Abstract
Objective
To analyse composition of coronary thrombus in vivo, in ST-elevation Myocardial Infarction (STEMI) patients.
Background
The dynamic process of intracoronary thrombus formation in STEMI patients is poorly understood.
Methods
Intracoronary thrombi (n=45) were obtained by thromboaspiration in 288 consecutive STEMI patients presenting for primary percutaneous intervention and analyzed using high definition pictures taken with a scanning electron microscope. Plasma biomarkers (TnI, CRPus, IL-6, PAI-1, sCD40 ligand and TNF-α) and plasma fibrin clots viscoelastic properties were measured simultaneously on peripheral blood.
Results
Thrombi were mainly composed of fibrin (55.9±18%) with platelets (16.8±18%), erythrocytes (11.5±9%), cholesterol crystal (5.2±8.4%) and leukocytes (1.3±2.0%). The median ischemic time was 175 min [IQR 140-297]. Ischemic time impacted thrombi composition, resulting in a positive correlation with intracoronary thrombus fibrin content, r=0.38, p=0.01 and a negative correlation with platelet content r=-0.34, p=0.02. Thus, fibrin content increased with ischemic time, ranging from 48.4±21% (<3 hours) up to 66.9±9% (>6 hours) (p=0.02), while platelet content decreased from 24.9±23% (<3 hours) to 9.1±6% (>6 hours) (p=0.07). Soluble CD40 ligand was positively correlated to platelet content in the thrombus (r=0.40, p=0.02) and negatively correlated with fibrin content (r=-0.36; p=0.04). Multivariate analysis indicated that ischemic time was the only predictor of thrombus composition with a 2-fold increase of fibrin-content per ischemic hour (adjusted OR2 [1.03-3.7] p=0.01).
Conclusions
In acute STEMI, platelet and fibrin contents of the occlusive thrombus are highly dependent of ischemic time, which may have a direct impact on the efficacy of drugs or devices used for coronary reperfusion.
Keywords: Thrombus, STEMI
INTRODUCTION
Acute coronary thrombosis resulting in total occlusion of a coronary artery leads to ST-segment elevation myocardial infarction (STEMI)(1). Exposition of the lipid-rich core after atherosclerosis plaque rupture / erosion into the arterial lumen triggers the formation of unstable platelet aggregates, which may lead to intermittent reduction in coronary flow and cause distal embolization (2). Early fibrin formation strengthens the platelet aggregate, favoring persistent flow obstruction and blood coagulation activation proximally and distally to the occlusion. The resulting red thrombus is comprised of erythrocytes and inflammatory cells entrapped by the fibrin network. The layered aspect highlights the repeated episodes of thrombus growth(3) (4) .
It remains unclear how time affects the dynamic process of thrombus formation in the coronary arteries of ST-elevation Myocardial Infarction (STEMI) patients (5). Data are also scarce on how thrombus architecture correlates with coronary reperfusion after primary percutaneous Intervention (PCI) or with peripheral blood biomarkers. The increase use of thromboaspiration in primary PCI for STEMI offers a unique opportunity to study thrombus composition, dynamic formation and architecture in vivo(6-9)
Ischemic time was hypothesized to be among the strongest independent correlates of thrombus architecture. We therefore evaluated the effect of the following plausible determinants of thrombus architecture (e.g., coronary anatomy and reperfusion, patient clinical presentation, peripheral blood biomarkers, fibrin clots viscoelastic properties and response to fibrinolysis). Our aim was to characterize the independent correlates of thrombus architecture and to assess whether thrombus architecture per se could affect myocardial reperfusion.
METHODS
Study design and thrombus collection
Between January 2007 and April 2008 we prospectively screened all the STEMI patients referred to the catheterization laboratory for primary PCI associated with thromboaspiration performed by a low-profile catheter (Export™ 6F, Medtronic, Santa Rosa, CA, USA). Thromboaspiration was performed whenever possible (when the anatomy of the coronary artery - curve and size- allowed it) in all patients with a TIMI Flow 0 and in all patients with a visible thrombus if TIMI Flow was 1 or more.
Collected thrombi were immediately washed with saline and fixed with 2% glutaraldehyde in 50mM Na-cacodylate buffer (pH 7.3).
Scanning electron microscope (SEM) analysis
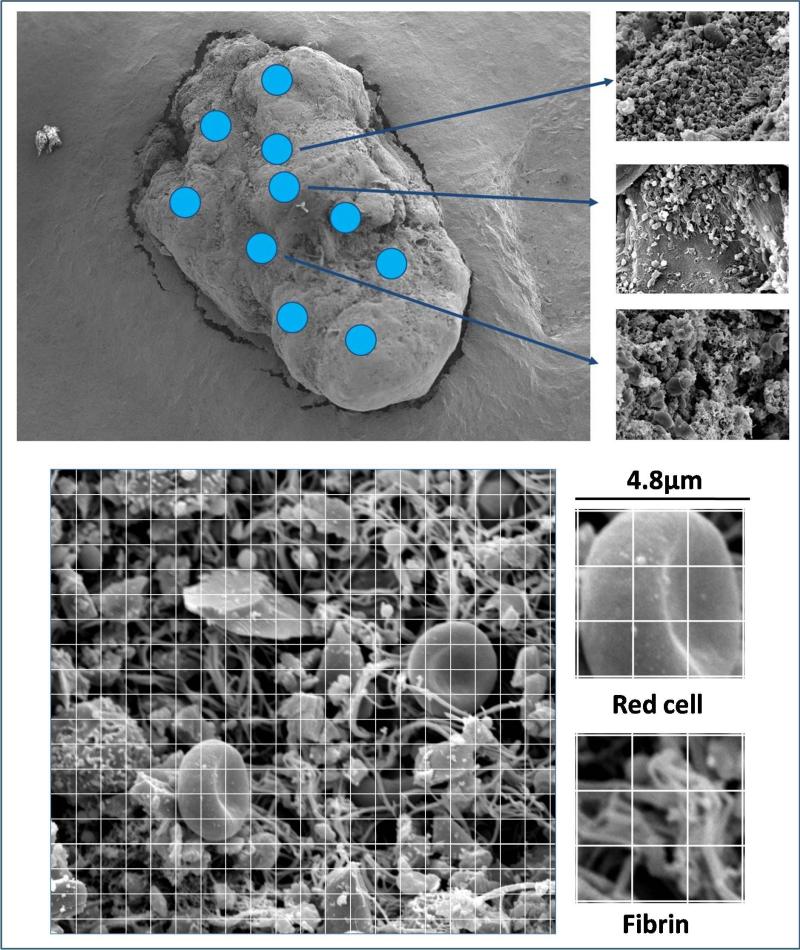
Sample fixation, dehydration and preparation were performed according to previously published method (10). High definition photographs (3000X magnification) were obtained using a Philips/FEI XL20 SEM (4nm resolution) (FEI, Hillsboro, OR, USA) (11). To control for composition heterogeneity in the analysis of the surface of thrombus, we covered several areas (at least 12 according to a grid and the size of the thrombus) for each thrombus (Figure 1). A semi-quantitative analysis and a visual approach were used for the first ten thrombi. For the semi-quantitative analysis, each picture was divided in 400 squares (Image J software) and the predominant composition (platelet, fibrin, erythrocytes, leukocytes, cholesterol crystal) of each square was noted (Figure 1) (12). All the structures visualized were easy to identify, and crystals like structures were characterized as cholesterol crystal (asterisks in fig 2 bis) on the basis of previous SEM images described in vitro (13,14) and the probability of their presence in the setting of a ruptured plaque exposing a lipid rich core.The visual approach consisted in a careful observation of each pictures with a subjective evaluation of the percentage of each component. Very low inter-individual variability was found in the thrombus composition analysis (<6%) between the two approaches, therefore the time-consuming semi-quantitative analysis was not performed for the remaining 35 thrombi (Figure 2 and 2bis). The two scientists performing SEM and analysis were blinded to the clinical data.
