagents have the inherent tendency to attack cells that rapidly proliferate and have a good blood supply. Furthermore, chemotherapeutic reagents travel in the blood system, which allows them to be used for cancers in multiple parts in the body. Doxorubicin is an antibiotic drug widely used in chemotherapy,14 and currently being tested in clinical trials for the treatment of several cancers.15–21 It functions by intercalating with DNA and thus inhibiting DNA replication. It is commonly used in the treatment of a wide range of cancers.
In the current study, we have explored the effect of doxorubicin on the antigen-specific immune responses generated in mice vaccinated with CRT/E6 and/or Ii-PADRE DNA. We observed that pretreatment with doxorubicin suppressed the E6-specific CD8+ T-cell immune responses generated by CRT/E6 DNA vaccination in vaccinated mice. In contrast, pretreatment with doxorubicin enhanced the PADRE-specific CD4+ T-cell immune responses generated by Ii-PADRE DNA vaccination. Furthermore, coadministration of Ii-PADRE DNA could not only reverse the suppression, but also enhanced the E6-specific CD8+ T-cell responses in CRT/E6-vaccinated mice pretreated with doxorubicin. Finally, treatment with doxorubicin followed by CRT/E6 combined with Ii-PADRE DNA vaccination led to enhanced antitumor effects and prolonged survival in TC-1 tumor-bearing mice. The clinical implications of the current study are discussed.
Materials and methods
Drug, antibodies, peptides, cell lines and mice
Doxorubicin-HCL (D1515, Sigma-Aldrich, St Louis, MO, USA) was reconstituted with 0.9% NaCl normal saline and kept at 4 °C in refrigerator. After 3 weeks of reconstitution, solution was abandoned. Antibodies against mouse CD3ε (NA/LE, clone 145-2C11), CD4 (phycoerythrin; PE-conjugated, clone L3T4), interferon (IFN)-γ (fluorescein isothiocyanate; FITC-conjugated, clone XMG1.2) and CD8a (PE-conjugated, clone Ly-1) were purchased from BD Pharmingen (San Diego, CA, USA). The HPV-16 E6 (YDFAFRDL) and PADRE (AKFVAAWTLKAAA) peptides were synthesized by Macromolecular Resources (Denver, CO, USA) at a purity of ≥70%. The production and maintenance of TC-1 have been described previously.22 Female C57BL/6 mice (6–8 weeks) were acquired from the National Cancer Institute (Frederick, MD, USA). All animals were maintained under specific pathogen-free conditions, and all procedures were done according to approved protocols and in accordance with recommendations for the proper use and care of laboratory animals.
Plasmid DNA constructs and DNA preparation
The generation of pcDNA3-Ii and pcDNA3-Ii-PADRE has been described previously.9 pcDNA3-CRT/E6 was generated as described previously.6 The DNA were amplified and purified as described previously.23
Treatment with doxorubicin combined with DNA vaccination using a gene gun
DNA-coated gold particles were prepared according to a previously described protocol.23 DNA-coated gold particles were delivered to the shaved abdominal region of mice, using a helium-driven gene gun (Bio-Rad, Hercules, CA, USA) with a discharge pressure of 400 lb per square inch. Doxorubicin (5 mg kg−1) or 0.9% NaCl normal saline as a control was injected to mice through tail vein prior to 1 week or 2 weeks before DNA vaccination. C57BL/6 mice were immunized with several combinations of DNA constructs (CRT/E6 alone, CRT/E6+pcDNA3, CRT/E6+Ii-chain CRT/E6+Ii-PADRE, Ii-chain, Ii-PADRE). Each cartridge contained 1 μg of plasmid DNA mixture and mice received two shots per mouse of the DNA mixtures by gene gun bombardment for a total of 2 μg per mouse. Each mouse received a booster of the same regimen 1 week later.
Intracellular cytokine staining and flow cytometry analysis
Splenocytes were harvested from mice (five per group) 1 week after the last vaccination. Prior to intracellular cytokine staining, 5 × 106 per mouse of pooled splenocytes from each vaccination group were incubated for 16 h with 1 μl ml−1 of E6 peptide (YDFAFRDL) containing an MHC class I (H-2Kb or Db) epitope (amino acid 50–57) or MHC class II (I-Ab) epitope PADRE (AKFVAAWTLKAAA) peptides for detecting antigen-specific CD8+ or CD4+ T-cell precursors in the presence of GolgiPlug (BD Pharmingen). Intracellular IFN-γ staining and flow cytometry analysis were performed as described previously.23 Analysis was performed on a FACScan with CELLQuest software (Becton Dickinson Immunocytometry System, Mountain View, CA, USA).
