Abstract
Oxidative stress markers and peroxiredoxins are connected to cancer. A large set of urinary bladder carcinomas were studied for the expression of nitrotyrosine and 8-hydroxydeguanosine (8OHdG) , two markers indicating oxidative damage. Serum and urine 8-OHdG were assessed in a subset of patients. We also analysed immunohisto-chemically the expression of nrf2, keap1, all six peroxiredoxins (prx) and thioredoxin (trx) in these tumors. 15 % of the cases showed 8OHdG and 36 % nitrotyrosine positivity. Expression of nitrotyrosine and 8OHdG associated with a poor prognosis (p=0.050, p=0.011, respectively). Peroxiredoxin positivity ranged from 39 % to 84 % lowest expression being for prx 4 and highest for prx 3. Prx 4 expression associated with a poor prognosis (p=0.025) with high grade (p=0.044) and larger tumors (p=0.023). Cytoplasmic trx positivity was seen in 91 % and nuclear in 59 % of tumors. Nuclear and cytoplasmic trx associated with each other (p<0.001), and nuclear trx associated with prx 6 (p=0.001), prx 2 (p<0.001), and prx 5 (p<0.001). 8OHdG associated with nuclear trx positivity (p=0.002), inversely with prx 1 (p=0.025) and with keap1 (p=0.020). Nuclear nrf2 was associated with nitrotyrosine (p=0.042). The results show that the amount of oxidative stress in urinary bladder tumors affects the prognosis of the patients. Of antioxidative enzymes, prx4 associated with an unfavourable prognosis. Selective inhibition of prx4 expression might then be one additional option of treatment of bladder cancer.
Keywords: Urinary bladder, carcinoma, oxidative stress, peroxiredoxin, nrf2, keap1
Introduction
Carcinoma of the urinary bladder is the 7th most common cancer in the world [1]. Most of the cases originate from the urothelial epithelium, and these are divided in two main groups; infiltrating urothelial carcinomas and non-invasive urothelial neoplasms [1]. Development of bladder cancer is associated with exposure to exogenous carcinogens, tobacco smoking and occupational exposure to aromatic amines being the most significant factors [1].
Tobacco smoke contains many carcinogens and many of these are able to induce formation of radicals and reactive oxygen species (ROS). Oxidative and xenobiotic stress in cells is sensed by nrf2 (Nuclear factor erythroid 2-related factor 2). In case of such a stress, nrf2 is released from cytoplasmic Keap1 (Kelch-like ECH-associated protein 1), and moves to the nucleus where it induces the expression of several genes involved in the antioxidative defence, such as peroxiredoxins (Prx) [2, 3]. Nrf2 bound to keap1 is complexed with Cullin 3-based E3 ubiquitin ligase complex, ubiquitinated and then degraded in proteosomes [2, 3].
Oxidative stress can be detected in histological sections with several antibodies [4, 5]. Nitrotyrosine is an antibody detecting nitrosative stress and anti-8OHdG antibodies (8-hydroxydegua-noside) detect DNA adduct formation mainly due to hydroxyl-induced stress [5]. Previous works have shown that these markers can be used in the assessment of oxidative stress in tissues and in ovarian carcinoma, where for example, high levels of 8OHdG have been associated with a poor prognosis [5-7].
Prx are hydrogen peroxide scavenging enzymes present in procaryotic and eucaryotic cells [8, 9] They are common proteins comprising of about 0.1-0.8 % of the protein contents of the cell [8]. In mammals there are six peroxiredoxins the genes of which reside in chromosomes 1, 4, 8, 19 and X, both prx 3 and 5 being found in chromosome 19 [8]. While prx1, 2 and 6 are found in the cytosol and nuclei of the cells, prx 4 is present in the endoplasmic reticulum [8, 10]. Prx 3 and 5 are found in mitochondria, prx 5 additionally in the peroxisomes [8]. Thus different types of peroxiredoxins take care of scavenging peroxides in different cellular compartments.
Hydrogen peroxide takes part in cellular signaling and peroxiredoxins may thus influence several cellular functions, such as proliferation, migration, differentiation or apoptosis [8-11]. Peroxiredoxins are divided in three subgroups based on their cysteine groups and catalytic mechanisms. Prx 1-4 belong to typical-2-Cys, Prx 5 to atypical-2-Cys and prx 6 to 1-Cys subgroup [8]. In the typical-2-Cys subgroup sulfhydryl groups in one molecule react with those in another molecule while in atypical-2-Cys peroxiredoxin the reaction takes place between two sulfhydryl sites in the same molecule [11]. In 1-Cys peroxiredoxin there is only one reactive sulfhydryl group which reacts with that of another molecule [11].
Aberrant expression of peroxiredoxins has been found in various cancers and antibodies to some peroxiredoxins, like prx 1 have been found in sera of cancer patients [8]. Peroxiredoxins are upregulated in oxidative stress and their level predicts the radiosensitivity of tumors [8]. In earlier studies, several malignancies like malignant mesotheliomas have been shown to express high amounts of peroxiredoxins, a fact which may partly explain their therapy resistance [10].
