Abstract
Objective
Customisation of birthweight-for-gestational-age standards for maternal characteristics assumes that variation in birth weight as a result of those characteristics is physiological, rather than pathological. Maternal height and parity are among the characteristics widely assumed to be physiological. Our objective was to test that assumption by using an association with perinatal mortality as evidence of a pathological effect.
Design
Population-based cohort study.
Setting
Sweden.
Population
A total of 952 630 singletons born at ≥28 weeks of gestation in the period 1992–2001.
Methods
We compared perinatal mortality among mothers of short stature (<160 cm) versus those of normal height (≥160 cm), and primiparous versus multiparous mothers, using an internal reference of estimated fetal weight for gestational age. The total effects of maternal height and parity were estimated, as well as the effects of height and parity independent of birthweight (controlled direct effects). All analyses were based on fetuses at risk, using marginal structural Cox models for the estimation of total and controlled direct effects.
Main outcome measures
Perinatal mortality, stillbirth, and early neonatal mortality.
Results
The estimated total effect (HR; 95% CI) of short stature on perinatal death among short mothers was 1.2 (95% CI 1.1–1.3) compared with women of normal height; the effect of short stature independent of birthweight (controlled direct effect) was 0.8 (95% CI 0.6–1.0) among small-for-gestational-age (SGA) births, but 1.1 (95% CI 1.0–1.3) among non-SGA births. Similar results were observed for primiparous mothers.
Conclusions
The effect of maternal short stature or primiparity on perinatal mortality is partly mediated through SGA birth. Thus, birthweight differences resulting from these maternal characteristics appear not only to be physiological, but also to have an important pathological component.
Keywords: Birthweight, directed acyclic graph, effect decomposition, gestational age, maternal height, parity perinatal mortality
Introduction
Small-for-gestational-age (SGA) birth is an indicator of suboptimal fetal growth, and is strongly associated with perinatal mortality and morbidity.1,2 Traditionally, sex-specific birthweight-for-gestational-age percentiles have been calculated based on an appropriate population reference, with SGA usually defined as a birth weight lower than the tenth percentile. Customised birth weight standards, first introduced by Gardosi et al.,3,4 are sex-specific birthweight-for-gestational-age standards that aim to account for variations in birth weight resulting from maternal characteristics, and have become increasingly popular.5–7 They have been recommended for clinical use by the practice guidelines of the British Royal College of Obstetricians and Gynaecologists.8
Maternal characteristics currently used in customisation include parity, height, pre-pregnancy weight (or body mass index, BMI), age, education, marital status, and ethnicity. Customisation assumes that the variations in birth weight resulting from differences in those characteristics are ‘physiological’, i.e. are not associated with adverse pathological consequences. This assumption is the basis for the claim that customisation better differentiates ‘small-but-healthy’ from growth-restricted infants, but has never been validated. In the past, smoking was even included as a maternal characteristic in customisation,4 but was later rejected as obviously pathological.9,10
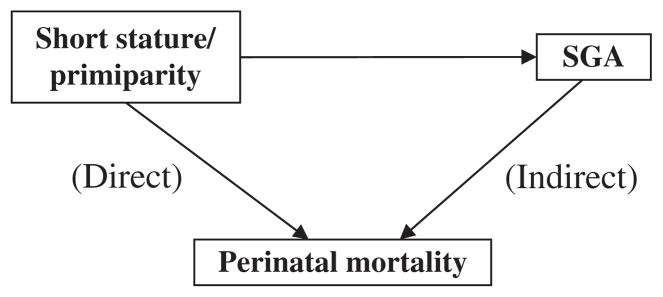
Infants born to short or primiparous mothers are known to be at higher risk of SGA than infants born to mothers of normal height or to multiparous mothers,11–14 and SGA is associated with an increased risk of fetal and neonatal death.15–17 It is also well documented that infants born to short or primiparous mothers are at slightly increased risk of perinatal death overall.18,19 These associations can be illustrated by a schematic directed acyclic graph (DAG) (see Fig. 1).20 If the small infant size is physiological (i.e. ‘normal’ or ‘expected’), SGA should not lie on the causal pathway to perinatal mortality, i.e. it should not be a causal intermediate. If, however, small size has a pathological component, then it should lie on the causal pathway, and adjustment for birth size should attenuate the association between maternal short stature or primiparity and perinatal mortality. In this study, we investigated whether the SGA associated with short maternal stature and primiparity should be regarded as solely physiological, or whether the association also has an important pathological component.21,22 We do so by estimating the effects of maternal short stature or primiparity on perinatal mortality, and the effects that are independent of birth weight for gestational age.
