Abstract
Context: Undervirilization in males, i.e. 46,XY disordered sex development (46,XY DSD), is commonly caused by either lack of androgen action due to mutant androgen receptor (AR) or deficient androgen synthesis, e.g. due to mutations in 17α-hydroxylase (CYP17A1). Like all other microsomal cytochrome P450 (CYP) enzymes, CYP17A1 requires electron transfer from P450 oxidoreductase (POR).
Objective: The objective of the study was to analyze the clinical and biochemical phenotype in a 46,XY individual carrying concomitant POR and AR mutations and to dissect their impact on phenotypic expression.
Methods: We characterized the clinical and biochemical phenotype, genetic identification, and functional analysis of POR missense mutation by yeast micrososomal coexpression assays for CYP17A1, CYP21A2 and CYP19A1 activities.
Results: The patient presented neonatally with 46,XY DSD and was diagnosed as partial androgen insensitivity syndrome carrying a disease causing AR mutation (p.Q798E). She was raised as a girl and gonadectomized at the age of 4 yr. At 9 yr progressive clitoral enlargement prompted reassessment. Urinary steroid analysis was indicative of POR deficiency, but surprisingly androgen production was normal. Genetic analysis identified compound heterozygous POR mutations (p.601fsX12/p.Y607C). In vitro analysis confirmed p.Y607C as a pathogenic mutation with differential inhibition of steroidogenic CYP enzymes.
Conclusion: Both mutant AR and POR are likely to contribute to the neonatal presentation with 46,XY DSD. Virilization at the time of adrenarche appears to suggest an age-dependent, diminishing disruptive effect of both mutant proteins. This case further highlights the importance to assess both gonadal and adrenal function in patients with 46,XY DSD.
Concomitant mutations in the POR and AR genes result in neonatal undervirilization and androgenization at adrenarche, indicating amelioration of the disruptive effect of the mutations.
Disordered sexual development (DSD) in genetic males (46,XY DSD) can be due to a number of distinct mutations compromising different stages of sex determination and differentiation (1,2). The most common cause of 46,XY DSD is androgen insensitivity syndrome (AIS) due to inactivating mutations of the androgen receptor (AR) gene, which has an incidence of 1:20,000 life births (2). More than 300 mutations are listed in the AR database (http://androgendb.mcgill.ca) leading to different degrees of androgen resistance from azoospermia to complete androgen insensitivity syndrome. Missense and nonsense mutations in specific regions of the AR gene have distinct effects on AR function and can affect ligand binding, transactivation or N-terminal/C-terminal interaction of the molecule (3,4,5). However, the in vitro assessment of AR function may not always match the observed clinical phenotype in patients with AIS, with variable degrees of undervirilization in different individuals carrying the same distinct AR mutation (2).
Upstream of AR action, androgen synthesis may be affected and result in 46,XY DSD (6). Five enzymes and six catalytic reactions are required for the conversion of cholesterol to the most potent androgen, 5α-dihydrotestosterone. Mutations in the genes required for these conversions (CYP11A1, CYP17A1, HSD17B3, HSD3B2, and SRD5A2) represent distinct causes of 46,XY DSD, manifesting with a broad phenotypic spectrum (1,7). The identification of inactivating mutations in the P450 oxidoreductase gene (POR) (8,9) has demonstrated that sex steroid synthesis may also be disrupted by mutations in cofactor enzymes. POR transfers electrons from nicotinamide adenine dinucleotide phosphate (NADPH) to all microsomal cytochrome P450 (CYP) enzymes, including key enzymes of glucocorticoid and sex steroid synthesis, 21-hydroxylase (CYP21A2), 17α-hydroxylase/17,20-lyase (CYP17A1), and aromatase (CYP19A1). Intriguingly, affected individuals of both sexes may present with DSD. Whereas loss of 17α-hydroxylase and particularly 17,20-lyase activity in POR deficiency (ORD) readily explains the undervirilization in male newborns, it remains more mysterious why female patients present with 46,XX DSD. This may be explained by the presence of an alternative pathway to androgens, circumventing the classic androgen pathway via dehydroepiandrosterone (DHEA) (8), which has been previously identified in the tammar wallaby pouch young (10).
Here we present an individual with 46,XY DSD and concomitant, disease-causing mutations in the AR and POR genes, both fully established causes of undervirilization in their own right.
Subjects and Methods
Case reports
The patient was born at term after an uneventful pregnancy as the first child of nonconsanguineous parents of Polish origin [birth weight 2850 g (−1.3 sd score), length 52 cm (0.89 sd score), Apgar score 5/8]. The postnatal adaptation went well and no neonatal complication occurred. However, at birth, the attending pediatrician noticed ambiguous genitalia. The external genitalia looked predominantly female, but the clitoris was enlarged and a common urogenital sinus and blind ending vaginal pouch were present. The gonads were palpable within the inguinal canal. No other abnormalities or malformations were noted. The karyotype was 46, XY.
At the age of 14 d, a slightly elevated serum 17-hydroxyprogesterone (17OHP) was measured (Table 1). Circulating androgens and androgen precursors were low and testosterone showed a poor response to human chorionic gonadotropin (hCG) stimulation, whereas the gonadotrophin response to LHRH stimulation was normal (Table 1). Urinary steroid profiling by gas chromatography/mass spectrometry (GC/MS) at the age of 14 d showed undetectable androsterone, normal levels of fetal adrenal zone steroids, normal cortisol and 17OHP metabolite excretion, and no evidence of 5α-reductase deficiency.
Table 1.