In this study we investigated a large set of bladder tumors for oxidative damage using immunohistochemical markers for nitrotyrosine and 8OHdG and compared the results with the expression of nrf2 and keap1 in these tumors. Additionally we analysed all six peroxiredoxins and thioredoxin in these tumors. These variables were associated with clinical parameters of the tumors, such as survival and TNM-stage. Additionally, a subpopulation of cases was studied for the expression of 8OHdG adducts in the urine and serum.
Materials and methods
Materials
The tissue material consisted of 252 urothelial bladder carcinomas collected from the files at Oulu University Hospital, Oulu, Finland during 1990-2005. 67 % of the tumors were low grade and 33 % high grade. 73 % were superficial and 27 % invasive. During follow-up, 66 underwent cystectomy and 50 received radiation therapy. 120 received instillation treatment, of these 91 received Bacillus Calmette-Guérin (BCG), 6 mitomycin, 6 farmorubicin, 15 epirubicin+ interferon -alpha2b (IFN), and 2 BCG+IFN. 68 % of cases had one or more recurrence. The specimens were fixed in neutral formalin and further embedded in paraffin blocks and stored at the Department of Pathology in the same institute. The patients were surgically staged according to the current TNM classification system and the degree of tumor differentiation was classified after WHO Classification of Tumours [1]. The grade and the TNM status of tumors were obtained from patient records. The study was approved by the Local Ethics Committee of the Ostrobothnia District of Nothern Finland.
Immunohistochemistry
Paraffin-embedded tissues were first sectioned to slides of 4 μm thickness and placed on SuperFrostPlus glass, fixed at 37° C overnight, and processed further within a few days. The sections were deparaffinized in xylene and then rehydrated in descending ethanol series, incubated in 10 mM citrate buffer (pH 6.0), boiled in a microwave oven for 10 minutes, and cooled properly at room temperature before adding the primary antibody. Negative controls were prepared using the same procedure except that the primary antibodies were replaced by PBS and serum isotype controls (Zymed Laboratories, Inc.). Previously known positive control samples were also used. Table 1 shows more details of the stainings for each antibody used. For Keap1, nrf2, prx 1-6 chromogen used was aminoethylcarbazole, for 8OHdG, nitrotyrosine and thioredoxin diaminobentzidine.
Table 1.
Immunohistochemical methods used in this study
| Antibody (Clone/Product code) | Dilution | Immunostaining method | Source of primary antibody |
|---|---|---|---|
| PRDX I | 1:1500 | Histostain-Plus Bulk Kit | Labfrontier, York, United Kingdom |
| PRDX II | 1:1000 | Histostain-Plus Bulk Kit | Labfrontier, York, United Kingdom |
| PRDX III | 1:500 | Histostain-Plus Bulk Kit | Labfrontier, York, United Kingdom |
| PRDX IV | 1:500 | Histostain-Plus Bulk Kit | Labfrontier, York, United Kingdom |
| PRDX V | 1:2000 | Histostain-Plus Bulk Kit | Labfrontier, York, United Kingdom |
| PRDX VI | 1:2000 | Histostain-Plus Bulk Kit | Labfrontier, York, United Kingdom |
| TRX | 1:200 | A biotinylated secondary anti-goat antibody; avidin-biotin-peroxidase complex | American Diagnostica, Greenwich, CT |
| 8-OHdG | 1:50 | Dako Envision Kit | JaICA, Fukuroi, Japan |
| Nrf2 | 1:100 | Histostain Plus Broad Spectrum Kit | Santa Cruz Biotechnology, Santa Cruz, USA |
| Keap1 | 1:100 | Biocare Medical HRP Polymer Kit | Santa Cruz |
The immunohistochemical results were devided semiquantitatively as follows; 0=negative, 1-5 % = weak, 5-25 %=moderate, 25-75 %= strong, over 75 %= very strong. Nuclear and cytoplasmic positivity were assessed separately. The analyses were performed by five experienced pathologists (YS, PH, VK, SK, KMH). In the analysis, the results were also divided in two groups; negative ≤5 %, positive >5 %.
Enzyme-linked immunosorbent assay (ELISA)
The serum values of 8-OHdG could be determined from 36 patients and urine 8-OHdG from 76 urinary bladder carcinoma patients. Serum and urine samples were taken within one week and immunohistochemical and serum/urine samples within eight weeks from one another. Serum and urine samples were stored in polypropylene tubes or polystyrene tubes at -80 ° C until the time of analysis. To determine serum 8 -OHdG levels, Highly Sensitive 8-OHdG Check ELISA kit and for urine assessments New 8-OHdG Check ELISA kit (both from Japan Institute for the Control of Aging, Fukuroi, Japan) were used. Both kits utilize the same highly specific monoclonal 8-OHdG antibody (clone N45.1), but differ in their assay ranges. All assays were done by following the manufacturer's instructions with a few modifications. We assayed duplicates of each sample and used also the outermost wells of the microtiter plate. If concentrations from duplicate samples differed by more than 10% the assay was repeated.