In-vivo tumor treatment experiment
For the tumor treatment experiment, C57BL/6 mice (five per group) were challenged with 5 × 104 per mouse of TC-1 tumor cells by subcutaneous injection in the right leg. At day 3 after challenge with TC-1 tumor cells, mice were administered with doxorubicin solution or 0.9% NaCl normal saline as a control through tail vein and waited for 1 week. DNA vaccination was started at day 10 with gene gun. Each designated plasmid DNA mixture (2 μg per mouse) was vaccinated three times with 4-day intervals. Tumor growth was monitored by visual inspection and palpation twice weekly as described previously.22
Tumor measurement and conditional survival
Three dimensional tumor sizes were measured two or three times per week with Vernier calipers. Tumor sizes were approximated by multiplying the measured lengths. From day 25 after challenging tumor cells, tumors were measured every other day, and mice with tumor sizes >19 mm in diameter or projected tumor volumes >10% body weight or >2700 mm3 were considered moribund and killed. Tumor volume was calculated using the following formula: V = (L × W × D), where V is tumor volume, L is length, W is width and D is depth. All of the animal studies were approved by the Institutional Animal Care and use Committee at Johns Hopkins Hospital (Baltimore, MD, USA).
Statistical analysis
All data expressed as mean ± s.e. are representative of at least two different experiments. Comparisons between individual data points were made using a Student’s t-test. Kaplan–Meier survival curves for tumor treatment and protection experiments were applied; for differences between curves, P-values were calculated using the log-rank test. The value of P<0.05 was considered significant.
Results
Pretreatment with doxorubicin suppresses the E6-specific CD8+ T-cell immune responses generated by CRT/E6 DNA vaccination in vaccinated mice
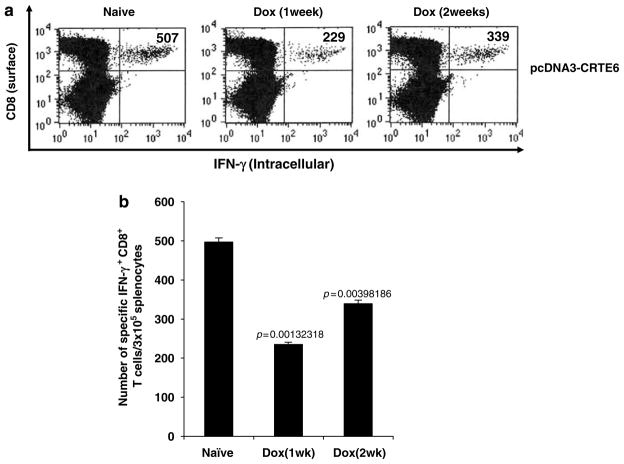
In order to determine the influence of doxorubicin on the E6-specific CD8+ T-cell immune response in CRT/E6 DNA-vaccinated mice, C57BL/6 mice (five per group) were treated with or without doxorubicin through tail vein. At 1 week or 2 weeks after doxorubicin treatment, mice were immunized with 2 μg per mouse of CRT/E6 DNA. At 1 week after the last vaccination, we measured the E6-specific CD8+ T-cell immune responses in vaccinated mice using intracellular IFN-γ staining followed by flow cytometry analysis. As shown in Figure 1, we observed that the E6-specific CD8+ T-cell responses were significantly reduced in vaccinated mice pretreated with doxorubicin compared to those in vaccinated mice without doxorubicin pretreatment. Furthermore, the mice pretreated with doxorubicin 2 weeks before DNA vaccination showed less reduction in the E6-specific CD8+ T-cell levels compared to mice pretreated with doxorubicin 1 week before DNA vaccination. We also calculated the percentage of the total CD8+ T cells in the spleen with or without doxorubicin treatment. We found that the percentage of total CD8+ T cells in the spleen does not have significant change with or without doxorubicin treatment (approximately 20–25% of the total splenocytes). Thus, our data indicate that pretreatment with doxorubicin suppressed the E6-specific CD8+ T-cell immune response generated in mice vaccinated with CRT/E6 DNA vaccine.
Figure 1.