Hormonal assessment in the patient at 1–2 months and at 9 yr
| 1–2 months | 9 yr | |||
|---|---|---|---|---|
| 17OHP (nmol/liter) | ||||
| At baseline | 9.38 | (1.8–7.5)a | 37.9 | (<6) |
| 60 min after ACTH 250 μg/m2 iv | — | 47.2 | ||
| Cortisol (nmol/liter) | ||||
| At baseline | — | 391 | (150–450) | |
| 60 min after ACTH 250 μg/m2 iv | — | 494 | (>550) | |
| DHEAS (μmol/liter) | 0.23 | (f: 0.04–1.32)b (m: 0.04–1.96) | 2.49 | (f: 0.23–2.37) (m: 0.42–2.13) |
| Androstenedione (nmol/liter) | ||||
| At baseline | 0.41 | (f: 0.7–1.9) (m: 1.3–4.25) | 0.73 | (f: 0.3–1.2) (m: 0.2–2.8) |
| 4 days after hCG 2000 IU/m2 | 2.15 | — | ||
| Testosterone (nmol/liter) | ||||
| At baseline | <0.20 | (f: 0.17–0.40) (m: 1.4–8.2) | <0.17 | (f: 0.17–0.30) (m: 0.15–0.65) |
| 4 days after hCG 2000 IU/m2 | 2.15 | — | ||
| ACTH (pg/ml) | — | 50.9 | (10–60) | |
| LH (U/liter) | ||||
| At baseline | 0.4 | (0.1–4) | — | |
| 60 min after LHRH 75 μg/m2 iv | 3.7 | (2–5 fold of baseline) | — | |
| FSH (U/liter) | ||||
| At baseline | 2.0 | (0.1–4) | — | |
| 60 min after LHRH 75 μg/m2 iv | 6.7 | (2–3 fold of baseline) | — | |
Bilateral gonadectomy had been carried out at the age of 4 yr. —, Not measured.
Age-specific normal reference range.
Age-specific reference ranges for androgens are listed for both girls (f) and boys (m).
The initial presentation with 46,XY DSD had prompted genetic analysis of the AR gene, which revealed the hemizygous mutation p.Q798E. Despite the finding of low circulating androgens, the diagnosis of partial AIS (PAIS) was made. The patient was assigned female gender and underwent bilateral removal of the inguinal gonads at the age of 4 yr; histopathological examination identified the removed tissue as immature testis.
Follow-up was inconsistent due to poor clinic attendance. However, at the age of 9 yr, the patient presented with progressive clitoral enlargement over the preceding 18 months. At examination, no other external signs of puberty were noticed (Tanner stages PH1, B1, A1); clitoral length was 3 cm. Except for the bilateral gonadectomy, no genital reconstruction surgery had been performed yet, largely due to parental doubts about the gender identification of her daughter (male hobbies and roles, aggressive behavior). Psychological assessment including thorough evaluation of her gender preference was offered but declined by the parents. Her growth chart showed normal linear growth along the 50th percentile, and the bone age was significantly delayed (−3 yr).
Hormonal assessment again revealed mildly elevated serum 17OHP. However, DHEA sulfate (DHEAS) levels were raised slightly above the age-specific reference ranges of both girls and boys (Table 1). Serum testosterone was below sensitivity of the used RIA (Table 1). Urinary steroid profiling with GC/MS was performed and showed a profile suggestive of combined inhibition of 21-hydroxylase and 17α-hydroxylase activities and thus indicative for ORD (for detailed analysis see Results). A short cosyntropin test revealed a normal baseline cortisol but an impaired cortisol response to ACTH stimulation (Table 1). Subsequently hydrocortisone replacement therapy for intercurrent stress, illness, and surgery was recommended, and the patient and parents were educated accordingly.
Urinary steroid metabolite analysis
Analysis of urinary steroid metabolite excretion was performed as described previously by a quantitative GC/MS selected ion-monitoring method (11). In brief, steroids were enzymatically released from conjugation and, after extraction, chemically derivatized before GC/MS selected ion-monitoring analysis. Steroids quantified included corticosterone metabolites [tetrahydrocorticosterone (THB), 5αTHB, tetrahydro-11-dehydrocorticosterone (THA), tetrahydro-deoxycorticosterone (TH-DOC)], the progesterone metabolite pregnanediol, 17-hydroxyprogesterone metabolites [pregnanetriol (PT), 17-hydroxypregnanolone (17HP)], the 17HP metabolite pregnenetriol (5-PT), the 21-desoxycortisol metabolite pregnanetriolone, cortisol metabolites [tetrahydrocortisol (THF), 5αTHF, and tetrahydrocortisone (THE)], and androgen metabolites [androsterone (An) and etiocholanolone (Et), DHEA, and 16-hydroxy-DHEA (16-OH DHEA)].
After quantification of steroid metabolites by GC/MS, we calculated the following substrate to product ratios to determine the approximate in vivo net activity of specific steroidogenic enzymes: corticosterone over cortisol metabolites (17α-hydroxylase; (THA+THB+5αTHB)/(THF+5αTHF+THE)], 17-hydroxyprogesterone over androgen metabolites [17,20-lyase; (17HP+PT)/(An+Et)], 17OHP over cortisol metabolites [21-hydroxylase (100 × pregnanetriolone/(THF+5αTHF+THE)], and the ratio of progesterone over cortisol metabolites [combined 21-hydroxylase and 17-hydroxylase activities, i.e. specific for ORD; PD/(THF+5αTHF+THE)]. These diagnostic ratios were compared with ratios obtained from urine analysis in a normal age-matched female reference cohort (n = 10).
Genetic analysis
DNA sequencing analysis was carried out with approval of the local research ethics committee after obtaining informed consent from patients and their parents. Direct sequencing of the coding region of the P450 oxidoreductase gene including 15 exons and exon-intron junctions (8) and exon 8 of the androgen receptor gene (3,12) was performed as previously described. Sequencing results were analyzed using Lasergene software (DNASTAR Inc., Madison, WI), and mutation numbering was carried out referring to the appropriate National Center for Biotechnology Information (Bethesda, MD) reference sequences [P450 oxidoreductase, NG_008930.1 (genomic; A of the ATG translation initiation codon is +1 bp) and NP_000932 (protein); the coding sequence variant of the AR was numbered according to M20132.1 (where A of the ATG translation initiation codon is +363 bp]; the protein mutation was numbered relative to AAA51729.1.