Statistical analysis
All statistical analyses were done using SPSS 17.0 for Windows (Chicago, IL). The significance of associations was defined using Fisher's exact test, chi-square test, Mann-Whitney test, and Kruskal-Wallis test. The log rank test, Breslow, Tarone-Ware and Kaplan-Meier curves were used in the survival analysis. Values ≤. 0.05 were considered statistically significant.
Results
The level of markers studied in tumors
In the bladder tumors studied (n=252 for prx 1-6, trx, 8OHdG and nitrotyrosine, n=173 for keap1 and nrf2), the expression of the studied proteins was as follows; cytoplasmic nrf2 positivity was seen in 87.7 % and nuclear positivity in 8% of cases. Keap1 positivity was seen in 36.6 % of cases (Figure 1). Trx cytoplasmic positivity was seen in 91.1 % and nuclear in 59.3 %. 14.9 % showed nuclear 8OHdG positivity and 36.2 % nitrotyrosine positivity (Figure 2 and 3). The extent of cytoplasmic positivity for prxs is shown in Table 2. Nuclear positivity was seen in 4.0 % of cases with prx 1, 5.0 % of cases with prx 2, 2.2 % of cases with prx 4 and 18.7 % of prx 6. Prx 3 and prx 5 were negative for nuclear staining.
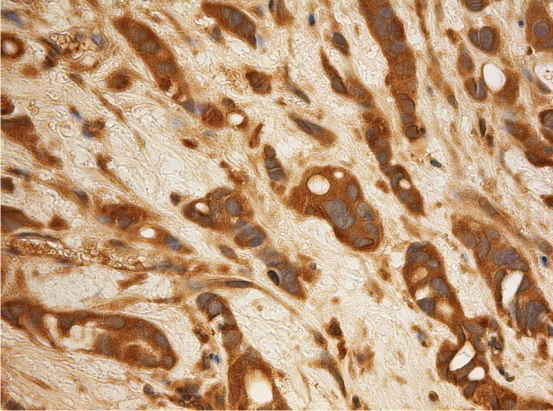
Figure 1.
Invasive urothelial carcinoma showing cyto-plasmic positivity for keap1. Chromogen used amino-ethylcarbazole.
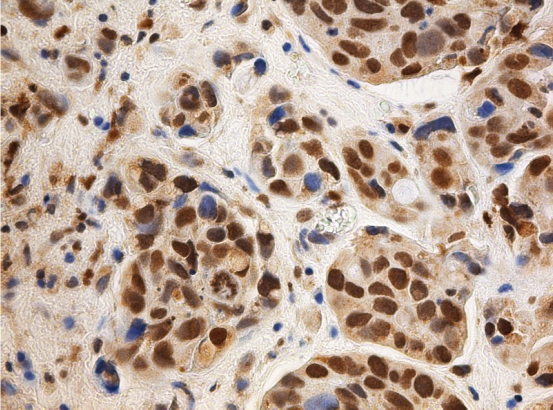
Figure 2.
In an invasive urothelial carcinoma nuclear expression for 8OHdG can be seen. Chromogen used diaminobentzidine.
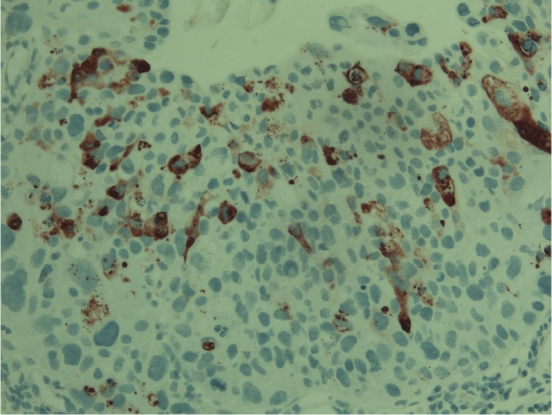
Figure 3.
Strong cytoplasmic expression for nitrotyrosine can be seen in this case of an invasive urothelial carcinoma. Chromogen used diaminobentzidine.
Table 2.
Percentage of expression of different prxs in bladder carcinoma
| prx1 | prx2 | prx3 | prx4 | prx5 | prx6 | |
|---|---|---|---|---|---|---|
| Neg | 36 | 40 | 17 | 52 | 39 | 35 |
| 1-5 % | 30 | 28 | 25 | 25 | 31 | 21 |
| 5-25 % | 19 | 9 | 25 | 11 | 14 | 22 |
| 25-75 % | 13 | 12 | 25 | 9 | 10 | 10 |
| >75% | 2 | 11 | 8 | 5 | 6 | 13 |
Associations between the variables in bladder tumors
The association of cytoplasmic prx with other variables is shown in Table 3. Prxs associated mostly with nitrotyrosine, nuclear trx and keap1. Curiously, no significant associations were found between prxs and 8OHdG or nrf2. Nitrotyrosine associated with nuclear prx 6 (p=0.001), nuclear 8OHdG with nuclear prx 6 (p=0.001) and prx4 (p=0.003), cytoplasmic trx (p=0.023) and with nuclear prx1. 8OHdG was associated with nuclear thioredoxin positivity (p=0.002), with nitrotyrosine (p=0.009), and inversely with keap1 (p=0.020). Nuclear nrf2 was associated with nitrotyrosine (p=0.042).
