Abstract
Functional deficiency of the X-linked methyl-CPG binding protein 2 (MeCP2) leads to the neurodevelopmental disorder Rett syndrome (RTT). Due to random X-chromosome inactivation (XCI), most RTT patients are females who are heterozygous for the MECP2 mutation and therefore mosaic in MeCP2 deficiency. Some MECP2 heterozygote females are found to have unbalanced XCI, which may affect the severity of neurological symptoms seen in these patients; however, whether MeCP2 deficiency affects XCI in the postnatal and adult brain is unclear. Here we developed a novel MeCP2 mosaic mouse model in which the X chromosome containing the wild-type Mecp2 expresses a green fluorescent protein (GFP) transgene, while the X chromosome harboring the mutant Mecp2 does not. Due to random XCI, the neurons in the female MeCP2 mosaic mice express either wild-type MeCP2 (GFP+) or mutant MeCP2 (GFP−), and the two can be distinguished by GFP fluorescence. Using this mouse model, we evaluated XCI in female heterozygote mice from 3 to 9 months after birth. We found that MeCP2 deficiency does not affect XCI at 3 months of age, but does alter the proportion of wild-type MeCP2-expressing neurons at later ages, suggesting that MeCP2 impacts XCI patterns in an age-dependent manner. Given the important function of MeCP2 in neuronal development, our data could shed light on how MeCP2 deficiency affects postnatal brain functions and the dynamic changes in the neurological symptoms of RTT.
INTRODUCTION
Loss-of-function mutations in the X-linked methyl-CPG binding protein 2 (MECP2) lead to Rett syndrome (RTT), a neurodevelopmental disorder that manifests itself during early postnatal development (1). RTT strikes 1 in 15 000 females, but mutations in the MECP2 and reduction of its protein product are also associated with many other neurodevelopmental disorders, including X-linked mental retardation, autism and Angelman syndrome (2). Reduced MeCP2 expression is frequent in the autism frontal cortex and correlates with aberrant MECP2 promoter methylation (3). The MECP2 gene encodes MeCP2 that binds methylated DNA and regulates gene transcription. MeCP2 plays a critical role in proper neuronal development, particularly during neuronal maturation (4–6). Several lines of Mecp2 knockout (KO) mice have been used to study the function of MeCP2 (7–10); however, most of these studies have focused on what happens in the total absence of MeCP2 in male mice, and little is known about the function of MeCP2 when it is expressed in female mice, which are heterozygous for the MECP2 mutation, reminiscent of human RTT.
Most RTT patients are females who are heterozygous for MeCP2 deficiency due to random X-chromosome inactivation (XCI). Mammalian female cells randomly inactivate one of the two X chromosomes in somatic cells, and the genes on the inactive X chromosome (with a few exceptions) are not expressed. XCI occurs before the completion of gastrulation during early embryonic development and is believed to be irreversible (11). Skewed XCI can occur when there is preferential inactivation of the maternal or paternal X chromosome and has been linked to both autism (12) and X-chromosome-linked mental retardation (13).
The severity of neurological deficits in these X-linked disorders, including RTT, is suspected of being partially dependent on XCI patterns. Interestingly, while some studies found skewed XCI in MECP2 heterozygous females (14–16), others showed skewed XCI was specific to older adult time points (17). The question is, why do Mecp2 heterozygote brains, with at least half of their neurons still expressing functional MeCP2, have such severe neurological deficits? There is a known survival advantage for neurons expressing the wild-type Mecp2 allele compared with those expressing the mutant allele, suggesting that the skewed ratio of mutant Mecp2-expressing neurons versus wild-type Mecp2-expressing neurons could be due to the survival disadvantage of mutant neurons (14). Nevertheless, there has been no significant neuronal death found in RTT brains (18), and MeCP2 protein expression levels in wild-type MeCP2-expressing neurons in both RTT patients and Mecp2 mutant mice are lower than in wild-type cells from normal brains (15). These observations suggest that mutation of MeCP2 in heterozygote females may affect the development of both neurons expressing the mutant allele and neurons expressing the wild-type allele. Thus, to understand the etiology of RTT, determining the characteristics of XCI in females that are heterozygous for MeCP2 deficiency is critical.
In this study, we developed a novel MeCP2 mosaic mouse model in which the X chromosome containing the wild-type Mecp2 also expresses a green fluorescent protein (GFP) transgene, while the X chromosome harboring the mutant Mecp2 does not. Due to random XCI, the neurons in the female MeCP2 mosaic mice express either wild-type MeCP2 (GFP+ cells) or mutant MeCP2 (GFP− cells), and the two can be distinguished by GFP fluorescence. Using this model, we found no difference in the proportion of wild-type MeCP2-expressing neurons in the MeCP2 mosaic females at age 3 months; however, at 6 and 9 months, the percentage of wild-type MeCP2-expressing neurons changes significantly compared with the GFP control mouse. These results show that MeCP2 deficiency in the MeCP2 mosaic mouse model may not affect primary XCI in early development, but does affect the proportion of neurons expressing the wild-type Mecp2 allele in a region- and age-dependent manner.
