Abstract
A case of metastatic balloon cell malignant melanoma (BCMM) is presented. The balloon melanoma cells (BMC) were absent in the shave biopsy of the primary lesion and present as a minor component in the wide and deep excision. A subsequent right neck lymph node metastasis showed complete replacement of the lymph node by large, foamy cells. Though the tumor was amelanocytic and Fontana-Masson stain failed to reveal melanin, it stained positively for S-100, HMB-45, and Melan-A. Ultrastructurally, the foamy cells were characterized by cytoplasmic vacuoliza-tion and a lack of melanosomes. The differential diagnosis of metastatic balloon cell malignant melanoma is broad, and clinicopathologic correlation may play a critical role in achieving the correct diagnosis.
Keywords: Malignant, melanoma, balloon cell, clear cell tumors
Introduction
Balloon cell malignant melanoma (BCMM) is the rarest histological type of primary cutaneous melanoma and is composed of large, polyhedral, foamy cells with abundant cytoplasmic vacuoles.[1] When BCMM metastasizes, it may present as a tumor consisting predominantly or entirely of foamy cells, also known as balloon melanoma cells (BMC), which may appear cytologically bland. Its benign morphologic appearance presents a challenge for accurate clinicopathologic diagnosis. This report presents a case of metastatic BCMM with a discussion on the multiple diagnostic modalities used, including CT scan, fine-needle aspiration, histochemical, immunohistochemical, utrastructural studies, and most importantly correlation with clinical history.
Case report
In 2010, a 79-year-old man presented with a 3.0 cm firm, mobile mass located in level III of the neck (below the hyoid bone and above the inferior border of the cricoid cartilage). The patient had a history of multiple skin cancers, including basal cell carcinoma, squamous cell carcinoma in situ, and an amelanocytic melanoma at the anterior base of the right neck diagnosed in 2007 on shave biopsy which was subsequently treated by wide and deep excision with sentinel lymph node biopsies. Clinically, the 2007 amelanocytic melanoma had appeared as a 1.0 × 0.6 cm erythematous papule.
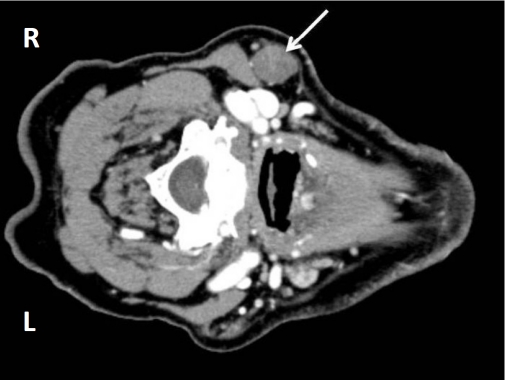
A computed tomography scan with contrast of the neck soft tissue revealed a well-circumscribed 2.3 × 1.7 × 1.6 cm round mass in the right neck, level ll/lll, abutting the inferior margin of the parotid gland (Figure 1). No additional lesions or masses were identified and other physical parameters were within normal limits.
Figure 1.
CT with contrast of the right neck mass showing a well circumscribed 2.3 × 1.7 × 1.6 cm mass (arrow).
An excisional biopsy showed a lymph node with nearly complete effacement by foamy cells. A diagnosis of metastatic balloon cell melanoma to lymph node was made after further clinical investigation as well as correlation with immunohistochemical staining and electron microscopy.
Cytopathology
Fine-needle aspiration of the right neck mass showed sheets of discohesive, large, polyhedral foamy cells with granular cytoplasm (Figure 2f). Cytoplasmic membranes were indistinct. These cells had bland nuclear features with a low nuclear to cytoplasmic ratio. Eccentrically placed nuclei were round to ovoid. Occasional intranuclear cytoplasmic inclusions and inconspicuous nucleoli were identified. No pigmentation was seen. A descriptive diagnosis was rendered and the differential diagnoses of metastatic granular cell tumor, oncocytoma, and histiocytic disorder were considered.