Figure 1.
Effect decomposition: direct and indirect effects.
Methods
Study sample
Our study is based on data from the Swedish Medical Birth Register. The data include information for all live births delivered at 22 completed weeks of gestation, or later, and all stillbirths occurring at 28 completed weeks of gestation, or later. The accuracy of gestational age (GA), birthweight, and stillbirth recorded in the Register has been previously validated.23 Gestational age was usually based on a second-trimester ultrasound; otherwise, information on the last menstrual period was used. In Sweden, all women since 1990 have been offered an ultrasonic scan performed no later than 18 completed weeks of gestation, and 95% of women accept this offer.23,24 The register contains information on demographic characteristics, reproductive history, and events and conditions occurring during delivery and the neonatal period; in particular, information on self-reported maternal height and pre-pregnancy weight, which is not available in most other countries, is included. A total of 989 203 births were recorded in the register between 1992 and 2001, of whom 959 446 were singletons. We excluded 1503 births with unknown GA, 3333 with unknown birthweight, and 28 with unknown sex. We further excluded 1952 live births with GAs of <28 weeks to ensure a comparable risk set for analysing fetal and early neonatal mortality. The study sample thus comprised 952 630 singleton births of ≥28 weeks of gestation from 1992 to 2001. Further details on the Swedish Medical Birth Register and the study sample have been described elsewhere.5,25,26
Classification of SGA
A population-based fetal weight standard was calculated based on the intrauterine (ultrasound) estimated fetal weight-for-gestational-age (EFWGA) percentile equation published by Hadlock.27 It has been shown that this standard improves the prediction of risk for perinatal mortality over the conventional population-based birthweight standard to a degree comparable with that of the customised standard.25,26 Briefly, sex-specific birthweight percentiles for 280 days of gestation were first calculated based on the study sample, and the sex-specific EFWGA z-scores and percentiles were then extrapolated by Hadlock’s proportionality formula.4 Further details have been described in our previous studies.25,26 Births were classified as SGA if the birthweight was less than the tenth percentile of the sex-specific EFWGA.
Statistical analysis
The primary outcomes in this study were perinatal mortality at ≥28 weeks of gestation (including stillbirth and early neonatal death) and preterm birth (<37 weeks of gestation). Maternal height was dichotomised as short (<160 cm) versus normal (≥160 cm), and parity as primiparous versus multiparous. We compared rates of SGA, large for gestational age (LGA; larger than the ninetieth percentile of the sex-specific EFWGA), stillbirth, and early neonatal death, as well as other demographic and clinical characteristics, by maternal height and by parity. We also compared the GA-specific birth rates and preterm birth rates between short mothers and those of normal stature, and between primiparous and multiparous mothers. As the classification of SGA births was based on the estimated fetal weight, our analysis used fetuses at risk as the denominator.28
Research questions regarding mediation of effects are common in epidemiologic research, and many techniques have been developed to address such questions. This procedure is typically referred to as effect decomposition, and involves teasing apart the direct and indirect effects of an exposure of interest. As shown in Fig. 1, we hypothesise that the total effect of maternal height or parity on perinatal mortality consists of a direct effect and an indirect effect (i.e. an effect mediated by SGA). However, estimates of direct and indirect effects from standard regression methods with adjustment for intermediate variables can be biased in the presence of an interaction between SGA and maternal short stature or primiparity.29,30 Marginal structural models, using inverse probability weighting for the exposure and intermediate variables, enables appropriate adjustment for confounding factors, and provides valid estimates of the controlled direct effects for both SGA and non-SGA infants.29–32 We tested interactions between maternal short stature or primiparity and SGA, and estimated total effects and effects independent of birthweight (controlled direct effects) for both SGA and non-SGA births using marginal structural Cox models. Weights were obtained by estimating two sets of weights, one for short stature or primiparity and one for SGA, by logistic regression. Weight models included infant sex, maternal age, maternal BMI, mother’s country of birth [Nordic (i.e. Denmark, Finland, Iceland, Norway and Sweden) versus non-Nordic], smoking, and maternal education (school years completed). Controlled direct effects were calculated using the methods described by Vander Weele, with confidence intervals obtained using robust standard errors.32
We also estimated controlled direct effects of maternal height and parity on perinatal mortality, mediated by continuous sex-specific EFWGA z-scores in place of dichotomous SGA, using marginal structural Cox models including a quadratic term for z-scores. Model weights for z-scores were obtained by linear regression and were truncated at the 99th percentile.33 We further estimated the controlled direct effect of maternal height as a continuous variable. All analyses were carried out using SAS v9.1 (SAS Institute Inc., Cary, NC, USA).
