Abstract
Treatment with the ω-3 polyunsaturated fatty acids (PUFAs) docosahexanoic acid (DHA) and eicosapentanoic acid (EPA) exerts cardioprotective effects, and suppresses Ca2+-induced opening of the mitochondrial permeability transition pore (MPTP). These effects are associated with increased DHA and EPA, and lower arachidonic acid (ARA) in cardiac phospholipids. While clinical studies suggest the triglyceride lowering effects of DHA and EPA are equivalent, little is known about the independent effects of DHA and EPA on mitochondria function. We compared the effects of dietary supplementation with the ω-3 PUFAs DHA and EPA on cardiac mitochondrial phospholipid fatty acid composition and Ca2+-induced MPTP opening. Rats were fed a standard lab diet with either normal low levels of ω-3 PUFA, or DHA or EPA at 2.5% of energy intake for 8 weeks, and cardiac mitochondria were isolated and analyzed for Ca2+-induced MPTP opening and phospholipid fatty acyl composition. DHA supplementation increased both DHA and EPA and decreased ARA in mitochondrial phospholipid, and significantly delayed MPTP opening as assessed by increased Ca2+ retention capacity and decreased Ca2+-induced mitochondria swelling. EPA supplementation increased EPA in mitochondrial phospholipids, but did not affect DHA, only modestly lowered ARA, and did not affect MPTP opening. In summary, dietary supplementation with DHA but not EPA, profoundly altered mitochondrial phospholipid fatty acid composition and delayed Ca2+-induced MPTP opening.
Keywords: cardiac, eicosapentaenoic acid, docosahexaenoic acid, fish oil, heart, mitochondrial permeability transition pore
1. Introduction
Cardiac mitochondria are important not only for maintaining sufficient ATP generation, but also for prevention of cell death and loss of cardiomyocytes. Opening of the mitochondrial permeability transition pore (MPTP) leads to cell death, and has been implicated in ischemic injury and the development and progression of heart failure [1,23], thus pharmacological prevention of MPTP is a target for cardioprotective therapy[5,13,17,34]. The MPTP is a large diameter, high conductance, voltage-dependent channel that allows passage of water, ions, and molecules up to ~1.5 kD[13,34]. Mitochondrial accumulation of high levels of Ca2+ triggers MPTP opening, while Ca2+ chelation causes it to rapidly close. The molecular components and structure of the pore are not precisely known[13], however recent data suggest MPTP opening is affected by the composition of mitochondrial membrane phospholipids[24].
Pharmacological doses of the ω-3 polyunsaturated fatty acids (PUFAs) docosahexanoic acid (DHA (22:6n3)) and eicosapentanoic acid (EPA (20:5n3)) from fish oils are used clinically to lower plasma triglycerides, however they may also decreased ischemic injury, and prevent the development and progression of heart failure[8,9,11,27]. The mechanisms for these effects are not clear, but may be dependent upon changes in cardiac phospholipid composition and improved mitochondrial tolerance to stress. We recently found that dietary supplementation with a mixture of EPA and DHA increased the mitochondrial phospholipid content of DHA and EPA, and decreased arachidonic acid (ARA; 20:4n6) [24]. ARA is the precursor to pro-inflammatory prostaglandins, thromboxanes and leukotrienes. Release of ARA from cell membranes can trigger MPTP opening and is implicated in cell death by either necrosis or apoptosis [18,26,29,35]. Both DHA and EPA can decrease ARA through inhibition of the elongation of linoleic acid[8]. We showed that dietary supplementation with DHA+EPA (70:30 ratio) prevented ventricular remodeling and cardiomyocyte apoptosis in rats with aortic banding, and increased DHA and EPA and decreased ARA in cardiac phospholipids[9]. DHA+EPA supplementation also decreased ARA and increased DHA in cardiolipin (CL)[31], an inner membrane tetra-acyl phospholipid comprised primarily of linoleic acid (18:2n6) that anchors cytochrome C to the membrane and inhibits apoptosis[21,25]. Dietary supplementation with DHA+EPA can increase total CL content in cardiac mitochondria[20,28], and prevent the decline in tetralinoleyl CL (L4CL), the primary moiety of CL, in hearts subjected to prolonged aortic constriction[31].
Little is known about the independent effects of DHA and EPA on cardiac mitochondria. While clinical studies show that the triglyceride lowering effects of DHA and EPA are equivalent [12], there is evidence that DHA supplementation is superior to EPA in changing the fatty acid composition of cardiac phospholipids [30]. DHA is readily shortened to form EPA, but there is minimal elongation of EPA to form DHA[22], and appears to not occur in the heart [15]. Thus DHA supplementation should be more effectively increasing total ω-3 PUFA and decreasing ARA in membrane phospholipids, particularly in the heart. We hypothesize that dietary supplementation with DHA causes a greater increase in total ω-3 PUFA and decrease in ω-6 PUFA in mitochondrial membrane phospholipids than does supplementation with EPA, and results in a delay in Ca2+-induced MPTP opening, while EPA does not.
2. Methods
2.1 Experimental Design
The animal protocol was conducted according to the Guideline for the Care and Use of Laboratory Animals (NIH publication 85-23) and was approved by the University of Maryland School of Medicine Institutional Animal Care and Use Committee. Investigators were blinded to treatment when measurements were performed. The animals were maintained on a reverse 12-h light–dark cycle and all procedures were performed in the fed state between 3 and 6 h from the start of the dark phase. Two series of experiments were performed. In the initial studies (Series 1) male Wistar rats weighing 190–200 g were fed a standard low fat diet (CTRL) or modified standard diet containing DHA or EPA at 2.5% of total caloric intake, which corresponds to a human intake of approximately 5.5 g/day (calculated assuming an energy intake of 2000 kcal/day and 9 kcal/g of fat). The rats were maintained on the diet for 8 weeks. Following dietary treatment, rats were anesthetized with isoflurane, blood was drawn, and the heart was harvested for biochemical analysis and mitochondrial isolation. Plasma from these animals was analyzed for free fatty acids and triglyceride levels, and cardiac mitochondria for respiration, Ca2+ retention capacity, VDAC and cyclophilin D, and membrane phospholipid composition as described below. Following completion of Series 1, a second series of animal studies (Series 2) were performed to assess mitochondrial swelling using a light scattering assay, and the effects of different respiratory substrates on Ca2+ retention capacity in mitochondria from control and DHA supplemented rats. Animals were treated for 10 weeks and were fed either CTRL or DHA (2.5% of total caloric intake).