RESULTS
GFP mosaic (TgGFPX+/−) mice express GFP in about 50% of cells in the hippocampus, cortex and striatum
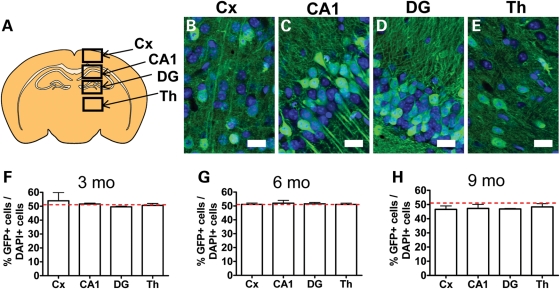
To determine the effect of MeCP2 on XCI, we used a unique line of mice that have an enhanced GFP (eGFP) transgene inserted into the X chromosome; eGFP expression is known to be subject to random XCI in a number of tissues (19). In our study, the GFP mosaic females (TgGFPX+/−) express GFP from the paternally contributed X chromosome. The use of GFP fluorescence as a readout for the active X chromosome in this mouse line has been validated by a number of studies (20–23). To determine whether female TgGFPX+/− female mice faithfully demonstrate random XCI in the adult brain, we quantified ∼50 cells per region to assess the proportion of GFP-positive cells in the cortex (Cx), CA1, dentate gyrus (DG) and thalamus (Th) (Fig. 1A–E) regions of the brain known to be affected in RTT (5,24–26). We analyzed mice at 3, 6 and 9 months of age and found that brain tissue was mosaic for GFP expression at all ages analyzed. Specifically, ∼50% of cells in all these regions expressed GFP at 3 months (n = 3, Cx = 53.90%, CA1 = 51.64%, DG = 49.56%, Th = 50.61%) and 6 months (n = 3, Cx = 51.18%, CA1 = 52.03%, DG = 51.55%, Th = 51.11%) (Fig. 1F and G). At 9 months of age, the proportion of GFP+ cells was slightly lower than 50% in all brain regions analyzed (n = 3, Cx = 46.63%, CA1 = 47.23%, DG = 46.86%, Th = 48.31%) (Fig. 1H). These data suggest that GFP mosaic TgGFPX+/− mice undergo random XCI and that GFP fluorescence can be used to track cells that express the active X chromosome, consistent with earlier observations in other tissues (19).
Figure 1.
GFP is expressed in half of total cells in the GFP mosaic control mice. (A) Illustration of a representative coronal section showing the brain regions analyzed. (B–E) Representative confocal images of brain regions used for analysis (GFP is green, DAPI is blue). (F–H) At 3, 6 and 9 months of age, the proportion of GFP+ cells among total cells in each brain region analyzed is ∼50% (red dotted lines indicate 50%). Note there is a slight decrease in the proportion of GFP+ cells at 9 months of age. Scale bar = 20 µm.
GFP is expressed in all major lineages of neural cells in the brain of GFP mosaic TgGFPX−/+ females
To determine whether GFP was expressed in all major lineages of cells in the adult brain, we analyzed control GFP mosaic (TgGFPX+/−) mice using cell-type-specific markers, including doublecortin (DCX) for immature neurons, neuronal nuclear antigen (NeuN) for mature neurons, glial fibrillary acidic protein (GFAP) and S100 calcium binding protein B (S100β) for astrocytes and oligodendrocyte marker O1 (O1) for oligodendrocytes. Using confocal microscopy, we saw that cells expressing all five markers could be colocalized with GFP (Fig. 2). In the adult cortex, GFP was found to be expressed in 52.92% of NeuN-positive neurons in the DG of the hippocampus (n = 11, SEM = 0.55) (Fig. 2D) and 38.76% of NeuN-positive neurons in the cortex (n = 13, SEM = 3.38, P < 0.01 one-sample t-test) (Fig. 2E). In the DG of the hippocampus, where new neurons are continuously generated, GFP was expressed in 55.47% (n = 3, SEM = 2.23) of DCX-positive immature neurons (Fig. 2I). Since the number of oligodendrocytes was very low in the DG, we chose the corpus callosum (CC) to analyze GFP expression in O1-positive oligodendrocytes; GFP was expressed in 48.21% (n = 3, SEM = 3.99) of O1-positive cells in the CC (Fig. 2V). Thus, GFP is expressed in approximately half of neurons and oligodendrocytes, which is consistent with the proportion of GFP-positive cells in the total cell population (Fig. 1).
Figure 2.
The proportion of GFP-expressing cells exhibit cell type-specific differences. (A–C) Sample confocal images used in analysis showing GFP (green) and NeuN (blue) in the DG. Arrowhead indicates a GFP+NeuN+ neuron. (D) The proportion of GFP+ neurons among total neurons (GFP+NeuN+/NeuN+) was 52.92% in the DG (E), but was only 38.76% in the CX. (F–H) Sample confocal images used in analysis showing GFP (green) and DCX (red) in the DG. Arrowhead indicates a DCX+ young neuron that was also GFP+. Arrow indicates a DCX+ young neuron that did not express GFP. (I) Proportion of GFP+ DCX+ neurons among total DCX+ neurons was 55.47%. (J–L) Sample confocal images used in analysis showing GFP (green) and S100β (red) in the DG. Arrowhead indicates an S100β+ astrocyte that was also GFP+. Arrow indicates an S100β+ astrocyte that did not express GFP. (M and N) The proportion of GFP+ astrocytes (GFP+S100β+/S100β+) was 29.94% in the DG (M), but was only 26.54% in the CX (N). (O–Q) Sample confocal images used in analysis showing GFP (green) and GFAP (blue) in the DG. Arrowhead indicates a GFAP+ astrocyte that did not express GFP. (R) Proportion of GFP+ cells was only 18.84% among GFAP+ cells. (S–U) Sample confocal images used in analysis showing GFP (green) and O1+ oligodendrocytes (red) in the CC. (V) Proportion of GFP+O1+ oligodendrocytes among total O1+ cells in the CC was 48.21% (CC, corpus callosum; Cx, cortex; DG, dentate gyrus) **P < 0.01, compared with 50%. Arrowhead indicates an O1+ oligodendrocyte that was also GFP+. Arrow indicates an O1+ oligodendrocyte that did not express GFP. Scale bar = 10 µm.
Interestingly, we found that GFP was expressed in only 18.84% of GFAP-positive cells (n = 3, SEM = 4.92, P < 0.01 one-sample t-test) (Fig. 2R). In addition, GFP was expressed in 29.94% (n = 3, SEM = 0.66, P < 0.01 one-sample t-test) of S100β-positive cells of the DG (Fig. 2M), and 26.54% (n = 3, SEM = 1.64, P < 0.01 one-sample t-test) of S100β-positive cells in the cortex (Fig. 2N). These data indicate that astrocytes may have a preference for expression of the maternal X chromosome.
