Abstract
Patients with peripheral arterial disease (PAD) undergoing percutaneous coronary intervention (PCI) are at high risk for adverse cardiovascular events. Trends over time in outcomes with advances in PCI and medical therapy are unknown. We evaluated 866 patients with PAD in the National Heart, Lung, and Blood Institute (NHLBI) Dynamic Registry undergoing PCI according to treatment eras: the early bare metal stent (BMS) era (Wave 1: 1997-1998, n=180), the BMS era (Waves 2 and 3; 1999 and 2001-2002; n=339), and the drug-eluting stent (DES) era (Waves 4 and 5: 2004 and 2006; n=347). We compared in-hospital and 1-year outcomes by recruitment era. In-hospital coronary artery bypass graft surgery (CABG) rates were significantly lower in the later eras (3.9%, 0.9%, 0.6%, early BMS, BMS, and DES eras respectively, ptrend=0.005), and an increasing percentage of patients were discharged on aspirin, beta blockers, statins, and thienopyridines (all ptrend<0.001). Cumulative 1-year event rates in patients with PAD in the early BMS era, BMS era, and DES era of death were 13.7%, 10.5%, and 9.8% (ptrend = 0.21), of myocardial infarction (MI) were 9.8%, 8.8%, and 10.0% (ptrend = 0.95), and repeat revascularization were 26.8%, 21.0%, and 17.2% (ptrend = 0.008). The 1-year adjusted hazard ratios (HR) of adverse events in patients with PAD using the early BMS era as the reference are as follows: Death: BMS era HR=0.84 (95% CI 0.46-1.55, p=0.58) and DES era HR=1.35 (95% CI 0.71-2.56, p=0.36); MI: BMS era HR=0.89 (95% CI 0.48-1.66, p=0.72) and DES era HR=1.02 (95% CI 0.55-1.87, p=0.95); and repeat revascularization: BMS era HR=0.63 (95% CI 0.41-0.97, p=0.04) and DES era HR=0.46 (95% CI 0.29-0.73, p=0.001). In conclusion, despite significant improvements in medical therapy and a reduction in repeat revascularization over time, patients with PAD who undergo PCI have a persistent high rate of death and MI.
Keywords: Peripheral arterial disease, stents, catheterization
In unselected patients undergoing percutaneous coronary intervention (PCI), the adverse outcomes of death and myocardial infarction (MI) have improved over time [1-3]. However, trends over time in outcomes specifically of patients with peripheral arterial disease (PAD), given advances in PCI including the use of drug-eluting stents (DES) and more aggressive medical therapy, are not known. Thus, utilizing the National Heart, Lung and Blood Institute (NHLBI) Dynamic Registry, we compared the in-hospital and one year outcomes of patients with PAD undergoing PCI across three different treatment eras: the early bare metal stent (BMS) era, the BMS era, and the DES era.
Methods
The specific methodology and characteristics of the NHLBI Dynamic Registry have been reported previously [2]. In brief, data were collected on approximately 2,000 consecutive patients undergoing PCI during five recruitment ‘waves’ across 27 clinical centers (Wave 1: July 1997-February 1998; Wave 2: February-June 1999; Wave 3: October 2001-March 2002; Wave 4: February-May 2004; Wave 5: February-August 2006). Only patients with PAD were evaluated and were grouped in 3 distinct treatment eras: the early (BMS) era (Wave 1), the BMS era (Waves 2 and 3), and the DES era (Waves 4 and 5). Patients were contacted via telephone interview at one year by trained nurse coordinators to assess vital status, symptoms, coronary events or cardiac-related hospitalizations. Informed consent was obtained for all patients and the study protocol was approved by Institutional Review Boards at the respective clinical sites and at the University of Pittsburgh data coordinating center.
Symptomatic PAD was defined as a history or presence of claudication either with rest or exertion, amputation for arterial vascular insufficiency, vascular reconstruction, bypass surgery or angioplasty to the extremities, or documented aortic aneurysm. Death was defined as all cause mortality. In waves 1 and 2, MI was defined as evidence of two or more of the following: (1) typical chest pain > 20 minutes duration not relieved by nitroglycerin, (2) serial electrocardiogram recordings showing changes from baseline or serially in ST-T and/or Q-waves in ≥ 2 contiguous leads, (3) serum enzyme elevation of creatinine kinase-myocardial band (CK-MB) > 5% (total creatinine kinase (CK) >2× normal, lactate dehydrogenase (LDH) subtype 1 > LDH subtype 2, or troponin > 0.2 μg/ml), or (4) new wall motion abnormalities. In waves 3-5, an MI had to satisfy at least one of the 2 following criteria: (1) evolutionary ST-segment elevation, development of new Q-waves in 2 or more contiguous electrocardiogram leads, or new or presumably new left bundle branch pattern on the electrocardiogram, (2) biochemical evidence of myocardial necrosis manifested as a) CK-MB ≥ 3 times the upper limit of normal, b) total CK ≥ 3 times the upper limit of normal (if CK-MB not available), or troponin level above the upper limit of normal. Elective coronary artery bypass graft surgery (CABG) was classified when surgery was deferred for >24 hours, urgent when required within 24 hours, and emergent when required immediately. Angiographic success was classified as either partial when some but not all attempted lesions were successfully treated or total when all attempted lesions were successfully treated. Procedural success was defined as either partial or total angiographic success without death, Q-wave MI, or emergency CABG. Major adverse cardiac event (MACE) was defined as death, MI, or repeat revascularization.