Results
Descriptive infant and maternal characteristics of our study population, stratified by maternal height and parity, are presented in Table 1. The mean birthweight of infants born to mothers of short stature was 220 g lower, and the mean GA was 2 days shorter, and these infants were more likely to be SGA (17.2 versus 9.3%) and preterm (6.1 versus 4.5%) than infants born to mothers of normal stature. Rates of stillbirth and early neonatal mortality were also higher among infants born to mothers of short stature compared with infants born to mothers of normal stature (3.7/1000 versus 3.0/1000 and 1.2/1000 versus 1.0/1000, respectively). Similarly, the mean birthweight of infants born to primiparous mothers was 172 g lower, and they were more likely to be SGA (14.1 versus 7.7%) and preterm (6.1 versus 4.0%) than those born to multiparous mothers. The stillbirth rate was also higher among infants born to primiparous than multiparous mothers (3.8/1000 versus 2.9/1000), but the early neonatal mortality rate was similar (1.1/1000).
Table 1.
Infant and maternal characteristics (% or mean ± SD) by maternal height and parity
| Maternal height |
Parity |
|||
|---|---|---|---|---|
| <160 cm (n = 111 133) | ≥160 cm (n = 753 746) | Primiparous (n = 400 505) | Multiparous (n = 552 099) | |
| Female sex (%) | 48.8 | 48.6 | 48.6 | 48.7 |
| Maternal height < 160 cm (%) | 12.3 | 13.2 | ||
| Primiparity (%) | 40.1 | 42.1 | ||
| Maternal age > 35 years (%) | 10.7 | 10.3 | 5.2 | 14.3 |
| Maternal BMI ≥ 25 (%) | 35.2 | 30.0 | 26.5 | 33.8 |
| Nordic ethnicity (%) | 65.7 | 90.0 | 87.2 | 85.6 |
| Smoking (%) | 16.9 | 16.2 | 15.4 | 17.1 |
| Maternal education completed (%) | ||||
| 0–9 years | 19.5 | 10.1 | 9.7 | 12.9 |
| 10–12 years | 54.3 | 53.8 | 52.7 | 54.0 |
| 13–14 years | 13.9 | 18.0 | 17.8 | 17.2 |
| 15 years or more | 12.4 | 18.1 | 19.8 | 15.9 |
| Birthweight (mean ± SD), g | 3373 ± 537 | 3593 ± 550 | 3460 ± 552 | 3632 ± 548 |
| SGA (%) | 17.2 | 9.3 | 14.1 | 7.7 |
| LGA (%) | 5.7 | 11.1 | 6.6 | 13.1 |
| Gestational age (mean ± SD), days | 277 ± 13 | 279 ± 12 | 279 ± 13 | 279 ± 11 |
| Preterm birth (%) | 6.1 | 4.5 | 6.1 | 4.0 |
| Stillbirth (per 1000) | 3.7 | 3.0 | 3.8 | 2.9 |
| Early neonatal death* (per 1000) | 1.2 | 1.0 | 1.1 | 1.1 |
Based on live births only.
Table 2 shows the estimated total and controlled direct effects of maternal short stature and primiparity on stillbirth, early neonatal death, perinatal mortality, and preterm birth. The total effects of maternal short stature indicate that infants born to mothers of short stature were at increased risk of perinatal death, including stillbirth and early neonatal death, and preterm birth. Primiparity was also associated (total effects) with an increased risk of all adverse birth outcomes under study. However, in analyses of both non-SGA and especially SGA infants, the controlled direct effects of maternal short stature and primiparity were attenuated. Among SGA infants, no controlled direct effects of short stature or primiparity were observed for any outcome. In contrast, among non-SGA births, a controlled direct effect of short maternal stature remained for perinatal mortality (HR 1.1; 95% CI 1.0–1.3) and preterm birth (HR 1.3; 95% CI 1.3–1.4). Among non-SGA births, primiparity had a controlled direct effect on perinatal mortality (HR 1.2; 95% CI 1.1–1.3), stillbirth (HR 1.2; 95% CI 1.1–1.3), and preterm birth (HR = 1.6; 95% CI 1.6–1.6) (Table 2). The differences in controlled direct effects on perinatal mortality and preterm birth between SGA and non-SGA births shown in Table 2 reflect significant interactions between both maternal short stature and primiparity and SGA (all P < 0.001).