Characterization of the E6-specific CD8+ T-cell immune response in CRT/E6 DNA-vaccinated mice with or without pretreatment with doxorubicin. C57BL/6 mice (five per group) were injected doxorubicin dissolved in 0.9% NaCl solution or 0.9% NaCl solution as a control through tail vein. At 1 week or 2 weeks after doxorubicin treatment, mice were immunized with 2 μg per mouse of CRT/E6 DNA twice with a 1-week interval. At 1 week after the last vaccination, splenocytes from treated mice were harvested and characterized for E6-specific CD8+ T cells using intracellular interferon (IFN)-γ staining followed by flow cytometry analysis. (a) Representative flow cytometry data for the E6-specific CD8+ T-cell immune responses. The numbers in the upper right-hand corner represent the number of E6-specific IFN-γ-secreting CD8+ T cells per 3 × 105 splenocytes. (b) Bar graphs depicting the numbers of E6-specific IFN-γ-secreting CD8+ T cells per 3 × 105 splenocytes (means ± s.e.). The data presented in this figure are from one representative experiment of two performed.
Pretreatment with doxorubicin enhances the PADRE-specific CD4+ T-cell immune responses generated by Ii-PADRE DNA vaccination in vaccinated mice
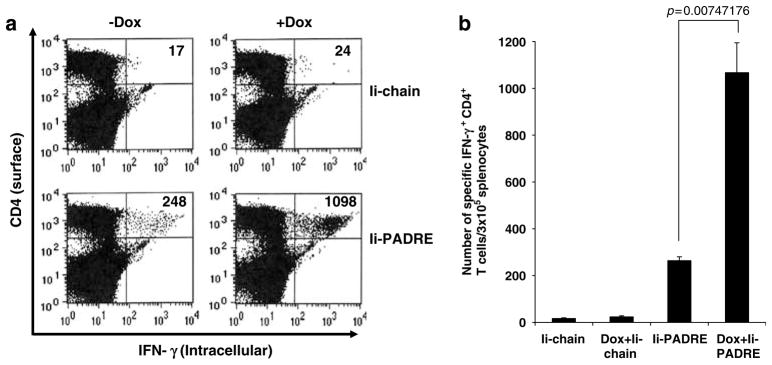
In order to determine if pretreatment with doxorubicin will also influence the PADRE-specific CD4+ T-cell immune responses generated by vaccination with Ii-PADRE DNA, C57BL/6 mice (five per group) were treated with or without doxorubicin through tail vein. At 1 week after doxorubicin treatment, mice were immunized with 2 μg per mouse of Ii-PADRE DNA or Ii-chain alone. At 1 week after the last vaccination, we measured the PADRE-specific CD4+ T-cell immune responses in vaccinated mice using intracellular IFN-γ staining followed by flow cytometry analysis. As shown in Figure 2a, doxorubicin pretreated mice vaccinated with Ii-PADRE DNA demonstrated a significant increase in the PADRE-specific CD4+ T-cell immune responses compared to mice vaccinated with Ii-PADRE DNA without doxorubicin pretreatment. A graphical representation of the number of PADRE-specific CD4+ T cells in the treated mice is depicted in Figure 2b. We also calculated the percentage of the total CD4+ T cells in the spleen with or without doxorubicin treatment. We found that the percentage of total CD4+ T cells in the spleen does not have significant change with or without doxorubicin treatment (approximately 20–25% of total splenocytes). Thus, our data suggest that pretreatment with doxorubicin led to enhancement, rather than reduction of PADRE-specific CD4+ T-cell immune responses generated by Ii-PADRE DNA vaccination in vaccinated mice.
Figure 2.
Characterization of the PADRE-specific CD4+ T-cell immune response in Ii-PADRE DNA-vaccinated mice with or without pretreatment with doxorubicin. C57BL/6 mice (five per group) were injected doxorubicin dissolved in 0.9% NaCl solution or 0.9% NaCl solution as a control through tail vein. At 1 week after doxorubicin treatment, mice were immunized with 2 μg per mouse of Ii-PADRE DNA or Ii-chain alone twice with a 1-week interval. At 1 week after the last vaccination, splenocytes from treated mice were harvested and characterized for PADRE-specific CD4+ T cells using intracellular interferon (IFN)-γ staining followed by flow cytometry analysis. (a) Representative flow cytometry data showing the numbers of activated PADRE-specific CD4+ T cells in the treated mice. (b) Bar graphs depicting the numbers of PADRE-specific CD4+ T cells per 3 × 105 splenocytes (means ± s.e.). The data presented in this figure are from one representative experiment of two performed.