Figure 1.
Analysis of clot surface with 10+ (or more) areas to average the thrombus heterogeneity (above) and perform semi quantitative analysis (below).
Figure 2 bis. Scanning electron micrograph at 3000x magnification.
This portion is mainly composed of platelet aggregates with a few cholesterol crystals (in yellow)
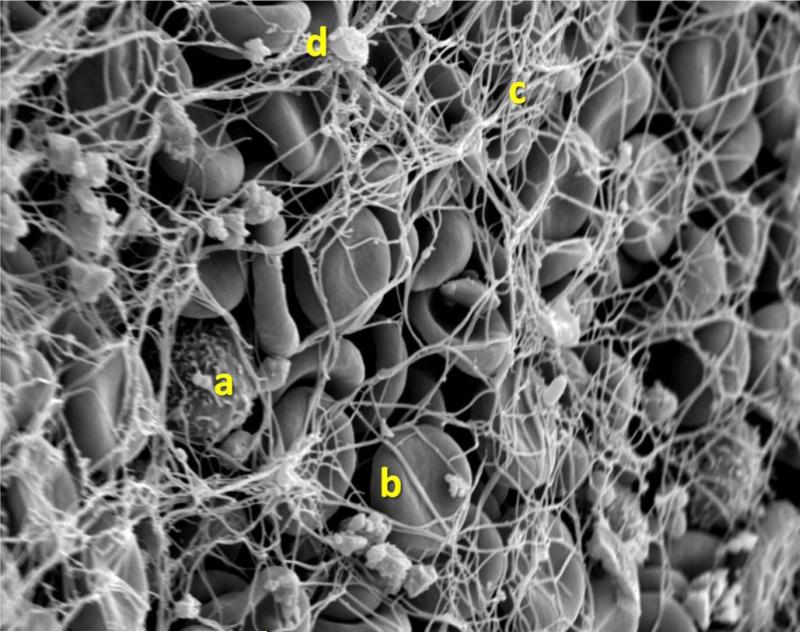
Figure 2. Scanning electron micrograph at 3000x magnification.
This portion is mainly composed of erythrocytes (b) trapped in a fibrin mesh (c) with few platelet aggregates (d) and rare white cells (a)
Blood samples and measurement
Blood samples were drawn from the arterial sheath just after insertion and were collected in Vacutainer tubes (Becton Dickinson) containing trisodium citrate 0.129 M (3.2% sodium citrate) for routine blood work and for biomarkers measurements.
Soluble CD40L levels were measured using a human sCD40L ELISA bioassay (Arcus Biological, Modena, Italy). TNF-α and IL-6 levels were measured by Quantikine sandwich enzyme inmunoassays (R&D Systems, Abingdon, UK) according to the manufacturer's instructions. CRP levels were quantified by commercially available ELISA kits (Zymutest,Hypen BioMed, Neuville sur Oise, France). The PAI-1 concentration was measured using an Asserachrom ® PAI-1 kit (Diagnostica Stago, Asnieres, France). All measures were assessed in duplicate.
Whole blood clot viscoelastics properties and lysis rate
Clots were formed in a thermostable 37°C plastic plate by recalcifying 700 μL of PPP with CaCl2 (10 mM final concentration) and 5 μL of purified recombinant human tissue factor (Innovin, Dade Behring). To facilitate lysis 10 μL of tissue plasminogen activator (rt-PA) (0.5 nM final concentration) (Boehringer Ingelheim, Germany) was also added. Plasma clots viscoelastic properties were measured with a Hemodyne RM-2 Analyser (Hemodyne, Richmond, VA). Fibrinolysis was assessed by continuous monitoring of the elastic modulus (EM) and was expressed as the lysis rate (s-1). The assay accuracy is reportedly similar to that of determination of the lysis front velocity (or fiber lysis rate). Clot lysis time (CLT) was defined as the time needed for the maximum elastic modulus to decrease by 50%. The intra-individual variability of this technique was published previously (15).
Data collection
All patients entered the e-PARIS registry, a prospective web-based registry regrouping clinical and biological data on STEMI patients treated by primary PCI. Our angiographic core laboratory reviewed all angiographic films and noted the size of the infarct-related artery, TIMI Frame Count (cTFC) and myocardial blush grade (MBG), as previously described(16). Clinical and biological data were analysed to identify predictors of thrombus composition. This study was approved by the Institutional Review Board of the Pitié-Salpêtrière Hospital in Paris (CPP), France.
Clinical Follow-Up
Patients were followed-up for 30 days through out-patient consultation. Physicians noted the occurrence of Major Cardiovascular and Cerebrovascular Events (MACCE) including death, stroke, recurrent MI, urgent revascularization and definite and probable stent thrombosis (Academic Research Consortium definition), as well as major and minor bleeding events.ST-segment resolution was collected from the patient file.