Patients were stratified by stent era and descriptive statistics were summarized as means for continuous variables and percentages for categorical variables. Temporal trends in baseline patient and lesion characteristics and outcomes at one year were assessed using the Cochran-Mantel-Haenszel test for dichotomous variables and the Jonckeheere-Terpstra test for continuous and nominal/ordinal variables. Cumulative event rates at one year were calculated by the Kaplan–Meier method and compared using the log rank statistic. Patients who did not experience the outcome of interest were censored at the last known date of contact or at one year if contact extended beyond one year.
Stepwise Cox regression was used to estimate one year risk ratios of clinical events in relation to stent era with the early BMS era as the reference. Covariates for multivariable models were identified from factors that differed significantly between the stent eras and also associated with one year outcome using stepwise Cox proportional hazards regression models (pentry_0.30, pstay_0.10). The proportionality assumption was assessed for all Cox proportional-hazards models graphically and statistically and assumptions were met for all models. All analyses were performed with SAS version 9.2 (SAS Institute Inc) and a two-sided p-value of 0.05 or less was considered to indicate statistical significance.
Results
A total of 866 patients with PAD were evaluated, with 180 in the early BMS era, 339 in the BMS era, and 347 in the DES era. In the more recent treatment eras, patients were less likely to have had a prior MI, but were more likely to have renal disease and be diagnosed with hypertension or hyperlipidemia (Table 1). While the rates of several other demographic features of high risk did not differ across the three waves, patients with PAD had high baseline rates of prior PCI, prior CABG, diabetes, and current tobacco use. Revascularization in patients with PAD was performed less commonly for unstable angina, but more commonly for myocardial infarction in later eras (Table 2). While the lesion length was longer in the more recent eras, there was a lower likelihood of thrombus in the treated lesion. Other characteristics of the lesion that would predict higher risk for PCI such as calcification, ulceration, or location at a bifurcation did not differ among the eras. Over 40% of patients in all three eras had three vessel coronary artery disease present on coronary angiography (early BMS era 42.8%, BMS era 45.4%, and DES era 42.1%, ptrend=0.68), and over 10% of patients had involvement of the left main coronary artery (early BMS era 15.6%, BMS era 10.0%, and DES era 15.0%, ptrend=0.79).
Table I. Demographics of Patients with Peripheral Arterial Disease by Treatment Era.
| Variable | Early BMS Era (N=180) |
BMS Era (N=339) |
DES Era (N=347) |
Ptrend |
|---|---|---|---|---|
| Mean age (years) | 67.9 | 67.6 | 68.9 | 0.23 |
| Female | 42.8% | 37.8% | 34.3% | 0.06 |
| White Race | 87.2% | 80.5% | 77.2% | 0.02 |
| Mean Body Mass Index (kg/m2) | 27.4 | 28.6 | 28.2 | 0.26 |
| Prior percutaneous coronary intervention | 40.0% | 40.1% | 46.5% | 0.18 |
| Prior coronary artery bypass graft | 32.2% | 33.6% | 34.5% | 0.87 |
| Prior myocardial infarction | 48.0% | 41.1% | 32.8% | <0.001 |
| Diabetes mellitus | 40.8% | 43.4% | 46.0% | 0.25 |
| Insulin treated diabetes mellitus | 17.2% | 21.2% | 17.6% | 0.85 |
| Hypertension* | 70.5% | 83.3% | 90.8% | <0.001 |
| Heart failure | 20.8% | 23.1% | 20.3% | 0.76 |
| Hypercholesterolemia** | 68.7% | 74.5% | 85.3% | <0.001 |
| Cerebrovascular disease | 18.9% | 18.3% | 21.3% | 0.41 |
| Pulmonary disease | 13.9% | 17.4% | 17.0% | 0.44 |
| Renal disease | 13.9% | 17.7% | 21.3% | 0.03 |
| Current Smoker | 20.5% | 27.3% | 24.4% | 0.24 |
Hypertension = blood pressure ≥ 140 mmHg systolic or ≥ 90 mmHg diastolic on 2 occasions or if the patient is currently on antihypertensive medications
Hypercholesterolemia = repeated values for serum cholesterol > 240mg/100ml or if a physician has medically treated the participant for high cholesterol
Table II. Angiographic and Procedural Characteristics of Patients with Peripheral Arterial Disease by Treatment Era.