Together, the histological characterization of the control GFP mosaic TgGFPX−/+ mice shows that GFP is expressed in approximately half of the total cell populations in the cortex, CA1, DG and Th (Fig. 1A) at 3, 6, and 9 months of age (Fig. 1B–D). Similarly, GFP is expressed in half of the neurons and oligodendrocytes analyzed (Fig. 2D, E, I, V). However, the proportions of GFP-expressing GFAP- and S100β-positive astrocytes were significantly lower (Fig. 2M, N, R).
In the MeCP2 mosaic mouse model, GFP-positive neurons express MeCP2
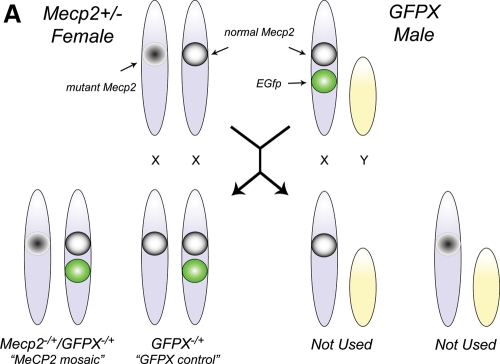
To determine the effect of MeCP2 deficiency on XCI, we created a MeCP2 mosaic mouse model by crossing a Mecp2+/− female with a TgGFPX+/y male mouse (Fig. 3). All the resulting female progeny were heterozygous for the paternal GFP transgene. Additionally, expression of GFP in all the female progeny is mosaic, which suggests that GFP+ cells indicate a paternally active X chromosome. About half the female progeny are wild-type for MeCP2 (TgGFPX+/−/Mecp2+/+), and we refer to them as the ‘GFPX controls.' The other half of the female progeny are heterozygous for MeCP2 deficiency (TgGFPX+/−/Mecp2+/−), and we refer to them as the ‘MeCP2 mosaic mice.' In these mice, GFP+ cells indicate that the paternal X chromosome, containing the GFP transgene and the wild-type Mecp2, is active.
Figure 3.
Breeding scheme for generating a MeCP2 mosaic mouse model. The cross between the Mecp2-/+ female mouse and the TgGFPX male mouse yielded female offspring that were either Mecp2-/+/TgGFPX-/+ (MeCP2 mosaic model) or Mecp2+/+/TgGFPX-/+ (GFP mosaic control). Male mice are not used in the analysis. Note that the paternal X chromosome of the MeCP2 mosaic model contains both the wild-type Mecp2 and the GFP transgene, while the maternal X chromosome has the mutated Mecp2. Thus, expression of GFP can be used to determine which X chromosome is active (or silenced).
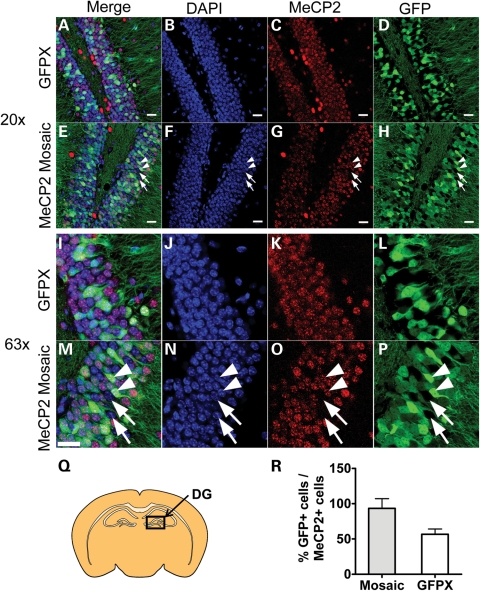
To assess whether GFP expression is linked to wild-type MeCP2 expression in the MeCP2 mosaic mice, we stained tissue with an anti-MeCP2 antibody. We found that GFP was expressed in 93.5% of MeCP2+ cells of the DG in the Mosaic mouse model (Fig. 4C, D, F) (n = 3, SEM = 13.75). As expected, GFP was expressed in ∼50% of MeCP2+ cells in the DG of GFP mosaic controls (n = 3, SEM = 7.49). These results indicate that wild-type MeCP2 and GFP are located on the same X chromosome in the MeCP2 mosaic mouse, allowing us to track the sex-specific origin of allele expression using GFP fluorescence.
Figure 4.
The GFP+ cells in the MeCP2 mosaic mouse express wild-type MeCP2. (A–P) Sample confocal images of the DG showing colocalization of MeCP2 and GFP. (A–D) ×20 image of the control GFP mouse. Note that nearly all the densely packed cells of the DG expressed MeCP2 in the control (E–H) and the MeCP2 mosaic mouse. Arrows indicate cells that are both GFP- and MeCP2-negative. Arrowheads indicate cells that are positive for both GFP and MeCP2 (I–L). ×63 image of the control GFP mouse and (M–P) MeCP2 mosaic mouse. (Q) Schematic brain section illustrating the region of the DG analyzed. (R) Approximately 94% of MeCP2+ cells expressed GFP in the MeCP2 mosaic mouse model, whereas 50% of MeCP2+ cells expressed GFP in a GFP mosaic control. Arrowhead indicates a MeCP2+ cells that were also GFP+. Arrow indicates a MeCP2− cells that did not express GFP. Scale bar = 20 µm.
MeCP2 deficiency leads to age-dependent alteration in the proportion of GFP+ neurons in MeCP2 mosaic brains
To characterize the effect of MeCP2 deficiency on XCI, we evaluated the MeCP2 mosaic and GFP mosaic control mice at 3, 6 and 9 months of age. Although the percentage of GFP+ neurons (GFP + NeuN+/NeuN+) in the cortex exhibited dynamic changes from 3 to 9 months of age, there was no significant difference between the MeCP2 mosaic mice and GFP mosaic controls at any of the three time points (Fig. 5D–F).
Figure 5.
