Abstract
We report the first genome-wide association study of habitual caffeine intake. We included 47,341 individuals of European descent based on five population-based studies within the United States. In a meta-analysis adjusted for age, sex, smoking, and eigenvectors of population variation, two loci achieved genome-wide significance: 7p21 (P = 2.4×10−19), near AHR, and 15q24 (P = 5.2×10−14), between CYP1A1 and CYP1A2. Both the AHR and CYP1A2 genes are biologically plausible candidates as CYP1A2 metabolizes caffeine and AHR regulates CYP1A2.
Author Summary
Caffeine is the most widely consumed psychoactive substance in the world. Although demographic and social factors have been linked to habitual caffeine consumption, twin studies report a large heritable component. Through a comprehensive search of the human genome involving over 40,000 participants, we discovered two loci associated with habitual caffeine consumption: the first near AHR and the second between CYP1A1 and CYP1A2. Both the AHR and CYP1A2 genes are biologically plausible candidates, as CYP1A2 metabolizes caffeine and AHR regulates CYP1A2. Caffeine intake has been associated with manifold physiologic effects and both detrimental and beneficial health outcomes. Knowledge of the genetic determinants of caffeine intake may provide insight into underlying mechanisms and may provide ways to study the potential health effects of caffeine more comprehensively.
Introduction
Caffeine (1,3,7-trimethylxanthine) is the most widely consumed psychoactive substance in the world with nearly 90% of adults reporting regular consumption of caffeine-containing beverages and foods [1], [2]. Although demographic and social factors have been linked to habitual caffeine consumption, twin studies report heritability estimates between 43 and 58% for caffeine use; 77% for heavy use, and 45, 40, and 35%, respectively, for caffeine toxicity, tolerance and withdrawal symptoms [3]. Genetic association studies focused on candidate genes related to the pharmacokinetic and pharmacodynamic properties of caffeine have identified genes encoding cytochrome P-450 (CYP)1A2, as the primary enzyme involved in caffeine metabolism [3], [4]. The genome-wide association approach has emerged as a powerful means for discovering novel loci related to habitual use of a second stimulant, tobacco [5], but has not yet clearly identified genes for other common behavioral traits, including caffeine consumption. To comprehensively examine the influence of common genetic variation on habitual caffeine consumption behavior we undertook a meta-analysis of genome-wide association studies (GWAS) from population-based cohorts. Our study confirms the important roles of CYP1A2 and AHR in determining caffeine intake, thus supporting the utility of the GWAS approach to the discovery of loci linked to this complex behavioral trait.
Results
We performed a meta-analysis of 47,341 individuals of European descent, derived from five studies within the US, the Atherosclerosis Risk in Communities (ARIC, N = 8,945) Study, the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO, N = 4,942), the Nurses' Health Study (NHS, N = 6,774), the Health Professionals Follow-Up Study (HPFS, N = 4,023), and the Women's Genome Health Study (WGHS, N = 22,658). Sample characteristics are presented in Table 1. Caffeine intake was assessed using semi-quantitative food frequency questionnaires (FFQ) that included questions on the consumption of caffeinated coffee, tea, soft drinks, and chocolate.
Table 1. Descriptive characteristics of studies participating in meta-analysis.* .
| Study | Description | N | Female, % | Age, years | Caffeine, mg/day | Current smokers, % | Platform |
| ARIC | Cohort | 8,945 | 52.8 | 54.3 (5.7) | 332.9 (311.1) | 24.4 | Affymetrix 6.0 |
| PLCO | Cohort: nested case-control** | 4,942 | 23.5 | 67.7 (5.4) | 491.1 (494.1) | 22.1 | Illumina 240KIllumina 310KIllumina 550kIllumina 610Q |
| NHS T2D | Cohort: nested T2D case-control | 3,135 | 100 | 51.1 (10.5) | 284.5 (206.3) | 14.8 | Affymetrix 6.0 |
| NHS CHD | Cohort: nested CHD case-control | 1,102 | 100 | 53.5(10.6) | 316.7 (218.0) | 30.0 | Affymetrix 6.0 |
| NHS KS | Cohort: nested KS case-control | 488 | 100 | 47.7 (11.7) | 264.4 (203.6) | 15.3 | Illumina 610Q |
| NHS BrC | Cohort: nested BrC case-control | 2,049 | 100 | 52.3 (9.6) | 286.5 (204.0) | 15.6 | Illumina 550k |
| HPFS T2D | Cohort: nested T2D case-control | 2,381 | 0 | 55.5 (8.4) | 250.9 (227.6) | 7.6 | Affymetrix 6.0 |
| HPFS CHD | Cohort: nested CHD case-control | 1,099 | 0 | 56.7 (8.7) | 243.2 (230.7) | 9.9 | Affymetrix 6.0 |
| HPFS KS | Cohort: nested KS case-control | 543 | 0 | 48.8 (6.8) | 230.5 (241.6) | 6.4 | Illumina 610Q |
| WGHS | Cohort | 22,658 | 100 | 54.7 (7.1) | 298.5 (232.9) | 11.5 | Illumina HumanHap300 Duo+ |
| Total | 47,341 |
*Values are mean (standard deviation) for age and caffeine; percent for female and current smokers.
**Includes samples from prostate cancer case-control (n = 1885), bladder cancer case-control (n = 572), glioma case-control (n = 3), lung cancer case-control (n = 1758), pancreatic cancer case-control (n = 299), renal cancer case-control study (n = 271).