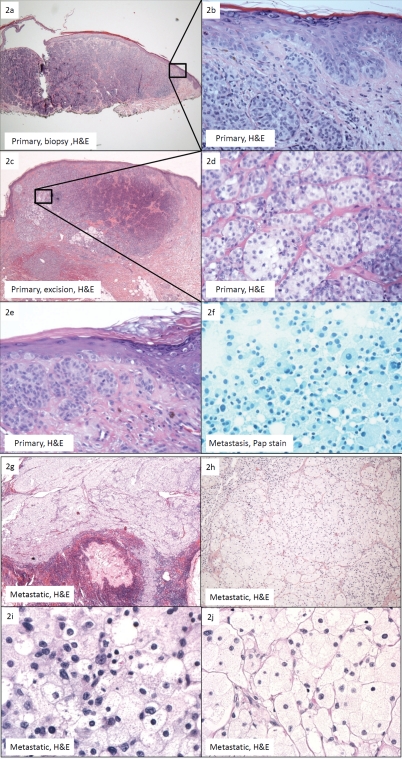
Figure 2.
(a)Primary melanoma, shave biopsy: confluent irregular nests within the dermis and as single units at the dermo-epidermal junction (H&E, ×4); (b) Close-up of Figure 2a. No clear cells or melanin pigment was identified (H&E, ×40); (c). Primary melanoma, wide and deep excision: note the minimal amount of balloon cells in a background of predominantly “basaloid” and spindle-shaped melanoma cells. The balloon cells occupy less than 50% of the tumor (H&E, 4×); (d) Primary melanoma, wide and deep excision: the polyhedral balloon cells are arranged in nests and cords separated by thin fibrous septae. They show discernabie cytopiasmic membranes and foamy to clear cytoplasm with occasional pale eosinophilic granules. They are minimally pleomorphic, variably sized, ranging from 40-100 microns in diameter, and show a central or eccentric nuclei. Few prominent nucleoi are seen (H&E, 40×); (e) Primary melanoma, wide and deep excision: intraepidermal pagetoid spread is present. Note the presence of a mitotic figure in the spindle-shaped population of melanoma cells. Hemosiderin is observed (H&E, 40×); (f) Metastatic balloon cell malignant melanoma (BCMM), fine-needle aspiration: sheets of discohesive, large, polyhedral foamy cells with granular cytoplasm. Cytopiasmic membranes are indistinct. These cells show bland nuclear features with a low nuclear to cytopiasmic ratio. Eccentrically placed nuclei are round to ovoid. Occasional intranuclear cytopiasmic inclusions and inconspicuous nucleoli are identified. No pigmentation is present (Pap, 40×); (g) Metastatic BCMM. The lymph node shows near complete replacement by balloon melanoma cells. No spindle-shaped or epithelioid melanoma cells are present H&E, 4×); (h) Metastatic BCMM. The balloon melanoma cells are arranged in nests separated by thin fibrous septae and are histologically identical to those seen in the primary lesion (H&E, 10×); (i) Metastatic BCMM. The tumor shows mild pleomorphism with focal areas of high cellular-ity and nuclear pleomorphism (H&E, 60×); (j) Metastatic BCMM. The majority of the tumor is cytologically bland with minimal pleomorphism (H&E, 60×).
Histopathology
The 2007 shave biopsy of the primary lesion from the anterior base of the right neck measured 0.7 × 0.4 × 0.2 cm and was diagnosed as melanoma with a Breslow thickness of 1.6 mm (Figure 2a). Atypical melanocytes were present as confluent irregular nests within the dermis and as single units at the dermo-epidermal junction. The tumor was composed of predominantly spindle-shaped and minimally epithelioid melanocytes (Figure 2b). No foamy cells or melanin was identified.
The subsequent wide and deep excision showed residual malignant melanoma extending to the reticular dermis with a Breslow thickness of 2.1mm. A population of polyhedral, foamy cells was identified in a background of predominantly “basaloid” and spindle-shaped atypical melanocytes (Figure 2c). Intraepidermal pagetoid spread and a mild lymphocytic reaction were observed (Figure 2e). No melanin was appreciated. The foamy cells were arranged in nests and cords separated by thin fibrous septae in 25% of the tumor (Figure 2d) [1]. The cells had discernable cytoplasmic membranes and foamy to clear cytoplasm with occasional pale eosinophilic granules. The BMC were variably sized, ranging from 40-80 microns in diameter, and had central or eccentric minimally pleomorphic nuclei. Intranuclear pseudoinclusions were identified. Prominent nucleoli were focally observed (Figure 2d). The overall mitotic count was less than 2 mitoses per 50 high power fields. Simultaneous right cervical sentinel lymph node biopsies showed seven negative lymph nodes with the aid of immunohistochemistry.