MeCP2 deficiency leads to age-dependent alteration in the proportion of GFP+ neurons in MeCP2 mosaic brains. (A–C) Percentage of GFP+ neurons (GFP+NeuN+/NeuN+) in the DG at 3 months (A), 6 months (B) and 9 months (C) of age. (D–F) Percentage of GFP+ neurons in the cortex at 3 months (D), 6 months (E) and 9 months (F) of age. (G) In the DG, the proportion of GFP+ neurons in the MeCP2 mosaic mice decreases at 6 months of age and increases at 9 months of age, whereas in the GFP mosaic control brains, the proportion of GFP+ neurons stays constant from 3 to 6 months, but decreases at 9 months of age. The proportion of GFP+ neurons in the cortex is not affected by Mecp2 deficiency (*P < 0.05, ***P < 0.001).
In contrast, the proportion of GFP+ neurons in the DG showed dynamic changes in an age-dependent manner. At 3 months, we found that the percentage of GFP+ neurons (GFP+NeuN+/NeuN+) in the DG is the same compared with GFPX controls (Fig. 5A, n = 11 mosaic, n = 14 control). However, at 6 months of age, the percentage of GFP+ neurons was significantly lower in the MeCP2 mosaic mice compared with the GFP mosaic controls (Fig. 5B, n = 12 mosaic, n = 19 control). To our surprise, at 9 months of age, the proportion of GFP+ neurons was significantly higher in the MeCP2 mosaic mice compared with GFP mosaic controls (Fig. 5C, n = 11 mosaic, n = 11 control).
These data suggest that MeCP2 deficiency does not impact XCI at younger ages (3 months) in either the cortex or the DG; however, a lack of MeCP2 does affect the proportion of neurons that express GFP and therefore functional MeCP2 specifically in the DG, but not in the cortex, in an age-dependent manner.
Parental origin-dependent XCI in MeCP2 mosaic mice is not affected by the GFP transgene
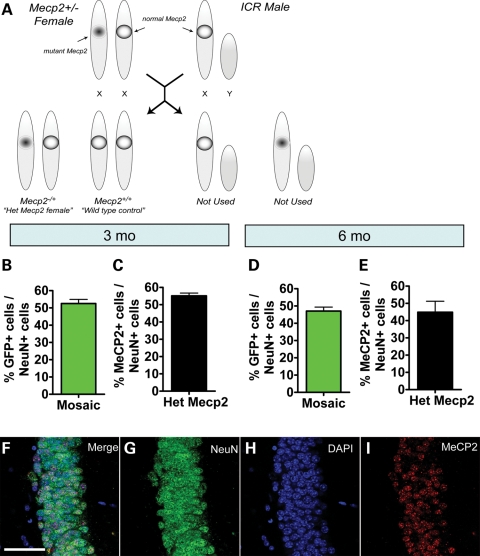
Because the insertion of the GFP transgene may affect expression of X-linked genes and alter XCI patterns, we assessed the percentage of MeCP2+ neurons among total NeuN+ neurons in Mecp2+/− females without the GFP transgene. We found that the percentage of MeCP2+ neurons (MeCP2+NeuN+/NeuN+) is no different from the GFP+ positive neurons (GFP+NeuN+/NeuN+) in the MeCP2 mosaic mouse model at 3 months (n = 11; one-sample t-test) (Fig. 6B and C) and 6 months of age (mosaic n = 11, Het Mecp2 n = 6; one-sample t-test) (Fig. 6D and E). Therefore, the GFP transgene insertion does not appear to affect XCI or the representation of GFP+ cells in either MeCP2 mosaic mice or GFP mosaic control mice.
Figure 6.
The proportion of MeCP2-expressing cells in the mosaic mouse model is not affected by the GFP transgene. (A) Schematic illustration showing the breeding between the Mecp2−/+ heterozygous female mouse and a wild-type ICR male mouse. The resulting progeny include the Mecp2−/+ (heterozygote Mecp2 female), and the wild-type female control. (B–E) Comparison of GFP expression in the MeCP2 mosaic mouse with MeCP2 expression in the Het Mecp2 mouse at (B and C) 3 months of age and (D–E) 6 months of age showed no difference. [Note that (B) and (D) are the same data shown in Figure 5A and B. They are presented here again for direct comparison with (C) and (E)]. (F–I) Sample confocal image demonstrating mosaic expression of MeCP2 in the Het Mecp2 mice (NeuN, green; DAPI, blue; MeCP2, red). Scale bar = 50 µm.
DISCUSSION
We have created a unique MeCP2 mosaic mouse model (Mecp2−/+/TgGFPX−/+) to study the pattern of XCI. Our data indicate this model can be used to determine the parental origin of the active (expressed) X chromosome based on GFP fluorescence. We found that GFP is expressed in approximately half the neurons in the DG and oligodendrocytes in the CC (Fig. 2D, E, I, V), suggesting that there is little bias in the parental origin-specific XCI in these cells. However, we saw that only ∼40% of NeuN+ neurons in the cortex were GFP+. In our study, the GFP-expressing X chromosome was contributed by the father; therefore, the lower representation of GFP-expressing neurons we observed may be due to preferential expression of the maternal chromosome. It is well known that extra-embryonic lineages of cells have non-random X inactivation, where imprinted X inactivation could result in preferential silencing of the parentally inherited X chromosome in females (27). Recently, preferential expression of the maternally inherited X chromosome was seen in glutamatergic neurons of the female cortex (23). This sex-specific origin of allelic expression in glutamatergic neurons of the cortex may explain why we observe a lower proportion of GFP-expressing neurons in the cortex of TgGFPX+/− mice. We also found that only 20–30% astrocytes in both the DG and cortex expressed the paternal X chromosome. It has been shown that astrocytes show monoallelic expression of the Gfap gene in the mouse cortex, and that the active allele is selected in a stochastic manner and maintained through cell division (28). The Gfap and S100β genes are not located on the X chromosome, and the XCI of astrocytes as well as its effect on expression of Gfap and S100β is largely unexplored. There is an overall lack of studies in published literature characterizing the parental origin of X-chromosome activation in glial cell types. Further investigation is needed to specify how Gfap, and other astrocyte markers might be related to parental selection of the active X-chromosome or XCI patterning in astrocytes. Since MECP2 mutations in sporadic cases of RTT are mostly inherited paternally (29), the impact of such preferential inactivation of the paternal X in astrocytes may lead to milder RTT pathology, an important possibility for further exploration.