| Early BMS Era (N=180) |
BMS Era (N=339) |
DES Era (N=347) |
Ptrend | |
|---|---|---|---|---|
| Patient Level | ||||
| Revascularization Reason | ||||
| Stable Angina Pectoris | 26.1% | 18.9% | 18.4% | 0.06 |
| Unstable Angina Pectoris | 52.2% | 46.6% | 38.9% | 0.002 |
| Acute Myocardial Infarction | 11.7% | 23.0% | 22.5% | 0.01 |
| Circumstances of Procedure | 0.35 | |||
| Elective | 63.3% | 51.3% | 56.8% | |
| Urgent | 26.7% | 42.8% | 33.1% | |
| Emergent | 10.0% | 5.9% | 10.1% | |
| Mean left ventricular ejection fraction | 50.6% | 48.1% | 49.5% | 0.90 |
| Mean Significant lesions | 3.8 | 3.9 | 3.8 | 0.81 |
| Any total occlusion | 49.4% | 50.1% | 51.9% | 0.57 |
| Mean number of lesions attempted | 1.5 | 1.5 | 1.4 | 0.004 |
|
Early BMS Era (N=275) |
BMS Era (N=503) |
DES Era (N=475) |
Ptrend | |
| Lesion Level | ||||
| Mean reference vessel size (mm) | 3.1 | 3.0 | 3.1 | 0.11 |
| Mean lesion length (mm) | 12.8 | 13.3 | 17.2 | <0.001 |
| Mean diameter % stenosis | 82.6% | 81.9% | 83.0% | 0.47 |
| Evidence of thrombus | 14.6% | 8.7% | 7.7% | 0.005 |
| Calcified | 39.0% | 23.0% | 37.6% | 0.57 |
| Ulcerated | 8.8% | 9.3% | 11.4% | 0.23 |
| Bifurcation | 10.7% | 11.1% | 10.3% | 0.82 |
| Ostial lesion | 13.8% | 9.0% | 10.8% | 0.35 |
| Lesion tortuosity | 0.07 | |||
| Moderate/Severe | 22.8% | 31.5% | 30.2% | |
| Lesion previously treated | 18.4% | 13.1% | 9.1% | 0.0002 |
| Stent use | 67.2% | 82.0% | 92.8% | <0.001 |
| Total angiographic success | 88.3% | 91.7% | 93.6% | 0.11 |
| Drug-eluting stent use* | -- | -- | 76.9% | 1 |
| Procedural success | 91.7% | 94.1% | 94.5% | 0.24 |
restricted to the DES era
In-hospital complications of death (early BMS era 3.3%, BMS era 2.7%, and DES era 2.0%, ptrend=0.35) and MI (early BMS era 5.0%, BMS era 3.2%, and DES era 3.2%, ptrend=0.34) did not differ by treatment era. Whereas in-hospital CABG rates were less frequent in later eras (early BMS era 3.9%, BMS era 0.9%, and DES era 0.6%, ptrend=0.005), there were no differences in major entry site complications (early BMS era 6.1%, BMS era 5.0%, and DES era 6.6%, ptrend=0.68). Procedural success rates were similar among the three groups (early BMS era 91.7%, BMS era 94.1% and DES era 94.5% ptrend=0.24).
