Abstract
Spinal muscular atrophy (SMA), an inherited disease of motor neuron dysfunction, results from insufficient levels of the survival motor neuron (SMN) protein. Movement of the SMN protein as granules within cultured axons suggests that the pathogenesis of SMA may involve defects in neuronal transport, yet the nature of axon transport vesicles remains enigmatic. Here we show that SMN directly binds to the α-subunit of the coat protein I (COPI) vesicle coat protein. The α-COP protein co-immunoprecipitates with SMN, small nuclear ribonucleoprotein-associated assembly factors and β-actin mRNA. Although typically Golgi associated, in neuronal cells α-COP localizes to lamellipodia and growth cones and moves within the axon, with a subset of these granules traveling together with SMN. Depletion of α-COP resulted in mislocalization of SMN and actin at the leading edge at the lamellipodia. We propose that neurons utilize the Golgi-associated COPI vesicle to deliver cargoes necessary for motor neuron integrity and function.
INTRODUCTION
Spinal muscular atrophy (SMA) is a neuromuscular disorder characterized by degeneration of lower motor neurons with resultant muscle atrophy. Homozygous deletion of SMN1 (survival motor neuron) is the most frequent cause of severe forms of SMA (1). SMN1 encodes the 294-amino acid nucleocytoplasmic SMN protein. Why motor neurons are selectively sensitive to reduced levels of the ubiquitous SMN protein has not been resolved (2). SMN forms a stable complex with Gemin2–8 and Unrip that bind to Sm proteins and snRNAs and is absolutely necessary for the assembly of small nuclear ribonucleoproteins (snRNPs) in all tissues (3–5). The role of SMN in snRNP assembly inferred that SMN deficiency might lead to abnormal RNA splicing of essential transcripts in motor neurons, and although there is evidence to support this hypothesis, this causality is not without controversy (6,7). An alternative proposal is that SMN deficiency alters mRNA transport in axons. SMN along with Gemin2 and Gemin3 have been visualized within the axon and growth cone (8). In SMA model organisms, mutations in SMN orthologs lead to partial defects in motor axon outgrowth and pathfinding, neuromuscular junction defects and progressive degeneration of motor neurons (9,10). These observations suggest that SMN may participate in axonal transport of proteins and/or RNA cargo molecules in motor neurons and consistent with this hypothesis, SMN is visualized in granules that oscillate in neuronal processes (11). Furthermore, SMN is required for efficient β-actin mRNA localization to growth cones of cultured motor neurons (12). Subtle changes in the transport of SMN or in SMN function in cargo transport could diminish motor neuron function and lead to the progressive neuromuscular degeneration observed in SMA. Interestingly, a compound that facilitates actin dynamics, but not snRNP function, has been reported to extend survival in SMA mice (13).
Coatomer is a conserved coat protein I (COPI) vesicle coat protein complex mediating intra-Golgi and Golgi to ER trafficking. This heptameric protein complex is comprised of α-COP (160 kDa), β-COP (110 kDa), β'-COP (110 kDa), γ-COP (98 kDa), δ-COP (61 kDa), ɛ-COP (36 kDa) and ζ-COP (20 kDa) (14–17). Interestingly, the coatomer subunits α-COP and β-COP associate with asialoglycoprotein receptor (ASGR) and κ-opioid receptor (kor) mRNAs, respectively, and it has been speculated that coatomer may be involved in translational regulation and transport and localization of mRNA molecules (18,19). We discovered α-COP is an SMN-interacting protein and present data that imply this highly conserved Golgi-associated vesicle coat complex are utilized by neuronal cells for axonal transport of SMN, Gemins and mRNAs. These findings offer new insights into the mechanism of cargo transport in neurons and the pathogenesis of motor neuron disease.
RESULTS
Interaction of SMN with α-COP
To identify novel neuronal SMN binding proteins, ∼4 million yeast colonies from a murine embryonic spinal cord yeast two-hybrid cDNA expression library were probed with murine Smn as bait. Three independent partially overlapping clones of the α-subunit of coatomer (α-COP) were isolated, the largest encoding amino acids 751–1224. Murine and human SMN proteins were confirmed to interact with α-COP by yeast two-hybrid experiments (Supplementary Material, Fig. S1). The conserved region of SMN encompassing exons 6 and 7 (amino acids 242–294) mediated association in vitro with α-COP; exon 2b (amino acids 52–91) also showed weak binding to α-COP (Supplementary Material, Fig. S2). Purified glutathione S-transferase (GST)-α-COP protein also bound to purified histidine-tagged SMN, confirming their direct interaction (Supplementary Material, Fig. S3). To extend these findings, we generated Flag epitope-tagged wild-type (amino acids 1–1224) and truncated (amino acids 1–750 and 751–1224) α-COP. Wild-type Flag-α-COP co-precipitated endogenous coatomer subunits COP (δ-COP, Fig. 1C; ɛ- and γ-COP, not shown). It is well established that coatomer rapidly assembles into a heptameric complex and is not present as free subunits except for a subfraction of ζ-COP (15,20). Importantly, endogenous SMN along with its binding partners Gemin2 and Gemin3 co-immunoprecipitated with Flag-α-COP but not with the negative control Flag-bacterial alkaline phosphatase (Fig. 1A). Flag-α-COP:751–1224 also co-precipitated endogenous SMN and Gemin2 (Fig. 1B).
Figure 1.
Coatomer associates with components of the SMN complex in vivo. (A) Flag-α-COP co-precipitated endogenous SMN, Gemin2 and Gemin3 proteins from HEK293 lysate (Flag-bacterial alkaline phosphatase). (B) The C-terminal half (amino acids 751–1224) of α-COP complexes with endogenous SMN and Gemin2. (C) Endogenous α-COP co-immunoprecipitated SMN, Gemin2, Gemin3 and δ-COP from SH-SY5Y cells, whereas control IgG or anti-clathrin antibody (TD.1) did not. (D) Anti-SMN rabbit antibodies co-precipitated α-COP from SH-SY5Y cells. As expected, Gemin2 and Gemin3 were also present. (E) RNA immunoprecipitation using anti-α-COP antibody or control IgG followed by RT–PCR for β-actin mRNA.
