Abstract
IL-15 is an important cytokine involved in the survival and function of CD8+ T cells and NK cells. IL-15 can be presented by IL-15Rα (IL-15RA) to bind with the shared IL-2/IL-15Rβ and common γ-chains, which activate signaling pathways on NK cells and CD8+ T cells. In the present study, we characterized the function of trans-presented IL-15 on NK cells and CD8+ T cells using TC-1 tumor cells transduced with a retrovirus encoding IL-15 linked to IL-15RA (IL-15/IL-15RA). We demonstrated that the expression of IL-15/IL-15RA on TC-1 cells led to increased percentages of tumor-infiltrating NK cells, NKT cells, and CD8+ T cells, resulting in the inhibition of tumor growth in challenged mice. Additionally, in vivo Ab depletion experiments demonstrated that NK1.1+ cells and CD8+ T cells were important in this inhibition of tumor growth. Furthermore, this accumulation of immune cells and inhibition of tumor growth was abolished by a single amino acid mutation in the common γ-chain binding site on IL-15. We also observed that IL-15/IL-15RA-transduced TC-1 cells led to the activation of STAT5 in NK and CD8+ T cells in trans, which was abolished in the mutated IL-15/IL-15RA-transduced TC-1 cells. Taken together, our data suggest that common γ-chain binding-dependent activation of the shared IL-15/IL-2Rβ/common γ signaling pathway may play an important role in the activation of NK cells and CD8+ T cells, resulting in IL-15/IL-15RA trans-presentation-mediated inhibition of tumor growth.
Interleukin-15 is a pleiotropic cytokine that plays an important role in both adaptive and innate immune responses. IL-15 is particularly important to the development, survival, and function of CD8+ T cells and NK cells (1, 2). IL-15−/− (knockout) mice are deficient in CD8+ T cells and NK cells (2). Furthermore, IL-15 administration via recombinant protein, viral vector, or plasmid has been shown to enhance NK cell and CD8+ T cell immune responses against a variety of pathogenic diseases, including tumors. The use of IL-15 alone or in conjunction with vaccination or drug treatments has proven effective in treating a variety of human and mouse tumors in animal models (3).
IL-15Rα (IL-15RA)3 is a high-affinity binding receptor for IL-15 (4, 5). Like IL-15 knockout mice, IL-15RA knockout mice are deficient in CD8+ T cells and NK cells (6). Several in vitro and in vivo studies suggest that IL-15RA on the surface of CD8+ T cells and NK cells may contribute to IL-15 signals (6–8). Alternatively, IL-15RA found on the surface of other cell types, including macrophages and dendritic cells, can bind and present IL-15 to NK and CD8 cells in trans (9, 10). It is now generally thought that IL-15RA expression by hematopoietic and nonhematopoietic cell types, as opposed to dispensable expression of IL-15RA by NK and CD8+ T cells, is critical for NK cell and memory CD8+ T cell homeostasis (11–13). Thus trans-presentation of IL-15 to CD8+ T cells and NK cells is considered the dominant mechanism involved in their homeostasis.
It has been shown that IL-15 can signal through the shared IL-2/IL-15Rβ and common γ (γc)-chains in the presence or absence of IL-15RA (5, 14, 15). IL-15 has been shown to activate the JAK-STAT pathway through the β/γc complex (16, 17). The β-chain of the β/γc complex recruits JAK1, which facilitates JAK1 activation and phosphorylation of STAT3 upon ligand binding. The γc-chain recruits JAK3, which facilitates JAK3 and posphorylation of STAT5 upon ligand binding (18, 19). Activation of STAT5 and/or STAT3 is critical for IL-15-mediated NK and T cell survival, proliferation, and function (20, 21). In addition to JAK-STAT signaling, multiple other pathways including the Ras/MAPK/ERK and PI3K-AKT pathways have been implicated in IL-15 signal transduction (22–24). IL-15 signaling pathways have been elucidated in experiments using soluble rather than trans-presented IL-15 to activate signaling in T cells and NK cells. Because biological responses to soluble IL-15 and trans-presented IL-15 are dependent on the presence of the receptor β/γc complex on responding cells in vitro (9, 10), it is likely that the same signal pathways activated by soluble IL-15 are also activated by IL-15 presented by IL-15RA in trans.
The role of the receptor β/γc complex in IL-15 trans-presentation and signaling in NK cells and/or CD8+ T cells has been difficult to address in vivo. In vivo administration of receptor β- or γc-chain-blocking Ab has been shown to inhibit responses attributed to IL-15 (25, 26). However, because multiple cytokines share the same receptor, it is unclear whether this is a direct result of blocking IL-15 interaction with the receptor β- or γc-chains, or secondary effects of coinhibiting the functions of other β- and/or γc-dependent cytokines, such as IL-2 or IL-7. The same difficulty applies to the use of receptor β or γc knockout cells, or the systemic administration of the soluble antagonistic IL-15 recombinant proteins (11, 12, 27). A more specific approach is therefore necessary to analyze the contribution of the β/γc complex in IL-15 trans-presentation in vivo.
In the present study, we study the role of β/γc signaling in IL-15 trans-presentation using TC-1 tumor cells expressing IL-15 linked to IL-15RA (IL-15/IL-15RA). We showed that the expression of the chimeric IL-15/IL-15RA on TC-1 tumor cells led to increased percentage of tumor-infiltrating NK cells, NKT cells, and CD8+ T cells and to the inhibition of tumor growth in challenged mice. Furthermore, we showed that NK1.1+ cells and CD8+ T cells are essential for the inhibition of tumor growth. The accumulation of NK cells, NKT cells, and CD8+ T cells and the inhibition of tumor growth was abolished by a single amino acid mutation in the γc binding site on IL-15 of the IL-15/IL-15RA molecule. We also observed that IL-15/IL-15RA-transduced TC-1 cells activated STAT5 phosphorylation in NK and CD8+ T cells. In contrast, the activation of STAT5 was abolished by a mutation in the γc-chain binding site on IL-15 of the IL-15/IL-15RA. Taken together, our data suggests that γc binding-dependent activation of NK cells and CD8+ cells through the β/γc signaling pathway may play a decisive role in IL-15/IL-15RA trans-presentation-mediated inhibition of tumor growth.
Materials and Methods
Reagents
All Abs were used according to manufacturer recommendations. The Abs to CD8A, Thy1.1, CD3ε, CD44, NK1.1, CD132, CD122, CD28, streptavidin-PE, and streptavidin-allophycocyanin, and 7-aminoactinomycin D and phosphorylated STAT1, STAT3, STAT5, AKT, and ERK1/2 and iso-type controls were purchased from BD Biosciences. The Abs against phosphorylated STAT1, STAT3, and STAT5 for flow cytometry analysis have been previously reported (28–30). Biotinylated IL-15 (catalog no. BAF447) and IL-15RA (catalog no. BAF551) Abs were purchased from R&D Systems. Murine NK cell and CD8 T cell-negative selection kits were purchased from Miltenyi Biotec. pORF-IL-15 was purchased from InvivoGen. Recombinant human IL-15 and anti-granzyme B Ab was purchased from eBioscience. Sterile filtered FACS buffer was made with 0.2% BSA in Dulbecco’s PBS (pH 7.4). Polybrene was purchased from Sigma-Aldrich.
Mice
C57BL/6J mice were acquired from the National Cancer Institute. OT-1 RAG−/− mice were purchased from The Jackson Laboratory. All animals were maintained under specific pathogen-free conditions, and all procedures were performed according to approved protocols and in accordance with recommendations for the proper use and care of laboratory animals.
Plasmids
IL-15RA was cloned from murine splenocyte cDNA into PCDNA3.1 as previously described (31). pORF IL-15 was subcloned into PCDNA3.1. The signal peptide of IL-15 was replaced with IL-2 signal peptide using the long primer BamHI (5′-ACC GGA TCC GCC GCC ACC ATG TAC AGC ATG CAG CTC GCA TCC TGT GTC ACA TTG ACA CTT GTG CTC CTT GTC AAC AGC AAC TGG ATA GAT GTA AGA-3′). IL-15 with the IL-2 signal peptide was cloned into BamH1/EcoRI sites in PCDNA3.1+. IL-15 with the IL-2 signal peptide was linked to IL-15RA using a strategy similar to one previously described linking human IL-15 to human IL-15RA (32). Recombinant PCR primers linker/IL-15RA (forward primer: 5′-CGG AGG TGG TGG TTC CGG TGG TGG TGG TAG TGG AGG TGG TAG TCT TCA GGG CAC CAC GTG TC) and linker/IL-15 (reverse primer: 5′-CAC CGG AAC CAC CAC CTC CGG AAC CGC CTC CAC CGG AAC ACC GCC GGA GGA CGT GTT GAT G) were used in a two-step PCR with outside primers IL-15RA (3′ end reverse primer) and IL-15 (5′ end forward primer) listed above, and the product was cloned into PCDNA3.1 using BamHI/EcoRI restriction sites. Q108D in IL-15 was mutated by recombinant PCR with the primers AGC TTT ATA CGC ATT GTC GAC ATG TTC ATC AAC ACG TCC and GGA CGT GTT GAT GAA CAT GTC GAC AAT GCG TAT AAA GCT. All constructs were subcloned via BamHI/XhoI into the pMIGII retroviral vector. The pMIGII vector has been previously described (33). Large-scale plasmid preparations were purified using Qiagen EndoFree Plasmid Maxi kits.