In vitro enzymatic activity assays
The cDNA of the POR missense mutant p.Y607C POR, generated by site-directed mutagenesis, was cloned into the yeast expression vector pDE2 and used for microsomal coexpression assays in comparison with wild-type (WT) POR as previously described (13). In brief, yeast microsomes coexpressing WT or mutant p.Y607C POR and WT human CYP17A1, CYP21A2, and CYP19A1, respectively, were incubated with 0.5–5 μm progesterone or 17-hydroxypregnenolone for 17α-hydroxylase and 17,20-lyase activities of CYP17A1, 0.5–5 μm progesterone for 21-hydroxylase (CYP21A2) activity, and 50–500 nm androstenedione for aromatase (CYP19A1) assays. Steroids were added to the final reaction volume of 200 μl in 4 μl ethanol also containing 10,000 cpm [H3] steroid substrate (all 55.4 Ci/mol). Purified recombinant cytochrome b5 (CYB5; Invitrogen, Paisley, UK) was added in a final concentration of 10 pm to the 17,20-lyase assays. All reactions were initiated by the addition of 200 nm NADPH and subsequently incubated at 37 C. Steroids were extracted with dichloromethane and separated by thin-layer chromatography on PE SIL G/UV silica gel plates (Whatman, Maidstone, UK) in a 3:1 chloroform to ethyl acetate solvent system (for aromatase assays 12:1 dichloromethane/acetone), and quantified by thin-layer chromatography scanner analysis (Bioscan 2000 image analyzer; Lablogic, Sheffield, UK). The data represent the results of three independent experiments carried out in triplicate and are expressed as mean ± sem.
Microsomal protein quantification was performed using the Bradford method (Bio-Rad, Hemel-Hempstead, UK), and the expression of similar amounts of protein was confirmed by Western blotting as previously described (13), using antibodies to human POR (Abcam, Cambridge, UK), human CYP17A1 (Santa Cruz Inc., Heidelberg, Germany), human CYP19A1 (Abcam), and CYP21A2 (Abcam).
Kinetic parameters were assessed by nonlinear regression, using the Michaelis-Menten equation to determine the Michaelis-Menten constant (Km) and maximal velocity (Vmax). Catalytic efficiency was defined as the ratio Vmax to Km and expressed as percentage of WT activity. Calculation of enzyme kinetic parameters and subsequent statistical analysis was performed using curve-fitting software (Enzfitter 2.0.9.1; Biosoft, Cambridge, UK).
Results
In vivo steroidogenesis as assessed by urinary steroid profiling
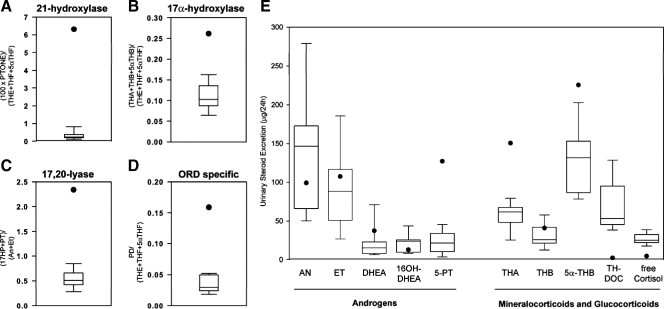
GC/MS analysis of urinary steroid metabolite excretion in our patient at the age of 9 yr revealed a pattern indicative of ORD, with diagnostic ratios demonstrating combined 21-hydroxylase, 17α-hydroxylase, and 17,20-lyase inhibition (Fig. 1, A–D). 21-Hydroxylation as the ratio of the 21-deoxycortisol metabolite pregnanetriolone over cortisol metabolites was significantly compromised compared with age-specific controls (Fig. 1A). Similarly, 17α-hydroxylation reflected by the ratio of corticosterone over cortisol metabolites was significantly impaired (Fig. 1B). 17,20-Lyase activity, as assessed by the ratio of 17OHP metabolites over active androgen metabolites was also compromised (Fig. 1C). Combined inhibition of 17α-hydroxylation and 21-hydroxylation, the hallmark biochemical finding in ORD, was reflected in our patient by a markedly increased ratio of progesterone over cortisol metabolites (Fig. 1D).
Figure 1.
In vivo steroidogenic enzyme activity in the patient at the age of 9 yr as determined by diagnostic substrate to product ratios (A–D) and total excretion (E) of 24-h urinary steroid metabolites measured by GC/MS and shown in comparison with an age-matched reference cohort (n = 10). Box plots represent interquartile ranges (25th to 75th percentile), whiskers the fifth and 95th percentile, respectively, of the normal reference cohort; the patient’s results are represented by a closed circle. For steroid abbreviations and definition of steroid substrate/product ratios, please see Subjects and Methods.
Twenty-four-hour urinary androsterone and etiocholanolone, the main metabolites of androstenedione, testosterone, and dihydrotestosterone, were within the age-specific mid-normal range (Fig. 1E). The excretion of the 17HP metabolite 5-PT and also DHEA were increased (Fig. 1E), indicative of up-regulation of adrenal androgen production. The corticosterone metabolites THB, 5αTHB, and THA were increased, and the excretion of free cortisol was decreased, reflecting 17α-hydroxylase and 21-hydroxylase inhibition, respectively.