Table 3.
Association between peroxiredoxins and other markers
| 8OHdG | Nitrotyrosine | Cytoplasmic Trx | Nuclear Trx | Keap1 | cytoplasmic nrf2 | nuclear nrf2 | |
|---|---|---|---|---|---|---|---|
| prx1 | 0.14 | 0.026* | 0.244 | 0.251 | 0.053 | 0.429 | 0.659 |
| prx2 | 0.48 | 0.127 | 0.348 | <0.001* | 0.025* | 0.097 | 0.449 |
| prx3 | 0.34 | 0.024* | 0.113 | 0.531 | 0.050* | 0.389 | 0.463 |
| prx4 | 0.07 | 0.001* | 0.413 | 0.025* | 0.363 | 0.498 | 0.671 |
| prx5 | 0.26 | 0.001* | 0.573 | 0.001* | 0.482 | 0.378 | 0.462 |
| prx6 | 0.12 | <0.001* | 0.307 | 0.042* | 0.001* | 0.273 | 0.306 |
=Significant association
Keap1 was positively associated with cytoplasmic nrf2 expression (p=0.030). There was no association between cytoplasmic thioredoxin expression and KEAP1 positivity (p=0.28), however, keap1 was associated with nuclear thioredoxin expression (p=0.023). Nitrotyrosine was strongly associated with keap1 (p=0.001).
Nuclear and cytoplasmic trx associated with each other (p<0.001). Prx 6 associated with prx 2 (p=0.001), prx 4 (p=0.040), prx 5 (p=0.001), prx 3 (p=0.008). Prx 1 associated with prx 4 (p=0.026), prx 2 with prx 5 (p=0.003) and prx 3 (p=0.001), prx 3 with prx 5 (0.021). Additionally, there were many near significant associations between peroxiredoxins.
Associations with clinical characteristics
In high grade tumors there was a significantly lower expression of keap 1 (p=0.032). Such associations were not found for nrf2 or trx (either nuclear or cytoplasmic positivity). 8OHdG (p=0.202) or nitrotyrosine (p=0.083) did not associate with the grade of the tumors or the pT stage (p=0.985, p=0.179, respectively). Of peroxiredoxins, cytoplasmic prx 5 expression was inversely associated with tumor grade (p=0.006). No other peroxiredoxin showed such association. Prx4, on the other hand, was more positive in T2-T3 tumors (p=0.023) and a similar tendency was observed for prx 2 (p=0.061).
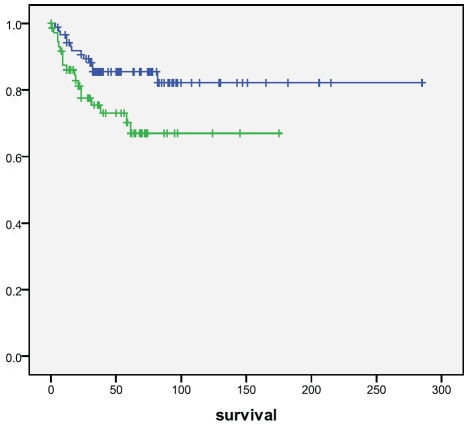
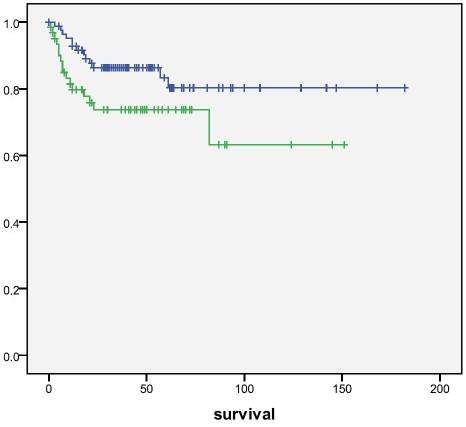
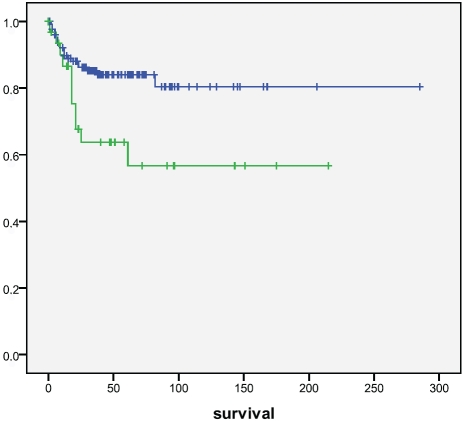
Cytoplasmic (p=0.25) or nuclear (p=0.55) nrf2 did not associate with survival, neither did keap1 (p=0.83). Of prxs,cytoplasmic prx4 positivity was associated with a worse survival (p=0.025, log rank) (Figure 4). Cases with nitrotyrosine positivity had a worse survival (p=0.050), which was even stronger with 8OHdG (p=0.011) (Figure 5 and 6). Also nuclear prx6 associated with a worse survival (p=0.03). In Cox multivariate analysis nitrotyrosine, 8OHdG or prx4 did not have independent prognostic value (data not shown).