2.2 Diets
All diets were custom-manufactured (Research Diets Inc., New Brunswick, NJ), and had 68% of total energy from carbohydrate (38% of total energy from cornstarch, 5% from maltodextrin and 25% from sucrose), 20% protein (casein supplemented with l-cystine) and 12% energy from fat. In Series 1 the CTRL diet the fat was made up of 35.3% cocoa butter, 39.8% lard, 16.6% soybean oil and 8.3% palm kernel oil (see Table 1 for fatty acid composition). The DHA diet contained 5.75% of total energy from algal oil that was comprised of 45.6% DHA by mass (DHASCO, Martek Inc, Columbia, MD, USA), with the balance from cocoa butter and soybean oil. The EPA diet had 2.6% of energy from purified fish oil comprised of 95.5% EPA by mass (KD Pharma, Bexbach, Germany), with the balance from cocoa butter, soybean oil, safflower oil and palm kernel oil. DHA and EPA oils contained ascorbyl palmitate (250ppm) and tocopherols (250ppm) to prevent peroxidation, which was less than 0.5meq/kg at the time of manufacture of the diet. All diets were supplemented with the same amount of vitamins (Vitamin Mix V10001, 10g/kg), minerals (Mineral Mix S10026, 10g/kg), cellulose (50g/kg) and choline (2g/kg). In Series 2, the DHA was again 2.5% of total energy in the diet, but the diets had 66% of total energy from carbohydrate (54% of total energy from cornstarch and 12% from maltodextrin), 20% protein (casein supplemented with l-cystine) and 14% energy from fat (see Table 1 for fatty acid composition). In the CTRL diet, the fat was made up of 71.5% cocoa butter, 17.1% soybean oil, 7.2% palm oil, 2.8% safflower oil and 1.4% linseed oil. In the DHA diet, 5.75% of algal oil partially replaced cocoa butter. Animals were treated for 8 weeks and mitochondria isolated as in Series 1.
Table 1.
Fatty acid compositions of the rodent diets expressed as the molar percent of total fatty acids in the diet. In Series 1 all diets had 12% of total energy from fat, 20% from protein and 68 % from carbohydrate, and in Series 2 all diets had 14% of total energy from fat, 20% from protein and 66 % from carbohydrate.
| Series 1 |
Series 2 |
||||
|---|---|---|---|---|---|
| Fatty Acid | CTRL | DHA | EPA | CRTL | DHA |
| C12:0 | 3.9 | 2.2 | 3.9 | 3.4 | 1.5 |
| C14:0 | 1.7 | 4.9 | 1.3 | 1.1 | 3.6 |
| C16:0 | 21.6 | 14.7 | 13.1 | 21.7 | 14.6 |
| C16:1 | 1.7 | 1.8 | - | 0.2 | 1.4 |
| C18:0 | 18.9 | 13.8 | 14.0 | 25.8 | 14.2 |
| C18:1n-9 | 34.9 | 29.7 | 31.6 | 30.3 | 28.8 |
| C18:2n-6 | 13.9 | 11.1 | 13.0 | 13.9 | 14.7 |
| C18:3n-3 | 1.8 | 1.3 | 1.3 | 2.2 | 2.2 |
| C20:5n-3 | - | - | 19.1 | - | - |
| C22:6n-3 | - | 19.1 | - | - | 17.8 |
2.3 Mitochondrial Preparation
Mitochondria were isolated as previously described[24]. LV tissue (400–500 mg) was minced and homogenized in 1:10 cold modified Chappel-Perry buffer (100mM KCl, 50mM MOPS, 5mM MgSO4, 1mM ATP, 1mM EGTA, 2mg/ml BSA), and the homogenates were centrifuged at 500 × g. Subsequent centrifugation allowed for separation and purification of the subsarcolemmal mitochondria. The concentration of mitochondrial protein was measured by the Lowry method using bovine serum albumin as a standard.
2.4 Metabolic and Biochemical Parameters
Free fatty acids and triglycerides were assessed in the plasma using commercially available kits (Wako, Richmond, VA). Mitochondrial proteins were separated by electrophoresis in 4–12% NuPage gels, transferred onto a nitrocellulose membrane, and incubated with specific antibodies to cyclophilin D and voltage-dependent anion channel (VDAC) (1:10,000 and 1:5000, respectively, both from Mitosciences, Eugene, OR). Fluorescence-conjugated secondary antibodies (IRDye 800, 1:10,000; LI-COR Bioscience) were used for incubation before the membranes were scanned with Odyssey® infrared imaging system (LI-COR Bioscience). The digitized image was analyzed with Odyssey® software.
2.5 Mitochondrial respiration
Mitochondrial oxygen consumption was measured using a Clark-type oxygen electrode (Qubit Systems, Ontario, Canada). Mitochondria (0.25 mg protein) were suspended in 0.5 ml solution consisting of 100 mM KCl, 50 mM MOPS, 5 mM KH2PO4 1 mM EGTA, and 0.5 mg fatty acid-free bovine serum albumin, at pH 7.4 and 37 °C. State 3 (ADP-stimulated) and state 4 (non-phosphorylating) respiration were measured with glutamate + malate (10 and 5 mM, respectively), pyruvate+malate (10 and 5 mM, respectively) and palmitoylcarnitine+malate (40 μM and 5 mM, respectively) to assess respiration through complex I–IV, while succinate+rotenone (10 mM and 7.5 μM, respectively), were used to assess respiration through complex II–IV of the ETC exclusively. State 4 respiration was also measured in the presence of oligomycin to inhibit the mitochondrial ATP synthase.
2.6 Ca2+ Retention Capacity
The capacity for mitochondrial to retain Ca2+, an established index of MPTP opening, was assessed in isolated mitochondrial as previously described in detail[24]. Briefly, mitochondria (0.5 mg protein) were suspended in respiration buffer in the absence of bovine serum albumin and the presence of 5μM EGTA, 1mM MgCl2, 10 mM glutamate and 5 mM malate. A 5 mM calcium solution was continuously infused at a rate of 5 μl/min for 20 min, and free Ca2+ was monitored by use of 0.7 μl Fura-6-F (0.07 mM) at 37 °C using a fluorescence spectrometer with excitation wavelengths for the free and calcium-bound forms of 340 and 380 nm, respectively, and emission wavelength of 550 nm. Opening of the MPTP was defined as the point where the extramitochondrial [Ca2+] reached twice baseline values[19].
For Series 2 a high throughput Ca2+ retention assay was developed to allow evaluation of the effects of mitochondrial respiratory substrates on the delay in MPTP induced by DHA supplementation. The assay was modified from Basso et al[4], and was performed using a 96-well fluorescence plate reader (FLUOstar Optima, BMG Labtech, Germany). Briefly, 25μg of mitochondria were resuspended in 200 μL of the same buffer used above, but with varying substrates; either glutamate + malate (10 and 5 mM, respectively), pyruvate+malate (10 and 5 mM, respectively), palmitoylcarnitine+malate (40 μM and 5 mM, respectively) or succinate (10 mM) with rotenone (7.5 μM). Extramitochondrial Ca2+ was monitored using 1μM Calcium Green 5N and fluorescence measured at 485 nm and 538 nm for excitation and emission wavelengths respectively. Automated additions of 25 nmoles Ca2+/mg mitochondrial protein were performed at regular 7 minute intervals and fluorescence measured every 17 seconds for 160 min at 37°C.