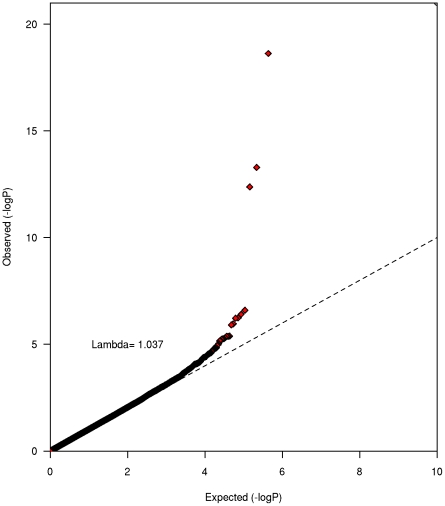
Study-level genomic inflation factors (λ) were low ranging from 1.00 (PLCO) to 1.03 (HPFS), suggesting that population stratification was well controlled (Figure S1). A total of 433,781 imputed and genotyped SNPs passed our stringent criteria for the meta-analysis. Test statistic inflation at the meta-analysis level revealed no evidence of notable underlying population substructure (λ = 1.04, Figure 1).
Figure 1. QQ plot for the genome-wide meta-analysis of caffeine consumption.
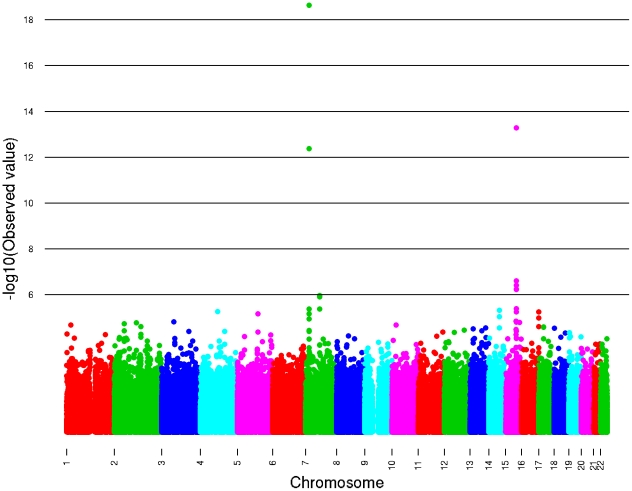
Two loci reached genome-wide significance with no evidence for significant between- study heterogeneity (Table 2, Figure 2 and Figure 3, Table S1). The strongest associated SNP (rs4410790, P = 2.4×10−19, Figure S2) is located at 7p21, 54 kb upstream of AHR (aryl hydrocarbon receptor). The second strongest associated SNP (rs2470893, P = 5.2×10−14, Figure S2) mapped to 15q24 within the bidirectional promoter of the CYP1A1-CYP1A2 locus [6], [7]. A synonymous coding SNP (rs2472304, P = 2.5×10−7) in CYP1A2 exon 7 that was highly correlated with 6 other SNPs but not correlated with rs2470893 (r2 = 0.18, HapMap CEU) was amongst the highest ranked loci in our meta-analysis (Table 2). Although we only considered variants that were imputed with high probability, we also conducted a sensitivity analysis restricting our sampling to individuals with genotyped data (Table 2). Regression coefficients remained essentially unchanged, but P-values were less significant reflecting the reduced sample size (rs4410790: P = 4.0×10−18; rs2470893 P = 9.5×10−8). Similar results were also observed when men and women were examined separately (Table S2). Had the analysis been performed instead by discovery at genome-wide significance (P<5×10−8) in the WGHS followed by replication in meta-analysis of the remaining cohorts, only SNPs at the same loci would have met Bonferroni corrected standards of significance. In a post-hoc investigation of study heterogeneity in which we compared WGHS to the remaining studies combined, there was significant heterogeneity for rs4410790 (P = 0.01), although this could be attributable to chance.
Table 2. Genome-wide meta-analytic results for caffeine consumption (P<10−6).
| Index SNP | Chr | Position (NCBI 36) | Closest gene(s) (±100 kb) | Total SNPs* (P<1×10−3) | EA | EAF | Imputed and Genotyped | Genotyped | |||||
| N | β (SE) | P | P het ** | N | β (SE) | P | |||||||
| rs4410790 | 7 | 17251102 | AHR | 1 | T | 0.38 | 36013 | −0.15 (0.02) | 2.4×10−19 | 0.14 | 25738 | −0.16 (0.02) | 4.0×10−18 |
| rs2470893 | 15 | 72806502 | LMAN1L, EDC3, CYP1A2, CYP1A1, CSK | 1 | T | 0.31 | 47341 | 0.12 (0.02) | 5.2×10−14 | 0.68 | 25738 | 0.10 (0.02) | 9.5×10−8 |
| rs2472304 | 15 | 72831291 | CYP1A2 | 4 | A | 0.65 | 47325 | 0.08 (0.01) | 2.5×10−7 | 0.06 | 30663 | 0.07 (0.02) | 3.2×10−4 |
| rs6495122 | 15 | 72912698 | ULK3, SCAMP2, MP1, LMAN1L, CYP1A2, CSK, COX5A, CPLX3, C14orf17 | 1 | A | 0.43 | 47341 | −0.07 (0.01) | 5.8×10−7 | 0.08 | 25738 | −0.05 (0.02) | 0.007 |
| rs12148488 | 15 | 73169595 | SCAMP5,PPCDC | 3 | T | 0.50 | 47341 | −0.07 (0.01) | 5.9×10−7 | 0.43 | 25738 | −0.06 (0.02) | 0.001 |
Chr, chromosome; EA, effect allele; EAF, effect allele frequency; SE standard error.
*Number of significant SNPs in LD (r2 >0.5) and/or located <250 kb from index SNP according to HapMap.
**P value for between study heterogeneity.
Figure 2. The –log10 P-plots for the genome-wide meta-analysis of caffeine consumption.
