Abstract
Spinal muscular atrophy (SMA) is an inherited motor neuron disease caused by the mutation of the survival motor neuron 1 (SMN1) gene and deficiency of the SMN protein. Severe SMA mice have abnormal motor function and small, immature myofibers early in development suggesting that SMN protein deficiency results in retarded muscle growth. Insulin-like growth factor 1 (IGF-1) stimulates myoblast proliferation, induces myogenic differentiation and generates myocyte hypertrophy in vitro and in vivo. We hypothesized that increased expression of IGF-1 specifically in skeletal muscle would attenuate disease features of SMAΔ7 mice. SMAΔ7 mice overexpressing a local isoform of IGF-1 (mIGF-1) in muscle showed enlarged myofibers and a 40% increase in median survival compared with mIGF-1-negative SMA littermates (median survival = 14 versus 10 days, respectively, log-rank P = 0.025). Surprisingly, this was not associated with a significant improvement in motor behavior. Treatment of both mIGF-1NEG and mIGF-1POS SMA mice with the histone deacetylase inhibitor, trichostatin A (TSA), resulted in a further extension of survival and improved motor behavior, but the combination of mIGF-1 and TSA treatment was not synergistic. These results show that increased mIGF-1 expression restricted to muscle can modulate the phenotype of SMA mice indicating that therapeutics targeted to muscle alone should not be discounted as potential disease-modifying therapies in SMA. IGF-1 may warrant further investigation in mild SMA animal models and perhaps SMA patients.
INTRODUCTION
Spinal muscular atrophy (SMA) is an autosomal recessive motor neuron disease with a carrier frequency of ∼1 in 40 in the population. In severe SMA patients, motor neuron degeneration and muscle atrophy result in severe weakness of proximal more than distal muscles and early death (1,2). SMA is caused by mutation of the survival motor neuron 1 (SMN1) gene and deficiency of the SMN protein (3). Currently, there is no disease-modifying treatment to offer to patients with this devastating disease.
Muscle from severe SMA patients shows widespread small myofibers, which have a developmentally arrested appearance (4) and an immature expression profile of myofibrillary proteins (5). Similarly, in severe SMAΔ7 mice, myofibers are hypotrophic and express an immature pattern of myosins before there is degeneration of innervating motor nerve (6,7). This muscle pathology may be simply secondary to early motor neuron dysfunction. Experiments in SMA mice in which SMN expression has been selectively restored in myofibers (8) or selectively depleted from motor neuron precursors (9) suggest that SMN deficiency in motor neurons is the principal driver of SMA disease pathology. Nonetheless, it is unresolved whether SMN protein deficiency in muscle, particularly in muscle precursor cells, causes intrinsic defects of muscle growth that contribute to the SMA phenotype. It has been suggested, for example, that SMN deficiency in satellite cells or myoblasts impairs myofiber growth and regeneration (10,11). It has also been proposed that SMN-deficient muscle fails to provide the appropriate retrograde signaling to motor nerve (12,13). In a drosophila SMA model, wishful thinking (wit), a type II bone morphogenetic protein receptor important for retrograde signaling from muscle to nerve at the neuromuscular junction (NMJ), modifies the SMA NMJ phenotype (14).
Insulin-like growth factor 1 (IGF-1), also known as somatomedin C, is a small polypeptidic hormone that belongs to the IGF family. IGF-1 is principally secreted by the liver into the blood in response to growth hormone (GH), but various tissues can also synthesize isoforms of IGF-1 locally, where they may play autocrine or paracrine roles. In muscle, IGF-1 plays an important role during muscle development and induces muscle regeneration after injury and denervation (15). Transgenic mice overexpressing a locally synthesized form of IGF-1 (class I Ea or mIGF-1) in skeletal muscle develop muscle hypertrophy resulting from increased proliferation of satellite cells (16). Local muscle IGF-1 expression attenuates age-related skeletal muscle atrophy (16) and improves muscle function in the mdx mouse model of muscular dystrophy (17). In addition, IGF-1 has been postulated to have retrogradely acting neurotrophic effects on motor neurons (18) and transgenic overexpression of mIGF-1 (19) or viral delivery of IGF-1 (20) to muscle delays the onset and progression of disease in amyotrophic lateral sclerosis (ALS) mice. Overexpression of mIGF-1 in muscle was also recently shown to markedly extend the survival of mice with spinal and bulbar muscular atrophy (SBMA), an X-linked adult form of SMA (21).
We hypothesized that overexpressing mIGF-1 in skeletal muscle would attenuate the disease features of severe SMA mice. We show that mIGF-1 improves muscle mass and extends the survival of severe SMA mice independent of SMN induction indicating that muscle-directed therapy remains an important consideration for the treatment of SMA patients.
RESULTS
SMAΔ7 mice overexpressing mIGF-1 show increased muscle mass
Transgenic mice overexpressing a rat, non-circulating, muscle-specific isoform of IGF-1 (mIGF-1) under control of the rat myosin light chain promoter have been previously described (16). SMAΔ7 mice (hSMN2+/+/SMNΔ7+/+/mSmn+/−) were bred to these mIGF-1 transgenic mice in order to obtain SMA mIGF-1POS (mIGF-1+/−/hSMN2+/+/SMNΔ7+/+/mSmn−/−) and SMA mIGF-1NEG (mIGF-1−/−/hSMN2+/+/SMNΔ7+/+/mSmn−/−) mice (See Material and Methods for details of breeding strategy). Heterozygous (Het) mIGF-1POS mice (mIGF-1+/−) were born with expected frequencies, but mIGF-1+/+ mice were never recovered suggesting that homozygousity of the transgenic mIGF-1 allele is embryonically lethal when on the FVB/NJ background.
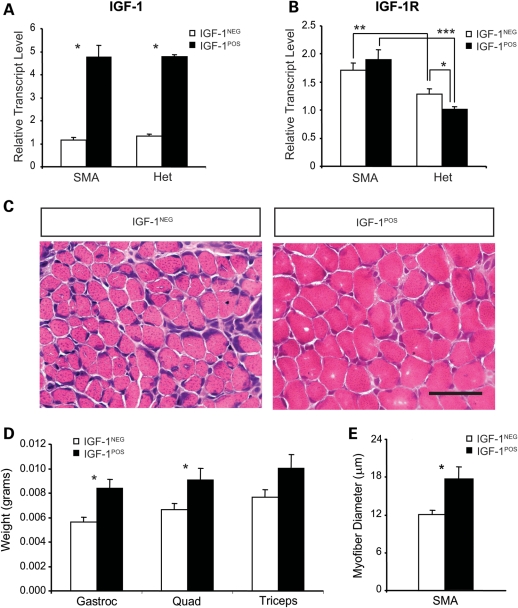
Quadriceps muscles were isolated from SMA and heterozygous littermate mIGF-1POS and mIGF-1NEG mice at postnatal day 10 (P10), and the expression levels of total IGF-1 transcripts (endogenous mouse + exogenous rat) were determined by quantitative reverse transcriptase-polymerase chain reaction (qRT–PCR). This verified an ∼5-fold increase in total IGF-1 transcript levels in mIGF-1POS mice (Fig. 1A). We also measured transcript levels of the IGF-1 receptor, IGFR1 (Fig. 1B), which mediates the biological activity of IGF-1. Both SMA mIGF-1NEG and mIGF-1POS mice showed increased IGFR1 expression compared with corresponding heterozygous littermates (1.3-fold increase in SMA mIGF-1NEG compared with Het mIGF-1NEG and 1.9-fold increase in the SMA mIGF-1POS compared with Het mIGF-1POS, P < 0.01 and P < 0.001, respectively). These data suggest a compensatory upregulation of the IGFR1 in SMA-diseased muscle. Interestingly, heterozygous littermate mIGF-1POS mice showed mildly reduced IGFR1 expression compared with heterozygous littermate mIGF-1NEG mice (1.29 ± 0.09 versus 1.01 ± 0.05, P < 0.05), suggesting a compensatory downregulation of IGFR1 in the presence of excess IGF-1 in healthy mice. This compensation did not occur in SMA mIGF-1POS mice.