Despite keen interest in MeCP2 deficiency, few studies have analyzed the dynamic changes of MeCP2 expression in heterozygote female mice at old age (17). In the present study, by analyzing adult animals from 3 months of age (young adult, pre-symptomatic females) to 9 months of age (aging, end-stage symptomatic females), we unearth new information that may shed light on the changing landscape of RTT pathology and symptoms. Using this MeCP2 mosaic mouse model, we show that XCI is not skewed in 3-month-old female mice, suggesting that primary XCI may be unaffected during early development. This is supported by an earlier study, where heterozygous Mecp2308 embryonic mice at E9.5 showed no difference in XCI patterns compared with wild-type controls (30). At 6 months of age, MeCP2 mosaic mice had fewer GFP+ wild-type neurons in the hippocampus compared with 3-month-old mice, whereas GFP+ neurons in the GFP mosaic controls stayed the same. Since XCI is likely irreversible and early XCI patterns are unaffected in embryonic Mecp2 heterozygous brains, the altered XCI patterns we see at 6 months of age are unlikely to be due to skewed XCI. One possible explanation for why GFP+ wild-type cells are reduced in MeCP2 mosaic mice at 6 months of age is that the Mecp2 mutation in neurons expressing the maternal allele may have a negative effect on the GFP+MeCP2+ WT neighbors, leading to reduced survival of GFP-positive WT cells from three to 6 months of age. Interestingly, while the proportion of GFP+ neurons was significantly reduced in the control GFP mosaic mice at 9 months of age, it was significantly higher in the MeCP2 mosaic mice. It is possible that there is epigenetic silencing of the cytomegalovirus (CMV) promoter driving the GFP transgene in the control GFP mosaic mice, resulting in fewer GFP-expressing neurons at 9 months of age. However, since this silencing of the CMV promoter is dependent upon functional MeCP2 (31), it may not happen properly in the MeCP2 mosaic mice, resulting in a higher representation of GFP-positive cells. In fact, a recent report demonstrated that MeCP2 directly associates with endogenous retrovirus long-interspersed nuclear element-1 (LINE-1) promoter regions (32). The MeCP2-mediated repression of LINE-1 and the regulation of CMV promoter by MeCP2 (31) suggest that MeCP2 may be required to properly silence the expression of retroviral genes. This may partially explain the elevated proportion of CMV-GFP expressing cells in MeCP2 mosaic animals at the 9-month time point in our study. This is possible because it has been shown that MeCP2 deficiency may have a powerful non-cell autonomous influence on surrounding neurons (33). Alternatively, MeCP2-deficient neurons may have a survival disadvantage compared with wild-type neurons. It has been shown that in MeCP2 heterozygote female mice, wild-type cells express lower levels of MeCP2 than in MeCP2 wild-type mice, which supports that the mutant cells can influence the phenotype of wild-type cells by a non-cell-autonomous mechanism (15). A previous study of females with X-linked mental retardation (XLMR) suggests that cells expressing the mutated X chromosome may have a selective disadvantage resulting in a higher proportion of cells expressing the normal X chromosome (13). Additionally, XCI patterns in various brain regions of Mecp2 mutant female mice are found to favor the wild-type allele, further supporting a selective advantage for cells expressing the wild-type allele (14,15). Further studies will help distinguish which of these hypothetical models is more accurate.
A number of X-linked genes are known to be associated with brain functions and human intellectual disabilities (34). The high proportion of autistic incidence in males versus females further support the involvement of X-linked genes in XLMR syndromes and others that share autistic phenotype. While MeCP2 deficiency is known to be responsible for > 90% RTT cases, mutations of MECP2 have also been found in a number of autistic females who do not meet the diagnostic criteria for RTT (35). Various XLMR-related genes have been implicated in the proper development and function of neuronal dendritic spines and synapses, processes that are affected in these patients (36). One puzzling aspect of these syndromes is the variability in the severities of the phenotypes presented by patients. It has been suggested that the severity of the RTT phenotype may be due in part to the degree of XCI skewing (14,17,30). Thus, it is possible that disease phenotypes in other XLMR and autistic disorders might be affected by XCI or the representation of the active X chromosome expressing the normal genes. Our studies show that heterozygotic deficiency of MeCP2 could affect the ratio of neurons expressing the normal genes. Further studies on differential XCI patterns in various brain regions, neuronal cell types and different age points will elucidate how differential XCI patterning impacts neuronal phenotype. Although it is highly speculative at this point, it would be interesting to know how the parental origin of the X chromosome could be manipulated in the disease-affected cell types/brain regions to improve or reverse the XLMR phenotype.
Studies using Mecp2 null male mice have led to major advances in our knowledge of RTT pathogenesis; however, most RTT patients are heterozygous females who are mosaic for the MeCP2 mutation. Thus, MeCP2 mosaic female mice can be used to determine XCI preference distinguished by GFP fluorescence (Fig. 4), giving us a unique model to study how the MeCP2 mutation may affect neuronal development in heterozygous females. Additionally, although MeCP2 is expressed at all developmental stages and in all brain cells (15), it can be difficult to detect using standard immunohistochemistry, as MeCP2 expression varies in neurons at early developmental stages and is low in non-neuronal cell types. Our MeCP2/GFP mosaic model allows researchers to identify a cell's genotype based on expression of GFP, which can be observed as early as E2.75 (19). In summary, a parental origin-specific preference for X-chromosome expression may play an important role in the severity of symptoms in RTT patients. Therefore, a better understanding of allelic preference in specific cell types and brain regions may give us valuable insight into the pathogenesis of RTT.