Three years later, the subsequent right neck lymph node metastasis showed a lymph node with nearly complete effacement by foamy balloon cells. Minimal lymphoid tissue was identified and no spindle-shaped or epithelioid melanoma cells were present with complete sampling (Figure 2g). Melanin was absent. The balloon cells were arranged in nests separated by thin fibrous septae and was histologically identical to those seen in the subset of balloon cells in the primary lesion (Figure 2h).
Immunohistochemistry and special stains
Immunohistochemical stains on the lymph node revealed reactivity to S-100, HMB-45, Melan-A, and vimentin, and there was no reactivity to CD10, CD68, cytokeratin, and CEA (Figures 3a-d). The Ki-67 nuclear antigen labeling index showed focal concentrations of up to 20%. The BMC were focally PAS positive and resisted diastase digestion. A Fontana-Masson stain failed to reveal melanin.
Figure 3.
Metastatic balloon cell malignant melanoma (BCMM). (a) Metastatic BCMM, positive for Melan-A (Melan-A, 40×). Inset shows primary melanoma, wide and deep excision: Melan-A showing a positive staining pattern similar to S-100. Transitional cells show darker cytopiasmic staining than balloon melanoma cells (Melan-A, 40×); (b) Metastatic BCMM, positive for S-100. Transitional cells show darker cytopiasmic staining than balloon melanoma cells (S-100, 40×); (c) Metastatic BCMM, positive for HMB-45 (HMB-45, 40×); (d) Metastatic BCMM, negative for CD68, ruling out granular cell tumor and xanthomatous lesion. However, some BCMM may be positive for CD68 (CD68, 40×).
Ultrastructural studies
Ultrastructural studies were performed on formalin fixed, deparaffinized tissue. The tumor cells showed cytoplasm filled with abundant vacuoles of variable sizes. No premelanosomes or melanosomes were identified. No cytoplasmic lipid was identified.
Discussion
The clinical presentations of BCMM are variable. BCMM have been characterized as nodular, ulcerated, pedunculated, polypoid, and papillomatous, with clinical differential diagnoses of halo nevus, fibromatous lesions, malignant melanoma, basal cell carcinoma, squamous cell carcinoma, and cutaneous adnexal tumors [1-2]. The primary lesion in our case appeared as an erythematous papule, and given the patient's history of multiple basal cell carcinomas, basal cell carcinoma had been clinically suspected.
In the absence of prior clinical and pathological history, numerous differential diagnoses were posed in this lesion composed exclusively of foamy to clear balloon cells, including renal cell carcinoma, adrenal tumors, malignant granular cell tumor, clear cell sarcoma, alveolar soft part sarcoma, perivascular epithelioid cell tumors (PECOMAS), liposarcoma, xanthomatous lesions, sebaceous lesions, hibernoma, and clear cell hidradenoma. The first case of balloon cell melanoma was reported in 1970 by Gardner [3]. As with several subsequent reports, the metastatic lesion was misinterpreted. In the case by Gardner, the lymph node metastasis was initially diagnosed as clear cell sarcoma. Ranchod reported a case in which a diagnosis of round cell liposarcoma was made on a lymph node metastasis [4]. Akslen reported a case in which the metastatic tumor mimicked clear cell renal carcinoma [5]. These cases, as well as our case, illustrate the importance of clinical investigation and correlation in reaching a diagnosis of BCMM.
Balloon cells have been reported in nevi, including intradermal, halo and combined nevi, but remain rare in melanoma[6-8]. BMC are cytologically distinct from balloon nevus cells. BMC show mild pleomorphism and may show mitotic figures. Focal areas of high mitotic count and nuclear pleomorphism can be identified (Figures 2i-j). Though rare, tumor necrosis, when seen, is supportive of melanoma. Balloon cell nevi have centrally located nuclei with no nuclear pleomorphism and no mitotic activity. Schrader suggested that the presence of multi-nucleated giant balloon cells was a feature of balloon cell nevi, but these were later found to be identified in BCMM as well [1, 6]. Maturation in balloon cell nevi is also an important clue to their benignity. Although intraepidermal pagetoid spread and a confluent junctional proliferation of BMC may be identified in BCMM, it is interesting to note that there are no reports of balloon cell melanoma in situ.