Statistical Analysis
Categorical variables were expressed as percentages and continuous variables as means ± SD or medians with the IQR [25th-75th percentiles] for time delay. Association between continuous variables was assessed by Pearson's correlation test. The variable ischemic time had an almost normal distribution. As complementary analyses using Pearson's correlation test after log-transformation and non parametric Spearman's test found similar results, the variable was considered normally distributed for all other analyses. Potential associations between clinical and biological parameters were tested by Student's t test and ANOVA test with Dunnett's correction for multiple comparisons. Multivariable logistic and mixed models were used to assess the relationship between ischemic time and both qualitative and quantitative thrombus composition. Co-variables (age, diabetes, renal insufficiency, admission troponin fibrinogen and platelet levels, pre-hospital abciximab use, clopidogrel pre-treatment, unfractionated heparin versus enoxaparin use and coronary artery reference diameter) were introduced in the models on top of ischemic time in a stepwise fashion. Diagnostics were systematically conducted for both logistic and mixed models to assess the normal distribution of residuals. Analyses were performed with SASv9.2 (SAS Institute, Cary, North Carolina).
RESULTS
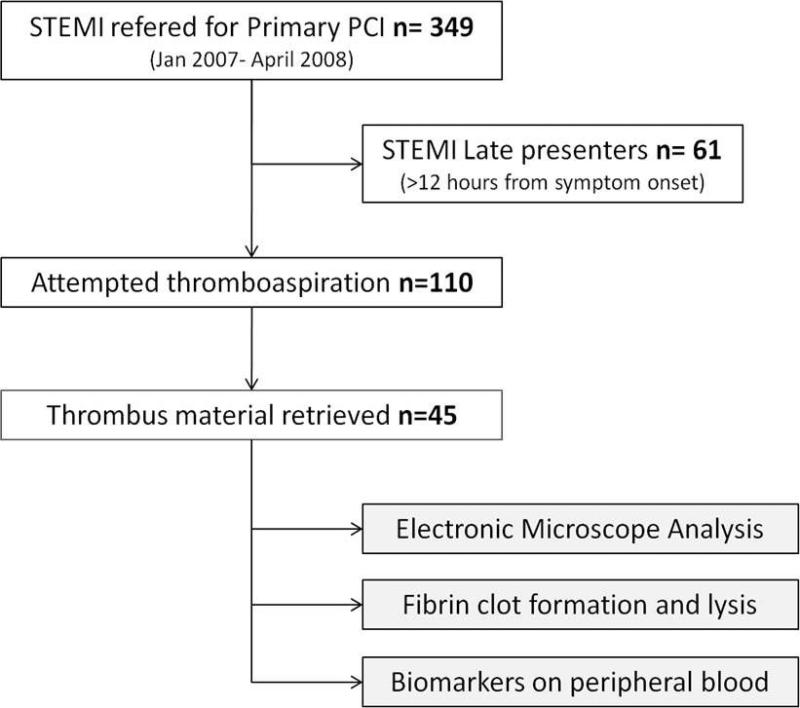
We prospectively screened 349 patients referred for primary PCI. Sixty-one late presenters were excluded because there was a time delay of >12 hours from symptom onset to PCI or because symptom onset could not be characterized. Thromboaspiration was used in 1/3 of the early STEMI presenters (38%) and significant coronary thrombi >1 mm3 were successfully retrieved in 1/3 of them (Figure 3). The baseline characteristics of the patients are shown in Table 1. Patients were treated aggressively with a high proportion receiving high loading doses of clopidogrel (at least 600 mg) and abciximab on top of aspirin and low-molecular weight or unfractionated heparin. Median ischemic time (from symptom onset to PCI) was 175 min [IQR 140-297]. Details of procedures, angiographic findings, biomarker distributions and viscoelastic measurements and fibrinolysis results are reported in Table 2.
Figure 3.
Flow chart of the study.
Table 1.
Baseline characteristics.
| Baseline characteristics |
Study Population |
|---|---|
| n=45 | |
| Demographics and Risk Factors | |
| Age | 57.9 ± 13.2 |
| Male |
38 (84.4%) |
| Active Smoker | 21 (46.6%) |
| Diabetes | 12(26.6%) |
| Hypertension | 16 (35.5%) |
| Dyslipidemia | 30 (66.6%) |
| BMI |
26.4 ± 4.1 |
| Past Medical History | |
| h/o ACS | 7 (15.5%) |
| h/o Angioplasty | 6 (13.3%) |
| h/o CABG |
1 (2.2%) |
| Clinical Presentation | |
| Anterior MI | 21 (46.6%) |
| Cardiac Arrest | 4 (11.1%) |
| TIMI risk Score | 3.0 ± 2.1 |
| Killip | 1.2 ± 0.6 |
| Cardiogenic Shock | 2 (4.4%) |
| Time delay in min | Median [IQR] |
| Symptom Onset - Medical Contact | 80 [55-179] |
| Medical Contact – Cath-lab | 66 [55-128] |
| Cath-lab - Sheath Insertion | 20 [10-33] |
| Symptom Onset - PCI |
175 [140-297] |
| Biomarkers on admission | |
| Troponin I (μg/mL) | 5.1 ± 13.8 |
| Negative troponin | 17 (37.7%) |
| CK (IU/mL) | 447 ± 828 |
| CRP (mg/L) | 13.7 ± 38 |
| Fibrinogen (g/L) | 3.7 ± 1.3 |
| Platelet (mm3) | 242 ± 93 |
| Creatinine Clearance (mL/min) |
98 ± 33 |
| Antithrombotic Treatment | |
| ASA | 45 (100%) |
| Clopidogrel | 37 (83.5%) |
| Unfractionned Heparin | 17 (37.7%) |
| Enoxaparin | 26 (62.3%) |
| GPIIb-IIIa Inhibitor | 41 (91.1%) |
| Prehospital | 8 (17.7%) |
| Cath-lab | 33 (73.3%) |
| Thrombolysis | 1 (2.2%) |
Table 2.
