Fibrin clot structure strongly affects fibrinolysis rate and clot stability, and certain characteristics have been correlated with premature myocardial infarction [1]. Consequently, many studies have investigated a variety of factors that influence clot structure under static conditions. However, almost nothing is known about the influence of flow on clot structure, even though intravascular clots and thrombi form in the presence of flowing blood. A few perfusion chamber investigations with flowing blood have shown that while platelet adhesion increases with increasing wall shear rate, the amount of fibrin deposited decreases [2–4]. Other studies have provided intriguing hints that flow may orient fibers along the direction of flow [5–7], but these studies provided no quantification and the conditions of clotting initiation were non-physiological. Polarized light microscopy images of ex vivo coronary artery or aortic thrombi reveal that such thrombi are composed of fibers that are significantly more oriented along the long axis of the vessel than fibers in clots formed in vitro under static conditions [8].
The increased orientation of fibrin in thrombi compared with in vitro clots could arise either because factors associated with the vessel wall trigger directional fibrin formation, or because blood flow influences fibrin formation along the direction of flow [8]. To simulate in vitro the physiological effects of blood flow and to quantify fibrin fiber orientation, we employed a collagen-coated perfusion chamber to study fibrin formation on platelets in the presence of flow.
Permanox slides (75 × 38 mm; Nunc, Thermo Fisher Scientific, Rochester, NY, USA) were cleaned with 1 M NaOH, rinsed, dried, then coated with 0.1 mg mL−1 collagen for 1 h at 4 °C, and blocked for 1 h with 0.35% bovine serum albumin (BSA). The chamber and gasket of the parallel plate flow chamber [9] were placed atop the slide and vacuum sealed. Human platelets freshly prepared by gel filtration [10] were recalcified with 20 mM (final conc.) CaCl2 for 1–2 min, then perfused through the collagen-coated reaction chamber at 0.444 mL min−1 for 3 min and left to incubate for 30 min. During this time, pooled, platelet-poor human plasma (PPP) [11] was recalcified with 20 mM (final conc.) CaCl2 for 20 min at room temperature, and then perfused through the reaction chamber for 10 min (replacing the platelet suspension) at 5 or 20 s−1 [laminar shear rate (s−1) = 6Q/(h2w), where Q is volumetric flow rate (mL/s), h is chamber height (cm), and w is chamber width (cm)]. Next, the clot was washed by perfusion of cacodylate buffer at 200 μL min−1. The slide with the clot was removed from the chamber, the direction of flow was marked, and samples were fixed with 2% glutaraldehyde. Samples were then prepared for scanning electron microscopy by dehydration and sputter-coating [12]. Control samples formed in the absence of flow were prepared by adding 0.5 U mL−1 thrombin and 20 mM CaCl2 (final conc.) to PPP and incubating for 1 h in microchambers.
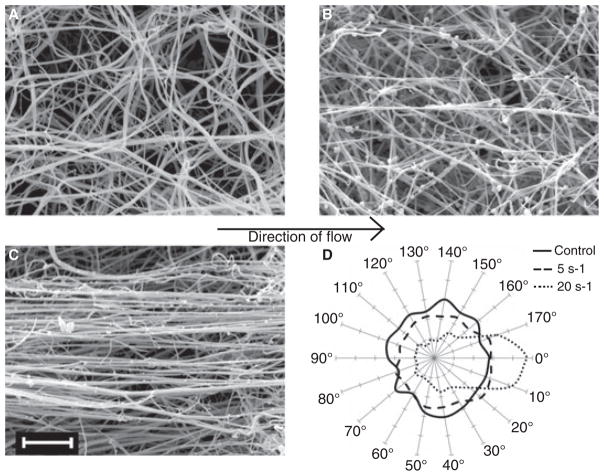
Scanning electron microscopy (Phillips/FEI XL20) revealed striking effects of flow on clot structure. Fibrin fibers were randomly oriented in the absence of flow, weakly oriented at 5 s−1, and strongly oriented when prepared at 20 s−1 shear (Fig. 1A–C). Although there were still some randomly oriented connecting fibers, the majority of fibers in the clot that was formed at 20 s−1 shear (Fig. 1C) aligned along the direction of flow (horizontally, as shown).
Fig. 1.
Scanning electron micrographs of clots formed under 0 s−1 (A), 5 s−1 (B) and 20 s−1 (C) shear. Micrographs were obtained at 5000× (magnification bar = 5 μm). Images are representative of 12 locations visualized on each clot from two to three experiments per condition. The direction of flow is from left to right, as shown by the arrow. This angle was considered 0°, and the orientation of each fiber in a set area was measured resulting in an angle from 0 to 180°. Measurements were placed in 10° bins and plotted on polar coordinates (D).
We analyzed fiber diameter and orientation quantitatively using images obtained from duplicate or triplicate experiments. To measure fiber orientation, a line was drawn tracing each fiber, the angle from the axis of flow (0–180°) was measured, and the frequency of fibers at each 10° interval was plotted on polar coordinates (Fig. 1D). In a clot formed without flow, the frequency of fibers oriented at any one angle was about the same as that of any other angle (solid line). When the clot was formed at low shear (5 s−1), more fibers oriented along the direction of flow (170–20°) than oriented perpendicular to this direction (70–120°) (dashed line). The asymmetric distribution of fiber orientation frequencies was striking for clots formed at higher shear (20 s−1) (dotted line). Twenty-six percent of all fibers measured (407 fibers) were in the two bins closest to the direction of flow (170–10°), whereas only 6% of fibers (94) were in the two bins perpendicular to the direction of flow.