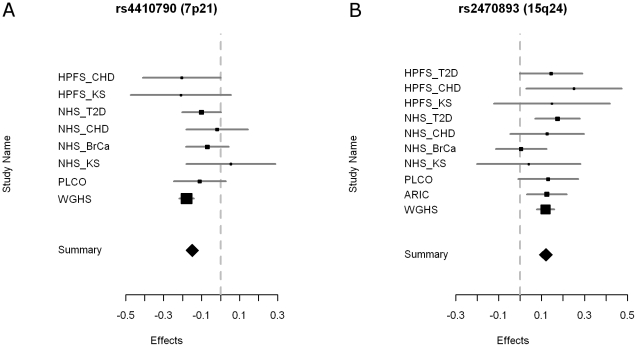
Figure 3. Forest plots of the meta-analysis for the two caffeine-associated loci.
A) rs4410790 and B) rs2470893. The contributing effect from each study is shown by a square, with confidence intervals indicated by horizontal lines. The contributing weight of each study to the meta-analysis is indicated by the size of the square. The meta-analysis estimate is shown at the bottom of each graph.
Based on the well-established biological link between smoking and AHR [8], and CYP1A2 [9] and caffeine consumption behavior [2], we explored the role of cigarette smoking (Table 3). Compared to our primary model that adjusted for smoking, a model not adjusted for smoking yielded slightly attenuated associations and when restricting analyses to ‘never smokers’ similar regression coefficients were observed as for the complete study population. These findings suggest that smoking is unlikely the cause of the associations observed in our GWAS of caffeine intake.
Table 3. Genome-wide meta-analysis of caffeine consumption (P<10−6): Smoking effects.
| Index SNP | Chr | EA | Not Adjusted for Smoking | Never Smokers | Current Smokers | |||||||||
| N | β | P | P het * | N | β | P | P het * | N | β | P | P het * | |||
| rs4410790 | 7 | T | 36150 | −0.15 | 8.2×10−18 | 0.18 | 16809 | −0.19 | 1.8×10−14 | 0.09 | 5058 | −0.10 | 0.02 | 0.96 |
| rs2470893 | 15 | T | 47612 | 0.12 | 5.0×10−13 | 0.70 | 21413 | 0.13 | 3.0×10−8 | 0.19 | 7466 | 0.06 | 0.16 | 0.56 |
| rs2472304 | 15 | A | 47596 | 0.07 | 2.4×10−6 | 0.15 | 21410 | 0.07 | 0.0019 | 0.03 | 7464 | 0.03 | 0.36 | 0.47 |
| rs6495122 | 15 | A | 47612 | −0.07 | 5.2×10−6 | 0.24 | 21413 | −0.07 | 0.0011 | 0.03 | 7466 | −0.01 | 0.75 | 0.38 |
| rs12148488 | 15 | T | 47612 | −0.07 | 1.9×10−6 | 0.63 | 21413 | −0.08 | 0.0001 | 0.07 | 7466 | −0.002 | 0.97 | 0.27 |
Chr, chromosome; EA, effect allele;
*P value for between study heterogeneity.
We further conducted 21 candidate gene analyses and found significant gene-based associations (Bonferroni corrected for the total number of human genes) between CYP2C9 (P = 0.023), and ADORA2A (P = 0.011) and caffeine intake in addition to CYP1A2 and AHR (Table 4).
Table 4. Candidate gene-based association results.* .
| Chr | Gene | #SNPs | #simulations | start position | stop position | Gene-based P |
| 1 | ADORA3 | 43 | 1000 | 111827492 | 111908120 | 0.69 |
| 1 | FMO3 | 26 | 1000 | 169326659 | 169353583 | 0.17 |
| 1 | ADORA1 | 43 | 1000 | 201363458 | 201403156 | 0.13 |
| 2 | XDH | 47 | 1000 | 31410691 | 31491115 | 0.22 |
| 5 | DRD1 | 33 | 100000 | 174800280 | 174803769 | 0.10 |
| 7 | AHR | 18 | 1000000 | 17304831 | 17352299 | <1×10−6 |
| 7 | CYP3A4 | 11 | 1000 | 99192539 | 99219744 | 0.56 |
| 7 | CYP3A43 | 3 | 1000 | 99263571 | 99302109 | 0.58 |
| 8 | NAT1 | 3 | 1000 | 18111894 | 18125100 | 0.52 |
| 8 | NAT2 | 32 | 1000 | 18293034 | 18303003 | 0.62 |
| 10 | CYP2C9 | 23 | 100000 | 96688404 | 96739138 | 0.023 |
| 10 | CYP2C8 | 20 | 100000 | 96786518 | 96819244 | 0.05 |
| 10 | CYP2E1 | 16 | 1000 | 135190856 | 135202610 | 0.23 |
| 11 | DRD2 | 34 | 100000 | 112785526 | 112851211 | 0.077 |
| 12 | TAS2R7 | 4 | 1000 | 10845397 | 10846493 | 0.96 |
| 12 | TAS2R14 | 1 | 1000 | 10982119 | 10983073 | 0.72 |
| 15 | CYP1A2 | 11 | 1000000 | 72828236 | 72835994 | <1×10−6 |
| 17 | ADORA2B | 15 | 1000 | 15788955 | 15819935 | 0.30 |
| 17 | PPP1R1B | 19 | 1000 | 35036704 | 35046404 | 0.74 |
| 19 | CYP2A6 | 45 | 1000 | 46041282 | 46048192 | 0.43 |
| 19 | CYP2A7 | 28 | 1000 | 46073183 | 46080497 | 0.60 |
| 22 | COMT | 41 | 1000 | 18309308 | 18336530 | 0.27 |
| 22 | ADORA2A | 8 | 100000 | 23153529 | 23168325 | 0.011 |
*Gene-based analyses were performed using VEGAS [37]. See Materials and Methods for details.