Sequencing analysis
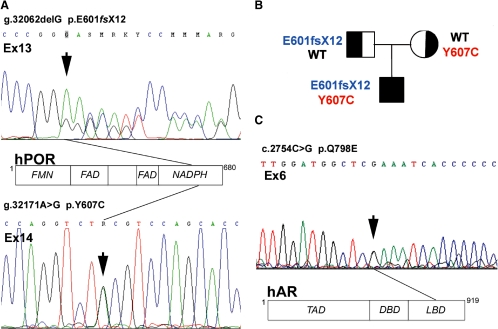
Sequencing of the coding region of the POR gene revealed compound heterozygosity for two POR mutations (Fig. 2A). We identified a deletion of guanine in exon 13 (g.32062delG) resulting in a stop codon and subsequent premature truncation of the POR protein 12 amino acids after the frameshift (p.E601fsX12). Second, we found a missense mutation in exon 14 (g.32171A>G) changing tyrosine at amino acid position 607 to cysteine (p.Y607C). Segregation analysis demonstrated that the p.Y607C mutation is located on the maternal allele, whereas the frameshift mutation is of paternal origin (Fig. 2B).
Figure 2.
Results of genetic analysis. A, Electropherogram depicting the compound heterozygous POR mutations in our patient. The deletion of the guanine in exon 13 (g.32,062delG) and the missense mutation in exon 14 (32,171 A>G) of the POR gene are marked by black arrows. The structure of the POR protein and the approximate location of the mutations are indicated in the schematic representation of the POR protein including its three functional domains, which bind the three partners of the electron transfer chain, FMN (flavin mononucleotide), FAD (flavin adenine dinucleotide), and NADPH. B, Pedigree of the index family with segregation analysis of the two identified POR mutations. C, Electropherogram depicting the missense mutation in exon 6 of the AR gene (c.2754C>G) marked with a black arrow. The translational effect is indicated in the schematic graph representing the AR protein including its functional domains TAD (transactivation domain), DBD (DNA binding domain), and LBD (ligand binding domain). hAR, Human AR.
We confirmed the presence of a missense mutation in the AR gene by direct sequencing, a glutamine to glutamic acid change (c.2754C>G), resulting in a missense mutation in position 798 within exon 6 of the AR protein (p.Q798E) (Fig 3C). This mutation was found in the patient and the mother.
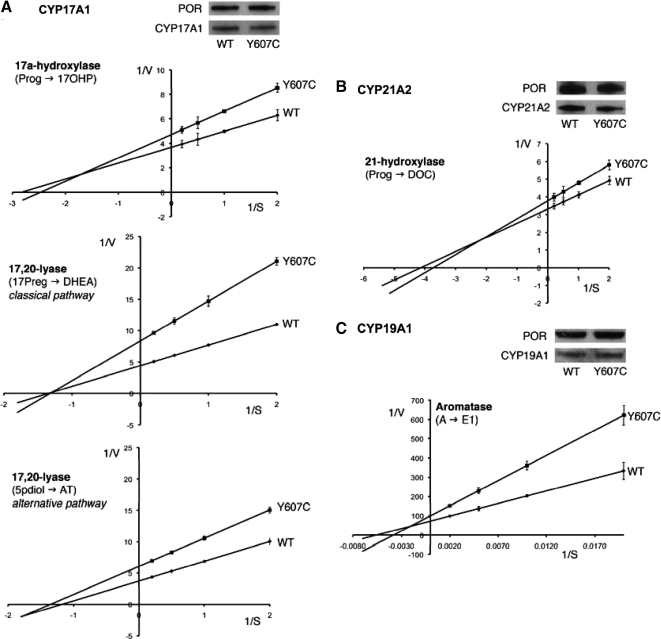
Figure 3.
Kinetic analysis of steroidogenic enzyme activities. Lineweaver-Burk plots of steroidogenic activities as assessed by incubation of yeast microsomes coexpressing human WT or mutant p.Y607C POR with human CYP17A1 (A), CYP21A2 (B), or CYP19A1 (C) with either 0.5–5 μm progesterone (for 17α-hydroxylase and 21-hydroxylase activities), 0.5–5 μm 17hydroxypregnenolone (for 17,20-lyase activity in the classic pathway), 0.5–5 μm 5-pregnanediolone (for 17,20-lyase activity in the alternative pathway), or 50–500 nm androstenedione (for aromatase activity). Representative Western blots demonstrate equal expression of POR and the respective CYP enzyme in the microsomal preparations used. AT, Androsterone; DOC, 11-deoxycortiocosterone; E1, estrone; A, androstenedione; 17Preg, 17-hydroxypregnenolone; 5pdiol, 5alpha-pregnanediolone; Prog, progesterone.
In vitro assessment of steroidogenic activities
We assessed the impact of the maternal p.Y607C POR mutation on steroidogenic microsomal CYP enzymes using yeast microsomal coexpression of WT or mutant POR with CYP17A1, CYP21A2 or CYP19A1.
p.Y607C POR decreased catalytic efficiencies of all three enzymes compared with WT protein (Fig. 3 and Table 2). However, a differential pattern of inhibition was observed. 17α-Hydroxylase activity of CYP17A1 showed significant inhibition with a residual catalytic efficiency of 56% compared with WT (Fig. 3A and Table 2). CYP17A1 17,20-lyase activity was also significantly compromised when looking at the classic pathway, with only 43% residual activity for the conversion of 17-hydroxypregnenolone to DHEA, whereas assessment of 17,20-lyase activity within the proposed alternative pathway demonstrated moderate impairment only, with 66% residual activity for the conversion of 5α-pregnanediolone to androsterone (Fig. 3A and Table 2).
Table 2.