To investigate endogenous α-COP association with SMN, we generated antisera against a highly conserved peptide (amino acids 398–414, Supplementary Material, Fig. S4a) and characterized the antibodies by immunoblot (Supplementary Material, Fig. S4b–e), immunoprecipitation (Supplementary Material, Fig. S4f and h) and immunofluorescence (Supplementary Material, Fig. S4i–l). This antibody (CPa-1) immunoprecipitated the endogenous coatomer complex (Supplementary Material, Fig. S4h). The α-COP antiserum but not pre-immune serum co-immunoprecipitated SMN from SH-SY5Y human neuroblastoma cell lysates along with Gemin2 and Gemin3 and δ-COP. Significantly, the functionally similar but unrelated clathrin vesicle coat protein did not co-immunoprecipitate SMN (Fig. 1C). A rabbit antibody to SMN also co-immunoprecipitated endogenous α-COP protein (Fig. 1D). These results demonstrated that SMN along with two snRNP assembly factors, Gemin2 and Gemin3, physically associate with the COPI vesicle coat protein complex coatomer. Direct binding of SMN with the α-COP protein mediates this association, at least in vitro.
The α-COP complex incorporates the β-actin mRNA
β-Cop (Copb1) has been reported to bind to the kor mRNA (19). The endogenous α-COP protein was immunoprecipitated from formaldehyde-treated SH-SY5Y cells and mRNAs were isolated (21). Because depletion of SMN has been reported to decrease β-actin mRNA localization in growth cones (12), we were particularly interested in testing for its presence in the α-COP complex. This was confirmed by polymerase chain reaction (PCR) amplification of the β-actin mRNA in the CPa-1 immunoprecipitation but not with irrelevant immunoglobulin G (IgG) antibody (Fig. 1E).
Co-localization of SMN and α-COP
Coatomer is a cytosolic protein complex that is recruited by the GTPase Arf1 to the outer Golgi membrane, where it is required for COPI vesicle budding (22). Our anti-α-COP antibodies revealed a perinuclear Golgi-like pattern with numerous small punctate structures scattered throughout the cell; this pattern was lost upon inclusion of the antigenic peptide (Supplementary Material, Fig. S4i and j). Furthermore, the detected pattern co-localized with GFP-ɛ-COP, which is known to enter into the Golgi coatomer complex (Supplementary Material, Fig. S4k) and was coincident with the Golgi marker protein GM130 (Supplementary Material, Fig. S4l) (23). Supplementary Material, Fig. S5 shows the large degree of co-localization in primary neurons including the perinuclear Golgi and extending into the neurites. Interestingly, the surrounding glial cells have primarily Golgi-distributed α-Cop with much less SMN, indicating the distribution and expression levels are quite different in neurons.
The β-COP subunit of coatomer is also visualized in the axon, suggesting additional functions for this complex in neurons (19). We first queried the subcellular address of α-COP in primary mouse hippocampal neurons cultured in vitro. We observed abundant α-COP in Golgi, cell body and within neurites and growth cones (Fig. 2A–C). Similarly, ectopically expressed α-COP fused to the mCherry fluorescent protein localized to the growth cone in these neurons (Fig. 2D). We tested for the presence of another subunit of coatomer, γ2-COP, which co-localized with the α-COP within the growth cone (Fig. 2E–G), implying the presence of assembled coatomer complexes. In human neuroblastoma SH-SY5Y cells differentiated with retinoic acid and brain-derived neurotrophic factor (BDNF), α-COP also localized to the growth cone (Fig. 2H and I).
Figure 2.
α-COP is transported within the axon toward the growth cone in primary neurons. (A–G) Cultured primary mouse hippocampal neurons (HPN). Endogenous α-COP in the cell body (arrowhead in A and B) and the growth cone (arrow in B and C) as detected by affinity-purified anti-α-COP antibody. Similarly, ectopically expressed α-COP: mCherry localized in the cell body (arrowhead) and the growth cone (arrow) in HPN (D). Anti-γ2-COP antibody detected endogenous γ2-COP in the growth cone (green, arrow in E and F) and co-localized with α-COP (red in G). (H and I) Human SH-SY5Y cells differentiated with retinoic acid and BDNF. α-COP localized in the cell body (green, arrowhead in H) and the growth cone (arrow in I). (J–O) Chicken primary RGC. Anti-α-COP detected α-COP in the cell body (green, arrowhead in J) and distributed as granules along the axon (green in K). It was also abundant in the growth cone (red, arrow in L and M). (N and O) Live chicken RGC neurons ectopically expressing α-COP: mCit. α-COP: mCit localized in the same perinuclear Golgi (black arrowhead in N) and moved toward the growth cone (O, arrow indicates the stationary granule; also see Supplementary Material, Movie S1).
To gain insight into the function of SMN association with α-COP, we studied their localization in early and late cultures of primary mouse hippocampal and motor neurons. Both concentrated to lamellipodia in immature neurons (<1 day in vitro) and weakly stained if at all at the perinuclear Golgi (Supplementary Material, Fig. S6a–i). In motor neurons (Fig. 3A–E) and mature hippocampal neurons (data not shown), α-COP and SMN were visualized in axonal growth cones. Extensive co-localization was prominent in early neurons that appeared to decrease as neurons matured.
Figure 3.
SMN and α-COP localization partially overlap in neurites and growth cones. (A–E) Primary murine motor neuron. Immunodetection of α-COP (red in C, green in E) and SMN (green in D, red in E). Co-localization of SMN with α-COP appears yellow in (B) and (E). (F–H) Nerve growth factor-differentiated PC12 cells expressing α-COP:mCherry and SMN: mCit. Abundant α-COP extends from the neurite into the growth cone. All SMN granules (green) are adjacent to and move with α-COP. SMN transiently dissociate and re-associate with α-COP as they move distally (Supplementary Material, Movie S2).
The CPa-1 antisera were raised to an evolutionarily highly conserved peptide. CPa-1 antibodies immunoprecipitated and detected the α-COP protein within Golgi, soma, axons and growth cones of cultured primary chicken retinal ganglion cell (RGC) neurons (Fig. 2J–M). The stained structures appeared like vesicles but were smaller and distinct from the typical dextran-fluorescein isothiocyanate-labeled macropinocytic vesicles (data not shown). To verify and image the distribution of α-COP in live RGC axons, we electroporated an expression construct for α-COP-monomeric citrine (mCit) fluorescent protein and performed time-lapse imaging. α-COP:mCit was visualized in Golgi and soma and as round speckles within the axon (Fig. 2N and O). Movement was observed 10 min following exposure to BDNF, with these particles primarily moving toward the axonal growth cone while some exhibited retrograde motion toward the cell body (Supplementary Material, Movie S1). These results confirm that α-COP localizes to the neurite and the growth cone in primary neurons and is transported toward the axonal growth cone upon stimulation by BDNF.