In BCMM, balloon cells are usually sparse in the primary melanoma, but have a potential of constituting the entire metastasis. In at least three cases, the primary melanoma did not exhibit any balloon cells and the tumor predominantly consisted of spindle-shaped and epithelioid cells [4, 9-10]. When balloon cells were present in the primary lesion, they were a minor component. Sondergaard reported a case of a 20-year-old man with a primary melanoma that was composed of spindle-shaped and epithelioid cells and only upon step-sectioning of the lesion did a small area of large nonpigmented clear cells with vesicular cytoplasm appear [11]. This discovery reiterates the importance of thorough examination of the entire tissue. When BMC are a minimal component of the primary lesion, a diagnosis of malignant melanoma with balloon cells is made, rather than BCMM. Kao has defined cutaneous balloon cell melanoma as a tumor composed of more than 50% foamy cells [1].
The main reason BCMM can be mistaken for a benign clear cell neoplasm is its bland cytology (Figure 2j). Another reason for the diagnostic difficulty is the general lack of melanin in BMC, in contrast to balloon nevus cells. In several case reports, the BMC contained no melanin pigment and stained negatively for Fontana-Masson [2, 4, 10, 12]. Melanin granules were found in sparse amounts in only nine of the 34 reported by Kao [1]. This characteristic is further exacerbated by the fact that when balloon cell melanoma metastasizes, the metastases are often composed entirely of balloon cells with no residual spindle-shaped or epithelioid component. However, if spindle-shaped and epithelioid cells are present in metastases, they are a supportive tool in the diagnosis of metastatic melanoma as these are the cells which may be pigmented and stain positively for melanin.
Immunohistochemistry is helpful in the diagnosis of BCMM, in conjunction with clinical history and other diagnostic modalities. Histological and IHC stains alone have limitations due to the wide differential diagnoses for BCMM. Notably, clear cell sarcoma also stains positively for S-100, HMB-45, Fontana-Masson, and PAS. However, whereas the tumor in our case is composed of nests of balloon cells divided by fibrous septae illustrated best by reticulin stain, clear cell sarcoma is composed of nests which are more packeted and contain fusiform cells with scattered wreath-like or syncytial giant cells. The nuclei in clear cell sarcoma are also uniform, lacking pleomorphism, and contain prominent central nucleoli. In addition, molecular analysis of clear cell sarcoma shows EWS-AFT1 fusion. Another possible diagnosis is perivascular epithelioid cell tumor (PECOMA) which stains positively for HMB-45, melan-A, tyrosinase, and in 30% of cases, positively for S-100. They also stain positively for smooth muscle actin, which may assist in differentiating the tumors from BCMM. Granular cell tumors have granular cytoplasm with abundant PAS-positive, diastase-resistant lysosomes. Granular cell tumors and xanthomatous lesions may be distinguished from BCMM by negative melan-A staining. S-100 is also negative in xanthomatous lesions. Sebaceous lesions have “bubbly” cytoplasm and the nucleus is crenated, showing a scalloped border. They are distinctly S-100 and HMB-45 negative and stain positively for epithelial membrane antigen. Renal clear cell carcinoma often shows prominent vascular stroma, hemorrhage, and tumor necrosis. It can be differentiated with positive staining for cytokeratin and CD10.
Electron microscopy of BMC shows abundant cytoplasmic membrane-bound vacuoles, scattered glycogen granules, along with mitochondria, ribosomes, and Golgi apparatus. While abnormal melanosomes may appear in the spindle-shaped and epithelioid cells, the balloon melanoma cell is often completely devoid of melanosomes or show only infrequent abnormal premelanosomes [4, 10-11, 13]. Rarely, small clusters of melanosomes and premelanosomes are identified [1].
Ranchod and Friedman both reported cases of patients surviving more than 10 and 20 years after the diagnosis of primary BCMM [4, 9]. The patient reported by Gardner lived 3 years after primary diagnosis [3]. In the case series reported by Kao, 78.9% of patients with tumors greater than 2.0 mm thick died of widespread metastasis [1]. Importantly, the prognosis of BCMM does not depend on the degree of balloon cell change, tumor size, nuclear atypia, or mitotic activity, but instead follows the prognosis patterns of conventional malignant melanoma correlating with tumor thickness. Kao reports a 37.5% overall 5-year survival rate in BCMM [1].