Discussion
In the first GWAS of caffeine intake in a total of 47,341 individuals from five U.S. studies, loci at 15q24 and 7p21 achieved genome-wide significance. CYP1A2 at 15q24 and AHR at 7p21 are attractive candidate genes for caffeine intake. At plasma concentrations typical of humans (<100 µM), caffeine is predominantly (∼95% of a dose) metabolized by CYP1A2 via N1-, N3-, and N7-demethylation to its three dimethylxanthines, namely, theobromine, paraxanthine, and theophylline, respectively [10]. CYP1A2 expression and activity vary 10- to 60-fold between individuals [11]. Human CYP1A2 is located immediately adjacent to CYP1A1 in reverse orientation and the two genes share a common 5′-flanking region [12]. At least 15 AHR response elements (AHRE) reside in this bidirectional promoter region and rs2470893 is located in AHRE6 (originally reported as AHRE5[7]) which correlates with transcriptional activation of both CYP1A1 and CYP1A2 [6], [7]. CYP1A1 expression in the liver (the target tissue for caffeine metabolism) is low and there is little evidence that this enzyme contributes to caffeine metabolism. This contrasts with the tissue specific expression of CYP1A2 in the liver, which suggests further evidence supporting its role in caffeine metabolism. The observation that a stronger association exists for SNPs upstream of the gene suggests that variation in CYP1A2 gene expression probably affects caffeine intake. The protein product of AHR, AhR, is a ligand–activated transcription factor that, upon binding, partners with ARNT and translocates to the nucleus where it regulates the expression of a number of genes including CYP1A1 and CYP1A2. There is marked variation in AhR binding affinity across populations, but so far no polymorphisms have been identified that account for this variation [13]. The most studied SNP, rs2066853 (R554K), is located in exon 10, a region of AHR that encodes the transactivation domain[13]. Although this SNP was associated with caffeine in the current study (P = 0.0004), our strongest signal mapped upstream of AHR, suggesting variation in AHR expression has a key role in propensity to consume caffeine. An interaction between CYP1A2 and AHR could be biologically plausible; however, we did not find any evidence supporting statistical interaction between the top two loci (data not shown).
Human and animal candidate gene studies for caffeine intake and related traits have focused on various other genes linked to caffeine's metabolism and targets of action. In our candidate gene analyses, we observed significant gene-based associations between CYP2C9 and ADORA2A and caffeine intake in addition to CYP1A2 and AHR. CYP2C9 catalyzes the N7-demethylation and C8-hydroxylation of caffeine to theophylline and 1,3,7-trimethyluric acid (a minor metabolite), respectively; but its role relative to CYP1A2 is generally small[10]. In amounts typically consumed from dietary sources, caffeine antagonizes the actions of adenosine at the adenosine A2A receptor (ADORA2A) [2], which plays an important role in the stimulating and reinforcing properties of caffeine [14], [15]. Polymorphisms of ADORA2A have been previously implicated in caffeine-induced anxiety as well as habitual caffeine intake[16], [17].
All studies contributing to our GWAS of caffeine intake were US-based. Consistent with the adult caffeine consumption pattern of this country, coffee contributed to well over 80% of caffeine intake. Previous studies suggest that some of the heritability underling specific caffeine sources (i.e. coffee and tea) may be distinct in relation to total caffeine intake [18]. To evaluate the robustness of findings, we conducted an additional GWAS analysis using caffeinated coffee intake as the outcome variable yielding the same strong signals (rs4410790: 1.4×10−29, rs2470893: 3.6×10−19).
Imprecision in phenotypic assessment and differences across studies could have limited the scope of our discovery. Although dietary intake obtained by FFQ is subject to misclassification, validation studies in subsamples of the included studies indicated that the consumption of caffeine-containing beverages is assessed with good accuracy [19], [20], [21]. The cubic root transformation we applied to reported caffeine intakes, however, limits interpretation of the effect estimates. The crude weighted mean difference in caffeine intake between homozygote genotypes was 44 mg/d for rs4410790 and 38 mg/d for rs2470893 (Table S3 and S4). The two SNPs together, however, explained between 0.06 and 0.72% of the total variation in caffeine intake across studies suggesting additional variants remain to be discovered [22]. Finally, our GWAS assumed an additive genetic model and based on study-level results (Figure 1 and Figure 2) potential non-linear effects will require confirmation in future studies.
Caffeine intake has been associated with pleotropic physiologic effects in relation to both detrimental and beneficial health outcomes [23]. Our current study provides insights into the primary pathways underlying caffeine intake. Knowledge of the genetic determinants of caffeine intake may provide insight into underlying mechanisms and may provide ways to study the potential health effects of caffeine more comprehensively by using genetic determinants as instrumental variables for caffeine intake or by taking into consideration caffeine-gene interactions. With the exception of nicotine dependency and the associated nicotinic receptor, genes that influence traits associated with dependency have been difficult to identify. The association of caffeine consumption with genes involved in metabolism or its regulation (CYP1A2 and AhR, respectively) illustrates that it is feasible to use GWAS to identify genetic determinants of other behavioral traits that are assessed with lower accuracy. We also recognize that the identified variants could influence regulation of their genomic elements distant from the known, high profile, neighboring candidate genes. In conclusion, we identified two loci related to caffeine consumption that will be worthy of further investigation with regard to both beneficial and toxic effects of caffeine as well as the extensive group of carcinogens, drugs, and xenobiotics also metabolized through action of the regulation of the gene products of CYP1A2 and AHR.
Material and Methods
Ethics Statement
This study was conducted according to the principles expressed in the Declaration of Helsinki. All participants in the contributing studies gave written informed consent including consent for genetic analyses. Local institutional review boards approved study protocols.
Study Populations
We conducted a meta-analysis of 47,341 individuals of European descent, sourced from Atherosclerosis Risk in Communities (ARIC, N = 8,976), the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO, N = 4,942), the Nurses' Health Study (NHS, N = 6,774), the Health Professionals Follow-Up Study (HPFS, N = 4,023), and the Women's Genome Health Study (WGHS, N = 22,658) to identify novel loci associated with habitual caffeine consumption. Study population descriptions and genotyping quality control for data generated with either the Affymetrix 6.0 or the Illumina Infinium arrays (HumanHap300, 550 or 610 arrays) are provided in Text S1 and Table S5 and S6.