Figure 1.
Overexpression of mIGF-1 results in increased muscle mass in SMA mice. (A) IGF-1 and (B) IGFR1 mRNA expression levels were determined by qRT–PCR in quadriceps muscles from P10 mIGF-1NEG and mIGF-1POS SMA and heterozygous (Het) littermate mice [*P < 0.05, **P < 0.01 and ***P < 0.001, n = 6 mice per group except for SMA mIGF-1NEG (n = 7)]. (C) H&E stained cross-sections of quadriceps muscle from P10 SMA mIGF-1NEG (left) and a P10 mIGF-1POS SMA mice (right). Scale bar = 50 µm. (D) Weights of gastrocnemius (gastroc), quadriceps (quad) and triceps muscles in P10 mIGF-1NEG and mIGF-1POS SMA mice (*P < 0.05, n = 7 mIGF-1NEG and n = 8 mIGF-1 + SMA mice). (E) Myofiber diameter in the P10 quadriceps muscle in SMA mIGF-1NEG and SMA mIGF-1POS mice (*P < 0.05, n = 3 mIGF-1NEG, and n = 4 mIGF-1POS SMA mice). Values represent average ± SEM.
In order to examine whether increased mIGF-1 expression resulted in increased muscle size, the muscle masses of the quadriceps, gastrocnemius and triceps muscles were determined in SMA mIGF-1NEG and mIGF-1POS mice at P10 (Fig. 1C and D). The wet weights of quadriceps and gastrocnemius were significantly increased in SMAΔ7 mIGF-1POS mice compared with SMAΔ7 mIGF-1NEG mice [quadriceps: SMAΔ7 mIGF-1POS = 9.98 ± 0.97 (n = 6) and SMAΔ7 mIGF-1NEG = 6.67 ± 0.49 mg (n = 7), P = 0.009 and gastrocnemius: SMAΔ7 mIGF-1POS = 8.36 ± 0.77 (n = 6) and SMAΔ7 mIGF-1NEG = 5.45 ± 0.21 mg (n = 7), P = 0.003]. Histological analysis revealed that myofiber diameter was also significantly increased in SMA mIGF-1POS quadriceps muscle by 1.5-fold (P < 0.05) (Fig. 1C and E). The average total body weights of the cohorts of mice used for muscle analysis were SMA mIGF-1NEG = 3.23 ± 0.20 g and SMA mIGF-1POS = 4.08 ± 0.38 g (P = 0.12).
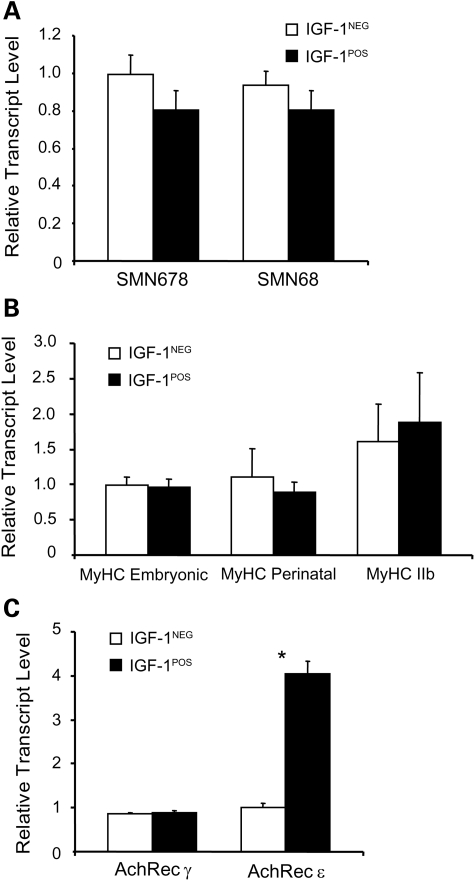
In order to evaluate whether IGF-1 expression altered the expression of SMN, we measured full-length (SMN678) and truncated forms of SMN (SMN68) transcripts in the quadriceps muscle of SMA mice at P10. There was no change in the expression levels of SMN in response to mIGF-1 overexpression (Fig. 2A). We also evaluated transcripts encoding myosin heavy chain (MyHC) isoforms and acetylcholine receptor (AChR) subunits. We have previously shown increased expression of perinatal and reduced expression of adult IIb MyHC as well as increased neonatal δ and reduced adult ɛ AChR subunit expression in SMA muscle consistent with a pattern of immaturity (7). SMA IGF-1POS muscle showed no statistical differences with SMA mIGF-1NEG muscle, except for the ɛ subunit of the AChR, which was 4-fold increased in mIGF-1POS muscle (Fig. 2B and C). Interestingly, Het mIGF-1POS mice also showed a 4-fold increase in the expression of the AChR ɛ subunit compared with Het mIGF-1NEG mice (data not shown) indicating that mIGF-1 overexpression in muscle activates expression of the mature subunit of AChR independent of disease state.
Figure 2.
Expression of SMN is not changed by mIGF-1 overexpression. (A) SMN678 and SMN68 mRNA levels were determined in quadriceps isolated from P10 SMA mIGF-1NEG (n = 7) and SMA mIGF-1POS mice (n = 6). (B) Embryonic, perinatal and adult MyHC and (C) AChR γ and AChR ε mRNA levels were measured in the same muscles (*P < 0.05). Values represent average ± SEM.