We reasoned that the COPI vesicle coat protein α-COP might be involved in SMN transport within the axon. Though primary chick retinal neurons showed α-COP trafficking, when human SMN was electroporated into these cells, virtually none of these neurons retained axons, so it was not possible to study SMN and α-COP co-trafficking in this model system. We therefore chose to use PC12 cells differentiated with nerve growth factor. SMN:mCit and α-COP:mCherry were co-transfected into PC12 cells. Time-lapse confocal images revealed that these proteins move together toward the growth cone and that the majority, if not all, of the SMN was associated with α-COP, whereas α-COP free of SMN association was common (Fig. 3F–H). Their association is dynamic as there was an apparent dissociation of SMN from α-COP followed by rapid re-association as these particles progressively transited distally within the neurite (Supplementary Material, Movie S2). Although α-COP is in a complex with COPI, we sought further evidence that SMN associates with the coatomer complex. Expression of SMN:mCherry and ɛ-COP:YFP revealed rapid association and dissociation as these proteins moved within the cell body and neurite (Supplementary Material, Movie S3). In summary, these results reveal a dynamic association of SMN with two components of the COPI complex and their co-trafficking distally from the soma into the distal neurite.
Effects of α-COP depletion on neurite outgrowth
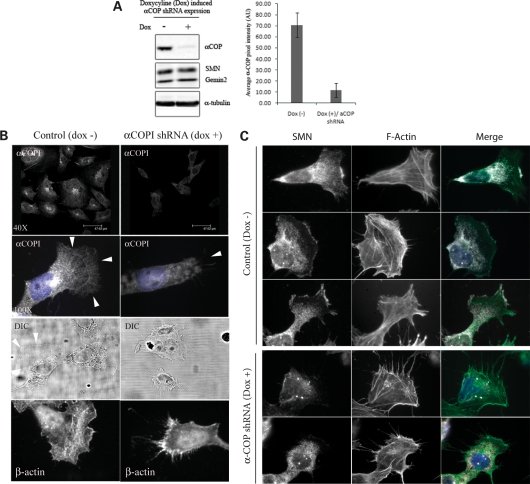
SH-SY5Y cells were neuroblastoma-derived and retain the ability to differentiate into neuronal N and S subtypes. We visualized abundant α-COP localization to growth cones in SH-SY5Y cells exposed to retinoic acid and BDNF (Fig. 2H and I) (24). To gain insight into the role of α-COP in neurite outgrowth, we established tet-inducible α-COP shRNA expression in these cells; however, unlike parental SH-SY5Y cells, the clonal-derivative cells (SH-α-COPkd) did not form prominent axons with the differentiation protocol. Induction of α-COP shRNA expression in this clone resulted in ∼80% reduction in α-COP protein expression with no detectable change in SMN and Gemin2 protein levels (Fig. 4A). After 72 h of α-COP shRNA induction, the cells exhibited a dramatically reduced lamellipodial surface area and instead demonstrated prominent filament-like extensions from the cells. These were confirmed to be filipodia by β-actin and F-actin staining (Fig. 4B and C, respectively). In control SH-SY5Y cells, α-COP localized to Golgi and extended toward the leading edge into the sheet-like region of lamellipodia. These observations are consistent with the involvement of SMN in actin metabolism (12,25,26). In uninduced SH-α-COPkd cells, SMN localized to nuclear gems and cytoplasm (Fig. 4C). Interestingly, a fraction of SMN was detectable at the leading edge and appeared just distal to the F-actin-rich region. When α-COP was depleted, SMN failed to localize to the leading edge and accumulated as granules in both cytoplasm and nucleus (Fig. 4C). Coincident with the depletion of α-COP and aberrant SMN localization, there was a noticeable increase in number and length of F-actin-rich filipodia. These results imply that α-COP is required for SMN transport to the leading edge.
Figure 4.
Depletion of α-COP alters SMN localization. (A) Doxycycline (dox)-induced α-COP shRNA expression in SH-SY5Y cells. Western blot revealed ∼80% reduction in the α-COP protein at 72 h, whereas SMN and Gemin2 levels were unchanged. (B) Top four images show a reduction in the fluorescence intensity of α-COP by immunofluorescence analysis of dox-induced α-COP shRNA cells. Note that controls cells (left) retained a spread lamellipodia with almost no filipodia, whereas cells expressing α-COP shRNA (right) showed a reduction in the lamellipodium area (arrows). The bottom four panels show that compared with uninduced control (left), cells that express α-COP shRNA (right) had prominent filipodia, visualized by differential interference contrast (DIC) microscopy, which were β-actin positive by immunofluorescence. (C) SMN and F-actin localized at the leading edge in control cells (top nine panels). α-COP knockdown induced a loss or reduction of SMN from leading edge, loss of lamellae and increased F-actin positive filipodia (bottom six panels).
DISCUSSION
The specialized function of the SMN protein that leads to motor neuron dysfunction in SMA is unknown. One proposal for the etiology of SMA is that deficiency of the SMN protein results in abnormal RNA splicing, primarily affecting U11/U12-dependent exon recognition (27). A second hypothesis is based on the observation of SMN protein motion in axons of cultured neurons (8,11) and posits a role in cargo transport. Our results reveal that SMN directly binds the α-COP component of coatomer, which defines the COPI vesicle. The identity of the structures that transport protein and mRNA within the axon remains elusive. The data we present here merge to form a new conceptual basis for this critical aspect of neurobiology.
Vesicles have been identified in the microtubule-rich central (28–30) and actin-rich peripheral regions (31,32) of axonal growth cones. The constituents of these vesicles are obscure. Typically COPI vesicles mediate Golgi to ER retrograde and intra-Golgi transport for post-translational processing of protein cargoes. Very little is known of their functional repertoire in neurons. The Golgi apparatus has long been recognized to be strategically positioned adjacent to the site where the axon sprouts (33). Asymmetric distribution of cellular proteins and localized addition of membranes are crucial for establishing and maintaining cell polarity and thus neuronal maturation and function (34,35). Our finding that α-COP proteins egress from Golgi to lamellipodia and into the axonal growth cone represents a novel, specialized utilization of these vesicles within neurons. The enrichment of α-COP in lamellipodia of immature neurons and growth cones of maturing neurons raises the tantalizing possibility that coatomer is involved in axonal morphogenesis analogous to the role of COPII vesicle coat proteins in dendrite, but not axon, formation (35). Consistent with this hypothesis, brefeldin A inhibits Arf1, the GTPase that recruits coatomer to COPI vesicles, and causes rapid arrest of axonal growth and elongation (36,37).