Pretreatment with doxorubicin enhances the E6-specific CD8+ T-cell immune responses generated by vaccination with CRT/E6 combined with Ii-PADRE DNA in vaccinated mice
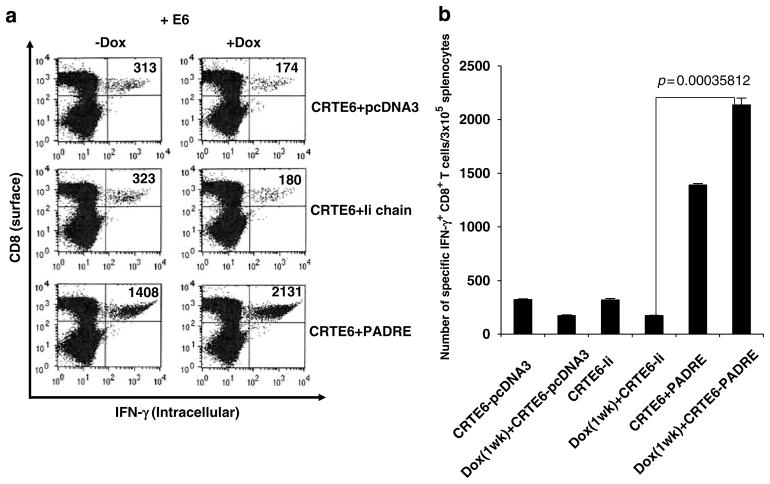
In order to determine if pretreatment with doxorubicin will also influence the E6-specific CD8+ T-cell immune responses generated by vaccination with CRT/E6 combined with Ii-PADRE DNA, C57BL/6 mice (five per group) were treated with or without doxorubicin through tail vein. At 1 week after doxorubicin treatment, mice were immunized with 2 μg per mouse of CRT/E6 and/or Ii-PADRE DNA or Ii-chain alone. At 1 week after the last vaccination, we measured the E6-specific CD8+ T-cell immune responses in vaccinated mice using intracellular IFN-γ staining followed by flow cytometry analysis. As shown in Figure 3, mice pre-treated with doxorubicin followed by vaccination with CRT/E6 and Ii-PADRE DNA generated a significantly higher E6-specific CD8+ T-cell immune responses compared to mice vaccinated with CRT/E6 and Ii-PADRE DNA without doxorubicin pretreatment. Thus, our data indicate that coadministration of Ii-PADRE DNA can not only reverse the suppression, but also enhance the E6-specific CD8+ T-cell responses in CRT/E6-vaccinated mice pretreated with doxorubicin.
Figure 3.
Characterization of the E6-specific CD8+ T-cell immune response in mice vaccinated with CRT/E6 and Ii-PADRE DNA with or without pretreatment with doxorubicin. C57BL/6 mice (five per group) were injected doxorubicin dissolved in 0.9% NaCl solution or 0.9% NaCl solution as a control through tail vein. At 1 week after doxorubicin treatment, mice were immunized with 2 μg per mouse of CRT/E6 DNA and Ii-PADRE DNA or Ii-chain alone twice with a 1-week interval. At 1 week after the last vaccination, splenocytes from treated mice were harvested and characterized for E6-specific CD8+ T cells using intracellular interferon (IFN)-γ staining followed by flow cytometry analysis. (a) Representative flow cytometry data showing the numbers of activated E6-specific CD8+ T cells in the treated mice. (b) Bar graphs depicting the numbers of E6-specific CD8+ T cells per 3 × 105 splenocytes (means ± s.e.). The data presented in this figure are from one representative experiment of two performed.