Overexpression of mIGF-1 in muscle increases survival of SMA mice
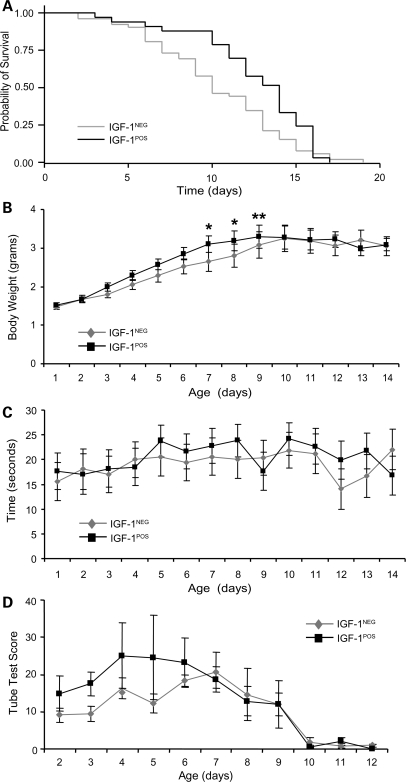
We next evaluated the survival of mIGF-1NEG and mIGF-1POS SMA mice. SMA mIGF-1NEG mice showed a median survival of 10 days (Fig. 3A). This is less than the approximate 14-day survival generally reported for SMAΔ7 mice (22) and may relate to mating the SMAΔ7 mice, which are on a mixed FVB background, to the mIGF-1 transgenic mice, which are on a pure FVB background. Supporting this idea is the observation that SMAΔ7 mice made congenic on a FVB background have a median survival of 10 days (23). Compared with SMA mIGF-1NEG mice, SMA mIGF-1POS mice had an increased median survival of 14 days indicating that mIGF-1 overexpression in muscle modestly extends survival by 40% (log-rank P = 0.025). Maximal survival of SMA mIGF-1POS mice was not increased, however. A survival benefit was more evident in SMA mIGF-1POS mice, when the mother was also mIGF-1POS (Supplementary Material, Fig. S1). Gender was not found to significantly influence survival (data not shown).
Figure 3.
Overexpression of mIGF-1 modestly improves survival of SMA mice. (A) Kaplan–Meier survival curves comparing mIGF-1NEG and mIGF-1POS SMA mice. Median survival in mIGF-1NEG SMA mice was 10 days and median survival in mIGF-1POS mice was 14 days (log-rank P = 0.025, n = 52 mIGF-1NEG and n = 33 mIGF-1POS SMA mice). (B) Body weight was increased in SMA mIGF-1POS mice on P7, P8 and P9 compared with SMA mIGF-1NEG mice (*P < 0.05 and **P < 0.01, as determined by multiple imputation test). (C) Average righting time showed no difference between SMA mIGF-1NEG and mIGF-1POS mice (n = 52 and n = 33, respectively). (D) The composite tube test score also did not show statistically significant differences between mIGF-1NEG and mIGF-1POS SMA mice (n = 8 mice per group). Values represent average ± SEM.
Like survival, weight gain and maximal weight achieved were reduced in SMA mIGF-1NEG mice compared with standard SMAΔ7 mice (Fig. 3B). However, weight gain was modestly improved in SMA mIGF-1POS mice compared with mIGF-1NEG mice, particularly toward the end of the first postnatal week suggesting that IGF-1 may have accelerated neonatal muscle growth in SMA mice. The maximal weight achieved was equivalent in the two groups, however, and occurred at P9. For SMA mIGF-1NEG mice, this was 3.24 ± 0.34 g (n = 22) and for SMA mIGF-1POS mice this was 3.29 ± 0.29 g (n = 26). Of note, an increase in body weight was not evident in mIGF-1POS compared with mIGF-1NEG control littermate mice until the fifth postnatal week (Supplementary Material, Fig. S2) indicating that mIGF-1 may not have maximal effects on muscle growth until this stage of postnatal development. These observations are in agreement with prior work indicating maximal muscle hypertrophy at 6 weeks in mIGF-1 transgenic mice (16).
We also examined motor function in SMA mIGF-1NEG and mIGF-1POS mice. Latency of time to right showed no statistically significant difference between groups (Fig. 3C). At P10, SMA mIGF-1POS mice had a righting time latency of 24.2 ± 3.5 s (n = 23) and in SMA mIGF-1NEG mice, it was 21.9 ± 3.7 s (n = 22), P = 0.48. The hind-limb suspension test (24) was also performed and a composite score of this test (25) showed no statistically significant differences between groups (Fig. 3D).
Trichostatin A increases survival of SMA mice
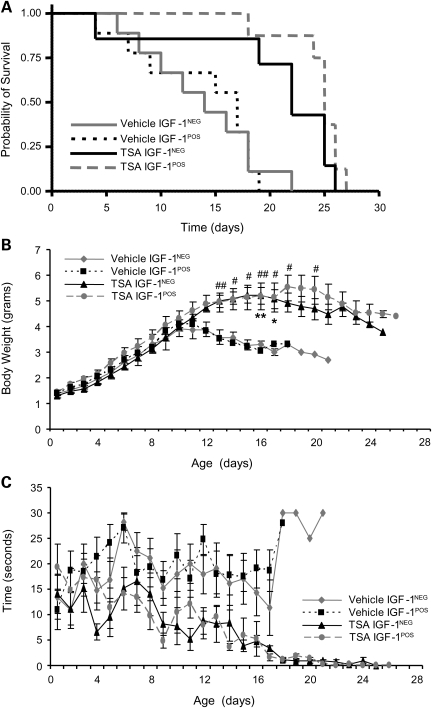
We next evaluated whether the therapeutic effects of IGF-1 targeted to muscle might be synergistic with a drug that induces SMN expression. We have previously shown that the histone deacetylase (HDAC) inhibitor, trichostatin A (TSA), activates SMN expression in spinal cord and muscle tissues (26,27) and increases the survival of SMAΔ7 mice by 40% when delivered starting at P2 (27). We have also shown that nutritional repletion can further enhance this therapeutic effect (27). Given this, we provided nutritional support to all animals starting at P3 (see Material and Methods for details) and culled all litters to 6 pups. SMA mIGF-1NEG and mIGF-1POS mice were treated with daily intraperitoneal injections of vehicle or TSA at a dose of 8 mg/kg. This dose was chosen rather than the 10 mg/kg dose used in prior studies (26,27) because toxicity was witnessed with TSA 10 mg/kg in these relatively more fragile mice. Culling and nutrition alone extended the median survival of SMA mIGF-1NEG mice from 10 to 14 days with these interventions alone (data not shown). Vehicle-treated SMA mIGF-1NEG mice showed a median survival of 14 days and TSA increased this survival to 22 days (median extension of survival = 57%, P = 0.059) (Fig. 4A). Vehicle-treated SMA mIGF-1POS mice had a median survival of 16 days and TSA increased this to 25 days (median extension of survival = 56%, P = 0.005) (Fig. 4A). Examination of body weights and latency to righting showed improvements in TSA-treated compared with vehicle-treated SMA mice; however, no further benefit was seen in SMA IGF-1POS mice treated with TSA (Fig. 4B and C).
Figure 4.
The benefits of TSA and mIGF-1 are not synergistic. (A) Kaplan–Meier survival curves comparing mIGF-1NEG and mIGF-1POS SMA mice receiving TSA. Median survival in vehicle-treated mIGF-1NEG mice was 14 days (n = 9) and in vehicle-treated mIGF-1POS mice was 17 days (n = 9) (P = 0.94). TSA treatment increased median survival of mIGF-1NEG mice (n = 7) to 22 days (P = 0.059) and of mIGF-1POS mice (n = 8) to 25 days (P < 0.005). (B) Body weights were increased in SMA mice receiving TSA compared with SMA mice receiving vehicle, but mIGF-1 did not result in a further increase as determined by the multiple imputation analysis test (vehicle mIGF-1NEG versus TSA mIGF-1NEG: *P < 0.05 and **P < 0.01 and vehicle mIGF-1POS versus TSA mIGF-1POS: #P < 0.05 and ##P < 0.01). (C) Righting time showed a trend toward improvement in TSA treated mice, but was not further increased by mIGF-1. Values represent average ± SEM.