Only a small fraction (we estimate <5%) of the total endogenous SMN protein is in complex with coatomer. It is relevant to note that COPI subunits rapidly assemble into the coatomer complex, so free proteins are not present except for ζ-COP (16,20). Pulse-chase experiments indicate α-COP rapidly assembles into the coatomer complex and this occurs before entry of SMN (data not shown). It seems plausible that cytoplasmically exposed α-COP on the outer Golgi membrane (22) attaches to cytoplasmic SMN and its binding partners Gemin2 and Gemin3. There is electron micrographic evidence for SMN localization to the Golgi apparatus in motor neurons (38). The dynamic dissociation and re-association of SMN among α-COP granules in the axon we observed implies that SMN is not inside the coat vesicle. The limited accessibility of a subset of SMN monoclonal antibodies to the α-COP protein in coatomer and our observation that detergents destabilized their immunoprecipitation are consistent with transient interaction of two large complexes.
Coatomer along with other COPI vesicle coat proteins participates in selecting and packaging cargo molecules into COPI vesicles (22). In this regard, SMN exon 2b, which binds α-COP, is highly conserved and resembles signal sequences found in cargo proteins that are recognized by coatomer (39). SMN and Gemin proteins have been previously visualized in neurites and growth cones (8,40,41). In binding to SMN, α-COP may coordinate loading of SMN and SMN-associated factors, such as Gemin2 and Gemin3, onto COPI vesicles. COPI vesicles are known to associate with motor proteins for their transport (22). Our studies raise the intriguing possibility that the SMN complex is transported into the axon by coatomer. These results clearly depict SMN co-trafficking with α-COP in neurites toward the growth cone. In transit, SMN appears to transiently dissociate from α-COP and then rapidly re-connect with α-COP as it progresses distally within the neurite, suggesting a dynamic mechanism to convey SMN cargoes toward the growth cone. The majority of the α-COP granules were not associated with SMN, implying only a subset of COPI particles are used for transport of SMN.
An important question is whether the COPI complex transports mRNAs. α-COP bound to the 3'-untranslated region of the ASGR mRNA in vitro (18) and β-COP (Copb1) immunoprecipitated the kor and actin mRNAs (19). The polyA-binding protein Pab1p was reported to associate with yeast coatomer (42). Although none of the coatomer subunits encode canonical RNA-binding domains, α-COP and β'-COP contain N-terminal tryptophan (W), aspartic acid (D) repeats, a motif recently reported to interact with mRNA (43). Our results demonstrate that α-COP is in a complex with β-actin mRNA. SMN, Gemin2 and Gemin3 have been shown to associate with β-actin mRNA (44). SMN has been reported to bind hnRNP-R, which directly binds to β-actin mRNA (12,45). The α-Cop (data not shown) and β-Cop subunits do not bind RNA (19). More likely, the vesicle binds to other factors that specify the RNA to be mobilized. As we demonstrate, α-Cop binds to SMN, which in turn binds to RNA-binding proteins including hnRNP-R and -Q, KSRP and HuD (46–48). Thus COPI probably represents the upstream scaffold for attachment of multiple RNA-binding proteins. The RNA-binding protein HuR is known to be present in coatomer (19). Coatomer has also been shown to regulate mRNA translation (18), and therefore associated mRNAs might be silenced by coatomer while in transit to lamellipodia, growth cone or distal axon. Taken together, these results suggest that components of the COPI vesicle coat protein complex participate in the transport and translational regulation of mRNA molecules. We are pursuing experiments to identify and quantify RNAs in the COPI complex in the presence and absence of SMN.
Reduced axon growth in SMN-deficient motoneurons correlated with decreased actin mRNA and protein in distal axons and growth cones (12). SMN is in a complex with the F-actin-bundling protein and SMA modifier plastin 3 and associates with the actin monomer-binding protein profilin (25,26). Smn deficiency increased profilin IIa levels leading to an inappropriate activation of the RhoA/ROCK pathway (49). These studies indicate a role for SMN in actin metabolism and suggested linkage between deregulation of actin dynamics and SMA pathogenesis (50). The lamellipodium is a dynamic sheet-like protrusive entity observed at the leading edge of migrating cells and neuronal growth cones. Actin polymerization is the driving force behind the lamellipodial protrusion. Importantly, the Golgi orients toward the leading edge in cells with migratory shape (51) and toward the axon in neuronal cells (33). Our results show that SMN is also in juxtaposition with the lamellipodium. The depletion of α-COP reduced SMN localization at the lamellipodia and either inhibited extension or induced regression of lamellipodia. These results suggest that the α-COP protein is critical for SMN localization, implying α-COP transports SMN to lamellipodia. SMN could participate in actin cytoskeletal dynamics in lamellipodia or axonal growth cone by direct interaction with the G-actin-binding protein profilin, plastin 3 or indirectly by localizing β-actin mRNA. Our data infer a novel function of coatomer in β-actin and SMN mobilization to the leading edge and growth cones of neurons. Further studies are necessary to investigate the relationship of the COPI pathway with axonal transport in SMA (52) and other motor neuron degenerative diseases such as ALS or Huntington's disease (53,54).
MATERIALS AND METHODS
Yeast two-hybrid screen
A murine embryonic spinal cord yeast two-hybrid library was from Stratagene. Murine and human SMN genes were cloned into EcoRI–PstI and EcoRI–Sal sites of pBD-GAL4-Cam plasmid, respectively. Murine SMN alone did not trans-activate the reporter genes (lacZ and HIS3) and was used as bait for identifying the interacting proteins by following the manufacturer's instructions.