MATERIALS AND METHODS
Animals
All animal procedures were performed according to protocols approved by the University of New Mexico Animal Care and Use Committee. The Mecp2 KO mice (Mecp2tm1.1Jae) used in this study were created by deleting exon 3 containing the MBD domain of MeCP2 (7). These mice have been bred over 40 generations onto an ICR background. They start to show neurological symptoms between 5 and 7 weeks of age and die around 10 weeks of age. TgGFPX transgenic mice were originally obtained from The Jackson Laboratory. The MeCP2 mosaic mouse was created by crossing a TgGFPX+/y male mouse with a Mecp2 heterozygous (Mecp2+/−) female. The female offspring were all heterozygous for GFPX; some were heterozygous for MeCP2 deficiency (Mecp2+/−), and others were wild-type for MeCP2 (Mecp2+/+) (See Fig. 3 for a breeding diagram). For histological analyses, mice were euthanized by intraperitoneal injection of sodium pentobarbital. Mice were then perfused with saline followed by 4% paraformaldehyde (PFA). Brains were dissected out, post-fixed overnight in 4% PFA, equilibrated in 30% sucrose and frozen in a −80°C freezer until further processing.
For the data presented in Figure 1, we used three GFP mosaic (TgGFPX+/−) female mice per age group for quantification, each from a different litter.
In Figure 2, we conduct the cell lineage analysis in the same group of 3-month-old GFPX mice used in Figure 1. The same numbers of mice were used as in Figure 1 for all markers, except for NeuN. The NeuN data combine the data from the same 3-month group and the GFP mosaic control group shown in Figure 5. For Figure 4, the same group of 3-month-old TgGFP-X+/− mice used in Figure 1 was used for control GFP mosaic (n = 3). Three 3-month-old ‘MeCP2 mosaic' mice were used.
For Figure 5, we used multiple littermates from different litters at each time point. At 3 months, we used 11 MeCP2 mosaic mice and 14 control GFP mice. At 6 months, we used 12 MeCP2 mosaic mice and 19 control GFP mice. At 9 months, we used 10 MeCP2 mosaic mice and 11 control GFP mice. For Figure 6, at 3 months of age, 11 animals from each genotype were used. At 6 months of age, 11 MeCP2 mosaic and 6 Het Mecp2 mice were used.
Immunohistochemistry
Forty micrometer brain sections were generated from frozen fixed brains using a sliding microtome. Immunostaining was conducted according to our published methods (5,37–39). Three brain tissue sections were taken from each animal. The primary antibodies used include mouse anti-NeuN (Chemicon 1:5000), chicken anti-MeCP2 (1:5000, a generous gift from Dr Janine LaSalle University of California, Davis), rabbit anti-DCX (Cell Signaling, 1:200), rabbit anti-GFAP (DAKO, 1:1000), rabbit S100β (DAKO, 1:1000), rabbit anti-O1 (vendor) and chicken anti-GFP (Molecular Probes, 1:500). The secondary antibodies used were AF488, AF568 and AF647 (Molecular Probes, 1:500). After primary and secondary antibody staining, the sections were counterstained with 4′-6-diamidino-2-phenylindole and mounted on glass slides using 1,4-diazabicyclo[2.2.2]octane-polyvinyl alcohol anti-fade mounting medium and stored in the dark and cold until imaged.
Confocal microscopy and quantification
Images of 40 μm thick brain sections were taken using a Zeiss LSM510META Confocal Microscope with a ×20/air or a ×63/1.4 Oil DIC Plan-apochronial lens. Z-stacks were taken at a 4 μm interval throughout the thickness of the tissue section. For quantification of GFP or MeCP2 immunoreactivity in each brain region, the Z-stack images were analyzed offline using MetaMorph image analysis software (Molecular Devices). Three concurrent frames from the confocal Z-stacks were chosen based upon image quality. These three frames were then stamped with a 600 × 600 counting frame, and the immunoreactivity of each cell within the counting frame was scored and recorded. The counts were performed as previously described (39).
For Figures 1 and 2, the mean and median number of cells assayed from each region was ∼50.
Statistical analysis
For the studies comparing the control GFP mice to the MeCP2 mosaic mice (Figs 4 and 5), or comparing Mecp2 Het mice to the MeCP2 mosaic mice (Fig. 6), statistical analyses were performed using an unpaired, two-tailed, Student's t-tests. The data bars and error bars indicate mean ± standard error mean (SEM). When characterizing XCI distribution in the control GFP (TgGFPX+/−) mice (Figs 1 and 2), a two-tailed one-sample t-test was used to test the null hypothesis that the mean was equal to half (50%) of total sampled cells.
FUNDING
This work was supported by the National Institutes of Health (MH080434 to X.Z., MH078972 to X.Z., a graduate student diversity supplement to R.D.S., GM060201-funded Initiatives to Maximize Student Diversity (IMSD) program to R.L.P.) and the International Rett Syndrome Foundation.