Cell culture, transduction, and proliferation
TC-1 and murine ovarian surface epithelial carcinoma (MOSEC) tumor cells were cultured as previously described (34, 35). For transduction, retroviral constructs or internal ribosomal entry site (IRES)-GFP empty vectors were transfected into Phoenix packaging cells and virions were harvested at 48 and 72 h after transfection. Virus was filtered using a 0.45-μm cellulose acetate syringe filter and concentrated 10-fold using an Millipore 100K cutoff centrifuge filter. TC-1 cells were infected with concentrated virus in the presence of 8 μg/ml polybrene for 24 h, after which media was replaced with normal media. Cells were sorted for GFP expression after a brief expansion. Cell doubling time was determined by plating 1 × 103 TC-1 cells/well in 6-well plates in triplicate. Four days later, cells were counted and doubling time was calculated using the formula Td = 96 × log2/log(no. of cells at 96 h/1 × 103).
In vitro T cell and NK stimulation
Purified NK cells and CD8 T cells from fresh mouse splenocytes were prepared using negative magnetic selection kits according to the manufacturer’s protocol (Miltenyi Biotec). T cells (5 × 104) or NK cells were mixed with 5 × 104 tumor cells in 96-well U-shaped plates. In some wells 50 ng/ml recombinant mouse IL-15 was added at the time of mixing. For AKT and ERK1/2 analysis, CD8+ T cells from OT-1 RAG−/− mice were first serum starved for 4 h and stimulations were done in the absence of serum. Where indicated, 0.1 μg/ml SIINFEKL peptide (OVA peptide), 1 μg/ml anti-CD28, and 10% serum were also added at the time of mixing. Cells were centrifuged to maximize contacts. Cells were incubated at 37°C in a 5% CO2 incubator for 45 min and harvested according to the manufacturer’s directions for intracellular staining of phosphorylated proteins.
Preparation of single-cell suspensions from solid tumors and cell staining
One week after s.c. injection of 2.5 × 106 TC-1 tumor cells, tumors were excised, placed in complete RPMI media, and incubated at 4°C overnight. Solid tumors were removed (set aside media) and minced into 1- to 2-mm pieces and immersed in HBSS containing 1 mg ml−1 collagenase D and 0.25 mg ml−1 DNase I (Roche), and incubated 45 min at 37°C. Undigested tissue fragments were removed by filtering through a 70-μm nylon filter mesh. The resultant single tumor-cell suspensions were combined with set-aside media and washed twice in HBSS followed by cell staining. Cells were stained according to directions and Ab concentration given by supplier. Analysis was performed on a FACScan with CellQuest software (BD Biosciences). Percentage of tumor-infiltrating lymphocytes was calculated by gating on the lymphocyte population according to forward scatter and side scatter properties and determining the percentages of CD4+, CD8+, NK1.1+, CD3−, or NK1.1+ CD3+ cells within this population.
Tumor challenge experiments
Unless otherwise stated, for tumor challenge, mice were injected s.c. with 5 × 104 transduced tumor cells/mouse in the right hindleg. Tumor growth was measured using calipers, and average tumor diameter was calculated by taking the average of the longest and shortest diameter of the tumor. Tumors were excised and weighed after sacrificing animals.
Ab depletion
Depletion was performed using blocking Abs for in vivo depletion of CD8, CD4, and NK cells using a protocol similar to one described previously (34). C57BL/6 mice (n = 5/group) were depleted of CD8, CD4, and NK1.1+ cells or CD8 and NK1.1+ cells using 200 μg in 200 μl of relevant anti-NK1.1, anti-CD4, or anti-CD8 Abs. Mice were injected with purified mAb GK1.5 (anti-CD4), mAb 2.43 (anti-CD8), and/or mAb PK136 (anti-NK1.1) i.p. four times every 3 days. Two days after the fourth Ab injection, the mice from the various depleted groups were s.c. challenged with 5 × 104/mouse of TC-1 tumor cells transduced with IL-15/IL-15RA into the left flank. Continued Ab depletion was performed in tumor-bearing mice 1 wk after tumor challenge. Tumor diameters were measured on days 0, 10, 14, 18, and 22.
Statistical analysis
Statistical analysis was performed in most cases using a one-sided Student’s t test with statistical significance at α = 0.05. In some cases where indicated, a two-sided Student’s t test was used. Unless otherwise stated, p-values shown are unadjusted, and statistical significance was determined after Bonferroni adjustment by dividing the p-value by number of independent statistical comparisons made.
Results
Expression of IL-15RA leads to surface expression of IL-15 on IL-15-transduced cells
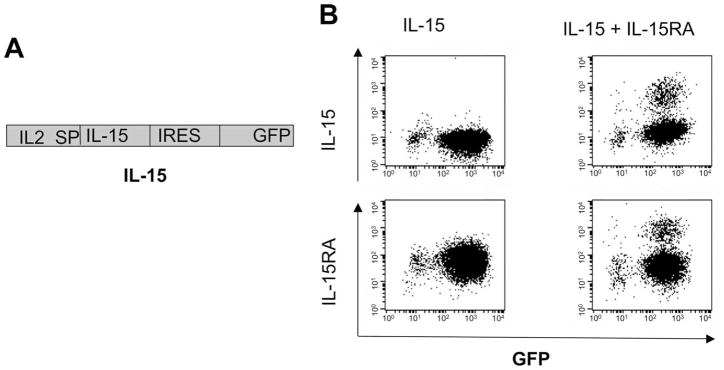
It has been shown that the intracellular interaction of IL-15RA with IL-15 leads to increased production and stabilization of IL-15 (36). Thus, to demonstrate if expression of IL-15RA could lead to surface expression of IL-15 on IL-15-transduced cells, we first transduced TC-1 cells with retrovirus encoding the IL-2 signal peptide (IL2SP) linked to IL-15 followed by IRES and GFP. This construct is denoted as IL-15. Fig. 1A depicts the schematic diagram of the construct. The IL-15 signal peptide was replaced with the stronger IL-2 signal peptide to enhance IL-15 production and secretion (37). The IRES allows cotranslation of IL-15 and GFP from the same transcript. The IL-15-transduced TC-1 cells were sorted for GFP expression, and a portion of GFP+ cells were further transduced with retrovirus encoding IL-15RA. Cells were analyzed for IL-15 or IL-15RA expression by flow cytometry analysis. As shown in Fig. 1B, transduction with IL-15RA in IL-15-transduced cells led to a significant increase of IL-15 surface expression (top right panel) compared with cells transduced with IL-15 alone (top left panel). We also noted that IL-15RA transduction into IL-15-expressing MOSEC cells demonstrated increased surface expression of IL-15 (data not shown). Thus, our data indicate that IL-15RA expression facilitated the expression of IL-15 on the surface of IL-15-transduced tumor cells.
FIGURE 1.
Characterization of the expression of IL-15 on TC-1 cells transduced with IL-15 and/or IL-15RA. A, Schematic diagram of the IL-15 construct. TC-1 cells were transduced with a retrovirus encoding the IL2SP linked to IL-15 followed by IRES and GFP. This construct is denoted as IL-15. The IL-15-transduced TC-1 cells were sorted for GFP expression, and a portion of GFP+ cells was further transduced with retrovirus-encoding IL-15RA. Cells were analyzed for IL-15 or IL-15RA expression by flow cytometry analysis. B, Representative flow cytometry data demonstrating the expression of IL-15 and IL-15RA in cells transduced with IL-15 and/or IL-15RA. Note that surface expression of IL-15 was significantly higher on the TC-1 cells transduced with both IL-15 and IL-15RA. The data shown here are from one representative experiment of two performed.