Figure 4.
Survival of patients according to prx 4 positivity in tumors. Patients with prx 4 positive tumors had a significantly worse survival compared to those that did not (p=0.025). Upper line=prx 4 negative, lower line=prx 4 positive. Survival given in months.
Figure 5.
Survival of patients according to nitrotyrosine positivity in tumors. Patients with nitrotyrosine positive tumors had a significantly worse survival compared to those that did not (p=0.050). Upper line= nitrotyrosine negative, lower line=nitrotyrosine positive. Survival given in months.
Figure 6.
Survival of patients according to 8OHdG positivity in tumors. Patients with 8OHdG positive tumors had a significantly worse survival compared to those that did not (p=0.011). Upper line=8OHdG negative, lower line=8OHdG positive. Survival given in months.
A significant difference was found in survival between low and high grade tumors (p<0.001) and between invasive and noninvasive ones (p<0.001), as expected. There were more keap1 negative cases in high grade tumors (p=0.043). Cytoplasmic prx 4 was more often positive in high grade tumors (p=0.044). Other factors did not show any significant associations with the grade of tumors.
Tumors positive for prx2 and prx 3 had a longer disease free interval (p=0.004 and p<0.001, log rank, respectively). Also, the presence of cytoplasmic nrf2 positivity predicted a longer DFS. The presence of recurrences did not, however, associate with any of the peroxiredoxins (Figure 5).
Studies with urinary and serum levels of 8OHdG
The mean creatinine value of the patients was 106 μmol/L (range 43-272 μmol/L). The mean serum 8OHdG value was 0.24 ng/ml (0.13-0.58 ng/ml) and urinary 12.20 ng/ml (1.66-62.96 ng/ml). The serum creatinine levels correlated with serum levels of 8OHdG (R=0.739, p<0.001) but not with urinary 8OHdG (R=-0.075, p=0.52) nor did serum or urinary 8OHdG correlate with each other (R=0.257, p=0.13). Higher serum 8OHdG levels associated with high grade tumors (p=0.028). There was a trend for higher urinary 8OHdG values to associate with strong Prx4 (p=0.07) and prx 5 (p=0.099) expression. No other associations were found (data not shown).
Discussion
In this study we investigated the presence of oxidative damage in bladder tumors by using histological assessment to evaluate nitrotyrosine and 8OHdG in the material and also serum and urinary levels of 8OdG in a small set of samples. Additionally, the expression of nrf2 and keap1 as sensors of oxidative damage and the presence of peroxiredoxin expression was evaluated in the tumors. We also assessed the expression of thioredoxin, an enzyme which is able to reduce peroxiredoxins back to a functional state.
14.9 % of cases showed nuclear 8OHdG positivity and 36.2 % nitrotyrosine positivity. Levels of 8OHdG and nitrotyrosine have previously been studied in tissue sections of breast and ovarian tumors [5, 12, 13]. Expression of 8OHdG and nitrotyrosine could already be detected in benign and borderline ovarian tumors and high levels of 8OHdG in ovarian tumors associated with aggressive behavior and poor survival [5, 13]. Our results show a similar phenomenon, tumors with high nitrotyrosine and 8OHdG expression had a significantly worse survival in bladder tumors. The expression of 8OHdG and nitrotyrosine was, however, higher in ovarian carcinomas, showing an over 80 % expression with both markers [5]. Studies indicate that nitrotyrosine and 8OHdG reflecting nitric oxide and hydroxyl mediated carcinogenesis take part in the carcinogenesis of bladder cancer. This is no surprise given that smoking is one of the risk factors leading to bladder cancer [1]. In line with this also high serum levels of 8OHdG predicted a short survival in resected small cell lung carcinomas [14]. Also, smokers have higher expression of 8OHdG in urine than non-smokers [15].
We also analysed a small set of samples for serum and urinary expression of 8OHdG and associated its expression with survival. The serum and urinary levels of 8OHdG did not associate with those found in the tissues. Reasons for this may be different anatomical vascular conditions in bladder tumors and a different urinary pooling in tumor cells between non- invasive and invasive tumors. In addition, it is likely that e.g. oxidative bursts of macrophages and neutrophils, oncological treatments and various inflammatory conditions may affect serum and urine 8-OHdG concentrations. Unfortunately we had only access to a small number of urinary and serum samples of the patients. The creatinine values of the patients, however, strongly correlated with serum 8-OHdG levels indicating that its excretion from the blood is associated with kidney function.
Peroxiredoxins are antioxidative enzymes which are able to neutralize hydrogen peroxide and other organic peroxides in the tissues [8, 11]. They may also function as chaperons and thus may increase cell survival in cellular stress [8, 11]. In cancer tissue the level of peroxiredoxins is usually upregulated [8]. Our results showed also a high expression of prxs in bladder carcinoma the highest levels being for prx3 and lowest for prx4. Of all prxs studied, prx 4 was associated with a worse survival and larger tumors. Prx4 is located in the endoplasmic reticulum and extracellular space of the cells [8]. It induces NF-kB activation in cells and induces proliferation in breast cancer cell lines [16, 17]. Overexpression of prx4 protects from TRAIL induced apoptosis [18]. It is overexpressed in pancreatic and prostate cancer and has been suggested as a marker for prostate cancer [8]. In previous studies bladder carcinoma cells have been shown to be sensitive to TRAIL induced apoptosis and part of the prx4's influence may be due to this effect [19]. This is emphasized by the role of TRAIL in sensitizing tumor cells to BCG treatment [20]. Antisense treatment of prx4 expression might then be one additional way of treating patients with urothelial cancer.