Conclusion
In conclusion, the macroscopic features of BCMM are variable. Histologically, BCMM can present with four cell types: 1. Balloon melanoma cells, 2. Spindle-shaped or epithelioid melanoma cells, 3. Transitional cells, and 4. Conventional nevi cells. Cutaneous balloon cell melanoma must be composed of more than 50% large clear or foamy cells [1]. Balloon cells are usually sparse or absent in the primary melanoma; however, when balloon cell melanoma metastasizes, the metastases are often composed entirely of balloon cells with no residual spindle-shaped or epithelioid component. While cytopathology and ultrastructural studies are supportive and immunohistochemistry is diagnostic, clinical history is most critical in the diagnosis of BCMM.
References
- 1.Kao GF, Helwig EB, Graham JH. Balloon cell malignant melanoma of the skin. A clinicopathologic study of 34 cases with histochemical, immunohistochemical, and ultrastructural observations. Cancer. 1992;69:2942–2952. doi: 10.1002/1097-0142(19920615)69:12<2942::aid-cncr2820691213>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 2.Aloi FG, Coverlizza S, Pippione M. Balloon cell melanoma: a report of two cases. J Cutan Pathol. 1988;15:230–233. doi: 10.1111/j.1600-0560.1988.tb00550.x. [DOI] [PubMed] [Google Scholar]
- 3.Gardner WA, Vazquez MD. Balloon Cell Melanoma. Archives of Pathology. 1970;89:470. &. [PubMed] [Google Scholar]
- 4.Ranchod M, Path MM. Metastatic Melanoma with Balloon Cell Changes. Cancer. 1972;30:1006. doi: 10.1002/1097-0142(197210)30:4<1006::aid-cncr2820300423>3.0.co;2-c. &. [DOI] [PubMed] [Google Scholar]
- 5.Akslen LA, Myking AO. Balloon Cell Melanoma Mimicking Clear Cell-Carcinoma. Pathology Research and Practice. 1989;184:548–550. doi: 10.1016/S0344-0338(89)80150-5. [DOI] [PubMed] [Google Scholar]
- 6.Schrader WA, Helwig EB. Balloon cell nevi. Cancer. 1967;20:1502–1514. doi: 10.1002/1097-0142(196709)20:9<1502::aid-cncr2820200918>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 7.Goette DK, Doty RD. Balloon Cell Nevus -Summary of Clinical and Histologic Characteristics. Arch of Dermatol. 1978;114:109–111. doi: 10.1001/archderm.114.1.109. [DOI] [PubMed] [Google Scholar]
- 8.Perez MT, Suster S. Balloon cell change in cellular blue nevus. Am J Dermatopathol. 1999;21:181–184. doi: 10.1097/00000372-199904000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Friedman M, Rao U, Fox S. The Cytology of Metastatic Balloon Cell Melanoma. Acta Cytol. 1982;26:39–43. [PubMed] [Google Scholar]
- 10.Mowat A, Reid R, Mackie R. Balloon Cell Metastatic Melanoma - an Important Differential in the Diagnosis of Clear-Cell Tumors. Histopathology. 1994;24:469–472. doi: 10.1111/j.1365-2559.1994.tb00556.x. [DOI] [PubMed] [Google Scholar]
- 11.Sondergaard K, Henschel A, Houjensen K. Metastatic Melanoma with Balloon Cell Changes - an Electron-Microscopic Study. Ultrastruct Pathol. 1980;1:357–360. doi: 10.3109/01913128009141437. [DOI] [PubMed] [Google Scholar]
- 12.Peters MS, Su WPD. Balloon Cell Malignant-Melanoma. J Am Acad Dermatol. 1985;13:351–354. doi: 10.1016/s0190-9622(85)70173-9. [DOI] [PubMed] [Google Scholar]
- 13.Nowak MA, Fatten SM, Campbell TE. Glycogen-rich malignant melanomas and glycogen-rich balloon cell malignant melanomas - Frequency and pattern of PAS positivity in primary and metastatic melanomas. Arch Pathol Lab Med. 1998;122:353–360. [PubMed] [Google Scholar]