DISCUSSION
Here, we have evaluated the effects of muscle-restricted overexpression of mIGF-1 on the phenotype of severe SMAΔ7 mice. SMA mice overexpressing mIGF-1 showed increased muscle mass and a 40% increase in median survival, but this was not accompanied by a measurable improvement in motor function. Combining this intervention with daily injections of the HDAC inhibitor TSA further extended survival, but this combination did not show significantly synergistic benefits.
SMA is caused by insufficient expression levels of the SMN protein. SMN plays a key role in the assembly of a specific class of RNA–protein complexes, the uridine-rich small ribonuclear proteins (U snRNPs) (28,29), which are central components of the spliceosome. Deficiency of SMN impairs assembly of Sm proteins onto snRNA (30,31) and thus is predicted to affect splicing. Splicing changes have been reported in various tissues of SMA mice with increased numbers of alterations present in older mice (31,32). The consequence of these splicing changes and their role in SMA has yet to be defined; however, their presence in multiple tissue types raises the possibility that although motor neurons are most susceptible to the molecular deficits caused by SMN deficiency, other tissues may also be affected. Indeed, recent studies have increasingly pointed to previously unrecognized abnormalities of the heart and vasculature in both SMA mice and humans (33–39). It has long been debated whether intrinsic abnormalities of skeletal muscle contribute to SMA disease manifestations. Determining whether treatments exclusively targeted to muscle, when given alone or in combination with other treatments, could be beneficial for SMA patients is a priority for SMA therapeutics development.
IGF-1 is essential for normal growth and development (reviewed in 15). Several transcript isoforms of IGF-1, which originate from alternative splicing and promoter usage, are divided into two classes (reviewed in 40). IGF-1 class II isoforms predominate in the liver, are circulating and are highly GH responsive. Class I isoforms are widely expressed, but are preferentially confined to the tissue of origin and act in autocrine/paracrine way. Class I Ea IGF-1 (mIGF-1) is synthesized locally in muscle. It is transiently increased in response to muscle damage and results in increased satellite cell activation and muscle growth. This isoform of IGF-1 is not thought to enter the circulation in significant quantities (16,41). Interestingly, we observed that the survival benefit in SMA IGF-1POS mice was more evident when the mother was also IGF-1POS (Supplementary Material, Fig. S1). Although this might relate to nonspecific effects of a healthier intra-uterine environment or less traumatic birth from heavier IGF-1POS mothers, it is also possible that low levels of circulating mIGF-1 from IGF-1POS mothers crossed the placenta and had beneficial effects on SMA pups in utero.
Although severe SMA mice overexpressing mIGF-1 showed a modest survival benefit, there was no motor behavioral improvement despite an increase in total weight and muscle mass. Muscle strength is difficult to evaluate in neonatal mice and our motor behavioral measures may not be sufficiently sensitive to detect small differences in muscle strength. Nonetheless, these results raise the possibility that increased survival is not a direct result of improved muscle power, but may result from other effects such as improved metabolic status. Type I SMA patients have severe muscle wasting and are at high risk for entering a fasting state with resulting hypoglycemia and muscle proteolysis (42,43). SMA mice have also been shown to be hypoglycemic (44) and show evidence of muscle breakdown as indicated by increased expression of muscle-specific ubiquitin ligases late in disease (personal unpublished data). Although mIGF-1 overexpression did not improve low glucose levels in SMA mice (Supplementary Material, Fig. S3), increased muscle bulk may have protected mIGF-1POS mice against muscle breakdown by as yet undefined mechanisms. Optimizing muscle bulk in SMA patients might have similar beneficial effects. Indeed, increased survival of type I SMA patients in recent years can be ascribed in part to aggressive nutritional support with gastrostomy feedings despite little change in muscle strength (45).
Although increasing muscle bulk may benefit SMA patients, the lack of improvement in motor behavior suggests that increasing muscle mass alone does not substantially improve the function of motor neurons in severe SMA mice. The results from this study are consistent with a previous study in which we reported that inhibition of myostatin increased muscle mass and myofiber diameter, but did not improve survival or motor function of severe SMA mice (46). This failure to increase muscle strength in the face of larger muscles may relate to impaired activity of motor neurons in SMA mice either due to intrinsic abnormalities of motor neuron excitability (47) or to impaired synaptic activation of motor neurons (48,49). Without restored excitation of the SMN-deficient motor neurons themselves, muscle may not be stimulated to contract regardless of muscle size and power capacity. This leaves open the possibility, however, that activation of SMN-deficient motor neurons (either by restoration of SMN in motor neurons or by other means) together with strategies to increase muscle bulk could give additive or even synergistic effects. Surprisingly, in this study, TSA treatment of SMA IGF-1POS mice did not result in significant synergistic effects.
In addition to promoting muscle growth, IGF-1 has been shown to have neurotrophic effects on motor neurons and therefore has been considered a promising therapy for motor neuron diseases (50). The mechanism for these neurotrophic effects is not known, but it is intriguing that we observed a 4-fold increase in the expression of the mature ɛ subunit of the AChR in IGF-1 overexpressing mice. The adult ɛ subunit-containing AChRs have distinct gating properties with a reduced channel open time and increased ion conductance compared with embryonic δ subunit-containing AChRs (51,52), and one might speculate that these properties play important roles in NMJ synapse stability particularly during disease. In two other motor neuron disease animal models, mIGF-1 overexpression was shown to have substantial effects on survival and motor function. When SBMA mice were bred to the same line of mIGF-1 overexpressing mice used in this study, median survival was increased by ∼200% and there was a marked improvement in grip strength and rotarod scores (21). SOD1G93A ALS mice overexpressing mIGF-1 showed a 24% increase in median survival and enhanced walking distance (19). Why are such effects not seen in severe SMA mice? Part of the answer may lie in the ability of diseased motor neuron axons to respond to IGF-1's ability to promote distal axonal sprouting. IGF-1 has been shown to induce sprouting of motor neurons in vitro (18) as well as in vivo when expressed in target muscle (18,53). Partial denervation with reinnervation of myofibers by sprouting of remaining intact intramuscular motor axons is a feature of both ALS and SBMA, but unlike in these adult-onset motor neuron diseases, there is little evidence of reinnervation in severe SMA human patients or mice. In contrast, milder SMA patients have robust reinnervation resulting in very large motor units evident on neurophysiological testing (2). Recently, it was demonstrated that mSmn+/− mice, which have a 40% reduction in anterior horn cell number, appear to compensate for this loss with collateral sprouting that prevents muscle weakness (54). This process is dependent, at least in part, on another neurotrophic factor, ciliary neurotrophic factor (54). Distal sprouting has also been reported in mild A2G SMA mice (55). These observations suggest that milder forms of SMA disease may show enhanced benefit from IGF-1.