Table 2.
Hazard ratios (95% CI) for stillbirth, early neonatal death, perinatal mortality, and preterm birth, estimated from marginal structural Cox models*
| Total effect | Controlled direct effect |
||
|---|---|---|---|
| SGA | Non-SGA | ||
| Maternal height <160 cm | |||
| Perinatal mortality | 1.2 (1.1–1.3) | 0.8 (0.6–1.0) | 1.1 (1.0–1.3) |
| Stillbirth | 1.2 (1.1–1.4) | 0.8 (0.6–1.1) | 1.1 (0.9–1.3) |
| Early neonatal death | 1.3 (1.0–1.5) | 0.8 (0.5–1.2) | 1.2 (0.9–1.6) |
| Preterm birth | 1.3 (1.3–1.4) | 0.9 (0.9–1.0) | 1.3 (1.3–1.4) |
| Primiparity | |||
| Perinatal mortality | 1.3 (1.3–1.4) | 0.9 (0.8–1.1) | 1.2 (1.1–1.3) |
| Stillbirth | 1.4 (1.3–1.5) | 1.0 (0.8–1.2) | 1.2 (1.1–1.3) |
| Early neonatal death | 1.1 (1.0–1.3) | 0.7 (0.5–1.0) | 1.0 (0.9–1.2) |
| Preterm birth | 1.6 (1.6–1.7) | 1.1 (1.1–1.2) | 1.6 (1.6–1.6) |
Inverse probability weights calculated based on the infant and maternal characteristics presented in Table 1, including infant sex, maternal age, maternal BMI, Nordic ethnicity, smoking, and maternal education.
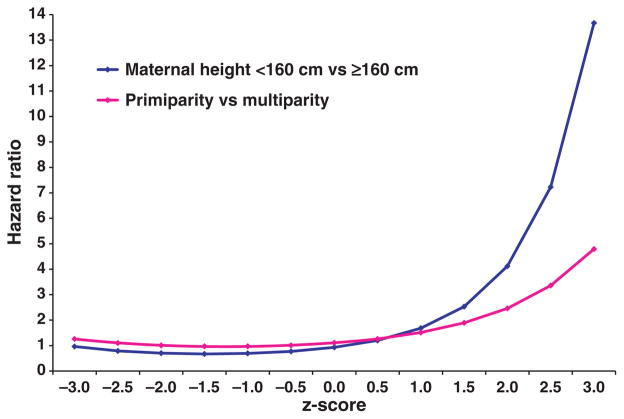
The results from our marginal structural Cox models with continuous z-scores were similar to those based on SGA. The controlled direct effects are plotted in Fig. 2 as functions of the z-scores. Significant interactions with z-scores were observed for both maternal short stature and primiparity. At lower z-scores (≤0), the controlled direct effect of short stature or primiparity (Fig. 2) on perinatal mortality was attenuated (below or close to 1). At large z-scores (≥2), however, we observed stronger controlled direct effects of both short stature and primiparity on perinatal mortality. The results from our marginal structural Cox models with both maternal height and continuous z-scores as continuous variables were also similar (data available upon request).
Figure 2.
Controlled direct effects: hazard ratio for perinatal mortality as a function of z-scores.