The expression of the chimeric IL-15/IL-15RA on TC-1 tumor cells inhibits tumor growth in tumor-challenged mice
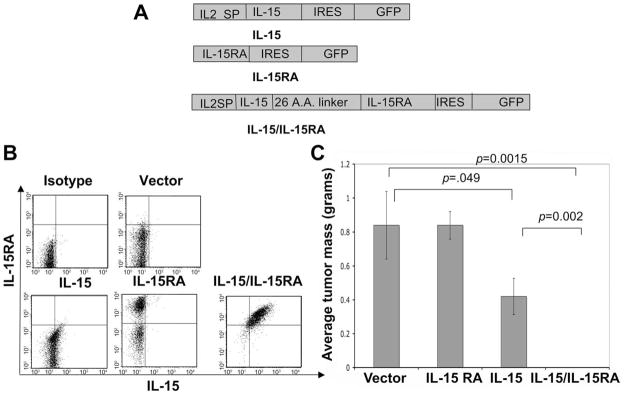
To investigate the effect of trans-presentation of IL-15 by IL-15RA on tumor cells and to allow expression of both IL-15 and IL-15RA from a single construct, we engineered TC-1 cells to express an IL-15/IL-15RA construct. We generated this chimeric construct linking IL-15 to IL-15RA using a strategy similar to one previously described (32). The expression of this chimeric construct would presumably lead to the expression of IL-15 physically bound to IL-15RA produced by the transduced cell. Fig. 2A depicts the schematic diagrams of the chimeric IL-15/IL-15RA construct. The IL-15/IL-15RA construct encodes the IL2SP linked to IL-15 attached to IL-15RA via a 26-aa poly-proline linker followed by IRES and GFP. The inclusion of GFP enabled us to determine the transduction efficiency and sort the tranduced cells. The weak IL-15 signal peptide was replaced with the stronger IL-2 signal peptide to enhance surface expression (37). As also for Fig. 1A, the IL-15 construct encodes the IL2SP linked to IL-15 followed by IRES and GFP. The IL-15RA construct encodes IL-15RA followed by IRES and GFP. We then transduced TC-1 cells with retrovirus encoding IL-15, IL-15RA, or the IL-15/IL-15RA constructs and compared the level of IL-15 and IL-15RA expression in the GFP+ sorted cells by flow cytometry analysis. As shown in Fig. 2B, TC-1 cells transduced with IL-15/IL-15RA demonstrated significantly higher expression of both IL-15 and IL-15RA compared with the cells transduced with IL-15 alone or IL-15RA alone. Furthermore, we observed that TC-1 cells transduced with IL-15 showed slightly higher expression of IL-15 compared with cells transduced with vector alone. Thus, our data indicate that TC-1 cells tranduced with IL-15/IL-15RA demonstrate significantly higher expression of both IL-15 and IL-15RA on the cell surface.
FIGURE 2.
Characterization of IL-15 and IL-15RA expression on transduced TC-1 cells and tumor growth in C57BL/6 mice. A, Schematic diagram of the IL-15, IL-15RA, and the chimeric IL-15/IL-15RA constructs. The IL-15 construct encodes the IL2SP linked to IL-15 followed by IRES and GFP. The IL-15RA construct encodes IL-15RA followed by IRES and GFP. The IL-15/IL-15RA construct encodes the IL2SP linked to IL-15 attached to IL-15RA via a 26-aa poly-proline linker followed by IRES and GFP. B, Flow cytometry data demonstrating IL-15 and IL-15RA expression on TC-1 cells transduced with IL-15, IL-15RA, or IL-15/IL-15RA. GFP+ cells were sorted before staining for IL-15 and IL-15RA. C, C57BL/6 mice (n = 5/group) were injected with 5 × 104 TC-1 tumor cells/mouse transduced with IL-15, IL-15RA, or IL-15/IL-15RA s.c. into the left flank. Eighteen days later, tumors were isolated and weighed. Bar graph represents the average tumor mass on day 18 after injection of transduced TC-1 cells. p-values were determined by one-sided Student’s t test. The data shown here are from one representative experiment of two performed.
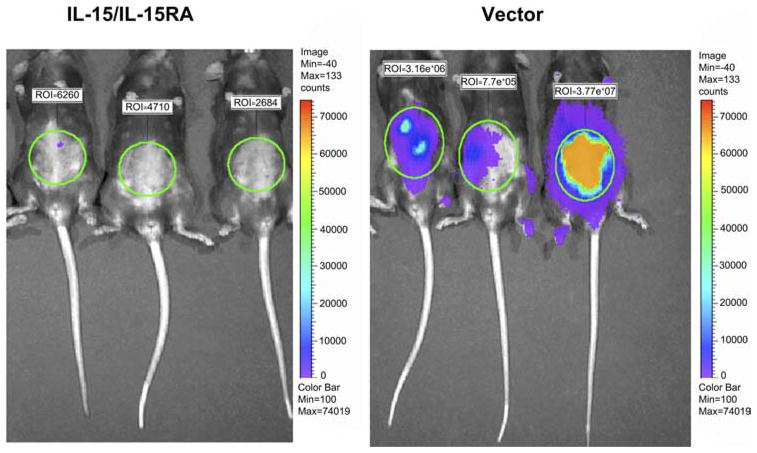
We further investigated the tumor growth in mice challenged with TC-1 cells transduced with each of these constructs. C57BL/6 mice (n = 5/group) were s.c. challenged with TC-1 tumor cells transduced with IL-15, IL-15RA, or IL-15/IL-15RA. Eighteen days after tumor challenge, tumors were isolated and weighed. As shown in Fig. 2C, mice challenged with TC-1 cells transduced with IL-15/IL-15RA demonstrated significant inhibition of tumor growth (almost undetectable) compared with mice challenged with TC-1 cells transduced with IL-15 or IL-15RA alone. Similarly, C57BL/6 mice challenged with MOSEC cells transduced with IL-15/IL-15RA demonstrated significant inhibition of tumor growth compared with mice challenged with vector-transduced MOSEC tumor cells (Fig. 3). Thus, our data indicate that mice challenged with different tumor cells transduced with IL-15/IL-15RA demonstrate significant inhibition in tumor growth compared with mice challenged with tumor cells transduced with IL-15 or IL-15RA alone.
FIGURE 3.
Luminescence imaging to demonstrate in vivo tumor growth of IL-15/IL-15RA-transduced luciferase-expressing MOSEC cells in C57BL/6 mice. C57BL/6 mice (n = 5/group) were injected with 1 × 105 luciferase-MOSEC tumor cells/mouse transduced with IL-15/IL-15RA or empty vector i.p. GFP+ cells were sorted before i.p. injection. Eighteen days later, tumor growth in challenged mice was assessed using luminescence imaging system using methods as previously described (47). The data shown here are from one representative experiment of two performed.
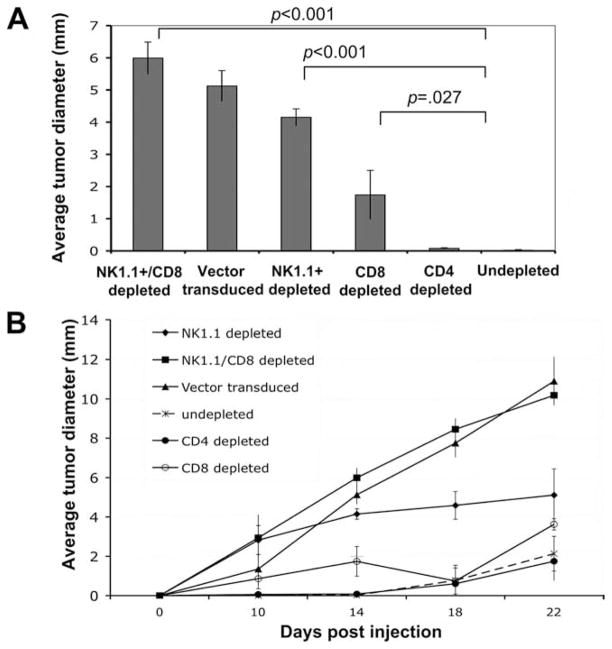
NK1.1+ cells and CD8+ T cells are important in the inhibition of tumor growth in mice challenged with IL-15/IL-15RA-transduced TC-1 cells
To determine the subset of lymphocytes important for the observed inhibition of tumor growth in mice challenged with IL-15/IL-15RA-transduced TC-1 cells, we performed Ab depletion experiments. C57BL/6 mice were depleted of NK1.1+ cells, CD4 cells, CD8 cells, or both NK1.1+ and CD8 cells as described in Materials and Methods. We have previously shown that depletion with these Abs results in efficient depletion at the concentrations used (34). Tumor growth was monitored on days 0, 10, 14, 18, and 22 after tumor challenge. As shown in Fig. 4A, depletion of NK1.1+ cells or CD8+ T cells alone in mice challenged with IL-15/IL-15RA-transduced TC-1 cells partially restored tumor growth on day 14 after tumor challenge. Furthermore, depletion of both NK1.1+ cells and CD8+ T cells in mice challenged with IL-15/IL-15RA-transduced TC-1 cells completely restored tumor growth on day 14 after tumor challenge. In contrast, there was no significant difference in the tumor growth in CD4+ T cell-depleted mice and undepleted mice. Fig. 4B depicts the tumor growth over time of IL-15/IL-15RA-transduced TC-1 cells in tumor-bearing mice depleted of the different subsets of lymphocytes. Thus, our data indicate that NK1.1+ cells and CD8+ T cells play an important role in the inhibition of tumor growth in mice challenged with IL-15/IL-15RA-transduced TC-1 cells.
FIGURE 4.
In vivo Ab depletion experiment. C57BL/6 mice (n = 5/group) were depleted of CD8, CD4, and NK cells or CD8 and NK cells using relevant anti-NK1.1, anti-CD4, anti-CD8, or anti-NK1.1 and anti-CD8 Abs i.p. three times every 2 days. Two days after the fourth Ab injection, the mice from the various depleted groups were challenged with 5 × 104/mouse of TC-1 tumor cells transduced with IL-15/IL-15RA s.c. into the left flank. TC-1 cells transduced with the empty vector as well as undepleted mice challenged with TC-1 cells transduced with IL-15/IL-15RA were used as negative and positive controls, respectively. Continued Ab depletion was performed in tumor-bearing mice 1 wk after tumor challenge. Tumor diameters were measured on days 0, 10, 14, 18, and 22. A, Average tumor diameter on day 14 after challenge with the IL-15/IL-15RA-transduced TC-1 cells in the various depleted groups. B, Growth kinetics of IL-15/IL-15RA-transduced TC-1 cells in Ab-depleted mice. p-values were determined by one-sided Student’s t test.