In this study, we have shown for the first time that a therapeutic directed specifically to muscle can improve the survival and weight of severe SMA mice. These effects are modest and not accompanied by improved motor behavioral outcomes, suggesting that targeting muscle alone does not directly improve the function of severely SMN-deficient motor neurons. Further studies are needed to address whether IGF-1 or similar muscle-directed therapeutic strategies will have better effects in milder models of SMA in which SMN-deficient motor neuron retain the capacity for distal reinnervation as seen in mild SMA patients. Furthermore, the potential benefits of IGF-1 delivered to the nervous system should also be explored in SMA disease models in the future.
MATERIALS AND METHODS
Animals
All experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Johns Hopkins University Animal Use Committee. Breeder pairs for SMAΔ7 mice (22) on a FVB background (Stock number 005025; FVB.Cg-Tg(SMN2 × delta7)4299Ahmb Tg(SMN2)89Ahmb Smn1tm1Msd/J) were purchased from Jackson Laboratories. These mice were genotyped by PCR of tail DNA as previously reported (26). SMA mice were null at the mSmn allele (mSmn−/−) and control littermates were heterozygous (mSmn+/−) or wild-type (mSmn+/+) at the mSmn allele. Transgenic mice on a FVB background expressing IGF-1 in muscle under control of the myosin light chain promoter have been described previously (16). The myosin light chain promoter is first active at embryonic day 9.5. Mice were genotyped by PCR of tail DNA using the primers IGF-1-F: 5′-TTCCTGTCTACAGTGTCTGTG-3′ and IGF-1-R: 5′-GAGCTGACTTTGTAGGCTTCA-3′ as previously described (16). To determine the copy number of mIGF-1 transgene, mice were genotyped by qPCR of genomic DNA. Quantitative primers to detect mIGF-1 (Mm00439560_m1) were purchased from Applied Biosystems. A primer recognizing the promoter sequence of the mSmngene, MSP0, previously reported in Kernochan et al. (56), was used as an endogenous control as its copy number is stable in all mice. qPCR reactions were run in triplicate using the ABI Prism 7900 Sequence Detector System (Applied Biosystems). As expected, we detected 1 copy of mIGF-1 transgene in mice known to have one copy and 0 copies in the SMAΔ7 mice without the mIGF-1 transgene. mIGF-1+/− transgenic mice were interbred. Of 75 pups resulting from 6 different breeding pairs, 44 were IGF-1 positive and all had a single copy of the IGF-1 transgene. No IGF-1+/+ mice were recovered.
IGF-1 transgenic mice were bred to SMAΔ7 mice in order to obtain IGF-1+/−/hSMN2+/+/SMNΔ7+/+/mSmn+/− mice. In order to obtain IGF-1POS SMA mice, IGF-1+/−/hSMN2+/+/SMNΔ7+/+/mSmn+/− males were bred to either IGF-1+/−/hSMN2+/+/SMNΔ7+/+/mSmn+/− or hSMN2+/+/SMNΔ7+/+/mSmn+/− females. In crosses with IGF-1POS females, 66 of 101 pups (66%) were IGF-1POS and none had two copies of the IGF-1 transgene. Although Barton et al. (17) previously described mdx:IGF-1+/+ mice, these mice were on a C57Bl/6 background.
Drug treatment, nutrition and behavior
All litters used for drug studies were culled at P3 to a total of 6 pups. SMA mice entered in a drug study were fed starting at P4 using a custom-made gavage apparatus made from a 20 gauge 1 in. long needle (Monoject), an adapter made from 1′ Cole-Parmer Tygon tubing fit over the needle and 12′ Clay Adams polyethylene tubing (PE10) attached to a 1 ml syringe. The neonatal mouse was held upright by holding the neck skin during feeding and the syringe was placed on the working surface. The tubing was guided by the mouth into the esophagus until the tip rested just above the esophageal sphincter. The formula was released at a slow, controlled rate. Between P4 and P8, the mice were fed once or twice a day with Zoologic 30/55 formula and from P9 onwards, twice a day with Enfamil Enfacare Lipil formula. Starting at P8, the mice were administered a subcutaneous injection of 0.05 ml/g of body weight of vitamin B dissolved in lactated ringer solution (0.5 ml Vitamin B Complex in 250 ml of lactated ringer solution) once a day. TSA was dissolved in dimethyl sulphoxide (DMSO) to a concentration of 4 µg/µl. Mice were injected by intraperitoneal injection at a dose of 8 mg/kg once a day starting on the second day of life. Vehicle consisted of DMSO alone.
Daily weights were measured starting from the day of birth (P1). The lower value of two trials of righting time was determined starting on P1 with a maximal time of 30 s per trial as previously described (26). The hind-limb suspension test was performed by suspending the mouse from the hind-limbs on the edge of a 50 ml conical between P2 and P12 as previously described by El-Khodor et al. (24). Average position score, total number of pulls and total latency to fall time (maximum 30 s) during two trials was determined. A composite score of this test described by Heier and DiDonato was used (25).
Histological analysis of muscle
Quadriceps, gastrocnemius and triceps muscles were dissected, weighed, embedded and flash frozen in liquid nitrogen. Twelve micrometer cryostat sections were obtained from the mid-belly of each muscle and stained with hematoxylin and eosin (H&E). Total muscle cross-section areas and muscle fiber diameters were quantified by an investigator blinded to the animal's treatment group using a Microcolor RGB-MS-C camera, OpenLab software and a Zeiss Axiophot microscope.
RNA extraction and quantification
Animals were anesthetized with isoflurane and euthanized. Quadriceps muscles were isolated and flash frozen in liquid nitrogen. Total RNA was isolated from homogenized quadriceps muscle using TRIzol reagent (Invitrogen), and converted to cDNA as previously described (26). Specific primers and probes were used to amplify SMN678, and SMNΔ7 mRNA as previously described (26). Probes and primers to amplify embryonic (Mm01332473_g1), perinatal (Mm00838799_g1), adult IIb (Mm01332516_g1) MyHC, AChR γ (Mm00437419_m1), AChR ɛ (Mm00437406_g1), IGF-1R (Rn01477918_m1), IGF-1 (Mm00710307_m1) and 18S (Hs99999901_s1) were purchased from Applied Biosystems. qRT–PCR reactions were run in triplicate using the ABI Prism 7900 Sequence Detector System (Applied Biosystems). The level of each transcript was quantified by the threshold cycle (Ct) method using 18S as an endogenous control. Values were normalized to the mean of the IGF-1−/− mice for each gene, which was assigned as 1.