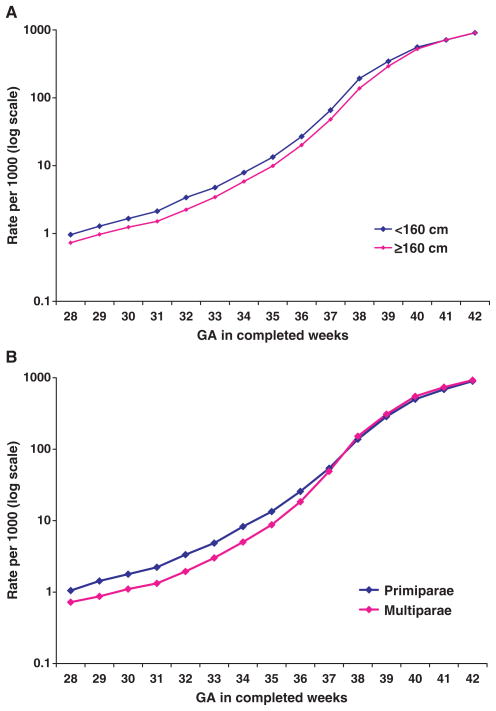
Gestational age-specific birth rates by maternal height and by parity are compared in Fig. 3. The birth rate among short mothers was higher than among mothers of normal height from 28 to 40 weeks of gestation, but not at 41 or 42 weeks of gestation (Fig. 3A). The birth rate was higher among primiparous versus multiparous mothers until 37 weeks of gestation, but then became lower after 37 weeks of gestation (Fig. 3B). The observed differences in birth rates at later gestations were quite large (>10 per 1000 among late preterm births), although the logarithmic scale of the graph visually obscures the magnitude of these differences at the high birth rates observed during the late preterm period. The interaction between SGA and maternal height or parity on preterm birth was also significant. Among the SGA births, preterm birth rates (9.7 and 10.0%, respectively) were similar between mothers of short stature and of normal height. However, among non-SGA births, preterm birth rates were quite different. Specifically, among non-SGA infants, those born to mothers of short stature were more likely to be preterm than those born to mothers of normal stature (5.4 versus 4.0%). Similar results were observed among infants born to primiparous and multiparous mothers: among SGA birth, preterm birth rates were 10.4 and 10.0%, respectively, whereas the rates were 5.3 and 3.6%, respectively, among non-SGA births.
Figure 3.
Gestational age-specific birth rate by maternal height and by parity.
Discussion
We observed significant effects of maternal short stature and primiparity on perinatal mortality independent of birthweight among non-SGA births, but not among SGA births. Thus, the reduced birthweight robustly observed in short or primiparous mothers cannot be considered entirely physiological, i.e. the reduction appears to have an important pathological component. We also observed a significant interaction between SGA and maternal height or parity. Specifically, among non-SGA births, preterm birth rates were substantially higher among infants born to short or primiparous mothers than among those born to mothers of normal height or to multiparous mothers. Among SGA births, however, preterm birth rates were similar among infants born to mothers of short and normal stature, and among those born to primiparous and multiparous mothers. These results help clarify our understanding of the effects of both physiological and pathological factors of fetal growth, and have implications for the interpretation of customised birthweight standards.
The associations we observed are consistent with past research showing an increased risk of perinatal mortality among primiparous or short women.18,19 To our knowledge, however, the effects of maternal height and parity on perinatal mortality independent of birthweight have received less attention. Our results suggest that the reduced birthweight among infants born to short or primiparous women has an important pathological component. Non-SGA fetuses, and especially macrosomic fetuses, are probably more susceptible to pathological impact, perhaps because of a reduced uterine capacity and blood supply, and the consequent reduction in the delivery of oxygen and nutrients to the developing fetus, which may both restrict fetal growth and increase the risk of stillbirth. Our results are similar whether birthweight is considered as a dichotomous variable (SGA versus non-SGA) or as a continuous z-score. The overall higher birth rate at preterm gestations among short or primiparous mothers (Fig. 3) that we have observed has been reported previously.18,34 The smaller uterine size associated with short stature or primiparity may lead to membrane stretching, cervical shortening, or other biomechanical factors that increase the likelihood of preterm delivery. The attenuated effect of both maternal short stature and primiparity on birth rate and risk of preterm birth among women with SGA fetuses, however, suggests that these underlying pathophysiologic mechanisms may not apply when the fetus is small.
Fetal growth is influenced both by physiological and pathological factors. In attempting to define an ‘abnormal’ birth weight for GA, these two types of influences should be distinguished. Proponents of birthweight standards customised according to maternal characteristics assume that the influence of those maternal characteristics on fetal growth is purely physiological, i.e. that variations caused by these characteristics are free of adverse consequences. If the effect of maternal height on birthweight is purely physiological, i.e. if smallness because of short stature is ‘normal’, SGA should not lie on the causal pathway to perinatal death, as depicted in Fig. 1. Recent evidence indicates that the superiority of customised over conventional standards of birthweight for gestational age in predicting perinatal mortality derives from using the estimated fetal weight (rather than the actual weight at birth) at preterm gestations, not from customising by maternal characteristics.25,26 At preterm gestations, the observed birth weights are low relative to the weights of unborn fetuses, and thus constitute a biased sample of fetal weights.26,35,36
Three types of standards should be distinguished: (1) a customised standard; (2) a population standard based on birth weights (conventional); and (3) a non-customised population standard based on a best estimate of intrauterine weights.26 Hadlock’s growth curve, based on a modest number of white fetuses, may not fit well with other ethnic populations. A large-scale US National Institutes of Health study of ultrasound scans of fetuses from a variety of ethnic origins is currently underway, and should provide additional data on normal fetal growth patterns.