Expression and purification of recombinant protein
For in vitro transcription, translation, expression and purification of GST fusion proteins, human SMN and α-COP cDNAs were cloned into EcoRI–NotI sites of pTNT and pGEX4T-3 plasmids. BL21-arginine (R), isoleucine (I), proline (P), leucine (L) codon plus Escherichia coli transformed with plasmids was induced with 0.5 mm isopropylthio-β-d-galactoside for 2 h at 30°C except α-COP mutants 1–357 and 358–722 at 37°C. GST and 6-His-tagged proteins were purified using glutathione agarose beads and TALON (Clontech's proprietary name for cobalt based immobilized metal affinity chromatography resins to purify histidine tagged proteins) metal affinity resin (Clontech Laboratories Inc.), respectively. In vitro transcription and translation for α-COP were at 25°C.
GST pull-down assays
Two micrograms of purified GST-SMN or GST-α-COP bound to glutathione beads was incubated with in vitro translated 35S-labeled SMN, α-COP or luciferase for 30 min in buffer B. The beads were washed with buffer A and bound proteins resolved by polyacrylamide gel electrophoresis and analyzed by fluorography. Buffer A: 50 mm Tris–HCl, pH 7.4, 150 mm NaCl, 1 mm MgCl2, 0.05% NP-40, 20 mm ethylenediaminetetraacetic acid (EDTA), 1 mm dithiothreitol (DTT) and 1× protease inhibitor cocktail. Buffer B: 10 mm N (2-hydroxyethyl) piperazine-N′-(2-ethanesulphonic acid), pH 7.0, 100 mm potassium chloride, 5 mm MgCl2, 25 mm EDTA, 0.5% NP-40, 2 mm DTT and 1× protease inhibitor cocktail.
Antisera
Rabbit antisera were raised against the α-COP peptide KDADSQNPDAPEGKRSSC conjugated to keyhole limpet hemocyanin. The rabbit SMN antibody was H-195 (Santa Cruz Biotechnology Inc.).
Co-immunoprecipitation assays
Human α-COP cDNA or mutants were cloned into pExchange-4a with a Kozak sequence and N-terminal FLAG epitope and transfected into HEK293 (human embryonic kidney) using Lipofectamine 2000. Twenty hours later, lysates were made in lysis buffer (1% NP-40, 50 mm Tris–HCl, pH 8.0, 150 mm NaCl, EDTA-free protease inhibitor cocktail), sonicated and clarified by centrifugation at 14 000g for 15 min. Lysates were incubated with protein A or G Dynabeads (Invitrogen) prebound to purified CPa-1 antibody or anti-Flag M2 beads (Sigma) for 2 h at 4°C and washed with lysis buffer. For endogenous immunoprecipitation, ∼10 million SH-SY5Y cells were suspended in 2 ml of PA-150 (150 mm KCl, 20 mm Tris–HCl, pH 7.7, 3 mm MgCl2 and 0.5 mm DTT), sonicated on ice for 20 s and Tween-20 added to 0.1% final concentration following which the lysate was centrifuged at 14 000g for 15 min. Incubate 25 μl of protein G Dynabeads plus 25 μl of affinity purified CPa-1 antibody in 1 ml of PA-150 (no DTT, no detergent) for 1 h in cold room, and wash the beads once with the same buffer. Add 600 µl of lysate to the beads and incubate for 5 h in cold room, wash three times in PA-150 (no DTT or no detergent). Bound proteins were denatured in sodium dodecyl sulfate loading buffer, resolved in 6–15% gradient gel, blots were cut into strips, immunoblotted using appropriate antibodies and developed using a Pierce Dura Kit.
Immunofluorescence
Cells were fixed in 4% paraformaldehyde for 15 min, permeabilized with 0.02% Triton X-100 in Dulbecco's phosphate-buffered saline (DPBS) for 3 min, washed in DPBS and blocked with 5% goat serum or albumen for 30 min. Antibody was added for 1 h and washed with DPBS. Alexa fluor 488, 555 or 633 conjugated secondary antibodies were added for 1 h, washed with DPBS and mounted onto the microscope slide using Prolong Gold with 4′,6′-diamidino-2-phenylindole or Fluromount G. Samples were imaged using a Leica TCS-SP2 confocal microscope.
Inducible shRNA experiments
SH-SY5Y cells were transduced with tet-R expressing lentivirus (55) and selected with 4 mg/ml of blasticidin S HCl. These cells were electroporated with α-COP shRNA (AACTGGATGCTTTATGTAACATT) in pSuper.puro using an Amaxa Nucleofector Kit. Cells stably expressing the shRNA were isolated after 4 weeks in puromycin. To test for α-COP knockdown, cells were grown in the presence or the absence of 5 µg/ml of doxycycline for 72 h. Clones that showed a significant decrease in α-COP protein levels upon doxycycline addition were identified by western blot analysis using CPa-1 antibody. Clone SH-α-COPkd was selected for subsequent experiments.
Live cell imaging
Chick retinal explants were transfected by electroporation and RGC axonal cultures prepared as described (56). Two days post-transfection and before imaging, cultures were treated with 10 ng/ml of BDNF for 10 min. Axons positive for α-COP: mCit expression were filmed on a heated stage using a 60× objective lens on an inverted Carl Zeiss Asiovert 200 microscope at a frequency of 5 s per frame. PC12 cells on matTak glass bottom culture dishes were transfected with SMN:mCit and α-COP: mCherry using Lipofectamine 2000. Twelve hours post-transfection, cells were exposed to 2.5 µg/ml of puromycin for 36 h and imaged using a spinning disk confocal microscope.
RNA immunoprecipitation
mRNA immunoprecipitation and reverse transcriptase-polymerase chain reaction (RT–PCR) were performed using CPa-1 antibody or IgG control and human β-actin primers as described (21).
SUPPLEMENTARY MATERIAL
Supplementary Material is available at HMG online.
FUNDING
This work was supported in part by Muscular Dystrophy Association, USA grant #68695 to E.J.A. and NIH RO1HD055835 to G.J.B.