Caffeine Intake Assessment
In the NHS, every 2 to 4 years of follow-up diet was assessed using a validated semi-quantitative food frequency questionnaire (FFQ) [24]. For the present analysis, we included the participants' mean caffeine intakes of the 1984 (first year in which caffeinated and decaffeinated coffee were differentiated) and 1986 FFQs. The following caffeine-containing foods and beverages were included in the FFQ: coffee with caffeine, tea, cola and other carbonated beverages with caffeine, and chocolate. For each item, participants were asked how often, on average, they had consumed a specified amount of each beverage or food over the past year. The participants could choose from nine frequency categories (never, 1–3 per month, 1 per week, 2–4 per week, 5–6 per week, 1 per day, 2–3 per day, 4–5 per day and 6 or more per day). Intakes of nutrients and caffeine were calculated using US Department of Agriculture food composition sources. In these calculations, we assumed that the content of caffeine was 137 mg per cup of coffee, 47 mg per cup of tea, 46 mg per can or bottle of cola or other caffeinated carbonated beverage, and 7 mg per 1 oz serving of chocolate candy. We assessed the total intake of caffeine by summing the caffeine content for the specified amount of each food multiplied by a weight proportional to the frequency of its use. In a validation among a subsample of this cohort, we obtained high correlations between intake of caffeinated coffee and other caffeinated beverages from the FFQ and four 1-week diet records (coffee, r = 0.78; tea, r = 0.93; and caffeinated sodas, r = 0.85)[21].
In the WGHS, caffeine intake was assessed at baseline (1991) using the same FFQ and caffeine algorithm as the NHS [25].
HPFS participants have been followed with repeated FFQs every 4 years. Caffeine-intake was assessed by the same methods as described above for the NHS cohort. In a validation study in a subsample of participants, we obtained high correlations between consumption of coffee and other caffeinated beverages estimated from the FFQ and consumption estimated from repeated 1-wk diet records (coffee: r = 0.83; tea: r = 0.62; low-calorie caffeinated sodas: r = 0.67; and regular caffeinated sodas: r = 0.56)[21]. For the present analysis, we included the participants mean caffeine intakes of the 1986 (baseline) and 1990 FFQs.
In the ARIC study, caffeine consumption was quantified at the baseline (1987–1989) examination from an interview-administered 66-item semi-quantitative FFQ[19], [20]. The Harvard Nutrition Database was used to assign caffeine (and nutrient) content to each of the food and beverage line items. Line items quantifying consumption of caffeine-containing beverages included sodas (regular and diet), coffee, and tea. The frequency of consumption of each of these items was multiplied by their caffeine content and summed across all beverages to obtain a total caffeine intake value.
Caffeine intake in the PLCO trial was assessed at the randomization phase (between 1992–2001) using responses from a FFQ developed at the National Cancer Institute called the Diet History Questionnaire (DHQ). The DHQ was previously validated against four 24 hour dietary recalls [26] and asks about consumption frequency of 124 food items over the past 12 months, including the primary sources of caffeine: coffee, tea, and soft drinks. For soft drinks, participants selected among 10 possible frequency response categories from “never” to “6+ times per day,” with three possible portion size response categories: <12 ounces or <1 can or bottle; 12–16 ounces or 1 can or bottle; or >16 ounces or >1 can or bottle. Frequency and portion size for coffee and tea were queried together as cups per unit time ranging from “none” to “6 or more cups per day.” For all three of the above beverages, participants were asked the proportion of the time each were consumed in decaffeinated form (almost never or never, about ¼ of the time, about ½ the time, about ¾ of the time, almost always or always). From these responses daily consumption of caffeine was computed taking into account the caffeine content, portion size, and frequency of intake. Caffeine estimates were derived from two 24-hour dietary recalls administered in the 1994-96 Continuing Survey of Food Intake by Individuals (CSFII)[27], a nationally representative survey conducted during the period when the DHQ was being administered. Individual foods/beverages reported on the recalls were placed in food groups consistent with items on the DHQ and weighted mean nutrient values based on survey data were derived for adults stratified by sex using methods previously described [28].
Imputation
Each study used either MACH [29] (ARIC, NHS, HPFS, WGHS) or IMPUTE [30] (PLCO) to impute up to ∼2.5 million autosomal SNPs with NCBI build 36 of Phase II HapMap CEU data (release 22) as the reference panel. Genotypes were imputed for SNPs not present in the genome-wide arrays or for those where genotyping had failed to meet the quality control criteria. Imputation results are summarized as an “allele dosage” (a fractional value between 0 and 2), defined as the expected number of copies of the minor allele at that SNP.
Phenotype Harmonization and Model Selection
The algorithm used for the calculation of caffeine intake was study-specific to allow for differences in questionnaires and consumption habits in different study populations. Raw caffeine-intake measures were skewed across studies and after exploring a variety of transformation options, we found that a cubic-root transformation was very close to the most optimal transformation identified by the Box-Cox procedure and was used to ensure normality of the residuals. Our final models were also adjusted for age (continuous), sex, case-control status (if applicable), study-site (if applicable), smoking status (never, former, and current: 2 categories), and study specific eigenvectors (see Table S5 for study-specific models). Adjustment for smoking status was appropriate given the strong correlation between smoking and caffeine intake that might impede our ability to uncover caffeine-specific loci. Each study collected information on smoking status at the time FFQ were administered. A flexible modeling approach was used to accommodate the different methods by which smoking was collected across studies, but all included never, former and two categories of current smokers. Further adjustments for body-mass-index did not change results appreciably.
Study-Level GWAS
Each study performed genome-wide association testing for normalized caffeine-intake across ∼2.5 million SNPs, based on linear regression under an additive genetic model. Analyses were adjusted for additional covariates as described above and further detailed in Table S5. Imputed data (expressed as allele dosage) were examined using ProbABEL[31] or R (scripts developed in-house). The genomic inflation factor λ for each study as well as the meta-analysis was estimated from the median χ2 statistic.