2.7. Ca2+-Induced Swelling
In Series 2 light scattering, an index of Ca2+-induced swelling was monitored using a 96 well spectrophotometric plate reader (SpectraMax, Molecular Devices, USA). Briefly, 25μg of mitochondria were resuspended in 200 μL the same buffer as used for the Ca2+ retention capacity assay. Baseline absorbance at 540nm was read at 7 second intervals for 2 min, then either 50 or 100 nmoles Ca2+ was rapidly added to the wells and the absorbance was read for 15 min at 37°C.
2.8 Membrane Lipid Composition
Cardiac phospholipid fatty acid composition was assessed in a subset of animals from Series 1 (n = 7–9/group) on isolated cardiac mitochondria homogenates by gas chromatography with a flame ionization detector according to a modification of the transesterification method as previously described [24]. CL composition was assessed on isolated cardiac mitochondria by electrospray ionization mass spectrometry using 1,1′,2,2′-tetramyristoyl CL as an internal standard as previously described (n = 9/group)[24,31,32].
2.9 Statistical Analyses
Mean values are presented ± SEM, and the level of significance was set at p < 0.05. Comparisons between groups was made with a one-way analysis of variance (ANOVA) and the Bonferoni post hoc test. Analysis of non-normal data sets was done with Kruskal-Wallis ANOVA on ranks and post hoc comparisons were made using Dunn’s method. A two-way repeated measure ANOVA with a Holm-Sidak post hoc test was performed when appropriate.
3. Results
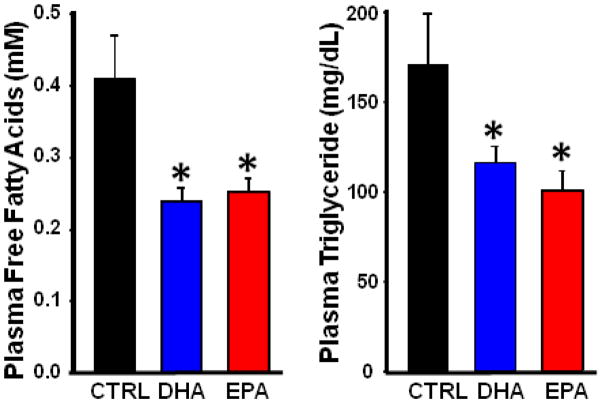
Body and cardiac masses were unaffected by diet (Table 2) and mitochondrial yield not was different among groups. EPA and DHA lowered plasma free fatty acid and triglyceride concentrations to a similar extent compared to CTRL (Figure 1), as previously shown in humans[12].
Table 2.
Body mass, organ mass, and mitochondrial yield.
| Control | DHA | EPA | |
|---|---|---|---|
| Terminal Body Mass (g) | 503 ± 15 | 501 ± 19 | 486 ± 29 |
| LV Mass (g) | 0.92 ± 0.04 | 0.94 ± 0.05 | 0.89 ± 0.07 |
| RV Mass (g) | 0.32 ± 0.01 | 0.30 ± 0.02 | 0.29 ± 0.03 |
| Biatrial Mass (g) | 0.09 ± 0.01 | 0.08 ± 0.01 | 0.09 ± 0.02 |
| Liver Mass (g) | 16.0 ± 1.1 | 14.8 ± 0.5 | 15.5 ± 1.5 |
| Mitochondrial Yield (mg mito protein/g wet wt) | 18.1 ± 3.0 | 18.1 ± 2.2 | 19.0 ± 1.5 |
Figure 1.
Serum triglyceride (Left) and free fatty acid concentration (Right). Data are means of n=6–8/group. *P < 0.05 vs. CTRL.
3.1 Mitochondrial Phospholipid Composition
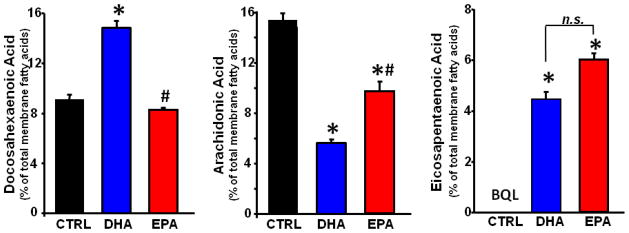
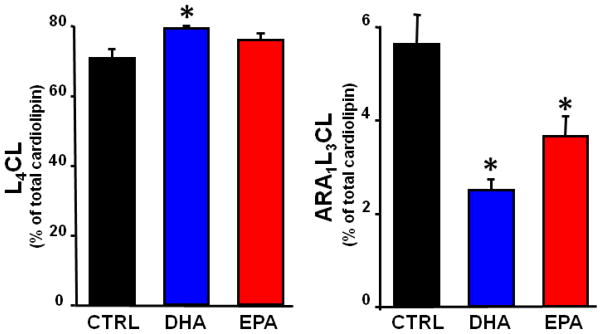
EPA was not detected in the CTRL group. The DHA diet significantly increased DHA and EPA, and decreased ARA in mitochondrial phospholipids. On the other hand, the EPA diet did not affect DHA levels, and only modestly decreased ARA levels, and increased EPA in a manner similar to treatment with DHA (Figure 2). Dihomogammalinolenic Acid (20:3n6), an intermediate in the synthesis of ARA from linoleic acid, was not detected in the CTRL group, but was increased to a similar extent by supplementation with either DHA or EPA (p<0.05)(Table 3). The composition of CL was altered by DHA, showing an increase in L4CL (Figure 3), the major and most critical species of CL[33]. In addition, there was a trend to increase total CL content in the DHA group (13.2±0.8 nmols/mg mito prot) compared to CTRL (10.7±0.8)(p<0.08), with no effect in the EPA treated animals (11.1±0.8). There was a decrease in CL species containing one ARA and three linoleic acid moieties (ARA1L3CL) in both DHA and EPA groups compared to CTRL (Figure 3).
Figure 2.
Cardiac mitochondrial phospholipid fatty acid composition expressed as percentage of total fatty acids. Data are means of n=7–8/group. *P < 0.001 vs. CTRL.
Table 3.