Statistical analysis
Survival data were analyzed using GraphPad Prism 5 software package. Statistical differences were compared with the log-rank test of Kaplan–Meier curves for two experimental groups, whereas comparisons of survival curves between three or more groups were done using the Bonferroni post hoc test. Behavioral, pathological and biochemical data were analyzed using Excel and SAS software packages, and statistical significance was determined using either Student's t-test or two-way ANOVA followed by Bonferroni multiple comparisons, as appropriate. Missing data from body weight and righting times curves were analyzed using multiple imputation assuming a simple multi-level model with random intercepts and later analyzed with two-way ANOVA followed by the Bonferroni test. Significance was set at a P-value of less than 0.05.
SUPPLEMENTARY MATERIAL
Supplementary Material is available at HMG online.
FUNDING
This was supported by National Institutes of Health (R01NS062869), Howard Hughes Medical Institute Physician Scientist Award, Families of Spinal Muscular Atrophy and Spinal Muscular Atrophy Foundation grants to C.J.S. and by 7FP-Myoage (Grant Agreement Number: 223576) to A.M.
ACKNOWLEDGEMENTS
We would like to thank Sergio Rey for his advice on the statistical analysis of the data.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Dubowitz V. Muscle Disorders in Childhood. Philadelphia: WB Saunders; 1995. [Google Scholar]
- 2.Crawford T.O., Pardo C.A. The neurobiology of childhood spinal muscular atrophy. Neurobiol. Dis. 1996;3:97–110. doi: 10.1006/nbdi.1996.0010. doi:10.1006/nbdi.1996.0010. [DOI] [PubMed] [Google Scholar]
- 3.Lefebvre S., Burglen L., Reboullet S., Clermont O., Burlet P., Viollet L., Benichou B., Cruaud C., Millasseau P., Zeviani M., et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80:155–165. doi: 10.1016/0092-8674(95)90460-3. doi:10.1016/0092-8674(95)90460-3. [DOI] [PubMed] [Google Scholar]
- 4.Fidzianska A., Goebel H.H., Warlo I. Acute infantile spinal muscular atrophy. Muscle apoptosis as a proposed pathogenetic mechanism. Brain. 1990;113:433–445. doi: 10.1093/brain/113.2.433. doi:10.1093/brain/113.2.433. [DOI] [PubMed] [Google Scholar]
- 5.Stevens L., Bastide B., Maurage C.A., Dupont E., Montel V., Cieniewski-Bernard C., Cuisset J.M., Vallee L., Mounier Y. Childhood spinal muscular atrophy induces alterations in contractile and regulatory protein isoform expressions. Neuropathol. Appl. Neurobiol. 2008;34:659–670. doi: 10.1111/j.1365-2990.2008.00950.x. doi:10.1111/j.1365-2990.2008.00950.x. [DOI] [PubMed] [Google Scholar]
- 6.Biondi O., Grondard C., Lecolle S., Deforges S., Pariset C., Lopes P., Cifuentes-Diaz C., Li H., della Gaspera B., Chanoine C., et al. Exercise-induced activation of NMDA receptor promotes motor unit development and survival in a type 2 spinal muscular atrophy model mouse. J. Neurosci. 2008;28:953–962. doi: 10.1523/JNEUROSCI.3237-07.2008. doi:10.1523/JNEUROSCI.3237-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kong L., Wang X., Choe D.W., Polley M., Burnett B.G., Bosch-Marce M., Griffin J.W., Rich M.M., Sumner C.J. Impaired synaptic vesicle release and immaturity of neuromuscular junctions in spinal muscular atrophy mice. J. Neurosci. 2009;29:842–851. doi: 10.1523/JNEUROSCI.4434-08.2009. doi:10.1523/JNEUROSCI.4434-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gavrilina T.O., McGovern V.L., Workman E., Crawford T.O., Gogliotti R.G., DiDonato C.J., Monani U.R., Morris G.E., Burghes A.H. Neuronal SMN expression corrects spinal muscular atrophy in severe SMA mice while muscle-specific SMN expression has no phenotypic effect. Hum. Mol. Genet. 2008;17:1063–1075. doi: 10.1093/hmg/ddm379. doi:10.1093/hmg/ddm379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park G.H., Maeno-Hikichi Y., Awano T., Landmesser L.T., Monani U.R. Reduced survival of motor neuron (SMN) protein in motor neuronal progenitors functions cell autonomously to cause spinal muscular atrophy in model mice expressing the human centromeric (SMN2) gene. J. Neurosci. 2010;30:12005–12019. doi: 10.1523/JNEUROSCI.2208-10.2010. doi:10.1523/JNEUROSCI.2208-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicole S., Desforges B., Millet G., Lesbordes J., Cifuentes-Diaz C., Vertes D., Cao M.L., De Backer F., Languille L., Roblot N., et al. Intact satellite cells lead to remarkable protection against Smn gene defect in differentiated skeletal muscle. J. Cell Biol. 2003;161:571–582. doi: 10.1083/jcb.200210117. doi:10.1083/jcb.200210117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shafey D., Cote P.D., Kothary R. Hypomorphic Smn knockdown C2C12 myoblasts reveal intrinsic defects in myoblast fusion and myotube morphology. Exp. Cell Res. 2005;311:49–61. doi: 10.1016/j.yexcr.2005.08.019. doi:10.1016/j.yexcr.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 12.Braun S., Croizat B., Lagrange M.C., Warter J.M., Poindron P. Constitutive muscular abnormalities in culture in spinal muscular atrophy. Lancet. 1995;345:694–695. doi: 10.1016/s0140-6736(95)90869-2. doi:10.1016/S0140-6736(95)90869-2. [DOI] [PubMed] [Google Scholar]
- 13.Guettier-Sigrist S., Coupin G., Braun S., Warter J.M., Poindron P. Muscle could be the therapeutic target in SMA treatment. J. Neurosci. Res. 1998;53:663–669. doi: 10.1002/(SICI)1097-4547(19980915)53:6<663::AID-JNR4>3.0.CO;2-3. doi:10.1002/(SICI)1097-4547(19980915)53:6<663::AID-JNR4>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 14.Chang H.C., Dimlich D.N., Yokokura T., Mukherjee A., Kankel M.W., Sen A., Sridhar V., Fulga T.A., Hart A.C., Van Vactor D., et al. Modeling spinal muscular atrophy in Drosophila. PLoS ONE. 2008;3:e3209. doi: 10.1371/journal.pone.0003209. doi:10.1371/journal.pone.0003209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duan C., Ren H., Gao S. Insulin-like growth factors (IGFs), IGF receptors, and IGF-binding proteins: roles in skeletal muscle growth and differentiation. Gen. Comp. Endocrinol. 2010;167:344–351. doi: 10.1016/j.ygcen.2010.04.009. doi:10.1016/j.ygcen.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Musaro A., McCullagh K., Paul A., Houghton L., Dobrowolny G., Molinaro M., Barton E.R., Sweeney H.L., Rosenthal N. Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat. Genet. 2001;27:195–200. doi: 10.1038/84839. doi:10.1038/84839. [DOI] [PubMed] [Google Scholar]