ACKNOWLEDGEMENTS
We thank our lab members for offering comments on this manuscript. We thank Eric Campeau for lentivirus expressing tet-repressor, Dr Paul Furcinitti for technical assistance, Dr Li-Na Wei for Flag-β-COP (Copb1) and Dr Jennifer Lippincott-Schwartz for ɛ-COPI-YFP.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Lefebvre S., Burglen L., Reboullet S., Clermont O., Burlet P., Viollet L., Benichou B., Cruaud C., Millasseau P., Zeviani M., et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80:155–165. doi: 10.1016/0092-8674(95)90460-3. doi:10.1016/0092-8674(95)90460-3. [DOI] [PubMed] [Google Scholar]
- 2.Monani U.R. Spinal muscular atrophy: a deficiency in a ubiquitous protein; a motor neuron-specific disease. Neuron. 2005;48:885–895. doi: 10.1016/j.neuron.2005.12.001. doi:10.1016/j.neuron.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Gubitz A.K., Feng W., Dreyfuss G. The SMN complex. Exp. Cell Res. 2004;296:51–56. doi: 10.1016/j.yexcr.2004.03.022. doi:10.1016/j.yexcr.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 4.Battle D.J., Kasim M., Yong J., Lotti F., Lau C.K., Mouaikel J., Zhang Z., Han K., Wan L., Dreyfuss G. The SMN complex: an assembly machine for RNPs. Cold Spring Harb. Symp. Quant. Biol. 2006;71:313–320. doi: 10.1101/sqb.2006.71.001. doi:10.1101/sqb.2006.71.001. [DOI] [PubMed] [Google Scholar]
- 5.Battle D.J., Lau C.K., Wan L., Deng H., Lotti F., Dreyfuss G. The Gemin5 protein of the SMN complex identifies snRNAs. Mol. Cell. 2006;23:273–279. doi: 10.1016/j.molcel.2006.05.036. doi:10.1016/j.molcel.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 6.Burghes A.H., Beattie C.E. Spinal muscular atrophy: why do low levels of survival motor neuron protein make motor neurons sick? Nat. Rev. Neurosci. 2009;10:597–609. doi: 10.1038/nrn2670. doi:10.1038/nrn2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chari A., Paknia E., Fischer U. The role of RNP biogenesis in spinal muscular atrophy. Curr. Opin. Cell Biol. 2009;21:387–393. doi: 10.1016/j.ceb.2009.02.004. doi:10.1016/j.ceb.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Zhang H., Xing L., Rossoll W., Wichterle H., Singer R.H., Bassell G.J. Multiprotein complexes of the survival of motor neuron protein SMN with Gemins traffic to neuronal processes and growth cones of motor neurons. J. Neurosci. 2006;26:8622–8632. doi: 10.1523/JNEUROSCI.3967-05.2006. doi:10.1523/JNEUROSCI.3967-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McWhorter M.L., Monani U.R., Burghes A.H., Beattie C.E. Knockdown of the survival motor neuron (Smn) protein in zebrafish causes defects in motor axon outgrowth and pathfinding. J. Cell Biol. 2003;162:919–931. doi: 10.1083/jcb.200303168. doi:10.1083/jcb.200303168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferri A., Melki J., Kato A.C. Progressive and selective degeneration of motoneurons in a mouse model of SMA. Neuroreport. 2004;15:275–280. doi: 10.1097/00001756-200402090-00013. doi:10.1097/00001756-200402090-00013. [DOI] [PubMed] [Google Scholar]
- 11.Zhang H.L., Pan F., Hong D., Shenoy S.M., Singer R.H., Bassell G.J. Active transport of the survival motor neuron protein and the role of exon-7 in cytoplasmic localization. J. Neurosci. 2003;23:6627–6637. doi: 10.1523/JNEUROSCI.23-16-06627.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rossoll W., Jablonka S., Andreassi C., Kroning A.K., Karle K., Monani U.R., Sendtner M. Smn, the spinal muscular atrophy–determining gene product, modulates axon growth and localization of β-actin mRNA in growth cones of motoneurons. J. Cell Biol. 2003;163:801–812. doi: 10.1083/jcb.200304128. doi:10.1083/jcb.200304128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowerman M., Beauvais A., Anderson C.L., Kothary R. Rho-kinase inactivation prolongs survival of an intermediate SMA mouse model. Hum. Mol. Genet. 2010;19:1468–1478. doi: 10.1093/hmg/ddq021. doi:10.1093/hmg/ddq021. [DOI] [PubMed] [Google Scholar]
- 14.Waters M.G., Serafini T., Rothman J.E. ‘Coatomer': a cytosolic protein complex containing subunits of non-clathrin-coated Golgi transport vesicles. Nature. 1991;349:248–251. doi: 10.1038/349248a0. doi:10.1038/349248a0. [DOI] [PubMed] [Google Scholar]
- 15.Stenbeck G., Harter C., Brecht A., Herrmann D., Lottspeich F., Orci L., Wieland F.T. β-COP, a novel subunit of coatomer. EMBO J. 1993;12:2841–2845. doi: 10.1002/j.1460-2075.1993.tb05945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuge O., Hara-Kuge S., Orci L., Ravazzola M., Amherdt M., Tanigawa G., Wieland F.T., Rothman J.E. zeta-COP, a subunit of coatomer, is required for COP-coated vesicle assembly. J. Cell Biol. 1993;123:1727–1734. doi: 10.1083/jcb.123.6.1727. doi:10.1083/jcb.123.6.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duden R., Kajikawa L., Wuestehube L., Schekman R. epsilon-COP is a structural component of coatomer that functions to stabilize alpha-COP. EMBO J. 1998;17:985–995. doi: 10.1093/emboj/17.4.985. doi:10.1093/emboj/17.4.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de La Vega L.A., Stockert R.J. The cytoplasmic coatomer protein COPI. A potential translational regulator. J. Biol. Chem. 1999;274:31135–31138. doi: 10.1074/jbc.274.44.31135. doi:10.1074/jbc.274.44.31135. [DOI] [PubMed] [Google Scholar]