Mitochondrial phospholipid fatty acid composition expressed as molar percent of total phospholipid fatty acid.
| Fatty Acid | Control | DHA | EPA |
|---|---|---|---|
| C16:0 | 12.2 ± 0.8 | 13.3 ± 0.5 | 10.8 ± 0.6 |
| C16:1 | BQL | BQL | 4.5 ± 1.8 |
| C18:0 | 20.0 ± 0.4 | 18.9 ± 0.5 | 19.8 ± 0.8 |
| C18:1n9 | 14.3 ± 0.8 | 13.0 ± 1.0 | 11.8 ± 1.0 |
| C18:1n7 | 4.5 ± 0.2 | 3.7 ± 0.2 * | 4.2 ± 0.2 |
| C18:2n6 | 22.0 ± 0.9 | 23.1 ± 1.0 | 22.4 ± 0.8 |
| C20:3n6 | BQL | 1.6 ± 0.2 * | 1.1 ± 0.2 * |
| C20:4n6 | 14.6 ± 1.0 | 5.7 ± 0.2 * | 9.8 ± 0.8 *# |
| C20:5n3 | BQL | 4.4 ± 0.3 * | 6.0 ± 0.2 * |
| C22:6n3 | 9.1 ± 0.5 | 14.8 ± 0.6 * | 8.3 ± 0.2 # |
| Total ω-3 PUFA | 9.1 ± 0.5 | 19.2 ± 0.5 * | 14.3 ± 0.3 *# |
Data are expressed as percent of total mitochondrial membrane phospholipid content. BQL, below the quantifiable limit (limit of detection = 0.41% of total phospholipid fatty acids). Data are the mean ± SEM. n = 7–8/group.
p < 0.05 compared to the control group;
p < 0.001 compared to DHA
Figure 3.
Cardiac mitochondrial cardiolipin (CL) content for L4CL and L3AA1 (molecular weights of 1448 and1472, respectively) expressed as percentage of total CL. Data are means of n=8/group. *P < 0.001 vs. CTRL.
3.2 Mitochondrial Respiration
State 3 respiration with glutamate+malate, pyruvate+malate, palmitoylcarnitine+malate, or succinate+rotenone as substrates was unaffected by dietary treatment. DHA treatment decreased state 4 respiration by 30% and the increased RCR by 70% with pyruvate+malate as the substrate in both the absence and presence of oligomycin to eliminate any ATP turnover (p<0.05)(Table 4); treatment with EPA had no effect. Neither state 4 respiration or the respiratory control ratio (RCR) with glutamate+malate, palmitoylcarnitine+malate, or succinate+rotenone as substrates were affected by treatment (Table 4). The P:O ratio (ADP added:Oxygen consumed) was not different among groups with any of the substrates (Table 4), indicating no change in respiratory coupling.
Table 4.
Mitochondrial Respiration
| Control | DHA | EPA | |
|---|---|---|---|
| Glutamate + Malate | |||
| State 3 | 120.2 ± 10.0 | 115.2 ± 16.2 | 119.3 ± 13.4 |
| State 4 (− oligomycin) | 34.9 ± 2.1 | 35.3 ± 2.9 | 32.1 ± 4.8 |
| State 4 (+ oligomycin) | 20.4 ± 1.8 | 17.2 ± 2.0 | 19.1 ± 3.3 |
| RCR | 7.2 ± 1.1 | 7.0 ± 0. 9 | 7.0 ± 1.0 |
| P:O | 2.57 ± 0.20 | 2.37 ± 0.18 | 2.76 ± 0.23 |
| Pyruvate + Malate | |||
| State 3 | 226.6 ± 19.5 | 240.4 ± 28.7 | 221.2 ± 31.0 |
| State 4 (− oligomycin) | 78.1 ± 4.4 | 54.0 ± 3.2* | 63.5 ± 6.4 |
| State 4 (+ oligomycin) | 50.1 ± 4.1 | 34.8 ± 4.2* | 43.3 ± 5.2 |
| RCR | 4.5 ± 0.2 | 7.7 ± 1.0* | 5.1 ± 0.3 |
| P:O | 2.60 ± 0.17 | 2.48 ± 0.09 | 2.44 ± 0.30 |
| Palmityl-carnitine + Malate | |||
| State 3 | 265.2 ± 28.2 | 265.7 ± 34.4 | 243.7 ± 28.1 |
| State 4 (− oligomycin) | 59.7 ± 4.2 | 42.6 ± 1.9 | 59.4 ± 7.0 |
| State 4 (+ Oligomycin) | 32.0 ± 3.5 | 24.9 ± 2.6 | 29.7 ± 3. 8 |
| RCR | 8.8 ± 1.0 | 11.4 ± 1. 8 | 9.5 ± 1.8 |
| P:O | 2.46 ± 0.13 | 2.50 ± 0.12 | 2.57 ± 0.14 |
| Succinate + Rotenone | |||
| State 3 | 316. 8 ± 31.1 | 325.2 ± 19.6 | 307.2 ± 37.1 |
| State 4 (− oligomycin) | 105.7 ± 9.7 | 99.3 ± 7.0 | 102.1 ± 11.5 |
| State 4 (+ oligomycin) | 82.5 ± 9.5 | 84.7 ± 10.1 | 80.3 ± 11.7 |
| RCR | 4.1 ± 0.4 | 4.1 ± 0.4 | 4.1 ± 0.5 |
| P:O | 1.57 ± 0.09 | 1.48 ± 0.06 | 1.48 ± 0.08 |
Data are the mean ± SEM. n = 7 or 8/group. All Rates are expressed in ng atoms O·mg−1·min−1.
p<0.05 compared to the control group.
The RCR, defined as the ratio of State 3 to State 4 respiration rate, was calculated from the State 4 rate with oligomycin. The P:O ratio was calculated from measurements made without oligomycin.
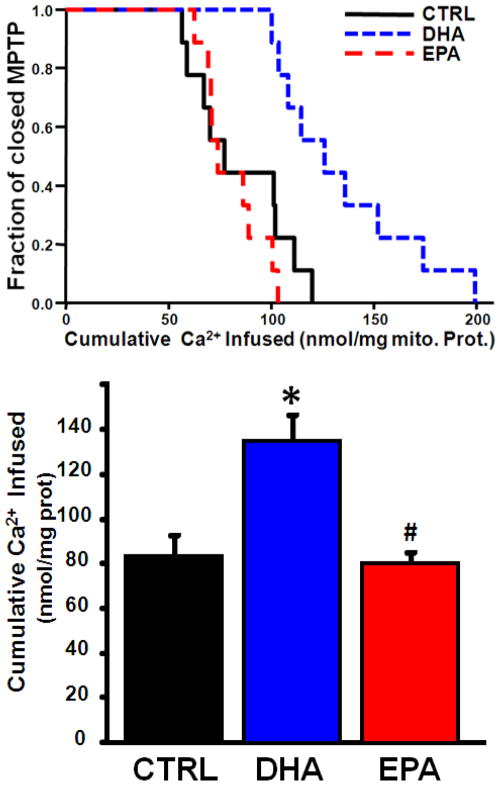
3.3 Ca2+ Retention Capacity
Compared to the CTRL and EPA treated groups, DHA significantly increased the Ca2+ retention capacity, an index of MPTP opening (Figure 4). As expected, addition of 100nM CsA lead to a significant increase in Ca2+ required to elicit MPTP in the CTRL group (83.0±8.7 nmol Ca2+/mg mito prot vs 144.8±20.0, p<0.05) and a similar effect in the EPA group (80.8±4.9 vs 130.6±12.8, p<0.05). There was no difference in the DHA group with the addition of CsA (134.9±11.4 vs 147.0±17.3, NS). Cyclophilin-D is a key regulatory component of the MPTP[3], however western blot analysis found no effect of any diet on cyclophilin-D protein expression (Table 5). The voltage-dependent anion channel (VDAC) has been proposed to play a role in regulation of the MPTP, however protein expression of VDAC1 and VDAC2 was similar among groups (Table 5).