Even though other peroxiredoxins are upregulated in cancer they did not seem to influence survival in bladder carcinoma. Regardless of this, other peroxiredoxins surely influence tumor behavior by combating oxidative stress. This is demonstrated by the association of most peroxiredoxins with nitrotyrosine expression with the exception of prx2 (Table 3). Prx2 also appeared to increase the disease free interval even though not affecting prognosis. Prx2 expression was observed in about half of the cases and its levels have previously been shown to be increased in breast and bladder cancer but reduced levels have been observed in the progression of bladder cancer and melanoma [8]. We did not analyse such sequencial data, but prx2 had a tendency to be more expressed in T2-T3 tumors suggesting its activation in more widespread bladder carcinomas. However, the influence on disease free interval remains obscure.
Prx3 and prx 5 are exceptional among peroxiredoxins in that they are located in mitochondria, where the oxidative stress is most abundant [8]. Prx3 was also the most frequently expressed peroxiredoxin in our material. In breast cancer prx3 has been associated to a better prognosis perhaps because it also associates with receptor positive tumors [21]. In our material prx3 associated with an extended disease free interval but not with prognosis. C-myc is known to regulate prx3 but its upregulation is not high enough in bladder cancer to account for the high expression state [22]. Prx 5 is also overexpressed in breast cancer and in mesothelioma but much is not known about prx5 in other tumors except for ovarian tumors where prx5 was associated with higher stage [5,23].
Prx1 has rather extensively been studied in cancer. Prx1 knockdown mice have an increased risk of cancer and prx 1 levels are increased in several cancer types such as breast, lung, ovarian, esophageal and pancreatic cancer [8, 12, 21, 23, 24]. In lung cancer, prx1 was an independent prognostic factor [8]. Quan et al studied the mRNA expression of prx1 and 6 in a set of 149 tumor specimens [25]. They found both prxs to be elevated in tumor tissue but their expression was enhanced in tumors showing no recurrence or progression compared to those that did [25]. In our immunohistochemical analysis we did not find such an association with any of the prxs (data not shown). Nuclear prx6 was, however, associated with a poor prognosis. This is in line with the emerging importance of peroxiredoxins also in nuclear redox signaling [26].
Thioredoxin reduces peroxiredoxins back to a functional state. Not surprisingly nuclear trx associated with prx 6 (p=0.001), prx 2 (p<0.001), and prx 5 (p<0.001). Previously, high levels of thioredoxin in urinary bladder carcinoma cell lines have induced resistance for chemotherapeutic agents such as cisplatin [27]. High expression of thioredoxin is not surprising given the role of tobacco smoking in the evolution of bladder carcinoma. In line with this trx was especially associated with 8OHdG expression. Thioredoxin is also able to influence the attachment of transcriptional factors such as p53, NF-kB and AP-2 to DNA [28]. It also stimulates HIF-1alpha and in this way induces angio-genesis [29]. High expression of thioredoxin in bladder carcinoma, as has been detected also in other carcinomas, has multiple effects on cell proliferation, differentiation, angiogenesis and redox regulation [29]. Regardless of this we found no evident influence on patient survival.
Oxidative and xenobiotic stress is sensed by nrf2, and such stress induces detachment of nrf2 from a complex with keap1 in the cytoplasm.Nrf2 is then moved to the nucleus where it attaches to antioxidant response elements in the promoter regions of antioxidative genes thus inducing expression of several antioxidative genes [30]. In line with this, nuclear expression of nrf2 was significantly associated with nitrotyrosine positivity implicating that in tumors with higher oxidative stress nrf2 is shifted to nucleus and protective mechanisms agaist oxidative stress are evoked. The proportion on tumors expressing nuclear positivity was, however, low. This might suggest that nrf2 might be dysregulated in a proportion of bladder cancers. There was, however, no mutation in a set of 29 urinary bladder samples studied by Kim et al [31]. Nrf2 cytoplasmic positivity was, however, observed in 87 % of cases and it associated with keap1 positivity suggesting a functional relationship between the two. Lack of nrf2 function however, appears to be relevant in N-nitrosobutyl(4-hydroxybutyl)amine- induced bladder carcinogenesis in mice [32]. Nuclear nrf2 expression was, however, associated with nitrotyrosine.
In conclusion, our results show that 8OHdG and nitrotyrosine are prognostic factors in urinary bladder carcinoma. All peroxiredoxins except prx2 associated with the expression of nitrotyrosine suggesting that peroxiredoxins are specifically elevated in nitric oxide mediated oxidative stress. Nuclear thioredoxin associated with prx2, 4, 5 and 6. Prx 4 was associated with a poor survival, a fact which might be related to its protection of bladder tumor cells from TRAIL-induced apoptosis.