- 19.Bi J., Tsai N.P., Lu H.Y., Loh H.H., Wei L.N. Copb1-facilitated axonal transport and translation of κ opioid-receptor mRNA. Proc. Natl Acad. Sci. USA. 2007;104:13810–13815. doi: 10.1073/pnas.0703805104. doi:10.1073/pnas.0703805104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lowe M., Kreis T.E. In vivo assembly of coatomer, the COP-I coat precursor. J. Biol. Chem. 1996;271:30725–30730. doi: 10.1074/jbc.271.48.30725. doi:10.1074/jbc.271.48.30725. [DOI] [PubMed] [Google Scholar]
- 21.Peritz T., Zeng F., Kannanayakal T.J., Kilk K., Eiriksdottir E., Langel U., Eberwine J. Immunoprecipitation of mRNA-protein complexes. Nat. Protoc. 2006;1:577–580. doi: 10.1038/nprot.2006.82. doi:10.1038/nprot.2006.82. [DOI] [PubMed] [Google Scholar]
- 22.Beck R., Rawet M., Wieland F.T., Cassel D. The COPI system: molecular mechanisms and function. FEBS Lett. 2009;583:2701–2709. doi: 10.1016/j.febslet.2009.07.032. doi:10.1016/j.febslet.2009.07.032. [DOI] [PubMed] [Google Scholar]
- 23.Presley J.F., Ward T.H., Pfeifer A.C., Siggia E.D., Phair R.D., Lippincott-Schwartz J. Dissection of COPI and Arf1 dynamics in vivo and role in Golgi membrane transport. Nature. 2002;417:187–193. doi: 10.1038/417187a. doi:10.1038/417187a. [DOI] [PubMed] [Google Scholar]
- 24.Encinas M., Iglesias M., Liu Y., Wang H., Muhaisen A., Cena V., Gallego C., Comella J.X. Sequential treatment of SH-SY5Y cells with retinoic acid and brain-derived neurotrophic factor gives rise to fully differentiated, neurotrophic factor-dependent, human neuron-like cells. J. Neurochem. 2000;75:991–1003. doi: 10.1046/j.1471-4159.2000.0750991.x. doi:10.1046/j.1471-4159.2000.0750991.x. [DOI] [PubMed] [Google Scholar]
- 25.Oprea G.E., Krober S., McWhorter M.L., Rossoll W., Muller S., Krawczak M., Bassell G.J., Beattie C.E., Wirth B. Plastin 3 is a protective modifier of autosomal recessive spinal muscular atrophy. Science. 2008;320:524–527. doi: 10.1126/science.1155085. doi:10.1126/science.1155085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giesemann T., Rathke-Hartlieb S., Rothkegel M., Bartsch J.W., Buchmeier S., Jockusch B.M., Jockusch H. A role for polyproline motifs in the spinal muscular atrophy protein SMN. Profilins bind to and colocalize with smn in nuclear gems. J. Biol. Chem. 1999;274:37908–37914. doi: 10.1074/jbc.274.53.37908. doi:10.1074/jbc.274.53.37908. [DOI] [PubMed] [Google Scholar]
- 27.Gabanella F., Butchbach M.E., Saieva L., Carissimi C., Burghes A.H., Pellizzoni L. Ribonucleoprotein assembly defects correlate with spinal muscular atrophy severity and preferentially affect a subset of spliceosomal snRNPs. PLoS One. 2007;2:e921. doi: 10.1371/journal.pone.0000921. doi:10.1371/journal.pone.0000921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Forscher P., Smith S.J. Actions of cytochalasins on the organization of actin filaments and microtubules in a neuronal growth cone. J. Cell Biol. 1988;107:1505–1516. doi: 10.1083/jcb.107.4.1505. doi:10.1083/jcb.107.4.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forscher P., Kaczmarek L.K., Buchanan J.A., Smith S.J. Cyclic AMP induces changes in distribution and transport of organelles within growth cones of Aplysia bag cell neurons. J. Neurosci. 1987;7:3600–3611. doi: 10.1523/JNEUROSCI.07-11-03600.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diefenbach T.J., Guthrie P.B., Stier H., Billups B., Kater S.B. Membrane recycling in the neuronal growth cone revealed by FM1–43 labeling. J. Neurosci. 1999;19:9436–9444. doi: 10.1523/JNEUROSCI.19-21-09436.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hazuka C.D., Foletti D.L., Hsu S.C., Kee Y., Hopf F.W., Scheller R.H. The sec6/8 complex is located at neurite outgrowth and axonal synapse-assembly domains. J. Neurosci. 1999;19:1324–1334. doi: 10.1523/JNEUROSCI.19-04-01324.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sabo S.L., McAllister A.K. Mobility and cycling of synaptic protein-containing vesicles in axonal growth cone filopodia. Nat. Neurosci. 2003;6:1264–1269. doi: 10.1038/nn1149. doi:10.1038/nn1149. [DOI] [PubMed] [Google Scholar]
- 33.de Anda F.C., Pollarolo G., Da Silva J.S., Camoletto P.G., Feiguin F., Dotti C.G. Centrosome localization determines neuronal polarity. Nature. 2005;436:704–708. doi: 10.1038/nature03811. doi:10.1038/nature03811. [DOI] [PubMed] [Google Scholar]
- 34.Horton A.C., Ehlers M.D. Neuronal polarity and trafficking. Neuron. 2003;40:277–295. doi: 10.1016/s0896-6273(03)00629-9. doi:10.1016/S0896-6273(03)00629-9. [DOI] [PubMed] [Google Scholar]
- 35.Ye B., Zhang Y., Song W., Younger S.H., Jan L.Y., Jan Y.N. Growing dendrites and axons differ in their reliance on the secretory pathway. Cell. 2007;130:717–729. doi: 10.1016/j.cell.2007.06.032. doi:10.1016/j.cell.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hess D.T., Smith D.S., Patterson S.I., Kahn R.A., Skene J.H., Norden J.J. Rapid arrest of axon elongation by brefeldin A: a role for the small GTP-binding protein ARF in neuronal growth cones. J. Neurobiol. 1999;38:105–115. doi: 10.1002/(sici)1097-4695(199901)38:1<105::aid-neu8>3.0.co;2-m. doi:10.1002/(SICI)1097-4695(199901)38:1<105::AID-NEU8>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 37.Jareb M., Banker G. Inhibition of axonal growth by brefeldin A in hippocampal neurons in culture. J. Neurosci. 1997;17:8955–8963. doi: 10.1523/JNEUROSCI.17-23-08955.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pagliardini S., Giavazzi A., Setola V., Lizier C., Di Luca M., DeBiasi S., Battaglia G. Subcellular localization and axonal transport of the survival motor neuron (SMN) protein in the developing rat spinal cord. Hum. Mol. Genet. 2000;9:47–56. doi: 10.1093/hmg/9.1.47. doi:10.1093/hmg/9.1.47. [DOI] [PubMed] [Google Scholar]