Pretreatment with doxorubicin enhances the PADRE-specific CD4+ T-cell immune responses generated by vaccination with CRT/E6 combined with Ii-PADRE DNA in vaccinated mice
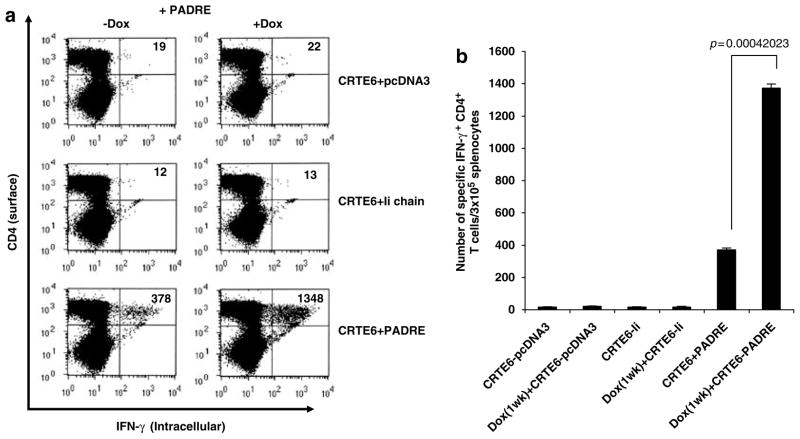
We also characterized the PADRE-specific CD4+ T-cell immune responses in mice pretreated with doxorubicin followed by vaccination with CRT/E6 combined with Ii-PADRE DNA. As shown in Figure 4, mice pretreated with doxorubicin vaccinated with CRT/E6 and Ii-PADRE DNA generated a significantly higher PADRE-specific CD4+ T-cell immune responses compared to mice vaccinated with CRT/E6 and Ii-PADRE DNA without doxorubicin pretreatment. Furthermore, there was no significant difference in PADRE-specific CD4+ T-cell immune responses between mice pretreated with doxorubicin and vaccinated with Ii-PADRE alone (1.83%) (See Figure 2) or Ii-PADRE+CRT/E6 (2.24%). Taken together, our data suggest that pretreatment with doxorubicin leads to enhanced PADRE-specific CD4+ T-cell immune responses in mice vaccinated with Ii-PADRE with or without CRT/E6 DNA.
Figure 4.
Characterization of the PADRE-specific CD4+ T-cell immune response in mice vaccinated with CRT/E6 and Ii-PADRE DNA with or without pretreatment with doxorubicin. C57BL/6 mice (five per group) were injected doxorubicin dissolved in 0.9% NaCl solution or 0.9% NaCl solution as a control through tail vein. At 1 week after doxorubicin treatment, mice were immunized with 2 μg per mouse of CRT/E6 DNA and Ii-PADRE DNA or Ii-chain alone twice with a 1-week interval. At 1 week after the last vaccination, splenocytes from treated mice were harvested and characterized for PADRE-specific CD4+ T cells using intracellular interferon (IFN)-γ staining followed by flow cytometry analysis. (a) Representative flow cytometry data showing the numbers of activated PADRE-specific CD4+ T cells in the treated mice. (b) Bar graphs depicting the numbers of PADRE-specific CD4+ T cells per 3 × 105 splenocytes (means ± s.e.). The data presented in this figure are from one representative experiment of two performed.
Treatment with doxorubicin followed by CRT/E6 combined with Ii-PADRE DNA vaccination leads to enhanced antitumor effects and prolonged survival in TC-1 tumor-bearing mice
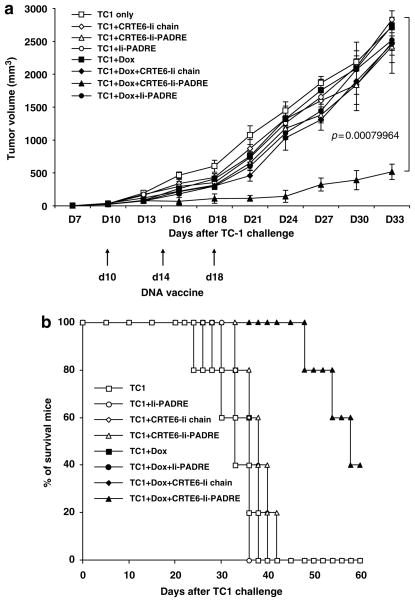
In order to determine if the enhanced E6-specific T-cell response generated by treatment with doxorubicin followed by CRT/E6 combined with Ii-PADRE DNA vaccination translates into therapeutic antitumor effects, we performed in-vivo tumor treatment experiments using an HPV-16 E6-expressing tumor model, TC-1. C57BL/6 mice were first challenged subcutaneously with TC-1 tumor cells and then, 3 days later, treated with or without doxorubicin through tail vein. At 1 week after doxorubicin treatment, mice were immunized with 2 μg per mouse of CRT/E6 and/or Ii-PADRE DNA or Ii-chain alone. The treated mice were monitored for tumor growth. As shown in Figure 5a, tumor-bearing mice treated with doxorubicin followed by CRT/E6 combined with Ii-PADRE DNA vaccination exhibited significantly decreased tumor growth compared to the tumor-bearing mice vaccinated with CRT/E6 combined with Ii-PADRE DNA without doxorubicin treatment (P<0.01). We also performed Kaplan–Meier survival analysis of the treated mice. As shown in Figure 5b, tumor-challenged mice treated with doxorubicin followed by CRT/E6 combined with Ii-PADRE DNA vaccination also exhibited significantly prolonged survival compared to the other treatment groups. Thus, our data indicate that treatment treated with doxorubicin followed by CRT/E6 combined with Ii-PADRE DNA vaccination leads to enhanced antitumor effects and prolonged survival in TC-1 tumor-bearing mice.