Figure 4.
Effect of diet on the Ca2+ retention capacity. Upper Panel: The fraction of preparations with the MPTP open plotted as a function of the cumulative amount of Ca2+ added to the cuvette containing isolated mitochondria. Lower panel: Mean Ca2+ infused to initiate MPTP opening. Data are means of n=8–9/group.
Table 5.
Western blot results for VDAC1, VDAC2 and cyclophilin D in isolated mitochondria as assessed by densitometry. There were no differences among groups.
| CTRL | DHA | EPA | |
|---|---|---|---|
| VDAC 1 | 1.00 ± 0.13 | 1.03 ± 0.22 | 0.87 ± 0.16 |
| VDAC 2 | 1.00 ± 0.15 | 1.02 ± 0.20 | 0.94 ± 0.17 |
| Cyclophilin D | 1.00 ± 0.08 | 1.01 ± 0.10 | 1.00 ± 0.08 |
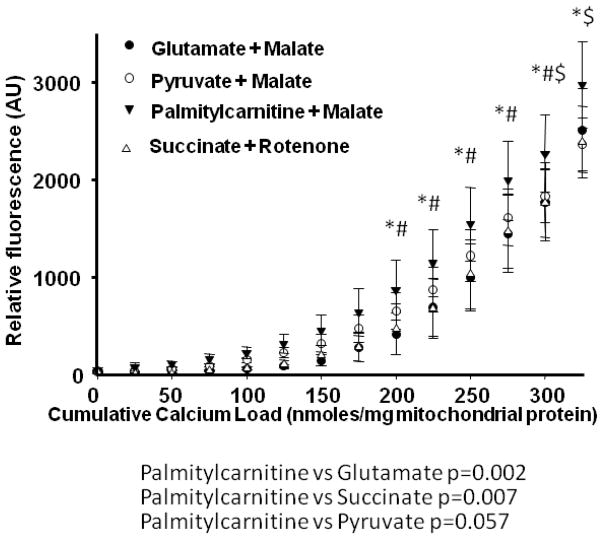
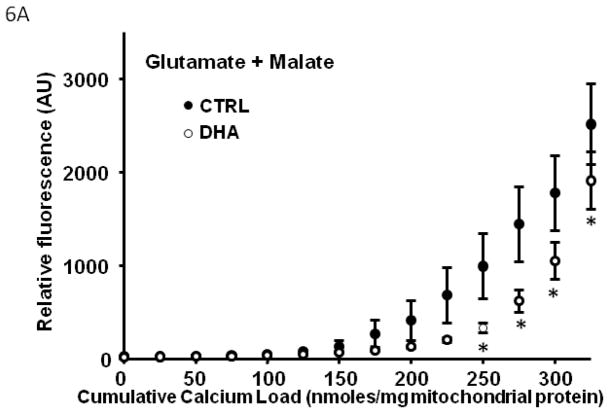
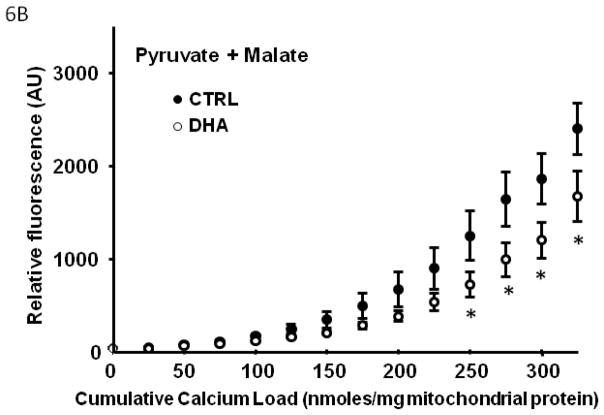
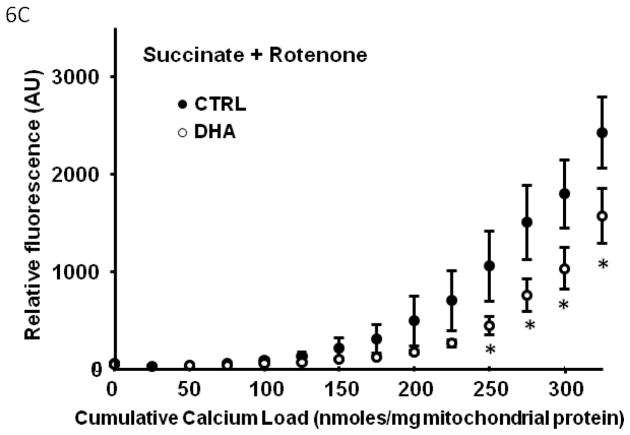
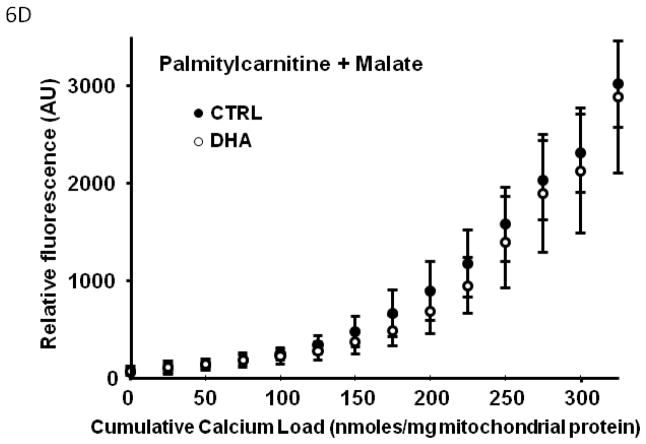
In Series 2, we used a high throughput assay to compare Ca2+ retention capacity of mitochondria from DHA supplemented hearts to CTRL, in the presence of different respiratory substrates. First, in the control diet, there was a decreased Ca2+ retention capacity with palmitoylcarnitine+malate when compared to glutamate+malate or succinate+rotenone (p<0.007; Figure 5), and a strong trend when compared to pyruvate+malate (p=0.057). Mitochondria from rats supplemented with DHA had significantly enhanced Ca2+ retention capacity compared to CTRL animals, as reflected in lower extramitochondrial Ca2+ for a given cumulative Ca2+ load with all substrates except palmitoylcarnitine+malate (Figure 6).
Figure 5.