Kinetic analysis of the POR mutant p.Y607C according to yeast microsomal coexpression assays of either WT or mutant POR with human CYP17A1, CYP21A2, and CYP19A1
| CYP17A1
|
CYP21A2
|
CYP19A1
|
|||
|---|---|---|---|---|---|
| 17α-Hydroxylase Prog → 17OHP | 17,20-Lyase (classic) 17Preg → DHEA | 17,20-Lyase (alternative) 5pdiolone → An | 21-Hydroxylase Prog → DOC | Aromatase A → E1 | |
| Vmax (pmol/μg · min) | |||||
| WT | 0.24 ± 0.02 | 0.22 ± 0.00 | 0.27 ± 0.01 | 0.30 ± 0.02 | 0.014 ± 0.001 |
| p.Y607C | 0.21 ± 0.02 | 0.13 ± 0.01 | 0.16 ± 0.00 | 0.27 ± 0.02 | 0.010 ± 0.001 |
| Km (μm) | |||||
| WT | 0.26 ± 0.10 | 0.71 ± 0.04 | 0.85 ± 0.14 | 0.24 ± 0.08 | 180 ± 44 |
| p.Y607C | 0.41 ± 0.13 | 0.95 ± 0.11 | 0.75 ± 0.06 | 0.27 ± 0.10 | 262 ± 54 |
| Catalytic efficiency Vmax/Km (% WT) | |||||
| p.Y607C | 56 ± 3 | 44 ± 4 | 66 ± 3 | 79 ± 2 | 56 ± 3 |
All assays were carried out in three independent triplicate experiments; results are presented as means ± sem. For CYP17A1, both 17α-hdyroxylase and 17,20-lyase activities within the classic and alternative androgen pathway were determined. Prog, Progesterone; 17Preg, 17-hydroxypregnenolone; 5pdiolone, 5-pregnanediolone; DOC, 11-deoxycortiocosterone; A, androstenedione; E1, estrone.
In contrast to the pronounced inhibition of 17α-hydroxylase activity, the POR mutant p.Y607C had only a minor effect on 21-hydroxylation, with 79% residual activity (Fig. 3B and Table 2). CYP19A1 aromatase activity was markedly decreased when coexpressed with p.Y607C POR with 56% residual activity compared with WT POR (Fig. 3C and Table 2). Western blotting determined equal amounts of POR and CYP enzyme protein in all used microsome preparations (Fig. 3, A–C).
Discussion
To our knowledge, this is the first report of combined ORD and AIS. At birth, our patient presented with severe 46,XY DSD prompting female gender assignment and bilateral gonadectomy following the diagnosis of AIS. However, at the age of 9 yr, i.e. around the time of adrenarche, progressive clitoral enlargement and near-normal androgen levels were detected.
The identified AR mutation, p.Q798E, has been previously associated with the clinical phenotype of PAIS (3,12,14,15,16). Interestingly, the same mutation was also identified in three patients with azoospermia but no evidence of 46,XY DSD (17,18,19). This phenotypic variability currently remains unexplained. The p.Q798E mutation is located in the middle of the AR ligand binding domain, residing in the loop between helix 7–8. Two in vitro studies failed to demonstrate impaired AR ligand binding for p.Q798E (3,17). Furthermore, there was no significant impairment of N-/C-terminal interaction, found to be an explanation for the phenotype in some patients with PAIS (5). This is remarkable as mutations in the AR ligand binding domain usually have significant functional impact. Luciferase reporter assays for p.Q798E AR function have shown reduced promoter transactivation in vitro (3,5,15,17). Of note, its transactivation is restored back to WT activity with increasing androgen concentrations (5,17), suggesting that increased availability of androgens could enhance mutant p.Q798E AR action. However, in the previously identified four individuals with p.Q798E no progressive virilization at time of adrenarche or puberty has been reported.
Whereas patients with AIS due to AR mutations usually have high normal or increased androgen levels, our patient presented neonatally with very low circulating androgens. This suggests an androgen biosynthesis defect. Indeed, this was supported by the results of urinary steroid metabolite analysis at the age of 9 yr, which were indicative of ORD. This diagnosis was confirmed by direct sequencing, revealing compound heterozygous mutations. The novel POR frameshift mutation p.E601fsX12 is highly likely to abolish function as previous studies have shown that a premature stop codon result in loss of activity in the p.R616X POR mutant (20,21). This indicates that early truncation of POR mRNA may result either in nonsense mediated RNA decay or that the integrity of the C terminus of the POR protein is crucial for electron transfer. The missense mutation p.Y607C POR has been previously identified when screening a large cohort of healthy Americans (22). However, this is the first time that this mutation has been found in a clinically affected ORD patient and hence has been confirmed as disease causing. Our coexpression assays with p.Y607C POR demonstrated only a mild impairment of 21-hydroxylase activity but significant inhibition of 17α-hydroxylase. Combined inhibition of both activities is consistent with the biochemical finding of normal baseline cortisol but impaired cortisol response to cosyntropin. Furthermore, preferential inhibition of 17α-hydroxylase over 21-hydroxylase explains the observation of mineralocorticoid metabolite accumulation in our patient, as previously described for p.A287P POR (13).
However, one of the most striking features in our patient is the reemergence of androgen production at the age of 9 yr, resulting in progressive clitoral hypertrophy, a phenomenon not yet in patients with ORD (8,9,23). This suggests that the disruptive effect of mutant POR on 17,20-lyase activity, resulting in low or nondetectable androgens during the neonatal period, had been partially overcome at time of adrenarche. In vitro assays suggested compromised 17,20-lyase activity due to p.Y607C for both the classic and alternative pathway, although to a lesser degree for the latter. However, clinical biochemical assessment certainly suggested androgen production via the classic pathway, with high-normal circulating concentrations of DHEAS and androstenedione, and urinary androgen metabolite specific for the alternative androgen pathway, e.g. 5α-17HP, were not found to be elevated in our patient.