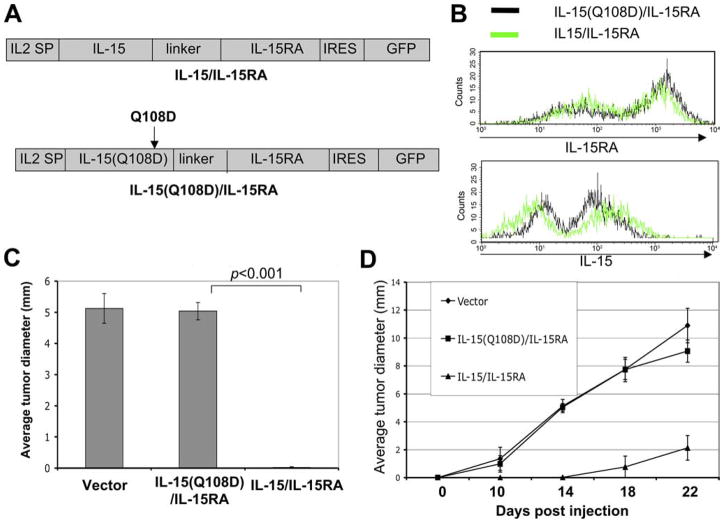
A single amino acid mutation in the γc binding site on IL-15 abolishes the inhibition of tumor growth in IL-15/IL-15RA-transduced TC-1 cells
It has been suggested that IL-15 can be presented by IL-15RA through the β/γc complex on NK and CD8+ T cells in trans (9–12). Additionally, the region of IL-15 that binds to the γc-chain has been defined. Furthermore, a single point mutation at the location of aa 108 in the γc-chain binding site of IL-15, which has been shown to be important in the interaction of IL-15 with the γc-chain, completely abolished the bioactivity of IL-15 in vitro (38, 39). To specifically define the role of receptor β/γc complex-mediated signaling in IL-15 trans-presentation, we generated a chimeric IL-15/IL-15RA construct with a point mutation (Q108D) on IL-15. Fig. 5A depicts a schematic diagram comparing the mutant IL-15(Q108D)/IL-15RA construct with the IL-15/IL-15RA construct. TC-1 cells were transduced with a retrovirus encoding IL-15/IL-15RA or IL-15(Q108D)/IL-15RA, and the level of IL-15 and IL-15RA expression was characterized in the GFP+ cells by flow cytometry analysis. As shown in Fig. 5B, IL-15(Q108D)/IL-15RA-transduced TC-1 cells expressed similar levels of IL-15RA (top panel) and IL-15 (bottom panel) surface expression compared with the IL-15/IL-15RA-transduced TC-1 cells. Thus, our data indicate that the IL-15/IL-15RA construct with the mutation in IL-15 does not affect the expression of the chimeric protein on the surface of the transduced tumor cells.
FIGURE 5.
In vivo tumor growth experiments in mice challenged with TC-1 cells transduced with IL-15/IL-15RA or IL-15(Q108D)/IL-15RA mutant construct. A, Schematic representation of the wild-type IL-15/IL-15RA construct and the IL-15(Q108D)/IL-15RA mutant construct. The IL-15/IL-15RA construct encodes the IL-2 signal peptide (SP) linked to IL-15 attached to IL-15RA via a 26-aa poly-proline linker followed by IRES and GFP. The IL-15(Q108D)/IL-15RA construct encodes the IL-2 signal peptide (SP) linked to IL-15 mutated at aa 108 attached to IL-15RA via a 26-aa poly-proline linker followed by IRES and GFP. TC-1 cells were transduced with a retrovirus encoding IL-15/IL-15RA or IL-15(Q108D)/IL-15RA. GFP+ cells were sorted and further analyzed for IL-15 or IL-15RA expression by flow cytometry analysis. B, Expression of IL-15RA (upper panel) and IL-15 (lower panel) on TC-1 cells transduced with IL-15/IL-15RA or IL-15(Q108D)/IL-15RA. C and D, C57BL/6 mice (n = 5/group) were injected with 5 × 104/mouse of TC-1 tumor cells transduced with IL-15/IL-15RA, IL-15(Q108D)/IL-15RA, or empty vector s.c. into the left flank. Tumor diameters were measured on days 0, 10, 14, 18, and 22. C, Average tumor diameter on day 14 after injection of TC-1 cells transduced with IL-15/IL-15RA, IL-15(Q108D)/IL-15RA, or empty vector. D, Growth kinetics of TC-1 cells transduced with IL-15/IL-15RA, IL-15(Q108D)/IL-15RA, or empty vector. p-values were determined by one-sided Student’s t test. The data shown here are from one representative experiment of two performed.
To define the role of β/γc complex-mediated signaling on the inhibition of tumor growth, we characterized the tumor growth in C57BL/6 mice challenged with TC-1 cells transduced with IL-15/IL-15RA or IL-15(Q108D)/IL-15RA. Tumor growth was monitored on days 0, 10, 14, 18, and 22 after tumor challenge. As shown in Fig. 5C, while mice challenged with IL-15/IL-15RA-transduced TC-1 cells demonstrated significant inhibition of tumor growth on day 14 after tumor challenge, mice challenged with IL-15(Q108D)/IL-15RA-transduced TC-1 cells demonstrated significant tumor growth. Furthermore, we observed that IL-15(Q108D)/IL-15RA-transduced TC-1 cells demonstrated similar tumor growth as the TC-1 tumor cells transduced with vector alone. Fig. 5D depicts the tumor growth over time of TC-1 cells transduced with either vector, IL-15/IL-15RA, or IL-15(Q108D)/IL-15RA in tumor-bearing mice. Thus, our data demonstrate that a single amino acid mutation on IL-15 at the γc binding site abolishes the inhibition of tumor growth mediated by the expression of the chimeric IL-15/IL-15RA protein. Our data suggest that the signaling pathway mediated by the β/γc complex may play a significant role in the inhibition of TC-1 tumor growth in tumor-challenged mice.
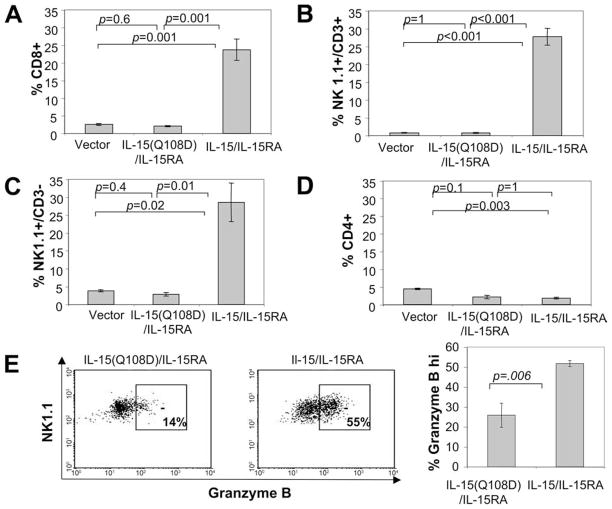
IL-15/IL-15RA-transduced TC-1 cells demonstrate increased activation and accumulation of tumor-infiltrating NK cells, NKT cells, and CD8+ T cells in tumor-infiltrating lymphocytes (TILs) from tumor-bearing mice
Since we observed that NK1.1+ cells and CD8+ T cells play an important role in the inhibition of tumor growth in mice challenged with IL-15/IL-15RA-transduced TC-1 cells (see Fig. 4), we further determined the percentage of CD8+ T cells, NK cells, and NKT cells in the TILs of these mice. To ensure growth of IL-15/IL-15RA-transduced TC-1 tumors in challenged mice, C57BL/6 mice (n = 5/group) were injected with 10× the previously used number of TC-1 cells transduced with IL-15/IL-15RA, IL-15(Q108D)/IL-15RA, or empty vector s.c. into the left flank. By using this large number of cells for tumor challenge, tumor sizes were similar between the different transduced TC-1 groups on day 10 after injection (our unpublished observations). Ten days after injection, tumors were analyzed by flow cytometry to determine the percentage of NK cells, NKT cells, and CD8+ T cells in the TILs. As shown in Fig. 6A–C, mice challenged with IL-15/IL-15RA-transduced TC-1 cells demonstrated significantly higher percentages of NK cells, NKT cells, and CD8+ T cells in the TILs compared with mice challenged with TC-1 cells transduced with IL-15(Q108D)/IL-15RA or empty vector. In contrast, we did not observe an increase in the percentage of CD4+ T cells in the TILs of IL-15/IL-15RA-transduced TC-1 cells (Fig. 6D). Furthermore, there was no significant difference in the percentage of NK cells, NKT cells, and CD8+ T cells in the TILs in TC-1 cells transduced with IL-15(Q108D)/IL-15RA compared with empty vector. Thus, our data indicate that mice challenged with IL-15/IL-15RA-transduced TC-1 cells demonstrate enhanced accumulation of NK cells, NKT cells, and CD8+ T cells, but not CD4+ T cells, in tumors in vivo. Furthermore, our data suggest that receptor β/γc complex-mediated signaling is responsible for the enhanced accumulation of NK cells, NKT cells, and CD8+ T cells in IL-15/IL-15RA-transduced TC-1 tumors.