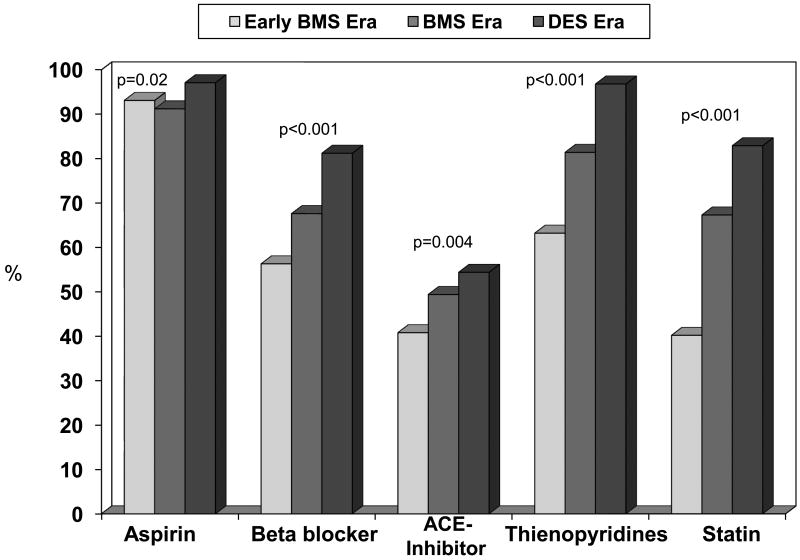
The percentage of patients discharged on medications including aspirin, beta-blockers, angiotensin converting enzyme inhibitors, thienopyridines and statins significantly increased over time across the three treatment waves (Figure 1). Additionally, the mean length of stay significantly decreased over time (early BMS era 3.3 days, BMS era 2.8 days, and DES era 2.2 days, ptrend=0.004).
Figure 1. Discharge Medication Post PCI by Treatment Era.
PCI = percutaneous coronary intervention, BMS = bare metal stent, DES = drug-eluting stent, ACE = angiotensin converting enzyme
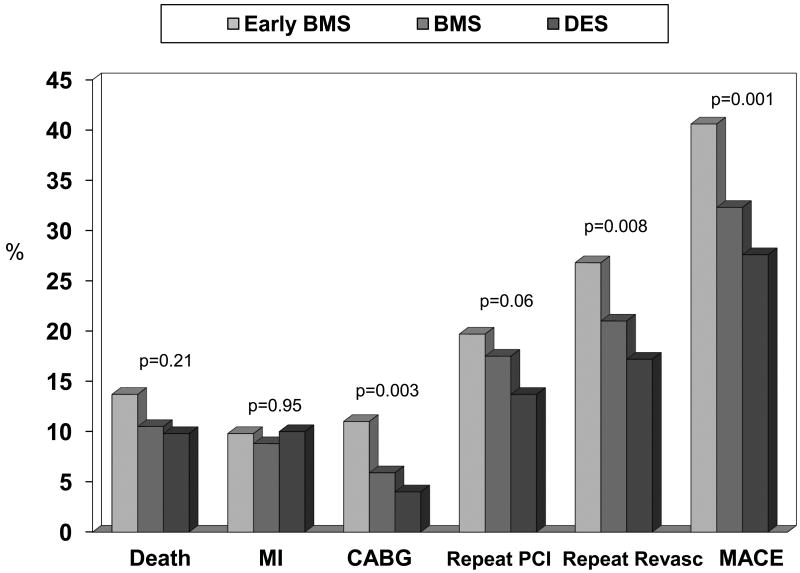
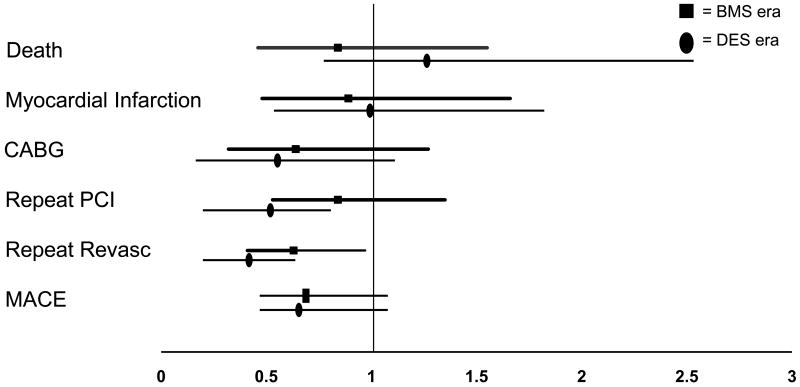
Cumulative 1-year adverse event rates revealed no differences in the incidence of death and MI across the treatment waves; however there was a significant decrease in CABG, repeat revascularization, MACE, and a trend toward a reduction in repeat PCI in the later treatment eras (Figure 2). Adjusted hazard ratios of one year adverse events in patients with PAD with the pre-BMS era as the reference are shown in Figure 3. Compared to patients with PAD in the pre BMS era, patients in the BMS era and the DES era were less likely to experience repeat revascularization at one year and patients in the DES era were less likely to undergo repeat PCI. However, there was no significant difference in the adjusted hazard ratios of death, MI, CABG, or MACE in the later waves.
Figure 2. Cumulative One Year Adverse Events Rates by Treatment Era.
BMS = bare metal stent, DES = drug-eluting stent, MI = myocardial infarction, CABG = coronary artery bypass graft surgery, PCI = percutaneous coronary intervention, MACE = major adverse cardiac events
Figure 3. Adjusted One Year Hazard Ratios of Adverse Events (Reference is Early BMS Era).