It is obvious that the observed increase in androgen production was of adrenal origin because the patient had undergone bilateral gonadectomy 5 yr earlier. It has been previously reported that adrenarche and gonadarche are distinct events and happen independently of each other (24,25), and our patient certainly illustrates this. Adrenarche is generally characterized by a marked increase of circulating DHEA and DHEAS levels between 6 and 8 yr of age, associated with pubertal hair growth, i.e. pubarche (26,27). At the time of adrenarche, there is a physiological increase in the expression of the cofactor enzyme CYB5 within the adrenal zona reticularis, the major site of adrenal androgen production (28,29). CYB5 expression is low in preadrenarchal adrenals but steadily increases after 5 yr of age to reach a plateau at the age of 13 yr (27). CYB5 serves as an allosteric facilitator for the interaction of POR and CYP17A1, stabilizing their interaction by forming a CYP-POR-CYB5 complex (30). There is currently no information on the exact interaction site of CYB5 with POR. Based on three-dimensional modeling, p.Y607C can be predicted to disrupt the binding of NADPH to the POR NADPH-binding domain (22,31). However, if one assumes that the hydrophobic surface area near p.Y607C could potentially be the interaction area with CYB5, it is conceivable that increasing concentrations of CYB5 at time of adrenarche could partially overcome the effect of the mutant. All microsomal assays in this study were carried in the presence of excess CYB5 concentrations, and consequently, it is likely that the disruptive effect of p.Y607C POR on 17,20-lyase activity would certainly be more significant in a milieu of relative CYB5 deficiency, i.e. before adrenarche. Intriguingly, it was recently demonstrated in the domestic ferret that the majority of gonadectomy-induced androgen-producing adrenal tumors express CYB5, which is physiologically not present in normal ferret adrenals (32). Gonadectomy-induced adrenal tumorigenesis has also been described in certain mouse strains and increased LH stimulation and altered activin/inhibin signaling have been implicated in the pathogenesis (33,34,35). In humans, increased androgen production after gonadectomy has not been reported, but if such a cross talk between the gonadal and adrenal axes also exists, it may contribute to the virilization observed in our patient.
Thus, we could speculate that the increase in both androgen production and action at the time of puberty is explained by a two-step model. First, the emerging CYB5 expression in the adrenal zona reticularis may ameliorate the disruptive effect of p.Y607C POR, resulting in an increase in 17,20-lyase activity and adrenal androgen production during adrenarche. Second, increasing levels of androgens could then potentially enhance the transactivation capacity of the AR mutant p.Q798E, as previously observed in vitro (5,17). This subsequently results in improved androgen action and the observed clitoral enlargement, which would represent phallic catch-up growth due to increased androgen sensitivity in an individual with a male genetic background, as observed previously in some 46,XY individuals with idiopathic micropenis (36). Of note, virilization at puberty and the subsequent change of gender identity is not uncommon in 46,XY DSD individuals with HSD17B3 or SRD5A2 deficiencies who where raised as girls (37,38,39). However, the mechanism in these conditions is different because testicular-derived androgens accumulate before the enzyme block (androstenedione in HSD17B3 and testosterone in SRD2A2) and are likely to be converted by other isoenzymes (38). The observed biological response to a relatively sudden increase in androgen levels in HSD17B3 and SRD5A2 deficiency patients reflects the susceptibility of individuals with a male karyotype to develop phenotypic virilization, similar to our case.
In conclusion, our patient illustrates the close interaction of factors involved in the regulation of androgen synthesis and androgen action, respectively. Furthermore, our case highlights that patients presenting with DSD also require thorough work-up of the adrenal axis and vice versa (40) to ensure that these patients are not exposed to the unrecognized risk of life-threatening adrenal crisis, which fortunately did not occur in our case until the conclusive diagnosis of adrenal insufficiency was established, 9 yr after the initial presentation with 46,XY DSD.
Acknowledgments
We gratefully acknowledge Vicki Pilfold-Wilkie (University of Cambridge, Cambridge, UK) for skillful technical help with sequencing analysis of the AR.
Footnotes
This work was supported by the Medical Research Council UK (Program Grant 0900567, to W.A.), the European Society for Pediatric Endocrinology (Research Fellowship, to J.I.), the European Community’s Seventh Framework Program (Collaborative Research Project EuroDSD, to W.A. and I.A.H.; Marie Curie Intra-European Fellowship PIEF-GA-2008-221058, to N.R.), the Wellcome Trust (Clinician Scientist Fellowship GR079865MA, to N.K.), and the National Institute for Health Research (NIHR) Cambridge Biomedical Research Centre (to J.D.D. and I.A.H.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online April 21, 2010
Abbreviations: AIS, Androgen insensitivity syndrome; An, androsterone; AR, androgen receptor; CYB5, cytochrome b5; CYP, cytochrome P450; DHEA, dehydroepiandrosterone; DHEAS, dehydroepiandrosterone sulfate; DSD, disordered sexual development; Et, etiocholanolone; GC/MS, gas chromatography/mass spectrometry; hCG, human chorionic gonadotropin; 17HP, 17-hydroxypregnanolone; Km, Michaelis-Menten constant; NADPH, nicotinamide adenine dinucleotide phosphate; 17OHP, 17-hydroxyprogesterone; ORD, POR deficiency; PAIS, partial AIS; PD, pregnanediol; POR, P450 oxidoreductase; PT, pregnanetriol; 5-PT, pregnenetriol; THA, tetrahydro-11-dehydrocorticosterone; THB, tetrahydrocorticosterone; THE, tetrahydrocortisone; THF, tetrahydrocortisol; Vmax, maximal velocity; WT, wild type.