Effect of different respiratory substrates on the Ca+ retention capacity of CTRL mitochondria. Data are means of n=10–11/group. * p<0.05, palmitoylcarnitine + malate vs glutamate + malate. $ p<0.05, palmitoylcarnitine + malate vs succinate + rotenone. # p<0.05, palmitoylcarnitine + malate vs pyruvate + malate.
Figure 6.
Effect of DHA on mitochondrial Ca2+ retention capacity in the presence of different respiratory substrates. Data are means of n=9–11/group. * p<0.05, CTRL vs DHA.
3.4 Mitochondrial Swelling
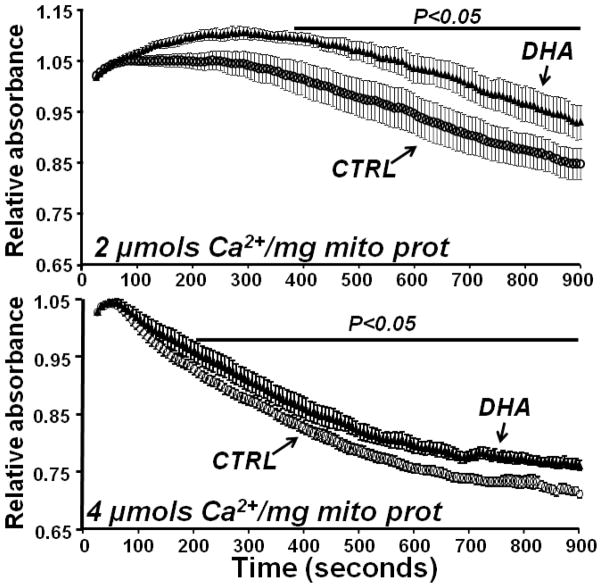
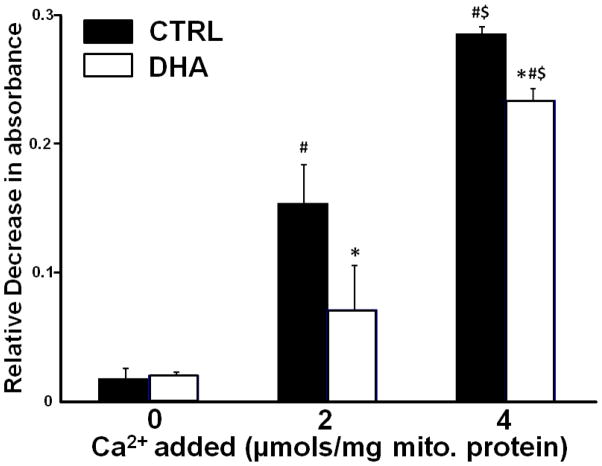
In the mitochondria from CTRL rats there was a dose-dependent decrease in absorbance at 540nm with the addition of Ca2+, which was significantly attenuated with DHA supplementation (Figure 7 and 8).
Figure 7.
Effect of DHA on mitochondrial Ca2+ induced swelling. Ca2+ was added at time zero. Vehicle treated wells had stable absorbance over the 15 minutes of observation (data not show). Data are means of n=9/group.
Figure 8.
Effects of DHA on Ca2+-induced mitochondrial swelling as assessed from the relative change in absorbance from 0 to 15 minutes. Data are means of n=9/group. * p<0.05, CTRL vs DHA within same Ca2+ dose. # p<0.05, no Ca2+ vs 2 μmoles Ca2+/mg mitochondrial protein. $ p<0.05, 2 μmoles Ca2+/mg mitochondrial protein vs 4 μmoles Ca2+/mg mitochondrial protein.
4. Discussion
The novel findings of this study are 1) DHA supplementation delayed MPTP opening in response to Ca2+ compared to animals fed the standard diet or supplemented with EPA, and 2) this effect is associated with a greater increase in total ω-3 PUFA in cardiac mitochondrial phospholipids with DHA supplementation compared to EPA, which corresponds with a greater reduction in the amount of ARA and an increase in L4CL. These differences between DHA and EPA occurred despite equivalent triglyceride lowering effects in the present investigation and in clinical studies[12,22], suggesting that the lipid lowering effects of DHA and EPA are independent of phospholipid remodeling, as previously proposed[16]. Thus the results of the present study show a novel and potentially important difference between DHA and EPA supplementation: DHA causes more extensive alterations in mitochondrial phospholipid fatty acid composition and delays Ca2+-induced MPTP opening, despite lipid lowering effects that are similar to EPA.
Opening of the MPTP contributes to cardiac pathology with acute stress associated with ischemia/reperfusion or with the chronic stress of heart failure, thus interventions that prevent MPTP opening may have profound clinical importance [17]. Long term treatment with pharmacological doses of DHA may prevent MPTP and cardiomyocyte apoptosis, and improve clinical outcome in ischemia heart disease and heart failure. We previously showed that dietary supplementation with a mixture of DHA+EPA (70:30) prevented left ventricular dysfunction and lowered cardiomyocyte apoptosis in rats subjected to chronic arterial pressure overload, suggesting that MPTP opening was prevented[9]. Additional studies are needed to assess the effect of treatment with DHA on MPTP, apoptosis and myocardial dysfunction and injury in hearts subjected to ischemia/reperfusion, and in models of heart failure.
The molecular mechanisms by which DHA supplementation delays Ca2+-induced MPTP opening are not clear. The structure of the MPTP and its interaction with membrane phospholipids environment are currently poorly understood [2,13]. ARA release fromrcell membranes has previously been implicated in MPTP opening[26,29], suggesting the association between the decline in ARA in mitochondrial phospholipids and delay in Ca2+-induced MPTP observed in the present investigation is causal. Inhibition of ARA release from membranes reduced myocardial infarct size following I/R, and this effect was lost by concurrent perfusion with free ARA[35]. Similarly, inhibition of ARA release in isolated renal mitochondria prevented MPTP opening and addition of free ARA restored MPTP[18]. Similar results are observed in liver mitochondria and cultured cells[26,29]. Clearly additional studies are needed to fully assess the role of depletion of ARA in possibly mediating the effects of DHA on MPTP opening in cardiac mitochondria. It is important to note that although EPA significantly decreased membrane ARA, it did not affect measures of Ca2+-induced MPTP opening. It is possible that a threshold content of ARA is necessary to elicit the differences observed with DHA feeding. Alternatively, the delay in calcium-induced MPTP might not be due to a decrease in membrane ARA, but rather attributable to an increase in membrane DHA, which is completely unaffected by EPA feeding.