Figure 5.
In-vivo tumor treatment experiments. C57BL/6 mice (five per group) were first challenged with 5 × 104 per mouse of TC-1 tumor cells by subcutaneous injection. At 3 days after tumor challenge, mice were injected doxorubicin dissolved in 0.9% NaCl solution or 0.9% NaCl solution as a control through tail vein. At 1 week after doxorubicin treatment, mice were immunized with 2 μg per mouse of CRT/E6 DNA and/or Ii-PADRE DNA or Ii-chain alone three times with 4-day intervals. The mice were monitored for evidence of tumor growth by inspection and palpation twice a week. Tumor volumes were measured starting from day 7 after tumor challenge. (a) Line graph depicting the tumor volumes in mice with different tumor treatments (means ± s.e.). (b) Kaplan–Meier survival analysis in mice treated in the various groups. The data shown here are from one representative experiment of two performed.
Discussion
In the current study, we have explored the effect of doxorubicin on the antigen-specific immune responses generated in mice vaccinated with CRT/E6 and/or Ii-PADRE DNA. We observed that pretreatment with doxorubicin suppressed the E6-specific CD8+ T-cell immune responses generated by CRT/E6 DNA vaccination in vaccinated mice. In contrast, pretreatment with doxorubicin enhanced the PADRE-specific CD4+ T-cell immune responses generated by Ii-PADRE DNA vaccination. Furthermore, coadministration of Ii-PADRE DNA could not only reverse the suppression, but also enhanced the E6-specific CD8+ T-cell responses in CRT/E6-vaccinated mice pretreated with doxorubicin. Finally, treatment with doxorubicin followed by CRT/E6 combined with Ii-PADRE DNA vaccination led to enhanced antitumor effects and prolonged survival in TC-1 tumor-bearing mice.
In our study, we observed that treatment with doxorubicin could not only specifically enhance PADRE-specific CD4+ T-cell immune responses, but also suppressed the levels of E6-specific CD8+ T cells. It is not however clear if treatment with doxorubicin will lead to a general enhancement of antigen-specific CD4+ T cells and reduction of antigen-specific CD8+ T cells in other antigenic systems. Furthermore, the mechanism by which doxorubicin impacts the antigen-specific CD4 and CD8+ T-cell immune responses remains to be illustrated. One possibility is that doxorubicin may influence the function of professional APCs and result in the preferential activation of PADRE-specific CD4+ T helper cells, but not E6-specific CD8+ T cells. Furthermore, previous studies have shown that treatment with doxorubicin may induce apoptosis of T cells.24 The specific mechanisms for the observed effects of doxorubicin on antigen-specific T cells warrant further investigation.
In our study, we observed that vaccination with Ii-PADRE could not only reverse the suppression, but also lead to a significant enhancement in the E6-specific CD8+ T-cell immune response. This may be related to the induction of IL-2 secreting PADRE-specific CD4+ T helper cells by Ii-PADRE DNA. In our previous study, we have shown that PADRE-specific CD4+ T cells stimulated by PADRE-loaded DCs secrete IL-2 that leads to the proliferation of antigen-specific CD8+ T cells.25 The IL-2 secreted by the activated PADRE-specific CD4+ T helper cells generated by vaccination with Ii-PADRE, at the vicinity of the antigen-specific CD8+ T cells may contribute to the enhancement of the E6-specific CD8+ T-cell immune response.
The employment of gene gun administration is essential for the success of the current strategy. The Ii-PADRE DNA strategy requires the induction of CD4+ T helper cells in the vicinity of antigen-specific CD8+ T cells in order to enhance T-cell activation. The CRT/E6 DNA vaccines also requires the direct delivery into the DCs in order to effectively influence the priming of the T cells. The antigen linked to CRT will be directly targeted to the ER of the DCs and thus enhance the antigen processing. Thus, the strategies employed in the current study rely heavily on the intradermal delivery of DNA constructs through gene gun.