References
- Mendonca BB, Domenice S, Arnhold IJ, Costa EM 2009 46,XY disorders of sex development (DSD). Clin Endocrinol (Oxf) 70:173–187 [DOI] [PubMed] [Google Scholar]
- Hughes IA 2008 Disorders of sex development: a new definition and classification. Best Pract Res Clin Endocrinol Metab 22:119–134 [DOI] [PubMed] [Google Scholar]
- Bevan CL, Brown BB, Davies HR, Evans BA, Hughes IA, Patterson MN 1996 Functional analysis of six androgen receptor mutations identified in patients with partial androgen insensitivity syndrome. Hum Mol Genet 5:265–273 [DOI] [PubMed] [Google Scholar]
- Ghali SA, Gottlieb B, Lumbroso R, Beitel LK, Elhaji Y, Wu J, Pinsky L, Trifiro MA 2003 The use of androgen receptor amino/carboxyl-terminal interaction assays to investigate androgen receptor gene mutations in subjects with varying degrees of androgen insensitivity. J Clin Endocrinol Metab 88:2185–2193 [DOI] [PubMed] [Google Scholar]
- Jääskeläinen J, Deeb A, Schwabe JW, Mongan NP, Martin H, Hughes IA 2006 Human androgen receptor gene ligand-binding-domain mutations leading to disrupted interaction between the N- and C-terminal domains. J Mol Endocrinol 36:361–368 [DOI] [PubMed] [Google Scholar]
- Krone N, Dhir V, Ivison H, Arlt W 2007 Congenital adrenal hyperplasia and P450 oxidoreductase deficiency. Clin Endocrinol (Oxf) 66:162–172 [DOI] [PubMed] [Google Scholar]
- Krone N, Arlt W 2009 Genetics of congenital adrenal hyperplasia. Best Pract Res Clin Endocrinol Metab 23:181–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlt W, Walker EA, Draper N, Ivison HE, Ride JP, Hammer F, Chalder SM, Borucka-Mankiewicz M, Hauffa BP, Malunowicz EM, Stewart PM, Shackleton CHL 2004 Congenital adrenal hyperplasia caused by mutant P450 oxidoreductase and human androgen synthesis: analytical study. Lancet 363:2128–2135 [DOI] [PubMed] [Google Scholar]
- Flück C, Tajima T, Pandey AV, Arlt W, Okuhara K, Verge C, Jabs EW, Mendonça BB, Fujieda K, Miller WL 2004 Mutant P450 oxidoreductase causes disordered steroidogenesis with and without Antley-Bixler syndrome. Nat Genet 36:228–230 [DOI] [PubMed] [Google Scholar]
- Wilson JD, Auchus RJ, Leihy MW, Guryev OL, Estabrook RW, Osborn SM, Shaw G, Renfree MB 2003 5α-Androstane-3α,17β-diol is formed in tammar wallaby pouch young testes by a pathway involving 5α-pregnane-3αa,17α-diol-20-one as a key intermediate. Endocrinology 144:575–580 [DOI] [PubMed] [Google Scholar]
- Shackleton C, Marcos J, Malunowicz EM, Szarras-Czapnik M, Jira P, Taylor NF, Murphy N, Crushell E, Gottschalk M, Hauffa B, Cragun DL, Hopkin RJ, Adachi M, Arlt W 2004 Biochemical diagnosis of Antley-Bixler syndrome by steroid analysis. Am J Med Genet A 128A:223–231 [DOI] [PubMed] [Google Scholar]
- Batch JA, Williams DM, Davies HR, Brown BD, Evans BA, Hughes IA, Patterson MN 1992 Androgen receptor gene mutations identified by SSCP in fourteen subjects with androgen insensitivity syndrome. Hum Mol Genet 1:497–503 [DOI] [PubMed] [Google Scholar]
- Dhir V, Ivison HE, Krone N, Shackleton CH, Doherty AJ, Stewart PM, Arlt W 2007 Differential inhibition of CYP17A1 and CYP21A2 activities by the P450 oxidoreductase mutant A287P. Mol Endocrinol 21:1958–1968 [DOI] [PubMed] [Google Scholar]
- Quigley CA, De Bellis A, Marschke KB, el-Awady MK, Wilson EM, French FS 1995 Androgen receptor defects: historical, clinical, and molecular perspectives. Endocr Rev 16:271–321 [DOI] [PubMed] [Google Scholar]
- Hiort O, Sinnecker GH, Holterhus PM, Nitsche EM, Kruse K 1996 The clinical and molecular spectrum of androgen insensitivity syndromes. Am J Med Genet 63:218–222 [DOI] [PubMed] [Google Scholar]
- Thai HT, Kalbasi M, Lagerstedt K, Frisén L, Kockum I, Nordenskjöld A 2005 The valine allele of the V89L polymorphism in the 5α-reductase gene confers a reduced risk for hypospadias. J Clin Endocrinol Metab 90:6695–6698 [DOI] [PubMed] [Google Scholar]
- Wang Q, Ghadessy FJ, Trounson A, de Kretser D, McLachlan R, Ng SC, Yong EL 1998 Azoospermia associated with a mutation in the ligand-binding domain of an androgen receptor displaying normal ligand binding, but defective trans-activation. J Clin Endocrinol Metab 83:4303–4309 [DOI] [PubMed] [Google Scholar]
- Hiort O, Holterhus PM, Horter T, Schulze W, Kremke B, Bals-Pratsch M, Sinnecker GH, Kruse K 2000 Significance of mutations in the androgen receptor gene in males with idiopathic infertility. J Clin Endocrinol Metab 85:2810–2815 [DOI] [PubMed] [Google Scholar]
- Ferlin A, Vinanzi C, Garolla A, Selice R, Zuccarello D, Cazzadore C, Foresta C 2006 Male infertility and androgen receptor gene mutations: clinical features and identification of seven novel mutations. Clin Endocrinol (Oxf) 65:606–610 [DOI] [PubMed] [Google Scholar]
- Flück CE, Nicolo C, Pandey AV 2007 Clinical, structural and functional implications of mutations and polymorphisms in human NADPH P450 oxidoreductase. Fundam Clin Pharmacol 21:399–410 [DOI] [PubMed] [Google Scholar]