Angiographic results, revascularization procedure and biological data.
| Angiography, Revascularization and Biological Data |
Study Population |
|---|---|
| n=45 | |
|
Radial Approach
|
42 (93.3%) |
| Diameter of the infarct-related artery | 3.15 ± 0.5 |
| Acute stent thrombosis | 5 (11.1%) |
| Patients with at least more than one significant lesion |
13 (28.8%) |
| Infarct related artery | |
| LAD | 21 (46.6%) |
| RCA | 17 (37.7%) |
| CX | 6 (13.3%) |
| CABG | 1 (2.2%) |
| Successful PCI | 41 (91.1%) |
| Ejection Fraction post MI (%) |
48.9 ± 12 |
|
Markers of reperfusion
|
|
| TIMI 3 Flow before PCI | 3 (6.6%) |
| TIMI 3 Flow after PCI | 40 (88.8%) |
| cTFC before PCI | 133 ± 45 |
| cTFC after PCI | 25 ± 22 |
| TIMI MBG3 before PCI | 4 (8.8%) |
| TIMI MBG3 after PCI | 18 (40%) |
| ST resolution >70% |
25 (55.3) |
|
Biomarkers
|
|
| CRP us (μg/mL) | 8.5 ± .3.9 |
| IL6 (pg/mL) | 14.2 ± 34.8 |
| PAI-1 (ng/mL) | 70.2 ± 51.2 |
| sCD40-Ligand (ng/mL) | 1.2 ± 0.6 |
| TNF-ALPHA (pg/ml) |
8.6 ± 1.1 |
|
Viscoelastic properties and response to fibrinolysis
|
|
| PCF (kdynes/sec) | 1.4 ± 1.4 |
| Elastic modulus max (kdynes/sec) | 8.7 ± 8.8 |
| Clotting Time (sec) | 691 ± 651 |
| Clot Lysis Time (sec) | 347 ± 204 |
| Lysis rate ×104 (sec-1) | 2.9 ± 4.1 |
Thrombus composition
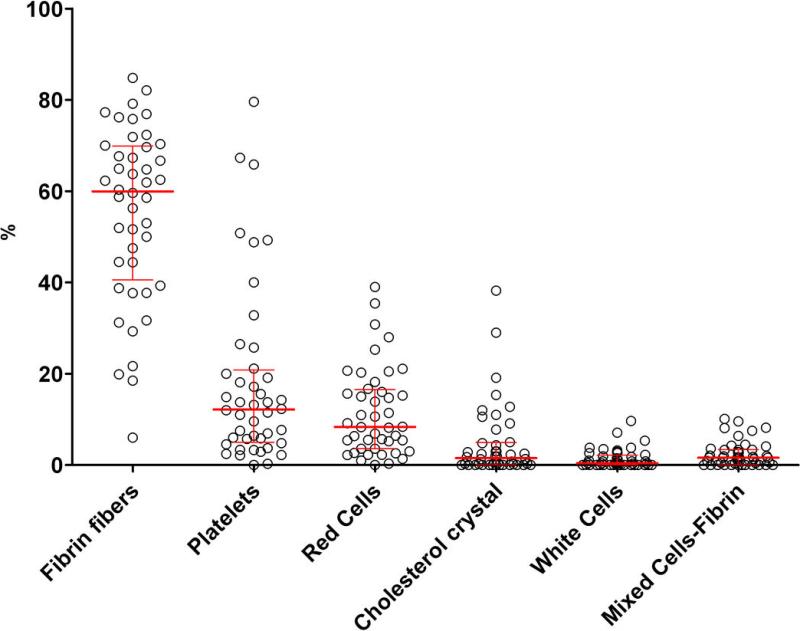
Analyses were performed on 44 of the 45 thrombi collected. One thrombus was not analyzed because the SEM preparation had failed. Fibrin fibers were the major thrombus component, representing more than 60% of their composition. Platelets, erythrocytes, cholesterol crystals and leukocytes together comprised the remaining 40% (Figure 4). Thrombus components were easily identified in our high definition SEM images. The two examples shown here are good representatives of the broad scope of thrombi components. These include a fibrin-and-erythrocyte composition typical of “old” fibrin-rich thrombi, usually found in patients with an ischemic time of > 3 hours (Figure 2) and a “fresh” platelet-rich thrombus of a patient presenting early, within the first hour following symptom onset (Figure 2 bis). Remarkably, we could identify in these high definition images an unexpected percentage (5%) of cholesterol crystals that were only described previously ex vivo(13).
Figure 4. Thrombi composition in n=44 STEMI patients (early presenters).
Red lines indicate median and IQR [25%-75%].
Effect of time on thrombus composition
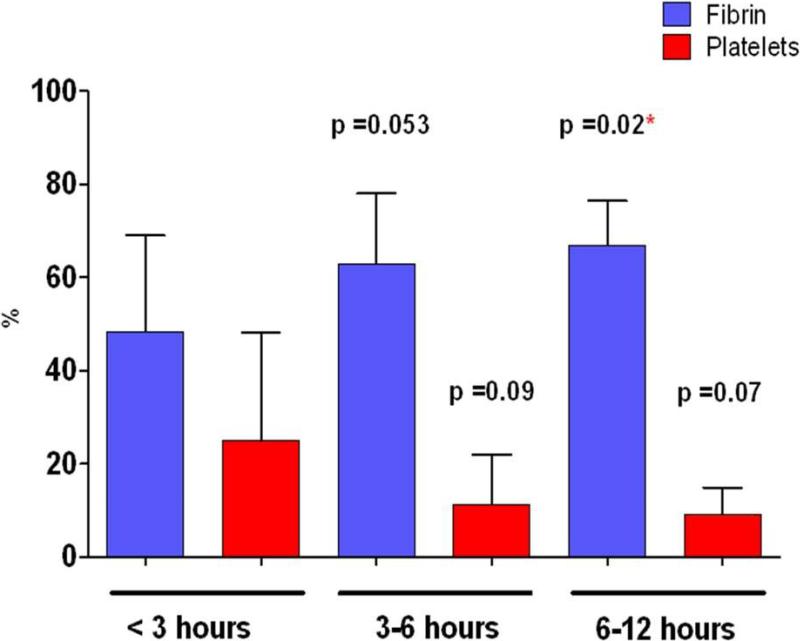
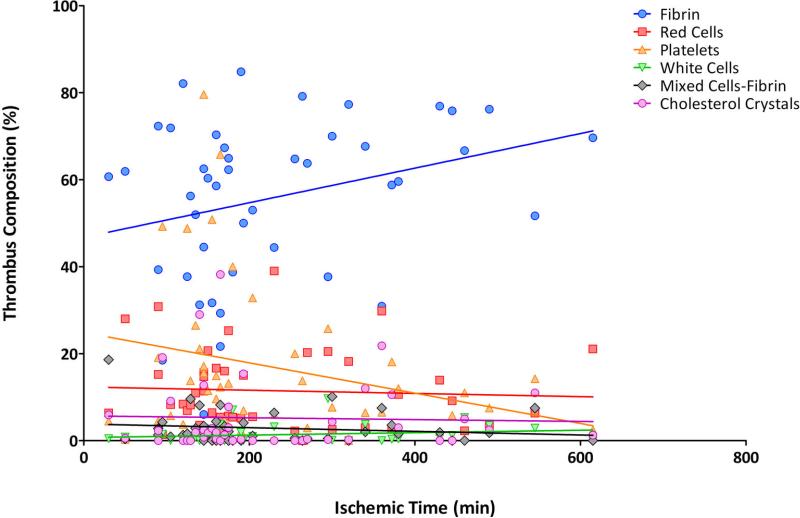
Ischemic time was found to have a high impact on thrombi composition resulting in a positive correlation with intracoronary thrombus fibrin content, r=0.38, p=0.01 and a negative correlation with platelet content r=-0.34, p=0.02.. Pearson's correlation test after log-transformation and Spearman's non parametric correlation test showed similar results (r=0.39, p=0.01; r=-0.34, p=0.025 and r=0.36, p=0.017; r=-0.30, p=0.05 respectively). Additionally, when ischemic time was divided in three categories (<3 hours, 3-6 hours and more than 6 hours) we observed a stepwise increase in fibrin content with respect to prolonged ischemic time ranging 48.4±21% (<3 hours) to 66.9±9% (>6 hours) (p=0.02). Platelet content decreased from 24.9±23% (<3 hours) to 9.1±6% (>6 hours) (p=0.07) (Figure 5). The evolution of each thrombus component relative to ischemic time is represented in Figure 6.