- 39.Goldberg J. Decoding of sorting signals by coatomer through a GTPase switch in the COPI coat complex. Cell. 2000;100:671–679. doi: 10.1016/s0092-8674(00)80703-5. doi:10.1016/S0092-8674(00)80703-5. [DOI] [PubMed] [Google Scholar]
- 40.Zhang H., Xing L., Singer R.H., Bassell G.J. QNQKE targeting motif for the SMN-Gemin multiprotein complex in neurons. J. Neurosci Res. 2007;80:2657–2667. doi: 10.1002/jnr.21308. [DOI] [PubMed] [Google Scholar]
- 41.Todd A.G., Morse R., Shaw D.J., Stebbings H., Young P.J. Analysis of SMN-neurite granules: Core Cajal body components are absent from SMN-cytoplasmic complexes. Biochem. Biophys. Res. Commun. 2010;397:479–485. doi: 10.1016/j.bbrc.2010.05.139. doi:10.1016/j.bbrc.2010.05.139. [DOI] [PubMed] [Google Scholar]
- 42.Trautwein M., Dengjel J., Schirle M., Spang A. Arf1p provides an unexpected link between COPI vesicles and mRNA in Saccharomyces cerevisiae. Mol. Biol. Cell. 2004;15:5021–5037. doi: 10.1091/mbc.E04-05-0411. doi:10.1091/mbc.E04-05-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lau C.K., Bachorik J.L., Dreyfuss G. Gemin5-snRNA interaction reveals an RNA binding function for WD repeat domains. Nat. Struct. Mol. Biol. 2009;16:486–491. doi: 10.1038/nsmb.1584. doi:10.1038/nsmb.1584. [DOI] [PubMed] [Google Scholar]
- 44.Todd A.G., Morse R., Shaw D.J., McGinley S., Stebbings H., Young P.J. SMN, Gemin2 and Gemin3 associate with β-actin mRNA in the cytoplasm of neuronal cells in vitro. J. Mol. Biol. 2010;401:681–689. doi: 10.1016/j.jmb.2010.06.058. doi:10.1016/j.jmb.2010.06.058. [DOI] [PubMed] [Google Scholar]
- 45.Glinka M., Herrmann T., Funk N., Havlicek S., Rossoll W., Winkler C., Sendtner M. The heterogeneous nuclear ribonucleoprotein-R is necessary for axonal β-actin mRNA translocation in spinal motor neurons. Hum. Mol. Genet. 2010;19:1951–1966. doi: 10.1093/hmg/ddq073. [DOI] [PubMed] [Google Scholar]
- 46.Hubers L., Valderrama-Carvajal H., Laframboise J., Timbers J., Sanchez G., Cote J. HuD interacts with survival motor neuron protein and can rescue spinal muscular atrophy-like neuronal defects. Hum. Mol. Genet. 2011;20:553–579. doi: 10.1093/hmg/ddq500. doi:10.1093/hmg/ddq500. [DOI] [PubMed] [Google Scholar]
- 47.Rossoll W., Kroning A.K., Ohndorf U.M., Steegborn C., Jablonka S., Sendtner M. Specific interaction of Smn, the spinal muscular atrophy determining gene product, with hnRNP-R and gry-rbp/hnRNP-Q: a role for Smn in RNA processing in motor axons? Hum. Mol. Genet. 2002;11:93–105. doi: 10.1093/hmg/11.1.93. [DOI] [PubMed] [Google Scholar]
- 48.Tadesse H., Deschenes-Furry J., Boisvenue S., Cote J. KH-type splicing regulatory protein interacts with survival motor neuron protein and is misregulated in spinal muscular atrophy. Hum. Mol. Genet. 2008;17:506–524. doi: 10.1093/hmg/ddm327. doi:10.1093/hmg/ddm327. [DOI] [PubMed] [Google Scholar]
- 49.Bowerman M., Shafey D., Kothary R. Smn depletion alters profilin II expression and leads to upregulation of the RhoA/ROCK pathway and defects in neuronal integrity. J. Mol. Neurosci. 2007;32:120–131. doi: 10.1007/s12031-007-0024-5. doi:10.1007/s12031-007-0024-5. [DOI] [PubMed] [Google Scholar]
- 50.Bowerman M., Anderson C.L., Beauvais A., Boyl P.P., Witke W., Kothary R. SMN, profilin IIa and plastin 3: a link between the deregulation of actin dynamics and SMA pathogenesis. Mol. Cell Neurosci. 2009;42:66–74. doi: 10.1016/j.mcn.2009.05.009. doi:10.1016/j.mcn.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 51.Mogilner A., Keren K. The shape of motile cells. Curr. Biol. 2009;19:R762–R771. doi: 10.1016/j.cub.2009.06.053. doi:10.1016/j.cub.2009.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nishimura A.L., Mitne-Neto M., Silva H.C., Richieri-Costa A., Middleton S., Cascio D., Kok F., Oliveira J.R., Gillingwater T., Webb J., et al. A mutation in the vesicle-trafficking protein VAPB causes late-onset spinal muscular atrophy and amyotrophic lateral sclerosis. Am. J. Hum. Genet. 2004;75:822–831. doi: 10.1086/425287. doi:10.1086/425287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Vos K.J., Grierson A.J., Ackerley S., Miller C.C. Role of axonal transport in neurodegenerative diseases. Annu. Rev. Neurosci. 2008;31:151–173. doi: 10.1146/annurev.neuro.31.061307.090711. doi:10.1146/annurev.neuro.31.061307.090711. [DOI] [PubMed] [Google Scholar]
- 54.Duncan J.E., Goldstein L.S. The genetics of axonal transport and axonal transport disorders. PLoS Genet. 2006;2:e124. doi: 10.1371/journal.pgen.0020124. doi:10.1371/journal.pgen.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Campeau E., Ruhl V.E., Rodier F., Smith C.L., Rahmberg B.L., Fuss J.O., Campisi J., Yaswen P., Cooper P.K., Kaufman P.D. A versatile viral system for expression and depletion of proteins in mammalian cells. PLoS One. 2009;4:e6529. doi: 10.1371/journal.pone.0006529. doi:10.1371/journal.pone.0006529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kolpak A.L., Jiang J., Guo D., Standley C., Bellve K., Fogarty K., Bao Z.Z. Negative guidance factor-induced macropinocytosis in the growth cone plays a critical role in repulsive axon turning. J. Neurosci. 2009;29:10488–10498. doi: 10.1523/JNEUROSCI.2355-09.2009. doi:10.1523/JNEUROSCI.2355-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.