This study has several key implications for clinical translation. The current strategy could potentially be applied to clinical arena in the case of cancer patients treated with doxorubicin. The strategy of vaccination with Ii-PADRE could reverse the suppression caused by doxorubicin treatment and potentially enhance the antigen-specific CD8+ T-cell responses and antitumor effects in patients receiving immunotherapy. It will be important to determine if the current strategy can be extended to other types of chemotherapeutic drugs and also other forms of DNA vaccination. The success in the current study suggests that application of Ii-PADRE DNA in conjunction with other DNA vaccines administered through gene gun may serve as a platform for cancer immunotherapy in patients receiving chemotherapy.
Acknowledgments
This work was supported by the Flight Attendant Medical Research Institute and National Cancer Institute SPORE in Cervical Cancer P50 CA098252 and the 1 RO1 CA114425-01.
References
- 1.Donnelly JJ, Ulmer JB, Liu MA. DNA vaccines. Life Sci. 1997;60:163–172. doi: 10.1016/s0024-3205(96)00502-4. [DOI] [PubMed] [Google Scholar]
- 2.Gurunathan S, Klinman DM, Seder RA. DNA vaccines: immunology, application, and optimization. Annu Rev Immunol. 2000;18:927–974. doi: 10.1146/annurev.immunol.18.1.927. [DOI] [PubMed] [Google Scholar]
- 3.Hung CF, Wu TC. Improving DNA vaccine potency via modification of professional antigen presenting cells. Curr Opin Mol Ther. 2003;5:20–24. [PubMed] [Google Scholar]
- 4.Tsen SW, Paik AH, Hung CF, Wu TC. Enhancing DNA vaccine potency by modifying the properties of antigen-presenting cells. Expert Rev Vaccines. 2007;6:227–239. doi: 10.1586/14760584.6.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gelebart P, Opas M, Michalak M. Calreticulin, a Ca2+-binding chaperone of the endoplasmic reticulum. Int J Biochem Cell Biol. 2005;37:260–266. doi: 10.1016/j.biocel.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 6.Peng S, Ji H, Trimble C, He L, Tsai YC, Yeatermeyer J, et al. Development of a DNA vaccine targeting human papillomavirus type 16 oncoprotein E6. J Virol. 2004;78:8468–8476. doi: 10.1128/JVI.78.16.8468-8476.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peng S, Tomson TT, Trimble C, He L, Hung CF, Wu TC. A combination of DNA vaccines targeting human papillomavirus type 16 E6 and E7 generates potent antitumor effects. Gene Ther. 2006;13:257–265. doi: 10.1038/sj.gt.3302646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castellino F, Germain RN. Cooperation between CD4+ and CD8+ T cells: when, where, and how. Annu Rev Immunol. 2006;24:519–540. doi: 10.1146/annurev.immunol.23.021704.115825. [DOI] [PubMed] [Google Scholar]
- 9.Hung CF, Tsai YC, He L, Wu TC. DNA vaccines encoding Ii-PADRE generates potent PADRE-specific CD4+ T-cell immune responses and enhances vaccine potency. Mol Ther. 2007;15:1211–1219. doi: 10.1038/sj.mt.6300121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emens LA, Jaffee EM. Leveraging the activity of tumor vaccines with cytotoxic chemotherapy. Cancer Res. 2005;65:8059–8064. doi: 10.1158/0008-5472.CAN-05-1797. [DOI] [PubMed] [Google Scholar]
- 11.Emens LA. Chemotherapy and tumor immunity: an unexpected collaboration. Front Biosci. 2008;13:249–257. doi: 10.2741/2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang TH, Lee JH, Song CK, Han HD, Shin BC, Pai SI, et al. Epigallocatechin-3-gallate enhances CD8+ T cell-mediated anti-tumor immunity induced by DNA vaccination. Cancer Res. 2007;67:802–811. doi: 10.1158/0008-5472.CAN-06-2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Machiels JP, Reilly RT, Emens LA, Ercolini AM, Lei RY, Weintraub D, et al. Cyclophosphamide, doxorubicin, and paclitaxel enhance the antitumor immune response of granulocyte/macrophage-colony stimulating factor-secreting whole-cell vaccines in HER-2/neu tolerized mice. Cancer Res. 2001;61:3689–3697. [PubMed] [Google Scholar]