BMS = bare metal stent, PCI = percutaneous coronary intervention, Revasc = revascularization
Discussion
Utilizing the subsequent waves of the NHLBI Dynamic Registry, we found no improvement over time in the rates of death and MI for patients with PAD undergoing PCI, despite advances in PCI and increased use of evidence-based medical therapy. Although there was an improvement in rates of CABG and repeat PCI for PAD patients in later treatment eras that was likely driven by the introduction of drug-eluting stents, there was no reduction in the 1-year adjusted hazard ratio of death or MI. An increasing number of patients with PAD were noted in subsequent waves, likely representing greater evaluation and recognition of this risk factor.
High adverse event rates for patients with PAD undergoing coronary revascularization was reported as early as the BARI trial, in which patients with multivessel CAD and lower extremity PAD randomized either to CABG or percutaneous coronary transluminal angioplasty had an adjusted relative risk of death 1.5 times greater than patients without PAD [4]. This observation that patients with PAD experience higher adverse events following PCI has subsequently been reported in multiple trials following BARI [5-12]. However, for the overall population of patients undergoing PCI, adverse event rates over time, including both in-hospital and 30 day mortality rates, following PCI have improved [1-3]. Williams and colleagues revealed that patients in the Dynamic registry undergoing PCI during 1997-1998 had lower rates of in-hospital mortality, myocardial infarction, and emergency CABG than patients undergoing PCI between 1985-1986 (4.9% vs. 7.9%, p=0.001) [2]. Additionally, Singh et al investigated the outcomes of patients with unstable angina undergoing PCI in 3 different treatment eras: 1979-1989, 1990-1993, and 1994-1998 [3]. One year event-free survival from death and MI was significantly higher in the more recent treatment groups compared to patients in the earlier groups (74% vs. 70% vs. 77%, p<0.001). Our study, examining outcomes over time after PCI in a real-world registry, reveals that the same is not true for patients with PAD. They continued to have an elevated risk of death and MI following PCI across all three treatment eras.
One explanation for the persistently elevated rates of adverse events in patients with PAD over time may be related to a discrepancy in medication prescription compared to patients without PAD. Brilakis and colleagues examined compliance with practice guidelines in the Get with the Guidelines-Coronary Artery Disease database [13]. Patients with a history of prior PAD who presented to the hospital with an acute coronary syndrome were less likely to receive treatment with appropriate lipid lowering therapy (79% vs. 89%, p<0.001), and were less likely to receive either an angiotensin converting enzyme inhibitor or angiotensin receptor blocker if they had left ventricular dysfunction (74% vs. 82%, p<0.001), as compared to patients without evidence of PAD. In comparison, in our study, a higher number of patients in the more recent treatment era were discharged home on statin therapy (82.9%); additionally, prescription rates for other guideline recommended therapy included 97.1% for aspirin, 96.8% for thienopyridines, and 81.2% for beta blockers. Although the discharge medication prescription rate for standard coronary artery disease medications has improved over the treatment eras as shown in our study, in comparison to patients without PAD, a considerable gap may still remain.
Another reason that may account for the high adverse event rates in PAD patients following PCI may relate to an inability of PAD patients to exercise and thus participate in cardiac rehabilitation programs, which have been associated with improved outcomes including improved quality of life and reduction in hospital readmission rates [14-15]. Additionally, PAD is associated with a higher atherosclerotic burden, including not only cardiovascular disease, but also cerebrovascular disease. Nikolsky and colleagues demonstrated that both the in-hospital and one year mortality rates following PCI were higher in patients with a history of PAD and cerebrovascular disease compared to patients with PAD alone [16]. Additionally, patients with PAD are more likely to experience an in-hospital neurologic event, including transient ischemia attack or cerebrovascular accident, than patients without PAD [16]. In our study, 19.6% of PAD patients undergoing PCI had a known diagnosis of cerebrovascular disease.