- 17.Barton E.R., Morris L., Musaro A., Rosenthal N., Sweeney H.L. Muscle-specific expression of insulin-like growth factor I counters muscle decline in mdx mice. J. Cell Biol. 2002;157:137–148. doi: 10.1083/jcb.200108071. doi:10.1083/jcb.200108071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caroni P., Grandes P. Nerve sprouting in innervated adult skeletal muscle induced by exposure to elevated levels of insulin-like growth factors. J. Cell Biol. 1990;110:1307–1317. doi: 10.1083/jcb.110.4.1307. doi:10.1083/jcb.110.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dobrowolny G., Giacinti C., Pelosi L., Nicoletti C., Winn N., Barberi L., Molinaro M., Rosenthal N., Musaro A. Muscle expression of a local Igf-1 isoform protects motor neurons in an ALS mouse model. J. Cell Biol. 2005;168:193–199. doi: 10.1083/jcb.200407021. doi:10.1083/jcb.200407021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaspar B.K., Llado J., Sherkat N., Rothstein J.D., Gage F.H. Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science. 2003;301:839–842. doi: 10.1126/science.1086137. doi:10.1126/science.1086137. [DOI] [PubMed] [Google Scholar]
- 21.Palazzolo I., Stack C., Kong L., Musaro A., Adachi H., Katsuno M., Sobue G., Taylor J.P., Sumner C.J., Fischbeck K.H., et al. Overexpression of IGF-1 in muscle attenuates disease in a mouse model of spinal and bulbar muscular atrophy. Neuron. 2009;63:316–328. doi: 10.1016/j.neuron.2009.07.019. doi:10.1016/j.neuron.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le T.T., Pham L.T., Butchbach M.E., Zhang H.L., Monani U.R., Coovert D.D., Gavrilina T.O., Xing L., Bassell G.J., Burghes A.H. SMNDelta7, the major product of the centromeric survival motor neuron (SMN2) gene, extends survival in mice with spinal muscular atrophy and associates with full-length SMN. Hum. Mol. Genet. 2005;14:845–857. doi: 10.1093/hmg/ddi078. doi:10.1093/hmg/ddi078. [DOI] [PubMed] [Google Scholar]
- 23.Kariya S., Park G.H., Maeno-Hikichi Y., Leykekhman O., Lutz C., Arkovitz M.S., Landmesser L.T., Monani U.R. Reduced SMN protein impairs maturation of the neuromuscular junctions in mouse models of spinal muscular atrophy. Hum. Mol. Genet. 2008;17:2552–2569. doi: 10.1093/hmg/ddn156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El-Khodor B.F., Edgar N., Chen A., Winberg M.L., Joyce C., Brunner D., Suarez-Farinas M., Heyes M.P. Identification of a battery of tests for drug candidate evaluation in the SMNDelta7 neonate model of spinal muscular atrophy. Exp. Neurol. 2008;212:29–43. doi: 10.1016/j.expneurol.2008.02.025. doi:10.1016/j.expneurol.2008.02.025. [DOI] [PubMed] [Google Scholar]
- 25.Heier C.R., DiDonato C.J. Translational readthrough by the aminoglycoside geneticin (G418) modulates SMN stability in vitro and improves motor function in SMA mice in vivo. Hum. Mol. Genet. 2009;18:1310–1322. doi: 10.1093/hmg/ddp030. doi:10.1093/hmg/ddp030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Avila A.M., Burnett B.G., Taye A.A., Gabanella F., Knight M.A., Hartenstein P., Cizman Z., Di Prospero N.A., Pellizzoni L., Fischbeck K.H., et al. Trichostatin A increases SMN expression and survival in a mouse model of spinal muscular atrophy. J. Clin. Invest. 2007;117:659–671. doi: 10.1172/JCI29562. doi:10.1172/JCI29562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Narver H.L., Kong L., Burnett B.G., Choe D.W., Bosch-Marce M., Taye A.A., Eckhaus M.A., Sumner C.J. Sustained improvement of spinal muscular atrophy mice treated with trichostatin a plus nutrition. Ann. Neurol. 2008;64:465–470. doi: 10.1002/ana.21449. doi:10.1002/ana.21449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Battle D.J., Kasim M., Yong J., Lotti F., Lau C.K., Mouaikel J., Zhang Z., Han K., Wan L., Dreyfuss G. The SMN complex: an assembly machine for RNPs. Cold Spring Harb. Symp. Quant. Biol. 2006;71:313–320. doi: 10.1101/sqb.2006.71.001. doi:10.1101/sqb.2006.71.001. [DOI] [PubMed] [Google Scholar]
- 29.Pellizzoni L. Chaperoning ribonucleoprotein biogenesis in health and disease. EMBO Rep. 2007;8:340–345. doi: 10.1038/sj.embor.7400941. doi:10.1038/sj.embor.7400941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gabanella F., Butchbach M.E., Saieva L., Carissimi C., Burghes A.H., Pellizzoni L. Ribonucleoprotein assembly defects correlate with spinal muscular atrophy severity and preferentially affect a subset of spliceosomal snRNPs. PLoS ONE. 2007;2:e921. doi: 10.1371/journal.pone.0000921. doi:10.1371/journal.pone.0000921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Z., Lotti F., Dittmar K., Younis I., Wan L., Kasim M., Dreyfuss G. SMN deficiency causes tissue-specific perturbations in the repertoire of snRNAs and widespread defects in splicing. Cell. 2008;133:585–600. doi: 10.1016/j.cell.2008.03.031. doi:10.1016/j.cell.2008.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baumer D., Lee S., Nicholson G., Davies J.L., Parkinson N.J., Murray L.M., Gillingwater T.H., Ansorge O., Davies K.E., Talbot K. Alternative splicing events are a late feature of pathology in a mouse model of spinal muscular atrophy. PLoS Genet. 2009;5:e1000773. doi: 10.1371/journal.pgen.1000773. doi:10.1371/journal.pgen.1000773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menke L.A., Poll-The B.T., Clur S.A., Bilardo C.M., van der Wal A.C., Lemmink H.H., Cobben J.M. Congenital heart defects in spinal muscular atrophy type I: a clinical report of two siblings and a review of the literature. Am. J. Med. Genet. A. 2008;146A:740–744. doi: 10.1002/ajmg.a.32233. doi:10.1002/ajmg.a.32233. [DOI] [PubMed] [Google Scholar]
- 34.Rudnik-Schoneborn S., Heller R., Berg C., Betzler C., Grimm T., Eggermann T., Eggermann K., Wirth R., Wirth B., Zerres K. Congenital heart disease is a feature of severe infantile spinal muscular atrophy. J. Med. Genet. 2008;45:635–638. doi: 10.1136/jmg.2008.057950. doi:10.1136/jmg.2008.057950. [DOI] [PubMed] [Google Scholar]