FIGURE 6.
Characterization of the percentage of tumor-infiltrating CD4+, CD8+, NK, and NK-T cells and activated NK1.1+ cells in mice challenged with TC-1 cells transduced with IL-15/IL-15RA or IL-15(Q108D)/IL-15RA. C57BL/6 mice (n = 4/group) were injected with 2.5 × 106/mouse of TC-1 tumor cells transduced with IL-15/IL-15RA, IL-15(Q108D)/IL-15RA or empty vector s.c. into the left flank. Ten days later, tumors were excised and tumor cell suspensions were stained for CD4, CD8, or NK1.1 and CD3. Cells were analyzed by flow cytometry, and the percentage of CD4+, CD8+, NK1.1+/CD3+ (NKT cells) or NK1.1+/CD3− cells of the total TILs was determined. A, Percentage of tumor-infiltrating lymphocytes staining CD8+. B, Percentage of tumor-infiltrating lymphocytes staining NK1.1+ and CD3+. C, Percentage of tumor-infiltrating lymphocytes staining NK1.1+ and CD3−. D, Percentage of tumor-infiltrating lymphocytes staining CD4+. E, Representative flow cytometry data (left two panels) and bar graph (right panel) demonstrating the percentage of NK1.1+ gated cells from TILs staining for high intracellular granzyme B expression. The data shown here are from one representative experiment of two performed. Data are represented as means ± SE. Indicated Bonferroni adjusted p-values are determined by two-sided Student’s t test.
Granzyme B plays an important role in NK cell killing. Multiple cytokines enhance intracellular granzyme B expression in NK cells, and intracellular granzyme B expression levels may be used as a marker for NK cell activation (40). Because NK1.1+ cells appeared to be the most critical cell type responsible for the inhibition of IL-15/IL-15RA-bearing tumor growth, we analyzed the activation status of NK1.1+ cells by staining for intracellular granzyme B in the TILs of tumor-bearing mice. As shown in Fig. 6E, the percentage of NK1.1+ cells expressing high levels of intracellular granzyme B was significantly increased in TILs from IL-15/IL-15RA-transduced tumors compared with TILs from IL-15(Q108D)/IL-15RA-transduced tumors. An increase in granzyme B levels was observed in both NK1.1+ CD3+ and NK1.1+ CD3− cells (data not shown). Therefore, IL-15/IL-15RA expression on TC-1 tumors increases the percentage of activated NK1.1+ cells in the TILs of tumor-bearing mice.
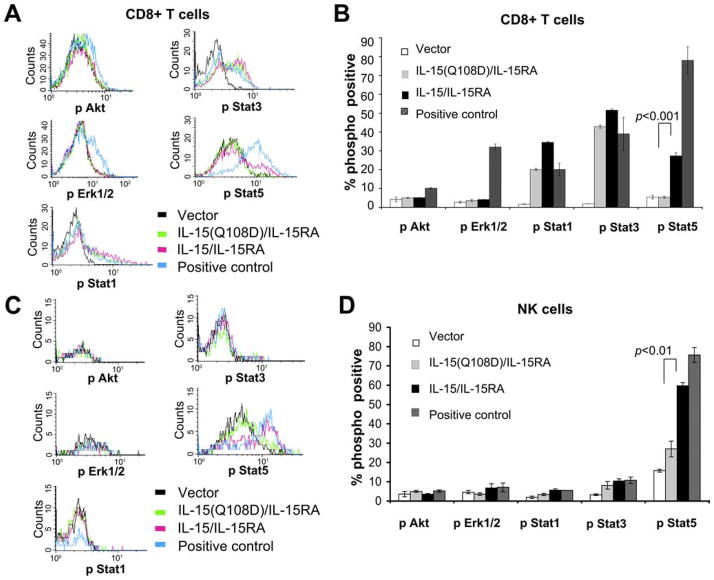
IL-15/IL-15RA-transduced TC-1 cells activate STAT5 phosphorylation in NK and CD8+ T cells
IL-15 presented by IL-15RA has been shown to activate the JAK-STAT pathway through the receptor β/γc complex (17). We hypothesized that TC-1 cells transduced with IL-15/IL-15RA would be able to activate JAK-STAT signaling in trans in CD8+ T cells and NK cells and that the mutant IL-15(Q108D)/IL-15RA would abolish this signaling. Thus, we characterized the activation of various STAT-signaling molecules implicated downstream of β/γc-chain signaling, including pSTAT1, pSTAT3, and pSTAT5 on purified, unstimulated CD8+ T cells after coincubation with TC-1 cells transduced with IL-15/IL-15RA, IL-15(Q108D)/IL-15RA, or empty vector. As a positive control, exogenous IL-15 was added to cultures of CD8+ T cells and NK cells incubated with TC-1 tumor cells transduced with IL-15(Q108D)/IL-15RA. As shown in Fig. 7, A and B, we observed that CD8+ T cells incubated with IL-15/IL-15RA-transduced TC-1 cells demonstrated significantly higher levels of STAT5 phosphorylation compared with CD8+ T cells incubated with the IL-15(Q108D)/IL-15RA-transduced TC-1 cells and vector-tranduced TC-1 cells (p < 0.001). In contrast, there was no significant difference in the levels of STAT5 phosphorylation in CD8+ T cells incubated with IL-15(Q108D)/IL-15RA-transduced TC-1 cells compared with vector-transduced cells. Furthermore, the addition of exogenous IL-15 to the CD8+ T cells cultured with IL-15(Q108D)/IL-15RA-transduced TC-1 cells was capable of restoring the levels of STAT5 phosphorylation. In similar experiments using a previously activated Ag nonspecific T cell line, we also observed activation of STAT5 phosphorylation by IL-15/IL-15RA-transduced TC-1 cells and not by vector or IL-15(Q108D)/IL-15RA-transduced TC-1 cells (data not shown). However, CD8+ T cells incubated with TC-1 cells transduced with either IL-15/IL-15RA or IL-15(Q108D)/IL-15RA demonstrated significantly higher levels of STAT1 and STAT3 phosphorylation compared with CD8+ T cells incubated with the vector-transduced TC-1 cells. Both fluorescence compensation controls and isotype staining controls remained at background levels of staining among groups (data not shown). These data suggest that IL-15(Q108D)/IL-15RA-transduced TC-1 cells lose the capacity to activate STAT5 phosphorylation in CD8+ T cells, yet they partially retain the ability to activate through other signaling pathways, including STAT1 and STAT3. This specific loss in the capacity to activate STAT5 phosphorylation is consistent with the notion that β-chain recruitment of JAK1 facilitates STAT3 phosphorylation whereas γc-chain recruitment of JAK3 facilitates STAT5 phosphorylation (18, 19).
FIGURE 7.
Characterization of the phosphorylation of signaling effectors in the IL-2/IL-15 receptor signal pathway. CD8+ cells or NK cells were isolated from naive C57BL/6 mice or OT-1 RAG−/− C57BL/6 mice. NK or CD8 cells were cocultured with TC-1 cells transduced with IL-15/IL-15RA, IL-15(Q108D)/IL-15RA, or empty vector at a ratio of 1:1 for 45 min. For AKT and ERK1/2 analysis, CD8+ OT-1 RAG−/− cells were similarly treated after 4 h of serum starvation. As positive controls, C57BL/6 CD8+ cells were cocultured with IL-15(Q108D)/IL-15RA-transduced TC-1 cells + 50 ng/ml IL-15, and OT-1 RAG−/− CD8+ cells were treated with OVA peptide (ERK1/2) or OVA peptide + anti-CD28 + serum (AKT). Cells were stained and analyzed for the phosphorylated forms of intracellular AKT, ERK1/2, STAT1, STAT3, or STAT5 using flow cytometry analysis. A and C, Staining intensity of indicated phosphorylated proteins in purified CD8+ T cells (A) and NK cells (C) after coincubation with the indicated transduced TC-1 cells. B and D, Percentage of CD8+ T cells (B) and NK cells (D) expressing the phosphorylated form of the indicated proteins after coincubation with the indicated transduced TC-1 cells. Bonferroni adjusted p-values are determined by two-sided student t test. The data shown here are from one representative experiment of two performed.