Figure 5. Impact of time on thrombus composition.
P-values are given for comparison with the group < 3 hours as a reference with multiple student t -test. * p value <0.05. Multiple comparison was done with the Dunnett's test resulting in a p value = 0.044 for fibrin and non significant for platelet content
Figure 6. Evolution of the percent thrombus composition for each component in % (Y axis) relative to ischemic time (min) (X axis).
Lines represent linear regression for correlation with ischemic time.
Effect of antithrombotic treatment on thrombus composition
Aspirin was administrated before thromboaspiration in 100% of patients and abciximab was used either in a pre-hospital setting or in the catheterization laboratory in 91% of patients, limiting the analysis of their impact on thrombus composition. Clopidogrel pre-treatment was administered in 29 patients (66%) and did not induced any significant change on platelet composition (24.1±6% vs. 15.5±3%; p=0.17) when compared to patients without pre-hospital inhibition of the P2Y12 receptor.
Biomarker distribution, viscoelastic properties and fibrinolysis
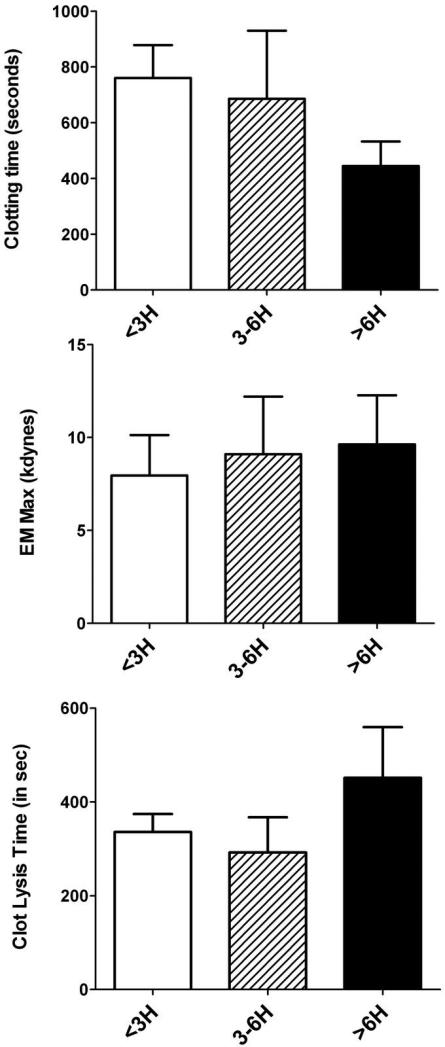
The peripheral measure of the platelet activation marker sCD40 ligand was positively correlated with platelet content in the thrombus (r=0.40, p=0.02) and negatively correlated with fibrin content (r=-0.36; p=0.04). The simultaneous measure of the more classical biomarker of myocardial infarction, namely troponin I, was a strong biomarker of ischemic time (r=0.56, p<0.01), but was not correlated with thrombus composition. No correlation was seen between ischemic time and inflammation biomarkers (IL-6, TNF-α, CRPus) or PAI-1 and neither platelet count nor fibrinogen level were correlated with thrombus composition (data not shown). Patients presenting after 6 hours had a non-significant trend towards faster clotting time, stiffer clot (as indicated from the elastic modulus) and longer clot lysis time (Figure 7).
Figure 7. Results of fibrin clot viscoelastic properties and response to fibrinolysis according to ischemic time.
No significant differences were found between groups with the Dunnett's test.
Independent correlates of thrombus composition and of reperfusion
Ischemic time was the only correlate of thrombus composition in the multivariable stepwise logistic analysis. None of the other co-variables met the 0.05 significance level for entering the model. Every additional ischemic hour led to a two-fold increase in the rates of fibrin rich thrombus -<30% platelet and >70% of fibrin- (adjOR 2 [1.03-3.7]) and a 50% reduction in platelet content (adjOR of 0.5 [0.27-0.94]) (p=0.001 for both). Similar results were obtained when the fibrin content (%) was analyzed quantitatively in a multivariable mixed model with the same co-variables introduced in the model on top of ischemic time. “Fresh” platelet-rich thrombi was defined as >30% platelet and <70% of fibrin and “old” fibrin-rich thrombi as <30% platelet and >70% of fibrin. No differences in reperfusion were seen between “fresh” platelet-rich thrombi and “old” fibrin-rich thrombi assessed by ST segment resolution and TIMI MBG. Independent correlates of successful reperfusion (ST segment resolution of >70%) included a low plasma fibrinogen level (adjOR 0.33 [0.12-0.91] (per g/L), p=0.003) and the use of enoxaparin versus UFH (adOR 10.3 [1.7-62], p=0.02). High and low counts for plasma fibrinogen level were 8g/L and 1.8g/L respectively.
DISCUSSION
Thrombus aspiration is a newly recommended technique that facilitates thrombus removal from the culprit coronary artery in myocardial infarction. The method reportedly improves mortality in STEMI patients presenting within 12 hours of symptoms onset when used in conjunction with primary PCI (7,8). This life-saving technique allowed us to study prospectively the composition of occlusive thrombi in STEMI patients admitted for mechanical coronary reper-fusion.