Meta-Analysis
Meta-analysis was conducted using a fixed effects model and inverse-variance weighting as implemented in METAL (see URLs in Text S1). The software also calculates the genomic control parameter and adjusts each study's standard errors. Fixed effects analyses are regarded as the most efficient method for discovery in the GWAS setting [32]. Heterogeneity across studies was investigated using the I2 statistic[33]. We applied stringent quality filters to imputed SNPs prior to meta-analysis; removing those with <0.02 MAF and/or with low imputation quality scores. The latter was defined as Rsq≤0.80 for SNPs imputed with MACH and proper_info≤0.7 for SNPs imputed with IMPUTE. X and Y chromosome, pseudosomal and mitochondrial SNPs were not included for the present analysis. We retained only SNP-phenotype associations that were based on results from at least 2 of the 10 participating studies and if greater than 50% of the samples contributing to the results were genotyped. Additional checks for experimental biases were implemented for notable associations including manual inspection of SNP (if imputed, an assayed SNP in high LD) cluster plots, and evaluation of HWE, and comparison of study MAFs to the HapMap CEPH panel. We considered P-values <5×10−8 to indicate genome-wide significance [34].
Candidate Gene–Based Analyses
We examined 515 SNPs in 23 genes (±50 kb) either previously studied or members of the key biological pathway: ‘Caffeine metabolism’ (KEGG [35], supplemented with candidates from[10], [36]) for association with caffeine consumption in our GWA meta-analysis sample. SNPs mapping to TAS2R10, 43 and 46, implicated in the oral detection of caffeine, did not pass our stringent QC criteria and thus were not included. Gene-based analyses were performed using VEGAS [37]. The software applies a test that incorporates information from a set of markers within a gene (or region) and accounts for LD between markers by using simulations from the multivariate normal distribution. The number of simulations per gene is determined adaptively. In the first stage, 1000 simulations are performed. If the resulting empirical P value is less than 0.1, 10000 simulations are performed. If the empirical P value from 10000 simulations is less than 0.0001, the program will perform 1000000 simulations. At each stage, the simulations are mutually exclusive. For computational reasons, if the empirical P value is 0, then no more simulations will be performed. An empirical P value of 0 from 1000000 simulations can be interpreted as P<10 E-6, which exceeds a Bonferroni-corrected threshold of P<2.8E-6 [∼0.05/17,787 (number of autosomal genes)].
Supporting Information
QQ plots for study-level GWAS of caffeine consumption. Results for genotyped and imputed SNPs denoted by red and blue points, respectively.
(TIFF)
Regional association plots of the two caffeine-associated loci. SNPs are plotted with their meta-analysis P-values (as -log10 values) as a function of genomic position (NCBI Build 36). In each panel, the index association SNP is represented by a diamond. Estimated recombination rates (taken from HapMap CEU) are plotted to reflect the local LD structure. SNP color indicates LD with the index SNP according to a scale from r2 = 0 to r2 = 1 based on pairwise r2 values from HapMap CEU. Plots were created using LocusZoom (see URLs).
(TIFF)
Genome-wide meta-analysis of caffeine consumption: All SNPs P<10−4.
(DOCX)
Genome-wide meta-analysis of caffeine consumption (P<10−6): Gender and study effects.
(DOCX)
Mean caffeine intakes (mg/d) by rs4410790 genotype.
(DOCX)
Mean caffeine intakes (mg/d) by rs2470893 genotype.
(DOCX)
Study-specific genotyping, imputation and statistical analysis.
(DOCX)
Sample quality control.
(DOCX)
Study population descriptions and URLS.
(DOC)
Acknowledgments
ARIC: The authors thank the staff and participants of the ARIC study for their important contributions.
NHS and HPFS: The NHS Breast Cancer GW scan was performed as part of the Cancer Genetic Markers of Susceptibility initiative of the NCI. We particularly acknowledge the contributions of R. Hoover, A. Hutchinson, K. Jacobs and G. Thomas. The NHS/HPFS type 2 diabetes GWAS is a component of a collaborative project that includes 13 other GWAS funded as part of the Gene Environment-Association Studies (GENEVA) under the NIH Genes, Environment and Health Initiative (GEI). Assistance with phenotype harmonization and genotype cleaning, as well as with general study coordination, was provided by the GENEVA Coordinating Center and Genotyping Centers (Broad Institute of MIT and Harvard, and Johns Hopkins University Center for Inherited Disease Research). We acknowledge the study participants in the NHS and HPFS for their contribution in making this study possible.
PLCO: The authors thank Drs. Christine Berg and Philip Prorok, Division of Cancer Prevention, National Cancer Institute, the Screening Center investigators and staff of the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial, Mr. Tom Riley and staff, Information Management Services, Inc., Ms. Barbara O'Brien and staff, Westat, Inc. We recognize Mr. Tim Sheehy and staff, of the DNA Extraction and Staging Laboratory, SAIC-Frederick, Inc, and Ms. Jackie King and staff, BioReliance, Inc. Most importantly, we acknowledge the study participants for their contributions to making this study possible.
Footnotes
The authors have declared that no competing interests exist.