Multiple other pathways, including the Ras/MAPK/ERK and PI3K-AKT pathways, have been implicated in IL-15 signal transduction. Under the conditions used above, we did not observe an increase in AKT or ERK1/2 phosphorylation after incubating CD8+ T cells with vector, IL-15/IL-15RA, or IL-15(Q108D)/IL-15RA, nor did we detect an increase after coincubation with IL-15(Q108D)/IL-15RA TC-1 cells + 50 ng/ml IL-15 that served above as a positive control for STAT activation (data not shown). To confirm that our flow cytometric assay could indeed detect AKT phosphorylation and ERK1/2 phosphorylation after activation, as a positive control we stimulated serum-starved OT-1 RAG−/− CD8+ T cells with OVA peptide (for ERK1/2 activation) or with OVA peptide + anti-CD28 + serum (for AKT activation). At the same time we cocultured serum-starved OT-1 CD8+ T cells with vector, IL-15(Q108D)/IL-15RA, or IL-15/IL-15RA TC-1 cells. Under these conditions we confirmed that STAT5 was still activated in CD8+ OT-1 T cells by IL-15/IL-15RA TC-1 cells and not in vector or IL-15(Q108D)/IL-15RA TC-1 cells (data not shown). As shown in Fig. 7, A and B, we noted a subtle yet significant increase in AKT phosphorylation (p < 0.05) and a significant increase in ERK1/2 phosphorylation (p < 0.01) in positive control-stimulated CD8+ T cells. However, we still did not detect an increase in AKT or ERK1/2 phosphorylation in CD8+ T cells after incubation with IL-15/IL-15RA TC-1 cells compared with IL-15(Q108D)/IL-15RA or vector TC-1 cells. We obtained similar results at 20 min of activation (data not shown).
As shown in Fig. 7, C and D, we observed that NK cells incubated with IL-15/IL-15RA-transduced TC-1 cells also demonstrated significantly higher levels of STAT5 phosphorylation compared with cells incubated with the IL-15(Q108D)/IL-15RA transduced TC-1 cells (p < 0.01), and the addition of IL-15 to IL-15(Q108D)/IL-15RA-transduced TC-1 cells restored the levels of STAT5 phosphorylation. Both fluorescence compensation controls and isotype staining controls remained at background levels of staining among groups (data not shown). We have not yet been able to detect a significant increase in STAT3, STAT1, AKT, or ERK1/2 phosphorylation in NK cells under the stimulating and assay conditions shown. We observed similar results using bone marrow-derived NK cells (data not shown).
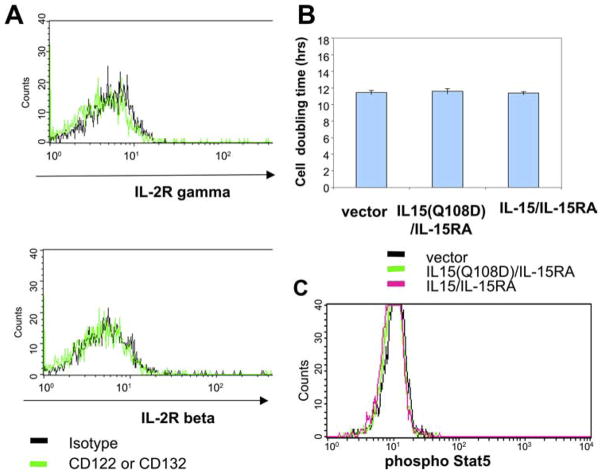
To rule out the potential contributions of IL-15/IL-15RA signaling among transduced TC-1 cells, we also compared STAT5 phosphorylation levels in TC-1 cells transduced with IL-15/IL-15RA, IL-15(Q108D)/IL-15RA, or empty vector. We observed that each of the various transduced TC-1 cells did not express detectable levels of β-or γc-chains (Fig. 8A). Furthermore, we did not observe any significant difference in cell proliferation or phosphorylation of STAT5 in TC-1 cells transduced with IL-15/IL-15RA or IL-15(Q108D)/IL-15RA or vector (Fig. 8, B and C). Thus, our data suggest that the transduced TC-1 cells most likely do not signal through the IL-15/IL-15RA signaling pathway among the tumor cells themselves. Taken together, our data suggest that γc binding-dependent activation of NK cells and CD8+ cells through the β/γc signaling pathway may play a decisive role in IL-15/IL-15RA trans-presentation-mediated inhibition of tumor growth.
FIGURE 8.
Characterization of the expression of β- and γc-chains, proliferation, and STAT5 phosphorylation on TC-1 cells transduced with IL-15/IL-15RA or IL-15(Q108D)/IL-15RA. A, γc- (top) or β-chain (bottom) expression on TC-1 cells transduced with IL-15/IL-15RA compared with the isotype control. Note that no detectable expression of β- or γc-chains was observed in the IL-15/IL-15RA-transduced TC-1 cells. Similarly, no detectable expression of β- or γc-chains was observed in the IL-15(Q108D)/IL-15RA-transduced TC-1 cells (data not shown). B, Comparison of the cell doubling time of TC-1 cells-transduced with IL-15(Q108)/IL-15R, IL-15/IL-15RA, or empty vector. C, Comparison of intra-cellular phosphorylated STAT5 staining in TC-1 cells transduced with IL-15(Q108)/IL-15RA, IL-15/IL-15RA, or empty vector.
Discussion
In the present study, we have demonstrated that expression of IL-15RA led to surface expression of IL-15 on IL-15-transduced cells. Additionally, we showed that the expression of the chimeric IL-15/IL-15RA on TC-1 tumor cells led to increased activation and percentage of tumor-infiltrating NK cells, NKT cells, and CD8+ T cells and the inhibition of tumor growth in tumor-challenged mice. This antitumor effect was shown to be abolished by a single amino acid mutation in the γc-chain binding site on IL-15 of the IL-15/IL-15RA. Additionally, it was observed that IL-15/IL-15RA-transduced TC-1 cells activate STAT5 phosphorylation in NK and CD8+ T cells and that a γc-chain-binding mutation in the IL-15 of IL-15/IL-15RA abolished activation of STAT5 phosphorylation in trans.
Our Ab depletion experiments demonstrate that the chimeric IL-15/IL-15RA expressed by tumor cells results in inhibition of tumor growth in an NK1.1+ -dependent fashion. While NK1.1 depletion can deplete NK cells (NK1.1+ CD3−), it can also lead to the depletion of a subset of activated CD8+ T cells that express the NK1.1 marker (NK1.1+ CD3+). Our data also demonstrate a significant increase in the percentage of both NK1.1+ CD3− cells (NK cells) and NK1.1+ CD3+ cells in TILs (see Fig. 6C). Furthermore, we also observed an increase in granzyme B expression in NK1.1+ CD3− and NK1.1+ CD3+ cells within TILs of IL-15/IL-15RA tumors (see Fig. 6E). Thus, the depletion of a subset of activated CD8+ T cells that express NK1.1 may also contribute to the loss of inhibition of tumor growth generated by depletion of NK1.1+ cells.
We have observed induction of STAT3 and STAT1 phosphorylation but not STAT5 phosphorylation by IL-15(Q108D)/IL-15RA TC-1 cells in primary CD8+ T cells (see Fig. 7, A and B). Our data suggest that trans-presentation of the γc-binding mutant IL-15(Q108D)/IL-15RA may lead to several possibilities including weak or atypical signaling in CD8+ T cells. For example, the trans-presentation of the γc-binding mutant may transduce a quantitatively weaker signal that results in undetectable STAT5 phosphorylation yet still detectable STAT1 and STAT3 phosphorylation. This quantitative interpretation is consistent with a previous report demonstrating that T cells, while not dependent on STAT3 for responses to high-dose IL-2, were defective in response to low-dose IL-2 in the absence of STAT3 (41). Alternatively, trans-presentation of the γc-binding mutant, by specific abrogation of interaction with the γ-chain but retained interaction with the β-chain, may transduce an asymmetric signal. A similar example of asymmetric STAT3/STAT5 signaling via the β/γc complex in lymphocytes was recently reported (42). In this study a fusion of IL-15 to GM-CSF (GIFT15) resulted in specific impairment of interaction of IL-15 with the γc subunit of the β/γc complex according to a best-fit model of GIFT15-receptor interaction. GIFT15-stimulated mouse splenocytes demonstrated suppressed STAT5 phosphorylation and enhanced STAT3 phosphorylation.
The β/γc complex has been shown to function by activation of the STAT3/STAT5 signaling pathways. Our data suggest that STAT5 may be a critical factor in the activation of the IL-15/IL-15RA signaling pathway in trans through the receptor β/γc complex for the inhibition of tumor growth. STAT5 has been shown to play an important role in a variety of cellular functions, including proliferation, differentiation, and effector function of T cells and NK cells (21, 43) (for reviews, see Refs. 44, 45). These functions may be potentially important for the observed inhibition of tumor growth of IL-15/IL-15RA-bearing tumors. Because several other pathways, including the AKT, ERK, STAT3, and STAT1 pathways, have also been implicated in the IL-15/IL-15RA signaling (22–24), we cannot completely rule out the possibility that these pathways are critical contributors to the observed inhibition of tumor growth. In our experiments, while we observed activation of AKT and ERK1/2 phosphorylation in CD8+ T cells after strong stimuli through the TCR, we did not observe significant enhancement of AKT or ERK1/2 in CD8+ T cells or NK cells in the presence of IL-15. It remains possible therefore that a more subtle induction of AKT and ERK1/2 phosphorylation by IL-15 stimulation in NK CD8+ T cells and STAT1 and STAT3 in NK cells is beyond the limit of detection in the assays used. We have used flow cytometry assays rather than more sensitive immunoblotting assays because these are cell-mixing experiments and we are interested in looking specifically at effector molecule phosphorylation in the responding cell (immune cell) and not the presenting cell (TC-1 tumor cell). Furthermore, we have noted that background AKT and ERK1/2 phosphorylation levels in the transduced TC-1 cells are fairly high, which makes cell-mixing immunoblotting assays rather difficult to interpret.