Poor outcomes for PAD patients following coronary revascularization are not only limited to PCI. Several studies have demonstrated higher adverse events in PAD patients undergoing CABG [17-25]. Collison and colleagues compared 1561 patients with PAD to 6328 patients without PAD undergoing CABG [19]. After multivariable adjustment, patients with PAD were more likely to experience pulmonary complications (OR 1.4; 95% CI 1.23-1.62; p=<0.001), low output (OR 1.3; 95% CI 1.09-1.45; p=0.001), and intraoperative complications (OR 1.39; 95% CI 1.06-1.83; p=0.02). PAD has also been associated with longer hospital stays [26], higher rates of neurologic complications [19, 25], and more renal failure [27] following CABG. Additionally, patients with PAD undergoing CABG have increased rates of both in-hospital and long term mortality compared to patients without PAD [17-18, 22, 24-25]. Thus, it appears that patients with PAD fare worse with either forms of coronary revascularization, PCI or CABG; however, the data examining whether one fares better than the other is limited. A substudy of the BARI trial examined the outcomes of 303 patients with non-coronary atherosclerosis undergoing either PTCA or CABG for multivessel CAD [4]. The adjusted relative risk of death for surgery versus PTCA was 0.87 (p=0.40). Limited power based on small numbers in the study was cited as the reason no difference was found. Rourke and colleagues compared CABG (n=964) versus PCI (n=341) in patients with PAD and multivessel CAD undergoing coronary revascularization from 1994-1996. Adjusted analysis revealed that patients undergoing CABG had better intermediate survival out to 3 yrs than similar patients undergoing PCI [28]. However, there are no recent studies examining whether benefits of PCI with drug-eluting stents translates into better outcomes for PAD patients compared to bypass surgery. Further studies are needed to determine the optimal revascularization strategy in this large subset of patients.
Potential limitations of this study include those inherent to all observational registries, including the existence of potential confounders. Despite the adjustment for differences among the three treatment eras in the multivariate analysis, it is possible that residual confounding exists. However, the strength of this study is that the patients and outcomes approximate real-world outcomes of patients with PAD undergoing PCI. Additionally, patients with PAD in this study were similar to those presenting in the real-world for invasive procedures. They were a heterogeneous group of patients with vascular disease. However, disease severity and cause of death were not identified, and moreover, patients were not individually screened for PAD.
Acknowledgments
This study was supported by grant number HL-33292 from the National Heart, Lung and Blood Institute of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Singh M, Peterson ED, Roe MT, Ou FS, Spertus JA, Rumsfeld JS, Anderson HV, Klein LW, Ho KKL, Holmes DR. Trends in the Association Between Age and In-Hospital Mortality After Experience Percutaneous Coronary Intervention: National Cardiovascular Data Registry. Circ Cardiovasc Intervent. 2009;2:20–26. doi: 10.1161/CIRCINTERVENTIONS.108.826172. [DOI] [PubMed] [Google Scholar]
- 2.Williams DO, Holubkov R, Yeh W, Bourassa MG, Al-Bassam M, Block PC, Coady P, Cohen H, Cowley M, Dorros G, Faxon D, Holmes DR, Jacobs A, Kelsey SF, King SB, 3rd, Myler R, Slater J, Stanek V, Vlachos HA, Detre KM. Percutaneous coronary intervention in the current era compared with 1985-1986: the National Heart, Lung, and Blood Institute Registries. Circulation. 2000;102:2945–2951. doi: 10.1161/01.cir.102.24.2945. [DOI] [PubMed] [Google Scholar]
- 3.Singh M, Rihal CS, Berger PB, Bell MR, Grill DE, Garratt KN, Barseness GW, Holmes DR., Jr Improving outcome over time of percutaneous coronary interventions in unstable angina. J Am Coll Cardiol. 2000;36:674–678. doi: 10.1016/s0735-1097(00)00768-3. [DOI] [PubMed] [Google Scholar]