Previous studies show that MPTP opening is delayed in cardiac mitochondria by the addition of CsA[13]. In the present investigation we observed that CsA delayed Ca2+-induced MPTP opening in rats fed either the standard diet or EPA, but not with DHA supplementation. This finding is in contrast to our recent observation that supplementation with DHA+EPA (70:30) delayed MPTP opening compared to a standard diet, with a further delay in pore opening in both groups with the addition of CsA. CsA inhibits MPTP by binding to cyclophilin-D[6,14], however in the present study we found no effect of DHA supplementation on mitochondrial cyclophilin-D content, suggesting the loss of the CsA effect on MPTP was not due to changes in cyclophilin-D. Furthermore, DHA supplementation had no effect on the Ca2+ retention capacity of mitochondria in the presence of palmitoylcarnitine+malate as respiratory substrates. However, this substrate also decreased the capacity of mitochondria to retain Ca2+ when compared to other substrates in animals fed the CTRL diet. Previous studies suggest that lipid mediators can induce pathological processes in mitochondria [7,10], and a high concentration of either palmitic or arachidonic acid can induce MPTP in isolated mitochondria [29]. It is possible that MPTP is sensitized and Ca2+ retention capacity is decreased by the relatively high concentration of palmitoylcarnititne (40 μM) required to support respiration in our preparation.
In summary, dietary supplementation with DHA but not EPA, profoundly altered mitochondrial phospholipid fatty acid composition by increasing DHA and depleting ARA, and delaying Ca2+-induced MPTP opening. This suggest novel and potentially important differences between DHA and EPA supplementation: DHA causes more extensive alterations in mitochondrial phospholipid fatty acid composition and delays Ca2+-induced MPTP opening, despite lipid lowering effects that are similar to EPA. These results suggest a novel pharmacological effect of DHA, and suggest that clinical treatment with DHA may exert greater cardioprotective benefit than treatment with standard fish oil formulations that are high in EPA.
Table 6.
Mitochondrial Cardiolipin (CL) Composition.
| CTRL | DHA | EPA | |
|---|---|---|---|
| CL Species (in mol% of total) | |||
| 1422 | 6.78 ± 0.38 | 6.24 ± 0.37 | 6.65 ± 0.37 |
| 1450 | 2.61 ± 1.13 | 0.44 ± 0.38 | 2.09 ± 1.20 |
| 1474 | 3.61 ± 0.24 | 2.98 ± 0.31 | 2.51 ± 0.27 |
| 1496 | 6.46 ± 1.01 | 5.24 ± 0.60 | 5.08 ± 0.61 |
| 1498 | 1.11 ± 0.54 | 0.67 ± 0.28 | 1.70 ± 0.68 |
| 1520 | 0.89 ± 0.13 | 0.76 ± 0.07 | 1.00 ± 0.11 |
| 1522 | 0.73 ± 0.11 | 0.91 ± 0.13 | 0.64 ± 0.11 |
| 1546 | 2.14 ± 0.41 | 1.73 ± 0.35 | 1.74 ± 0.29 |
| Total CL (in nmol/mg mito protein) | 10.72 ± 0.82 | 13.19 ± 0.85 | 11.10 ± 0.76 |
Acknowledgments
Funding. This work was supported by National Institutes of Health grants HL074237, HL091307 and HL101434.
The authors thank Kelly O’Connell and Bethany Brown for assistance with the biochemical measurements.
Abbreviations
- ARA
arachidonic acid
- CsA
cycolosporin A
- DHA
docosahexanoic acid
- EPA
eicosapentanoic acid
- MPTP
mitochondrial permeability transition pore
- PUFA
polyunsaturated fatty acid
- VDAC
voltage-dependant anion channel
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baines CP. The mitochondrial permeability transition pore and ischemia-reperfusion injury. Basic Res Cardiol. 2009;104:181–188. doi: 10.1007/s00395-009-0004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baines CP. The molecular composition of the mitochondrial permeability transition pore. J Mol Cell Cardiol. 2009;46:850–857. doi: 10.1016/j.yjmcc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 4.Basso E, Petronilli V, Forte MA, Bernardi P. Phosphate is essential for inhibition of the mitochondrial permeability transition pore by cyclosporin A and by cyclophilin D ablation. J Biol Chem. 2008;283:26307–26311. doi: 10.1074/jbc.C800132200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burelle Y, Khairallah M, Ascah A, Allen BG, Deschepper CF, Petrof BJ, des RC. Alterations in mitochondrial function as a harbinger of cardiomyopathy: lessons from the dystrophic heart. J Mol Cell Cardiol. 2010;48:310–321. doi: 10.1016/j.yjmcc.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarke SJ, McStay GP, Halestrap AP. Sanglifehrin A acts as a potent inhibitor of the mitochondrial permeability transition and reperfusion injury of the heart by binding to cyclophilin-D at a different site from cyclosporin A. J Biol Chem. 2002;277:34793–34799. doi: 10.1074/jbc.M202191200. [DOI] [PubMed] [Google Scholar]
- 7.Di PM, Cocco T, Lorusso M. Ceramide interaction with the respiratory chain of heart mitochondria. Biochemistry. 2000;39:6660–6668. doi: 10.1021/bi9924415. [DOI] [PubMed] [Google Scholar]
- 8.Duda MK, O’shea KM, Stanley WC. omega-3 polyunsaturated fatty acid supplementation for the treatment of heart failure: mechanisms and clinical potential. Cardiovasc Res. 2009;84:33–41. doi: 10.1093/cvr/cvp169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duda MK, O’shea KM, Tintinu A, Xu W, Khairallah RJ, Barrows BR, Chess DJ, Azimzadeh AM, Harris WS, Sharov VG, Sabbah HN, Stanley WC. Fish oil, but not flaxseed oil, decreases inflammation and prevents pressure overload-induced cardiac dysfunction. Cardiovasc Res. 2009;81:319–327. doi: 10.1093/cvr/cvn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia-Ruiz C, Colell A, Paris R, Fernandez-Checa JC. Direct interaction of GD3 ganglioside with mitochondria generates reactive oxygen species followed by mitochondrial permeability transition, cytochrome c release, and caspase activation. FASEB J. 2000;14:847–858. doi: 10.1096/fasebj.14.7.847. [DOI] [PubMed] [Google Scholar]
- 11.Gissi-Hf I. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1223–1230. doi: 10.1016/S0140-6736(08)61239-8. [DOI] [PubMed] [Google Scholar]
- 12.Grimsgaard S, Bonaa KH, Hansen JB, Nordoy A. Highly purified eicosapentaenoic acid and docosahexaenoic acid in humans have similar triacylglycerol-lowering effects but divergent effects on serum fatty acids. Am J Clin Nutr. 1997;66:649–659. doi: 10.1093/ajcn/66.3.649. [DOI] [PubMed] [Google Scholar]