Acknowledgments
The expert technical assistance of Mr Manu Tuovinen is appreciated. The study was supported by the KEVO foundation of Kuopio University Hospital. The assistance and comments of Mr Pasi Hirvikoski, MD, PhD and Ms Saila Kauppila, MD, PhD is greatly appreciated.
References
- 1.Eble JN, Sauter G, Epstein JI, Sesterhenn I. WHO Classification of tumours. Lyon, France: IARC Press; 2004. Tumours of the Urinary System and Male Genital Organs. [Google Scholar]
- 2.Itoh K, Mimura J, Yamamoto M. Discovery of the Negative Regulator of Nrf2, Keap1: A Historical Overview. Antioxid Redox Signal. 2010 doi: 10.1089/ars.2010.3222. (in press) [DOI] [PubMed] [Google Scholar]
- 3.Lau A, Villeneuve NF, Sun Z, Wong PK, Zhang DD. Dual roles of Nrf2 in cancer. Pharmacol Res. 2008;58:262–70. doi: 10.1016/j.phrs.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karihtala P, Soini Y. Reactive oxygen species and antioxidant mechanisms in human tissues and their relation to malignancies. APMIS. 2007;115:81–103. doi: 10.1111/j.1600-0463.2007.apm_514.x. [DOI] [PubMed] [Google Scholar]
- 5.Karihtala P, Soini Y, Vaskivuo L, Bloigu R, Puistola U. DNA adduct8-hydroxydeoxyguanosine, a novel putative marker of prognostic significance in ovarian carcinoma. Int J Gynecol Cancer. 2009;19:1047–51. doi: 10.1111/IGC.0b013e3181ad0f0d. [DOI] [PubMed] [Google Scholar]
- 6.Murtas D, Piras F, Minerba L, Ugalde J, Floris C, Maxia C, Demurtas P, Perra MT, Sirigu P. Nuclear 8-hydroxy-2'-deoxyguanosine as survival biomarker in patients with cutaneous melanoma. Oncol Rep. 2010;23(2):329–35. Feb. [PubMed] [Google Scholar]
- 7.Sheridan J, Wang LM, Tosetto M, Sheahan K, Hyland J, Fennelly D, O'Donoghue D, Mulcahy H, O'Sullivan J. Nuclear oxidative damage correlates with poor survival in colorectal cancer. Br J Cancer. 2009;100(2):381–8. doi: 10.1038/sj.bjc.6604821. Jan 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang B, Wang Y, Su Y. Peroxiredoxins, a novel target in cancer radiotherapy. Cancer Lett. 2009;286:154–60. doi: 10.1016/j.canlet.2009.04.043. [DOI] [PubMed] [Google Scholar]
- 9.Knoops B, Loumaye E, Van Der Eecken V. Evolution of the peroxiredoxins. Subcell Biochem. 2007;44:27–40. doi: 10.1007/978-1-4020-6051-9_2. [DOI] [PubMed] [Google Scholar]
- 10.Kinnula VL, Lehtonen S, Sormunen R, Kaarteenaho-Wiik R, Kang SW, Rhee SG, Soini Y. Over-expression of peroxiredoxins I, II, III, V, and VI in malignant mesothelioma. J Pathol. 2002;196:316–323. doi: 10.1002/path.1042. [DOI] [PubMed] [Google Scholar]
- 11.Barranco-Medina S, Lázaro JJ, Dietz KJ. The oligomeric conformation of peroxiredoxins links redox state to function. FEBS Lett. 2009;583:1809–16. doi: 10.1016/j.febslet.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 12.Sova H, Jukkola-Vuorinen A, Puistola U, Kauppila S, Karihtala P. 8-Hydroxydeoxyguanosine: a new potential independent prognostic factor in breast cancer. Br J Cancer. 2010;102:1018–23. doi: 10.1038/sj.bjc.6605565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pylväs M, Puistola U, Kauppila S, Soini Y, Karihtala P. Oxidative stress-induced antioxidant enzyme expression is an early phenomenon in ovarian carcinogenesis. Eur J Cancer. 2010;46:1661–7. doi: 10.1016/j.ejca.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Shen J, Deininger P, Hunt JD, Zhao H. 8-Hydroxy -2'-deoxyguanosine (8-OH-dG) as a potential survival biomarker in patients with nonsmallcell lung cancer. Cancer. 2007;109:574–80. doi: 10.1002/cncr.22417. [DOI] [PubMed] [Google Scholar]