- Agrawal V, Huang N, Miller WL 2008 Pharmacogenetics of P450 oxidoreductase: effect of sequence variants on activities of CYP1A2 and CYP2C19. Pharmacogenet Genomics 18:569–576 [DOI] [PubMed] [Google Scholar]
- Huang N, Agrawal V, Giacomini KM, Miller WL 2008 Genetics of P450 oxidoreductase: sequence variation in 842 individuals of four ethnicities and activities of 15 missense mutations. Proc Natl Acad Sci USA 105:1733–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma K, Hasegawa T, Nagai T, Adachi M, Horikawa R, Fujiwara I, Tajima T, Takeda R, Fukami M, Ogata T 2006 Urine steroid hormone profile analysis in cytochrome P450 oxidoreductase deficiency: implication for the backdoor pathway to dihydrotestosterone. J Clin Endocrinol Metab 91:2643–2649 [DOI] [PubMed] [Google Scholar]
- Counts DR, Pescovitz OH, Barnes KM, Hench KD, Chrousos GP, Sherins RJ, Comite F, Loriaux DL, Cutler Jr GB 1987 Dissociation of adrenarche and gonadarche in precocious puberty and in isolated hypogonadotropic hypogonadism. J Clin Endocrinol Metab 64:1174–1178 [DOI] [PubMed] [Google Scholar]
- Sklar CA, Kaplan SL, Grumbach MM 1980 Evidence for dissociation between adrenarche and gonadarche: studies in patients with idiopathic precocious puberty, gonadal dysgenesis, isolated gonadotropin deficiency, and constitutionally delayed growth and adolescence. J Clin Endocrinol Metab 51:548–556 [DOI] [PubMed] [Google Scholar]
- Hopper BR, Yen SS 1975 Circulating concentrations of dehydroepiandrosterone and dehydroepiandrosterone sulfate during puberty. J Clin Endocrinol Metab 40:458–461 [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Gang HX, Suzuki T, Sasano H, Rainey WE 2009 Adrenal changes associated with adrenarche. Rev Endocr Metab Disord 10:19–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Sasano H, Takeyama J, Kaneko C, Freije WA, Carr BR, Rainey WE 2000 Developmental changes in steroidogenic enzymes in human postnatal adrenal cortex: immunohistochemical studies. Clin Endocrinol (Oxf) 53:739–747 [DOI] [PubMed] [Google Scholar]
- Auchus R, Rainey W 2004 Adrenarche—physiology, biochemistry and human disease. Clin Endocrinol (Oxf) 60:288–296 [DOI] [PubMed] [Google Scholar]
- Auchus RJ, Lee TC, Miller WL 1998 Cytochrome b5 augments the 17,20-lyase activity of human P450c17 without direct electron transfer. J Biol Chem 273:3158–3165 [DOI] [PubMed] [Google Scholar]
- Wang M, Roberts DL, Paschke R, Shea TM, Masters BS, Kim JJ 1997 Three-dimensional structure of NADPH-cytochrome P450 reductase: prototype for FMN- and FAD-containing enzymes. Proc Natl Acad Sci USA 94:8411–8416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S, Kiupel M, Peterson 2nd RA, Heikinheimo M, Wilson DB 2008 Cytochrome b5 expression in gonadectomy-induced adrenocortical neoplasms of the domestic ferret (Mustela putorius furo). Vet Pathol 45:439–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuschlein F, Looyenga BD, Bleasdale SE, Mutch C, Bavers DL, Parlow AF, Nilson JH, Hammer GD 2003 Activin induces x-zone apoptosis that inhibits luteinizing hormone-dependent adrenocortical tumor formation in inhibin-deficient mice. Mol Cell Biol 23:3951–3964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman NA, Kiiveri S, Rivero-Müller A, Levallet J, Vierre S, Kero J, Wilson DB, Heikinheimo M, Huhtaniemi I 2004 Adrenocortical tumorigenesis in transgenic mice expressing the inhibin α-subunit promoter/simian virus 40 T-antigen transgene: relationship between ectopic expression of luteinizing hormone receptor and transcription factor GATA-4. Mol Endocrinol 18:2553–2569 [DOI] [PubMed] [Google Scholar]
- Bernichtein S, Petretto E, Jamieson S, Goel A, Aitman TJ, Mangion JM, Huhtaniemi IT 2008 Adrenal gland tumorigenesis after gonadectomy in mice is a complex genetic trait driven by epistatic loci. Endocrinology 149:651–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PA, Houk CP 2004 Outcome studies among men with micropenis. J Pediatr Endocrinol Metab 17:1043–1053 [DOI] [PubMed] [Google Scholar]
- Rösler A, Silverstein S, Abeliovich D 1996 A (R80Q) mutation in 17β-hydroxysteroid dehydrogenase type 3 gene among Arabs of Israel is associated with pseudohermaphroditism in males and normal asymptomatic females. J Clin Endocrinol Metab 81:1827–1831 [DOI] [PubMed] [Google Scholar]
- Lee YS, Kirk JM, Stanhope RG, Johnston DI, Harland S, Auchus RJ, Andersson S, Hughes IA 2007 Phenotypic variability in 17β-hydroxysteroid dehydrogenase-3 deficiency and diagnostic pitfalls. Clin Endocrinol (Oxf) 67:20–28 [DOI] [PubMed] [Google Scholar]
- Cohen-Kettenis PT 2005 Gender change in 46,XY persons with 5α-reductase-2 deficiency and 17β-hydroxysteroid dehydrogenase-3 deficiency. Arch Sex Behav 34:399–410 [DOI] [PubMed] [Google Scholar]
- Metherell LA, Naville D, Halaby G, Begeot M, Huebner A, Nürnberg G, Nürnberg P, Green J, Tomlinson JW, Krone NP, Lin L, Racine M, Berney DM, Achermann JC, Arlt W, Clark AJ 2009 Nonclassic lipoid congenital adrenal hyperplasia masquerading as familial glucocorticoid deficiency. J Clin Endocrinol Metab 94:3865–3871 [DOI] [PMC free article] [PubMed] [Google Scholar]