We report the results of a new analysis method for intracoronary thrombi obtained in vivo that allows the determination of their precise composition using scanning electron microscopy, associated here with an analysis of cardiac biomarkers, fibrin clot viscoelastic properties and fibrinolysis obtained simultaneously from patients’ peripheral blood. The analysis of high-definition scanning electron microscopy images that we produced with respect to ischemic time (from symptom onset to thrombus retrieval) revealed that the dynamic formation of the thrombus during an acute coronary occlusion is a fast evolving process. The platelet and fibrin fiber components of the thrombus which are both therapeutic targets, changed rapidly with respect to ischemic time. These observations of thrombus structure and formation in vivo demonstrate the relationship between thrombus composition and ischemic time, which was found to be the only major predictors of thrombus composition. Our results also demonstrate that thrombus composition is an indicator of coronary occlusion time and therefore of the beginning of myocardial damage.
Our findings add to the body of knowledge on thrombus formation in acute myocardial infarction. First, they confirm, at the pathophysiological level, the generally accepted pathogenesis of clot formation in vivo in STEMI patients. “Fresh” thrombi have the highest proportion of platelets, while the proportion of fibrin fibers increases over time, as the level of thrombin increases, leading to “older” fibrin-rich thrombi. These results confirm previous in vivo animal studies showing the very early stage of thrombus formation, just after endothelial injury, when the initial thrombus is primarily composed of activated platelets, rapidly stabilized by fibrin fibers, with a decreasing proportion of platelets over time (17-19). At a clinical level, our results are along the same lines as those reported by Kramer et al., who showed that the thrombus composition itself seems to be a prognostic marker and is linked to short and long-term mortality in a large study in STEMI (20). In this previous study, thrombus composition was evaluated by classical light microscopy showing two types of thrombi, “fresh thrombi” (<1 day) composed of layered patterns of fibrin and intact platelets, erythrocytes, and granulocytes and “older thrombi” (>1 day) comprised of lytic and/or organized areas. Our own analysis differs from this previous classification by the age of clots (earlier period), the method used (scanning electronic microscope), the structure analyzed (we studied the thrombus surface which reflects more the latter layers of thrombus formation), and the results reported (focused on the proportion of each component). The high resolution imaging facilitated the detailed characterization of all structures present in the thrombi, despite the difficulties of this task because of their remarkable heterogeneity. This allowed us for example to identify cholesterol crystals that were most likely released by the ruptured plaque. It should also be noted that fibrin is a major component of the thrombus even at the relatively early stages studied here. Additionally, simultaneous measurements of biomarkers allow us to confirm the role of sCD40 ligand on platelet accumulation in the intracoronary thrombus at the early phase of formation.
Our findings also help interpret results obtained from previous clinical trials. Indeed, fibrinolysis treatment has been demonstrated to have a narrow therapeutic time window and seems to be most efficient during the first 3 hours of STEMI (21,22). According to our study, this represents a period when the clot has a small and accessible amount of fibrin fibers and when fibrinolysis has not yet been overcome by the intense fibrin formation. Furthermore, the fast accumulation of fibrin fibers in the thrombus followed by its stabilization by FXIII cross-linking is likely to increase clot stiffness resulting in resistance to fibrinolysis as shown in vitro (15,23). Regarding antiplatelet agents, even though glycoprotein (Gp) IIb–IIIa inhibitors, such as abciximab, have been shown to reduce mortality in primary PCI (versus placebo), the potential benefit is mostly observed with an early administration (24-29). In contrast, negative studies with GPIIbIIa inhibitors seem to relate to late presentation of patients (30,31). These clinical results suggest a time limit under which these molecules are efficient, and correspond according to our study to the time when the occlusive thrombus contains a high proportion of platelets, shortly before it evolves into a compact fibrin-rich structure.
In our study, the effect of antithrombotic pre-treatment by aspirin and abciximab was initially included in the analysis, but due to the fact that almost all patients received these two fast acting agents before thromboaspiration, we could not demonstrate any potential effect of such drugs on thrombus composition. We believe that the impact of prehospital P2Y12 inhibition on platelet composition should be studied in a dedicated study.
This study has several limitations. Firstly, although erythrocytes were the second most common cell identified in our thrombi, we were surprised not to find an increased percentage of erythrocytes with time. Erythrocytes in clots were the origin of the word “red” thrombi for old thrombi as opposed to recent platelet-rich “white” thrombi. Erythrocytes may contribute more to thrombus composition at later stages and not in the time window of acute reperfusion of STEMI. The role of erythrocytes in thrombi remains to be studied more extensively, since they also appear to mediate the formation of thicker fibrin fibers and influence clot viscoelasticity by increasing the viscous component of the sample relative to the elastic component(32). Secondly due to the in vivo nature of our study, the analysis was performed on the main aspirated piece of the thrombus and did not include the total volume of retrieved fragments or the distal embolic debris. Because the retrieval of the thrombus is done by aspiration through the catheter lumen, the high definition analysis, made by scanning the surface of the piece of thrombus, can correspond in fact to any part of the thrombus (head or tail, surface or core, luminal or abluminal) turning this potential limitation into a major strength of this approach. To our knowledge there is no known technique (beside surgical removal or post-mortem autopsy) that can analyze the whole thrombi, including the part of the thrombus that might have embolized, or allows analysing the three-dimensional structure of a thrombus. Additionally the number of thrombus retrieved and the random localization of the pictures that were analyzed, should guarantee an unbiased measurement and control for composition heterogeneity in the pieces of thrombi that were obtained. Thirdly, potential distortion of the samples might have occurred during thrombo-aspiration. However we believe that damage was limited, knowing that thrombi are stiffer than in vitro clots and that fibrin fibers and clots bear outstanding elastic properties (33,34). The preserved appearance of cells and fibrin fibers in the microscopy images confirmed that distortion was minimal. Fourthly, the choice of scanning electron microscopy can be criticized since material cannot be stained as in histopathological analysis, but was motivated by its ability to provide high resolution images of thrombi in humans suffering from myocardial infarction (5). Fifthly, the aspirated thrombus may be older than expected from the duration of the ischemic time and younger thrombus could be superimposed with an older thrombus, thereby potentially confusing our observations (20). Finally, even if thrombi were retrieved randomly in a subset of patients representative of our STEMI population, the absence of successful thromboaspiration in all STEMI presenters could have biased our results. A methodological limitation also needs to be underlined as the multivariable analysis should be considered as only exploratory, because the model is not parsimonious.
Our work helps characterize thoroughly the components of coronary thombi in STEMI and thereby contributes to the current understanding of coronary thrombus formation, which is relevant to the efficacy of various reperfusion treatments. The composition of the aspirated thrombus could also be a potential surrogate endpoint in clinical trial assessing the benefit of new antithrombotic regimen (new drugs or new combination of drugs). Ongoing research should elucidate the mechanistic relationships between thrombus composition, myocardial perfusion and microvascular obstruction.