It has been shown that many tumors naturally express appreciable levels of IL-15 and IL-15RA (for review, see Ref. 23). Our studies demonstrate that IL-15 expression alone in TC-1 tumor cells partially inhibits tumor growth whereas IL-15 and IL-15RA expression together completely inhibit tumor growth in an NK1.1+ cell- and CD8+ T cell-dependent manner. Our results raise the intriguing possibility that coexpression of IL-15 and IL-15RA in the same cell may be an important innate immune response recognition factor in antitumor immunosurveillance. Previous studies using another tumor model have demonstrated that expression of IL-15RA on the surface of MC38 tumor cells prevented metastasis formation in wild-type mice but not in IL-15 knockout mice (46). Furthermore, NK1.1+ cell depletion restored the metastatic ability of IL-15RA-transduced MC38 tumor cells. These findings are consistent with the present study and demonstrate that trans-presentation of IL-15 by IL-15RA (either derived from surrounding cells or endogenously produced) to NK cells may potentially play a role in tumor immunosurveillance.
The inhibition of tumor growth in IL-15/IL-15RA-transduced TC-1 cells is not likely due to the immune response against the IL-15/IL-15RA fusion construct. The IL-15/IL-15RA fusion represents a novel protein that may potentially be recognized as a foreign Ag. However, given the fact that a single point mutation in IL-15/IL-15RA completely rescued tumor growth, we do not think that immune recognition of IL-15/IL-15RA as a foreign Ag plays a significant role in the inhibition in tumor growth observed in IL-15/IL-15RA-expressing TC-1 tumor cells. Furthermore, the ability of IL-15/IL-15RA-transduced TC-1 cells, but not IL-15(Q108D)/IL-15RA-transduced TC-1 cells, to activate STAT5 phosphorylation in NK and CD8 cells and to bring about increased NK and CD8+ T cell infiltrates in tumors supports this conclusion.
In our study, we found that a chimeric IL-15/IL-15RA construct expressed in TC-1 tumor cells was capable of inhibiting tumor growth more potently than expression of IL-15 alone in TC-1 cells. The potent ability of cellular expression of this construct to activate NK cells and T cells in trans may prove useful in potential clinical applications. For example, activation of NK cells around the tumor may lead to tumor cell death, resulting in the release and uptake of tumor-specific Ags and consequent cross-priming of tumor Ag-specific immune responses. Thus, it would be of interest to explore if vaccination with irradiated tumor cells expressing the chimeric IL-15/IL-15RA construct is capable of generating tumor-specific immunity and/or systemic antitumor effects against parental tumors in the future. This information would serve as a foundation for future clinical translation.
Footnotes
This work was supported by the National Cancer Institute SPOREs (Specialized Programs of Research Excellence) in Cervical Cancer Grants P50 CA098252 and 1 RO1 CA114425-01.
Abbreviations used in this paper: IL-15RA, IL-15Rα; γc, common γ IL2SP, IL-2 signal peptide; IRES, internal ribosomal entry site; MOSEC, murine ovarian surface epithelial carcinoma; TIL, tumor-infiltrating lymphocyte.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Goldrath AW, Sivakumar PV, Glaccum M, Kennedy MK, Bevan MJ, Benoist C, Mathis D, Butz EA. Cytokine requirements for acute and basal homeostatic proliferation of naive and memory CD8+ T cells. J Exp Med. 2002;195:1515–1522. doi: 10.1084/jem.20020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, Matsuki N, Charrier K, Sedger L, Willis CR, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 4.Giri JG, Kumaki S, Ahdieh M, Friend DJ, Loomis A, Shanebeck K, DuBose R, Cosman D, Park LS, Anderson DM. Identification and cloning of a novel IL-15 binding protein that is structurally related to the alpha chain of the IL-2 receptor. EMBO J. 1995;14:3654–3663. doi: 10.1002/j.1460-2075.1995.tb00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson DM, Kumaki S, Ahdieh M, Bertles J, Tometsko M, Loomis A, Giri J, Copeland NG, Gilbert DJ, Jenkins NA, et al. Functional characterization of the human interleukin-15 receptor α chain and close linkage of IL15RA and IL2RA genes. J Biol Chem. 1995;270:29862–29869. doi: 10.1074/jbc.270.50.29862. [DOI] [PubMed] [Google Scholar]
- 6.Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, Ma A. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 7.Berard M, Brandt K, Bulfone-Paus S, Tough DF. IL-15 promotes the survival of naive and memory phenotype CD8+ T cells. J Immunol. 2003;170:5018–5026. doi: 10.4049/jimmunol.170.10.5018. [DOI] [PubMed] [Google Scholar]
- 8.Oh S, Perera LP, Burke DS, Waldmann TA, Berzofsky JA. IL-15/IL-15Rα -mediated avidity maturation of memory CD8+ T cells. Proc Natl Acad Sci USA. 2004;101:15154–15159. doi: 10.1073/pnas.0406649101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Rα recycles and presents IL-15 in trans to neighboring cells. Immunity. 2002;17:537–547. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 10.Koka R, Burkett P, Chien M, Chai S, Boone DL, Ma A. Cutting edge: Murine dendritic cells require IL-15Rα to prime NK cells. J Immunol. 2004;173:3594–3598. doi: 10.4049/jimmunol.173.6.3594. [DOI] [PubMed] [Google Scholar]
- 11.Schluns KS, Klonowski KD, Lefrançois L. Transregulation of memory CD8 T-cell proliferation by IL-15Rα+ bone marrow-derived cells. Blood. 2004;103:988–994. doi: 10.1182/blood-2003-08-2814. [DOI] [PubMed] [Google Scholar]
- 12.Sandau MM, Schluns KS, Lefrançois L, Jameson SC. Cutting edge: Transpresentation of IL-15 by bone marrow-derived cells necessitates expression of IL-15 and IL-15Rα by the same cells. J Immunol. 2004;173:6537–6541. doi: 10.4049/jimmunol.173.11.6537. [DOI] [PubMed] [Google Scholar]
- 13.Burkett PR, Koka R, Chien M, Chai S, Boone DL, Ma A. Coordinate expression and trans presentation of interleukin (IL)-15Rα and IL-15 supports natural killer cell and memory CD8+ T cell homeostasis. J Exp Med. 2004;200:825–834. doi: 10.1084/jem.20041389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giri JG, Ahdieh M, Eisenman J, Shanebeck K, Grabstein K, Kumaki S, Namen A, Park LS, Cosman D, Anderson D. Utilization of the beta and gamma chains of the IL-2 receptor by the novel cytokine IL-15. EMBO J. 1994;13:2822–2830. doi: 10.1002/j.1460-2075.1994.tb06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carson WE, Giri JG, Lindemann MJ, Linett ML, Ahdieh M, Paxton R, Anderson D, Eisenmann J, Grabstein K, Caligiuri MA. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med. 1994;180:1395–1403. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnston JA, Wang LM, Hanson EP, Sun XJ, White MF, Oakes SA, Pierce JH, O’Shea JJ. Interleukins 2, 4, 7, and 15 stimulate tyrosine phosphorylation of insulin receptor substrates 1 and 2 in T cells: potential role of JAK kinases. J Biol Chem. 1995;270:28527–28530. doi: 10.1074/jbc.270.48.28527. [DOI] [PubMed] [Google Scholar]
- 17.Fehniger TA, Caligiuri MA. Interleukin 15: biology and relevance to human disease. Blood. 2001;97:14–32. doi: 10.1182/blood.v97.1.14. [DOI] [PubMed] [Google Scholar]
- 18.Lin JX, Migone TS, Tsang M, Friedmann M, Weatherbee JA, Zhou L, Yamauchi A, Bloom ET, Mietz J, John S, et al. The role of shared receptor motifs and common Stat proteins in the generation of cytokine pleiotropy and redundancy by IL-2, IL-4, IL-7, IL-13, and IL-15. Immunity. 1995;2:331–339. doi: 10.1016/1074-7613(95)90141-8. [DOI] [PubMed] [Google Scholar]
- 19.Miyazaki T, Kawahara A, Fujii H, Nakagawa Y, Minami Y, Liu ZJ, Oishi I, Silvennoinen O, Witthuhn BA, Ihle JN, et al. Functional activation of Jak1 and Jak3 by selective association with IL-2 receptor subunits. Science. 1994;266:1045–1047. doi: 10.1126/science.7973659. [DOI] [PubMed] [Google Scholar]