ACKNOWLEDGEMENTS
We would like to thank Ms Cheryl T. Strauss and Ms Andrea L. McQuate for editing the manuscript, Dr Peng Jin for providing us the TgGFPX mice, Dr Sundeep Kalantry for consultation on XCI analysis method, Dr Albee Messing for information on allelic specific GFAP expression and members of the Zhao lab for their helpful discussions.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Amir R.E., Van den Veyver I.B., Wan M., Tran C.Q., Francke U., Zoghbi H.Y. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 1999;23:185–188. doi: 10.1038/13810. doi:10.1038/13810. [DOI] [PubMed] [Google Scholar]
- 2.Hammer S., Dorrani N., Dragich J., Kudo S., Schanen C. The phenotypic consequences of MECP2 mutations extend beyond Rett syndrome. Ment. Retard. Dev. Disabil. Res. Rev. 2002;8:94–98. doi: 10.1002/mrdd.10023. doi:10.1002/mrdd.10023. [DOI] [PubMed] [Google Scholar]
- 3.Nagarajan R.P., Hogart A.R., Gwye Y., Martin M.R., LaSalle J.M. Reduced MeCP2 expression is frequent in autism frontal cortex and correlates with aberrant MECP2 promoter methylation. Epigenetics. 2006;1:e1–e11. doi: 10.4161/epi.1.4.3514. doi:10.4161/epi.1.1.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bienvenu T., Chelly J. Molecular genetics of Rett syndrome: when DNA methylation goes unrecognized. Nat. Rev. Genet. 2006;7:415–426. doi: 10.1038/nrg1878. doi:10.1038/nrg1878. [DOI] [PubMed] [Google Scholar]
- 5.Smrt R.D., Eaves-Egenes J., Barkho B.Z., Santistevan N.J., Zhao C., Aimone J.B., Gage F.H., Zhao X. Mecp2 deficiency leads to delayed maturation and altered gene expression in hippocampal neurons. Neurobiol. Dis. 2007;27:77–89. doi: 10.1016/j.nbd.2007.04.005. doi:10.1016/j.nbd.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzales M.L., LaSalle J.M. The role of MeCP2 in brain development and neurodevelopmental disorders. Curr. Psychiatry Rep. 2010;12:127–134. doi: 10.1007/s11920-010-0097-7. doi:10.1007/s11920-010-0097-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen R.Z., Akbarian S., Tudor M., Jaenisch R. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat. Genet. 2001;27:327–331. doi: 10.1038/85906. doi:10.1038/85906. [DOI] [PubMed] [Google Scholar]
- 8.Guy J., Hendrich B., Holmes M., Martin J.E., Bird A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat. Genet. 2001;27:322–326. doi: 10.1038/85899. doi:10.1038/85899. [DOI] [PubMed] [Google Scholar]
- 9.Shahbazian M., Young J., Yuva-Paylor L., Spencer C., Antalffy B., Noebels J., Armstrong D., Paylor R., Zoghbi H. Mice with truncated MeCP2 recapitulate many Rett syndrome features and display hyperacetylation of histone H3. Neuron. 2002;35:243–254. doi: 10.1016/s0896-6273(02)00768-7. doi:10.1016/S0896-6273(02)00768-7. [DOI] [PubMed] [Google Scholar]
- 10.Pelka G.J., Watson C.M., Radziewic T., Hayward M., Lahooti H., Christodoulou J., Tam P.P. Mecp2 deficiency is associated with learning and cognitive deficits and altered gene activity in the hippocampal region of mice. Brain. 2006;129:887–898. doi: 10.1093/brain/awl022. doi:10.1093/brain/awl022. [DOI] [PubMed] [Google Scholar]
- 11.Plath K., Mlynarczyk-Evans S., Nusinow D.A., Panning B. Xist RNA and the mechanism of X chromosome inactivation. Annu. Rev. Genet. 2002;36:233–278. doi: 10.1146/annurev.genet.36.042902.092433. doi:10.1146/annurev.genet.36.042902.092433. [DOI] [PubMed] [Google Scholar]
- 12.Talebizadeh Z., Bittel D.C., Veatch O.J., Kibiryeva N., Butler M.G. Brief report: non-random X chromosome inactivation in females with autism. J. Autism Dev. Disord. 2005;35:675–681. doi: 10.1007/s10803-005-0011-z. doi:10.1007/s10803-005-0011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plenge R.M., Stevenson R.A., Lubs H.A., Schwartz C.E., Willard H.F. Skewed X-chromosome inactivation is a common feature of X-linked mental retardation disorders. Am. J. Hum. Genet. 2002;71:168–173. doi: 10.1086/341123. doi:10.1086/341123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young J.I., Zoghbi H.Y. X-chromosome inactivation patterns are unbalanced and affect the phenotypic outcome in a mouse model of rett syndrome. Am. J. Hum. Genet. 2004;74:511–520. doi: 10.1086/382228. doi:10.1086/382228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braunschweig D., Simcox T., Samaco R.C., LaSalle J.M. X-Chromosome inactivation ratios affect wild-type MeCP2 expression within mosaic Rett syndrome and Mecp2−/+ mouse brain. Hum. Mol. Genet. 2004;13:1275–1286. doi: 10.1093/hmg/ddh142. doi:10.1093/hmg/ddh142. [DOI] [PubMed] [Google Scholar]
- 16.Shahbazian M.D., Zoghbi H.Y. Rett syndrome and MeCP2: linking epigenetics and neuronal function. Am. J. Hum. Genet. 2002;71:1259–1272. doi: 10.1086/345360. doi:10.1086/345360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Metcalf B.M., Mullaney B.C., Johnston M.V., Blue M.E. Temporal shift in methyl-CpG binding protein 2 expression in a mouse model of Rett syndrome. Neuroscience. 2006;139:1449–1460. doi: 10.1016/j.neuroscience.2006.01.060. doi:10.1016/j.neuroscience.2006.01.060. [DOI] [PubMed] [Google Scholar]
- 18.Armstrong D.D. Neuropathology of Rett syndrome. Ment. Retard. Dev. Disabil. Res. Rev. 2002;8:72–76. doi: 10.1002/mrdd.10027. doi:10.1002/mrdd.10027. [DOI] [PubMed] [Google Scholar]
- 19.Hadjantonakis A.K., Gertsenstein M., Ikawa M., Okabe M., Nagy A. Non-invasive sexing of preimplantation stage mammalian embryos. Nat. Genet. 1998;19:220–222. doi: 10.1038/893. doi:10.1038/893. [DOI] [PubMed] [Google Scholar]
- 20.Wang J., Mager J., Chen Y., Schneider E., Cross J.C., Nagy A., Magnuson T. Imprinted X inactivation maintained by a mouse Polycomb group gene. Nat. Genet. 2001;28:371–375. doi: 10.1038/ng574. doi:10.1038/ng574. [DOI] [PubMed] [Google Scholar]