- 13.Halestrap AP. What is the mitochondrial permeability transition pore? J Mol Cell Cardiol. 2009;46:821–831. doi: 10.1016/j.yjmcc.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 14.Halestrap AP, Pasdois P. The role of the mitochondrial permeability transition pore in heart disease. Biochim Biophys Acta. 2009 doi: 10.1016/j.bbabio.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 15.Igarashi M, Ma K, Chang L, Bell JM, Rapoport SI. Rat heart cannot synthesize docosahexaenoic acid from circulating alpha-linolenic acid because it lacks elongase-2. J Lipid Res. 2008;49:1735–1745. doi: 10.1194/jlr.M800093-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobson TA. Role of n-3 fatty acids in the treatment of hypertriglyceridemia and cardiovascular disease. Am J Clin Nutr. 2008;87:1981S–1990S. doi: 10.1093/ajcn/87.6.1981S. [DOI] [PubMed] [Google Scholar]
- 17.Javadov S, Karmazyn M. Mitochondrial permeability transition pore opening as an endpoint to initiate cell death and as a putative target for cardioprotection. Cell Physiol Biochem. 2007;20:1–22. doi: 10.1159/000103747. [DOI] [PubMed] [Google Scholar]
- 18.Kinsey GR, McHowat J, Patrick KS, Schnellmann RG. Role of Ca2+-independent phospholipase A2gamma in Ca2+-induced mitochondrial permeability transition. J Pharmacol Exp Ther. 2007;321:707–715. doi: 10.1124/jpet.107.119545. [DOI] [PubMed] [Google Scholar]
- 19.Kristian T, Gertsch J, Bates TE, Siesjo BK. Characteristics of the calcium-triggered mitochondrial permeability transition in nonsynaptic brain mitochondria: effect of cyclosporin A and ubiquinone O. J Neurochem. 2000;74:1999–2009. doi: 10.1046/j.1471-4159.2000.0741999.x. [DOI] [PubMed] [Google Scholar]
- 20.McMillin JB, Bick RJ, Benedict CR. Influence of dietary fish oil on mitochondrial function and response to ischemia. Am J Physiol. 1992;263:H1479–H1485. doi: 10.1152/ajpheart.1992.263.5.H1479. [DOI] [PubMed] [Google Scholar]
- 21.McMillin JB, Dowhan W. Cardiolipin and apoptosis. Biochim Biophys Acta. 2002;1585:97–107. doi: 10.1016/s1388-1981(02)00329-3. [DOI] [PubMed] [Google Scholar]
- 22.Mori TA, Woodman RJ. The independent effects of eicosapentaenoic acid and docosahexaenoic acid on cardiovascular risk factors in humans. Curr Opin Clin Nutr Metab Care. 2006;9:95–104. doi: 10.1097/01.mco.0000214566.67439.58. [DOI] [PubMed] [Google Scholar]
- 23.Nakayama H, Chen X, Baines CP, Klevitsky R, Zhang X, Zhang H, Jaleel N, Chua BH, Hewett TE, Robbins J, Houser SR, Molkentin JD. Ca2+-and mitochondrial-dependent cardiomyocyte necrosis as a primary mediator of heart failure. J Clin Invest. 2007;117:2431–2444. doi: 10.1172/JCI31060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’shea KM, Khairallah RJ, Sparagna GC, Xu W, Hecker PA, Robillard-Frayne I, des RC, Kristian T, Murphy RC, Fiskum G, Stanley WC. Dietary omega-3 fatty acids alter cardiac mitochondrial phospholipid composition and delay Ca2+-induced permeability transition. J Mol Cell Cardiol. 2009;47:819–827. doi: 10.1016/j.yjmcc.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ostrander DB, Sparagna GC, Amoscato AA, McMillin JB, Dowhan W. Decreased cardiolipin synthesis corresponds with cytochrome c release in palmitate-induced cardiomyocyte apoptosis. J Biol Chem. 2001;276:38061–38067. doi: 10.1074/jbc.M107067200. [DOI] [PubMed] [Google Scholar]
- 26.Penzo D, Petronilli V, Angelin A, Cusan C, Colonna R, Scorrano L, Pagano F, Prato M, Di LF, Bernardi P. Arachidonic acid released by phospholipase A(2) activation triggers Ca(2+)-dependent apoptosis through the mitochondrial pathway. J Biol Chem. 2004;279:25219–25225. doi: 10.1074/jbc.M310381200. [DOI] [PubMed] [Google Scholar]
- 27.Pepe S, McLennan PL. Cardiac membrane fatty acid composition modulates myocardial oxygen consumption and postischemic recovery of contractile function. Circulation. 2002;105:2303–2308. doi: 10.1161/01.cir.0000015604.88808.74. [DOI] [PubMed] [Google Scholar]
- 28.Pepe S, Tsuchiya N, Lakatta EG, Hansford RG. PUFA and aging modulate cardiac mitochondrial membrane lipid composition and Ca2+ activation of PDH. Am J Physiol. 1999;276:H149–H158. doi: 10.1152/ajpheart.1999.276.1.H149. [DOI] [PubMed] [Google Scholar]
- 29.Scorrano L, Penzo D, Petronilli V, Pagano F, Bernardi P. Arachidonic acid causes cell death through the mitochondrial permeability transition. Implications for tumor necrosis factor-alpha aopototic signaling. J Biol Chem. 2001;276:12035–12040. doi: 10.1074/jbc.M010603200. [DOI] [PubMed] [Google Scholar]
- 30.Sergiel JP, Martine L, Raederstorff D, Grynberg A, Demaison L. Individual effects of dietary EPA and DHA on the functioning of the isolated working rat heart. Can J Physiol Pharmacol. 1998;76:728–736. doi: 10.1139/cjpp-76-7-8-728. [DOI] [PubMed] [Google Scholar]
- 31.Shah KB, Duda MK, O’shea KM, Sparagna GC, Chess DJ, Khairallah RJ, Robillard-Frayne I, Xu W, Murphy RC, des RC, Stanley WC. The cardioprotective effects of fish oil during pressure overload are blocked by high fat intake: role of cardiac phospholipid remodeling. Hypertension. 2009;54:605–611. doi: 10.1161/HYPERTENSIONAHA.109.135806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sparagna GC, Johnson CA, McCune SA, Moore RL, Murphy RC. Quantitation of cardiolipin molecular species in spontaneously hypertensive heart failure rats using electrospray ionization mass spectrometry. J Lipid Res. 2005;46:1196–1204. doi: 10.1194/jlr.M500031-JLR200. [DOI] [PubMed] [Google Scholar]
- 33.Sparagna GC, Lesnefsky EJ. Cardiolipin remodeling in the heart. J Cardiovasc Pharmacol. 2009;53:290–301. doi: 10.1097/FJC.0b013e31819b5461. [DOI] [PubMed] [Google Scholar]
- 34.Weiss JN, Korge P, Honda HM, Ping P. Role of the mitochondrial permeability transition in myocardial disease. Circ Res. 2003;93:292–301. doi: 10.1161/01.RES.0000087542.26971.D4. [DOI] [PubMed] [Google Scholar]
- 35.Williams SD, Gottlieb RA. Inhibition of mitochondrial calcium-independent phospholipase A2 (iPLA2) attenuates mitochondrial phospholipid loss and is cardioprotective. Biochem J. 2002;362:23–32. doi: 10.1042/0264-6021:3620023. [DOI] [PMC free article] [PubMed] [Google Scholar]