The diameters of all foreground fibers were measured in one randomly selected 9.5 × 9.5 μm square of a grid (202–297 individual fibers per image), by drawing a perpendicular line across the fiber, plotting the brightness of pixels along this line and measuring the distance across the fiber. Diameters were averaged for 12 images of each sample, with the standard error of the mean calculated from the number of independent experiments. We found no significant difference in the average diameter between fibers formed at 5 s−1 shear (0.21 ± 0.03 μm, n = 3 experiments) and those formed at 20 s−1 shear (0.19 ± 0.04 μm, n = 2 experiments) (mean diameter ± standard error of the mean) (P = 0.23). Because fiber diameter is dependent upon thrombin concentration, we did not compare these diameters with those of control samples as they were prepared by addition of exogenous thrombin.
In conclusion, we have shown that in a parallel plate flow chamber, newly formed fibrin fibers orient in the direction of flow, with increasing alignment as the flow rate increased. Our results are consistent with previous studies [5–7], but were carried out under more physiological conditions, clotting was initiated by platelets on a collagen surface, and the observations were quantified. Because we found similar fiber orientation in our purified in vitro system to the alignment observed in thrombi within coronary arteries, we conclude that the flow of blood serves to orient the fibers that form inside a vessel. The striking degree of orientation even at low shear rates demonstrates the profound effect of flow on clot structure, which must be considered in understanding in vivo clotting and thrombosis.
Footnotes
Disclosure of Conflict of Interests
The authors state that they have no conflict of interest.
References
- 1.Collet JP, Allali Y, Lesty C, Tanguy ML, Silvain J, Ankri A, Blanchet B, Dumaine R, Gianetti J, Payot L, Weisel JW, Montalescot G. Altered fibrin architecture is associated with hypofibrinolysis and premature coronary atherothrombosis. Arterioscler Thromb Vasc Biol. 2006;26:2567–73. doi: 10.1161/01.ATV.0000241589.52950.4c. [DOI] [PubMed] [Google Scholar]
- 2.Orvim U, Roald HE, Stephens RW, Roos N, Sakariassen KS. Tissue factor-induced coagulation triggers platelet thrombus formation as e3ciently as fibrillar collagen at arterial blood flow conditions. Arterioscler Thromb. 1994;14:1976–83. doi: 10.1161/01.atv.14.12.1976. [DOI] [PubMed] [Google Scholar]
- 3.Sakariassen KS, Joss R, Muggli R, Kuhn H, Tschopp TB, Sage H, Baumgartner HR. Collagen type III induced ex vivo thrombogenesis in humans. Role of platelets and leukocytes in deposition of fibrin. Arteriosclerosis. 1990;10:276–84. doi: 10.1161/01.atv.10.2.276. [DOI] [PubMed] [Google Scholar]
- 4.Weiss HJ, Turitto VT, Baumgartner HR. Role of shear rate and platelets in promoting fibrin formation on rabbit subendothelium. Studies utilizing patients with quantitative and qualitative platelet defects. J Clin Invest. 1986;78:1072–82. doi: 10.1172/JCI112663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirchhofer D, Sakariassen KS, Clozel M, Tschopp TB, Hadvary P, Nemerson Y, Baumgartner HR. Relationship between tissue factor expression and deposition of fibrin, platelets, and leukocytes on cultured endothelial cells under venous blood flow conditions. Blood. 1993;81:2050–8. [PubMed] [Google Scholar]
- 6.Kuijper PH, Gallardo Torres HI, Lammers JW, Sixma JJ, Koender-man L, Zwaginga JJ. Platelet and fibrin deposition at the damaged vessel wall: cooperative substrates for neutrophil adhesion under flow conditions. Blood. 1997;89:166–75. [PubMed] [Google Scholar]
- 7.Neeves KB, Illing DA, Diamond SL. Thrombin flux and wall shear rate regulate fibrin fiber deposition state during polymerization under flow. Biophys J. 2010;98:1344–52. doi: 10.1016/j.bpj.2009.12.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whittaker P, Przyklenk K. Fibrin architecture in clots: a quantitative polarized light microscopy analysis. Blood Cells Mol Dis. 2009;42:51–6. doi: 10.1016/j.bcmd.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ji JY, Jing H, Diamond SL. Shear stress causes nuclear localization of endothelial glucocorticoid receptor and expression from the GRE promoter. Circ Res. 2003;92:279–85. doi: 10.1161/01.res.0000057753.57106.0b. [DOI] [PubMed] [Google Scholar]
- 10.Goel MS, Diamond SL. Neutrophil enhancement of fibrin deposition under flow through platelet-dependent and -independent mechanisms. Arterioscler Thromb Vasc Biol. 2001;21:2093–8. doi: 10.1161/hq1201.100255. [DOI] [PubMed] [Google Scholar]
- 11.Gersh KC, Nagaswami C, Weisel JW. Fibrin network structure and clot mechanical properties are altered by incorporation of erythrocytes. Thromb Haemost. 2009;102:1169–75. doi: 10.1160/TH09-03-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weisel JW, Nagaswami C. Computer modeling of fibrin polymeriza-tion kinetics correlated with electron microscope and turbidity observations: clot structure and assembly are kinetically controlled. Biophys J. 1992;63:111–28. doi: 10.1016/S0006-3495(92)81594-1. [DOI] [PMC free article] [PubMed] [Google Scholar]