- 21.Kalantry S., Mills K.C., Yee D., Otte A.P., Panning B., Magnuson T. The Polycomb group protein Eed protects the inactive X-chromosome from differentiation-induced reactivation. Nat. Cell Biol. 2006;8:195–202. doi: 10.1038/ncb1351. doi:10.1038/ncb1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalantry S., Purushothaman S., Bowen R.B., Starmer J., Magnuson T. Evidence of Xist RNA-independent initiation of mouse imprinted X-chromosome inactivation. Nature. 2009;460:647–651. doi: 10.1038/nature08161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gregg C., Zhang J., Butler J.E., Haig D., Dulac C. Sex-specific parent-of-origin allelic expression in the mouse brain. Science. 2010;329:682–685. doi: 10.1126/science.1190831. doi:10.1126/science.1190831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Armstrong D.D., Deguchi K., Antallfy B. Survey of MeCP2 in the Rett syndrome and the non-Rett syndrome brain. J. Child Neurol. 2003;18:683–687. doi: 10.1177/08830738030180100601. doi:10.1177/08830738030180100601. [DOI] [PubMed] [Google Scholar]
- 25.Kaufmann W.E., Moser H.W. Dendritic anomalies in disorders associated with mental retardation. Cereb. Cortex. 2000;10:981–991. doi: 10.1093/cercor/10.10.981. doi:10.1093/cercor/10.10.981. [DOI] [PubMed] [Google Scholar]
- 26.Asaka Y., Jugloff D.G., Zhang L., Eubanks J.H., Fitzsimonds R.M. Hippocampal synaptic plasticity is impaired in the Mecp2-null mouse model of Rett syndrome. Neurobiol. Dis. 2006;21:217–227. doi: 10.1016/j.nbd.2005.07.005. doi:10.1016/j.nbd.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Payer B., Lee J.T. X chromosome dosage compensation: how mammals keep the balance. Annu. Rev. Genet. 2008;42:733–772. doi: 10.1146/annurev.genet.42.110807.091711. doi:10.1146/annurev.genet.42.110807.091711. [DOI] [PubMed] [Google Scholar]
- 28.Takizawa T., Gudla P.R., Guo L., Lockett S., Misteli T. Allele-specific nuclear positioning of the monoallelically expressed astrocyte marker GFAP. Genes Dev. 2008;22:489–498. doi: 10.1101/gad.1634608. doi:10.1101/gad.1634608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trappe R., Laccone F., Cobilanschi J., Meins M., Huppke P., Hanefeld F., Engel W. MECP2 mutations in sporadic cases of Rett syndrome are almost exclusively of paternal origin. Am. J. Hum. Genet. 2001;68:1093–1101. doi: 10.1086/320109. doi:10.1086/320109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watson C.M., Pelka G.J., Radziewic T., Shahbazian M.D., Christodoulou J., Williamson S.L., Tam P.P. Reduced proportion of Purkinje cells expressing paternally derived mutant Mecp2308 allele in female mouse cerebellum is not due to a skewed primary pattern of X-chromosome inactivation. Hum. Mol. Genet. 2005;14:1851–1861. doi: 10.1093/hmg/ddi191. doi:10.1093/hmg/ddi191. [DOI] [PubMed] [Google Scholar]
- 31.Mehta A.K., Majumdar S.S., Alam P., Gulati N., Brahmachari V. Epigenetic regulation of cytomegalovirus major immediate-early promoter activity in transgenic mice. Gene. 2009;428:20–24. doi: 10.1016/j.gene.2008.09.033. doi:10.1016/j.gene.2008.09.033. [DOI] [PubMed] [Google Scholar]
- 32.Muotri A.R., Marchetto M.C., Coufal N.G., Oefner R., Yeo G., Nakashima K., Gage F.H. L1 retrotransposition in neurons is modulated by MeCP2. Nature. 2010;468:443–446. doi: 10.1038/nature09544. doi:10.1038/nature09544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ballas N., Lioy D.T., Grunseich C., Mandel G. Non-cell autonomous influence of MeCP2-deficient glia on neuronal dendritic morphology. Nat. Neurosci. 2009;12:311–317. doi: 10.1038/nn.2275. doi:10.1038/nn.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ropers H.H., Hamel B.C. X-linked mental retardation. Nat. Rev. Genet. 2005;6:46–57. doi: 10.1038/nrg1501. doi:10.1038/nrg1501. [DOI] [PubMed] [Google Scholar]
- 35.Carney R.M., Wolpert C.M., Ravan S.A., Shahbazian M., Ashley-Koch A., Cuccaro M.L., Vance J.M., Pericak-Vance M.A. Identification of MeCP2 mutations in a series of females with autistic disorder. Pediatr. Neurol. 2003;28:205–211. doi: 10.1016/s0887-8994(02)00624-0. [DOI] [PubMed] [Google Scholar]
- 36.Smrt R.D., Zhao X. Epigenetic regulation of neuronal dendrite and dendritic spine development. Front. Biol. 2010;5:304–323. doi: 10.1007/s11515-010-0650-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silber J., Lim D.A., Petritsch C., Persson A.I., Maunakea A.K., Yu M., Vandenberg S.R., Ginzinger D.G., James C.D., Costello J.F., et al. miR-124 and miR-137 inhibit proliferation of glioblastoma multiforme cells and induce differentiation of brain tumor stem cells. BMC Med. 2008;6:14. doi: 10.1186/1741-7015-6-14. doi:10.1186/1741-7015-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barkho B.Z., Munoz A.E., Li X., Li L., Cunningham L.A., Zhao X. Endogenous matrix metalloproteinase (MMP)-3 and MMP-9 promote the differentiation and migration of adult neural progenitor cells in response to chemokines. Stem Cells. 2008;26:3139–3149. doi: 10.1634/stemcells.2008-0519. doi:10.1634/stemcells.2008-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao X., Ueba T., Christie B.R., Barkho B., McConnell M.J., Nakashima K., Lein E.S., Eadie B.D., Willhoite A.R., Muotri A.R., et al. Mice lacking methyl-CpG binding protein 1 have deficits in adult neurogenesis and hippocampal function. Proc. Natl Acad. Sci. USA. 2003;100:6777–6782. doi: 10.1073/pnas.1131928100. doi:10.1073/pnas.1131928100. [DOI] [PMC free article] [PubMed] [Google Scholar]