- 4.Sutton-Tyrrell K, Rihal C, Sellers MA, Burek K, Trudel J, Roubin G, Brooks MM, Grogan M, Sopko G, Keller N, Jandová R. Long-term prognostic value of clinically evident noncoronary vascular disease in patients undergoing coronary revascularization in the Bypass Angioplasty Revascularization Investigation (BARI) Am J Cardiol. 1998;8:375–381. doi: 10.1016/s0002-9149(97)00934-x. [DOI] [PubMed] [Google Scholar]
- 5.Singh M, Lennon RJ, Darbar D, Gersh BJ, Holmes DR, Jr, Rihal CS. Effect of peripheral arterial disease in patients undergoing percutaneous coronary intervention with intracoronary stents. Mayo Clin Proc. 2004;79:1113–1118. doi: 10.4065/79.9.1113. [DOI] [PubMed] [Google Scholar]
- 6.Saw J, Bhatt DL, Moliterno DJ, Brener SJ, Steinhubl SR, Lincoff AM, Tcheng JE, Harrington RA, Simoons M, Hu T, Sheikh MA, Kereiakes DJ, Topol EJ. The influence of peripheral arterial disease on outcomes: a pooled analysis of mortality in eight large randomized percutaneous coronary intervention trials. J Am Coll Cardiol. 2006;48:1567–1572. doi: 10.1016/j.jacc.2006.03.067. [DOI] [PubMed] [Google Scholar]
- 7.Nikolsky E, Mehran R, Mintz GS, Dangas GD, Lansky AJ, Aymong ED, Negoita M, Fahy M, Moussa I, Roubin GS, Moses JW, Stone GW, Leon MB. Impact of symptomatic peripheral arterial disease on 1-year mortality in patients undergoing percutaneous coronary interventions. J Endovasc Ther. 2004;11:60–70. doi: 10.1177/152660280401100108. [DOI] [PubMed] [Google Scholar]
- 8.Chiu JH, Topol EJ, Whitlow PL, Hsu AP, Tuzcu EM, Franco I, Moliterno DJ. Peripheral vascular disease and one-year mortality following percutaneous coronary revascularization. Am J Cardiol. 2003;92:582–583. doi: 10.1016/s0002-9149(03)00726-4. [DOI] [PubMed] [Google Scholar]
- 9.Berger JS, Petersen JL, Brown DL. Percutaneous Coronary Intervention in New York State Vascular Disease Burden and In-Hospital Outcomes Among Patients Under. Circ Cardiovasc Intervent. 2009;2:317–322. doi: 10.1161/CIRCINTERVENTIONS.108.847459.108.847459. [DOI] [PubMed] [Google Scholar]
- 10.Nallamothu BK, Chetcuti S, Mukherjee D, Eagle KA, Grossman PM, Giri K, McKechnie RS, Kline-Rogers E, Moscucci M. Long-term prognostic implication of extracardiac vascular disease in patients undergoing percutaneous coronary intervention. Am J Cardiol. 2003;92:964–966. doi: 10.1016/s0002-9149(03)00978-0. [DOI] [PubMed] [Google Scholar]
- 11.Mukherjee D, Eagle KA, Smith DE, Kline-Rogers EM, Chetcuti S, Grossman PM, Nallamothu B, O'Donnell M, DeFranco A, Maxwell-Eward A, McGinnity J, Meengs WM, Patel K, Moscucci M, Blue Cross & Blue Shield of Michigan Cardiovascular Consortium (BMC2) Impact of extracardiac vascular disease on acute prognosis in patients who undergo percutaneous coronary interventions (data from the Blue Cross & Blue Shield of Michigan Cardiovascular Consortium [BMC2]) Am J Cardiol. 2003;92:972–974. doi: 10.1016/s0002-9149(03)00981-0. [DOI] [PubMed] [Google Scholar]
- 12.Naidu SS, Vlachos H, Faxon D, Jacobs AK, Selzer F, Detre K, Wilensky RL. Usefullness of non-coronary vascular disease in predicting adverse events in the year following percutaneous coronary intervention. Am J Cardiol. 2005;95:575–580. doi: 10.1016/j.amjcard.2004.10.036. [DOI] [PubMed] [Google Scholar]
- 13.Brilakis ES, Hernandez AF, Dai D, Peterson ED, Banerjee S, Fonarow GC, Cannon CP, Bhatt DL. Quality of Care for Acute Coronary Syndrome Patients With Known Atherosclerotic Disease. Results From the Get With the Guidelines Program. Circulation. 2009;120:560–567. doi: 10.1161/CIRCULATIONAHA.109.877092. [DOI] [PubMed] [Google Scholar]
- 14.Williams MA, Ades PA, Hamm LF, Keteyian SJ, LaFontaine TP, Roitman JL, Squires RW. Clinical evidence for a health benefit from cardiac rehabilitation: an update. Am Heart J. 2006;152:835–841. doi: 10.1016/j.ahj.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 15.Belardinelli R, Paolini I, Cianci G, Piva R, Georgiou D, Purcaro A. Exercise training intervention after coronary angioplasty: the ETICA Trial. J Am Coll Cardiol. 2001;37:1891–1900. doi: 10.1016/s0735-1097(01)01236-0. [DOI] [PubMed] [Google Scholar]