A strength of our analysis is the use of fetuses at risk as the denominator (rather than live births).37 This is the methodologically appropriate denominator for immediate birth outcomes such as perinatal mortality.38 The use of live births as the denominator at preterm GAs is biased, because birth weights at preterm gestations are suboptimal compared with unborn fetuses at the same GA. Our analysis included both term and preterm infants; excluding pre-term births would have diluted the pathological impact of maternal height or parity on the birth outcomes investigated in this study.
Furthermore, when an interaction is present (here, between maternal height or parity and SGA), standard methods for estimating direct effects are inappropriate. Marginal structural models specifically address these issues in effect decomposition, and account for the observed interaction. Our results show a complex relationship in which birthweight acts as both a mediator (intermediate factor) and an effect modifier (interaction). Birthweight is considered a mediator, as birthweight lies on the causal pathway between parity or short stature and perinatal mortality. However, the mediating effect of birthweight also varies with birthweight, and thus birth weight is also an effect modifier.
Several limitations of our study merit further discussion. Although we were able to account for many factors known to affect fetal growth, such as maternal smoking, BMI, and sociodemographic factors, we were unable to account for maternal pregnancy complications (e.g. pre-eclampsia). Residual confounding by socio-economic status is also possible, as we were only able to control for maternal education. However, if only one social variable can be chosen, education is often recommended,39 as maternal education is an important determinant of many pregnancy outcomes.40 Although appropriate methods for effect decomposition were used in our analysis, the observed interaction between birthweight and maternal height or parity prevented an estimation of indirect effects. When an interaction is present, controlled direct effects can be estimated, but the total effect cannot be decomposed into controlled direct and indirect effects. In addition, the methods do not allow us to estimate conditional hazard ratios, as are conventionally presented; only marginal effects can be estimated.
Conclusion
We conclude that factors known to reduce fetal growth, such as maternal short stature or primiparity, have both physiological and pathological effects on perinatal mortality and other adverse birth outcomes. By ignoring pathological variation in birth weight for gestational age caused by maternal characteristics, customising birth weight standards can lead to incorrect causal inferences.
Acknowledgments
Funding
Supported by a grant from the Canadian Institutes of Health Research. EFS and SLM are supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health.
Footnotes
Disclosure of interests
We have no conflicts of interest concerning the topic or contents of this article.
Contribution to authorship
XZ wrote the study protocol, performed the analyses (with SLM), and wrote the first draft of the manuscript. SLM, EFS, SC, and MSK all contributed to the study design, interpretation of results, and manuscript revisions.
Details of ethics approval
Not applicable.