CONCLUSION
Thrombus aspiration, an integral component of mechanical reperfusion of acute MI, allowed us to describe in detail the composition of the occlusive thrombus and its changes over time. Our study shows that the platelet and fibrin contents of the occlusive thrombi of acute STEMI evolve rapidly. Even within a population of patients treated with aggressive antithrombotic regimens, ischemic time is a key determinant of thrombus composition.
ACKNOWLEDGEMENTS
We thank Sophie Galier and Ghalia Anzaha for their expert technical assistance.
Funding: This study was supported by grants from the “Fédération Française de Cardiologie”, the “Institut de l’Athérothrombose” and NIH HL30954, HL090774, and T32 HL07439.
ABBREVIATIONS
- ACS
Acute Coronary Syndrome
- cTFC
corrected TIMI Frame Count
- IQR
InterQuartile Range
- LMWH
Low Molecular Weight Heparin
- MACCE
Major Cardiovascular and Cerebrovascular Events
- PCI
Percutaneous Coronary Intervention
- STEMI
ST Segment Elevation Myocardial Infarction
- TIMI
Timing in Myocardial Infarction
- TIMI MBG
TIMI Myocardial Blush Grade (MBG)
- UFH
Unfractionned Heparin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
J. Silvain – Research grants: Daiichi-Sankyo, Eli Lilly, Sanofi-Aventis, INSERM, Federation Francaise de Cardiologie, Societe Francaise de Cardiologie
Consultant fees: Daiichi-Sankyo, Eli Lilly
Lecture Fees: AstraZeneca, Daiichi-Sankyo, Eli Lilly
G. Cayla – Reearch grants: Fédération Française de Cardiologie
Consulting fees: Eli Lilly, Daiichi-Sankyo, Servier, Abbott, CLS Behring, AstraZeneca
F. Beygui – Lecture fees: Roche, Sanofi-Aventis, Pfizer, Astellas
**See RWI forms for Montalescot and Collet
REFERENCES
- 1.DeWood MA, Spores J, Notske R, et al. Prevalence of total coronary occlusion during the early hours of transmural myocardial infarction. N Engl J Med. 1980;303:897–902. doi: 10.1056/NEJM198010163031601. [DOI] [PubMed] [Google Scholar]
- 2.Falk E. Coronary thrombosis: pathogenesis and clinical manifestations. Am J Cardiol. 1991;68:28B–35B. doi: 10.1016/0002-9149(91)90382-u. [DOI] [PubMed] [Google Scholar]
- 3.Maseri A, Chierchia S, Davies G. Pathophysiology of coronary occlusion in acute infarction. Circulation. 1986;73:233–9. doi: 10.1161/01.cir.73.2.233. [DOI] [PubMed] [Google Scholar]
- 4.Mruk JS, Zoldhelyi P, Webster MW, et al. Does antithrombotic therapy influence residual thrombus after thrombolysis of platelet-rich thrombus? Effects of recombinant hirudin, heparin, or aspirin. Circulation. 1996;93:792–9. doi: 10.1161/01.cir.93.4.792. [DOI] [PubMed] [Google Scholar]
- 5.Beygui F, Collet JP, Nagaswami C, Weisel JW, Montalescot G. Images in cardiovascular medicine. Architecture of intracoronary thrombi in ST-elevation acute myocardial infarction: time makes the difference. Circulation. 2006;113:e21–3. doi: 10.1161/CIRCULATIONAHA.105.551705. [DOI] [PubMed] [Google Scholar]
- 6.Vlaar PJ, Svilaas T, van der Horst IC, et al. Cardiac death and reinfarction after 1 year in the Thrombus Aspiration during Percutaneous coronary intervention in Acute myocardial infarction Study (TAPAS): a 1-year follow-up study. Lancet. 2008;371:1915–20. doi: 10.1016/S0140-6736(08)60833-8. [DOI] [PubMed] [Google Scholar]
- 7.Burzotta F, De Vita M, Gu YL, et al. Clinical impact of thrombectomy in acute ST-elevation myocardial infarction: an individual patient-data pooled analysis of 11 trials. Eur Heart J. 2009;30:2193–203. doi: 10.1093/eurheartj/ehp348. [DOI] [PubMed] [Google Scholar]
- 8.De Luca G, Dudek D, Sardella G, Marino P, Chevalier B, Zijlstra F. Adjunctive manual thrombectomy improves myocardial perfusion and mortality in patients undergoing primary percutaneous coronary intervention for ST-elevation myocardial infarction: a meta-analysis of randomized trials. Eur Heart J. 2008;29:3002–10. doi: 10.1093/eurheartj/ehn389. [DOI] [PubMed] [Google Scholar]
- 9.Bavry AA, Kumbhani DJ, Bhatt DL. Role of adjunctive thrombectomy and embolic protection devices in acute myocardial infarction: a comprehensive meta-analysis of randomized trials. Eur Heart J. 2008;29:2989–3001. doi: 10.1093/eurheartj/ehn421. [DOI] [PubMed] [Google Scholar]
- 10.Weisel JW, Nagaswami C. Computer modeling of fibrin polymerization kinetics correlated with electron microscope and turbidity observations: clot structure and assembly are kinetically controlled. Biophys J. 1992;63:111–28. doi: 10.1016/S0006-3495(92)81594-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braet F, De Zanger R, Wisse E. Drying cells for SEM, AFM and TEM by hexamethyldisilazane: a study on hepatic endothelial cells. J Microsc. 1997;186:84–7. doi: 10.1046/j.1365-2818.1997.1940755.x. [DOI] [PubMed] [Google Scholar]
- 12.Collins TJ. ImageJ for microscopy. Biotechniques. 2007;43:25–30. doi: 10.2144/000112517. [DOI] [PubMed] [Google Scholar]
- 13.Abela GS, Aziz K. Cholesterol crystals rupture biological membranes and human plaques during acute cardiovascular events--a novel insight into plaque rupture by scanning electron microscopy. Scanning. 2006;28:1–10. doi: 10.1002/sca.4950280101. [DOI] [PubMed] [Google Scholar]
- 14.Abela GS, Aziz K, Vedre A, Pathak DR, Talbott JD, Dejong J. Effect of cholesterol crystals on plaques and intima in arteries of patients with acute coronary and cerebrovascular syndromes. Am J Cardiol. 2009;103:959–68. doi: 10.1016/j.amjcard.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 15.Collet JP, Allali Y, Lesty C, et al. Altered fibrin architecture is associated with hypofibrinolysis and premature coronary atherothrombosis. Arterioscler Thromb Vasc Biol. 2006;26:2567–73. doi: 10.1161/01.ATV.0000241589.52950.4c. [DOI] [PubMed] [Google Scholar]