- 14.Di Marco A, Gaetani M, Scarpinato B. Adriamycin (NSC-123, 127): a new antibiotic with antitumor activity. Cancer Chemother Rep. 1969;53:33–37. [PubMed] [Google Scholar]
- 15.Ning YM, He K, Dagher R, Sridhara R, Farrell AT, Justice R, et al. Liposomal doxorubicin in combination with bortezomib for relapsed or refractory multiple myeloma. Oncology (Williston Park) 2007;21:1503–1508. discussion 1511, 1513, 1516 passim. [PubMed] [Google Scholar]
- 16.Giotta F, Lorusso V, Maiello E, Filippelli G, Valerio MR, Caruso M, et al. Liposomal-encapsulated doxorubicin plus cyclophosphamide as first-line therapy in metastatic breast cancer: a phase II multicentric study. Ann Oncol. 2007;18(Suppl 6):vi66–vi69. doi: 10.1093/annonc/mdm228. [DOI] [PubMed] [Google Scholar]
- 17.Andreopoulou E, Gaiotti D, Kim E, Volm M, Oratz R, Freedberg R, et al. Feasibility and cardiac safety of pegylated liposomal doxorubicin plus trastuzumab in heavily pretreated patients with recurrent HER2-overexpressing metastatic breast cancer. Clin Breast Cancer. 2007;7:690–696. doi: 10.3816/CBC.2007.n.028. [DOI] [PubMed] [Google Scholar]
- 18.Pectasides D, Xiros N, Papaxoinis G, Aravantinos G, Sykiotis C, Pectasides E, et al. Gemcitabine and pegylated liposomal doxorubicin alternating with cisplatin plus cyclophosphamide in platinum refractory/resistant, paclitaxel-pretreated, ovarian carcinoma. Gynecol Oncol. 2008;108:47–52. doi: 10.1016/j.ygyno.2007.08.061. [DOI] [PubMed] [Google Scholar]
- 19.Alberts DS, Liu PY, Wilczynski SP, Clouser MC, Lopez AM, Michelin DP, et al. Randomized trial of pegylated liposomal doxorubicin (PLD) plus carboplatin versus carboplatin in platinum-sensitive (PS) patients with recurrent epithelial ovarian or peritoneal carcinoma after failure of initial platinum-based chemotherapy (Southwest Oncology Group Protocol S0200) Gynecol Oncol. 2008;108:90–94. doi: 10.1016/j.ygyno.2007.08.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mellqvist UH, Lenhoff S, Johnsen HE, Hjorth M, Holmberg E, Juliusson G, et al. Cyclophosphamide plus dexamethasone is an efficient initial treatment before high-dose melphalan and autologous stem cell transplantation in patients with newly diagnosed multiple myeloma: results of a randomized comparison with vincristine, doxorubicin, and dexamethasone. Cancer. 2008;112:129–135. doi: 10.1002/cncr.23145. [DOI] [PubMed] [Google Scholar]
- 21.O’Brien ME. Single-agent treatment with pegylated liposomal doxorubicin for metastatic breast cancer. Anticancer Drugs. 2008;19:1–7. doi: 10.1097/CAD.0b013e3282f14a00. [DOI] [PubMed] [Google Scholar]
- 22.Lin KY, Guarnieri FG, Staveley-O’Carroll KF, Levitsky HI, August JT, Pardoll DM, et al. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996;56:21–26. [PubMed] [Google Scholar]
- 23.Chen CH, Wang TL, Hung CF, Yang Y, Young RA, Pardoll DM, et al. Enhancement of DNA vaccine potency by linkage of antigen gene to an HSP70 gene. Cancer Res. 2000;60:1035–1042. [PubMed] [Google Scholar]
- 24.Zaleskis G, Berleth E, Verstovsek S, Ehrke MJ, Mihich E. Doxorubicin-induced DNA degradation in murine thymocytes. Mol Pharmacol. 1994;46:901–908. [PubMed] [Google Scholar]
- 25.Kim D, Monie A, He L, Tsai YC, Hung CF, Wu T-C. Role of IL-2 secreted by PADRE-specific CD4+ T Cells in enhancing E7-specific CD8+ T cell immune responses. Gene Therapy. 2008;15:677–687. doi: 10.1038/sj.gt.3303102. [DOI] [PMC free article] [PubMed] [Google Scholar]