- 20.Kelly J, Spolski R, Imada K, Bollenbacher J, Lee S, Leonard WJ. A role for Stat5 in CD8+ T cell homeostasis. J Immunol. 2003;170:210–217. doi: 10.4049/jimmunol.170.1.210. [DOI] [PubMed] [Google Scholar]
- 21.Imada K, Bloom ET, Nakajima H, Horvath-Arcidiacono JA, Udy GB, Davey HW, Leonard WJ. Stat5b is essential for natural killer cell-mediated proliferation and cytolytic activity. J Exp Med. 1998;188:2067–2074. doi: 10.1084/jem.188.11.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zambricki E, Shigeoka A, Kishimoto H, Sprent J, Burakoff S, Carpenter C, Milford E, McKay D. Signaling T-cell survival and death by IL-2 and IL-15. Am J Transplant. 2005;5:2623–2631. doi: 10.1111/j.1600-6143.2005.01075.x. [DOI] [PubMed] [Google Scholar]
- 23.Budagian V, Bulanova E, Paus R, Bulfone-Paus S. IL-15/IL-15 receptor biology: a guided tour through an expanding universe. Cytokine Growth Factor Rev. 2006;17:259–280. doi: 10.1016/j.cytogfr.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Zhang C, Zhang J, Niu J, Zhang J, Tian Z. Interleukin-15 improves cytotoxicity of natural killer cells via up-regulating NKG2D and cytotoxic effector molecule expression as well as STAT1 and ERK1/2 phosphorylation. Cytokine. 2008;42:128–136. doi: 10.1016/j.cyto.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Li XC, Demirci G, Ferrari-Lacraz S, Groves C, Coyle A, Malek TR, Strom TB. IL-15 and IL-2: a matter of life and death for T cells in vivo. Nat Med. 2001;7:114–118. doi: 10.1038/83253. [DOI] [PubMed] [Google Scholar]
- 26.Cooper MA, Bush JE, Fehniger TA, VanDeusen JB, Waite RE, Liu Y, Aguila HL, Caligiuri MA. In vivo evidence for a dependence on interleukin 15 for survival of natural killer cells. Blood. 2002;100:3633–3638. doi: 10.1182/blood-2001-12-0293. [DOI] [PubMed] [Google Scholar]
- 27.Kim YS, Maslinski W, Zheng XX, Stevens AC, Li XC, Tesch GH, Kelley VR, Strom TB. Targeting the IL-15 receptor with an antagonist IL-15 mutant/Fcγ 2a protein blocks delayed-type hypersensitivity. J Immunol. 1998;160:5742–5748. [PMC free article] [PubMed] [Google Scholar]
- 28.Krutzik PO, Nolan GP. Intracellular phosphoprotein staining techniques for flow cytometry: monitoring single cell signaling events. Cytometry A. 2003;55:61–70. doi: 10.1002/cyto.a.10072. [DOI] [PubMed] [Google Scholar]
- 29.Kimura A, Naka T, Kishimoto T. IL-6-dependent and -independent pathways in the development of interleukin 17-producing T helper cells. Proc Natl Acad Sci USA. 2007;104:12099–12104. doi: 10.1073/pnas.0705268104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kimura A, Naka T, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of Th17 cells. Proc Natl Acad Sci USA. 2008;105:9721–9726. doi: 10.1073/pnas.0804231105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bulfone-Pau SS, Bulanova E, Pohl T, Budagian V, Durkop H, Ruckert R, Kunzendorf U, Paus R, Krause H. Death deflected: IL-15 inhibits TNF-α -mediated apoptosis in fibroblasts by TRAF2 recruitment to the IL-15Rα chain. FASEB J. 1999;13:1575–1585. doi: 10.1096/fasebj.13.12.1575. [DOI] [PubMed] [Google Scholar]
- 32.Mortier E, Quemener A, Vusio P, Lorenzen I, Boublik Y, Grotzinger J, Plet A, Jacques Y. Soluble interleukin-15 receptor α (IL-15Rα)-sushi as a selective and potent agonist of IL-15 action through IL-15Rβ/γ: hyperagonist IL-15 × IL-15Rα fusion proteins. J Biol Chem. 2006;281:1612–1619. doi: 10.1074/jbc.M508624200. [DOI] [PubMed] [Google Scholar]
- 33.Holst J, Vignali KM, Burton AR, Vignali DA. Rapid analysis of T-cell selection in vivo using T cell-receptor retrogenic mice. Nat Methods. 2006;3:191–197. doi: 10.1038/nmeth858. [DOI] [PubMed] [Google Scholar]
- 34.Chen CH, Wang TL, Hung CF, Yang Y, Young RA, Pardoll DM, Wu TC. Enhancement of DNA vaccine potency by linkage of antigen gene to an HSP70 gene. Cancer Res. 2000;60:1035–1042. [PubMed] [Google Scholar]
- 35.Roby KF, Taylor CC, Sweetwood JP, Cheng Y, Pace JL, Tawfik O, Persons DL, Smith PG, Terranova PF. Development of a syngeneic mouse model for events related to ovarian cancer. Carcinogenesis. 2000;21:585–591. doi: 10.1093/carcin/21.4.585. [DOI] [PubMed] [Google Scholar]
- 36.Bergamaschi C, Rosati M, Jalah R, Valentin A, Kulkarni V, Alicea C, Zhang GM, Patel V, Felber BK, Pavlakis GN. Intracellular interaction of interleukin-15 with its receptor α during production leads to mutual stabilization and increased bioactivity. J Biol Chem. 2008;283:4189–4199. doi: 10.1074/jbc.M705725200. [DOI] [PubMed] [Google Scholar]
- 37.Bamford RN, DeFilippis AP, Azimi N, Kurys G, Waldmann TA. The 5′ untranslated region, signal peptide, and the coding sequence of the carboxyl terminus of IL-15 participate in its multifaceted translational control. J Immunol. 1998;160:4418–4426. [PubMed] [Google Scholar]
- 38.Olsen SK, Ota N, Kishishita S, Kukimoto-Niino M, Murayama K, Uchiyama H, Toyama M, Terada T, Shirouzu M, Kanagawa O, Yokoyama S. Crystal structure of the interleukin-15.interleukin-15 receptor α complex: insights into trans and cis presentation. J Biol Chem. 2007;282:37191–37204. doi: 10.1074/jbc.M706150200. [DOI] [PubMed] [Google Scholar]
- 39.Pettit DK, Bonnert TP, Eisenman J, Srinivasan S, Paxton R, Beers C, Lynch D, Miller B, Yost J, Grabstein KH, Gombotz WR. Structure-function studies of interleukin 15 using site-specific mutagenesis, polyethylene glycol conjugation, and homology modeling. J Biol Chem. 1997;272:2312–2318. doi: 10.1074/jbc.272.4.2312. [DOI] [PubMed] [Google Scholar]
- 40.Fehniger TA, Cai SF, Cao X, Bredemeyer AJ, Presti RM, French AR, Ley TJ. Acquisition of murine NK cell cytotoxicity requires the translation of a pre-existing pool of granzyme B and perforin mRNAs. Immunity. 2007;26:798–811. doi: 10.1016/j.immuni.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 41.Akaishi H, Takeda K, Kaisho T, Shineha R, Satomi S, Takeda J, Akira S. Defective IL-2-mediated IL-2 receptor α chain expression in Stat3-deficient T lymphocytes. Int Immunol. 1998;10:1747–1751. doi: 10.1093/intimm/10.11.1747. [DOI] [PubMed] [Google Scholar]
- 42.Rafei M, Wu JH, Annabi B, Lejeune L, Francois M, Galipeau J. A GMCSF and IL-15 fusokine leads to paradoxical immunosuppression in vivo via asymmetrical JAK/STAT signaling through the IL-15 receptor complex. Blood. 2007;109:2234–2242. doi: 10.1182/blood-2006-07-037473. [DOI] [PubMed] [Google Scholar]
- 43.Kelly JA, Spolski R, Kovanen PE, Suzuki T, Bollenbacher J, Pise-Masison CA, Radonovich MF, Lee S, Jenkins NA, Copeland NG, Morse HC, 3rd, Leonard WJ. Stat5 synergizes with T cell receptor/antigen stimulation in the development of lymphoblastic lymphoma. J Exp Med. 2003;198:79–89. doi: 10.1084/jem.20021548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wittig I, Groner B. Signal transducer and activator of transcription 5 (STAT5), a crucial regulator of immune and cancer cells. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5:449–463. doi: 10.2174/156800805774912999. [DOI] [PubMed] [Google Scholar]
- 45.Buitenhuis M, Coffer PJ, Koenderman L. Signal transducer and activator of transcription 5 (STAT5) Int J Biochem Cell Biol. 2004;36:2120–2124. doi: 10.1016/j.biocel.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 46.Kobayashi H, Dubois S, Sato N, Sabzevari H, Sakai Y, Waldmann TA, Tagaya Y. Role of trans-cellular IL-15 presentation in the activation of NK cell-mediated killing, which leads to enhanced tumor immunosurveillance. Blood. 2005;105:721–727. doi: 10.1182/blood-2003-12-4187. [DOI] [PubMed] [Google Scholar]
- 47.Hung CF, Tsai YC, He L, Wu TC. Control of mesothelin-expressing ovarian cancer using adoptive transfer of mesothelin peptide-specific CD8+ T cells. Gene Ther. 2007;14:921–929. doi: 10.1038/sj.gt.3302913. [DOI] [PMC free article] [PubMed] [Google Scholar]