- 15.Yao QH, Mei SR, Weng QF, Zhang PD, Yang Q, Wu CY, Xu GW. Determination of urinary oxidative DNA damage marker 8-hydroxy-2'-deoxyguanosine and the association with cigarette smoking. Talanta. 2004;63:617–23. doi: 10.1016/j.talanta.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 16.Lee SU, Rhee M, Min YK, Kim SH. Involvement of peroxiredoxin IV in the 16alphahydroxyestrone-induced proliferation of human MCF-7 breast cancer cells. Cell Biol Int. 2008;32:401–5. doi: 10.1016/j.cellbi.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Jin DY, Chae HZ, Rhee SG, Jeang KT. Regulatory role for a novel human thioredoxin peroxidase in NF-kappaB activation. J Biol Chem. 1997;272:30952–61. doi: 10.1074/jbc.272.49.30952. [DOI] [PubMed] [Google Scholar]
- 18.Wang HQ, Du ZX, Liu BQ, Gao YY, Meng X, Guan Y, Zhang HY. TNF-related apoptosis-inducing ligand suppresses PRDX4 expression. FEBS Lett. 2009;583:1511–5. doi: 10.1016/j.febslet.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Steele LP, Georgopoulos NT, Southgate J, Selby PJ, Trejdosiewicz LK. Differential susceptibility to TRAIL of normal versus malignant human urothelial cells. Cell Death Differ. 2006;13:1564–76. doi: 10.1038/sj.cdd.4401846. [DOI] [PubMed] [Google Scholar]
- 20.Kresowik TP, Griffith TS. Bacillus Calmette-Guerin immunotherapy for urothelial carcinoma of the bladder. Immunotherapy. 2009;1:281–8. doi: 10.2217/1750743X.1.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karihtala P, Mäntyniemi A, Kang SW, Kinnula VL, Soini Y. Peroxiredoxins in breast carcinoma. Clin Cancer Res. 2003;9:3418–24. [PubMed] [Google Scholar]
- 22.Zaharieva B, Simon R, Ruiz C, Oeggerli M, Mihatsch MJ, Gasser T, Sauter G, Toncheva D. High-throughput tissue microarray analysis of CMYC amplificationinurinary bladder cancer. Int J Cancer. 2005;117:952–6. doi: 10.1002/ijc.21253. [DOI] [PubMed] [Google Scholar]
- 23.Kinnula VL, Lehtonen S, Sormunen R, Kaarteenaho-Wiik R, Kang SW, Rhee SG, Soini Y. Overexpression of peroxiredoxins I, II, III, V, and VI in malignantmesothelioma. J Pathol. 2002;196:316–23. doi: 10.1002/path.1042. [DOI] [PubMed] [Google Scholar]
- 24.Cao J, Schulte J, Knight A, Leslie NR, Zagozdzon A, Bronson R, Manevich Y, Beeson C, Neumann CA. Prdx1 inhibits tumorigenesis via regulating PTEN/AKTactivity. EMBO J. 2009;28:1505–17. doi: 10.1038/emboj.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quan C, Cha EJ, Lee HL, Han KH, Lee KM, Kim WJ. Enhanced expression of peroxiredoxin I and VI correlates with development, recurrence and progression of human bladder cancer. J Urol. 2006;175:1512–6. doi: 10.1016/S0022-5347(05)00659-2. [DOI] [PubMed] [Google Scholar]
- 26.Lukosz M, Jakob S, Büchner N, Zschauer TC, Altschmied J, Haendeler J. Nuclear redox signaling. Antioxid Redox Signal. 2010;12:713–42. doi: 10.1089/ars.2009.2609. [DOI] [PubMed] [Google Scholar]
- 27.Yokomizo A, Ono M, Nanri H, Makino Y, Ohga T, Wada M, Okamoto T, Yodoi J, Kuwano M, Kohno K. Cellular levels of thioredoxin associated with drug sensitivity to cisplatin, mitomycin C, doxorubicin, and etoposide. Cancer Res. 1995;55:4293–6. [PubMed] [Google Scholar]
- 28.Watson WH, Yang X, Choi YE, Jones DP, Kehrer JP. Thioredoxin and its role intoxicology. Toxicol Sci. 2004;78:3–14. doi: 10.1093/toxsci/kfh050. [DOI] [PubMed] [Google Scholar]
- 29.Holmgren A, Lu J. Thioredoxin and thioredoxin reductase: current research with special reference to human disease. Biochem Biophys Res Commun. 2010;396:120–4. doi: 10.1016/j.bbrc.2010.03.083. [DOI] [PubMed] [Google Scholar]
- 30.Itoh K, Mimura J, Yamamoto M. Discovery of the negative regulator of Nrf2,Keap1: a historical overview. Antioxid Redox Signal. 2010;13:1665–78. doi: 10.1089/ars.2010.3222. [DOI] [PubMed] [Google Scholar]
- 31.Kim YR, Oh JE, Kim MS, Kang MR, Park SW, Han JY, Eom HS, Yoo NJ, Lee SH. Oncogenic NRF2 mutations in squamous cell carcinomas of oesophagus and skin. J Pathol. 2010;220:446–51. doi: 10.1002/path.2653. [DOI] [PubMed] [Google Scholar]
- 32.Iida K, Itoh K, Maher JM, Kumagai Y, Oyasu R, Mori Y, Shimazui T, Akaza H, Yamamoto M. Nrf2 and p53 cooperatively protect against BBNinduced urinarybladder carcinogenesis. Carcinogenesis. 2007;28:2398–403. doi: 10.1093/carcin/bgm146. [DOI] [PubMed] [Google Scholar]