ARIC is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute (NHLBI) contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, N01-HC-55022, R01HL087641, R01HL59367, and R01HL086694; National Human Genome Research Institute contract U01HG004402; and NIH contract HSN268200625226C. Infrastructure was partly supported by Grant Number UL1RR025005, a component of the NIH and NIH Roadmap for Medical Research. The NHS Breast Cancer GW scan was performed as part of the Cancer Genetic Markers of Susceptibility initiative of the NCI. We particularly acknowledge the contributions of R. Hoover, A. Hutchinson, K. Jacobs, and G. Thomas. The current research is supported by CA 40356 and U01-CA98233 from the NCI. The NHS/HPFS type 2 diabetes GWAS (U01HG004399) is a component of a collaborative project that includes 13 other GWAS funded as part of the Gene Environment-Association Studies (GENEVA) under the NIH Genes, Environment, and Health Initiative (GEI) (U01HG004738, U01HG004422, U01HG004402, U01HG004729, U01HG004726, 01HG004735, U01HG004415, U01HG004436, U01HG004423, U01HG004728, AHG006033) with additional support from individual NIH Institutes (NIDCR: U01DE018993, U01DE018903; NIAAA: U10AA008401; NIDA: P01CA089392, 01DA013423; NCI: CA63464, CA54281, CA136792, Z01CP010200). Assistance with genotype cleaning and general study coordination, was provided by the GENEVA Coordinating Center (U01HG004446). Assistance with data cleaning was provided by the NCBI. Genotyping was performed at the Broad Institute of MIT and Harvard, with funding support from the NIH GEI (U01HG04424), and Johns Hopkins University Center for Inherited Disease Research, with support from the NIH GEI (U01HG004438) and the NIH contract "High throughput genotyping for studying the genetic contributions to human disease" (HHSN268200782096C). Additional funding for the current research was provided by the NCI (P01CA087969, P01CA055075) and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK, R01DK058845). The NHS/HPFS CHD GWAS was supported by HL35464 and CA55075 from the NIH with additional support for genotyping from Merck/Rosetta Research Laboratories, North Wales, PA. The NHS/HPFS Kidney GWAS was supported by NIDDK: 5P01DK070756. PLCO was supported the Intramural Research Program of the Division of Cancer Epidemiology and Genetics and by contracts from the Division of Cancer Prevention, National Cancer Institute, NIH, DHHS. The WGHS is supported by HL 043851 and HL69757 from the NHLBI and CA 047988 from the NCI, the Donald W. Reynolds Foundation, and the Fondation Leducq, with collaborative scientific support and funding for genotyping provided by Amgen. MCC is a recipient of a Canadian Institutes of Health Research Fellowship. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Frary CD, Johnson RK, Wang MQ. Food sources and intakes of caffeine in the diets of persons in the United States. J Am Diet Assoc. 2005;105:110–113. doi: 10.1016/j.jada.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 2.Fredholm BB, Battig K, Holmen J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51:83–133. [PubMed] [Google Scholar]
- 3.Yang A, Palmer AA, de Wit H. Genetics of caffeine consumption and responses to caffeine. Psychopharmacology (Berl) 2010 doi: 10.1007/s00213-010-1900-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferre S. An update on the mechanisms of the psychostimulant effects of caffeine. J Neurochem. 2008;105:1067–1079. doi: 10.1111/j.1471-4159.2007.05196.x. [DOI] [PubMed] [Google Scholar]
- 5.Tobacco and Genetics Consortium. Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat Genet. 2010;42:441–447. doi: 10.1038/ng.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jorge-Nebert LF, Jiang Z, Chakraborty R, Watson J, Jin L, et al. Analysis of human CYP1A1 and CYP1A2 genes and their shared bidirectional promoter in eight world populations. Hum Mutat. 2010;31:27–40. doi: 10.1002/humu.21132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ueda R, Iketaki H, Nagata K, Kimura S, Gonzalez FJ, et al. A common regulatory region functions bidirectionally in transcriptional activation of the human CYP1A1 and CYP1A2 genes. Mol Pharmacol. 2006;69:1924–1930. doi: 10.1124/mol.105.021220. [DOI] [PubMed] [Google Scholar]
- 8.Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol. 2003;43:309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- 9.Zhou SF, Wang B, Yang LP, Liu JP. Structure, function, regulation and polymorphism and the clinical significance of human cytochrome P450 1A2. Drug Metab Rev. 2010;42:268–354. doi: 10.3109/03602530903286476. [DOI] [PubMed] [Google Scholar]
- 10.Kot M, Daniel WA. The relative contribution of human cytochrome P450 isoforms to the four caffeine oxidation pathways: an in vitro comparative study with cDNA-expressed P450s including CYP2C isoforms. Biochem Pharmacol. 2008;76:543–551. doi: 10.1016/j.bcp.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 11.Gunes A, Dahl ML. Variation in CYP1A2 activity and its clinical implications: influence of environmental factors and genetic polymorphisms. Pharmacogenomics. 2008;9:625–637. doi: 10.2217/14622416.9.5.625. [DOI] [PubMed] [Google Scholar]
- 12.Corchero J, Pimprale S, Kimura S, Gonzalez FJ. Organization of the CYP1A cluster on human chromosome 15: implications for gene regulation. Pharmacogenetics. 2001;11:1–6. doi: 10.1097/00008571-200102000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Harper PA, Wong JY, Lam MS, Okey AB. Polymorphisms in the human AH receptor. Chem Biol Interact. 2002;141:161–187. doi: 10.1016/s0009-2797(02)00071-6. [DOI] [PubMed] [Google Scholar]
- 14.Huang ZL, Qu WM, Eguchi N, Chen JF, Schwarzschild MA, et al. Adenosine A2A, but not A1, receptors mediate the arousal effect of caffeine. Nat Neurosci. 2005;8:858–859. doi: 10.1038/nn1491. [DOI] [PubMed] [Google Scholar]