- 35.Araujo Ade Q., Araujo M., Swoboda K.J. Vascular perfusion abnormalities in infants with spinal muscular atrophy. J. Pediatr. 2009;155:292–294. doi: 10.1016/j.jpeds.2009.01.071. doi:10.1016/j.jpeds.2009.01.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bevan A.K., Hutchinson K.R., Foust K.D., Braun L., McGovern V.L., Schmelzer L., Ward J.G., Petruska J.C., Lucchesi P.A., Burghes A.H., et al. Early heart failure in the SMN{Delta}7 model of spinal muscular atrophy and correction by postnatal scAAV9-SMN delivery. Hum. Mol. Genet. 2010;19:3895–3905. doi: 10.1093/hmg/ddq300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shababi M., Habibi J., Yang H.T., Vale S.M., Sewell W.A., Lorson C.L. Cardiac defects contribute to the pathology of spinal muscular atrophy models. Hum. Mol. Genet. 2010;19:4059–4071. doi: 10.1093/hmg/ddq329. doi:10.1093/hmg/ddq329. [DOI] [PubMed] [Google Scholar]
- 38.Heier C.R., Satta R., Lutz C., DiDonato C.J. Arrhythmia and cardiac defects are a feature of spinal muscular atrophy model mice. Hum. Mol. Genet. 2010;19:3906–3918. doi: 10.1093/hmg/ddq330. doi:10.1093/hmg/ddq330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rudnik-Schoneborn S., Vogelgesang S., Armbrust S., Graul-Neumann L., Fusch C., Zerres K. Digital necroses and vascular thrombosis in severe spinal muscular atrophy. Muscle Nerve. 2010;42:144–147. doi: 10.1002/mus.21654. doi:10.1002/mus.21654. [DOI] [PubMed] [Google Scholar]
- 40.Barton E.R. The ABCs of IGF-I isoforms: impact on muscle hypertrophy and implications for repair. Appl. Physiol. Nutr. Metab. 2006;31:791–797. doi: 10.1139/h06-054. doi:10.1139/H06-054. [DOI] [PubMed] [Google Scholar]
- 41.Shavlakadze T., Chai J., Maley K., Cozens G., Grounds G., Winn N., Rosenthal N., Grounds M.D. A growth stimulus is needed for IGF-1 to induce skeletal muscle hypertrophy in vivo. J. Cell Sci. 2010;123:960–971. doi: 10.1242/jcs.061119. doi:10.1242/jcs.061119. [DOI] [PubMed] [Google Scholar]
- 42.Bruce A.K., Jacobsen E., Dossing H., Kondrup J. Hypoglycaemia in spinal muscular atrophy. Lancet. 1995;346:609–610. doi: 10.1016/s0140-6736(95)91439-0. doi:10.1016/S0140-6736(95)91439-0. [DOI] [PubMed] [Google Scholar]
- 43.Orngreen M.C., Zacho M., Hebert A., Laub M., Vissing J. Patients with severe muscle wasting are prone to develop hypoglycemia during fasting. Neurology. 2003;61:997–1000. doi: 10.1212/01.wnl.0000086813.59722.72. [DOI] [PubMed] [Google Scholar]
- 44.Butchbach M.E., Rose F.F., Jr, Rhoades S., Marston J., McCrone J.T., Sinnott R., Lorson C.L. Effect of diet on the survival and phenotype of a mouse model for spinal muscular atrophy. Biochem. Biophys. Res. Commun. 2010;391:835–840. doi: 10.1016/j.bbrc.2009.11.148. doi:10.1016/j.bbrc.2009.11.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oskoui M., Levy G., Garland C.J., Gray J.M., O'Hagen J., De Vivo D.C., Kaufmann P. The changing natural history of spinal muscular atrophy type 1. Neurology. 2007;69:1931–1936. doi: 10.1212/01.wnl.0000290830.40544.b9. doi:10.1212/01.wnl.0000290830.40544.b9. [DOI] [PubMed] [Google Scholar]
- 46.Sumner C.J., Wee C.D., Warsing L.C., Choe D.W., Ng A.S., Lutz C., Wagner K.R. Inhibition of myostatin does not ameliorate disease features of severe spinal muscular atrophy mice. Hum. Mol. Genet. 2009;18:3145–3152. doi: 10.1093/hmg/ddp253. doi:10.1093/hmg/ddp253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Biondi O., Branchu J., Sanchez G., Lancelin C., Deforges S., Lopes P., Pariset C., Lecolle S., Cote J., Chanoine C., et al. In vivo NMDA receptor activation accelerates motor unit maturation, protects spinal motor neurons, and enhances SMN2 gene expression in severe spinal muscular atrophy mice. J. Neurosci. 2010;30:11288–11299. doi: 10.1523/JNEUROSCI.1764-10.2010. doi:10.1523/JNEUROSCI.1764-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ling K.K., Lin M.Y., Zingg B., Feng Z., Ko C.P. Synaptic defects in the spinal and neuromuscular circuitry in a mouse model of spinal muscular atrophy. PLoS ONE. 2010;5:e15457. doi: 10.1371/journal.pone.0015457. doi:10.1371/journal.pone.0015457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mentis G.Z., Liu W., Blivis D., Drobac E., Crowder M.E., Kong L., Alvarez F.J., Sumner C.J., O'Donovan M.J. Early functional impairment of sensory-motor connectivity in a mouse model of spinal muscular atrophy. Neuron. 2010;69:453–467. doi: 10.1016/j.neuron.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Musaro A., Dobrowolny G., Rosenthal N. The neuroprotective effects of a locally acting IGF-1 isoform. Exp. Gerontol. 2007;42:76–80. doi: 10.1016/j.exger.2006.05.004. doi:10.1016/j.exger.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 51.Sakmann B., Brenner H.R. Change in synaptic channel gating during neuromuscular development. Nature. 1978;276:401–402. doi: 10.1038/276401a0. doi:10.1038/276401a0. [DOI] [PubMed] [Google Scholar]
- 52.Fischbach G.D., Schuetze S.M. A post-natal decrease in acetylcholine channel open time at rat end-plates. J. Physiol. 1980;303:125–137. doi: 10.1113/jphysiol.1980.sp013275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Messi M.L., Delbono O. Target-derived trophic effect on skeletal muscle innervation in senescent mice. J. Neurosci. 2003;23:1351–1359. doi: 10.1523/JNEUROSCI.23-04-01351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simon C.M., Jablonka S., Ruiz R., Tabares L., Sendtner M. Ciliary neurotrophic factor-induced sprouting preserves motor function in a mouse model of mild spinal muscular atrophy. Hum. Mol. Genet. 2010;19:973–986. doi: 10.1093/hmg/ddp562. doi:10.1093/hmg/ddp562. [DOI] [PubMed] [Google Scholar]
- 55.Monani U.R., Pastore M.T., Gavrilina T.O., Jablonka S., Le T.T., Andreassi C., DiCocco J.M., Lorson C., Androphy E.J., Sendtner M., et al. A transgene carrying an A2G missense mutation in the SMN gene modulates phenotypic severity in mice with severe (type I) spinal muscular atrophy. J. Cell Biol. 2003;160:41–52. doi: 10.1083/jcb.200208079. doi:10.1083/jcb.200208079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kernochan L.E., Russo M.L., Woodling N.S., Huynh T.N., Avila A.M., Fischbeck K.H., Sumner C.J. The role of histone acetylation in SMN gene expression. Hum. Mol. Genet. 2005;14:1171–1182. doi: 10.1093/hmg/ddi130. doi:10.1093/hmg/ddi130. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.