References
- 1.World Health Organization. Physical status: the use and interpretation of anthropometry. Geneva. World Health Organ Tech Rep Ser. 1995;854:121–60. [PubMed] [Google Scholar]
- 2.Hay WW, Catz CS, Grave GD, Yaffe SJ. Workshop summary: fetal growth: its regulation and disorders. Pediatrics. 1997;99:585–91. doi: 10.1542/peds.99.4.585. [DOI] [PubMed] [Google Scholar]
- 3.Gardosi J, Chang A, Kalyan B, Sahota D, Symonds E. Customised antenatal growth charts. Lancet. 1992;339:283–7. doi: 10.1016/0140-6736(92)91342-6. [DOI] [PubMed] [Google Scholar]
- 4.Gardosi J, Mongelli M, Wilcox M, Chang A. An adjustable fetal weight standard. Ultrasound Obstet Gynecol. 1995;6:168–74. doi: 10.1046/j.1469-0705.1995.06030168.x. [DOI] [PubMed] [Google Scholar]
- 5.Clausson B, Gardosi J, Francis A, Cnattingius S. Perinatal outcome in SGA births defined by customised versus population-based birthweight standards. Br J Obstet Gynaecol. 2001;108:830–4. doi: 10.1111/j.1471-0528.2001.00205.x. [DOI] [PubMed] [Google Scholar]
- 6.McCowan L, Harding J, Stewrad A. Customised birthweight centiles predict SGA pregnancies with perinatal mortality. Br J Obstet Gynaecol. 2005;112:1026–33. doi: 10.1111/j.1471-0528.2005.00656.x. [DOI] [PubMed] [Google Scholar]
- 7.Ego A, Subtil D, Grange G, Thiebaugeorges O, Senat M, Vayssiere C, et al. Customized versus population-based birth weight standards for identifying growth restricted infants: a French multicenter study. Am J Obstet Gynecol. 2006;194:1042–9. doi: 10.1016/j.ajog.2005.10.816. [DOI] [PubMed] [Google Scholar]
- 8.Royal College of Obstetricians and Gynaecologists. The Investigation and Management of the Small-for-Gestational-age Fetus. London: RCOG; 2002. Guideline No. 31. [Google Scholar]
- 9.Steer PJ. Br J Obstet Gynaecol. Vol. 114. 2007. Editor’s Choice; pp. i–ii. [Google Scholar]
- 10.Ego A, Subtil D, Grange G, Thiebauaugeorges O, Senat MV, Vayssiere C, et al. Should parity be included in customised fetal weight standards for identifying small-for-gestational age babies? Results from a French multicentre study. Br J Obstet Gynaecol. 2008;115:1156–64. doi: 10.1111/j.1471-0528.2008.01855.x. [DOI] [PubMed] [Google Scholar]
- 11.Kramer MS. Determinants of low birth weight: methodological assessment and meta-analysis. Bull World Health Organ. 1987;65:663–737. [PMC free article] [PubMed] [Google Scholar]
- 12.Billewicz WZ, Thomson AM. Birthweights in consecutive pregnancies. J Obstet Gynaecol Br Common. 1973;80:491–8. doi: 10.1111/j.1471-0528.1973.tb15969.x. [DOI] [PubMed] [Google Scholar]
- 13.Wilcox MA, Chang AMZ, Johnson IR. The effects of parity on birthweight using successive pregnancies. Acta Obstet Gynecol Scand. 1996;75:459–63. doi: 10.3109/00016349609033354. [DOI] [PubMed] [Google Scholar]
- 14.Niswander K, Jackson EC. Physical characteristics of the gravida and their association with birth weight and perinatal death. Am J Obstet Gynecol. 1974;119:306–13. doi: 10.1016/0002-9378(74)90289-0. [DOI] [PubMed] [Google Scholar]
- 15.Kramer MS, Olivier M, McLean FH, Willis DM, Usher RH. Impact of intrauterine growth retardation and body proportionality on fetal and neonatal outcome. Pediatrics. 1990;85:707–13. [PubMed] [Google Scholar]
- 16.Ananth CV, Platt RW. Reexamining the effects of gestational age, fetal growth, and maternal smoking on neonatal mortality. BMC Pregnancy Childbirth. 2004;4:22. doi: 10.1186/1471-2393-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joseph KS, Fahey J, Platt RW, Liston RM, Lee SK, Sauve R, et al. An outcome-based approach for the creation of fetal growth standards: do singletons and twins need separate standards? Am J Epidemiol. 2009;169:616–24. doi: 10.1093/aje/kwn374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joseph KS, Liu S, Demissie K, Wen SW, Platt RW, Ananth CV, et al. For the Fetal and Infant Health Study Group of the Canadian Perinatal Surveillance System. A parsimonious explanation for intersecting perinatal mortality curves: understanding the effect of plurality and of parity. BMC Pregnancy Childbirth. 2003;3:3. doi: 10.1186/1471-2393-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cnattingius S, Haglund B, Kramer MS. Differences in late fetal death rates in association with determinants of small for gestational age fetuses: population based cohort study. BMJ. 1998;316:1483–7. doi: 10.1136/bmj.316.7143.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pearl J. Causality: Models, Reasoning and Inference. Cambridge University Press; Cambridge: 2000. [Google Scholar]