- 15.Ledent C, Vaugeois JM, Schiffmann SN, Pedrazzini T, El Yacoubi M, et al. Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature. 1997;388:674–678. doi: 10.1038/41771. [DOI] [PubMed] [Google Scholar]
- 16.Rogers PJ, Hohoff C, Heatherley SV, Mullings EL, Maxfield PJ, et al. Association of the anxiogenic and alerting effects of caffeine with ADORA2A and ADORA1 polymorphisms and habitual level of caffeine consumption. Neuropsychopharmacology. 2010;35:1973–1983. doi: 10.1038/npp.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cornelis MC, El-Sohemy A, Campos H. Genetic polymorphism of the adenosine A2A receptor is associated with habitual caffeine consumption. Am J Clin Nutr. 2007;86:240–244. doi: 10.1093/ajcn/86.1.240. [DOI] [PubMed] [Google Scholar]
- 18.Luciano M, Kirk KM, Heath AC, Martin NG. The genetics of tea and coffee drinking and preference for source of caffeine in a large community sample of Australian twins. Addiction. 2005;100:1510–1517. doi: 10.1111/j.1360-0443.2005.01223.x. [DOI] [PubMed] [Google Scholar]
- 19.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 20.Stevens J, Metcalf P, Dennis B, Tell G, Shimakawa T, et al. Reliability of a food frequency questionnaire by ethnicity, gender, age and education. Nutrition Research. 1996;16:735–745. [Google Scholar]
- 21.Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93:790–796. doi: 10.1016/0002-8223(93)91754-e. [DOI] [PubMed] [Google Scholar]
- 22.Park JH, Wacholder S, Gail MH, Peters U, Jacobs KB, et al. Estimation of effect size distribution from genome-wide association studies and implications for future discoveries. Nat Genet. 2010;42:570–575. doi: 10.1038/ng.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higdon JV, Frei B. Coffee and health: a review of recent human research. Crit Rev Food Sci Nutr. 2006;46:101–123. doi: 10.1080/10408390500400009. [DOI] [PubMed] [Google Scholar]
- 24.Willett WC. New York: Oxford University Press; 1998. Nutritional Epidemiology. [Google Scholar]
- 25.Ridker PM, Chasman DI, Zee RY, Parker A, Rose L, et al. Rationale, design, and methodology of the Women's Genome Health Study: a genome-wide association study of more than 25,000 initially healthy american women. Clin Chem. 2008;54:249–255. doi: 10.1373/clinchem.2007.099366. [DOI] [PubMed] [Google Scholar]
- 26.Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the Eating at America's Table Study. Am J Epidemiol. 2001;154:1089–1099. doi: 10.1093/aje/154.12.1089. [DOI] [PubMed] [Google Scholar]
- 27.Tippett K, Cypel Y. U.S. Department of Agriculture, Agricultural Research Service; 1997. Design and Operation: The Continuing Survey of Food Intakes by Individuals and the Diet and Health Knowledge Survey, 1994-96. [Google Scholar]
- 28.Subar AF, Midthune D, Kulldorff M, Brown CC, Thompson FE, et al. Evaluation of alternative approaches to assign nutrient values to food groups in food frequency questionnaires. Am J Epidemiol. 2000;152:279–286. doi: 10.1093/aje/152.3.279. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Abecasis GR. Mach 1.0: Rapid haplotype reconstruction and missing genotype inference. Am J Hum Genet. 2006;S79:2290. [Google Scholar]
- 30.Marchini J, Howie B, Myers S, McVean G, Donnelly P. A new multipoint method for genome-wide association studies by imputation of genotypes. Nat Genet. 2007;39:906–913. doi: 10.1038/ng2088. [DOI] [PubMed] [Google Scholar]
- 31.Aulchenko YS, Struchalin MV, van Duijn CM. ProbABEL package for genome-wide association analysis of imputed data. BMC Bioinformatics. 2010;11:134. doi: 10.1186/1471-2105-11-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pereira TV, Patsopoulos NA, Salanti G, Ioannidis JP. Discovery properties of genome-wide association signals from cumulatively combined data sets. Am J Epidemiol. 2009;170:1197–1206. doi: 10.1093/aje/kwp262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ioannidis JP, Patsopoulos NA, Evangelou E. Heterogeneity in meta-analyses of genome-wide association investigations. PLoS ONE. 2007;2:e841. doi: 10.1371/journal.pone.0000841. doi: 10.1371/journal.pone.0000841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pe'er I, Yelensky R, Altshuler D, Daly MJ. Estimation of the multiple testing burden for genomewide association studies of nearly all common variants. Genet Epidemiol. 2008;32:381–385. doi: 10.1002/gepi.20303. [DOI] [PubMed] [Google Scholar]
- 35.Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyerhof W, Batram C, Kuhn C, Brockhoff A, Chudoba E, et al. The molecular receptive ranges of human TAS2R bitter taste receptors. Chem Senses. 2010;35:157–170. doi: 10.1093/chemse/bjp092. [DOI] [PubMed] [Google Scholar]
- 37.Liu JZ, McRae AF, Nyholt DR, Medland SE, Wray NR, et al. A versatile gene-based test for genome-wide association studies. Am J Hum Genet. 2010;87:139–145. doi: 10.1016/j.ajhg.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
QQ plots for study-level GWAS of caffeine consumption. Results for genotyped and imputed SNPs denoted by red and blue points, respectively.
(TIFF)
Regional association plots of the two caffeine-associated loci. SNPs are plotted with their meta-analysis P-values (as -log10 values) as a function of genomic position (NCBI Build 36). In each panel, the index association SNP is represented by a diamond. Estimated recombination rates (taken from HapMap CEU) are plotted to reflect the local LD structure. SNP color indicates LD with the index SNP according to a scale from r2 = 0 to r2 = 1 based on pairwise r2 values from HapMap CEU. Plots were created using LocusZoom (see URLs).
(TIFF)
Genome-wide meta-analysis of caffeine consumption: All SNPs P<10−4.
(DOCX)
Genome-wide meta-analysis of caffeine consumption (P<10−6): Gender and study effects.
(DOCX)
Mean caffeine intakes (mg/d) by rs4410790 genotype.
(DOCX)
Mean caffeine intakes (mg/d) by rs2470893 genotype.
(DOCX)
Study-specific genotyping, imputation and statistical analysis.
(DOCX)
Sample quality control.
(DOCX)
Study population descriptions and URLS.
(DOC)