- 16.van 't Hof AW, Liem A, Suryapranata H, Hoorntje JC, de Boer MJ, Zijlstra F. Angiographic assessment of myocardial reperfusion in patients treated with primary angioplasty for acute myocardial infarction: myocardial blush grade. Zwolle Myocardial Infarction Study Group. Circulation. 1998;97:2302–6. doi: 10.1161/01.cir.97.23.2302. [DOI] [PubMed] [Google Scholar]
- 17.Shand RA, Butler KD, Davies JA, Menys VC, Wallis RB. The kinetics of platelet and fibrin deposition on to damaged rabbit carotid arteries in vivo: involvement of platelets in the initial deposition of fibrin. Thromb Res. 1987;45:505–15. doi: 10.1016/0049-3848(87)90313-6. [DOI] [PubMed] [Google Scholar]
- 18.Balasubramanian V, Grabowski E, Bini A, Nemerson Y. Platelets, circulating tissue factor, and fibrin colocalize in ex vivo thrombi: real-time fluorescence images of thrombus formation and propagation under defined flow conditions. Blood. 2002;100:2787–92. doi: 10.1182/blood-2002-03-0902. [DOI] [PubMed] [Google Scholar]
- 19.Sim D, Flaumenhaft R, Furie B. Interactions of platelets, blood-borne tissue factor, and fibrin during arteriolar thrombus formation in vivo. Microcirculation. 2005;12:301–11. doi: 10.1080/10739680590925682. [DOI] [PubMed] [Google Scholar]
- 20.Kramer MC, van der Wal AC, Koch KT, et al. Presence of older thrombus is an independent predictor of long-term mortality in patients with ST-elevation myocardial infarction treated with thrombus aspiration during primary percutaneous coronary intervention. Circulation. 2008;118:1810–6. doi: 10.1161/CIRCULATIONAHA.108.780734. [DOI] [PubMed] [Google Scholar]
- 21.Boersma E. Does time matter? A pooled analysis of randomized clinical trials comparing primary percutaneous coronary intervention and in-hospital fibrinolysis in acute myocardial infarction patients. Eur Heart J. 2006;27:779–88. doi: 10.1093/eurheartj/ehi810. [DOI] [PubMed] [Google Scholar]
- 22.Steg PG, Bonnefoy E, Chabaud S, et al. Impact of time to treatment on mortality after prehospital fibrinolysis or primary angioplasty: data from the CAPTIM randomized clinical trial. Circulation. 2003;108:2851–6. doi: 10.1161/01.CIR.0000103122.10021.F2. [DOI] [PubMed] [Google Scholar]
- 23.Ariens RA, Lai TS, Weisel JW, Greenberg CS, Grant PJ. Role of factor XIII in fibrin clot formation and effects of genetic polymorphisms. Blood. 2002;100:743–54. doi: 10.1182/blood.v100.3.743. [DOI] [PubMed] [Google Scholar]
- 24.Montalescot G, Antoniucci D, Kastrati A, et al. Abciximab in primary coronary stenting of ST-elevation myocardial infarction: a European meta-analysis on individual patients’ data with long-term follow-up. Eur Heart J. 2007;28:443–9. doi: 10.1093/eurheartj/ehl472. [DOI] [PubMed] [Google Scholar]
- 25.De Luca G, Suryapranata H, Ottervanger JP, Antman EM. Time delay to treatment and mortality in primary angioplasty for acute myocardial infarction: every minute of delay counts. Circulation. 2004;109:1223–5. doi: 10.1161/01.CIR.0000121424.76486.20. [DOI] [PubMed] [Google Scholar]
- 26.Montalescot G, Borentain M, Payot L, Collet JP, Thomas D. Early vs late administration of glycoprotein IIb/IIIa inhibitors in primary percutaneous coronary intervention of acute ST-segment elevation myocardial infarction: a meta-analysis. Jama. 2004;292:362–6. doi: 10.1001/jama.292.3.362. [DOI] [PubMed] [Google Scholar]
- 27.Montalescot G. Mechanical reperfusion: treat well, treat on time too. Lancet. 2008;372:509–10. doi: 10.1016/S0140-6736(08)61211-8. [DOI] [PubMed] [Google Scholar]
- 28.Herrmann HC, Lu J, Brodie BR, et al. Benefit of facilitated percutaneous coronary intervention in high-risk ST-segment elevation myocardial infarction patients presenting to nonpercutaneous coronary intervention hospitals. JACC Cardiovasc Interv. 2009;2:917–24. doi: 10.1016/j.jcin.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 29.Van't Hof AW, Ten Berg J, Heestermans T, et al. Prehospital initiation of tirofiban in patients with ST-elevation myocardial infarction undergoing primary angioplasty (On-TIME 2): a multicentre, double-blind, randomised controlled trial. Lancet. 2008;372:537–46. doi: 10.1016/S0140-6736(08)61235-0. [DOI] [PubMed] [Google Scholar]
- 30.Ellis SG, Tendera M, de Belder MA, et al. Facilitated PCI in patients with ST-elevation myocardial infarction. N Engl J Med. 2008;358:2205–17. doi: 10.1056/NEJMoa0706816. [DOI] [PubMed] [Google Scholar]
- 31.Mehilli J, Kastrati A, Schulz S, et al. Abciximab in patients with acute ST-segment-elevation myocardial infarction undergoing primary percutaneous coronary intervention after clopidogrel loading: a randomized double-blind trial. Circulation. 2009;119:1933–40. doi: 10.1161/CIRCULATIONAHA.108.818617. [DOI] [PubMed] [Google Scholar]
- 32.Gersh KC, Nagaswami C, Weisel JW. Fibrin network structure and clot mechanical properties are altered by incorporation of erythrocytes. Thromb Haemost. 2009;102:1169–75. doi: 10.1160/TH09-03-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Collet JP, Shuman H, Ledger RE, Lee S, Weisel JW. The elasticity of an individual fibrin fiber in a clot. Proc Natl Acad Sci U S A. 2005;102:9133–7. doi: 10.1073/pnas.0504120102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown AE, Litvinov RI, Discher DE, Purohit PK, Weisel JW. Multiscale mechanics of fibrin polymer: gel stretching with protein unfolding and loss of water. Science. 2009;325:741–4. doi: 10.1126/science.1172484. [DOI] [PMC free article] [PubMed] [Google Scholar]