- 16.Nikolsky E, Mehran R, Dangas GD, Lasic Z, Mintz GS, Negoita M, Lansky AJ, Stone GW, Moussa I, Iyer S, Na Y, Moses JW, Leon MB. Prognostic significance of cerebrovascular and peripheral arterial disease in patients having percutaneous coronary interventions. Am J Cardiol. 2004;93:1536–1539. doi: 10.1016/j.amjcard.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 17.Aboyans V, Lacroix P, Postil A, Guilloux J, Rollé F, Cornu E, Laskar M. Subclinical peripheral arterial disease and incompressible ankle arteries are both long-term prognostic factors in patients undergoing coronary artery bypass grafting. J Am Coll Cardiol. 2005;46:815–820. doi: 10.1016/j.jacc.2005.05.066. [DOI] [PubMed] [Google Scholar]
- 18.Birkmeyer JD, O'Connor GT, Quinton HB, Ricci MA, Morton JR, Leavitt BJ, Charlesworth DC, Hernandez F, McDaniel MD. The effect of peripheral vascular disease on in-hospital mortality rates with coronary artery bypass surgery. Northern New England Cardiovascular Disease Study Group. J Vasc Surg. 1995;2:445–452. doi: 10.1016/s0741-5214(95)70286-5. [DOI] [PubMed] [Google Scholar]
- 19.Collison T, Smith JM, Engel AM. Peripheral vascular disease and outcomes following coronary artery bypass graft surgery. Arch Surg. 2006;141:1214–1218. doi: 10.1001/archsurg.141.12.1214. [DOI] [PubMed] [Google Scholar]
- 20.Kunadian B, Dunning J, Millner RW. Modifiable risk factors remain significant causes of medium term mortality after first time Coronary artery bypass grafting. J Cardiothorac Surg. 2007;2:51. doi: 10.1186/1749-8090-2-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leavitt BJ, Sheppard L, Maloney C, Clough RA, Braxton JH, Charlesworth DC, Weintraub RM, Hernandez F, Olmstead EM, Nugent WC, O'Connor GT, Ross CS, Northern New England Cardiovascular Disease Study Group Effect of diabetes and associated conditions on long-term survival after coronary artery bypass graft surgery. Circulation. 2004;110:41–44. doi: 10.1161/01.CIR.0000138197.07051.e7. [DOI] [PubMed] [Google Scholar]
- 22.Mehta RH, Honeycutt E, Shaw LK, Milano CA, Smith PK, Harrington RA, Sketch MH., Jr Clinical and angiographic correlates of short- and long-term mortality in patients undergoing coronary artery bypass grafting. Am J Cardiol. 2007;100:1538–1542. doi: 10.1016/j.amjcard.2007.06.053. [DOI] [PubMed] [Google Scholar]
- 23.Mesh CL, Cmolik BL, Van Heekeren DW, Lee JH, Whittlesey D, Graham LM, Geha AS, Bowlin SJ. Coronary bypass in vascular patients: a relatively high-risk procedure. Ann Vasc Surg. 1997;11:612–619. doi: 10.1007/s100169900099. [DOI] [PubMed] [Google Scholar]
- 24.Minakata K, Konishi Y, Matsumoto M, Aota M, Sugimoto A, Nonaka M, Yamada N. Influence of peripheral vascular occlusive disease on the morbidity and mortality of coronary artery bypass grafting. Jpn Circ J. 2000;64:905–908. doi: 10.1253/jcj.64.905. [DOI] [PubMed] [Google Scholar]
- 25.Rihal CS, Sutton-Tyrrell K, Guo P, Keller NM, Jandova R, Sellers MA, Schaff HV, Holmes DR., Jr Increased incidence of periprocedural complications among patients with peripheral vascular disease undergoing myocardial revascularization in the bypass angioplasty revascularization investigation. Circulation. 1999;100:171–177. doi: 10.1161/01.cir.100.2.171. [DOI] [PubMed] [Google Scholar]
- 26.Herman C, Karolak W, Yip AM, Buth KJ, Hassan A, Legare JF. Predicting prolonged intensive care unit length of stay in patients undergoing coronary artery bypass surgery - development of an entirely preoperative scorecard. Interact Cardiovasc Thorac Surg. 2009;4:654–658. doi: 10.1510/icvts.2008.199521. [DOI] [PubMed] [Google Scholar]
- 27.Brown JR, Cochran RP, Leavitt BJ, Dacey LJ, Ross CS, MacKenzie TA, Kunzelman KS, Kramer RS, Hernandez F, Jr, Helm RE, Westbrook BM, Dunton RF, Malenka DJ, O'Connor GT, Northern New England Cardiovascular Disease Study Group Multivariable prediction of renal insufficiency developing after cardiac surgery. Circulation. 2007;116:139–143. doi: 10.1161/CIRCULATIONAHA.106.677070. [DOI] [PubMed] [Google Scholar]
- 28.O'Rourke DJ, Quinton HB, Piper W, Hernandez F, Morton J, Hettleman B, Hearne M, Nugent W, O'Connor GT, Malenka DJ, Northern New England Cardiovascular Disease Study Group Survival in patients with peripheral vascular disease after percutaneous coronary intervention and coronary artery bypass graft surgery. Ann Thorac Surg. 2004;78:466–470. doi: 10.1016/j.athoracsur.2004.01.044. [DOI] [PubMed] [Google Scholar]