- 21.Susser M. Causal Thinking in the Health Sciences: Concepts and Strategies in Epidemiology. Oxford University Press; New York: 1973. [Google Scholar]
- 22.Szklo M, Nieto FJ. Epidemiology: Beyond the Basics. Aspen Publishers; Gaithersburg, MD: 2000. [Google Scholar]
- 23.Cnattingius S, Ericson A, Gunnarskog J, Kallen B. A quality study of a medical birth registry. Scand J Soc Med. 1990;118:143–8. doi: 10.1177/140349489001800209. [DOI] [PubMed] [Google Scholar]
- 24.The Swedish Council on Technological Assessment in Health Care. Report no. 139 [in Swedish] Stockholm, Sweden: The Swedish Council on Technological Assessment in Health Care; 1998. p. 48. [Google Scholar]
- 25.Zhang X, Platt R, Cnattingius S, Joseph KS, Kramer M. The use of customized vs population-based birth weight standards in predicting perinatal mortality. Br J Obstet Gynaecol. 2007;114:474–7. doi: 10.1111/j.1471-0528.2007.01273.x. [DOI] [PubMed] [Google Scholar]
- 26.Hutcheon J, Zhang X, Cnattingius S, Kramer M, Platt R. Customised birthweight percentiles: does adjusting for maternal characteristics matter? Br J Obstet Gynaecol. 2008;115:1397–404. doi: 10.1111/j.1471-0528.2008.01870.x. [DOI] [PubMed] [Google Scholar]
- 27.Hadlock FP, Harrist RB, Martinez-Poyer J. Inutero analysis of fetal growth: a sonographic weight standard. Radiology. 1991;181:129–33. doi: 10.1148/radiology.181.1.1887021. [DOI] [PubMed] [Google Scholar]
- 28.Joseph KS, Demissie K, Platt RW, Ananth CV, McCarthy BJ, Kramer MS. A parsimonious explanation for intersecting perinatal mortality curves: understanding the effects of race and of maternal smoking. BMC Pregnancy Childbirth. 2004;4:7. doi: 10.1186/1471-2393-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robins JM, Greenland S. Identifiability and exchangeability for direct and indirect effects. Epidemiology. 1992;3:143–55. doi: 10.1097/00001648-199203000-00013. [DOI] [PubMed] [Google Scholar]
- 30.Kaufman JS, MacLehose RF, Kaufman S. A further critique of the analytic strategy of adjusting for covariates to identify biologic mediation. Epidemiol Perspect Innov. 2004;1:4. doi: 10.1186/1742-5573-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–60. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 32.Vander Weele TJ. Marginal structural modes for the estimation of direct and indirect effects. Epidemiology. 2009;20:18–26. doi: 10.1097/EDE.0b013e31818f69ce. [DOI] [PubMed] [Google Scholar]
- 33.Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168:656–64. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kramer MS, McLean FH, Eason EL, Usher RH. Maternal nutrition and spontaneous preterm birth. Am J Epidemiol. 1992;136:574–83. doi: 10.1093/oxfordjournals.aje.a116535. [DOI] [PubMed] [Google Scholar]
- 35.Secher N, Hansen P, Thomsen B, Keiding N. Growth retardation in preterm infants. Br J Obstet Gynaecol. 1987;94:115–20. doi: 10.1111/j.1471-0528.1987.tb02336.x. [DOI] [PubMed] [Google Scholar]
- 36.Cooke RWI. Conventional birth weight standards obscure fetal growth restriction in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2007;92:189–92. doi: 10.1136/adc.2005.089698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yudkin PL, Wood L, Redman CWG. Risk of unexplained still birth at different gestational ages. Lancet. 1987;1:1192–4. doi: 10.1016/s0140-6736(87)92154-4. [DOI] [PubMed] [Google Scholar]
- 38.Joseph KS. Incidence-based measures of birth, growth restriction and death can free perinatal epidemiology from erroneous concepts of risk. J Clin Epidemiol. 2004;57:889–97. doi: 10.1016/j.jclinepi.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 39.Kunst AE, Bos V, Mackenback JP. Guidelines in Monitoring Socio-economic Inequalities in Health in European Countries. Rotterdam: Erasmus University; 2001. [Google Scholar]
- 40.Arntzen A, Mortensen L, Schnor O, Cnattingius S, Gissler M, Andersen AMN. Neonatal and postneonatal mortality by maternal education – a population-based study of trends in the Nordic countries, 1981–2000. Eur J Public Health. 2008;18:245–51. doi: 10.1093/eurpub/ckm125. [DOI] [PubMed] [Google Scholar]